Abstract
Vasoactive intestinal polypeptide (VIP) signaling is critical for circadian rhythms. For example, the expression of VIP and its main receptor, VPAC2R, is necessary for maintaining synchronous daily rhythms among neurons in the suprachiasmatic nucleus (SCN), a master circadian pacemaker in animals. Where and when VPAC2R protein is expressed in the SCN and other brain areas has not been examined. Using immunohistochemistry, we characterized a new antibody and found that VPAC2R was highly enriched in the SCN and detectable at low levels in many brain areas. Within the SCN, VPAC2R was circadian, peaking in the subjective morning, and abundantly expressed from the rostral to caudal margins with more in the dorsomedial than ventrolateral area. VPAC2R was found in nearly all SCN cells including neurons expressing either VIP or vasopressin (AVP). SCN neurons mainly expressed VPAC2R in their somata and dendrites, not axons. Finally, constant light increased VIP and AVP expression, but not VPAC2R. We conclude that the circadian clock, not the ambient light level, regulates VPAC2R protein localization. These results are consistent with VPAC2R playing a role in VIP signaling at all times of day, broadly throughout the brain and in all SCN cells. J. Comp. Neurol. 520:2730–2741, 2012.
INDEXING TERMS: circadian rhythm, synchrony, entrainment, neuropeptide, G-protein coupled receptor
The suprachiasmatic nucleus (SCN) acts as a master circadian pacemaker in mammals generating daily rhythms in various physiological processes. Although individual SCN neurons harbor cell-autonomous, molecular feedback loops to generate circadian cycling (Herzog, 2007; Welsh et al., 2010), they depend on neuropeptides to reliably synchronize to the light–dark cycle and coordinate daily rhythms between cells (Aton et al., 2005; Maywood et al., 2006, 2011; Vosko et al., 2007; Hughes et al., 2011).
Vasoactive intestinal polypeptide (VIP) and its receptor, vasoactive intestinal polypeptide receptor 2 (VPAC2R) are known to be critical for circadian synchrony. VIP entrains and phase shifts the PER2 gene expression and firing rate rhythms in vitro (Reed et al., 2001; An et al., 2011). Mice lacking VIP (Vip−/−) or VPAC2R (Vipr2−/−) show weak or no circadian rhythms in locomotor activity, body temperature, hormonal release, and heart rate (Harmar et al., 2002; Colwell et al., 2003; Aton et al., 2005; Loh et al., 2008; Schroeder et al., 2011) and a loss of synchrony among SCN cells (Aton et al., 2005; Maywood et al., 2006; Brown et al., 2007). Vip−/− and Vipr2−/− mice have aberrant responses to light including the smaller phase shift and altered phase relation with light (Harmar et al., 2002; Colwell et al., 2003; Hughes et al., 2004). In contrast, knocking out Pac1r, a receptor modestly sensitive to VIP, produced mild defects in circadian rhythms (Hannibal et al., 2008). Given the similar phenotypes between Vip−/− and Vipr2−/− mice, it has been suggested that VIP signals primarily through VPAC2R to regulate circadian behaviors (Aton et al., 2005).
Little is known about the specifics of when and where VIP signaling occurs in the SCN. Approximately 10% of SCN cells located mainly in ventrolateral SCN synthesize VIP (Abrahamson and Moore, 2001). These neurons project throughout the SCN, suggesting direct communication with all SCN cells. VIP is released rhythmically from cultured SCN (Shinohara et al., 1993, 1995), but there is conflicting evidence for circadian VIP release in vivo (Laemle et al., 1995; Francl et al., 2010). VIP mRNA and protein decrease in the light portion of a 12:12-hour light–dark (LD) cycle, whereas they are constant in continuous darkness (Shinohara et al., 1993, 1999). The release of VIP appears to be stimulated by light in vivo (Shinohara et al., 1998; Francl et al., 2010). Vipr2 mRNA and Vipr2-reporter transgene expression have been found throughout the SCN and enriched in the dorsomedial SCN, where vasopressin (AVP)-expressing neurons are located (Usdin et al., 1994; Kalamatianos et al., 2004; Kallo et al., 2004). Two studies report that SCN Vipr2 mRNA levels are rhythmic in a 12:12-hour LD cycle but are constant or biphasic in constant conditions although they differ in the timing of and number of peaks in expression (Cagampang et al., 1998; Shinohara et al., 1999). Daily VPAC2R protein expression in the brain has not been analyzed.
In this study, we characterized the spatiotemporal expression of VPAC2R in the SCN by using a specific antibody. We found VPAC2R throughout the SCN with levels that varied by time of day, but not by light exposure.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice (4–8 weeks old) of three genotypes (wild-type, Vip−/− and Vipr2−/−) were housed individually in a 12:12-hour LD cycle in the Danforth Animal Facility (Washington University) for at least 20 days. Vip−/− (Colwell et al., 2003) mice were derived from founders generously provided by C. Colwell and J. Waschek (University of California-Los Angeles) and Vipr2−/− (Harmar et al., 2002) mice were derived from founders generously provided by A. Harmar (University of Edinburgh, Scotland). Groups of mice were killed according to time of the day in a light cycle (LD) or in constant darkness (DD) or in constant light (LL). In a light cycle, Zeitgeber Times (ZT) defined the time of perfusion relative to light onset (ZT0 and 24, 7:00 am) and offset (ZT12, 7:00 pm). For circadian experiments in DD and LL, running wheel activity was used as an indicator of subjective time where the daily onset of locomotion was defined as Circadian Time (CT) 12. Other circadian times were calculated in circadian hours from CT12. All procedures were approved by the Animal Care and Use Committee at Washington University and conformed to National Institutes of Health (NIH) guidelines.
Tissue preparation
After anesthesia with 2.5% tribromoethanol (Avertin), animals were perfused intracardially with 0.9% saline followed by chilled 4% paraformaldehyde (PFA; pH 7.2). Mice killed during the night or subjective night were anesthetized and perfused under dim red light. Brains were kept in PFA for 24 hours, andthen transferred to 30% sucrose in phosphate-buffered saline (PBS; pH 7.2) for 3 days until the brains sunk. Brains were quickly frozen with 2-methylbutane (Sigma, St. Louis, MO), maintained at −35°C, and stored at −80°C. Coronal sections (30 µm thick) were obtained with a cryostat (CM1850; Leica, Maryland Heights, MO) and stored as six sets in Watson’s cryoprotectant solution, pH 7.2, at −20°C for at least 48 hours.
For the analysis of intracellular localization of VPAC2R, dissociated SCN neuronal cultures were used. SCN punches were taken from coronal hypothalamic slices of C57Bl/6 pups (postnatal days 1–5), and cells were digested with papain at 37°C for 40 minutes. After brief centrifugation, the papain solution was replaced with media and cells were triturated by gentle pipetting. Cells were plated on glass coverslips coated with poly-D-lysine (Sigma) and laminin (Sigma) at a density of 3,000 cells/mm2. After a week in vitro, cells were fixed with chilled 4% PFA solution for 15 minutes.
