Abstract
Vascular calcification is present in arterial vessels used for dialysis vascular access creation prior to surgical creation. Calcification in the veins used to create a new vascular access has not previously been documented. The objective of this study was to describe the prevalence of venous calcification in samples collected at the time of vascular access creation.
67 vein samples were studied. A von Kossa stain was performed to quantify calcification. A semi-quantitative scoring system from 0–4+ was used to quantify the percentage positive area for calcification as a fraction of total area (0=0; 1+ = 1–10%; 2+ =11–25%; 3+ = 26–50%; 4+ >50% positive).
22/67(33%) samples showed evidence of venous calcification. Histologic examination showed varying degrees of calcification within each cell layer. Among the subset of patients with calcification, 4/22 (18%), 19/22 (86%), 22/22 (100%), and 7/22 (32%) had calcification present within the endothelium, intima, media, and adventitia, respectively. The mean semi-quantitative scores of the 22 samples with calcification were 0.18±0.08, 1.2±0.14, 1.6±0.13, and 0.36±0.12 for the endothelium, intima, media, and adventitia, respectively.
Our results demonstrate that vascular calcification is present within veins used to create new dialysis vascular access, and located predominately within the neointimal and medial layers.
Keywords: Vascular Calcification, Hemodialysis Vascular Access, Vascular Access Stenosis
Introduction
Aggressive venous neointimal hyperplasia is the most common histologic lesion seen in arteriovenous fistula (AVF) and graft (AVG) failure 1–6. While the majority of the research in vascular access dysfunction has focused on the mechanisms of neointimal hyperplasia development after AV access creation, recently, our group and others have reported that the health of the vessel (artery and vein) may play an important role in the short and long-term outcomes of AVFs 7–9. Progressive arterial calcification due to uremia plays an important role in accelerated cardiovascular mortality in end stage renal disease patients compared to the general population10–12. Emerging evidence has shown that vascular calcification in arteries used to create new vascular accesses may play an important role in vascular access failure13, 14. However, venous stenosis is the most common lesion in vascular access dysfunction and there have been no previous publications describing presence of venous calcification in the vessels used to create a new vascular access. Thus, the main objective of this study was to describe the prevalence of venous calcification and its distribution within the venous wall, in samples collected at the time of dialysis vascular access creation.
Methods
Study Population
67 patients requiring new vascular access placement, from 2008–2010, were recruited in our vascular access clinic for evaluation into this study. Prior to each evaluation a pre-operative ultrasound mapping of both extremities, or angiography, was performed to evaluate vessel diameters and stenosis. Patients were consented in our vascular access clinic, during access placement evaluation, to obtain venous tissue specimens at the time of vascular access surgery. Demographic data was collected at the time of recruitment. Data pertaining to the site of access placement and specific vessel obtained was collected at the time of surgery. Institutional Review Board approval was obtained to conduct this study.
Specimen Collection and Processing
Venous tissue specimens were collected at the time of surgical creation of vascular access. During the surgery, an approximately 8–10mm circumferential segment of vein was removed near the planned anastomosis site in each patient and immediately fixed in formalin.
Each venous tissue sample, fixed in formalin, was embedded and cut into 2–3 tissue blocks of 3–4 mm thickness using previously described techniques 2, 7. Each piece was paraffin-embedded and then sliced into 4µm sections for histological and histochemistry studies.
Histochemistry Studies
Sections from each tissue block were evaluated for the presence of calcification with von Kossa staining using standard techniques. In brief, deparaffinized slides were placed in 5% silver nitrate for 10 to 60 minutes with exposure to an ultraviolet light or 100 watt incandescent desk lamp, then rinsed and placed in 5% sodium thiosulfate for 2 to 3 minutes. Finally, the slides were rinsed and stained with a nuclear fast red stain. A brown or black color on the specimen indicated a positive stain.
The degree of calcification, based on the intensity of the Von Kossa stain, was scored by an independent investigator blinded to the identity of the tissues. A semi-quantitative scoring system from 0–4+ was used to quantify the percentage positive area for calcification as a fraction of total area (0=0; 1+ = 1–10% positive; 2+ =11–25% positive; 3+ = 26–50% positive; 4+ >50% positive) for each cell layer, endothelium, intima, media, and adventitia. Mean values for calcification for all samples were calculated.
Statistics
The distribution of study variables was characterized according to means ± S.E. and proportions. All statistical analyses were performed using JMP® 8.0 (Cary, NC) statistical software package.
