Abstract
Hemorrhagic stroke, including intracerebral hemorrhage (ICH), is a devastating subtype of stroke; yet, effective clinical treatment is very limited. Accumulating evidence has shown that mild to moderate hypothermia is a promising intervention for ischemic stroke and ICH. Current physical cooling methods, however, are less efficient and often impractical for acute ICH patients. The present investigation tested pharmacologically induced hypothermia (PIH) using the second generation neurotensin receptor (NTR) agonist HPI-201 (formerly known as ABS-201) in an adult mouse model with ICH. Acute or delayed administrations of HPI-201 (2 mg/kg bolus injection followed by 2 injections of 1 mg/kg, i.p.) were initiated at 1 or 24 hrs after ICH. HPI-201 induced mild hypothermia within 30 min and maintained body and brain temperatures at 32.7±0.4°C for at least 6 hrs without causing observable shivering. With the 1 hr delayed treatment, HPI-201-induced PIH significantly reduced ICH-induced cell death and brain edema compared to saline-treated ICH animals. When HPI-201-induced hypothermia was initiated 24 hrs after the onset of ICH, it still significantly attenuated brain edema, cell death and blood brain barrier breakdown. HPI-201 significantly decreased the expression of MMP-9, reduced caspase-3 activation, and increased Bcl-2 expression in the ICH brain. Moreover, ICH mice received 1-hr delayed HPI-201 treatment performed significantly better in the neurological behavior test 48 hrs after ICH. All together, these data suggest that systemic injection of HPI-201 is an effective hypothermic strategy that protects the brain from ICH injury with a wide therapeutic window. The protective effect of this PIH therapy is partially mediated through the alleviation of apoptosis and neurovascular damage. We suggest that pharmacological hypothermia using the newly developed neurotensin analogs is a promising therapeutic treatment for ICH.
Keywords: Intracerebral hemorrhage, pharmacological hypothermia, PIH, neurotensin receptor, ABS-201, HPI-201
INTRODUCTION
Stroke is a leading cause of human death and disability in the US and across the world (Baldwin et al., 2010). Intracerebral hemorrhage comprises 10–15% of all stroke cases. Patients suffering from ICH often have abysmal outcomes which are worse than ischemic stroke, with 30-day mortality estimated as high as 44%, and survivors typically suffer life-limiting disability (Broderick et al., 1993). Despite over three decades of research, the only FDA approved therapy for acute stroke is tissue plasminogen activator (tPA), which is limited to ischemic stroke with a narrow therapeutic window of only 4.5 hrs (Baldwin et al., 2010). To date, there has been no effective drug therapy for hemorrhagic stroke. Several clinical trials of potential treatments for hemorrhagic stroke to prevent hematoma expansion, control blood pressure, and remove clots have proven unsuccessful or induced inconsistent results (Broderick et al., 1993, Sutherland CS, 2008, Diringer MN, 2010). Effective therapies for this catastrophic subtype of stroke are urgently needed.
Mild to moderate hypothermia, also known as therapeutic hypothermia, that reduces body temperature by 3–5°C, is neuroprotective in pre-clinical and clinical studies for ischemic and hemorrhagic stroke subtypes (Hu et al., 2008, Theodorsson et al., 2008, Zhang et al., 2008, Fingas et al., 2009a, Torok et al., 2009). Therapeutic hypothermia protects the brain via multiple mechanisms. It reduces necrotic and apoptotic cell death (Maier et al., 1998), attenuates intracranial pressure (Schwab et al., 1998), increases cerebral perfusion pressure (Schwab et al., 1998), alleviates brain edema (Thome et al., 2005, Linares and Mayer, 2009), decreases inflammatory cytokine secretion (Sutcliffe et al., 2001, Yanagawa et al., 2002, Han et al., 2003), and improves the overall cerebral metabolic status in part by modulating ATP depletion (Linares and Mayer, 2009). Additionally, therapeutic hypothermia reduces the risk of hemorrhagic conversion and protects against hemorrhage-induced brain damage including fever that often accompanies ICH (Thome et al., 2005, Linares and Mayer, 2009). Clinically, hypothermia has been considered an approved therapy for patients after cardiac arrest and in children with hypoxic-ischemic encephalopathy (Nagel et al., 2008). In a recent pilot study on 12 patients with large spontaneous ICH, treatment using physical cooling-induced mild hypothermia (35°C) for 10 days prevented peri-hemorrhagic edema and improved 90 day survival (Kollmar et al., 2010). Another pilot study on 24 hemorrhagic stroke patients (8 in the hypothermic group and 18 in the control group) showed that mild hypothermia (34°C for 24 hrs, within 48 hrs after stroke) resulted in statistically significant improvement at 0.5 and 1 year follow up examinations using the modified Rankin Scale score (Abdullah and Husin, 2011). In a more recent clinical study, 25 patients with large supra-tentorial ICH (volume > 25 ml) were treated with mild hypothermia of 35°C for 8–10 days (Staykov et al., 2013). The body temperature in this trial was controlled using endovascular cooling catheters. Compared to a historical group of 25 patients with large ICH, peri-hemorrhagic edema volumes in the hypothermia group remained stable. Mortality rate was 8.3% in the hypothermia group versus 16.7% in the control group after 3 months and 28% versus 44 % after 1 year. This study suggests that mild hypothermia prevents the development of edema and reduces mortality rate in patients with large hemorrhagic stroke (Staykov et al., 2013). More active and coordinated efforts have been made to initiate a number of pilot studies on therapeutic hypothermia and a pan-European multicenter randomized controlled trial is underway (van der Worp et al., 2010).
A major dilemma that has limited the clinical application of therapeutic hypothermia in stroke patients is the inefficient and often impractical methods of physical cooling currently used to reduce body/brain temperatures. Physical cooling triggers undesirable shivering and vasoconstriction, both events make reduction and accurate control of temperature very challenging (Schwab et al., 1998, Staykov et al., 2013). With most advanced therapeutic hypothermia protocols, patients are admitted to the intensive care unit, typically sedated and mechanically ventilated. This rigorous approach exposes patients to multiple procedures and increased risk of side effects. In the search for a more efficient and practical hypothermia therapy, we have turned to a new concept of “regulated hypothermia” pharmacologically targeting central “thermoreceptors” and reducing the “set-point” in the thermoregulatory center, located in the hypothalamus (Katz et al., 2004). To this end, we have synthesized a group of neurotensin receptor agonists that showed marked effect in reducing body and brain temperatures in rodents and monkeys (Tyler et al., 1999, Fantegrossi et al., 2005). We refer to this approach as pharmacologically induced hypothermia (PIH) or pharmacological hypothermia and have recently reported neuroprotective effects of the novel neurotensin analog HPI-201 (formerly ABS-201) against ischemic stroke in mice (Choi et al., 2012).
Neurotensin (NT) is a tri-decapeptide found in the central nervous system (CNS). It is a well-accepted regulator of central temperature control as well as analgesia through its interaction with neurotensin receptors (NTRs) (Tyler-McMahon et al., 2000, Feifel et al., 2010). Neurotensin itself does not normally cross the blood-brain barrier (BBB) and is rapidly degraded by peptidases. There are two major subtypes of neurotensin receptors, NTR1 and NTR2; both are G protein coupled receptors. NTR1 receptor activation is associated with lowering body temperature and induction of analgesia. The clinical efficacy of first generation neurotensin analogs was limited by their inability to penetrate the BBB. Additionally, oral preparations lacked stability which further limited their utility (Dubuc et al., 1992). Later pharmaceutical research has determined that the 8–13 fragment of neurotensin [NT(8–13)] is the active moiety, and second generation neurotensin analogs that are biologically stable and can penetrate the BBB have been developed by Dix’s group (Kokko et al., 2005). NT[8–13] analogs were initially developed as potential antipsychotics (Hadden et al., 2005) and analgesics (Hughes et al., 2010), but were also noted for their hypothermic effect mediated through NTR1 in the brain (Dubuc et al., 1999). Our novel neurotensin derivatives such as HPI-201 have the following chemical structure: CH3-homolys-Arg-Pro-Tyr-tertLeu-Leu-COOH (Hadden et al., 2005). They cross the BBB, show high affinity for human NTR1 and effectively induce regulated hypothermia (Hadden et al., 2005, Orwig et al., 2009). HPI-201 induces regulated hypothermia by intravenous, intraperitoneal or oral administration (Hadden et al., 2005). Along with our previous investigation of the PIH therapy in ischemic stroke (Choi et al., 2012), the present study is the first effort to test the second generation hypothermic compound HPI-201 for early and delayed treatments of intracerebral hemorrhage in a rodent model. These investigations support that PIH therapy using novel compounds such as HPI-201 provides an effective and clinically feasibly approach after both ischemic and hemorrhagic strokes.
MATERIALS AND METHODS
Animals
C57/BL6 male mice, 10-weeks-old and approximately 25 g body weight, were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Animals were housed at room temperature with a 12-hr light/dark cycle in the pathogen-free Laboratory Animal Center for Research at Emory University. All the experimental protocols were approved by the Institutional Animal Care and Use Committee. Animals were randomly assigned to control or experimental groups and data analysis was performed under blinded or double blinded conditions. In total, 185 animals were used in the present investigation with approximately a 20% mortality rate due to ICH injury and surgery failure. Dead animals were eliminated from data analysis. Control and experimental groups normally contained 6 to 11 animals, which is common and suggested to be appropriate in hypothermia stroke therapy investigations based on estimates on variability and effect sizes (Kollmar et al., 2007, Crystal L MacLellan, 2012). Western blot analyses contained brain samples from 4 controls (n=4) and 6 drug-treated (n=6) animals.
Intracerebral hemorrhage stroke model
Intracerebral hemorrhage injury was achieved following published protocols (Rynkowski et al., 2008). Briefly, mice were anesthetized with isoflurane (3% induction, 1.5% maintenance) and 30 μL of autologous blood was drawn from the facial vein. A scalp incision was made along the midline and a 1-mm burr hole was then drilled on the right side of the skull. A 25G needle was vertically inserted into the brain through the bur hole (0.4 mm anterior to Bregma, 2.5 mm lateral, and 3.7 mm deep) and the blood was injected using a Hamilton syringe (Hamilton Company USA, Reno, NV). The injection rate was approximately 5 μL per min for the first 2 min and then 5 μL per min after an interval of 5 min. The syringe needle was left in place for an additional 10 min to prevent blood backflow. During the surgery and blood injection, animal body temperature was kept at 37±0.5°C using a homeothermic blanket control unit (Harvard Apparatus Limited, UK). Incisions were then closed using surgical glue (3M Corporate, St. Paul, MN). Except for the hypothermia group treated at 1 hr after stroke, all animals were kept in an incubator to maintain body temperature at 37±0.5°C for at least 4 hrs after surgery. To avoid postsurgical dehydration, 0.5 mL normal saline was given to each mouse by subcutaneous injection after surgery. There was no significant difference in mortality rate (~20%) between saline and HPI-201-treated groups.
