Abstract
Significance: Heme oxygenase-1 (HO-1) converts heme to biliverdin, carbon monoxide, and ferrous ions, but its cellular functions are far beyond heme metabolism. HO-1 via heme removal and degradation products acts as a cytoprotective, anti-inflammatory, immunomodulatory, and proangiogenic protein, regulating also a cell cycle. Additionally, HO-1 can translocate to nucleus and regulate transcription factors, so it can also act independently of enzymatic function. Recent Advances: Recently, a body of evidence has emerged indicating a role for HO-1 in postnatal differentiation of stem and progenitor cells. Maturation of satellite cells, skeletal myoblasts, adipocytes, and osteoclasts is inhibited by HO-1, whereas neurogenic differentiation and formation of cardiomyocytes perhaps can be enhanced. Moreover, HO-1 influences a lineage commitment in pluripotent stem cells and maturation of hematopoietic cells. It may play a role in development of osteoblasts, but descriptions of its exact effects are inconsistent. Critical Issues: In this review we discuss a role of HO-1 in cell differentiation, and possible HO-1-dependent signal transduction pathways. Among the potential mediators, we focused on microRNA (miRNA). These small, noncoding RNAs are critical for cell differentiation. Recently we have found that HO-1 not only influences expression of specific miRNAs but also regulates miRNA processing enzymes. Future Directions: It seems that interplay between HO-1 and miRNAs may be important in regulating fates of stem and progenitor cells and needs further intensive studies. Antioxid. Redox Signal. 20, 1827–1850.
Introduction
Stem and progenitor cells
Stem cells (SCs) are defined by two major features. First, they can differentiate into diverse specialized cell types. Second, they are capable of self-renewal—after asymmetric division each SC can generate one daughter cell with a stemness characteristic, and one daughter cell that differentiates. In this manner SCs can maintain their own population. They can also undergo a symmetric division to self-renew and expand (193).
SCs can be classified according to their differentiation potency to (i) totipotent, (ii) pluripotent, (iii) multipotent, and (iv) unipotent (monopotent). Totipotent SCs can give rise to all embryonic as well as extraembryonic tissues (trophoblast and placenta). This feature is evident only for zygote (which itself is not an SC, as does not self-renew), and cells within the first couple of divisions after fertilization (193).
Pluripotent embryonic stem cells (ESCs) appear at later stage of embryogenesis and their in vitro cultures are established from epiblast tissue of the inner cell mass of a blastocyst. They can develop into all cell types in embryo, regardless of the germ layer, and can form all cells of the organisms, except of the placenta. Among markers characteristic for pluripotent SCs are Oct4, Nanog, SSEA-1 (in mouse), or SSEA-4 (in human). In 2006 a breakthrough research demonstrated that pluripotent cells can be obtained from terminally differentiated somatic cells by an enforced expression of Oct4 and Sox2, accompanied by Klf4 and Myc or Nanog and Lin28. Reprogramming of human somatic cells was demonstrated a year later. Cells created this way are termed the induced pluripotent stem cells (iPS cells) and resemble ESCs, as they are able to differentiate in all somatic tissues as well as contribute to germline (18, 164, 193).
Multipotent SCs can differentiate into tissues of a single germ layer. Mesenchymal stem cells (MSCs) are regarded as multipotent, since they can differentiate into osteoblasts, chondrocytes, and adipocytes. Additionally, MSCs were postulated to give rise to functional endothelial cells or cardiomyocytes; however, this concept has not been conclusively proved so far (21, 22, 193). Despite suggestions that MSCs can be found in different adult tissues (e.g., adipose-derived MSCs and skeletal-muscle-derived MSCs), it appears that they develop only in the bone marrow stromal compartment. Here, together with osteoblasts and adipocytes, MSCs create a niche for the other multipotent SCs—hematopoietic stem cells (HSCs). HSCs are capable of differentiation into erythroid, lymphoid, and myeloid lineages and give rise to all types of mature blood cells (21, 22, 193).
Monopotent (or unipotent) SCs are progenitor cells of a single tissue, and can differentiate into only one cell type. They still posses, however, a self-renewal ability. Muscle satellite cells (mSCs) are examples of adult SCs for skeletal muscle. They display a monopotent characteristic; although, mSCs, when stimulated properly, can be reprogrammed to the other mesenchymal cell types—osteoblasts and adipocytes (10, 22, 173, 193).
microRNAs
microRNAs (miRNAs), the novel mediators governing SC commitment and differentiation, have been intensively studied over the last few years. They negatively regulate gene expression (19, 42, 45). Interestingly, genes involved in cell differentiation, which must be temporally downregulated, posses many miRNA binding sites (83).
miRNAs are short (19–25 nt) single-stranded RNAs. These regulatory molecules are encoded in genome and transcribed like other genes by the RNA polymerase II or III, what leads to the formation of long pri-miRNAs, with a hairpin structure and a fragment of double-stranded RNA (Fig. 1) (8, 198, 217). A significant proportion of miRNAs is not encoded by their own genes, but they are generated during the alternative splicing of mRNA genes (63). These so-called mirtrons are encoded in introns, and may regulate the expression of the gene from which they originate (32, 47, 218, 219).
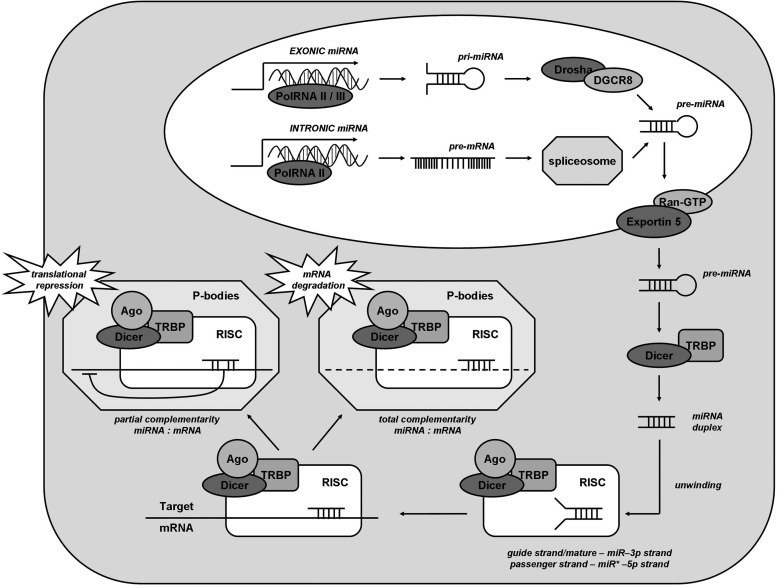
FIG. 1.