Immunostaining
Antibody characterization
The following antibodies were used in this study (Table 1):
VPAC2R: We tested the specificity of three antibodies against VPAC2R: rabbit anti-VPAC2R (AbCam, Cambridge, MA), goat anti-VPAC2R (Santa Cruz Biotechnology, Santa Cruz, CA), and mouse anti-VPAC2R (AbCam). A previous report claimed that it characterized VPAC2R protein expression in brain with goat anti-VPAC2R (Joo et al., 2004). However, we found that the goat anti-VPAC2R and mouse anti-VPAC2R antibodies produced nonspecific staining in the brains of both wild-type and Vipr2−/− mice. In contrast, we found that the rabbit anti-VPAC2R antibody revealed intense staining in the SCN of wild-type mice and background levels in Vipr2−/− SCN (Fig. 1). Similarly, preadsorption of the antibody with rat VIP (Bachem, Torrence, CA) produced background levels of staining. We tested the rabbit anti-VPAC2R at different concentrations and chose 1:15,000 for immunostaining using 3′,3-diaminobenzidine (DAB) and 1:1,000 for immunofluorescence. We concluded that the rabbit anti-VPAC2R specifically recognizes VPAC2R and used it for further study.
VIP: Both rabbit anti-VIP (Immunostar, Hudson, WI) and sheep anti-VIP (Millipore, Billerica, MA) yielded intense staining in wild-type SCN, but not in the Vip−/− SCN, with little background staining. We concluded that both antibodies specifically bind to VIP.
AVP, MAP2, and Tau-1: The specificity of these antibodies has been reported (Francon et al., 1982; Ben-Barak et al., 1985; Oliva et al., 2006). Specificity of the anti-AVP antibody (termed PS41) was originally based on Western blots for vasopressin-associated neurophysin, liquid phase radioimmunoassay, which was competed with neurophysin and not oxytocin, and lack of immunolabeling in AVP-deficient Brattleboro rats (Ben-Barak et al., 1985). Here, specificity was also confirmed by the distinctive staining pattern of AVP-expressing cells in the SCN, paraventricular nucleus, and supraoptic nucleus. Specificity of the anti-MAP2 antibody was originally based on Western blots that exclusively immunolabeled a high molecular weight (280-kDa) band corresponding to MAP2a and MAP2b isoforms and two low molecular weight (70-kDa) bands corresponding to the MAP2c isoform (Oliva et al., 2006). Specificity of the anti-Tau-1 antibody was originally based on Western blots that exclusively immunolabeled five bands (52–68 kDa) for tau-1, -2, -3, and -4 (Francon et al., 1982). Here, the absence of MAP2 and Tau-1 staining in cultured glia provided an additional, negative control for their neuron-specific labeling.
TABLE 1.
Primary and Secondary Antibodies Used in This Study
| Antigen | Immunogen | Source | Dilution |
|---|---|---|---|
| Vasoactive intestinal polypeptide receptor 2 (VPAC2R) | Synthetic peptide conjugated to KLH, amino acids 419–438 of human VPAC2R: LQFHRGSRAQSFLQTETSVI | Abcam (Cambridge, MA), rabbit polyclonal, #ab28624 | 1:1,000 (fluorescence) 1:15,000 (DAB) |
| VPAC2R | Synthetic peptide within amino acids 300–350 of human VPAC2R | Santa Cruz Biotechnology (Santa Cruz, CA), goat polyclonal, #sc-15960 | 1:500 (fluorescence) |
| VPAC2R | Synthetic peptide conjugated to KLH, amino acids 105–122 of human VPAC2R: FVDACGYSDPEDESKITF | Abcam, mouse monoclonal, # ab16156 | 1:1,000 (fluorescence) |
| Vasoactive intestinal polypeptide (VIP) | Synthetic full-length VIP coupled to bovine thyroglobulin with CDI linker | Immunostar (Hudson, WI), rabbit polyclonal, #20077 | 1:2,000 (fluorescence) 1:5,000 (DAB) |
| VIP | Synthetic peptide conjugated to KLH with glutaraldehyde, amino acids 1–28 | Millipore (Billerica, CA), sheep polyclonal, #AB1581, NG1839540 | 1:1,000 (fluorescence) |
| Vasopressin (AVP)-associated neurophysin | Acid-soluble extract of rat pituitary intermediate lobe | Dr. H. Gainer (NIH) or ATCC.org, mouse monoclonal, CRL-1799 | 1:50 (fluorescence) 1:5,000 (DAB) |
| Microtubule-associated protein 2 (MAP2) | Cow brain MAP2 purified from the material that pellets with GTP polymerized tubulin | Abcam, chicken polyclonal, # ab5392 | 1:2,000 (fluorescence) |
| MAP tau-1 (Tau-1) | Purified denatured bovine microtubule-associated proteins | Millipore, mouse monoclonal, #MAB3420, NG1743745, Clone PC1C6 | 1:2,000 (fluorescence) |
Figure 1.
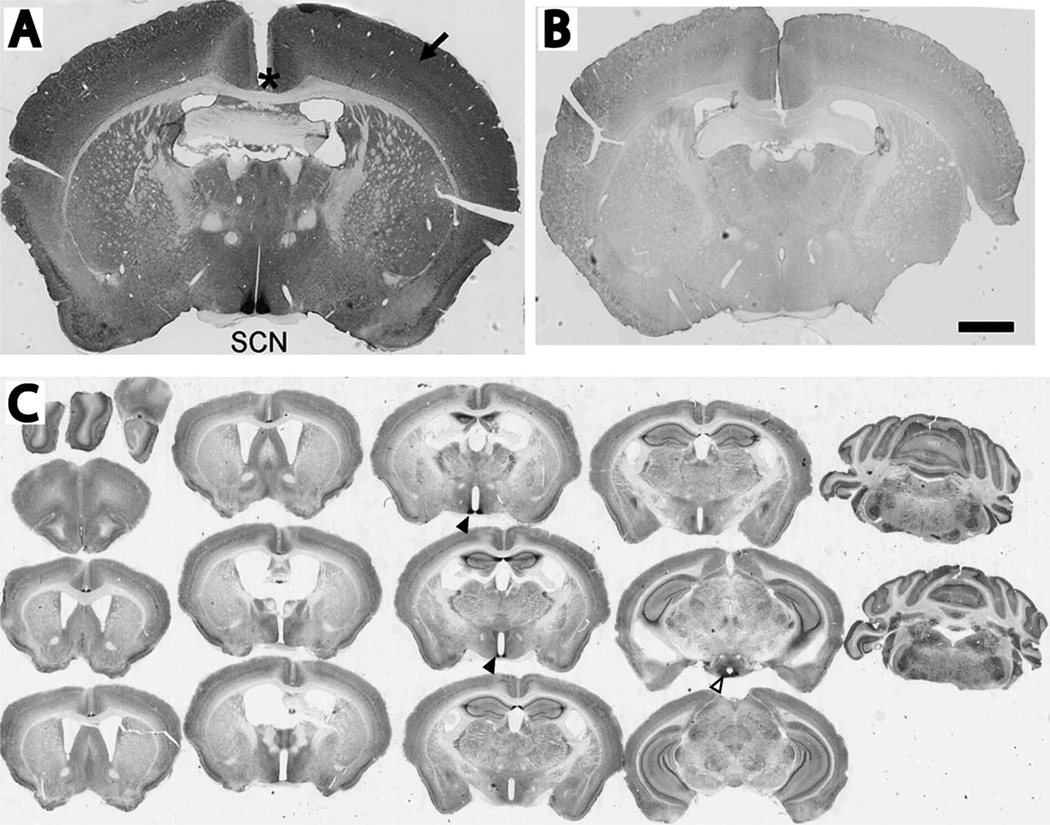
VPAC2R was strongly expressed in the suprachiasmatic nucleus (SCN). A: A representative coronal section of a wild-type mouse brain showing intense immunostaining in the SCN, moderate to weak staining in the neocortex (arrow) and no staining in axonal projections like the corpus callosum (asterisk). B: A representative brain section from a Vipr2−/− mouse showing little or no VPAC2R immunoreactivity. Sections in A and B were imaged, processed and displayed using identical protocols. C: Serial coronal sections of a mouse brain immunolabeled for VPAC2R. These sections were processed to reveal the intense staining in the SCN (closed triangles) and moderate staining in the lateral posterior arcuate nucleus (open triangles). Less intense labeling is seen broadly throughout the brain including the hippocampus, neocortex, and cerebellum. Scale bar = 1 mm in B (applies to A,B)
