Abstract
Object
The ability to predict seizure occurrence is extremely important to trigger abortive therapies and to warn patients and their caregivers. Optical imaging of hemodynamic parameters such as blood flow, blood volume and tissue and hemoglobin oxygenation has already been shown to successfully localize epileptic events with high spatial and temporal resolution. The ability to actually predict seizure occurrence using hemodynamic parameters is less well explored.
Method
In this paper, we will critically review the literature on data from neocortical epilepsy using optical imaging and discus these pre-ictal hemodynamic changes findings and its application in neurosurgery.
Result
Recent optical mapping studies have demonstrated pre-ictal hemodynamic changes in both human and animal neocortex.
Conclusion
Optical measurements of blood flow and oxygenation may become increasingly important for predicting as well as localizing epileptic events. The ability to successfully predict ictal onsets may be useful to trigger closed-loop abortive therapies.
Keywords: epilepsy, seizure, optical imaging, neurosurgery
Introduction
Hemodynamic signals derived from perfusion and oximetry significantly correlate spatially with brain function. It is the fundamental basis of functional neuroimaging. In recent years, there has been great interest in using a variety of brain mapping techniques that measure those hemodynamic responses to assist in clinical diagnosis and management of neurological disorders. In particular, seizures have been shown to elicit a large increase in metabolism, utilization of oxygen and increases in blood flow and blood volume in epileptic cortex. More recently, it has become apparent that these hemodynamic changes may actually precede seizure onset and be useful at seizure prediction. Here, we will focus on reviewing the literature on functional optical imaging methods and explore recent data on the timing and significance of anticipatory pre-ictal hemodynamic changes and potential their application in neurosurgery.
Prediction of seizure onset
Epilepsy is a clinical term referring to a disease that affects between 1 and 2% of the population of the United States involving recurrent seizures. Seizures can be sudden and occur without warning, which can cause significant injuries. The possibility of identifying events, whether they are behavioral, electrographic or hemodynamic, that reliably occur before epileptic seizures would have a dramatic impact on our ability to warn patients and their families of an upcoming event, thereby giving patients the ability to remove themselves from harm’s way. Attempts have been made to forecast epileptic seizures using a variety of methodologies such as electroencephalogram (EEG)52, functional magnetic resonance imaging (fMRI)8,12, single-photon emission computer tomography (SPECT)42, among others30. Such pre-ictal signals could also provide information for ‘closed-loop’ abortive therapies such as cortical stimulation24, focal drug perfusion11,45, cooling7, and optical inhibition 25,49,53. Additionally, seizure prediction mechanisms can offer insights into epileptogenesis 16.
Traditional analysis of EEG signals has not shown any obvious or consistent pre-ictal changes 23,34. Complex non-linear mathematic algorithms for electrographic data can be used to predict seizure with increasing reliability 13,26. Recently, our laboratory reported a ~20s focal hemodynamic change before seizure onset in human lesional neocortical case. In addition, we have shown pre-ictal vessel constriction as early as 5 s prior to seizure onset in an animal model using a two-photon microscope57,58.
Intrinsic optical mapping of neurovascular coupling during epilepsy
Neurovascular coupling concerns the relationship between neuronal activity, metabolism, tissue oxygenation, and blood flow. Adequate coupling is critical to supply the energy demands of the brain during normal physiological function as well as pathological conditions. Seizures create a large focal increase in metabolism and result in a dramatic increase in cerebral blood flow (CBF) to the ictal focus to provide adequate oxygenation. Whether or not CBF is adequate to meet the demands of an epileptic event has been a long-standing debate.
Optical imaging of intrinsic signals (ORIS) is a technique for measuring hemodynamic changes in the brain, based on enhanced light absorption of active neural tissue, which is caused by focal increases in cerebral blood flow (CBF), deoxygenation of hemoglobin and enhanced scattering of light15,29,33. At wavelengths such as 570 nm, an isosbestic wavelength of hemoglobin, ORIS provides a direct measure of total hemoglobin (Hbt). Hbt is equivalent to cerebral blood volume (CBV) if the hematocrit remains constant, and CBV is proportional to CBF. At 610 nm, deoxygenated hemoglobin (Hbr) absorbs light more strongly than oxygenated hemoglobin (HbO2) and it is possible to directly quantify both Hbr and Hbt with the appropriate calculation41. At 800 nm, or near infrared wavelengths, the optical signal is largely derived from light scattering related to cell swelling as well as intra- and extracellular fluid shifts, which provide an indirect representation of neuronal activity, less influenced by the changes in cerebral blood volume and hemoglobin oxygenation that dominate the intrinsic signal at lower wavelengths.