Biogenesis of miRNA. Gene encoding exonic miRNA is transcribed by an RNA polymerase II or III to pri-miRNA, which is further processed to pre-miRNA by Drosha/DGCR8 complex. Other miRNAs (intronic miRNA) are spliced from the introns of mRNA-coding genes. Pre-miRNA is then translocated to the cytoplasm, where Dicer-TRBP converts it to miRNA duplex, devoid of hairpin structure. Due to helicase activity of Dicer, the double-stranded structure is unwinded and subsequently incorporated into RISC complex (including Argonaute protein), where it binds to target mRNA. After localization in P-bodies, depending on the complementarity between miRNA and targeted sequence, mRNA is either degraded or its translation is suppressed. miRNA, microRNA; TRBP, TAR-RNA binding protein.
Processing of pri-miRNAs is conducted first in the nucleus, where RNase type III (Drosha enzyme) together with heme protein DGCR8 form pre-miRNAs that are 60–100-nt-long hairpins (53, 238) (Fig. 1). Mirtrons however omit this stage, and after alternative splicing, together with pre-miRNA, they are transported to the cytoplasm by Exportin 5 and RanGTP (47, 63). Afterward, pre-miRNAs are processed by Dicer nuclease (which is an RNase III-type endonuclease), complexed with TAR-RNA binding protein (TRBP, which recruits and binds pre-miRNA) (198, 213). Following cleavage, a miRNA duplex of desired length of ∼21 nt is formed, which is subsequently incorporated into RNA-induced silencing complex, consisting of Dicer, TRBP, and Argonaute protein (198, 213). The duplex is unwinded by a helicase domain of Dicer into passenger strand (typically degraded by RISC) and mature miRNA strand (198, 213). Then, the RISC complex delivers miRNA to the target mRNA. Binding occurs between seed sequence of miRNA (between nt 2 and 8) and usually 3′UTR of mRNA (213), although association with 5′UTR, promoter regions, and open reading frame is also possible (47). When the paring is perfect, mRNA is degraded by endonuclease Argonaute2 in RNA processing bodies (P-bodies), containing deadenylases and decapping enzymes (198, 213, 217). However, in case of incomplete miRNA-mRNA binding, repression of translation is induced. Argonaute proteins upon binding to 3′UTR may compete with eIF4F for m7G sequence on mRNA, and therefore inhibit initiation of translation (198, 217). Post-initiation repression is also possible, when Argonaute proteins lead to disassembly of ribosome complex and degradation of nascent protein (198, 217). Finally, degradation of mRNA occurs, initiated by polyA tail destabilization, resulting with translational repression (198, 217) (Fig. 1).
Heme oxygenase-1
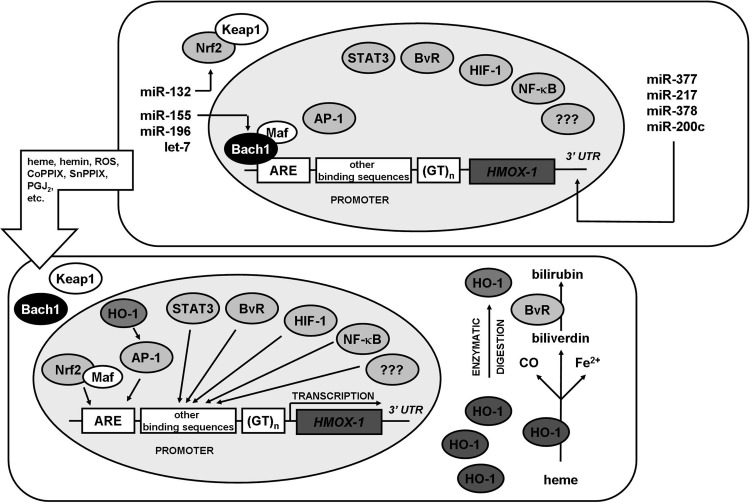
Heme oxygenase-1 (HO-1, encoded by HMOX1 gene) is regulated mainly at the transcriptional level (133, 178) (Fig. 2). However, as it was recently shown, expression of HO-1 is also regulated directly and indirectly by miRNAs (Fig. 2). Thus, miR-377 and miR-217 decrease HO-1 level in endothelial cells by interaction with 3′UTR of HMOX1 mRNA (17). Additionally, miR-122 was shown to reduce HO-1 expression (172), whereas our recent work highlights a negative regulation of HO-1 by miR-200c in renal proximal tubular epithelial cells (195) and by miR-378 in lung cancer cells (189). The other important mediator is also miR-155 that targets Bach1 mRNA and thereby increases expression of HO-1 in endothelial cells and macrophages by enhancing Nrf2 activity (109, 171), as well as miR-196 and let-7, which have similar effects in hepatocytes (74, 75). miR-132 by silencing Nrf2 mRNA can decrease HO-1 expression (195). It is important to note that regulation of HO-1 by miRNAs can be cell-type specific, as, for example, neither miR-217 nor miR-377 is expressed in kidney proximal tubular epithelial cells (195).
FIG. 2.
Regulation of HO-1 expression. In a steady state, transcription of HMOX1 gene is suppressed by Bach1 repressor that binds to ARE sequence and blocks binding of transcription factors. Stimulation of cell with various compounds leads to (i) dissociation of Bach1 repressor from ARE sequence; (ii) dissociation of Nrf2/Keap1 complex; (iii) association of Nrf2 to Maf proteins and binding to ARE sequence; (iv) activation of other transcription factors and their binding to other sequences within HMOX1 promoter; (v) transcription of HMOX1. When a protein is produced, it can catalyze degradation of heme to CO, Fe2+, and biliverdin, the latter converted to bilirubin by biliverdin reductase. HMOX1 protein can be processed by proteolysis to become an enzymatically inactive form that translocates to the nucleus where it affects activity of AP1 or NFκB transcription factors. Additionally, miR-377, miR-378, miR-200c, and miR-217 were shown to decrease HO-1 expression, whereas miR-155, miR-196 and let-7, as well as miR-132 regulate HO-1 expression indirectly, by affecting Bach1 and Nrf2, respectively. In human cells efficacy of transcription depends also on number of GT repeats in HMOX1 promoter. ARE, antioxidant responsive element; HO-1, heme oxygenase-1.
It is not surprising that HMOX1 expression can be regulated by miRNAs. However, our recent study revealed that both specific miRNA expression and global miRNA biogenesis can be regulated by HO-1 (103). This stresses additionally the significance of HO-1 as an important modulator of gene expression.
HO-1 is well known for its cytoprotective, proangiogenic, and anti-inflammatory properties, as well as for ability to regulate cell cycle. Although those effects were examined mostly in a cardiovascular bed (44, 84, 133), it has been shown that, apart from endothelium, smooth muscle cells, and immune cells, the HO-1 affects also other cell types, including stem and progenitor cells (62). At early stages of embryogenesis, it exerts proangiogenic, antioxidant, and anti-inflammatory activities in ESCs (104, 128, 214, 230, 251). Similar effects were observed in MSCs where HO-1 was induced, leading to improved transplantation outcome in the heart (82, 111, 186, 205, 216, 246), lungs (126), and kidneys (243). Moreover, HO-1 haploinsufficiency resulted in elevated mortality of HSCs (31) and was also important for cellular reprogramming, as its lack exacerbated oxidative-stress-induced cell death of murine iPS cells (128). HO-1 improves viability, proliferation, and production of proangiogenic factors in muscle progenitor cells (mSCs and myoblasts) (103, 112) and in so-called endothelial progenitor cells (EPCs; currently recognized as a heterogeneous population; see “Endothelial progenitor cells” sub-chapter) where, additionally, it facilitates migration (52). Eventually, also the MSC-, HSC-, and EPC-derived mature cells are affected by HO-1. Antiapoptotic, anti-inflammatory, and proproliferative effects of HO-1 were reported in osteoclasts (179, 256), osteoblasts (34, 41, 43), myoblasts (80, 168, 182), leukocytes (234), and endothelial cells (133).