Immunofluorescence for VPAC2R with VIP, AVP, MAP2, and Tau-1
To co-localize VPAC2R with neuropeptides or dendritic and axonal markers, fixed free-floating sections or cell cultures were rinsed with PBS three times followed by incubation with 3% Triton X-100 for 30 minutes at 37°C, blocking solution (10% bovine serum albumin [BSA], 0.25% Triton X-100; pH 7.2) for 1 hour at room temperature and then rabbit anti-VPAC2 antibody (1:1,000, AbCam) overnight at 4°C. Then samples were incubated in 1:200 donkey anti-rabbit Cy2 (Jackson ImmunoResearch, West Grove, PA) for 2 hours at room temperature. For double-staining with the VPAC2R antibody, samples were co-incubated with either mouse anti-AVP antibody (1:50, a gift from Dr. H. Gainer, NINDS, Bethesda, MD), sheep anti-VIP antibody (1:1,000, Millipore), chicken anti-MAP2 antibody (1:2,000, AbCam), or mouse anti-Tau-1 antibody (1:2,000, Millipore). To label for VPAC2R and VIP in SCN slices, samples were incubated with the rabbit anti-VPAC2R antibody followed by the donkey anti-rabbit Cy2 (1:200, Jackson ImmunoResearch), and then incubated with rabbit anti-VIP antibody (1:2,000, Immunostar) followed by goat anti-rabbit Cy3 (1:200, Jackson ImmunoResearch). All samples were incubated with 4′,6-diamidino-2-phenylindole (DAPI) for 10 minutes to label cell nuclei and to distinguish the boundary of the SCN.
Sections were mounted on slides (12-550-14; Fisher Scientific, Waltham, MA), dried overnight, and coverslipped with Permount (SP15 500, Fisher Scientific). In each experiment, SCN-containing sections or cultures from Vipr2−/− mice and sections or cultures incubated without primary antibody were used as negative controls.
Immunostaining for VPAC2R, VIP, and AVP using DAB
To quantify levels of VPAC2R and neuropeptides as a function of time of day and light exposure, brain sections were incubated with 3% H2O2 in PBS at 4°C for 30 minutes, washed with PBS, blocked at room temperature for 1 hour, and incubated with VPAC2R antibody (1:15,000) or VIP antibody (1:5,000, Immunostar) or AVP antibody (1:1,000) at 4°C for 2 days with agitation. Following three washes in PBS, sections were incubated with biotinylated goat anti-rabbit antibody or goat antimouse antibody (1:200, Standard ELITE ABC kit, Vectastatin, Vector, Burlingame, CA) at 4°C for 1 hour. Then sections were incubated with an avidin–biotin complex (Vector) at 4°C for 2 hours. Sections were rinsed three times with PBS, rinsed with 50 mM Tris-HCl (pH 7.2), and then incubated for 10 minutes with 0.05% DAB, 0.01% H2O2, 0.03% NiCl2 in 50 mM Tris-HCl. Sections were washed with PBS overnight, and mounted onto glass Superfrost slides (Fisher Scientific) the next day. The mounted sections were dehydrated in a series of increasing concentrations of ethanol and xylene. For all comparisons between brains collected at different ZTs or CTs, sections were processed together to minimize differences in treatment.
Image processing
Digitized images were taken by using an epifluorescence microscope and cooled CCD camera (Retiga 1350EX; QImaging, Burnaby, BC, Canada), a confocal microscope (Nikon A1, Nikon Instruments, Melville, NY), or a slide scanner (Nanozoomer Digital Pathology, Hamamatsu, Bridgewater, NJ). For brightness and contrast, the images were processed identically with ImageJ (http://rsbweb.nih.gov/ij). To quantify the intensity of expression, pixel intensity of immunoreactivity was averaged in the bilateral SCN and subtracted from the average background intensity of a brain area outside of the SCN.
Data analysis
The average pixel intensity of the VPAC2R staining in the medial, bilateral SCN was determined after subtracting the staining intensity of the brain area outside of the SCN. The intensity of background brain areas remained constant throughout the day (data not shown). Day and night differences of VPAC2R expression were assessed by one-way ANOVA (Origin 7.0, OriginLab, Northampton, MA). An observer blinded to experimental conditions counted VPAC2R- and AVP-expressing cells in dispersed cultures, and measured the intensity of AVP, VIP, and VPAC2R immunostaining.
RESULTS
VPAC2R was broadly expressed in brain and highly enriched in the SCN
We found intense labeling for VPAC2R in the SCN and weaker labeling throughout the brain including the neocortex, olfactory bulb, hippocampus, cerebellum, ventrolateral thalamus, arcuate nucleus of hypothalamus, and bed nucleus of the stria terminalis in the amygdala (Fig. 1). The staining intensity in the SCN was about seven times higher than that in the neocortex. We found no immunostaining in axonal tracts including the corpus callosum and anterior commissure. VPAC2R immunoreactivity was strong through the rostral to caudal extent of the SCN, with higher intensity in the dorsal area of the medial sections (Fig. 2). We conclude that VPAC2R is abundant in the brain, with the highest expression throughout the SCN.
Figure 2.
Broad VPAC2R expression in the SCN. VPAC2R immunoreactivity was present throughout the rostral (top left) to caudal extent (bottom right) of the SCN. Note that VPAC2R labeling tended to be more intense in the dorsomedial than ventrolateral SCN. Scale bar = 1 mm.
