Abstract
Objective: Allergen-specific immunotherapy (SIT) is the unique modifying treatment of atopic diseases. Dysregulation of T-cell apoptosis plays a crucial role in the in the development of asthma. Nevertheless, the effect of sublingual immunotherapy (SLIT) on T-cell apoptosis has not been elucidated. The aim of the study was to evaluate the influence of 1 year of SIT in atopic children on the frequency of Th1 and Th2 cells in peripheral blood, on T-cell apoptosis, and on the response of basophils to allergen challenge.
Methods: Children suffering from bronchial asthma were treated with SLIT for 12 months (Staloral 300). Basophil activation was evaluated by measurement of CD203c antigen expression. Th1 and Th2 cells frequencies and their associated frequencies of apoptosis by the expression of Bcl-2 were evaluated with flow cytometry.
Results: Basophil activation showed no difference in response before and after therapy. The frequency of Th1 cells increased (p=0.01, n=19), whereas the frequency of Th2 cells remained stable. Additionally, a significant increase of Bcl-2 positive Th1 cells was found (p=0.0465, n=19).
Conclusions: We conclude that the increase in frequency of Th1 cells secondary to SLIT might be associated with increased resistance to apoptotic signals. The basophil activation is not useful in evaluation of patient desensitization.
Introduction
Allergen-specific immunotherapy (SIT) based on the administration of increasing doses of allergen is the unique treatment of atopic diseases modifying the immune response.1–5 Currently SIT may be administered as subcutaneous (SCIT) or sublingual (SLIT). SLIT appears to be associated with lower incidence of systemic reactions.5,6 The clinical efficacy of SLIT is well established for both rhinitis and asthma.7 Some studies also show efficacy of SLIT in food allergy.8 Successful immunotherapy is associated with reduction in symptoms and medication scores, as well as improved quality of life.4 The knowledge regarding the exact mechanism underlying the clinical improvement upon SLIT has increased in the last decade. Nevertheless, the precise effect of SLIT on the immunological system of children suffering from asthma needs to be elucidated.
SLIT causes anergy and/or deletion of allergen-specific T-cells, skews allergen-specific responses from Th2 to a more protective Th1 phenotype, and downregulates allergen-specific immunoglobulin class E (asIgE) production.9–12 Subsequently, interferon gamma (IFN-γ) produced by Th1 cells inhibits proliferation and differentiation of Th2 cells, and induces production of allergen-specific immunoglobulin class G (asIgG4) and secretory immunoglobulin class A (IgA).4,8,13–15 To date, there are many publications regarding the clinical efficacy of SLIT, although very few consider laboratory analyses of SLIT action.16–20 Most of these concern the evaluation of inflammatory mediators and cytokine concentrations in bronchoalveolar lavage fluid and in serum obtained from asthmatic subjects treated with SLIT.13,17–19 The influence of SLIT on basophil surface antigens as activation markers is limited. In this study, we analyze the changes in CD203c expression on basophils in response to allergen challenge in patients sensitized to house dust mite or grass pollen allergens before and after 1 year of SLIT.
The purpose of the present study was also to assess the effect of 1 year of SLIT for the treatment of atopic asthma on the frequency of Th1 and Th2 cells and their susceptibility to apoptosis measured by the expression of Bcl-2 protein before and after therapy. It is postulated that the apoptotic pathway of Bcl-2 and Bax proteins is significantly disrupted in the course of asthma.21 Misbalance between anti-apoptotic Bcl-2 and pro-apoptotic Bax in T-cells has been reported in patients suffering from atopic asthma.21,22 The influence of SLIT on T-cell apoptosis has not yet been evaluated.