Results
In total, 67 vein specimens were collected for this study. 22/67 (33%) samples showed evidence of venous calcification (Figure 1). Histologic examination showed varying degrees of calcification within each cell layer. Among the subset of vein samples with calcification (n=22), 4/22 (18%), 19/22 (86%), 22/22 (100%), and 7/22 (32%) had calcification present within the endothelium, intima, media, and adventitia, respectively (Table 1). The mean semiquantitative scores of the 22 samples with calcification were 0.18±0.08, 1.2±0.14, 1.6±0.13, and 0.36±0.12 for the endothelium, intima, media, and adventitia, respectively (Table 1). The average calcification scores were significantly higher in the media compared to the intima (p=0.0064).
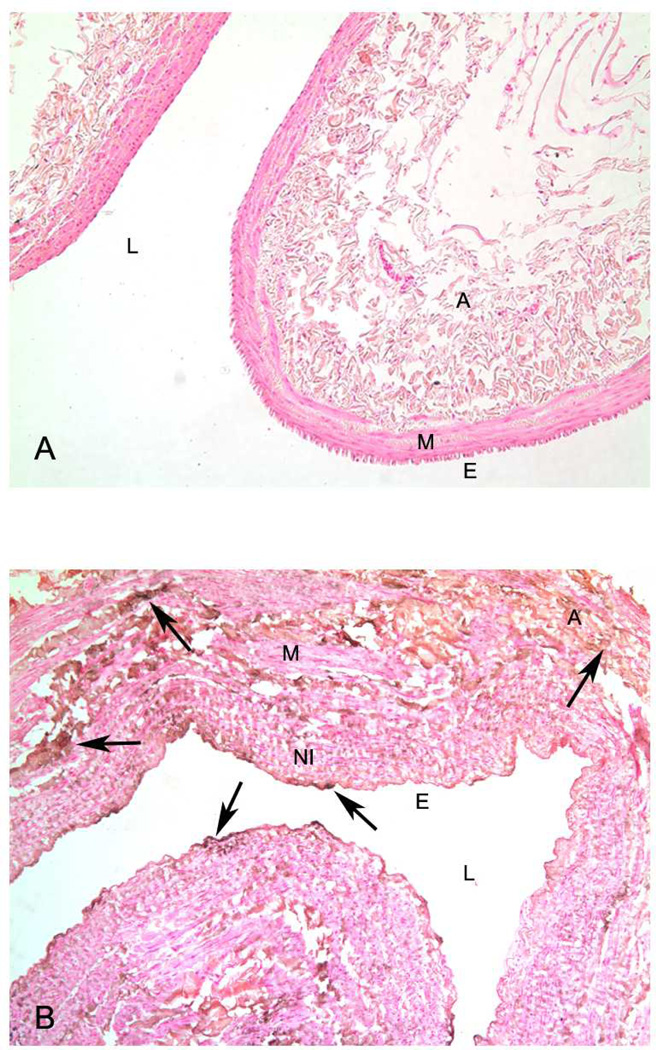
Figure 1.
Panel A. Representative histological section of normal vein. Note thin media (M) and no neointima present. There is also no calcification present after Von Kossa stain. Panel B. Representative histological section of vein obtained at the time of fistula creation with Von Kossa stain. Note presence of calcification (brown and black stain) in endothelium (E), neointima (NI), media (M), and adventitia (A).
Table 1.
Proportion of Vascular Calcification and Mean Semiquantitative Score by Cell Layer Among Patient Samples with Calcification
| Endothelium | Intima | Media | Adventitia | |
|---|---|---|---|---|
| Proportion of Vascular Calcification | 4/22(18%) | 19/22 (86%) | 22/22 (100%) | 7/22 (32%) |
| Semiquantitative Score | 0.18±0.08 | 1.2±0.14 | 1.6±0.13 | 0.36±0.12 |
Discussion
Aggressive venous neointimal hyperplasia is the most common histologic lesion seen in AVF and AVG dysfunction2, 3, 5, 6, 15. However, our group has recently reported that severe preexisting vascular changes is present in the veins used to create new vascular access prior to surgery7. Specifically, in a small group of patients, we found that venous neointimal hyperplasia is present in the majority of veins used to create new vascular accesses and may be associated with lower AVF and AVG maturation7. Furthermore, Wasse et. al have also recently reported that pre-existing venous neointimal hyperplasia is present in the majority of end stage renal disease and chronic kidney disease patients receiving a new vascular access16. Given the potential importance pre-existing vascular changes may play in access maturation, specifically in AVFs, understanding and elucidating new mechanisms that are associated with vascular vasodilation will play a key role in development of novel therapies for vascular access stenosis. In this descriptive study, we focused on reporting the prevalence of venous calcification and the location of the calcification within the vein.