Interestingly, a growing body of evidence suggests a direct role of HO-1 in differentiation of stem and progenitor cells. In this review we attempt to summarize recent findings in this field, focusing on the molecular pathways that mediate the differentiation-related effects of HO-1, especially those involving miRNAs.
Embryogenesis and HO-1
Role of HO-1 in embryogenesis and differentiation of totipotent or pluripotent ESCs has not been studied extensively, but some indications begun to emerge suggesting that HO-1 may be important in embryonic and early fetal development.
First, although mice that lack HO-1 are viable, they are born from HO-1+/− parents with frequency of 2–20% (depending on the genetic background, usually below 10%), less than predicted by Mendelian distribution (170, 251). In humans only two cases of complete HO-1 deficiency have been described (87, 236). These data suggest a mortality of murine and human embryos when HO-1 expression is missing. Indeed, HO-1, a cytoprotective (128) and immunomodulatory (214) enzyme, was postulated as a therapeutic target in immunological murine pregnancy complications (244), and its overexpression was shown to improve pregnancy outcome in mice (245). Interestingly, analysis of HMOX1 promoter polymorphism in group of women affected by idiopathic recurrent miscarriage revealed the higher frequency of genotypes associated with increased HO-1 level, indicating that too high HO-1 expression or its too strong inducibility can also be deleterious (51).
Influence of HO-1 on fetal growth has been usually ascribed to its role in placental vascularization (104, 230, 251). In fact, lack of HO-1, inhibition of its activity or stimulation of HO-1 in placenta by means of adenoviral vectors, resulted respectively in the reduced or improved growth of fetus in in vivo murine and rat models (104, 251). HO-1 was also necessary for proper trophoblast invasion in an in vitro model of growth of human cytotrophoblast cells on matrigel (142). Accordingly, in mice (226, 251), rats (104, 227), and humans (141), HO-1 can be detected both in embryonic and extra-embryonic tissues, starting from early stages of pregnancy. Its generation in trophoblast peaks when placenta is being formed, and then declines toward the end of pregnancy, remaining however still higher than in embryo (226, 227). Increased expression during embryogenesis, followed by its gradual downregulation when pregnancy progresses, may suggest that HO-1 plays a role in early developmental processes.
Placental or trophoblastic HO-1 may affect embryogenesis via regulation of insulin-like growth factor (IGF) binding protein-1, what was shown in an in vivo experiment performed in rats (104). IGF was shown to enhance the blastocyst formation, increase the number of blastomeres in cultured murine embryos, and facilitate the establishment of stem cell lines (131). Recently Lin et al. proved that HO-1 can directly affect differentiation of pluripotent SCs (128). They exploited a model of embryonic fibroblasts, isolated from HO-1+/+, HO-1+/−, and HO-1−/− mice, and then reprogrammed to iPS cells through enforced expression of c-Myc, Oct3/4, Klf4, and Sox2. At the beginning, the cells did not differ, displaying similar level of alkaline phosphatase, Oct4, and SSEA-1. Prolonged cell culture showed however, that lack of HO-1 augments the spontaneous differentiation, as evidenced by reduced expression of pluripotency markers in HO-1−/− cells. These results were further confirmed, when genetic or pharmacological silencing of HO-1 was performed in HO-1+/+ iPS cell cultures (128). Inhibition of spontaneous differentiation can be possibly mediated by HO-1-derived carbon monoxide, as treatment with CO could restore Oct4 expression in HO-1-deficient cells (128). Indeed, CO has already been demonstrated to be important for embryogenesis (250). The role of reactive oxygen species (ROS) has to be considered in those settings, with low levels preserving the self-renewal of SCs while higher ROS levels initiating differentiation (199). One can also suggests that the Erk1/2 kinases, upregulated in HO-1−/− iPS cells (128), may play a role. This signaling pathway is involved in differentiation of ESCs (110, 197), and its inhibition prevents neural or mesodermal maturation while promotes self-renewal of ESCs (153).
The other possible mediators through which HO-1 could affect the maturation of ESCs are miRNAs. Recently miRNAs have been demonstrated to regulate the pluripotency maintenance or lineage commitment in ESCs (19). Since a link between HO-1 and miRNAs in ESCs has not been studied yet, we can only extrapolate the results obtained in other cell types. For instance, in the myoblast cell line, HO-1 is a potent negative regulator of DGCR8 expression, leading to a decrease in a total miRNA pool (103). Importantly, deletion of DGCR8 (224), similarly as deletion of Dicer (152), resulted in disturbed differentiation of ESCs. Also, HO-1 overproduction in myoblasts inhibited expression of Lin28 and Let7 (103), two factors necessary for maintenance of stemness and for promoting the cell cycle exit (136, 145). Eventually, HO-1 in myoblasts reduced expression of miR-34a (103)—which belongs to a cluster that negatively regulates Oct4, Sox2, Nanog, and Klf4 (235)—and inhibited production of miR-290, miR-293, and miR-295 (103) - the cluster of miRNAs that restrains the early differentiation in murine ESCs (127). In contrast, miR-145, which also inhibits the expression of pluripotency markers (235), was upregulated in response to HO-1 in murine myoblast cell line (103). Thus, one can hypothesize that, apart from relatively well-documented role of HO-1 in formation of placenta, the proper activity of this enzyme may be necessary for early embryogenesis and regulation of lineage commitment of pluripotent cells. This supposition, however, has to be verified in a future work.
Hematopoiesis and HO-1
Unraveling the biology of hematopoietic stem and progenitor cells (HSPCs) began in 1960s (187, 209). Since then, extensive research on HSPCs allowed to understand better the main aspects of their activity, leading to first successful clinical application of SCs (208, 228). Recently, a growing body of evidence indicates that some mechanisms of self-renewal and differentiation found in HSPCs are shared by other tissue-specific SCs (25). Therefore, uncovering the role of HO-1 in hematopoiesis may also suggest how this enzyme is involved in SC-driven processes in other tissues.
It is estimated that 106 new blood cells have to be produced every second to provide their steady-state levels in human peripheral blood (25). Maintenance of this dynamic homeostasis is possible due to the activity of bone marrow HSPCs. Differentiation and expansion of these cells follow defined hierarchical scheme and are strictly controlled. HO-1 was reported to play a role in regulation of this process, including stages of early stem and progenitor cell differentiation as well as maturation of effector cells.