Precise localization of neocortical epileptic foci is very important for the neurosurgeon to identify and remove the seizure onset zone in order to achieve the best surgical outcome. Several studies have shown that ORIS can be used to map the onset and spread of epileptic events via their hemodynamic sequellae with very high spatial and temporal resolution, as well as high spatial sampling [25, 34–40]. Epileptic events initiate a large focal increase in metabolism and cerebral blood flow (CBF) at the seizure focus. In contrast, decreases in CBF have been demonstrated surrounding the focus, the etiology of which is unknown. The relationship between these events and neuronal activity and metabolism are also unknown. Studies using techniques with limited spatial and temporal resolution such as fMRI, PET, SPECT and autoradiography have shown that the relative increase in CBF more than meets the increase in metabolism leading to an increase in blood oxygenation. But studies using higher temporal resolution techniques such as near infra-red spectroscopy (NIRS) and ORIS showed that CBF is inadequate to meet the metabolic demands of the epileptic tissue leading to a decrease in both tissue and hemoglobin oxygenation (see review40 )
Optical signals and seizure prediction
Mapping pre-ictal changes in human epilepsy
Optical imaging has been intraoperatively done to map both human epileptic focus and human brain function activity during surgery3,18,19,35,39,46. Beyond localizing human physiological and pathological activity, it was also used to predict the pre-ictal changes in human epilepsy.
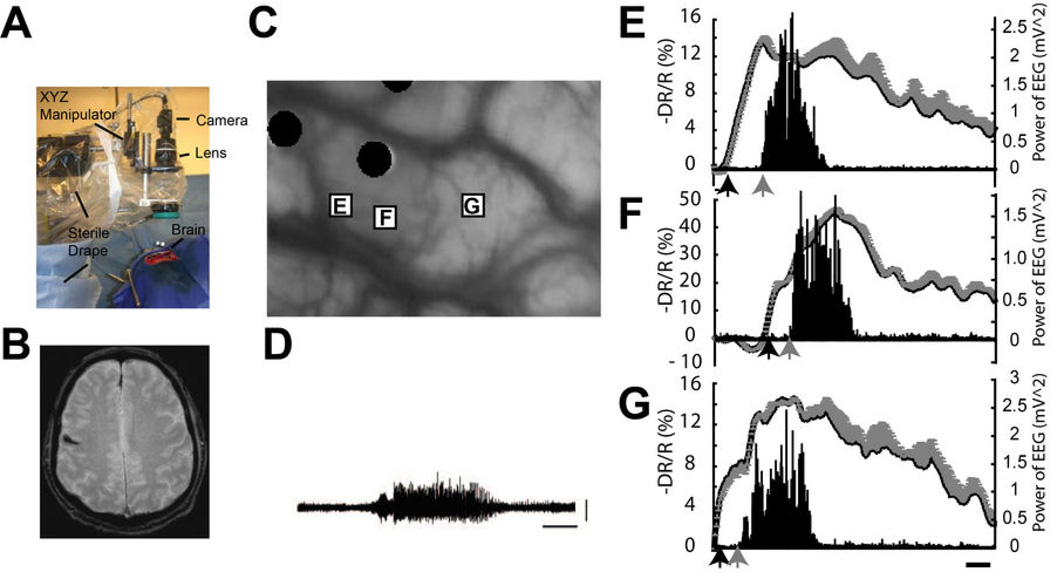
The idea of pre-ictal vascular reactivity predicting the seizure onset was proposed (mistakenly at the time) as early as 1933 by Gibbs17. More recently, studies have found increases in cerebral perfusion 20 min before focal and generalized spike-and-wave events using transcranial Doppler10. Fortuitously, we recorded ORIS from human cortex intraoperatively in a patient with recurrent focal seizures arising from a cavernous malformation58. We found that focal changes in cerebrovascular hemodynamics preceded the seizure onset by ~20 s, and occurred focally over the known location of the lesion and the seizure onsets (Fig. 1). Three spontaneous seizures were successfully recorded, two at 610 nm and one at 570 nm, providing data on Hbr and CBV. Each seizure was accompanied by a dramatic, focal change in the intrinsic signal. At 610 nm, a significant increase light reflectance began 23.74 ± 8.67 s prior to the electrographic onset of the seizure (Fig. 1 D and F). The spatial maps of the two seizures recorded at 610 nm were remarkably similar. At 570 nm, a significant decrease in light reflectance began 15.0 s prior to the electrographic onset of the seizure (Fig. 1 E), again restricted to the known epileptic gyrus consistent with a focal drop in CBV. Prior to the onset of the seizures, the signal inverted to a significant increase in light reflectance (increase in CBV), which reached maximum amplitude of 46.2%, peaking 58.1 s after the onset of the seizure. Again the signal was restricted to the known epileptic gyrus consistent with a focal drop in CBV. Prior to the onset of the seizures, the signal inverted to a significant increase in light reflectance (increase in CBV), which reached maximum amplitude of 46.2%, peaking 58.1 s after the onset of the seizure. This pre-ictal finding from spontaneous human epilepsy suggests that optical measurements may be useful to predict the seizure onset and location prior to any electrographic changes.