Determination of body and brain temperatures
Core body temperature was measured as rectal temperature using the temperature data acquisition system Thermes USB (Physitemp Instruments, Inc., NJ). Six rectal probes were simultaneously connected to six mice and temperature data from all mice was continuously collected on-line by a receiver and the operation software (Dasylab Lite version 10; Physitemp Instruments, Inc.). Temperature data was automatically collected at an interval of 1 to 10 min and data was stored in an IBM computer for on-line and off-line analysis. This system spares the animals the anxiety and potential injury caused by frequent insertions of a temperature probe into the rectum. Brain temperature was monitored using a brain temperature probe (Harvard Apparatus, MA) placed on the surface of the cerebral cortex via the same route used for autologous blood injection, as described in previous reports with some modifications (Miyazawa and Hossmann, 1992). Body and brain temperature data taken every 15 to 30 mins are shown in this report. Temperature monitoring was performed for 6–9 hrs after the administration of saline or HPI-201.
Nissl staining
10-μm thick fresh frozen brain sections containing target lesions were fixed with a 1:1 mixture of 10% formalin and acetic acid for 10 min. After washing with distilled water for 5 min, slices were stained with a working solution containing buffer solution (0.1 M acetate acid and 0.1 M sodium acetate, 94:6) and Cresyl violet sodium at a ratio of 5:1. The sections were then dehydrated in 100% ethyl alcohol and mounted for use before rinsing quickly in 70% and 90% ethyl alcohol.
Measurement of acute brain edema
Brain water content was determined by calculating the percentage of dry to wet brain weight. Two hemispheres and cerebella from the control and experimental mice were dried at 110°C for 48 hrs, reweighed, and water content was calculated using the formula: (wet - dry brain weight) / wet brain weight.
Hemispheric enlargement was considered as the index of brain swelling and quantified using the formula: (ipisilateral hemisphere – contralateral hemisphere) / contralateral hemisphere. The whole area of hemisphere was measured with Image J software (NIH, Bethesda, MD) after Nissl staining.
Brain atrophy measurement
To evaluate brain atrophy, the area of ipsilateral and contralateral ventricles were measured in six brain sections with Nissl staining at different levels 100 μm apart. The ratio of ipsilateral to contralateral ventricles was calculated using the sum of the total ventricle area in each hemisphere.. Image J (NIH) was used for the measurement. The ventricle ratio is representative of the brain atrophy.
Western blot analysis
Fresh frozen brain tissue surrounding the hematoma was homogenized on ice at 4°C, followed by the addition of 1 ml RIPA buffer and 10 μl PMSF per 100 mg of brain tissue sample. After centrifuging at 17000 rpm for 15 min, protein concentration was determined by a UV spectrophotometer. Samples were preserved at −80°C before use. For immunoblotting, proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to PVDF membrane. 0.2% Tween in Tris-buffered saline (TBS-T) containing 5% BSA was used to block the membrane. Blotting membranes were incubated at 4°C overnight with primary antibody against cleaved caspase-3 (1:500; Cell Signaling, Danvers, MA), Bcl-2 (1:1000; Cell Signaling), or MMP-9 (1:1000; Millipore, Billerica, Massachusetts). After 3 washes with TBS-T, membranes were incubated with AP-conjugated secondary antibodies (Promega) or HRP-conjugated secondary antibodies (GE Healthcare, Piscataway, NJ, USA) for 2 hrs at room temperature. After a final wash with TBS-T, signals were detected with bromochloroindolyl phosphate/nitroblue tetrazolium (BCIP/NBT) solution (Sigma) or film. Signal intensity was measured using Image J (NIH) and normalized to the β-actin signal intensity.
Immunohistochemical staining
Preparation of brain sections was performed as previously described (Li et al., 2008b). Briefly, 10-μm thick coronal fresh frozen sections were prepared using a cryostat Vibratome (Ultapro 5000; St. Louis, MO). The slides were completely air-dried and then fixed in 10% buffered formalin phosphate for 10 min. After incubation in −20°C ethanol/acetic acid (2:1) solution for 12 min and 0.2% Triton-X 100 for 5 min, slides were blocked in 1% fish gelatin (Sigma) diluted in PBS at room temperature for 1 hr, and then incubated with the primary antibodies occludin (1:500, Invitrogen, Grand Island, NY) or Collagen IV (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) and diluted in phosphate buffered saline (PBS) overnight at 4°C. Slides were washed with PBS, incubated with secondary antibodies and then mounted using ProLong AntiFade (Invitrogen). Fluorescence was detected by fluorescence microscopy (BX61; Olympus, Tokyo, Japan). Hoechst 33342 (Molecular Probes, Eugene, OR) was used as a nuclear marker and background staining.
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining of cell death
Detection of cell death was carried out using the TUNEL staining kit (DeadEnd™ Fluorometric TUNEL system, Promega, Madison, USA) as previously described (Li et al., 2008a). Brain sections were prepared and incubated with nucleotide mix and the terminal deoxynucleotidyl transferase recombinant enzyme (rTdT).enzyme in equilibration buffer at 37°C for 1 hr, and the reaction was stopped with 2x SSC. TUNEL positive cells show fluorescence by catalytically incorporating fluorescein-12-dUTP at the 3′-OH DNA ends using rTdT. Pictures were taken using a BX61 Olympus fluorescence microscope (Tokyo, Japan).
Counting immunoreactive cells
Cell count was performed following a modification of the principles of design based stereology. Systematic random sampling was employed to ensure accurate and non-redundant cell counting (Schmitz and Hof, 2005). Every section under analysis was at least 90 μm away from the next section. For each animal, six 10-μm thick sections spanning the entire region of interest were randomly selected for cell counting. Counting was performed on 6 randomly selected non over-lapping fields per section. Sections from different animals represent the same area in the anterior-posterior direction.
Behavioral evaluation
A modified neurological stroke scale (NSS) was applied to evaluate differences in behavior among treated and untreated animals as previously described (Chen et al., 2001). Briefly, this test consists of four parts: motor, sensory, balance beam tests, and the presence of reflexes or abnormal movements. Neurologic functional status was scored on a scale of 0 to 18 (fully intact, 0; maximum severity, 18). One point was scored for the inability to complete the test, or if the tested reflex was absent. Behavioral testing started 48 hrs after ICH in all experimental groups. The NSS was evaluated up to 14 days after stroke.
Statistical analysis
Results are shown as mean ± SEM. Statistical analysis was conducted using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA). Comparisons between two groups were achieved by the Student’s t test. For multiple comparisons, one-way ANOVA was applied. For the modified neurological stroke scale (NSS) test, we present raw data and the median and data distribution at each time point. The normal Gaussian distribution of the NSS score was verified using the Minitab statistical software (Minitab Inc., State College PA). Statistical analysis of non-parametric data was performed using the Kruskl-Wallis test followed by Dunn post-hoc correction for multiple group comparisons (GraphPad Software, Inc.) (Auriat and Colbourne, 2008, Fingas et al., 2009b). To facilitate comparisons to many previous reports that used Analysis of Variance, the values of mean and SEM of NSS scores are also presented and analyzed using one-way ANOVA with Bonferroni’s post-hoc correction. Significant difference was defined as p<0.05.
RESULTS
Drug administration and body temperature control
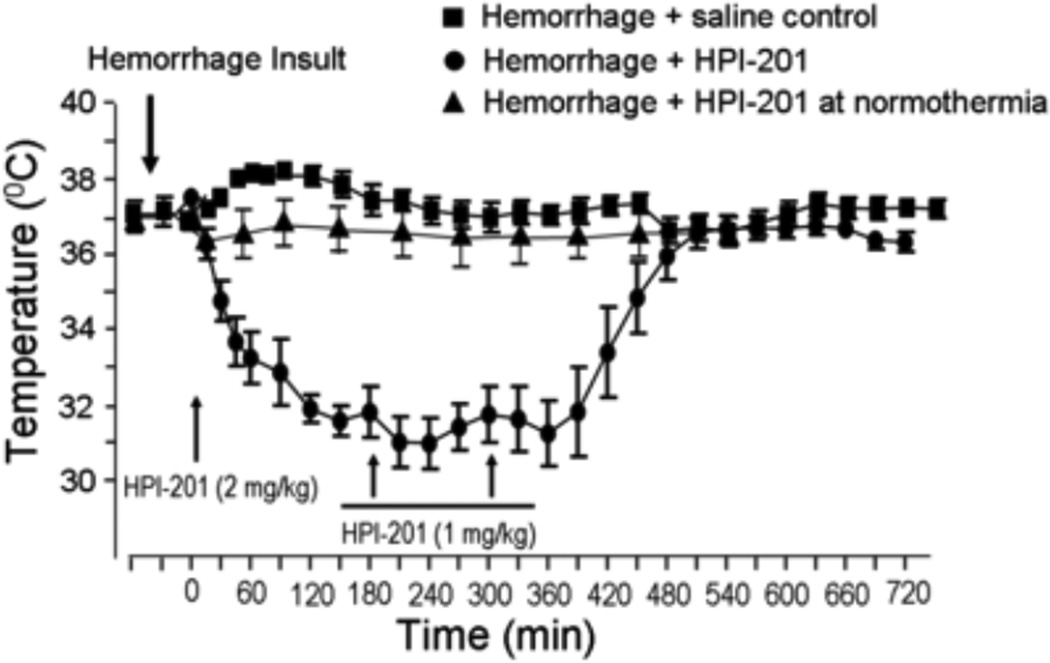
HPI-201 was administrated by intraperitoneal injection to induce hypothermia in ICH mice. The dosage was selected based on our previous investigation on ischemic stroke mice to induce a mild to moderate hypothermic effect (2–6°C reduction) (Choi et al., 2012). After one HPI-201 injection at 2 mg/kg, a hypothermic effect was seen within 15 min without triggering observable shivering responses. The hypothermic effect after the bolus injection lasted for at least 2 hrs (Fig. 1). Two additional injections of HPI-201 (1 mg/kg, i.p., interval of 1.5–2 hrs) maintained the temperature at 32.7±0.4°C for at least 6 hrs (Fig. 1). Comparable reductions in brain temperature was confirmed by an intracranially inserted temperature probe (data not shown, but see (Choi et al., 2012). Body temperature gradually returned to normal levels after the last injection at a rate of ~0.04°C/min and there was no temperature overshoot thereafter (Fig. 1).