VPAC2R was expressed in nearly all SCN cells
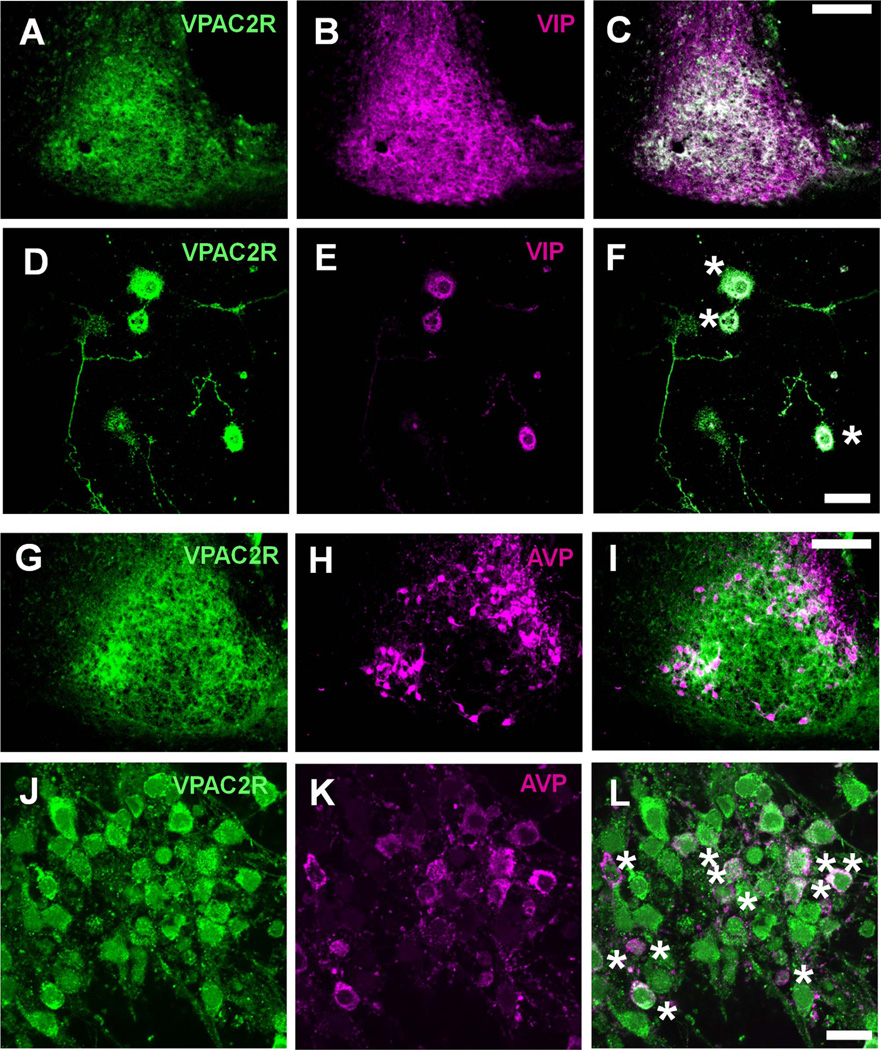
We further characterized the SCN cell types expressing VPAC2R by using double-label immunohistochemistry. We found that two non-overlapping SCN populations, VIP-or AVP-ergic neurons, expressed VPAC2R in vivo and in vitro (Fig. 3). Although VPAC2R was widespread, some AVP cells, especially in the medial SCN, showed weak to undetectable VPAC2R expression (Fig. 3I). Consistent with this heterogeneous distribution, some dispersed SCN cells expressed intense puncta of VPAC2R whereas others were less intensely labeled (Fig. 3F,L). We found that 94.4 ± 2.0% (mean ± SEM) of SCN cells in culture (n = 285 cells in four cultures) had detectable VPAC2R (Fig. 3J). We conclude that VPAC2R is expressed in most, if not all, SCN cells.
Figure 3.
VPAC2R expression overlapped with VIP and AVP expression in the SCN. A–L: Representative images of SCN cells co-stained for VPAC2R and VIP (A–F) or VPAC2R and AVP (G–L). Images show either the left SCN of a coronal brain section (A–C, G–I) or SCN cells 1 week after being plated at low density in vitro (D–F, J–L). The composite images show the extensive overlap (yellow) between VPAC2R (green) and VIP (red), and VPAC2R and AVP (red) expression (C,F,I,L). Dissociated SCN neurons expressing both VPAC2R and either neuropeptide were marked with an asterisk (F and L). Scale bar = 100 µm in C (applies to A–C) and I (applies to G–I); 20 µm in F (applies to D–F) and L (applies to J–L).
VPAC2R was expressed primarily in dendrites and cell bodies
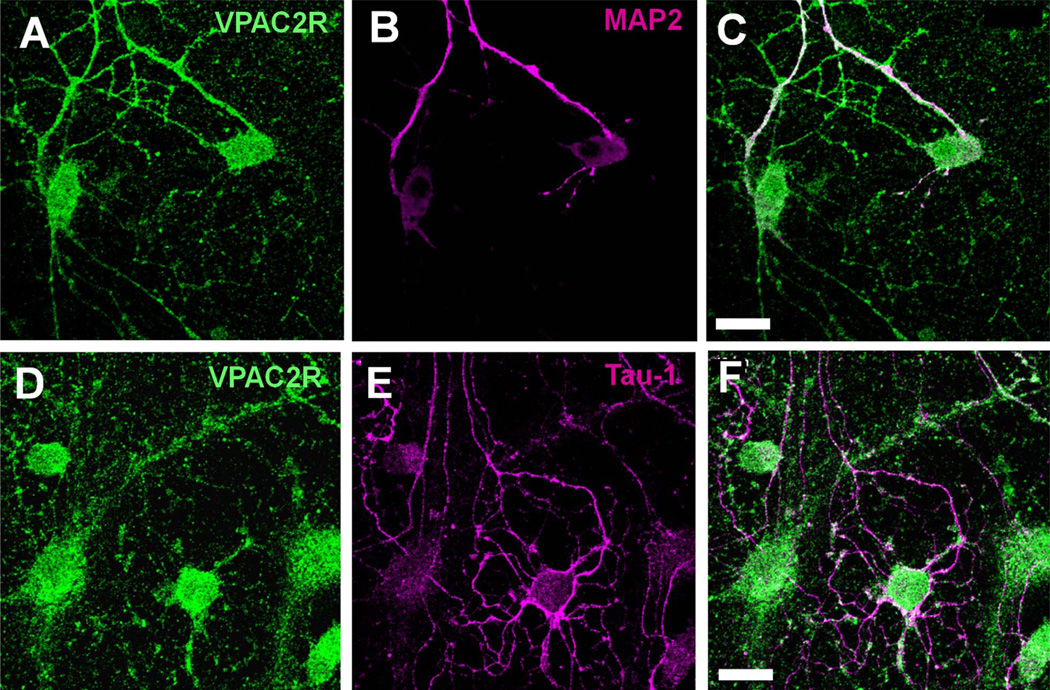
To identify the anatomical sites for VIP–VPAC2R interactions, we examined the intracellular localization of VPAC2R by staining dispersed SCN cultures for VPAC2R and markers for either dendrites (anti-MAP2) or axons (anti-Tau-1). VPAC2R mainly overlapped with the dendritic marker, with little to no overlap with the axonal marker (Fig. 4). These results are consistent with VIP signaling through VPAC2R along the soma and dendrites of neurons.
Figure 4.
VPAC2R co-localized with dendritic markers but not with axonal markers in the SCN. A–F: Representative images of dissociated SCN neurons stained for VPAC2R (A,D) and MAP2, a dendritic marker protein (B), or Tau-1, an axonal marker protein (E). Note that VPAC2R (green) staining colocalized with MAP2 (red) along processes (C) but not with Tau-1 (red) staining (F). Scale bar = 20 µm in C (applies to A–C) and F (applies to D–F).