Figure 1.
Pre-ictal changes of optical signal in human epilepsy. (A) A custom camera holder with x–y–z gross and fine manipulators suspends a camera, lens, and ring illuminator, draped in sterile plastic, over the exposed human cortex.
(B) Gradient echo axial MRI scan demonstrates a small cavernous malformation the right motor strip. (C) Surface of the brain under glass footplate. The black circles highlight the location of the recording electrodes. The rectangles demonstrate three regions of interest (ROIs), which contained the pixel values with the most statistically significant changes for each of the three seizures. The label on the rectangle corresponds to the graphs within this figure. (D) ECoG recording of a typical seizure. Scale bars: 20 seconds and 1 millivolt. The time course of (E and F) oximetry and (G) perfusion related intrinsic optical signal calculated as -ΔR/R (%) during each seizure from each ROI in (C) is graphed along with the power of the ECoG. Error bars represent SD of pixel values from each ROI. The onset of statistically significant optical signal changes is indicated with a black arrow and the onset of significant change in the power of the ECoG is indicated with a gray arrow. (see detail in [18])
Optical imaging of pre-ictal changes in animal model
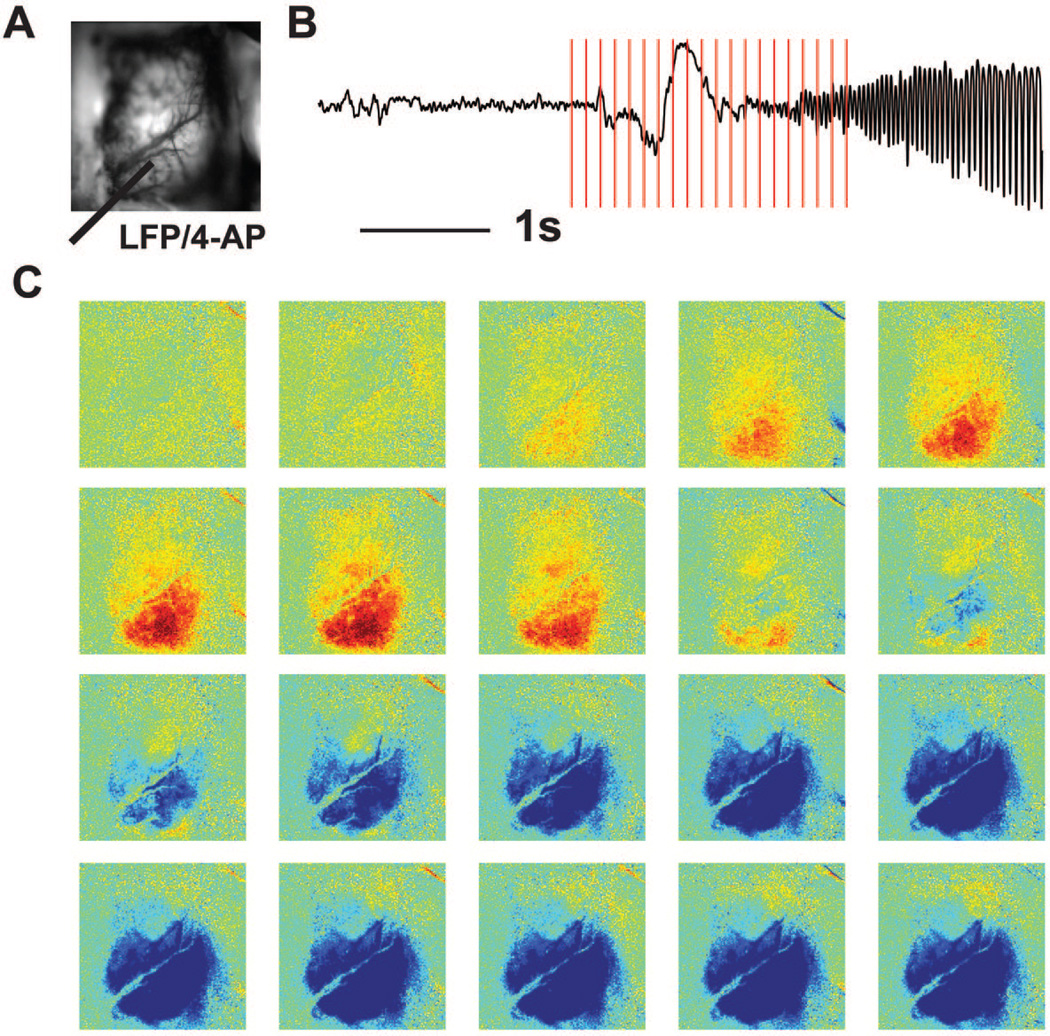
Despite the discovery of pre-ictal optical signal in human spontaneous epilepsy, many of our animal studies in pharmacologically-induced recurrent focal neocortical seizures using 4-aminopyridine injection (4-AP) did not find any pre-ictal changes in intrinsic optical imaging, autofluorescence flavoprotein metabolism or direct tissue measurements 2,28,47,56,57. In another study, however, we divided these 4-AP seizures into two groups based on their electrographic onset pattern. While some seizures began with a large population spike, followed by low-voltage fast activity (LVFA), others began with LVFA without an initial spike. Of the 67 seizures, 47 began with an initial spike and 20 began without the initial spike. Using ORIS to record CBV, when an initiating spike occurred, increases were identified 0.653 ± 0.482 s after the initial spike. However, for the 20 seizures that did not begin with an initial spike but a LVFA recruiting rhythm, CBV increases occurred 1.525 ± 1.218 s before the first significant change in the LFP27. Thus, pre-ictal increases in CBV can depend on pattern of seizure onset (Fig. 2).