Figure 1. Hypothermic effect of HPI-201 in hemorrhagic stroke mice.
Bolus injection of HPI-201 (2 mg/kg, i.p.) was initiated at 1 or 24 hrs after the onset of intracerebral hemorrhage insult in adult male mice. The line graph shows rectal temperature changes in ICH animals before and after 1 hr delayed injection of HPI-201. In saline injection control animals, body temperatures measured by a rectal thermometer remained relatively stable around 37°C (■). In mice that received HPI-201, body temperatures decreased soon after the injection and reached a mild to moderate hypothermia level within 30–60 min (●).
To maintain the hypothermic effect around 32°C, two additional doses (1 mg/kg) of HPI-201 were injected every 2 hrs (arrows) with 0.5 hr time variation depending on the temperature measured at any given time point. The duration of hypothermia in this investigation was maintained for at least 6 hrs. No overshoot of the temperature occurred during and after rewarming. As a control for the study of the essential role of low temperature in HPI-201-induced brain protection, a group of animals in later experiments received the same dosage of HPI-201 while their body temperature was forced to maintained at 37°C using a heating blanket (▲). N=8 per group, except n=7 in HPI-201 at normothermia group.
Acute brain protection with pharmacological hypothermia after ICH
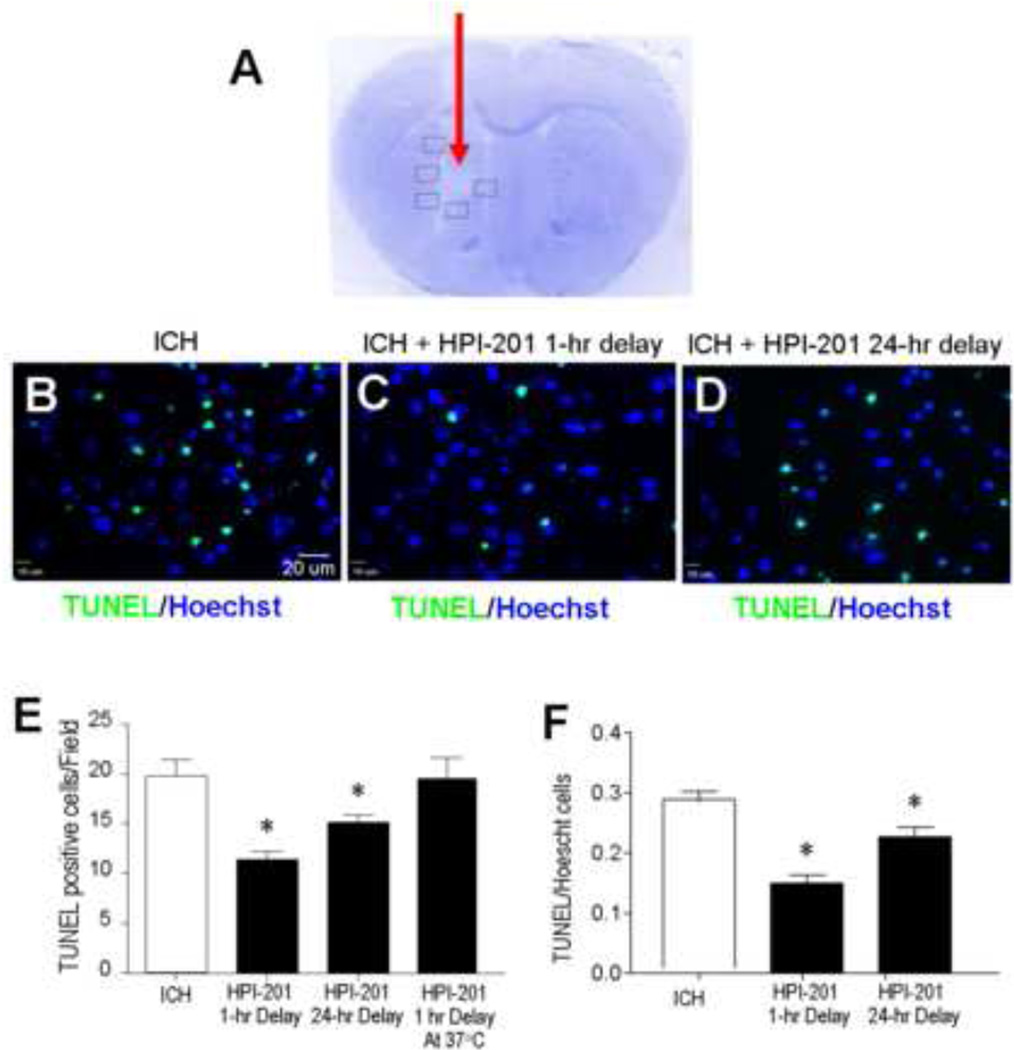
Noticeable brain tissue damage in and around the hematoma was seen in the intracerebral hemorrhage model 2–3 days after autologous blood injection (Fig. 2A). Mice received saline or HPI-201 treatment with 1 or 24 hrs delay after the onset of ICH. Cell death in the peri-hemorrhagic region was assessed using TUNEL staining 48 hrs after ICH. TUNEL-positive cells were reduced by 30–50% counted either by absolute number per survey field or by the percentage of total Hoechst 33342-positive cells in the field (Fig. 2B - 2F). On the other hand, the cytoprotective effect of HPI-201 was eliminated if, during HPI-201 treatment, the body temperature was physically maintained at ~37°C using a heating pad (Fig. 2E). This control experiment demonstrates that the protective effect of HPI-201 was achieved via its hypothermic effect. Similar results were also observed in our previous study in ischemic stroke (Choi et al., 2012).
Figure 2. Intracerebral hemorrhage-induced brain damage, cell death and effect of pharmacological hypothermia.
A. Nissl staining revealed tissue damage in the hematoma area 3 days after autologous blood injection (arrow). Boxed frames show the location of assay areas of TUNEL-positive cells. No similar tissue damage was seen in contralateral hemispheres. B - D. TUNEL staining of DNA damage and cell death in the perihemorrhage region of brain sections 48 hrs after ICH. Immunofluorescent images showing TUNEL-positive cells (green) and total cells stained with Hoechst 33342 (blue) in the three experimental groups: ICH control, ICH plus 1 hr delayed treatment with HPI-201 and ICH plus 24 hr delayed treatment with HPI-201. E. The number of TUNEL-positive cells in randomly selected survey fields. HPI-201 treatment initiated either 1 hr or 24 hr after ICH significantly decreased the amount of cell death. As a control that was performed after the initial experiments, additional animals that received HPI-201 injection were kept at 37°C using a heating blanket with feedback control (see Figure 1). These animals showed similar cell death as ICH only controls. F. The ratio of TUNEL-positive cells against total cells was compared in hemorrhage mice that received saline or delayed HPI-201 treatments. *. p<0.05 vs. ICH control; n=7 and 8 in 1 hr and 24 hr delay groups, respectively; n=7 in HPI-201 at 37°C group. All ICH control animals received saline injection in this investigation.
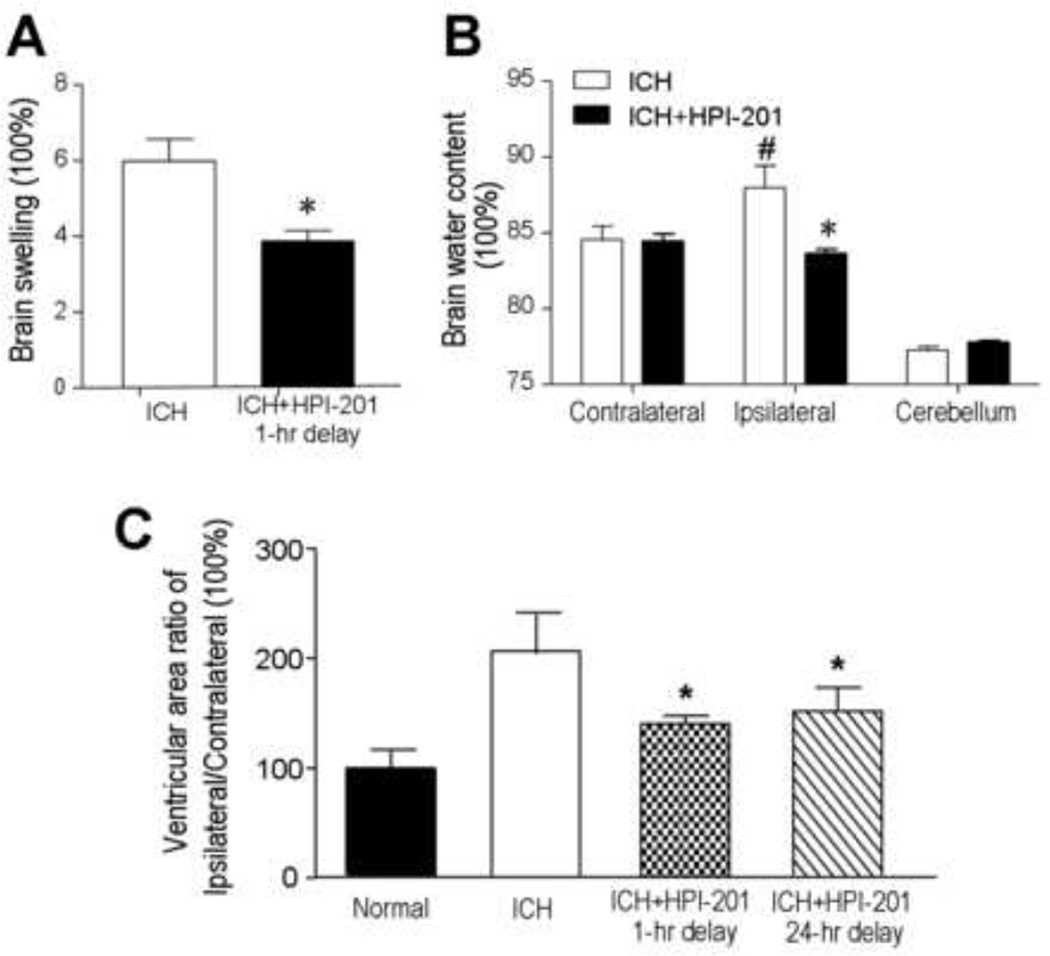
In the brain edema assay, areas of contralateral and ipsilateral hemispheres were compared. An enlargement of the ipsilateral hemisphere was seen in the ICH group 24–48 hrs after ICH, while HPI-201 treatment significantly attenuated this increase (Fig. 3A). To confirm that the enlarged brain area was due to brain edema, brain water content was measured 24 hrs after stroke in ICH plus saline controls or ICH plus HPI-201 mice. The wet and dry weights of ipsilateral and contralateral hemispheres and the cerebella were measured. No difference was detected in water content of the contralateral hemispheres between ICH controls and HPI-201-treated mice. Similarly, no difference was seen in the water content of cerebella of both sides between control and HPI-201-treated mice (Fig. 3B). The water content of the ipsilateral hemisphere was significantly increased in ICH mice. In ICH plus HPI-201 mice, however, this increase was prevented (Fig. 3B). In long-term experiments, either the 1- or 24-hr delayed treatment with HPI-201 showed a marked effect of preventing the development of brain atrophy measured 21 days after ICH (Fig. 3C).