Materials and Methods
Patients and samples
Twenty-five patients aged 8.1±3.1 years, 21 boys and 4 girls, were included in the study. All participants in the studied group were physician diagnosed with allergic rhinitis and well-controlled asthma (Table 1). Diagnosis was made in accordance to Expert Panel Report 3 Guidelines for the diagnosis and management of asthma.23 Atopy was confirmed by skin prick test and/or the presence of asIgE. The prick tests scored positive if the wheal obtained was similar or larger than the wheal elicited by the positive control (histamine hydrochloride, 10 mg/mL). Staloral 300 (Ewopharma AG, France) at a concentration of 300 IR/mL was used. The administration included an 11 day induction phase followed by a maintenance phase of 240 IR of allergen solution twice per week (Table 2). Children were treated with specific allergen solution according to the results of their skin/specific IgE tests. The group of patients suffering from house dust mite allergy was administered a vaccine containing Dermatophagoides pteronyssinus and D. farinae allergens in continuous schedule (all year treatment). Children with an allergy to pollen were administered a drug containing extracts of grass pollen and wheat pollen in pre-coseasonal schedule. Exclusion criteria were a history of other immunological or hematological disorders, severe infectious diseases, or systemic corticosteroids administered less than 4 weeks before the blood collection. Evaluation of basophil activation, Th1 and Th2 cell frequency, as well as assessment of Bcl-2 expression in T-cells were performed at baseline and after 12 months of SLIT.
Table 1.
Baseline Characteristics of Study Patients and Controls
| Parameter | Study group | Control group |
|---|---|---|
| Age (range, years) | 8.13±3.08 (5–15) | 9.83±3.37 (5.5–14.5) |
| Gender (n) | ||
| Boys | 21 | 7 |
| Girls | 4 | 8 |
| Bronchial asthma | 100% | 0% |
| Allergic rhinitis | 100% | 0% |
| Allergen-specific IgE | ||
| (>0.7 kU/L) | 88% | 0% |
| Grass pollen | 56% | 0% |
| Birch pollen (Betula verrucossa) | 28% | 0% |
| Dermatophagoides pteronyssinus | 28% | 0% |
| D. farinae | 28% | 0% |
| Family history of atopic diseases | 52% | 0% |
Table 2.
The Immunotherapeutic Protocol During the Induction and Maintenance Phase of Therapy
| Time | Allergen concentration | Number of doses | Dose administered |
|---|---|---|---|
| Day 1 | 10 IR/mL | 1 | 1 IR |
| Day 2 | 2 | 2 IR | |
| Day 3 | 4 | 4 IR | |
| Day 4 | 6 | 6 IR | |
| Day 5 | 8 | 8 IR | |
| Day 6 | 10 | 10 IR | |
| Day 7 | 300 IR/mL | 1 | 30 IR |
| Day 8 | 2 | 60 IR | |
| Day 9 | 4 | 120 IR | |
| Day 10 | 6 | 180 IR | |
| Day 11 | 8 | 240 IR | |
| Twice a week | 300 IR/mL | 8 | 240 IR |
Fifteen healthy individuals served as a control group (Table 1). Healthy individuals were characterized by a negative quantitative IgE test and negative history of asthma. Exclusion criteria for the control group also included recent respiratory disorders or any atopic/allergic diseases. Blood was obtained by venipuncture in EDTA-containing tubes. The study protocol was approved by the Ethics Committee of the Medical University of Warsaw, and written informed consent was obtained from all parents or legal guardians.
Flow cytometry of lymphocyte subpopulations
For flow cytometric analysis, 50 μL of ethylenediaminetetraacetic acid (EDTA) blood was stained for 25 minutes in the dark and at room temperature using 10 μL of the following antibodies: phycoerythrin-cyanine 5 (PC5)-conjugated anti-CD4 antibody (Beckman Coulter, Poland), phycoerythrin (PE)-conjugated anti-CRTh2 antibody (Beckman Coulter), and PE-conjugated anti-CCR5 antibody (Becton Dickinson, Poland). Intracellular staining for Bcl-2 was performed using an Intraprep Kit (Beckman Coulter) according to the manufacturer's instructions. Flow cytometric analysis was performed on a Cytomics FC500 (Beckman Coulter).
Basophil activation test
All tests were carried out within 2 hours of blood collection, in accordance with the recommendation regarding the time between blood collection and processing the basophils.24 The percentage of basophils in peripheral blood was assessed after complete blood count analysis. A residual sample was used for the basophil activation test. CD203c induced expression was evaluated using the Allergenicity Kit (Beckman Coulter) according to the manufacturer's instructions, as previously described.25 A measured expression of CD203c was used as the activation monitor for basophils. While McGowan et al. have suggested that both CD203c and CD63 be used,24 research from our laboratory has shown CD203c to be sufficient in this case.25 Allergens (Stallergenes, France) in a concentration of 0.2 IR/mL in phosphate-buffered saline (PBS) were used for the in vitro challenge.