Figure 2.
Pre-ictal decrease in CBV precede seizure onset. (A) Image of cortical surface to demonstrate location of 4-AP and LFP electrode (gray bar). (B) LFP recording of one seizure. The vertical lines showed the frame markers in (C). CBV images at selected time points (B) with respect to seizure onset show pre-ictal decrease in CBV then increase in CBV after seizure onset (light indicates the decrease of CBV and the dark indicates the increase of CBV.
Surrounding vasoconstriction of pre-ictal signals
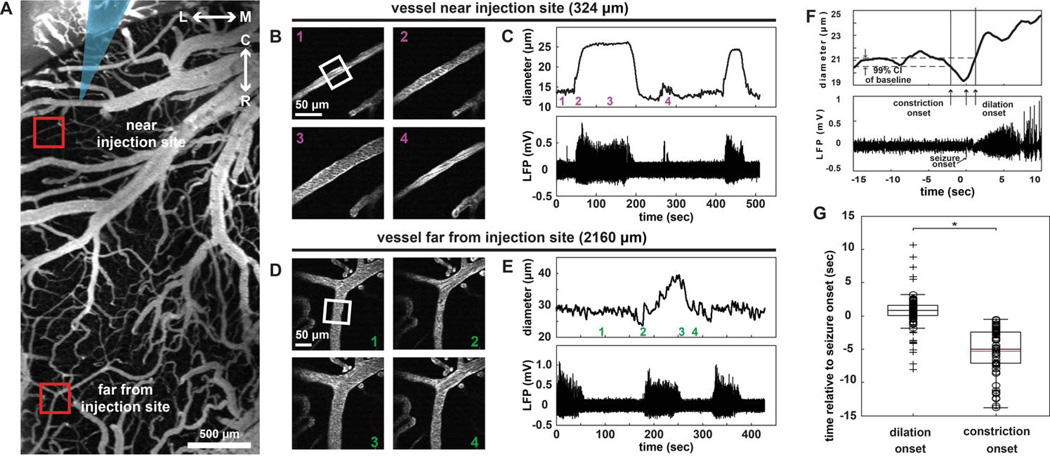
To determine the etiology of the pre-ictal vascular signals, we recently measured changes of arteriolar diameter during acute 4-AP seizures using 2-photon imaging and found pre-ictal vasoconstriction in cortex surrounding an ictal focus57. In vivo images of cortical vasculature were used to measure vessel diameter (Fig. 3A). High-magnification movies of individual arterioles allowed for tracking diameter changes during seizure activity near (Fig. 3B–C) and far (Fig. 3D–E) from the seizure focus (Fig. 3 A–E). We found that arterioles dilated in response to the seizure in the focus, with a decreasing amount of dilation with increasing distance from the 4-AP injection site (n = 4 rats, 71 vessels, 45 seizures, 143 measurements). Plotting the temporal profile of vasodilation compared with vasoconstriction (Fig. 3F and G), we determined that vasodilation in the focus occurred 0.5 ± 0.1 s after seizure onset, whereas vasoconstriction in the surround occurred 5.3 ± 0.5 s before seizure onset. Note that all vasoconstriction was observed to occur before seizure onset.
Figure 3.