Figure 3. Attenuation of hemorrhage-induced brain edema and brain atrophy with pharmacological hypothermia.
Brain edema was assessed 24 or 48 hrs after intracerebral hemorrhage. A. PIH therapy using HPI-201 was initiated 1 hr or 24 hrs after ICH and hemispheric enlargement, as an index of brain swelling, was quantified by the formula: (ipsilateral hemisphere - contralateral hemisphere) / contralateral hemisphere. PIH therapy noticeably reduced brain swelling in the hemorrhagic brains. B. Brain water content was measured in the experimental group with 24 hr delayed treatment of HPI-201. Forty eight hrs after ICH, there was no difference in water content of contralateral hemispheres and cerebella between ICH controls and ICH plus HPI-201 groups. Increased water content, however, was seen in the ipsilateral hemispheres of ICH mice and this increase was completely blocked by the PIH therapy using HPI-201. *. p<0.05 vs. ipsilateral side, #. P<0.05 vs. contralateral side; n=6 and n=4 in A and B, respectively. C. The long-term effect of HPI-201 on brain atrophy was measured 21 days after ICH as the ratio of areas of ipsilateral to contralateral ventricles. Both 1 hr and 24 hrs delayed treatment with HPI-201 prevented brain atrophy shown as reduced ventricular area ratio in HPI-201 treated animals. N=10, 11, 9 and 11 for sham, ICH control, ICH with 1 hr delay, ICH with 24 hr delay, respectively; *. p<0.05 vs. ICH control.
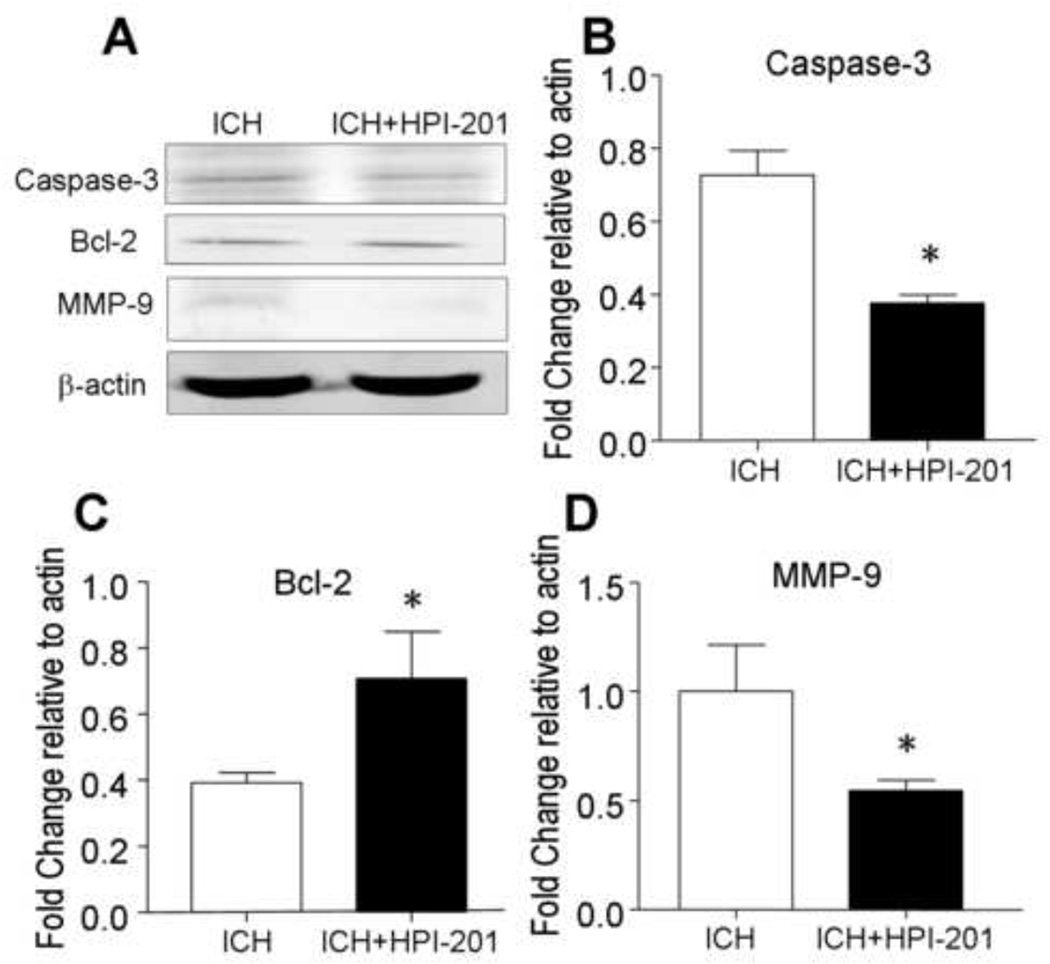
Around the hematoma in the ICH brain, noticeable caspase-3 activation was observed in Western blot analysis, indicating an apoptotic component of cell death (Fig. 4A). HPI-201 treatment 24 hrs after ICH significantly decreased caspase-3 activation (Fig. 4A and 4B). Coincidentally, the anti-apoptotic protein Bcl-2 expression level was elevated in hypothermia treated brains (Fig. 4C). Meanwhile, the matrix metallopeptidase-9 (MMP-9) expression, a marker for tissue breakdown and inflammatory brain injury after ICH (Rosenberg et al., 2001, Xue et al., 2009), was reduced by HPI-201 (Fig. 4D). This is consistent with previous knowledge that hypothermia reduces MMP-9 expression and blood brain barrier permeability after ischemic and traumatic brain injury (Hamann et al., 2004, Lee et al., 2005, Truettner et al., 2005).
Figure 4. Effects of pharmacological hypothermia on hemorrhage-induced cell death signaling.
Western blot analysis was used to examine apoptotic mechanisms and cell death signals 48 hrs after ICH and 24 hrs after delayed HPI-201 treatment. A. Western blotting gel images showed a decreased expression of activated caspase-3 and an up-regulation of Bcl-2 in the ischemic brain treated with HPI-201, both expected to attenuate apoptotic cell death. Hypothermia therapy also decreased MMP-9 expression level, suggesting reduced tissue breakdown. B - D. Quantified data analysis for expression levels of caspase-3 (B), Bcl-2 (C), and MMP-9 (D). *. p<0.05 vs. ICH control; n=4, 6, and 6, respectively.
Delayed pharmacological hypothermia attenuated blood brain barrier disruption
Collagen IV is the major component of basal lamina essential for maintenance of the endothelial permeability barrier. The 24-hr delayed treatment with HPI-201 did not show a significant effect on Collagen IV density area in the perihemorrhagic zone (Fig. 5C). However, when fragmented collagen IV staining was counted, there was a significant decrease in the number of fragmented pieces of collagen IV positive vessels, suggesting attenuated breakdown of microvessels in the HPI-201-treated post-hemorrhage brain (Fig. 5D). Occludin is a tetraspan integral membrane protein in epithelial and endothelial tight junction structures and its down-regulation/degradation contributes to endothelial barrier disruption (Wacker et al., 2012). Occludin expression in the ipsilateral region of the post-ICH brain was substantially reduced compared to the contralateral side (Fig. 6). HPI-201 treatment significantly prevented ICH-associated decreases in occludin expression in the brain (Fig. 6).
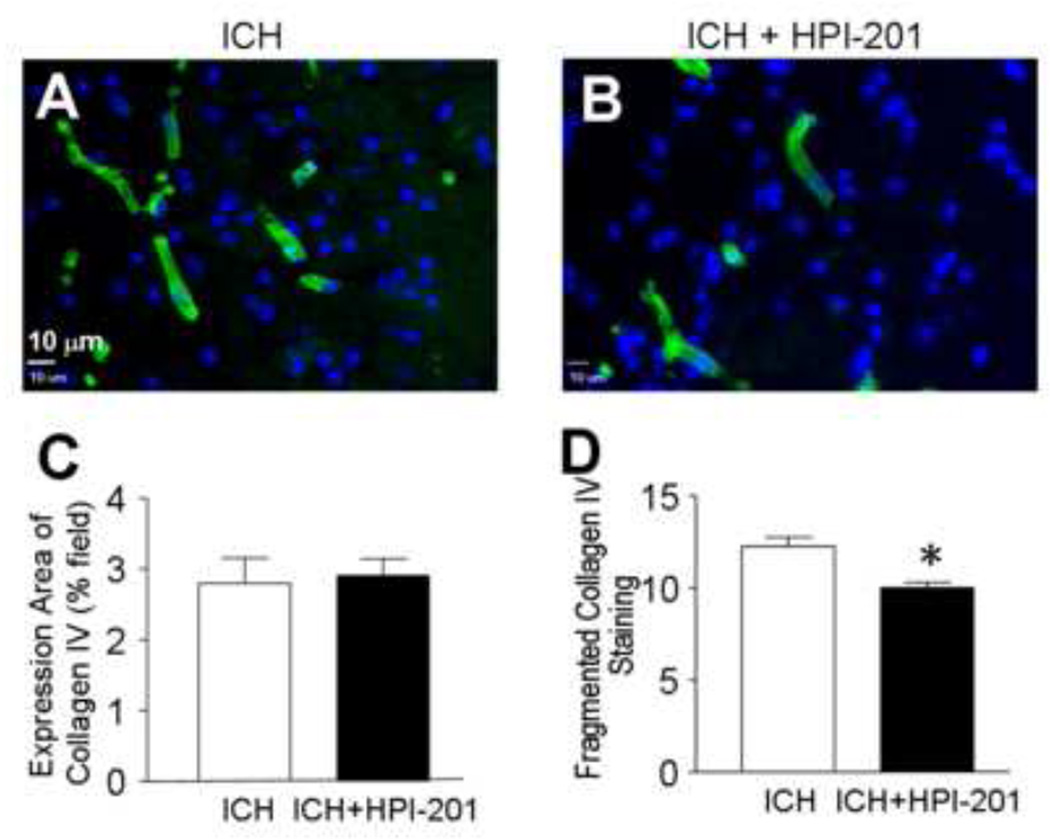
Figure 5. Protective effects of pharmacological hypothermia on vessels in the post-hemorrhagic brain.