Cardiovascular mortality, secondary to accelerated intimal and medial calcification, in ESRD patients is dramatically increased when compared to the general population13. Arterial vascular calcification likely not only contributes to the high prevalence of cardiovascular disease in ESRD patients, but also may play a major role in vascular access dysfunction. A recent study by Wang et. al has shown that one-third of uremic radial arteries collected at the time of AVF creation had arterial calcification predominately in the media of the vessel14. While the artery plays an important role in AVF maturation, the most common lesion seen in AVF nonmaturation is venous stenosis in the juxta-anastomotic region17. In our study we report that one-third of veins used to create new vascular accesses already have calcification present at the time of surgery. Furthermore, similar to Wang et al in arteries14, we have found that the predominant area of calcification in the vein is also within the media.
What is the clinical significance of calcification in veins in the context of vascular access stenosis and maturation prior to access creation? In arteries, medial calcification leads to increased stiffness and decreased vascular reactivity18, 19. However, veins respond to vascular injury differently from arteries. At a molecular and physiologic level veins produce less nitric oxide and prostacyclin compared to arteries1, 20. Thus, pre-existing venous calcification may further reduce the vein’s capacity to vasodilate after AV access creation, an essential process in AVF maturation. Furthermore, the mechanisms that lead to development of venous calcification are currently unknown. In arteries it has been hypothesized that vascular smooth muscle cells derive from a common mesenchymal precursor cell, and vascular smooth muscle cells, under different pathologic conditions, transform into osteoblast-like cells, which then play a critical role in recruitment and production of mediators that lead to calcium deposition14, 21, 22. In veins, it is likely a similar process occurs, and determining the origins of these osteoblast-like cells and whether they migrate from the adventitia, perhaps in a similar fashion as neointimal cells1, will play a critical role in understanding AVF maturation and developing future therapies to treat AVF non-maturation.
Conclusions
We have shown that vascular calcification is present within veins used to create new dialysis vascular access, and predominately located within the intima and media layers. Future studies are needed to evaluate the role of pre-existing venous calcification and AVF maturation and mechanisms of venous calcification in patients with advanced CKD.
Acknowledgements
Dr. Lee is supported by NIH 5K23DK083528-03 and National Kidney Foundation Franklin McDonald/Fresenius Medical Care Young Investigator Clinical Research Award. Dr. Roy-Chaudhury is supported by NIH 5U01-DK82218, NIH 5U01-DK82218S (ARRA), NIH 5R01-EB004527, NIH 1R21-DK089280-01, a VA Merit Review, two University of Cincinnati NIH/NCCR UL1RR026314 CTSA grants, and industry grants.
Footnotes
Disclosure
Dr. Lee is a consultant for Proteon Therapeutics. Dr. Roy-Chaudhury is on the advisory board/consultant for Pervasis Therapeutics, Inc., Proteon Therapeutics, WL Gore, Bioconnect Systems, Philometron and NanoVasc and receives research support from BioConnect Systems, Shire, Proteon Therapeutics, and WL Gore. These funding sources had no involvement in the design or execution of this study.
References
- 1.Roy-Chaudhury P, Sukhatme VP, Cheung AK. Hemodialysis vascular access dysfunction: a cellular and molecular viewpoint. J Am Soc Nephrol. 2006;17:1112–1127. doi: 10.1681/ASN.2005050615. [DOI] [PubMed] [Google Scholar]
- 2.Roy-Chaudhury P, Arend L, Zhang J, Krishnamoorthy M, Wang Y, Banerjee R, Samaha A, Munda R. Neointimal hyperplasia in early arteriovenous fistula failure. Am J Kidney Dis. 2007;50:782–790. doi: 10.1053/j.ajkd.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 3.Roy-Chaudhury P, Kelly BS, Miller MA, Reaves A, Armstrong J, Nanayakkara N, Heffelfinger SC. Venous neointimal hyperplasia in polytetrafluoroethylene dialysis grafts. Kidney Int. 2001;59:2325–2334. doi: 10.1046/j.1523-1755.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Terry CM, Blumenthal DK, Kuji T, Masaki T, Kwan BC, Zhuplatov I, Leypoldt JK, Cheung AK. Cellular and morphological changes during neointimal hyperplasia development in a porcine arteriovenous graft model. Nephrol Dial Transplant. 2007;22:3139–3146. doi: 10.1093/ndt/gfm415. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Krishnamoorthy M, Banerjee R, Zhang J, Rudich S, Holland C, Arend L, Roy-Chaudhury P. Venous stenosis in a pig arteriovenous fistula model--anatomy, mechanisms and cellular phenotypes. Nephrol Dial Transplant. 2008;23:525–533. doi: 10.1093/ndt/gfm547. [DOI] [PubMed] [Google Scholar]