Seizures induce spatially-dependent vascular changes. (A) 2-photon image of fluorescently-labeled surface vasculature (Gray arrow, implanted electrode. Boxed areas, near and far regions from the seizure focus highlighted in parts B–E). L↔M, lateral-medial axis; C↔R, caudal-rostral axis. Scale bar: 500µm. (B) Example of a representative vessel adjacent to the injection site in the focus demonstrates vascular dilation concurrent with seizure onset and evolution. (C) Plot of vessel diameter (above) in the white box in (B) during ictal events (below). Note vascular dilation with each event. Numbered timestamps in (C) top correspond to images in (B). (D) 2-photon images in located vessel > 2 mm from the injection site reveals a transient constriction of arterioles at the onset of the seizure. (E) Plot of vessel diameter (above) in the white box in (D) during ictal events (below). Note vascular constriction at the onset of each event, followed by dilation. Numbered timestamps in (E) top correspond to images in (D). (F) Representative example of vascular diameter (top panel) and simultaneous LFP recording (bottom panel) of seizure onset demonstrate pre-ictal vasoconstriction and post-ictal vasodilation. A 99% confidence interval about the mean diameter is shown in (F). Arrows indicate constriction (first arrow), seizure onset (second arrow), and dilation (third arrow). (G) Boxplot of dilation and constriction onset times relative to seizure onset. Gray and black lines represent mean and median respectively. Circles are individual data points and cross hairs are statistical outliers. *: p < 1.0E-7. (n= 4 rats, 71 vessels, 45 total seizures, 143 total measurements).57
In previous studies, we had demonstrated an inverted ORIS signal in the surround consistent with a decrease in CBV 56. It was not clear from these studies whether the surrounding decrease in CBF or CBV was caused by a passive shunting of blood into the ictal focus or by active shunting of blood due to vasoconstriction on the surrounding brain tissue. Using the 2-photon microscope to look directly at the arterioles, we demonstrated active pre-ictal vasoconstriction, indicating that ictal onset may be preceded by vasoconstriction in small arterioles surrounding an ictal focus. The etiology of pre-ictal surround vasoconstriction is unclear. One possibility is the active shunting of oxygenated blood to the imminent seizure focus. Another possibility is that the vasoconstriction is reaction to pre-ictal surround inhibition in the ‘ictal penumbra’38,50.
Discussion
Pre-ictal optical imaging studies in both human and animal seizures provides converging evidences for the existence of anticipatory changes in cerebral blood flow and hemoglobin oxygenation. Although the etiology of those changes is currently unknown, ORIS can be used as a method to detect these early events. Whether these vascular events are truly pre-ictal or rather represent subtle underlying neuronal or glial events is also unclear and will require more sensitive measurements of both signals. For example, fast ripples (very high frequency activity) and microseizures have been recorded in human epilepsy using high-resolution ECoG techniques6,22,36,37,43,44, which were not applied in the above studies. Ictal change in the ECoG or the LFP are a reflection of synchronous dendritic activity in large groups of neurons. ORIS recordings mostly arise from sub-threshold activity in an area 5–10 times larger than the area of spiking cells. Hence, subtle pre-ictal activity may not be recorded by the ECoG or LFP electrode but clearly recorded by optical methods. Additionally, pre-ictal signals may not be elicited by neurons but rather astrocyte- or pericyte-medicated signaling or local potassium and local neurotransmitter/neuropeptide release14,20,21,32,51. Hemodynamic changes may also be influenced by glia, which are not directly recorded with standard electrophysiological methods.
What is the significance of the pre-ictal optical signal? The ultimate goal of optical seizure prediction is not only to warn of an impending seizure but also to prevent seizure from occurring. New novel epilepsy therapies such as cortical stimulation, local short-acting, powerful drug application, and focal cooling have been investigated to stop seizures7,11,24,31,45. All of these methodologies would be more efficacious if those closed-loop intervention systems can predict the onset of seizures.
Currently, most of those systems use recording electrodes to provide ongoing feedback of cortical physiology. Number and location of electrodes may be important to provide a sufficiently early detection of an ongoing seizure. Complex mathematical algorithms applied to electrographic data for seizure prediction have recently garnered much attention 26. The pre-ictal optical signals we describe here may provide an alternative method. Additionally, the optical method is an non-invasive measurement, which can avoid the brain damage caused by implanted electrodes9. New neuroimaging and neuro-modulatory techniques such as 'optogenetics', which combines optical and genetic techniques, have emerged as a popular tool to probe and control neuronal function with light1,4,5,54,55. Recently, optical suppression of epilepsy has been studied by using optogenetic techniques and caged compounds 49,53. The combination of optical pre-ictal detection and optical control would be a novel optical device to terminate seizures.
Pre-ictal optical imaging may also help to identify the ictal focus in patients with non-lesional epilepsy. Non-lesional epilepsy surgery usually has a lower chance of seizure-free outcome than lesional epilepsy surgery48. Precise location of a pre-ictal and ictal onset zone in pre-surgical planning would result more effective neurosurgical resections.