During acquisition, basophils were selected as CD203c-positive/CRTH2 high/CD3-negative population using FL1/FL2 and SS/FL5 dot plots. The negative control threshold for positivity was set at less than 5% of activated cells according to the literature data.18,19 The threshold for positivity as well as sensitivity and specificity of the test was set with receiver operating characteristic (ROC) analysis for specific allergens.
Statistical analysis
The Wilcoxon matched pairs test was used to compare nonparametric data from patients before and after 1 year of SLIT. A p value of <0.05 was considered as being significant for all statistical analyses. The results of cell frequencies are presented as the median (first quartile; third quartile).
Results
Basophil numbers and activation
The frequency of basophils in peripheral blood was 0.65% (0.48; 0.93) before immunotherapy, and 0.60% (0.50; 0.80) after 1 year of treatment (p=0.97, n=19).
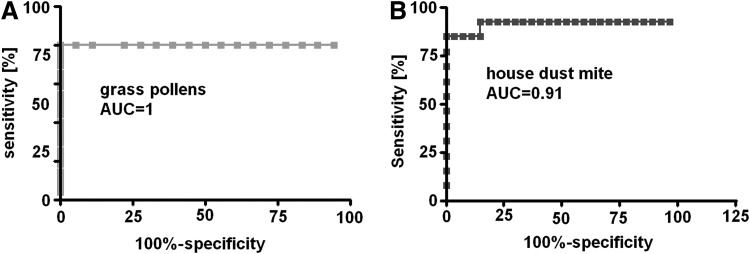
Basophils were activated with allergens in a concentration of 0.2 IR/mL. The optimal dilution of the suspension was assessed in a pilot study.21 ROC analysis was performed to assess an optimal threshold for positive and negative results of basophil activation after grass pollen and house dust mite allergen challenge. The threshold for positivity in grass pollen challenge was 10% of CD203c-positive basophils. Sensitivity and specificity of the test was 100%. Area under the curve was 1.0 (p<0.0001; Fig. 1a). The threshold for positive reaction after house dust mite allergen stimulation was obtained for 12.81% of activated basophils. Specificity of the test was 100% and sensitivity 84.62%. Area under the curve was 0.9131 (p<0.0001; Fig. 1b).
FIG. 1.
Receiver operating characteristic for basophil activation test based on the detection of surface CD203c antigen expression after (A) grass pollen allergens and (B) Dermatophagoides pteronyssinus (house dust mite) allergens challenge.
Basophil activation in asthmatic patients before and after 1 year of SLIT
The mean fluorescence channel (MFC) of basophils stained with PE-conjugated monoclonal antibody against CD203c in the sample incubated with PBS (negative control) was used as a marker of spontaneous degranulation. In the group of children before immunotherapy, the MFC of basophils after incubation with PBS was 5.50 (4.48; 8.72). After 1 year of SLIT, the MFC in the negative control was 5.29 (3.97; 7.59) (p=0.16, n=19).
Grass pollen stimulation of peripheral blood from children sensitized to grass pollen before immunotherapy resulted in activation of 47.07% (30.04; 60.71) of basophils. After 1 year of SLIT, 40.08% (30.99; 58.27) of basophils degranulated after grass pollen allergen challenge (p=0.63, n=19).
Allergen challenge of basophils from asthmatic children sensitized to D. pteronyssinus before immunotherapy resulted in activation of 35.78% (15.99; 39.74) of basophils, whereas after 1 year of SLIT 28.86% (5.63; 35.77) of basophils degranulated (p=0.16, n=19).