Collagen IV staining was used to evaluate and quantify vessel rupture of tissues around the hematoma 48 hours after ICH and 24 hrs after delayed HPI-201 treatment. A and B. Immunohistochemical images of collagen IV (green). Blue color is Hoechst 33342. C. The area of collagen IV expression was measured and its percentage of the total survey field was calculated. There was no difference between ICH and ICH plus HPI-201 groups. N=5 per group. D. The number of fragmented pieces of collagen IV positive vessel was reduced in ICH plus HPI-201 compared to ICH without the treatment. N=5 and 6 per group, respectively. *. p<0.05 vs. ICH control.
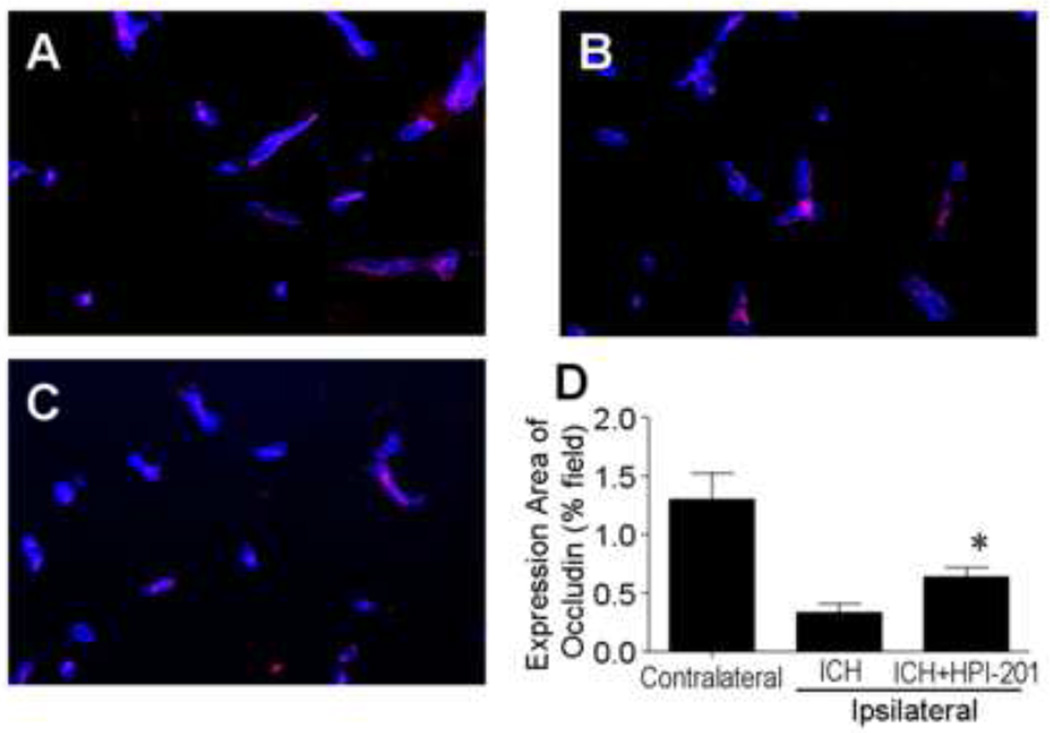
Figure 6. Attenuation of blood brain barrier disruption in the ICH brain by pharmacological hypothermia.
Blood brain barrier was evaluated as the expression of occludin in fresh frozen slices 48 hrs after ICH and 24 hrs after HPI-201 treatment. A - C. Immunostaining images showing occludin expression (red) in the contralateral (A), ipsilateral sides saline control (B) and ipsilateral side treated with HPI-201 (C). Blue color is collagen IV. D. Quantified data of occludin expression shows partial recovery of occludin in the hypothermia-treated group. N=4 animals in each group, *. p<0.05 vs. contralateral side.
The effect of HPI-201-induced hypothermia on functional recovery after ICH
To evaluate functional benefits of the PIH therapy in post-ICH mice, the modified neurological stroke scale (NSS) was tested different days after ICH. In ICH mice that received HPI-201 treatment with 1 hr delay, the NSS score showed a statistically significant benefit of reducing functional deficits at 2 days after ICH (n=9 and 12 in ICH control and ICH plus HPI-201 groups, respectively; p<0.05 by Kruskal-Wallis test and one-way ANOVA) (Fig. 7). The trend of this functional effect of HPI-201 continued for up to 14 days after ICH, but was not statistically significant for the time points of 7 and 14 days after ICH. This result indicated that with 1-hr delayed treatment, HPI-201 could at lease accelerate functional recovery after ICH. In mice that received the 24-hr delayed treatment of HPI-201, NSS scores did not show clear indication of functional benefits, although a seemingly better average score was seen at 14 days after ICH (n=9 and 9 in ICH control and ICH plus HPI-201 mice, respectively, p>0.05; Fig. 7).
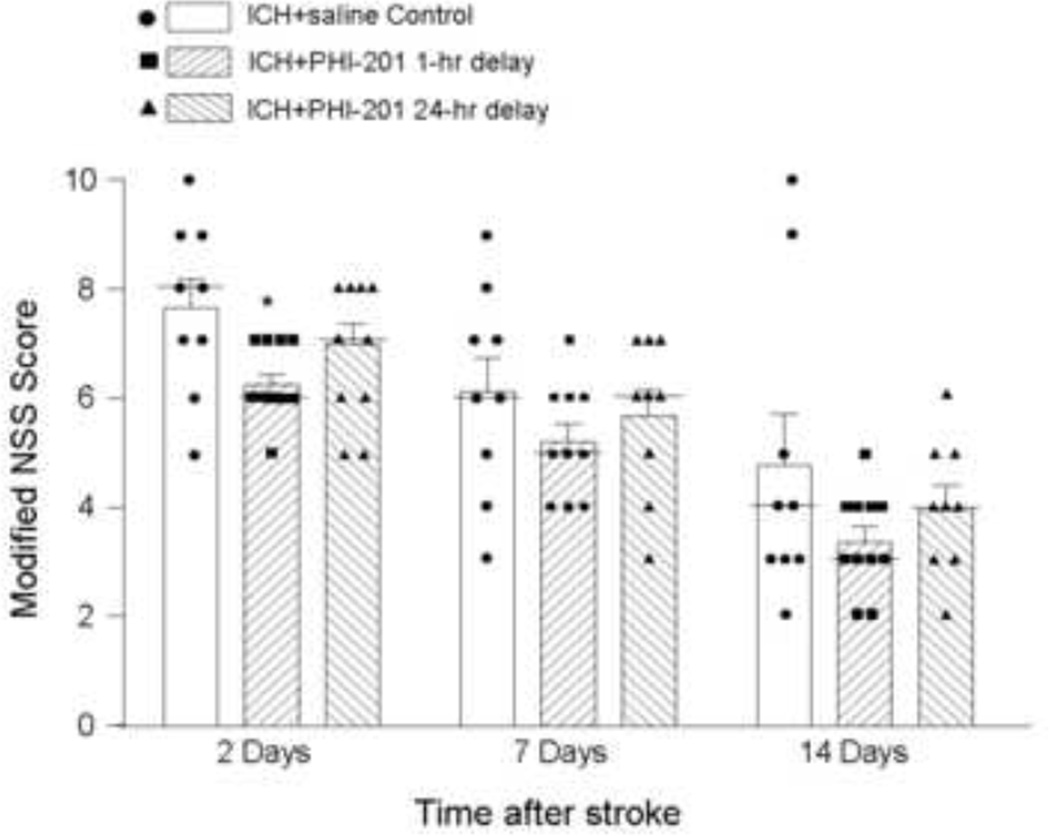
Figure 7. Functional recovery after hemorrhagic stroke.
The behavioral test was performed at different days in ICH control mice and ICH mice treated with HPI-201 with 1 hr or 24 hr delay. An 18-grade modified NSS test was used to evaluate functional damage and recovery of experimental animals. Both score distribution plus the median (a thin bar located among the scattered data) and calculated mean and SEM (bar graph) are shown in the figure. All data were verified for normal Gaussian distribution before statistical analysis. The Kruskal-Wallis test and one-way ANOVA test were used for comparisons of the three experimental groups, and both methods showed a significant effect of reducing the functional deficits 2 days after ICH in mice received 1-hr delayed HPI-201 treatment. The effect of 24 hr-delayed HPI-201 treatment was not statistically significant when the NSS scores were compared with ICH controls. On the other hand, there was no statistical difference between the NSS scores of 24 hr and 1-hr delayed HPI-201 treatment. The same animals showed trends of improved behavioral recovery at 7 and 14 days after ICH, but statistical analyses showed no significant difference. *. p<0.05 vs. ICH control, n=9, 12, and 9 for the three group, respectively.
DISCUSSION
The present investigation demonstrates the effectiveness of a pharmacologically induced hypothermia therapy using the neurotensin derivative compound HPI-201 in a mouse hemorrhagic stroke model. We showed that HPI-201 effectively reduced body temperature to a neuroprotective level and antagonized ICH-induced brain edema and cell death in the post-hemorrhage brain. Blood brain barrier and blood vessel damage were significantly attenuated by the HPI-201 treatment. Moreover, HPI-201 maintained its brain protective effects even when the PIH therapy was initiated as late as 24 hrs after ICH. In addition, the PIH therapy showed a potential of promoting or accelerating functional recovery after ICH. These observations are the first evidence suggesting a successful PIH therapy for the treatment of ICH.