In summary, new novel optical imaging techniques show clear pre-ictal optical signals from both human epilepsy and acute pharmacologically induced seizures in animal models. Optical measurements of blood flow and oxygenation may be extremely useful tools for predicting and localizing seizure onset, which can have a myriad of uses in warning patients and triggering abortive therapies. Those methods have great potential to assist the neurosurgeon not only to localize the seizure onset but also to trigger a closed-loop real time on-line device to predict and terminate seizures.
Acknowledgments
This work was supported by the NINDS RO1 NS49482 (T.H.S), CURE Taking Flight Award (H.M.), the Clinical and Translational Science Center (CTSC) Grant UL1 RR 024996 Pilot Grant (M.Z), and the Cornell University Ithaca-WCMC seed grant (M.Z.).
Footnotes
Disclosure:
The authors declare that there is no conflict of interest.
References
- 1.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bahar S, Suh M, Zhao M, Schwartz TH. Intrinsic optical signal imaging of neocortical seizures: the 'epileptic dip'. Neuroreport. 2006;17:499–503. doi: 10.1097/01.wnr.0000209010.78599.f5. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia S, Ragheb J, Johnson M, Oh S, Sandberg DI, Lin W-C. The role of optical spectroscopy in epilepsy surgery in children. Neurosurgical Focus. 2008;25:E24. doi: 10.3171/FOC/2008/25/9/E24. [DOI] [PubMed] [Google Scholar]
- 4.Boison D. Adenosine and Epilepsy: From Therapeutic Rationale to New Therapeutic Strategies. Neuroscientist. 2005;11:25–36. doi: 10.1177/1073858404269112. [DOI] [PubMed] [Google Scholar]
- 5.Boison D. Adenosine kinase, epilepsy and stroke: mechanisms and therapies. 2006;27:652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton JM, Peebles GA, Binder DK, Rothman SM, Smyth MD. Transcortical Cooling Inhibits Hippocampal-kindled Seizures in the Rat. Epilepsia. 2005;46:1881–1887. doi: 10.1111/j.1528-1167.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhary UJ, Duncan JS, Lemieux L. Mapping hemodynamic correlates of seizures using fMRI: A review. Human Brain Mapping. 2011 doi: 10.1002/hbm.21448. :n/a-n/a, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox MP, Ma H, Bahlke ME, Beck JH, Schwartz TH, Kymissis I. LED-based optical device for chronic in vivo cerebral blood volume measurement. IEEE Transactions on Electron Devices. 2010;57:174–177. doi: 10.1109/TED.2009.2033652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diehl B, Knecht S, Deppe M, Young C, Stodieck SR. Cerebral hemodynamic response to generalized spike-wave discharges. Epilepsia. 1998;39:1284–1289. doi: 10.1111/j.1528-1157.1998.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 11.Eder HG, Stein A, Fisher RS. Interictal and ictal activity in the rat cobalt/pilocarpine model of epilepsy decreased by local perfusion of diazepam. Epilepsy Research. 1997;29:17–24. doi: 10.1016/s0920-1211(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 12.Federico P, Abbott DF, Briellmann RS, Harvey AS, Jackson GD. Functional MRI of the pre-ictal state. Brain. 2005;128:1811–1817. doi: 10.1093/brain/awh533. [DOI] [PubMed] [Google Scholar]
- 13.Feldwisch-Drentrup H, Staniek M, Schulze-Bonhage A, Timmer J, Dickten H, Elger CE, et al. Identification of preseizure States in epilepsy: a data-driven approach for multichannel EEG recordings. Frontiers in Computational Neuroscience. 2011;5:32. doi: 10.3389/fncom.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- 15.Frostig RD, Lieke EE, Ts'o DY, Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990;87:6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavaret M, McGonigal A, Badier JM, Chauvel P. Physiology of frontal lobe seizures: pre-ictal, ictal and inter-ictal relationships. Supplements to Clinical neurophysiology. 2004;57:400–407. doi: 10.1016/s1567-424x(09)70377-0. [DOI] [PubMed] [Google Scholar]
- 17.Gibbs FA. Cerebral blood flow preceding and accompanying experimental convulsions. Arch Neurol Psychiatry. 1933;30:1003–1010. [Google Scholar]
- 18.Haglund MM. Optical imaging of visual cortex epileptic foci and propagation pathways. Epilepsia. 2012;53:87–97. doi: 10.1111/j.1528-1167.2012.03479.x. [DOI] [PubMed] [Google Scholar]
- 19.Haglund MM, Ojemann GA, Hochman DW. Optical imaging of epileptiform and functional activity in human cerebral cortex. Nature. 1992;358:668–671. doi: 10.1038/358668a0. [DOI] [PubMed] [Google Scholar]