Th1 and Th2 lymphocytes in peripheral blood of patients
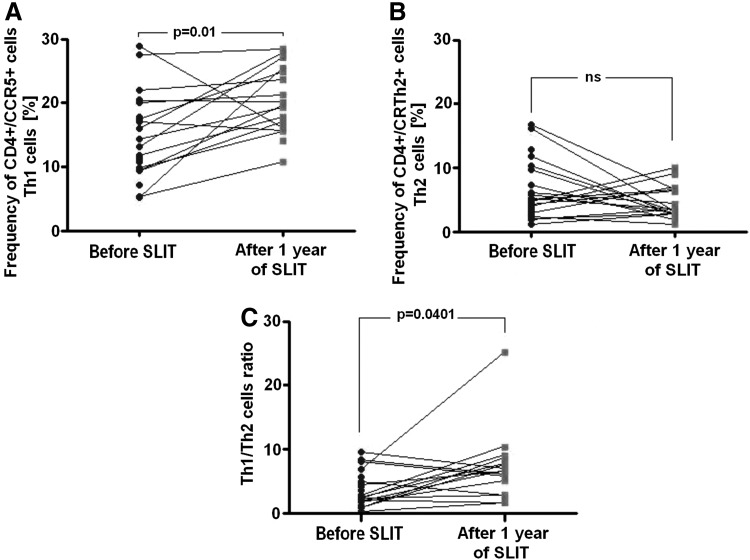
Th1 cells were defined as CD4+CCR5+lymphocytes, whereas Th2 cells were defined as CD4+CRTh2+lymphocytes. In asthmatic children before immunotherapy, Th1 cell frequency in peripheral blood was 13.22% (10.34; 18.95). As shown in Figure 2a, the frequency of these cells as CD4+CCR5+increased significantly to 19.86% (16.37; 24.52) after 1 year of SLIT (p=0.01, n=19).
FIG. 2.
Time-course analysis of the frequency of (A) Th1 cells, (B) Th2 cells, and (C) Th1/Th2 cell ratios before and after 1 year of sublingual immunotherapy (SLIT). Statistical significant differences (Wilcoxon matched pairs test) are shown; ns, not significant.
The frequency of Th2 cells in peripheral blood of asthmatic children was 4.96% (3.29; 7.86) at baseline. CD4+CRTH2+cell frequency showed a reduction to 3.2% (2.75; 6.42) after 1 year of treatment, but did not reach statistical significance (p=0.21, n=19; Fig. 2b). As a result, a significant increase in the Th1/Th2 cell ratio occurred: 2.68 (1.87; 4.68) at baseline to 6.33 (3.36; 7.65) after 1 year of SLIT (p=0.0401, n=19; Fig. 2c).
Bcl-2 expression on Th1 and Th2 lymphocytes in peripheral blood
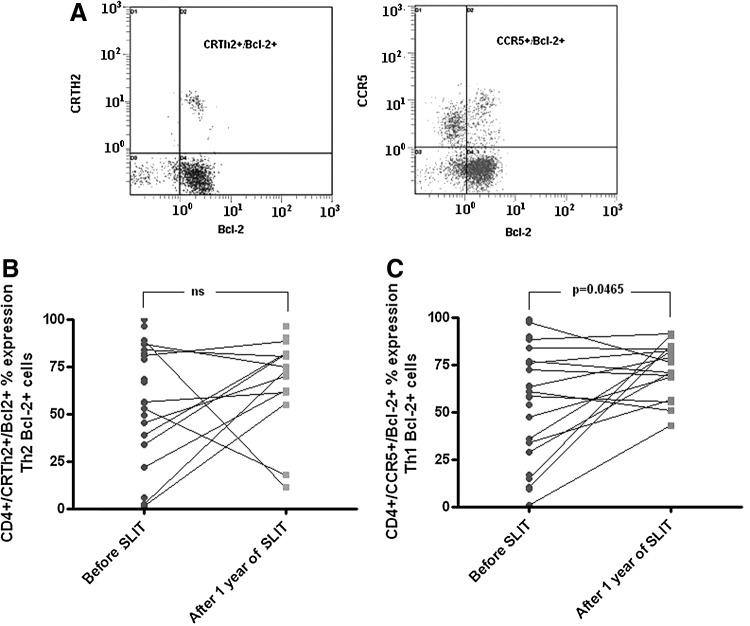
The expression of Bcl-2 in Th1 and Th2 cells is shown in Figure 3a. The quantitative analysis of Th1 Bcl-2+cell frequency demonstrated a significant increase after 1 year of SLIT. At baseline, 58.34% (31.23; 76.28) of Th1 cells expressed Bcl-2, whereas 73.61% (68.47; 82.43) of Th1 cells did express Bcl-2 after SLIT (p=0.0465, n=19; Fig. 3b).
FIG. 3.