Forced cooling methods to attain hypothermia have shown effectiveness in brain protection in ischemic and some hemorrhagic stroke models (Barone et al., 1997, Yenari et al., 2002, Konstas et al., 2006, Fingas et al., 2009a). To overcome the slow process of forced cooling and its associated side effects, recent studies have developed several new approaches for inducing and maintaining physical cooling effects. These methods require sophisticated equipment and/or invasive techniques and thus may benefit only a limited number of stroke patients. For years, pharmacological hypothermia has been pursued for the treatment of stroke and other CNS injuries. Neurotensin receptors are associated with analgesic, hypothermic and antipsychotic activities and NTR1 is thought to be the receptor target responsible for hypothermia (Dubuc et al., 1992). Since HPI-201 is BBB permeable and biologically stable, it can act on the central hypothalamic thermoregulatory center without causing shivering and hypertension which are known adverse responses associated with physical cooling. The mechanism for the lack of shivering is not fully understood but it is likely due to a “resetting” of the thermoregulatory “set point” (Handler et al., 1994). HPI-201 induced cooling has a reasonable re-warming rate and shows no overshoot in temperature and no adverse effects on blood glucose, pH, and local cerebral blood flow (Choi et al., 2012).
In the treatment of hemorrhagic stroke, HPI-201 appears to have a therapeutic window of up to 24 hrs after the hemorrhagic insult. In ischemic stroke models, on the other hand, our previous study showed neuroprotective effects with one-hour delayed HPI-201 treatment and others showed a therapeutic window of 2–4 hrs using physical cooling methods (Colbourne et al., 2000, Maier et al., 2001, Ohta et al., 2007, Clark et al., 2008, Choi et al., 2012). From available data, the therapeutic window of hypothermia therapy is likely much wider for hemorrhagic stroke than for ischemic stroke. This is most likely due to the fact that most brain injury in hemorrhagic stroke comes from the secondary damage after bleeding, which is a relatively slow process compared to an acute ischemic insult that can trigger injurious cellular and molecular responses within minutes (Siesjo et al., 1991, Li et al., 2000, Robinson et al., 2009).
The fact that HPI-201 quickly induces hypothermia without shivering and shows brain protective effects in both ischemic and hemorrhagic strokes should be of imperative significance in clinical settings. It would be an ideal situation, for example, to initiate PIH therapy on-site by paramedical personnel without having to identify the ischemic or hemorrhagic nature of the stroke attack. Given the reasonable cooling duration of about 2 hrs after the bolus injection of HPI-201, regulated hypothermia can be terminated or continued when the patient is more fully evaluated later by a stroke team. In regards to the early administration of therapeutic hypothermia, a report from MacLellan et al. cautioned that physical cooling initiated at 1 hr after the collagenase ICH model could aggravate bleeding presumably resulting from reduced activity of clotting enzymes and elevated blood pressure (MacLellan et al., 2004). This adverse effect of hypothermia does not occur in the antologous blood injection model of ICH (MacLellan et al., 2006). Whether different consequences of early administration of hypothermic treatment occur in human patients needs to be carefully evaluated. This consideration may be especially important for some ICH patients with protracted bleeding or re-bleeding.
While it remains to be determined, early initiation of PIH therapy may also prolong the therapeutic window for tPA treatment, offering a further advantage for ischemic stroke patients. This idea is strongly supported by a recent investigation in thromboembolic stroke rats that mild hypothermia of 34°C induced by a cooling pad showed a synergistic effect in reducing infarct volume when applied in combination with tPA. In neurological functional assessments, animals of the combination therapy performed better than the thrombolysis alone group (Kallmunzer et al., 2012). On the other hand, as mentioned above, cautions will be needed when considering hypothermia treatments for different stroke types. A recent report shows that, although therapeutic hypothermia is protective against mild hypoxia-ischemia brain injury in a neonatal rat model, immediate physical hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats (Sabir et al., 2012). Considering the reported adverse effect of early hypothermia in the collagenase ICH model reported by MacLellan et al. (MacLellan et al., 2004), it is necessary to specifically examine how the PIH therapy may be combined with tPA treatment after acute stroke.
Using HPI-201, we are able to show a marked neuroprotective effect with 6-hr duration of hypothermia after either ischemic or hemorrhagic strokes (Choi et al., 2012). In previous investigations using physical cooling methods, neuroprotective effects were observed in as short as a 4-hr duration of hypothermia at a temperature of 34°C (Kollmar et al., 2007). Many studies have shown neuroprotection with prolonged hypothermia up to 12–24 hrs or even days in rodent models and in humans (MacLellan et al., 2004, Kollmar et al., 2010). Currently, the duration of necessary cooling required for therapeutic benefits is not well defined. It most likely depends on the severity and type of the insult, mechanisms involved, timing of the treatment, progression of pathophysiological events and inter-species differences. It is possible that prolonged pharmacological hypothermia up to 12–24 hrs may result in enhanced brain protective effects. This can be achieved by additional drug injections and closer observation of possible side effects associated with prolonged hypothermia such as pneumonia (Kollmar et al., 2010). To this end, it is necessary to verify that the hypothermic compounds do not cause tolerance or desensitization with repeated administrations.
The protective mechanism of hypothermia therapy is multifaceted. As seen in this study, HPI-201-induced hypothermia alleviated apoptosis and reduced cerebral edema as well as brain atrophy. It is recognized that apoptotic cell death occurs in the perihemorrhagic zone (Hwang et al., 2011). Edema formation and herniation may lead to significant mass deformation that may further increase intracerebral and/or intraventricular pressure, contributing to late deterioration in the course of ICH (Ropper, 1986, Zazulia et al., 1999, Xi et al., 2006). It may be necessary to point out that edema alone may not be a determining factor in hemorrhagic stroke because some evidence suggested that attenuated edema (reduction from 5% to 2% increase in brain water content) did not result in histological or behavioral benefits (Fingas et al., 2007). As shown in an autologous blood injection rat model, in addition to attenuated brain edema, multiple mechanisms including reduced BBB disruption, inflammation and oxidative damage, may mediated the protective effect of brain hypothermia (Kawanishi et al., 2008). Another possibility is that edema reduction in that particular investigation was not large enough to establish therapeutic benefits. Clinically, the role of perihemmorrhagic brain edema and the benefits of reducing this delayed pathological event have not been well elucidated and contradictory data have been reported (Gebel et al., 2002, Arima et al., 2009). For example, recent data from the Intensive Blood Pressure Reduction in Acute Cerebral Hemorrhage (INTERACT) study did not show any influence of edema on morbidity and mortality (Arima et al., 2009). Some clinical studies looked at acute edema (1 hr after ICH) but did not consider the development of edema that peaks several days after ICH (Gebel et al., 2002). In patients with large intracerebral hemorrhage, edema may double its size from day 1 to day 14, while physical cooling induced 12 hrs after symptom onset and lasted for 10 days could prevent this edema expansion (Kollmar et al., 2010). In the present investigation on an experimental ICH model, we examined edema 1 to 2 days after the onset of ICH. We demonstrated that delayed treatment with PIH therapy even 24 hrs after ICH was successful in attenuating edema formation. This is highly consistent with the notion that edema formation is a relatively delayed and slow event and therefore pharmacological intervention may have a wider therapeutic window than that after ischemic stroke. As one of many brain edema assessments, brain water content was measured in our study and a relatively higher value of ~84% was observed in the contralateral hemispheres. This is consistent with the notion that brain water content is age and body weight dependent. The relatively younger animals used in our study normally have higher water content than older animals while adult mice brains usually contain about 81% water (Agrawal et al., 1968).
Functional outcomes were significantly better in the hypothermia group with 1 hr delayed HPI-201 treatment. Although the improvement in the NSS score appears modest, it is possible that even a small functional benefit may be clinically important for improving life quality of stroke patients. These functional benefits are encouraging because it has long been recognized that functional improvements using neuroprotective approaches are difficult after ICH due to the lack of penumbral tissue (Herweh et al., 2007). More importantly, and to the best of our knowledge, there have been only a limited number of animal studies that could show any functional benefits of hypothermia for the treatment of ICH (MacLellan et al., 2004, MacLellan et al., 2006, Fingas et al., 2009a). In addition to the pathological nature of the lack of penumbra in ICH damage, the type of behavioral and functional tests in ICH animal models may also affect the result of these assessments. Finally, although ICH does occur in young and middle age patients, it will be essential to evaluate the functional changes in ICH models of aged animals.
There are several limitations in the present investigation. The blood injection-induced ICH model may not fully reflect the pathology of clinical hemorrhagic stroke. In this case, the use of the collagenase-induced ICH model might be an alternative methodology to investigate the mechanism of the PIH effect (Manaenko et al., 2011). Additionally, the duration of hypothermia was 6 hrs in our experiments. This time course was selected based on previous investigations that hypothermia as short as 4-hrs using physical cooling was sufficient for antagonizing ischemia-induced infarct formation and edema (Kollmar et al., 2007). A longer duration of hypothermia (≥12–24 hrs) may show even greater neuroprotective effect as has been demonstrated in other hypothermia studies (Fingas et al., 2009a, MacLellan et al., 2009). A further point of clarification might come from a head-to-head comparison between PIH and alternate, more conventional models of hypothermia. We did not include an alternate model in this current investigation since our goal was to determine the efficacy of HPI-201 against ICH. On the other hand, we did test HPI-201 in animals whose body temperature was physically maintained at the normal temperature level. This experiment allowed us to confirm that the protective effects of HPI-201 were indeed due to its hypothermic action.
In summary, this research demonstrates the therapeutic potential of the second generation neurotensin NTR1 analog HPI-201 to show a central effect that reliably and rapidly induces therapeutic hypothermia. According to this and our recent investigations, pharmacologically induced hypothermia achieved by using HPI-201 provides a novel approach that can be utilized in both ischemic and hemorrhagic stroke subtypes. We suggest that HPI-201 and its derivatives are appealing reagents for drug development in acute CNS injuries.
Highlights.
Pharmacologically induced hypothermia (PIH) was achieved in an intracerebral hemorrhage (ICH) stroke model of the mouse.
PIH initiated either 1 or 24 hr after ICH reduced brain edema, BBB disruption, and cell death in the ICH brain.
PIH decreased the expression of MMP-9 and caspase-3 and increased the bcl-2 level in the ICH brain.
PIH treatment showed potential of improving functional recovery after hemorrhage stroke.