- 20.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- 21.Hamel E, Day BW, Miller JH, Jung MK, Northcote PT, Ghosh AK, et al. Synergistic effects of peloruside A and laulimalide with taxoid site drugs, but not with each other, on tubulin assembly. Mol Pharmacol. 2006;70:1555–1564. doi: 10.1124/mol.106.027847. [DOI] [PubMed] [Google Scholar]
- 22.Jiruska P, Finnerty GT, Powell AD, Lofti N, Cmejla R, Jefferys JGR. Epileptic high-frequency network activity in a model of non-lesional temporal lobe epilepsy. Brain. 2010;133:1380–1390. doi: 10.1093/brain/awq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz A, Marks DA, McCarthy G, Spencer SS. Does interictal spiking change prior to seizures? Electroencephalography and Clinical Neurophysiology. 1991;79:153–156. doi: 10.1016/0013-4694(91)90054-8. [DOI] [PubMed] [Google Scholar]
- 24.Kossoff EH, Ritzl EK, Politsky JM, Murro AM, Smith JR, Duckrow RB, et al. Effect of an External Responsive Neurostimulator on Seizures and Electrographic Discharges during Subdural Electrode Monitoring. Epilepsia. 2004;45:1560–1567. doi: 10.1111/j.0013-9580.2004.26104.x. [DOI] [PubMed] [Google Scholar]
- 25.Ledri M, Nikitidou L, Erdelyi F, Szabo G, Kirik D, Deisseroth K, et al. Altered profile of basket cell afferent synapses in hyper-excitable dentate gyrus revealed by optogenetic and two-pathway stimulations. European Journal of Neuroscience. 2012;36:1971–1983. doi: 10.1111/j.1460-9568.2012.08080.x. [DOI] [PubMed] [Google Scholar]
- 26.Litt B, Echauz J. Prediction of epileptic seizures. Lancet Neurol. 2002;1:22–30. doi: 10.1016/s1474-4422(02)00003-0. [DOI] [PubMed] [Google Scholar]
- 27.Ma H, Zhao M, Schwartz TH. Dynamic Neurovascular Coupling and Uncoupling during Ictal Onset, Propagation, and Termination Revealed by Simultaneous In Vivo Optical Imaging of Neural Activity and Local Blood Volume. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma H, Zhao M, Suh M, Schwartz TH. Hemodynamic surrogates for excitatory membrane potential change during interictal epileptiform events in rat neocortex. J Neurophysiol. 2009;101:2550–2562. doi: 10.1152/jn.90694.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- 30.Mormann F, Andrzejak RG, Elger CE, Lehnertz K. Seizure prediction: the long and winding road. Brain. 2007;130:314–333. doi: 10.1093/brain/awl241. [DOI] [PubMed] [Google Scholar]
- 31.Motamedi GK, Salazar P, Smith EL, Lesser RP, Webber WRS, Ortinski PI, et al. Termination of epileptiform activity by cooling in rat hippocampal slice epilepsy models. Epilepsy Research. 2006;70:200–210. doi: 10.1016/j.eplepsyres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prakash N, Uhlemann F, Sheth SA, Bookheimer S, Martin N, Toga AW. Current trends in intraoperative optical imaging for functional brain mapping and delineation of lesions of language cortex. NeuroImage. 2009;47(Supplement 2):T116–T126. doi: 10.1016/j.neuroimage.2008.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogowski Z, Gath I, Bental E. On the prediction of epileptic seizures. Biological Cybernetics. 1981;42:9–15. doi: 10.1007/BF00335153. [DOI] [PubMed] [Google Scholar]
- 35.Sato K, Nariai T, Sasaki S, Yazawa I, Mochida H, Miyakawa N, et al. Intraoperative Intrinsic Optical Imaging of Neuronal Activity from Subdivisions of the Human Primary Somatosensory Cortex. Cerebral Cortex. 2002;12:269–280. doi: 10.1093/cercor/12.3.269. [DOI] [PubMed] [Google Scholar]
- 36.Schevon CA, Goodman RR, McKhann GJ, Emerson RG. Propagation of Epileptiform Activity on a Submillimeter Scale. Journal of Clinical Neurophysiology. 2010;27:406–411. doi: 10.1097/WNP.0b013e3181fdf8a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schevon CA, Ng SK, Cappell J, Goodman RR, McKhann GJ, Waziri A, et al. Microphysiology of Epileptiform Activity in Human Neocortex. Journal of Clinical Neurophysiology. 2008;25:321–330. doi: 10.1097/WNP.0b013e31818e8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schevon CA, Weiss SA, McKhann G, Jr, Goodman RR, Yuste R, Emerson RG, et al. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commun. 2012;3:1060. doi: 10.1038/ncomms2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz TH, Chen LM, Friedman RM, Spencer DD, Roe AW. Intraoperative optical imaging of human face cortical topography: a case study. Neuroreport. 2004;15:1527–1531. doi: 10.1097/01.wnr.0000131006.59315.2f. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz TH, Hong S-B, Bagshaw AP, Chauvel P, Bénar C-G. Preictal changes in cerebral haemodynamics: Review of findings and insights from intracerebral EEG. Epilepsy research. 2011;97:252–266. doi: 10.1016/j.eplepsyres.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 41.Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Hageman N, et al. Columnar specificity of microvascular oxygenation and volume responses: implications for functional brain mapping. J Neurosci. 2004;24:634–641. doi: 10.1523/JNEUROSCI.4526-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.So EL, O'Brien TJ. Chapter 27 - Peri-ictal single-photon emission computed tomography: principles and applications in epilepsy evaluation. In: Hermann S, William HT, editors. Handbook of Clinical Neurology. Vol Volume 107. Elsevier: 2012. pp. 425–436. [DOI] [PubMed] [Google Scholar]
- 43.Staba RJ, Frighetto L, Behnke EJ, Mathern GW, Fields T, Bragin A, et al. Increased fast ripple to ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–2138. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- 44.Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer FB, et al. Microseizures and the spatiotemporal scales of human partial epilepsy. Brain. 2010;133:2789–2797. doi: 10.1093/brain/awq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stein AG, Eder HG, Blum DE, Drachev A, Fisher RS. An automated drug delivery system for focal epilepsy. Epilepsy research. 2000;39:103–114. doi: 10.1016/s0920-1211(99)00107-2. [DOI] [PubMed] [Google Scholar]
- 46.Suh M, Bahar S, Mehta AD, Schwartz TH. Blood volume and hemoglobin oxygenation response following electrical stimulation of human cortex. NeuroImage. 2006;31:66–75. doi: 10.1016/j.neuroimage.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 47.Suh M, Ma H, Zhao M, Sharif S, Schwartz TH. Neurovascular coupling and oximetry during epileptic events. Mol Neurobiol. 2006;33:181–197. doi: 10.1385/MN:33:3:181. [DOI] [PubMed] [Google Scholar]
- 48.Téllez-Zenteno JF, Ronquillo LH, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: A systematic review and meta-analysis. Epilepsy research. 2010;89:310–318. doi: 10.1016/j.eplepsyres.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Tonnesen J, Sorensen AT, Deisseroth K, Lundberg C, Kokaia M. Optogenetic control of epileptiform activity. Proc Natl Acad Sci U S A. 2009;106:12162–12167. doi: 10.1073/pnas.0901915106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trevelyan AJ, Schevon CA. How inhibition influences seizure propagation. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Lou N, Xu Q, Tian G-F, Peng WG, Han X, et al. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 2006;9:816–823. doi: 10.1038/nn1703. [DOI] [PubMed] [Google Scholar]
- 52.Williamson JR, Bliss DW, Browne DW, Narayanan JT. Seizure prediction using EEG spatiotemporal correlation structure. Epilepsy & Behavior. 2012;25:230–238. doi: 10.1016/j.yebeh.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 53.Yang XF, Schmidt BF, Rode DL, Rothman SM. Optical suppression of experimental seizures in rat brain slices. Epilepsia. 2010;51:127–135. doi: 10.1111/j.1528-1167.2009.02252.x. [DOI] [PubMed] [Google Scholar]
- 54.Zayat L, Noval MG, Campi J, Calero CI, Calvo DJ, Etchenique R. A new inorganic photolabile protecting group for highly efficient visible light GABA uncaging. ChemBioChem. 2007;8:2035–2038. doi: 10.1002/cbic.200700354. [DOI] [PubMed] [Google Scholar]
- 55.Zhang F, Wang L-P, Brauner M, Liewald JF, Kay K, Watzke N, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 56.Zhao M, Ma H, Suh M, Schwartz TH. Spatiotemporal dynamics of perfusion and oximetry during ictal discharges in the rat neocortex. J Neurosci. 2009;29:2814–2823. doi: 10.1523/JNEUROSCI.4667-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao M, Nguyen J, Ma H, Nishimura N, Schaffer CB, Schwartz TH. Preictal and Ictal Neurovascular and Metabolic Coupling Surrounding a Seizure Focus. The Journal of Neuroscience. 2011;31:13292–13300. doi: 10.1523/JNEUROSCI.2597-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao M, Suh M, Ma H, Perry C, Geneslaw A, Schwartz TH. Focal increases in perfusion and decreases in hemoglobin oxygenation precede seizure onset in spontaneous human epilepsy. Epilepsia. 2007;48:2059–2067. doi: 10.1111/j.1528-1167.2007.01229.x. [DOI] [PubMed] [Google Scholar]