CRTh2, CCR5, and Bcl-2 expression in CD4+lymphocytes. (A) Double positive (Th2/Bcl-2+ and Th1/Bcl-2+) populations are marked. Time-course analysis of the frequency of (B) Bcl-2+ Th1 cells and (C) Bcl-2+ Th2 cells before and after 1 year of SLIT. Statistically significant differences (Wilcoxon matched pairs test) are shown; ns, not significant.
The frequency of Th2 cells expressing Bcl-2 did not show a significant change after 1 year of SLIT: 66.63% (42.11; 82.69) at baseline, and 73.79% (61.60; 81.79) after SLIT (p=0.23, n=19; Fig. 3c).
Discussion
A flow cytometric basophil activation test based on the detection of surface activation markers is found to be a reliable test to confirm sensitization in allergic subjects. As CD203c and CD63 are upregulated after allergen challenge in sensitized patients, it has been suggested as an alternative tool for allergy diagnosis. The usefulness of the test was also reported with regard to the detection of biological changes associated with the efficacy of SIT.1,4,9 Furthermore, change in T-helper cell frequencies, especially Th2 cell decrease, was reported as marker of SLIT influence on the immunological system of atopic patients.17
Although it can be partly explained by the restoration of the ratio between Th1 and Th2 cells, the mechanism of action of SLIT is not fully understood. We have found that SLIT increased the frequency of Th1 cells in peripheral blood of atopic asthmatics, but did not significantly change the Th2 cell numbers. Our findings agree with a study reporting no influence of SLIT on Th2 cell numbers in peripheral blood of asthmatic children.26 Alternatively, O'Hehir et al. found a significant decrease in Th2 (CD4+lymphocytes expressing IL-5) cells demonstrated after 6 months of SLIT in subjects sensitized to house dust mites.27 In another study, in children with allergic rhinitis but not bronchial asthma who were given 2 years of SLIT, a significant decrease of IL-5 mRNA expression was observed.28 However, the results of both studies are not fully comparable to our results because they examine a group of nonasthmatic adults and children.
During inflammation in the bronchi, macrophages release IL-18, inducing Th2 cell proliferation.29 This might explain why no change in Th2 cell frequency is observed, despite increased Th1 numbers after SLIT. Probably, SLIT triggers the release of cytokines driving Th1 proliferation, and parallel IL-18 promote Th2 cell differentiation. Indeed, SLIT increases the frequency of Th1 cells, although other studies were performed with different groups of patients, where an increase in peripheral blood Th1 cells was also reported.30,31
The percentages of Th2 cells and those of peripheral basophils were not affected. These findings may be directly related, as IL-4 and IL-13 are both released from Th2 cells and are both responsible for the stimulation proliferation and differentiation of basophils in bone marrow.32 Neither percent change in spontaneous basophil activation nor in their sensitivity to specific allergens was identified following SLIT. This lack of change may be associated with the permanent contact of cells with allergens used in SLIT. One year of SLIT also did not decrease the percentage of activated basophils after allergen challenge. A similar observation was reported by Horak et al. They evaluated basophil activation through CD203c antigen upregulation after 4 months of SLIT in a group of 45 adults sensitized to grass pollen. There was no difference in CD203c expression on basophils stimulated with grass pollen allergens before and after treatment,33 while the same group also showed that basophil activation based on the detection of CD203c is not indicative of immunotherapy efficacy.34 There are also limited reports regarding changes of surface expression of basophil activation surface markers after SCIT. Although it concerned a group of nine patients, a shift of CD203c antigen expression on basophils from subjects sensitized to Japanese cedar pollen after rush immunotherapy was demonstrated, and it was postulated that these changes were a reliable marker of basophil desensitization and immunotherapy effectiveness.35 On the other hand, Swamy et al. observed a decrease of basophil activation after stimulation with specific allergens in a group of patients receiving dual SLIT (house dust mite and timothy grass) for 2 years.36
Lack of changes in basophil sensitivity to specific allergens after 1 year of SLIT is thought to be associated with high affinity IgE receptor (FcɛRI) expression on the surface of basophils. It is postulated that B-cells primarily react to SLIT by increasing asIgE production. On the other hand, asIgE is found to increase FcɛRI expression on effector cells.37 These suggestions may be confirmed after asIgE evaluation and measurement of FcɛRI expression on basophils in the peripheral blood of children after 1 year of SLIT.