HSCs with their multipotent character and self-renewal capabilities stand at the top of hematopoietic hierarchy (Fig. 3). Despite the very low number and a quiescent character of these cells, their function is critical to maintain hematological homeostasis (25). It is understandable that during evolution HSCs have been equipped in many mechanisms that protect them from stress conditions during postnatal differentiation. Cao et al. evidenced that HO-1 is one of these crucial protective factors (31). Paradoxically, HO-1+/− heterozygous mice showed more rapid recovery in the model of 5-fluorouracil myelotoxic injury or better reconstitution at initial time points after bone marrow transplantation. However, when the HO-1+/− animals were exposed to chronic stress or HO-1+/− HSPCs were serially transplanted, significant decrease of hematopoietic recovery and exhaustion of HSPC reserve was finally observed (31). These results suggest an important role of HO-1 in regulating the balance between self-renewal and differentiation of HSPCs under environmental stress. It was proposed that reported HO-1-dependent inhibition of stress-induced HSPC proliferation is reliant on CO. Reduced HO-1 expression and lowered CO concentration led to a decreased activation of p38-MAPK pathway and, consequently, to low levels of p21 in cycling cells (31).
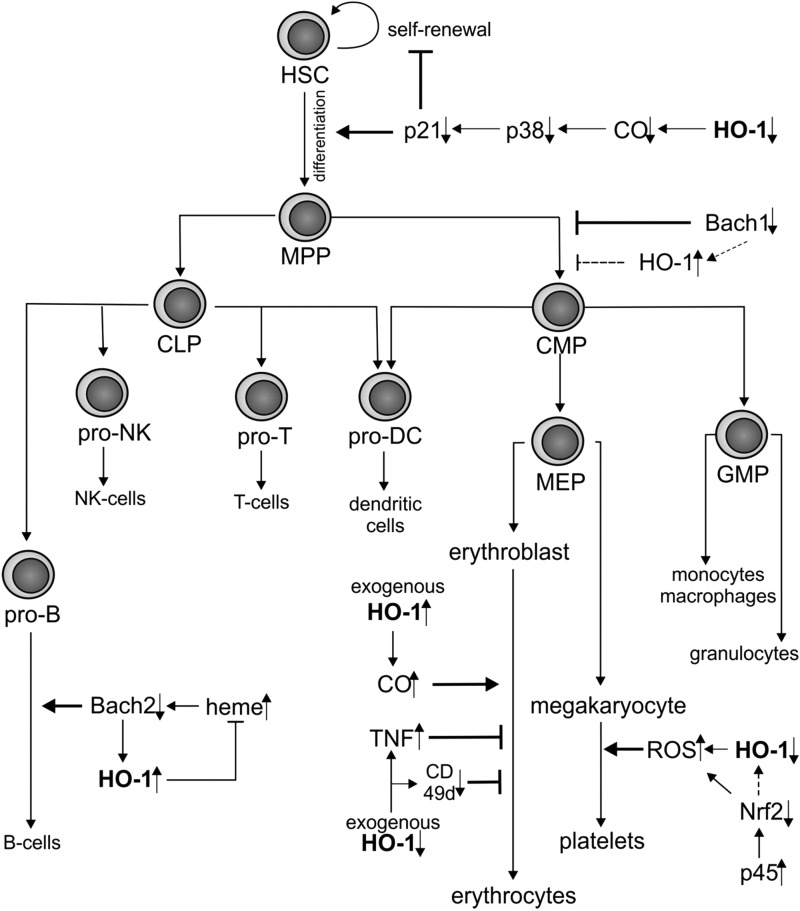
FIG. 3.
Modulation of hematopoiesis by HO-1. HO-1 deficiency accelerates differentiation but inhibits self-renewal of HSCs under stress conditions. Downregulation of Bach1 suppresses the MPP/CMP transition, but confirmation of whether this effect is HO-1 dependent requires further studies (dotted line). HO-1 activity in central macrophages of erythroblastic islands results in elevated CO concentration and regulates the erythroblast maturation acting through modulation of TNFα and α-subunit of α4β1 integrin. Decreased expression of HO-1 in megakaryocytes leads to increased generation of ROS what enhances production of platelets. By regulating the heme level in B-cell progenitors, HO-1 affects B-cell differentiation in a Bach2-dependent manner. CLP, common lymphoid progenitor; CMP, common myeloid progenitor; GMP, granulocyte–macrophage progenitor; HSC, hematopoietic stem cell; MEP, megakaryocyte–erythroid progenitor; MPP, multipotent progenitor; ROS, reactive oxygen species; TNFα, tumor necrosis factor α.
Similarly, decrease in a long-term blood chimerism was observed after bone marrow transplantation from Nrf2−/− mice (146). Although Nrf2 is one of the main transcription factors driving expression of HO-1, the HO-1 mRNA levels in HSPCs from Nrf2−/− mice were not altered, suggesting that mechanism of this effect is independent of HO-1 activity (146). However, the effect of Nrf2 may be similar, as recent study demonstrated that HSC and progenitor cell compartment was expanded in Nrf2-deficient mice at the expense of HSC quiescence and self-renewal (215).
In next stage of hematopoiesis, HSCs differentiate to multipotent progenitors (MPPs) and subsequently to common myeloid progenitors (CMPs) or common lymphoid progenitors (CLPs) (Fig. 3). The transition to CLPs might be affected by HO-1 as indicated by studies done in Bach1−/−HO-1−/− mice (190). Such mice exhibited higher numbers of HSC and MPP fractions in bone marrow, but lower numbers of CMPs and, consequently, CMP-derived progeny, including dendritic-cell-restricted progenitors, common dendritic cell progenitors, monocytes, macrophages, and dendritic cells. It suggests that Bach1/HO-1 pathway may be important in committing HSCs to CMPs (190). Nevertheless, the discussed event in Bach1−/−HO-1−/− mice is, at least to some extent, HO-1 independent, as Bach1−/−HO-1+/+ mice show similar decrease in macrophage and dendritic cells as Bach1−/−HO-1−/− phenotype. Therefore, the exact role of HO-1 in HSC/CMP transition has to be clarified in a model of Bach+/+HO-1−/− mice. Other pathways dependent on Bach-1/Nrf2 may play a role (215).