VPAC2R levels in the SCN changed with time of day
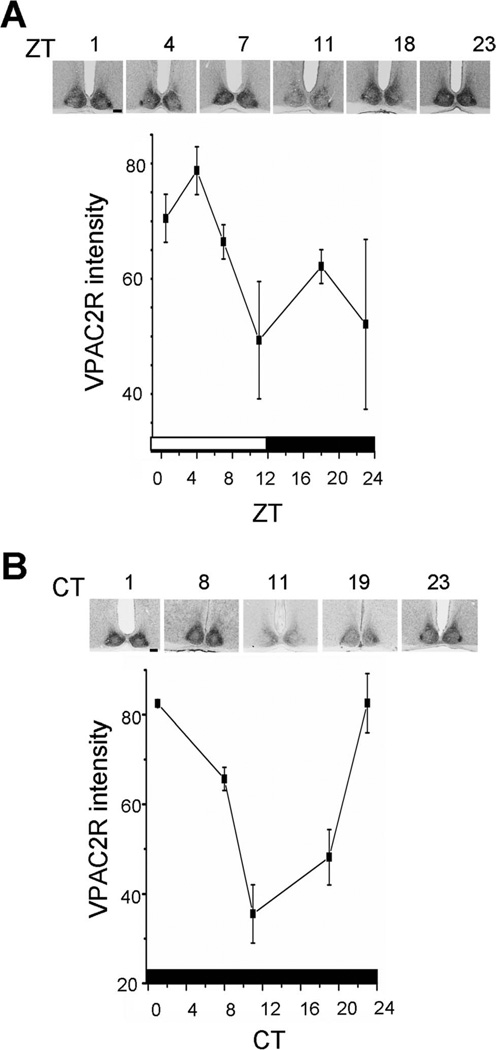
Because Vipr2 mRNA levels have been found to vary in the SCN with time of day, we examined VPAC2R protein levels from mice housed under a 12:12-hour light/dark cycle (LD) or in constant darkness (DD). We found that VPAC2R expression tended to be higher in the day and lower at night in an LD cycle, although this did not reach significance, apparently due to greater interindividual variability around dusk and dawn (Fig. 5; Kruskal-Wallis ANOVA, F, P = 0.12, n = 3 mice per time point). The rhythmic pattern was significant in DD, with a maximum near subjective dawn and minimum around subjective dusk (P < 0.005, one-way ANOVA, F4,10 = 16.54; n = 3 mice per time point). These results indicate that the VPAC2R expression is circadian in constant conditions.
Figure 5.
VPAC2R expression oscillated in a light–dark cycle and in constant darkness. Data points represent the mean ± SEM of three brains at each time point. A: In LD, VPAC2R expression was rhythmic, with a peak after dawn (ZT1) and trough around dusk (ZT11). B: In DD, the pattern was similar, with peak VPAC2R expression around subjective dawn (CT1) and trough near subjective dusk (CT11). Insets show sections of the SCN from representative mice at each time point. The bars at the bottom of each plot show the times of lights on (white) and off (black) experienced by the mice. Scale bar = 100 µm in A,B.
Constant light did not change VPAC2R levels in the SCN
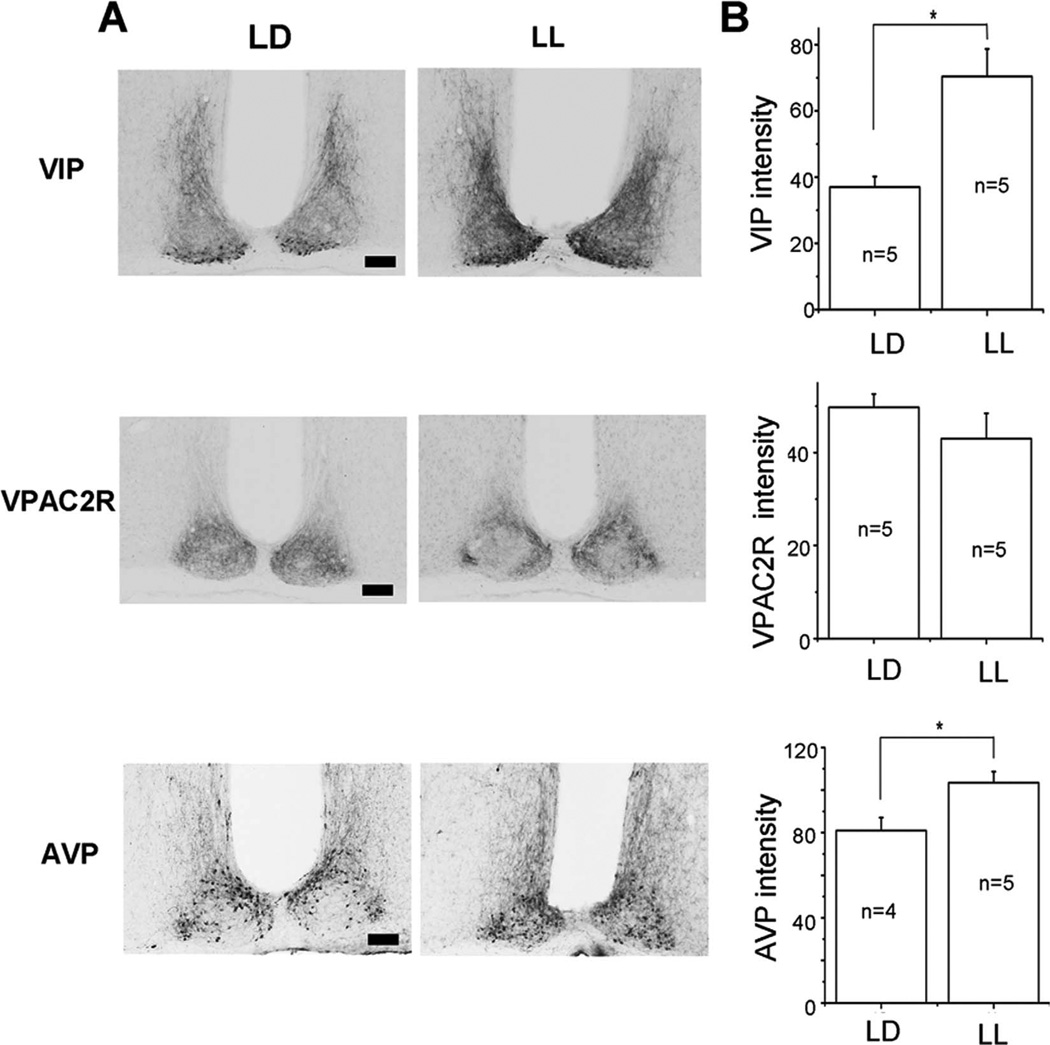
Rodents exposed to constant light increase the period of their daily activity compared with LD or DD activity and, in some cases, lose circadian rhythms in behavior (Ohta et al., 2005). The SCN of these animals shows a similar phenotype with arrhythmic behavior associated with a loss of synchrony among circadian cells (Ohta et al., 2005). Because VIP and VPAC2R are required for circadian synchrony in the SCN, we hypothesized that the effects of LL may involve changes in VIP–VPAC2R signaling. We found that, after 30 days of LL, the level of VPAC2R in the SCN did not change significantly compared with that in LD (P > 0.05, Student’s t-test; CT 5 (LL) compared with ZT 5 (LD); n = 5 mice in each condition; Fig. 6). In contrast, VIP and AVP levels increased in LL (P < 0.05, Student’s t-test; Fig. 6). These results suggest that light changes VIP–VPAC2R signaling in the SCN primarily through changes in VIP, not VPAC2R, abundance.