Previously, the effects of SLIT on Th1 cells have been explained by the observation that treatment increases the frequency of CD25+CD4+FoxP3+T regulatory cells, releasing IL-10 and transforming growth factor beta (TGF-β), which induce Th1 differentiation.27 Our findings point to a possible different mechanism, in which SLIT may improve the T-helper cell ratio. We postulate that the decrease of Th1 cell susceptibility to apoptosis may play a crucial role in restoring the immunological balance between T-helper cells in atopic diseases. There are to date no reports on the expression of apoptotic Bcl-2 proteins during SLIT. Furthermore, there are no data available on either SLIT or SCIT action, nor on any pro- or anti-apoptotic proteins of Th1 cells. It is of interest that Frisella et al. found that D. pteronyssisus allergens from the immunotherapy vaccine may induce apoptosis and stimulate TGF-β 1 release from human alveolar adenocarcinoma cells (A549 cell line).38 They also reported that after in vitro allergen challenge, apoptotic Th2 cells from asthmatic children sensitized to house dust mite increase in the group treated with SCIT.38 The mechanism of this increase is unknown, as the pathway of apoptosis induction was not investigated in either study.38,39 According to the results of the current study, this effect may be related to the prolonged life span of Bcl-2-positive Th1 cells.
In summary, the immune mechanisms leading to clinical tolerance after SLIT have not yet been fully elucidated. Although flow cytometric evaluation of basophil sensitivity to degranulation after allergen challenge is found to be a reliable method in allergy diagnosis, our results do not confirm previous reports that suggest a decrease of degranulation in response to short-term SLIT. Recent reports provide evidence that allergen-specific Th2 response is under the control of Th1-polarizing (IFNs, IL-12) and immunosuppressive cytokines (IL-10, TGF-β), both of which are produced in response to chronic stimulation of the immune system. SLIT shifts allergic-specific CD4+ T-cell responses from Th2 to Th1, with the stimulation of IFNγ-producing Th1 cells.40 Our study supports this observation. The shift from Th2 to Th1 response has been attributed to the action of immunoregulatory cells. However, an alternative approach of immunodeviation should be also taken into consideration. Our data confirm that the major systemic effect of SLIT—the regulation of the Th1/Th2 balance—can not only be modulated by an immunoregulatory loop but can also be related to the resistance of Th1 cells to apoptosis.
Acknowledgments
This project is co-financed by the Mazovia PhD Scholarship (2008–2009) funded by Mazovia Voivodeship, Polish Government.
Author Disclosure Statement
The authors declare that they do not have any conflicts of interest to report.
References
- 1.Majak P, Kaczmarek-Woźniak J, Brzozowska A, Bobrowska-Korzeniowska M, Jerzynska J, Stelmach I. One-year follow-up of clinical and inflammatory parameters in children allergic to grass pollen receiving high-dose ultrarush sublingual immunotherapy. J Investig Allergol Clin Immunol 2010; 20:602–606 [PubMed] [Google Scholar]
- 2.Okubo K, Gotoh M, Fujieda S, et al. A randomized double-blind comparative study of sublingual immunotherapy for cedar pollinosis. Allergol Int 2008; 57:265–275 [DOI] [PubMed] [Google Scholar]
- 3.Pfaar O, Kleine-Tebbe J, Hörmann K, Klimek L. Allergen-specific immunotherapy: which outcome measures are useful in monitoring clinical trials? Immunol Allergy Clin N Am 2011; 31:289–309 [DOI] [PubMed] [Google Scholar]
- 4.Matsuoka T, Shamji MH, Durham SR. Allergen immunotherapy and tolerance. Allergol Int 2013; 62:403–413 [DOI] [PubMed] [Google Scholar]