Several reports evidenced the involvement of HO-1 in differentiation of lineage committed progenitors (Fig. 3). The first studies regarding its role in maturation of hematopoietic progenitors concerned erythropoiesis (2, 77). Erythroblasts differentiate in the bone marrow and spleen within the defined niches called erythroblastic islands (20, 36). These structures were characterized as a central macrophage with many surrounding and interacting erythroid cells at different stages of maturation. Interestingly, the lack of HO-1 expression in erythroblasts is well described in both K562 erythroid cell line (7) and primary human bone marrow–derived erythroid progenitors (7). Oppositely, HO-1 is constitutively expressed in macrophages; therefore, it has been suggested that HO-1 activity in these cells is crucial to regulate erythropoiesis (211). Indeed, exogenous CO accelerated the differentiation of K562 cells in vitro (211). Moreover, in the in vivo model of HO-1+/− bone marrow transplantation, the differentiation of erythroblasts was impaired (30). This effect was mostly visible in the spleen as a prevailing site of erythropoiesis under stress conditions and to lesser extent in the bone marrow. Interestingly, higher percentage of tumor necrosis factor α (TNFα)–expressing macrophages was found in the spleen of HO-1+/−mice. As TNFα inhibits erythropoiesis, this may explain the erythropoietic defect in HO-1+/− individuals (30). Further, HO-1-deficient proerythroblasts displayed a decreased surface expression of α-subunit of α4β1 integrin (CD49d) that is crucial for adhesion to central macrophages, what might contribute to alterations of erythropoiesis.
HO-1 may also regulate thrombopoiesis (Fig. 3) (150, 159). Treatment of Meg-01 human megakaryoblastic cell line or primary human cord blood–derived megakaryocytes with prostaglandin-J2 (PGJ2) led to platelets release and concomitantly upregulated HO-1 expression. However, when HO-1 activity was inhibited by tin protoporphyrin IX (SnPPIX) before PGJ2 stimulation, the release of platelets was further increased. Additionally, PGJ2-induced platelet production was accompanied by elevation of oxidative stress. Hence, it is likely that the well-known antioxidant properties of HO-1 may inhibit the platelet production (159). Similar effect may be exerted by Nrf2 that shares DNA-binding specificities with NF-E2 p45, an essential regulator of megakaryopoiesis. Accordingly, it has been demonstrated that p45 through competing with Nrf2 promotes increased oxidative status, what enhances platelet gene expression and megakaryocytic maturation (150).
Contribution of HO-1 to development of murine lymphoid lineages has been suggested in case of B-cell maturation (Fig. 3). Accordingly, HO-1 expression increases with stages of B-cell differentiation, being the lowest in pre-B, moderate in immature-B, higher in mature-B, and the highest in plasma cells (225). As proposed, HO-1 regulates intracellular heme levels that affect the humoral immunity in Bach1- and Bach2-dependent mechanism. In turn, Bach1 and Bach2 modulate expression of HO-1, indicating a presence of negative feedback loop (159).
Despite increasing number of studies, many aspects of how HO-1 influences hematopoiesis need to be further elucidated. A growing body of evidence underlines the crucial role of stromal cells within HSC niche in controlling the HSC self-renewal and quiescence (223). It remains unclear whether HO-1 modulates HSC potential by regulating the niche components. Moreover, the nuclear form of HO-1 that lacks an enzymatic activity has been recently described (130). That raises a question whether the involvement of HO-1 in hematopoiesis described until now is solely dependent on its enzymatic activity or to some extent also on its not well-characterized non-enzymatic functions.
Importantly, recent results strongly support the hypothesis that miRNAs are crucial factors shaping the HSPC development (160). Taking into consideration that HO-1 may globally affect the miRNA production (103), including particular miRNAs known to regulate hematopoiesis, it is reasonable to hypothesize that a role of HO-1 in HSPC differentiation is at least partially miRNA dependent. One of the potential candidates is miR-146a, whose forced overexpression caused disturbed kinetics of differentiation and decreased engraftment and survival of HSCs (196). The levels of miRNA-146a seem to be strictly connected with HO-1 expression, as overexpression of the enzyme led to increased levels of miR146a in murine myoblast cell line (C2C12 cells) (103), while T regulatory cells from spleens of HO-1−/− mice showed decreased 146a expression (unpublished data). Moreover, the miRNAs 15a (100, 249), 142-3p (23), 155 (161), and 196b (169), whose knockdown or overexpression deregulates hematopoiesis, may be also regulated by HO-1 (103). Therefore, the potential influence of HO-1 and miRNA cross-talk on hematopoietic differentiation is particularly interesting and needs to be further studied.
HO-1 Role in Cancer SCs
Increasing understanding of SC properties led to discovery that certain fraction of cancer cells display similar characteristics to the tissue SC populations (174). Many reports evidenced that solid tumors and leukemias contain a small fraction of quiescent cancer cells that show enhanced self-renewal capacity and long-term tumor-initiating potential. These cells were called cancer SCs and are thought to be the most therapy-resistant fraction of tumor cells (49). As HO-1 was shown to regulate stress response and differentiation of adult SCs, it has been hypothesized that HO-1 activity may play an important role in cancer SC biology.
There are several reports showing that high HO-1 expression selects the most resistant tumor cells (15). Subclones of human lung adenocarcinoma cell line (CL3) with highest HO-1 expression were highly insensitive to toxic effects of arsenite (117). Further, HO-1 was shown to be a crucial factor responsible for chemotherapy resistance of human cholangiocarcinoma cells (101). Other studies demonstrated that HO-1 protects chronic myeloid leukemia against cell death induced by BCR/ABL inhibitors (139, 140). The resistance of multiple myeloma to bortezomib and lenalidomide is also linked to high expression of HO-1 (15). Consistently, silencing of HO-1 increased the sensitivity of the acute myeloid leukemia (AML) cells to cytarabine and daunorubicin chemotherapeutics (69). Apart from HO-1-dependent chemotherapy and drug resistance, high HO-1 expression may provide protection of cancer cells against photodynamic therapy (158).
The majority of studies that prove the cytoprotective role of HO-1 in cancer cells claimed that mechanism of HO-1-dependent resistance is connected with regulation of ROS (15, 69, 98, 101, 117, 188). Additional pathway has been proposed in case of AML cells; HO-1 counteracted induction of apoptosis caused by NFκB inhibition (176) and provided resistance to TNFα-induced cell death (177). Importantly, many cytoprotective effects ascribed to Nrf-2 were shown to be dependent on HO-1 activation (96, 134).
There is a body of evidence that HO-1 enhances survival and drug resistance in neoplasms. Drug resistance and tumor reoccurrence after therapy may result from presence of cancer SCs. Therefore, it is justified to postulate that HO-1 is important in this rare cell fraction. The first observation that supports this hypothesis came from work by Herrmann et al. (70), who studied primary AML cells isolated from patients. They sorted AML cells with CD34+CD38− and CD34+CD38+ phenotypes, which previously were shown to be enriched in leukemia stem and progenitor cells (206), and confirmed high HO-1 expression within these subpopulations (70). The water-soluble inhibitors of HO-1, SMA-zinc protoporphiryn IX (ZnPPIX) and PEG-ZnPPIX (154), inhibited in vitro growth of CD34+CD38− and CD34+CD38+ AML cells in a dose-dependent manner (70). Accordingly, incubation of AML cells with SMA-ZnPPIX reduced the occurrence of leukemia in vivo after xenotransplantation to immunocompromised mice (70), what confirmed the possibility to use HO-1 inhibitors to block leukemia-initiating cells.