- 6.Kelly BS, Heffelfinger SC, Whiting JF, Miller MA, Reaves A, Armstrong J, Narayana A, Roy-Chaudhury P. Aggressive venous neointimal hyperplasia in a pig model of arteriovenous graft stenosis. Kidney Int. 2002;62:2272–2280. doi: 10.1046/j.1523-1755.2002.00684.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee T, Chauhan V, Krishnamoorthy M, Wang Y, Arend L, Mistry MJ, El-Khatib M, Banerjee R, Munda R, Roy-Chaudhury P. Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011 doi: 10.1093/ndt/gfq733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YO, Choi YJ, Kim JI, Kim YS, Kim BS, Park CW, Song HC, Yoon SA, Chang YS, Bang BK. The impact of intima-media thickness of radial artery on early failure of radiocephalic arteriovenous fistula in hemodialysis patients. J Korean Med Sci. 2006;21:284–289. doi: 10.3346/jkms.2006.21.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YO, Song HC, Yoon SA, Yang CW, Kim NI, Choi YJ, Lee EJ, Kim WY, Chang YS, Bang BK. Preexisting intimal hyperplasia of radial artery is associated with early failure of radiocephalic arteriovenous fistula in hemodialysis patients. Am J Kidney Dis. 2003;41:422–428. doi: 10.1053/ajkd.2003.50051. [DOI] [PubMed] [Google Scholar]
- 10.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2003;18:1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 11.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. The New England journal of medicine. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 12.Adragao T, Pires A, Lucas C, Birne R, Magalhaes L, Goncalves M, Negrao AP. A simple vascular calcification score predicts cardiovascular risk in haemodialysis patients. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2004;19:1480–1488. doi: 10.1093/ndt/gfh217. [DOI] [PubMed] [Google Scholar]
- 13.Schlieper G, Kruger T, Djuric Z, Damjanovic T, Markovic N, Schurgers LJ, Brandenburg VM, Westenfeld R, Dimkovic S, Ketteler M, Grootendorst DC, Dekker FW, Floege J, Dimkovic N. Vascular access calcification predicts mortality in hemodialysis patients. Kidney international. 2008;74:1582–1587. doi: 10.1038/ki.2008.458. [DOI] [PubMed] [Google Scholar]
- 14.Wang N, Yang J, Yu X, Hu J, Xing C, Ju X, Shen X, Qian J, Zhao X, Wang X. Radial artery calcification in end-stage renal disease patients is associated with deposition of osteopontin and diminished expression of alpha-smooth muscle actin. Nephrology. 2008;13:367–375. doi: 10.1111/j.1440-1797.2008.00941.x. [DOI] [PubMed] [Google Scholar]
- 15.Roy-Chaudhury P, Wang Y, Krishnamoorthy M, Zhang J, Banerjee R, Munda R, Heffelfinger S, Arend L. Cellular phenotypes in human stenotic lesions from haemodialysis vascular access. Nephrol Dial Transplant. 2009;24:2786–2791. doi: 10.1093/ndt/gfn708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wasse H, Rivera AA, Huang R, Martinson DE, Long Q, McKinnon W, Naqvi N, Husain A. Increased Plasma Chymase Concentration and Mast Cell Chymase Expression in Venous Neointimal Lesions of Patients with CKD and ESRD. Seminars in dialysis. 2011 doi: 10.1111/j.1525-139X.2011.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beathard GA, Arnold P, Jackson J, Litchfield T. Aggressive treatment of early fistula failure. Kidney Int. 2003;64:1487–1494. doi: 10.1046/j.1523-1755.2003.00210.x. [DOI] [PubMed] [Google Scholar]
- 18.Nitta K, Akiba T, Uchida K, Otsubo S, Otsubo Y, Takei T, Ogawa T, Yumura W, Kabaya T, Nihei H. Left ventricular hypertrophy is associated with arterial stiffness and vascular calcification in hemodialysis patients. Hypertension research : official journal of the Japanese Society of Hypertension. 2004;27:47–52. doi: 10.1291/hypres.27.47. [DOI] [PubMed] [Google Scholar]
- 19.London GM, Marchais SJ, Guerin AP, Metivier F. Arteriosclerosis, vascular calcifications and cardiovascular disease in uremia. Current opinion in nephrology and hypertension. 2005;14:525–531. doi: 10.1097/01.mnh.0000168336.67499.c0. [DOI] [PubMed] [Google Scholar]
- 20.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 21.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circulation research. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. Journal of the American Society of Nephrology : JASN. 2004;15:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]