- 5.Aryan Z, Comapalati E, Canonica GW, Rezaei N. Allergen-specific immunotherapy in asthmatic children: from basis to clinical applications. Expert Rev Vaccines 2013; 12:639–659 [DOI] [PubMed] [Google Scholar]
- 6.Calderón MA, Simons FER, Malling H-J, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy 2011; 67:302–311 [DOI] [PubMed] [Google Scholar]
- 7.Cox L. Sublingual immunotherapy in pediatric allergic rhinitis and asthma: efficacy, safety, and practical considerations. Curr Allergy Asthma Rep 2007; 7:410–420 [DOI] [PubMed] [Google Scholar]
- 8.Akdis CA, Akdis M, Mechanisms of allergen-specific immunotherapy. J Allergy Clin Immunol 2011; 127:18–27 [DOI] [PubMed] [Google Scholar]
- 9.Ciprandi G, Tosca MA, Marseglia GL. Sublingual immunotherapy mechanisms of action: the role of Th1 response. Int J Immunopathol Pharmacol 2009; 22:S9–12 [PubMed] [Google Scholar]
- 10.Angelini F, Pacciani V, Corrente S, et al. Dendritic cells modification during sublingual immunotherapy in children with allergic symptoms to house dust mites. World J Pediatr 2011; 7:24–30 [DOI] [PubMed] [Google Scholar]
- 11.Moingeon P, Mascarell L. Induction of tolerance via the sublingual route: mechanisms and application. Clin Dev Immunol 2012; Article ID 623474, 10.1155/2012/623474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderon MA, Simons FER, Malling M-J, Lockey RF, Moingeon P, Demoly P. Sublingual allergen immunotherapy: mode of action and its relationship with the safety profile. Allergy 2012; 67:302–311 [DOI] [PubMed] [Google Scholar]
- 13.Ciprandi G, De Amici M, Tosca MA, Pistorio A, Marseglia GL. Sublingual immunotherapy affects specific antibody and TGF-beta serum levels in patients with allergic rhinitis. Int J Immunopathol Pharmacol 2009; 22:1089–1096 [DOI] [PubMed] [Google Scholar]
- 14.Shamji MH, Durham SR. Mechanisms of immunotherapy to aeroallergens. Clin Exp Allergy 2011; 41:1235–1246 [DOI] [PubMed] [Google Scholar]
- 15.Fujita H, Soyka MB, Akdis M, Akdis CA. Mechanisms of allergen-specific immunotherapy. Clin Transl Allergy 2012; 2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bahceciler NN, Arikan C, Taylor A, et al. Impact of sublingual immunotherapy on specific antibody levels in asthmatic children allergic to house dust mites. Int Arch Allergy Immunol 2005; 136:287–294 [DOI] [PubMed] [Google Scholar]
- 17.Bohle B, Kinciyan T, Gerstmayr M, Radakovics A, Jahn-Schmid B, Ebner C. Sublingual immunotherapy induces IL-10-producing T regulatory cells, allergen specific T-cell tolerance, and immune deviation. J Allergy Clin Immunol 2007; 120:707–713 [DOI] [PubMed] [Google Scholar]
- 18.Burastero SE, Mistrello G, Falagiani P, et al. Effect of sublingual immunotherapy with grass monomeric allergoid on allergen-specific T-cell proliferation and interleukin 10 production. Ann Allergy Asthma Immunol 2008; 100:343–350 [DOI] [PubMed] [Google Scholar]
- 19.Cosmi L, Santarlasci V, Angeli R, et al. Sublingual immunotherapy with Dermatophagoides monomeric allergoid down-regulates allergen-specific immunoglobulin E and increases both interferon gamma and interleukin-10-production. Clin Exp Allergy 2006; 36:261–272 [DOI] [PubMed] [Google Scholar]
- 20.Leatherman BD, Owen S, Parker M, et al. Sublingual Immunotherapy: past, present, paradigm for the future? A review of the literature. Otolaryngol Head Neck Surg 2007; 136:S1–20 [DOI] [PubMed] [Google Scholar]
- 21.Abdulamir AS, Kadhim HS, Hafidh RR, et al. Severity of asthma: the role of CD25+, CD30+, NF-κB and apoptotic markers. J Invest Allergol Clin Immunol 2009; 19:218–224 [PubMed] [Google Scholar]
- 22.Abdulamir AS, Hafidh RR, Abubakar F, Abbas KS. Changing survival, memory cell compartment, and T-helper balance of lymphocytes between severe and mild asthma. BMC Immunol 2008; 9:73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Expert Panel Report 3: Guidelines for the diagnosis and management of asthma National Heart, Lung, and Blood Institute. U.S. Department of Health and Human Services, National Institutes of Health; 2007 [Google Scholar]