Over last decade, the involvement of miRNAs in cancer pathophysiology has been investigated profoundly (210). Apart from functioning as an oncogene or a tumor suppressor, some miRNAs were revealed to be associated with self-renewing and therapy-resistant population of cancer cells (234), including the leukemic SCs (40). For example, miR-196b has been suggested to take part not only in self-renewal of hematopoietic progenitors, but also in leukemic SCs. It was less abundant in more differentiated cells, while its overexpression increased the replating potential of leukemic progenitors and partially blocked their differentiation (169). On the other hand, in AML patients, miR-204 was downregulated (58). Interestingly, upregulation of miR-196b and downregulation of miR-204 were observed in C2C12 cells in response to HO-1 overexpression (103). Such cells injected to immunodeficient mice behaved like tumor cells, did not differentiate, and formed lung metastasis (102).
Among miRNAs that are connected with HO-1 expression, miR-29a, miR-34a, miR-126, miR-145, miR-146a, mir-155, and miR-378 were found to be deregulated in various cancer SC populations (40, 50). Their functions and mechanisms that regulate their expression can, however, differ in SCs derived from distinct tumors.
Summing up, it appears that HO-1 activity in cancer SCs could result in tumor resistance to therapy. Therefore, the HO-1 inhibitors have grown into potential anticancer drugs. However, more studies on HO-1 role in strictly defined cancer SCs are needed. The possible influence of HO-1 on self-renewal and differentiation of cancer SCs seems to be particularly interesting.
Osteoclastogenesis and HO-1
Bone is a dynamic structure, whose constant remodeling in postnatal life is a consequence of balance between bone production by osteoblasts and bone resorption by osteoclasts, the specialized cells formed by fusion of monocyte-macrophage precursors. Osteoclastogenesis is tightly regulated by several cytokines (Fig. 4). Macrophage colony-stimulating factor promotes differentiation of HSCs into preosteoclasts, and their fusion into multinucleated, mature osteoclasts. Receptor activator for NFκB ligand (RANKL), which is a member of TNFα family, by binding to its receptor (receptor activator for NFκB [RANK]) and afterward to TNFα receptor associated factor 6, activates several signaling pathways during differentiation, including Erk1/2, p38, JNK, and PI3K/Akt kinases. This leads to activation of numerous transcription factors, such as NFκB and c-Fos, which induce expression of another transcription factor NFATc1 (NFAT2). It in turn acts as a cofactor of AP1 transcription factor and activates expression of target genes, specific for osteoclasts: tartrate resistant acid phosphatase (TRAP), cathepsin K, or calcitonin receptor. The major inhibitor of this process is osteoprotegerin, a soluble ligand for RANK, which inhibits RANKL-dependent signaling (24, 207, 252). Osteoclastogenesis can also be augmented by proinflammatory cytokines, for example, TNFα (24, 207, 252). Recently, the high mobility group box 1 (HMGB1) nuclear transcription factor, which can be released by damaged cells, was shown to upregulate maturation of osteoclasts by binding to advanced glycation endproduct receptors (254).
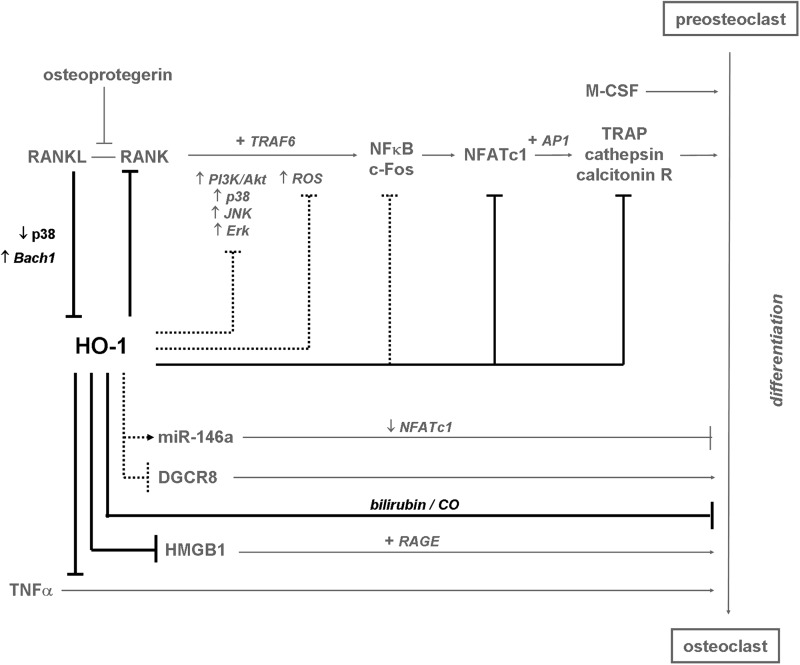
FIG. 4.
HO-1 as an inhibitor of osteoclastogenesis. HO-1 is downregulated during differentiation of osteoclasts by RANKL-dependent signaling. Elevated HO-1 inhibits osteoclastogenesis probably via inhibiting c-Fos and NFATc1 transcriptional activities. Thereby it decreases expression of markers of terminally differentiated osteoclasts (e.g., TRAP) and prevents bone resorption. HO-1 abolishes also secretion of HMGB1 and downregulates signaling from TNFα, which are known to induce osteoclast maturation. Similarly, inhibition of osteclastogenesis is also exerted by bilirubin and CO. Additionally, HO-1 may decrease activity of p38 and Erk1/2 kinases or DGCR8, and downregulates ROS (involved in upregulation of RANKL/RANK signaling pathway), all of them known as regulators of osteoclast maturation, whereas it may upregulate expression of miR-146a that is an inhibitor of osteoclastogenesis. Black—effects exerted by HO-1; gray—known pathways of osteoclastogenesis; spots—hypothetical pathways regulated by HO-1 in differentiating osteoclasts. (→)—induction; ( )—inhibition; (+)—in cooperation with. HMGB1, high mobility group box 1; RANK, receptor activator for NFκB; RANKL, receptor activator for NFκB ligand; TRAP, tartrate resistant acid phosphatase.
)—inhibition; (+)—in cooperation with. HMGB1, high mobility group box 1; RANK, receptor activator for NFκB; RANKL, receptor activator for NFκB ligand; TRAP, tartrate resistant acid phosphatase.
Several lines of evidence indicate that HO-1 plays a role in osteoclast maturation during postnatal life (Fig. 4). In contrast to preosteoclasts, the mature osteoclasts do not respond to hemin stimulation and do not upregulate HO-1 level, suggesting that HO-1 affects rather osteoclast differentiation than function. Accordingly, HO-1 expression is decreased in mature osteoclasts, and possibly such downregulation is necessary for proper osteoclastogenesis (256). This supposition has been confirmed recently in two independent studies, which showed that HO-1 is inhibited during osteoclast differentiation induced by stimulation of RANK with RANKL (68, 179). HO-1 inhibition in differentiating osteoclasts may depend on Bach1 transcriptional repressor, as this effect was less pronounced in the Bach1−/− cells (68). Additionally, the RANKL-dependent HO-1 downregulation can be mediated by declined p38 signaling (179). It appears that suppression of HO-1 enables the release of pro-osteoclastogenic HMGB1 (179). Moreover, ROS were demonstrated to be critical in RANKL-dependent signaling, as their presence is necessary for proper activation of downstream signaling pathways (Akt, p38, JNK, and ERK1/2 kinases) (67, 114), while HO-1 is known to affect differentiation of other cell types via inhibition of O2•− (14). Thus, it seems that inhibition of HO-1 may result in elevation of ROS, required for the RANKL/RANK signaling (179).