Figure 6.
Constant light increased VIP and AVP, but not VPAC2R expression in the SCN. A: Representative micrographs illustrate VIP-immunoreactive cell bodies in the ventral SCN and their dense projections into the dorsomedial SCN in light–dark (LD; left) and constant light (LL; right). Note the darker projections and cell bodies in LL. Similarly, AVP immunoreactivity increased, whereas VPAC2R labeling did not change in LL B: Mean ± SEM of the staining intensity in the SCN above background for VIP, VPAC2R, and AVP from mice maintained in LD or LL. Asterisks indicate significance, at P < 0.05, and n indicates the number of mice used. Scale bar = 100 µm in A.
DISCUSSION
The SCN is distinct in its VPAC2R expression
In the brain, VPAC2R protein has been reported in the SCN (Joo et al., 2004) and in gonadotropin-releasing neurons (Smith et al., 2000), although the reports showed only limited proof for the specificity of their antibodies. By using a specific antibody against VPAC2R, we found weak to moderate staining throughout the brain and intense staining in the SCN of mice, corroborating the reported SCN enrichment of Vipr2 mRNA (Usdin et al., 1994; Kalamatianos et al., 2004) and human Vipr2 transgene expression (Kallo et al., 2004). Although this distribution is consistent with broad and specific VIP–VPAC2R synaptic communication in the brain, it highlights the SCN as a unique nexus for VIP–VPAC2R signaling.
In general, VIP cell bodies are found clustered within the SCN and sparsely distributed in brain areas including the retina, olfactory bulb, amygdala, hippocampus, lateral septum, superior colliculus, periaqueductal gray, and layers within the neocortex (Staun-Olsen et al., 1985; Gall et al., 1986; Okamoto et al., 1992). VIP neurons project, however, to a wide range of targets including the lateral geniculate nucleus, neocortex, hippocampus, amygdala, median eminence, superior colliculus, and medial preoptic nucleus and throughout the medial hypothalamus to the paraventricular nucleus of the thalamus (Abrahamson and Moore, 2001; Heintz, 2004), all areas where we found VPAC2R immunoreactivity. The broad distribution of VPAC2R is also consistent with reported actions of VIP on firing rates in different brain areas (Yang et al., 2009), learning and memory (Gozes et al., 1993; Yamaguchi and Kobayashi, 1994), lactation (Harney et al., 1996; Smith et al., 2000; Gerhold et al., 2001), and, interestingly, as a neurotrophic factor (Cavallaro et al., 1996), but does not resolve why VPAC2R levels are so dramatically enriched in the SCN. It may be simply that the SCN differs from other brain areas in its reliance on VPAC2R over other VIP receptors (e.g., VPAC1R and PAC1R) (Otto et al., 2001; Jolivel et al., 2009).
We postulate that the distribution of VPAC2R supports the process of synchronizing circadian cells within the SCN, but likely serves alternate functions in other brain areas. In addition to the extensive evidence that the SCN, a master circadian pacemaker, depends on VIP–VPAC2R signaling to coordinate cellular and behavioral rhythms (Vosko et al., 2007), this study provides evidence that VPAC2R levels in the SCN are modulated by the circadian clock and that constant light can both alter the effects of circadian rhythms in behavior and increase VIP levels in SCN neurons.
Functional implications for circadian changes in VPAC2R abundance
Two previous reports (Cagampang et al., 1998; Shinohara et al., 1999) showed Vipr2 mRNA oscillations in a light–dark cycle with a pattern similar to the pattern we found for VPAC2R protein, falling from a peak around dawn to a trough around dusk. Here, we found that VPAC2R is also more abundant around subjective dawn than subjective dusk in constant darkness. This circadian modulation of VPAC2R protein differs from the arrhythmic or multipeak expression of Vipr2 mRNA reported previously (Cagampang et al., 1998; Shinohara et al., 1999), suggesting that circadian regulation of VPAC2R abundance happens post-transcriptionally. For example, VPAC2R proteins may be translated, stabilized, internalized, or degraded by the circadian clock. Regardless of how the rhythm arises, the presence of VPAC2R at all times of day in the SCN is consistent with the phase-shifting effects of VIP on SCN, with delays during most of the day and advances during the late night and early morning (An et al., 2011).
Because overexpression of VPAC2R shortens the circadian period of mouse locomotor activity (Shen et al., 2000), it seems likely that circadian changes in receptor abundance impact SCN function. Although the methods used here do not distinguish between active and inactive forms of the receptor, the majority of measured VPAC2R appeared near the plasma membrane and, thus, is likely active. We found the highest expression of VPAC2R in the late night and early morning, suggesting times when the SCN would be most sensitive to VIP. Consistent with this result, a previous report showed that less VIP was required to induce maximal phase shifts in the late night and early morning compared with the rest of the day (An et al., 2011). However, VIP has been reported to induce firing rate changes in more neurons (Reed et al., 2002) and larger phase shifts around dusk (An et al., 2011), when we found VPAC2R levels at their minimum in the SCN. This apparent contradiction could indicate enhancement of VIP signaling downstream of VPAC2R levels to produce larger physiological effects around dusk. Taken together, the discovery of circadian changes in receptor abundance thus raises important questions about the regulation of the receptor and its impact on intercellular signal transduction.
Evidence for signaling through proximate VPAC2R
We found that VPAC2R immunoreactivity was concentrated in cell bodies and dendrites and was low in axonal projections in vivo and in vitro. As with other members of the class B family of G-protein–coupled receptors, it is unclear whether VIP–VPAC2R signaling occurs primarily through proximate synaptic or paracrine communication. In flies, for example, pigment-dispersing factor receptor (PDFR), a functional homolog of mammalian VPAC2R (Im and Taghert, 2010), appears to be activated, at least in some cases, by local PDF release (Shafer et al., 2008). Activation of PDFR, like VPAC2R, increases intracellular cAMP levels (Shafer et al., 2008; An et al., 2011). The combination of VPAC2R expression on AVP cells in the dorsal SCN and VIP projections into this area implicates proximate, synaptic communication from the ventral to dorsal SCN via VIP–VPAC2R signaling. This is consistent with the observation that cells within the dorsomedial SCN desynchronize from each other when separated from the ventrolateral SCN (Yamaguchi et al., 2003; Buhr et al., 2010). The expression of VPAC2R on VIP neurons supports the hypothesis that VIP neurons synchronize each other through proximate VIP–VPAC2R signaling (Aton and Herzog, 2005). It is unclear, however, whether VIP signals to VPAC2R exclusively through proximate synapses, or also uses paracrine or humoral transmission.