- 24.McGowan EC, Saini S. Update on the performance and application of basophil activation tests. Curr Allergy Asthma Rep 2013; 13:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potapinska O, Gorska E, Demkow U, Zawadzka-Krajewska A, Kulus M, Wasik M. The usefulness of CD203c expression measurement on basophils after activation with grass pollen and Dermatophagoides pteronyssinus antigens. Preliminary study. Pneumonol Alergol Pol 2009; 77:138–144 [PubMed] [Google Scholar]
- 26.Eifan AO, Akkoc T, Yildiz A, et al. Clinical efficacy and immunological mechanisms of sublingual and subcutaneous immunotherapy in asthmatic/rhinitis children sensitized to house dust mite: an open randomized controlled trial. Clin Exp Allergy 2010; 40:922–932 [DOI] [PubMed] [Google Scholar]
- 27.O'Hehir RE, Gardner LM, de Leon MP, et al. House dust mite sublingual immunotherapy: the role for transforming growth factor-beta and functional regulatory T-cells. Am J Respir Crit Care Med 2009; 180:936–947 [DOI] [PubMed] [Google Scholar]
- 28.Savolainen J, Jacobsen L, Valovirta E. Sublingual immunotherapy in children modulates allergen-induced in vitro expression of cytokine mRNA in PBMC. Allergy 2006; 61:1184–1190 [DOI] [PubMed] [Google Scholar]
- 29.Kamiński M, Kłoda K, Pawlik A. The role of interleukin-18 in the pathogenesis of bronchial asthma and other allergic diseases and in activation of basophils and mastocytes. Pneumonol Alergol Pol 2008; 76:432–436 [PubMed] [Google Scholar]
- 30.Cosmi L, Santarlasci V, Angeli R, et al. Sublingual immunotherapy with Dermatophagoides monomeric allergoid down-regulate allergen-specific immunoglobulin E and increases both interferon gamma and interleukin-10-production. Clin Exp Allergy 2006; 36:261–272 [DOI] [PubMed] [Google Scholar]
- 31.Kim EH, Bird JA, Kulis M, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol 2011; 127:640–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol 2002; 38:881–885 [DOI] [PubMed] [Google Scholar]
- 33.Horak F, Zieglmayer P, Zieglmayer R, et al. Early onset of action of a 5-grass-pollen 300-IR sublingual immunotherapy tablet evaluated in an allergen challenge chamber. J Allergy Clin Immunol 2009; 124:471–477 [DOI] [PubMed] [Google Scholar]
- 34.Van Overtvelt L, Baron-Bodo V, Horiot S, et al. Changes in basophil activation during grass-pollen sublingual immunotherapy do not correlate with clinical efficacy. Allergy 2011; 66:1530–1537 [DOI] [PubMed] [Google Scholar]
- 35.Fujisawa T, Nagao M, Hiraguchi Y, et al. Biomarkers for allergen immunotherapy in cedar pollinosis. Allergol Int 2009; 58:163–170 [DOI] [PubMed] [Google Scholar]
- 36.Swamy RS, Reshamwala N, Hunter T, et al. Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. J Allergy Clin Immunol 2012; 130:215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shim JY, Kim BS, Cho SH, Min KU, Hong SJ. Allergen-specific conventional immunotherapy decreases immunoglobulin E-mediated basophil histamine releasability. Clin Exp Allergy 2003; 33:52–57 [DOI] [PubMed] [Google Scholar]
- 38.Frisella PD, Silverberg J, Joks R, Frieri M. Transforming growth factor beta: a role in the upper airway and rhinosinusitis-Dermatophagoides pteronyssinus-induced apoptosis with pulmonary alveolar cells. Am J Rhinol Allergy 2011; 25:231–235 [DOI] [PubMed] [Google Scholar]
- 39.Tsai YG, Chien JW, Chen WL, Shieh JJ, Lin CY. Induced apoptosis of TH2 lymphocytes in asthmatic children treated with Dermatophagoides pteronyssinus immunotherapy. Pediatr Allergy Immunol 2005; 16:602–608 [DOI] [PubMed] [Google Scholar]
- 40.Maggi E, Vultaggio A, Matucci A. T-cell responses during allergen-specific immunotherapy. Curr Opin Allergy Clin Immunol 2012; 12:1–6 [DOI] [PubMed] [Google Scholar]