Induction of HO-1 with hemin inhibits osteoclastogenesis in in vitro culture of primary murine preosteoclasts or RAW-D osteoclast precursors (179, 256) and, as a result, in vitro bone resorption (256). These effects were reversed by siRNA-mediated HO-1 silencing or upregulated after HO-1 gene transfer (179), and to some extent, they could be mimicked by HO-1 byproducts—bilirubin and CO (179, 256). Since proliferation of osteoclast precursor cells was increased and apoptosis was decreased, hemin-induced differences in the number of mature osteoclasts as well as reduced transcription of TRAP and calcitonin were attributed to impaired differentiation (179, 256).
The detailed mechanisms by which HO-1 regulates osteoclastogenesis have not been elucidated so far. It has been shown that induction of HO-1 expression with hemin reduced the expression of RANK (256) and release of HMGB1 (179) during in vitro differentiation of primary preosteoclasts. Similarly, in the Bach1-deficient primary preosteoclast cells, inhibition of differentiation was accompanied by attenuated RANKL/RANK signaling and decreased activity of c-Fos and NFATc1 (68). HO-1 may, to some extent, mediate these effects, as it is upregulated in Bach1−/− preosteoclasts, and its silencing partially restores osteoclastogenesis (68).
Increase in HO-1 expression may also be responsible for inhibition of osteoclast differentiation of murine primary bone marrow–derived macrophages cultured in vitro and treated with inducers of Nrf2: tert-butylhydroquinone (237), deltamethrin (180), and kahweol (56). Upregulated HO-1 expression was parallel to decreased HMGB1 release (56, 237), inhibition of Erk1/2 activity (56, 180), and attenuation of NFATc1-dependent transcription (56, 237). One can hypothesize that these pathways are involved in regulation of osteoclastogenesis by HO-1.
Inhibitory effect of HO-1 on osteoclast maturation and bone resorption was confirmed in vivo. HO-1 induced by cobalt protoporphiryn IX (CoPPIX) abolished inflammatory osteoclastogenesis mediated by TNFα (256), and TNFα-induced bone loss was decreased in Bach1-deficient mice (68). On the other hand, Nrf2−/− animals were reported to develop severe joint injury and spontaneous bone fracture, what might be due to increased formation of osteoclasts (231). Moreover, reduced expression of HO-1 was demonstrated in patients with rheumatoid arthritis, which is an inflammatory disease characterized by increased osteoclast activity and bone destruction (54, 256). It appears that high HO-1 activity leading to elevated bilirubin level can protect bone from erosion (256).
Like many other processes, osteoclastogenesis is regulated by miRNAs (Fig. 4). Despite few studies that report the role of miR-223, miR-155, or miR-21 in murine in vitro culture of preosteoclast cell line and primary cells (85, 233), this issue has not been investigated sufficiently yet. Some observations suggest, however, that miRNAs might mediate effects of HO-1 in osteoclasts. First, decreased expression of Dicer or DGCR8 proteins suppresses biogenesis of miRNAs in osteoclasts and slowdowns osteoclastogenesis (148, 200). Similar influence on DGCR8 expression and miRNA production can result from HO-1 activation in murine myoblast C2C12 cell line (103). Next, in C2C12 cells, HO-1 was shown to upregulate miR-146a (103), known to abolish osteoclast differentiation in vitro via inhibition of NFATc1 transcription factor (155). The crosstalk between miRNAs and HO-1 in osteoclastogenesis is, however, hypothetical and needs examination.
In conclusion, HO-1 inhibits osteoclastogenesis and expression of proteins characteristic for terminally differentiated osteoblasts via decrease in RNAK/RANKL signaling, decrease in HMGB1 secretion, and maybe through miRNA-dependent pathways (Fig. 4).
Osteoblastogenesis and HO-1
New bone tissue is formed by osteoblasts, the cells of mesenchymal origin. Following lineage commitment, osteoprogenitors proliferate intensively and differentiate into mature osteoblasts, generating osteogenic extracellular matrix and conducting bone mineralization. When trapped and isolated in the bone matrix, osteoblasts give rise to mitotically inactive osteocytes, which can further activate their precursors in a paracrine manner (81, 119).
Commitment of MSCs into osteoblastic lineage during postnatal life is induced by cooperation of Wnt/β-catenin, bone morphogenic protein 2 (BMP2), and runt-related transcription factor 2 (Runx2) (Fig. 5). Runx2 is a main transcription factor controlling osteoblastogenesis during skeletal development and postnatal life, and its deficiency precludes formation of mineralized skeleton. It is both sufficient and obligatory to promote osteoblastic differentiation of MSCs. Runx2 binding sequences are located in the promoters of several genes characteristic for osteoblasts, such as collagen 1α, osteopontin, bone sialoprotein, osteocalcin, osteonectin, or alkaline phosphatase. Expression of Runx2 is precisely controlled, and decreases during terminal stages of differentiation, what is necessary for proper bone development. It is potentiated by BMP2 or Wnt/β-catenin signaling pathways, while inhibited by Sox9 or Nkx3.2, the inducers of chondrogenesis. Transcriptional activity of Runx2 is controlled by the plethora of coactivators. Namely, CCAAT/enhancer binding protein α and β (c/EBPα and c/EBPβ) upregulate, together with Runx2, the expression of bone-specific growth factors or their receptors. Activating transcription factor 4, whose expression is required at later steps of osteoblast maturation, interacts with Runx2 or FoxO1 to induce transcription of genes typical for terminally differentiated osteoblasts. Interestingly, Runx2 is activated by FoxO1 in differentiating MSCs, whereas in mature osteoblasts or committed osteoblastic progenitors such an interaction can be opposite. This may depend on production of ROS, which are known to reduce bone mass and osteoblast differentiation through activation of FoxO proteins. Finally, Runx2 increases expression of osterix, another transcription factor that promotes osteoblastogenesis. Osterix elevates expression of major osteoblastogenic genes and, together with its cofactor NFATc1, activates the Wnt/β-catenin signaling pathway, which can be additionally stimulated by BMP2 and transforming growth factor β (6, 81, 105, 119, 125, 137, 229).
FIG. 5.