How constant light changes circadian behavior
Constant illumination leads initially to a lengthening of circadian period in mice and ultimately can result in two or more peaks in daily activity, which correlates nicely with changes in the periodicity of the SCN (Ohta et al., 2005). We found that LL increases VIP and AVP levels, but not VPAC2R levels, in the SCN. Because we measured VPAC2R levels at one time in LL, we cannot rule out the possibility that prolonged light affects VPAC2R levels or localization at other times of day. However, because the promoters of both the VIP and AVP genes have cAMP response elements (Hahm et al., 1999; Kim et al., 2001) and VIP signaling increases intracellular cAMP (Rea, 1990; An et al., 2011), prolonged light could cause the release of VIP, activate VPAC2R, and increase intracellular cAMP to ultimately upregulate the abundance of these neuropeptides. Interestingly, 6 hours of light has been reported to reduce VIP content in the rat SCN (Shinohara et al., 1998). We postulate that relatively short light exposures will decrease VIP content in the SCN by increasing release whereas longer exposures will increase synthesis of VIP and AVP. This could be important for signaling photoperiod in the SCN. In constant light, this positive feedback could lead to constitutive VIP–VPAC2R signaling and disrupted circadian rhythms in the SCN and behavior.
Taken together, our results indicate that VPAC2R is well positioned for specialized synaptic communication from VIP neurons in the ventral SCN to cells throughout the SCN at all times of day. This VIP–VPAC2R signaling is likely to play a role in signaling day length and differs from elsewhere in the brain where VPAC2R levels are relatively low.
ACKNOWLEDGMENTS
The authors thank Tatiana Simon for expert technical assistance, and Gary London, the Alafi Imaging Facility staff, and the members of the Herzog and Taghert labs for valuable discussions.
Grant sponsor: National Institute of Mental Health; Grant number: 63419; Grant sponsor: National Institutes of Health; Grant number: P30 NS057105; Grant sponsor: Imaging Sciences; Grant number: Training Grant T90 DA022871.
LITERATURE CITED
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- An S, Irwin RP, Allen CN, Tsai CA, Herzog ED. Vasoactive intestinal polypeptide requires parallel changes in adenylate cyclase and phospholipase C to entrain circadian rhythms to a predictable phase. J Neurophysiol. 2011;105:2289–2296. doi: 10.1152/jn.00966.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Herzog ED. Come together, right…now: synchronization of rhythms in a mammalian circadian clock. Neuron. 2005;48:531–534. doi: 10.1016/j.neuron.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Barak Y, Russell JT, Whitnall MH, Ozato K, Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. I. Production and characterization of monoclonal antibodies. J Neurosci. 1985;5:81–97. doi: 10.1523/JNEUROSCI.05-01-00081.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T, Colwell CS, Waschek J, Piggins HD. Disrupted neuronal activity rhythms in the suprachiasmatic nuclei of vasoactive intestinal polypeptide-deficient mice. J Neurophysiol. 2007;97:2553–2558. doi: 10.1152/jn.01206.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhr ED, Yoo SH, Takahashi JS. Temperature as a universal resetting cue for mammalian circadian oscillators. Science. 2010;330:379–385. doi: 10.1126/science.1195262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagampang FR, Sheward WJ, Harmar AJ, Piggins HD, Coen CW. Circadian changes in the expression of vasoactive intestinal peptide 2 receptor mRNA in the rat suprachiasmatic nuclei. Brain Res Mol Brain Res. 1998;54:108–112. doi: 10.1016/s0169-328x(97)00327-6. [DOI] [PubMed] [Google Scholar]
- Cavallaro S, Copani A, D’Agata V, Musco S, Petralia S, Ventra C, Stivala F, Travali S, Canonico PL. Pituitary adenylate cyclase activating polypeptide prevents apoptosis in cultured cerebellar granule neurons. Mol Pharmacol. 1996;50:60–66. [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Liu X, Waschek JA. Disrupted circadian rhythms in VIP and PHI deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R939–R949. doi: 10.1152/ajpregu.00200.2003. [DOI] [PubMed] [Google Scholar]
- Francl JM, Kaur G, Glass JD. Regulation of vasoactive intestinal polypeptide release in the suprachiasmatic nucleus circadian clock. Neuroreport. 2010;21:1055–1059. doi: 10.1097/WNR.0b013e32833fcba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francon J, Lennon AM, Fellous A, Mareck A, Pierre M, Nunez J. Heterogeneity of microtubule-associated proteins and brain development. Eur J Biochem. 1982;129:465–471. doi: 10.1111/j.1432-1033.1982.tb07072.x. [DOI] [PubMed] [Google Scholar]
- Gall C, Seroogy KB, Brecha N. Distribution of VIP- and NPY-like immunoreactivities in rat main olfactory bulb. Brain Res. 1986;374:389–394. doi: 10.1016/0006-8993(86)90436-1. [DOI] [PubMed] [Google Scholar]
- Gerhold LM, Horvath TL, Freeman ME. Vasoactive intestinal peptide fibers innervate neuroendocrine dopaminergic neurons. Brain Res. 2001;919:48–56. doi: 10.1016/s0006-8993(01)02993-6. [DOI] [PubMed] [Google Scholar]
- Gozes I, Glowa J, Brenneman DE, McCune SK, Lee E, Westphal H. Learning and sexual deficiencies in transgenic mice carrying a chimeric vasoactive intestinal peptide gene. J Mol Neurosci. 1993;4:185–193. doi: 10.1007/BF02782501. [DOI] [PubMed] [Google Scholar]
- Hahm S, Mizuno TM, Wu TJ, Wisor JP, Priest CA, Kozak CA, Boozer CN, Peng B, McEvoy RC, Good P, Kelley KA, Takahashi JS, Pintar JE, Roberts JL, Mobbs CV, Salton SR. Targeted deletion of the Vgf gene indicates that the encoded secretory peptide precursor plays a novel role in the regulation of energy balance. Neuron. 1999;23:537–548. doi: 10.1016/s0896-6273(00)80806-5. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Brabet P, Fahrenkrug J. Mice lacking the PACAP type I receptor have impaired photic entrainment and negative masking. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2050–R2058. doi: 10.1152/ajpregu.90563.2008. [DOI] [PubMed] [Google Scholar]
- Harmar AJ, Marston HM, Shen S, Spratt C, West KM, Sheward WJ, Morrison CF, Dorin JR, Piggins HD, Reubi JC, Kelly JS, Maywood ES, Hastings MH. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell. 2002;109:497–508. doi: 10.1016/s0092-8674(02)00736-5. [DOI] [PubMed] [Google Scholar]
- Harney JP, Scarbrough K, Rosewell KL, Wise PM. In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology. 1996;137:3696–3701. doi: 10.1210/endo.137.9.8756535. [DOI] [PubMed] [Google Scholar]
- Heintz N. Gene expression nervous system atlas (GENSAT) Nat Neurosci. 2004;7:483. doi: 10.1038/nn0504-483. [DOI] [PubMed] [Google Scholar]
- Herzog ED. Neurons and networks in daily rhythms. Nat Rev Neurosci. 2007;8:790–802. doi: 10.1038/nrn2215. [DOI] [PubMed] [Google Scholar]