Acknowledgements
This work is supported by the NIH grants NS0458710 (SPY), R41NS073378 (SPY/TD), NS 057255 (LW), NS075338 (LW), NS062097 (LW), AHA Established Investigator Award (LW), and a Yerkes National Primate Center/NIH P51 grant (SPY). This work was also supported by the NIH grant C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. CH was supported by NIH grant R25 NS065739.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullah JM, Husin A. Intravascular hypothermia for acute hemorrhagic stroke: a pilot study. Acta Neurochir Suppl. 2011;111:421–424. doi: 10.1007/978-3-7091-0693-8_72. [DOI] [PubMed] [Google Scholar]
- Agrawal HC, Davis JM, Himwich WA. Developmental changes in mouse brain: weight, water content and free amino acids. J Neurochem. 1968;15:917–923. doi: 10.1111/j.1471-4159.1968.tb11633.x. [DOI] [PubMed] [Google Scholar]
- Arima H, Wang JG, Huang Y, Heeley E, Skulina C, Parsons MW, Peng B, Li Q, Su S, Tao QL, Li YC, Jiang JD, Tai LW, Zhang JL, Xu E, Cheng Y, Morgenstern LB, Chalmers J, Anderson CS. Significance of perihematomal edema in acute intracerebral hemorrhage: the INTERACT trial. Neurology. 2009;73:1963–1968. doi: 10.1212/WNL.0b013e3181c55ed3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auriat AM, Colbourne F. Influence of amphetamine on recovery after intracerebral hemorrhage in rats. Behavioural brain research. 2008;186:222–229. doi: 10.1016/j.bbr.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Baldwin K, Orr S, Briand M, Piazza C, Veydt A, McCoy S. Acute ischemic stroke update. Pharmacotherapy. 2010;30:493–514. doi: 10.1592/phco.30.5.493. [DOI] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ, White RF. Brain cooling during transient focal ischemia provides complete neuroprotection. Neurosci Biobehav Rev. 1997;21:31–44. doi: 10.1016/0149-7634(95)00080-1. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Choi KE, Hall CL, Sun JM, Wei L, Mohamad O, Dix TA, Yu SP. A novel stroke therapy of pharmacologically induced hypothermia after focal cerebral ischemia in mice. FASEB J. 2012;26:2799–2810. doi: 10.1096/fj.11-201822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DL, Penner M, Orellana-Jordan IM, Colbourne F. Comparison of 12, 24 and 48 h of systemic hypothermia on outcome after permanent focal ischemia in rat. Exp Neurol. 2008;212:386–392. doi: 10.1016/j.expneurol.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Colbourne F, Corbett D, Zhao Z, Yang J, Buchan AM. Prolonged but delayed postischemic hypothermia: a long-term outcome study in the rat middle cerebral artery occlusion model. J Cereb Blood Flow Metab. 2000;20:1702–1708. doi: 10.1097/00004647-200012000-00009. [DOI] [PubMed] [Google Scholar]
- Crystal L, MacLellan RPaFC. A critical appraisal of experimental intracerebral hemorrhage research. J Cereb Blood Flow & Met. 2012;32:612–627. doi: 10.1038/jcbfm.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diringer MNSB, Mayer SA, Steiner T, Davis SM, Brun NC, Broderick JP. Thromboembolic events with recombinant activated factor VII in spontaneous intracerebral hemorrhage: results from the Factor Seven for Acute Hemorrhagic Stroke (FAST) trial. Stroke. 2010;41:48–53. doi: 10.1161/STROKEAHA.109.561712. [DOI] [PubMed] [Google Scholar]
- Dubuc I, Costentin J, Doulut S, Rodriguez M, Martinez J, Kitabgi P. JMV 449: a pseudopeptide analogue of neurotensin-(8–13) with highly potent and long-lasting hypothermic and analgesic effects in the mouse. Eur J Pharmacol. 1992;219:327–329. doi: 10.1016/0014-2999(92)90314-t. [DOI] [PubMed] [Google Scholar]
- Dubuc I, Sarret P, Labbe-Jullie C, Botto JM, Honore E, Bourdel E, Martinez J, Costentin J, Vincent JP, Kitabgi P, Mazella J. Identification of the receptor subtype involved in the analgesic effect of neurotensin. J Neuroscience. 1999;19:503–510. doi: 10.1523/JNEUROSCI.19-01-00503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Ko MC, Woods JH, Richelson E. Antinociceptive, hypothermic, hypotensive, and reinforcing effects of a novel neurotensin receptor agonist, NT69L, in rhesus monkeys. Pharmacol Biochem Behav. 2005;80:341–349. doi: 10.1016/j.pbb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Feifel D, Goldenberg J, Melendez G, Shilling PD. The acute and subchronic effects of a brain-penetrating, neurotensin-1 receptor agonist on feeding, body weight and temperature. Neuropharmacology. 2010;58:195–198. doi: 10.1016/j.neuropharm.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingas M, Clark DL, Colbourne F. The effects of selective brain hypothermia on intracerebral hemorrhage in rats. Exp Neurol. 2007;208:277–284. doi: 10.1016/j.expneurol.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Fingas M, Penner M, Silasi G, Colbourne F. Treatment of intracerebral hemorrhage in rats with 12 h, 3 days and 6 days of selective brain hypothermia. Exp Neurol. 2009a;219:156–162. doi: 10.1016/j.expneurol.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Fingas M, Penner M, Silasi G, Colbourne F. Treatment of intracerebral hemorrhage in rats with 12 h, 3 days and 6 days of selective brain hypothermia. Exp Neurol. 2009b;219:156–162. doi: 10.1016/j.expneurol.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Gebel JM, Jr, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, Spilker J, Tomsick TA, Duldner J, Broderick JP. Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2636–2641. doi: 10.1161/01.str.0000035283.34109.ea. [DOI] [PubMed] [Google Scholar]
- Hadden MK, Orwig KS, Kokko KP, Mazella J, Dix TA. Design, synthesis, and evaluation of the antipsychotic potential of orally bioavailable neurotensin (8–13) analogues containing non-natural arginine and lysine residues. Neuropharmacology. 2005;49:1149–1159. doi: 10.1016/j.neuropharm.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Hamann GF, Burggraf D, Martens HK, Liebetrau M, Jager G, Wunderlich N, DeGeorgia M, Krieger DW. Mild to moderate hypothermia prevents microvascular basal lamina antigen loss in experimental focal cerebral ischemia. Stroke. 2004;35:764–769. doi: 10.1161/01.STR.0000116866.60794.21. [DOI] [PubMed] [Google Scholar]
- Han HS, Karabiyikoglu M, Kelly S, Sobel RA, Yenari MA. Mild hypothermia inhibits nuclear factor-kappaB translocation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:589–598. doi: 10.1097/01.WCB.0000059566.39780.8D. [DOI] [PubMed] [Google Scholar]
- Handler CM, Bradley EA, Geller EB, Adler MW. A study of the physiological mechanisms contributing to neurotensin-induced hypothermia. Life Sciences. 1994;54:95–100. doi: 10.1016/0024-3205(94)00779-9. [DOI] [PubMed] [Google Scholar]
- Herweh C, Juttler E, Schellinger PD, Klotz E, Jenetzky E, Orakcioglu B, Sartor K, Schramm P. Evidence against a perihemorrhagic penumbra provided by perfusion computed tomography. Stroke. 2007;38:2941–2947. doi: 10.1161/STROKEAHA.107.486977. [DOI] [PubMed] [Google Scholar]
- Hu WW, Du Y, Li C, Song YJ, Zhang GY. Neuroprotection of hypothermia against neuronal death in rat hippocampus through inhibiting the increased assembly of GluR6-PSD95-MLK3 signaling module induced by cerebral ischemia/reperfusion. Hippocampus. 2008;18:386–397. doi: 10.1002/hipo.20402. [DOI] [PubMed] [Google Scholar]
- Hughes FM, Jr, Shaner BE, May LA, Zotian L, Brower JO, Woods RJ, Cash M, Morrow D, Massa F, Mazella J, Dix TA. Identification and functional characterization of a stable, centrally active derivative of the neurotensin (8–13) fragment as a potential first-in-class analgesic. J Med Chem. 2010;53:4623–4632. doi: 10.1021/jm100092s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BY, Appelboom G, Ayer A, Kellner CP, Kotchetkov IS, Gigante PR, Haque R, Kellner M, Connolly ES. Advances in neuroprotective strategies: potential therapies for intracerebral hemorrhage. Cerebrovasc Dis. 2011;31:211–222. doi: 10.1159/000321870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallmunzer B, Schwab S, Kollmar R. Mild hypothermia of 34 degrees C reduces side effects of rt-PA treatment after thromboembolic stroke in rats. Experimental & Translational Stroke Med. 2012;4:3. doi: 10.1186/2040-7378-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LM, Young AS, Frank JE, Wang Y, Park K. Regulated hypothermia reduces brain oxidative stress after hypoxic-ischemia. Brain Res. 2004;1017:85–91. doi: 10.1016/j.brainres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Kawanishi M, Kawai N, Nakamura T, Luo C, Tamiya T, Nagao S. Effect of delayed mild brain hypothermia on edema formation after intracerebral hemorrhage in rats. J Stroke Cerebrovasc Dis. 2008;17:187–195. doi: 10.1016/j.jstrokecerebrovasdis.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Kokko KP, Hadden MK, Price KL, Orwig KS, See RE, Dix TA. In vivo behavioral effects of stable, receptor-selective neurotensin[8–13] analogues that cross the blood-brain barrier. Neuropharmacology. 2005;48:417–425. doi: 10.1016/j.neuropharm.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Blank T, Han JL, Georgiadis D, Schwab S. Different degrees of hypothermia after experimental stroke: short- and long-term outcome. Stroke. 2007;38:1585–1589. doi: 10.1161/STROKEAHA.106.475897. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Staykov D, Dorfler A, Schellinger PD, Schwab S, Bardutzky J. Hypothermia reduces perihemorrhagic edema after intracerebral hemorrhage. Stroke. 2010;41:1684–1689. doi: 10.1161/STROKEAHA.110.587758. [DOI] [PubMed] [Google Scholar]
- Konstas AA, Choi JH, Pile-Spellman J. Neuroprotection for ischemic stroke using hypothermia. Neurocrit Care. 2006;4:168–178. doi: 10.1385/NCC:4:2:168. [DOI] [PubMed] [Google Scholar]