- Hughes AT, Fahey B, Cutler DJ, Coogan AN, Piggins HD. Aberrant gating of photic input to the suprachiasmatic circadian pacemaker of mice lacking the VPAC2 receptor. J Neurosci. 2004;24:3522–3526. doi: 10.1523/JNEUROSCI.5345-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AT, Guilding C, Piggins HD. Neuropeptide signaling differentially affects phase maintenance and rhythm generation in SCN and extra-SCN circadian oscillators. PLoS One. 2011;6:e18926. doi: 10.1371/journal.pone.0018926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SH, Taghert PH. PDF receptor expression reveals direct interactions between circadian oscillators in Drosophila. J Comp Neurol. 2010;518:1925–1945. doi: 10.1002/cne.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolivel V, Basille M, Aubert N, de Jouffrey S, Ancian P, Le Bigot JF, Noack P, Massonneau M, Fournier A, Vaudry H, Gonzalez BJ, Vaudry D. Distribution and functional characterization of pituitary adenylate cyclase-activating polypeptide receptors in the brain of non-human primates. Neuroscience. 2009;160:434–451. doi: 10.1016/j.neuroscience.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, Cha CI. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- Kalamatianos T, Kallo II, Piggins HD, Coen CW. Expression of VIP and/or PACAP receptor mRNA in peptide synthesizing cells within the suprachiasmatic nucleus of the rat and in its efferent target sites. J Comp Neurol. 2004;475:19–35. doi: 10.1002/cne.20168. [DOI] [PubMed] [Google Scholar]
- Kallo II, Kalamatianos T, Wiltshire N, Shen S, Sheward WJ, Harmar AJ, Coen CW. Transgenic approach reveals expression of the VPAC receptor in phenotypically defined neurons in the mouse suprachiasmatic nucleus and in its efferent target sites. Eur J Neurosci. 2004;19:2201–2211. doi: 10.1111/j.0953-816X.2004.03335.x. [DOI] [PubMed] [Google Scholar]
- Kim JK, Summer SN, Wood WM, Schrier RW. Role of glucocorticoid hormones in arginine vasopressin gene regulation. Biochem Biophys Res Commun. 2001;289:1252–1256. doi: 10.1006/bbrc.2001.6114. [DOI] [PubMed] [Google Scholar]
- Laemle LK, Ottenweller JE, Fugaro C. Diurnal variations in vasoactive intestinal polypeptide-like immunoreactivity in the suprachiasmatic nucleus of congenitally anophthalmic mice. Brain Res. 1995;688:203–208. doi: 10.1016/0006-8993(95)00507-m. [DOI] [PubMed] [Google Scholar]
- Loh DH, Abad C, Colwell CS, Waschek JA. Vasoactive intestinal peptide is critical for circadian regulation of glucocorticoids. Neuroendocrinology. 2008;88:246–255. doi: 10.1159/000140676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O’Neill JS, O’Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Chesham JE, O’Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc Natl Acad Sci U S A. 2011;108:12883–13884. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- Okamoto S, Okamura H, Terubayashi H, Akagi Y, Okamoto H, Ibata Y. Localization of vasoactive intestinal peptide (VIP) messenger RNA (mRNA) in amacrine cells of rat retina. Curr Eye Res. 1992;11:711–715. doi: 10.3109/02713689209000744. [DOI] [PubMed] [Google Scholar]
- Oliva AA, Jr, Atkins CM, Copenagle L, Banker GA. Activated c-Jun N-terminal kinase is required for axon formation. J Neurosci. 2006;26:9462–9470. doi: 10.1523/JNEUROSCI.2625-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C, Martin M, Wolfer DP, Lipp HP, Maldonado R, Schutz G. Altered emotional behavior in PACAP-type-I-receptor-deficient mice. Brain Res Mol Brain Res. 2001;92:78–84. doi: 10.1016/s0169-328x(01)00153-x. [DOI] [PubMed] [Google Scholar]
- Rea MA. VIP-stimulated cyclic AMP accumulation in the suprachiasmatic hypothalamus. Brain Res Bull. 1990;25:843–847. doi: 10.1016/0361-9230(90)90179-4. [DOI] [PubMed] [Google Scholar]
- Reed E, Cutler DJ, Brown TM, Brown J, Coen CW, Piggins HD. Effects of vasoactive intestinal polypeptide on neurones of the rat suprachiasmatic nuclei in vitro. J Neuroendocrinol. 2002;14:639–646. doi: 10.1046/j.1365-2826.2002.00826.x. [DOI] [PubMed] [Google Scholar]
- Reed HE, Meyer-Spasche A, Cutler DJ, Coen CW, Piggins HD. Vasoactive intestinal polypeptide (VIP) phase-shifts the rat suprachiasmatic nucleus clock in vitro. Eur J Neurosci. 2001;13:839–843. doi: 10.1046/j.0953-816x.2000.01437.x. [DOI] [PubMed] [Google Scholar]
- Schroeder A, Loh DH, Jordan MC, Roos KP, Colwell CS. Circadian regulation of cardiovascular function: a role for vasoactive intestinal peptide. Am J Physiol Heart Circ Physiol. 2011;300:H241–H250. doi: 10.1152/ajpheart.00190.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Spratt C, Sheward WJ, Kallo I, West K, Morrison CF, Coen CW, Marston HM, Harmar AJ. Overexpression of the human VPAC2 receptor in the suprachiasmatic nucleus alters the circadian phenotype of mice. Proc Natl Acad Sci U S A. 2000;97:11575–11580. doi: 10.1073/pnas.97.21.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Tominaga K, Isobe Y, Inouye ST. Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat: daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide. Y. J Neurosci. 1993;13:793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Honma S, Katsuno Y, Abe H, Honma KI. Two distinct oscillators in the rat suprachiasmatic nucleus in vitro. Proc Natl Acad Sci U S A. 1995;92:7396–7400. doi: 10.1073/pnas.92.16.7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara K, Tominaga K, Inouye ST. Luminance-dependent decrease in vasoactive intestinal polypeptide in the rat suprachiasmatic nucleus. Neurosci Lett. 1998;251:21–24. doi: 10.1016/s0304-3940(98)00491-1. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Funabashi T, Kimura F. Temporal profiles of vasoactive intestinal polypeptide precursor mRNA and its receptor mRNA in the rat suprachiasmatic nucleus. Brain Res Mol Brain Res. 1999;63:262–267. doi: 10.1016/s0169-328x(98)00289-7. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Jiennes L, Wise PM. Localization of the VIP2 receptor protein on GnRH neurons in the female rat. Endocrinology. 2000;141:4317–4320. doi: 10.1210/endo.141.11.7876. [DOI] [PubMed] [Google Scholar]
- Staun-Olsen P, Ottesen B, Gammeltoft S, Fahrenkrug J. The regional distribution of receptors for vasoactive intestinal polypeptide (VIP) in the rat central nervous system. Brain Res. 1985;330:317–321. doi: 10.1016/0006-8993(85)90691-2. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Bonner TI, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135:2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- Vosko AM, Schroeder A, Loh DH, Colwell CS. Vasoactive intestinal peptide and the mammalian circadian system. Gen Comp Endocrinol. 2007;152:165–175. doi: 10.1016/j.ygcen.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Kobayashi H. Effects of vasoactive intestinal peptide (VIP) on scopolamine-induced amnesia in the rat. Neuropeptides. 1994;26:153–158. doi: 10.1016/0143-4179(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Yang K, Trepanier CH, Li H, Beazely MA, Lerner EA, Jackson MF, MacDonald JF. Vasoactive intestinal peptide acts via multiple signal pathways to regulate hippocampal NMDA receptors and synaptic transmission. Hippocampus. 2009;19:779–789. doi: 10.1002/hipo.20559. [DOI] [PMC free article] [PubMed] [Google Scholar]