- Lee JE, Yoon YJ, Moseley ME, Yenari MA. Reduction in levels of matrix metalloproteinases and increased expression of tissue inhibitor of metalloproteinase-2 in response to mild hypothermia therapy in experimental stroke. Journal of neurosurgery. 2005;103:289–297. doi: 10.3171/jns.2005.103.2.0289. [DOI] [PubMed] [Google Scholar]
- Li J, Lu Z, Li WL, Yu SP, Wei L. Cell death and proliferation in NFkappaB p50 knockout mouse after cerebral ischemia. Brain Res. 2008a;1230:281–289. doi: 10.1016/j.brainres.2008.06.130. [DOI] [PubMed] [Google Scholar]
- Li PA, Shuaib A, Miyashita H, He QP, Siesjo BK, Warner DS. Hyperglycemia enhances extracellular glutamate accumulation in rats subjected to forebrain ischemia. Stroke. 2000;31:183–192. doi: 10.1161/01.str.31.1.183. [DOI] [PubMed] [Google Scholar]
- Li WL, Yu SP, Ogle ME, Ding XS, Wei L. Enhanced neurogenesis and cell migration following focal ischemia and peripheral stimulation in mice. Dev Neurobiol. 2008b;68:1474–1486. doi: 10.1002/dneu.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares G, Mayer SA. Hypothermia for the treatment of ischemic and hemorrhagic stroke. Crit Care Med. 2009;37:S243–S249. doi: 10.1097/CCM.0b013e3181aa5de1. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Clark DL, Silasi G, Colbourne F. Use of prolonged hypothermia to treat ischemic and hemorrhagic stroke. J Neurotrauma. 2009;26:313–323. doi: 10.1089/neu.2008.0580. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Davies LM, Fingas MS, Colbourne F. The influence of hypothermia on outcome after intracerebral hemorrhage in rats. Stroke. 2006;37:1266–1270. doi: 10.1161/01.STR.0000217268.81963.78. [DOI] [PubMed] [Google Scholar]
- MacLellan CL, Girgis J, Colbourne F. Delayed onset of prolonged hypothermia improves outcome after intracerebral hemorrhage in rats. J Cereb Blood Flow Metab. 2004;24:432–440. doi: 10.1097/00004647-200404000-00008. [DOI] [PubMed] [Google Scholar]
- Maier CM, Ahern K, Cheng ML, Lee JE, Yenari MA, Steinberg GK. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke. 1998;29:2171–2180. doi: 10.1161/01.str.29.10.2171. [DOI] [PubMed] [Google Scholar]
- Maier CM, Sun GH, Kunis D, Yenari MA, Steinberg GK. Delayed induction and long-term effects of mild hypothermia in a focal model of transient cerebral ischemia: neurological outcome and infarct size. J Neurosurgery. 2001;94:90–96. doi: 10.3171/jns.2001.94.1.0090. [DOI] [PubMed] [Google Scholar]
- Manaenko A, Chen H, Zhang JH, Tang J. Comparison of different preclinical models of intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:9–14. doi: 10.1007/978-3-7091-0693-8_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa T, Hossmann KA. Methodological requirements for accurate measurements of brain and body temperature during global forebrain ischemia of rat. J Cereb Blood Flow Metab. 1992;12:817–822. doi: 10.1038/jcbfm.1992.113. [DOI] [PubMed] [Google Scholar]
- Nagel S, Papadakis M, Hoyte L, Buchan AM. Therapeutic hypothermia in experimental models of focal and global cerebral ischemia and intracerebral hemorrhage. Expert Rev Neurother. 2008;8:1255–1268. doi: 10.1586/14737175.8.8.1255. [DOI] [PubMed] [Google Scholar]
- Ohta H, Terao Y, Shintani Y, Kiyota Y. Therapeutic time window of postischemic mild hypothermia and the gene expression associated with the neuroprotection in rat focal cerebral ischemia. Neurosci Res. 2007;57:424–433. doi: 10.1016/j.neures.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Orwig KS, Lassetter MR, Hadden MK, Dix TA. Comparison of N-terminal modifications on neurotensin(8–13) analogues correlates peptide stability but not binding affinity with in vivo efficacy. J Med Chem. 2009;52:1803–1813. doi: 10.1021/jm801072v. [DOI] [PubMed] [Google Scholar]
- Robinson SR, Dang TN, Dringen R, Bishop GM. Hemin toxicity: a preventable source of brain damage following hemorrhagic stroke. Redox report: communications in free radical research. 2009;14:228–235. doi: 10.1179/135100009X12525712409931. [DOI] [PubMed] [Google Scholar]
- Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. The New England journal of medicine. 1986;314:953–958. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- Rosenberg GA, Cunningham LA, Wallace J, Alexander S, Estrada EY, Grossetete M, Razhagi A, Miller K, Gearing A. Immunohistochemistry of matrix metalloproteinases in reperfusion injury to rat brain: activation of MMP-9 linked to stromelysin-1 and microglia in cell cultures. Brain Res. 2001;893:104–112. doi: 10.1016/s0006-8993(00)03294-7. [DOI] [PubMed] [Google Scholar]
- Rynkowski MA, Kim GH, Komotar RJ, Otten ML, Ducruet AF, Zacharia BE, Kellner CP, Hahn DK, Merkow MB, Garrett MC, Starke RM, Cho BM, Sosunov SA, Connolly ES. A mouse model of intracerebral hemorrhage using autologous blood infusion. Nat Protoc. 2008;3:122–128. doi: 10.1038/nprot.2007.513. [DOI] [PubMed] [Google Scholar]
- Sabir H, Scull-Brown E, Liu X, Thoresen M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats. Stroke. 2012;43:3364–3370. doi: 10.1161/STROKEAHA.112.674481. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Schwab S, Schwarz S, Aschoff A, Keller E, Hacke W. Moderate hypothermia and brain temperature in patients with severe middle cerebral artery infarction. Acta Neurochir Suppl. 1998;71:131–134. doi: 10.1007/978-3-7091-6475-4_39. [DOI] [PubMed] [Google Scholar]
- Siesjo BK, Memezawa H, Smith ML. Neurocytotoxicity: pharmacological implications. Fundamental & Clinical Pharmacol. 1991;5:755–767. doi: 10.1111/j.1472-8206.1991.tb00765.x. [DOI] [PubMed] [Google Scholar]
- Staykov D, Wagner I, Volbers B, Doerfler A, Schwab S, Kollmar R. Mild Prolonged Hypothermia for Large Intracerebral Hemorrhage. Neurocrit Care. 2013;18:178–183. doi: 10.1007/s12028-012-9762-5. [DOI] [PubMed] [Google Scholar]
- Sutcliffe IT, Smith HA, Stanimirovic D, Hutchison JS. Effects of moderate hypothermia on IL-1 beta-induced leukocyte rolling and adhesion in pial microcirculation of mice and on proinflammatory gene expression in human cerebral endothelial cells. J Cereb Blood Flow Metab. 2001;21:1310–1319. doi: 10.1097/00004647-200111000-00007. [DOI] [PubMed] [Google Scholar]
- Sutherland CSHM, Kaufmann AM, Silvaggio JA, Demchuk AM, Sutherland GR. Recombinant factor VIIa plus surgery for intracerebral hemorrhage. Can J Neurol Sci. 2008;35:567–572. doi: 10.1017/s0317167100009343. [DOI] [PubMed] [Google Scholar]
- Theodorsson A, Holm L, Theodorsson E. Hypothermia-induced increase in galanin concentrations and ischemic neuroprotection in the rat brain. Neuropeptides. 2008;42:79–87. doi: 10.1016/j.npep.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Thome C, Schubert GA, Schilling L. Hypothermia as a neuroprotective strategy in subarachnoid hemorrhage: a pathophysiological review focusing on the acute phase. Neurol Res. 2005;27:229–237. doi: 10.1179/016164105X25252. [DOI] [PubMed] [Google Scholar]
- Torok E, Klopotowski M, Trabold R, Thal SC, Plesnila N, Scholler K. Mild hypothermia (33 degrees C) reduces intracranial hypertension and improves functional outcome after subarachnoid hemorrhage in rats. Neurosurgery. 2009;65:352–359. doi: 10.1227/01.NEU.0000345632.09882.FF. [DOI] [PubMed] [Google Scholar]
- Truettner JS, Alonso OF, Dalton Dietrich W. Influence of therapeutic hypothermia on matrix metalloproteinase activity after traumatic brain injury in rats. J Cereb Blood Flow Metab. 2005;25:1505–1516. doi: 10.1038/sj.jcbfm.9600150. [DOI] [PubMed] [Google Scholar]
- Tyler-McMahon BM, Stewart JA, Farinas F, McCormick DJ, Richelson E. Highly potent neurotensin analog that causes hypothermia and antinociception. Eur J Pharmacol. 2000;390:107–111. doi: 10.1016/s0014-2999(99)00877-8. [DOI] [PubMed] [Google Scholar]
- Tyler BM, Douglas CL, Fauq A, Pang YP, Stewart JA, Cusack B, McCormick DJ, Richelson E. In vitro binding and CNS effects of novel neurotensin agonists that cross the blood-brain barrier. Neuropharmacology. 1999;38:1027–1034. doi: 10.1016/s0028-3908(99)00011-8. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Macleod MR, Kollmar R. Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials? J Cereb Blood Flow Metab. 2010;30:1079–1093. doi: 10.1038/jcbfm.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker BK, Freie AB, Perfater JL, Gidday JM. Junctional protein regulation by sphingosine kinase 2 contributes to blood-brain barrier protection in hypoxic preconditioning-induced cerebral ischemic tolerance. J Cereb Blood Flow Metab. 2012;32:1014–1023. doi: 10.1038/jcbfm.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurology. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Xue M, Fan Y, Liu S, Zygun DA, Demchuk A, Yong VW. Contributions of multiple proteases to neurotoxicity in a mouse model of intracerebral haemorrhage. Brain. 2009;132:26–36. doi: 10.1093/brain/awn215. [DOI] [PubMed] [Google Scholar]
- Yanagawa Y, Kawakami M, Okada Y. Moderate hypothermia alters interleukin-6 and interleukin-1alpha reactions in ischemic brain in mice. Resuscitation. 2002;53:93–99. doi: 10.1016/s0300-9572(01)00499-3. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Iwayama S, Cheng DY, Sun GH, Fujimura M, Morita-Fujimura Y, Chan PH, Steinberg GK. Mild hypothermia attenuates cytochrome C release but does not alter Bcl-2 expression or caspase activation after experimental stroke. J Cerebr Blood F Met. 2002;22:29–38. doi: 10.1097/00004647-200201000-00004. [DOI] [PubMed] [Google Scholar]
- Zazulia AR, Diringer MN, Derdeyn CP, Powers WJ. Progression of mass effect after intracerebral hemorrhage. Stroke. 1999;30:1167–1173. doi: 10.1161/01.str.30.6.1167. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ren C, Gao X, Takahashi T, Sapolsky RM, Steinberg GK, Zhao H. Hypothermia blocks beta-catenin degradation after focal ischemia in rats. Brain Res. 2008;1198:182–187. doi: 10.1016/j.brainres.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]