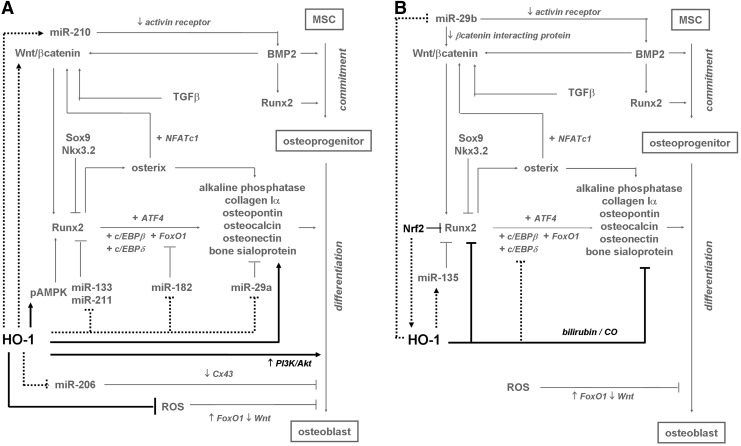
HO-1 as a stimulator (A) and inhibitor (B) of osteoblastogenesis. HO-1 activates PI3K/Akt and pAMPK signaling pathway and reduces ROS level, inducing differentiation and viability of osteoblasts. Additionally, HO-1 may induce osteoblastogenesis via activation of Wnt/β-catenin signaling. It could also interfere with differentiation process by potentially decreasing miRNAs involved in regulation of transcriptional cascade of Runx2 (miR-133, miR-211, miR182, and miR-29a). By modulating the level of miR-210 and miR-206, HO-1 could induce BMP2 signaling and expression of Cx43. On the other hand, HO-1 decreases expression of Runx2 and markers of differentiated osteoblasts, what is mediated by CO and bilirubin, and could be also achieved by decreased activity of c/EBPδ. HO-1 may possibly induce miR-135, a repressor of Runx2, and reduce miR-29b, what induces differentiation by affecting Wnt/β-catenin and BMP2 signaling. Nrf2, an HO-1 inducer, abolishes Runx2 activity. Black—effects exerted by HO-1; gray—known pathways of osteoblastogenesis; spots—hypothetical mechanisms regulated by HO-1 in differentiating osteoblasts. (→)—induction; ( )—, inhibition; (+)—in cooperation with. BMP2, bone morphogenic protein 2.
)—, inhibition; (+)—in cooperation with. BMP2, bone morphogenic protein 2.
Role of HO-1 in postnatal osteoblastogenesis has been studied in different models, but still there is no consensus whether it induces, inhibits, or has no effect on this process (Fig. 5). Some reports showed a slight increase in HO-1 expression during in vitro osteoblastic differentiation of human MSCs (14), and an enhanced bone mineralization after in vitro stimulation of mesenchymal osteoblast precursor cells (14, 221) or primary osteoblasts isolated from osteoarthritic patients (41) with CoPPIX. Markers of osteogenesis (BMP2, Runx2, and osteonectin) were, however, unchanged after pharmacological stimulation of HO-1 expression or inhibition of its activity (14, 41). Yet, their upregulation or reduction was observed upon CoPPIX or tin mesoporphiryn IX (SnMPIX) treatment, respectively, when cells were incubated additionally with high concentration of glucose (14) or with interleukin-1β (IL-1β) (41). Similar effect was observed when HO-1-overexpressing primary osteoblasts were stimulated with IL-1β (41). HO-1 was also suggested to be a mediator of in vitro osteoblastogenesis of primary rat or human MSCs induced by curcumin (64) and osteoprotegerin (221), or as a factor protecting MSCs from nicotine-dependent inhibition of osteogenic differentiation (92).
Induction of HO-1 expression with CoPPIX, especially under hyperglycemic conditions, leads to activation of AMPK (14, 221) and Akt (221) kinases in human primary MSCs cultured in vitro (Fig. 5), what can be reversed by SnMPIX (221). Both these signaling pathways upregulate osteoblastic differentiation (94), and can be proposed as mediators of osteoblastogenic activity of HO-1. Another possible mechanism of augmented maturation of human osteoblasts is HO-1-dependent decrease in production of O2•− (6, 14). Forced expression of HO-1 by means of lentiviral vectors induces also Wnt10 in vivo in a murine adipose tissue (29). Wnt10 was shown to increase osteoblast differentiation (105) and may be responsible for some effects of HO-1 activation, similarly to other factors that may promote osteoblastogenesis instead of adipogenesis (1, 60, 202, 203).
HO-1 was reported to support differentiation of human dental pulp cells into odontoblasts (99), which share many similarities with osteoblasts. They also originate from mesenchymal progenitors, rely on Runx2 as a regulator of differentiation, and express the same components of extracellular matrix (28). Pharmacological stimulation of HO-1 with CoPPIX upregulated the odontoblast differentiation markers, whereas its pharmacological or genetic inhibition, as well as scavenging the CO or ferrous iron, exerted opposite effects (99). Accordingly, pharmacological inhibition of HO-1 activity with ZnPPIX or SnPPIX blocked in vitro osteogenesis induced by pachymic acid (118), substance P (102), mechanical stress (116), or simvastatin (147), and reduced the expression of odontoblast differentiation markers, such as alkaline phosphatase, osteonectin, osteocalcin, osteopontin, and bone sialoprotein (102, 116, 118, 147).
On the other hand, Nrf2 was shown to repress transcriptional activity of Runx2 and inhibit osteoblastogenesis (71). This is in line with inhibitory role of HO-1 in osteoblast in vitro differentiation observed for primary osteoblastic cells isolated from rat calvaria, where genetic overexpression or pharmacological activation of HO-1 reduced mineralization and decreased expression of alkaline phosphatase, osteocalcin, and Runx-2, with only a slight decrease in cell viability (132). In accordance, treatment with bilirubin or CO-releasing molecule reduced osteoblast differentiation rate, the effect reversed by ZnPPIX (132). Additionally, HO-1/ferritin axis was reported to inhibit smooth muscle cell calcification and osteoblastic differentiation (242). As HO-1-derived CO potently decreases activity of c/EBPδ in murine myoblast C2C12 cell line (103), one can suppose that influence of HO-1 on osteoblastogenesis may be partially mediated by cEBP-dependent pathways. Finally, some experiments showed that neither overexpression of HO-1 (253) nor its deficiency in the primary MSCs isolated from HO-1−/− mice (243) modulated the rate of osteoblastic maturation in vitro.
Recent studies have underlined a role of miRNAs in formation of osteoblasts from both MSCs and C2C12 cell line (76). In the latter cells, we have demonstrated that miRNA expression profile is significantly influenced by high level of HO-1 activity (103). Thus, one can expect that similar changes might be induced during osteoblastogenesis, if expression of HO-1 is simultaneously increased. Overexpression of HO-1 in C2C12 cells caused a decrease in expression of miR-133a/b, miR-182, miR-206, miR-211, and miR-29a (which have been demonstrated to inhibit osteoblast maturation of C2C12 cells, murine MSCs, and primary murine osteoblasts isolated from rodent calvaria in vitro), while upregulated miR-210 (known to induce in vitro differentiation of murine stromal cells into osteoblasts) (76, 97, 103). Attenuating the osteoblastic maturation by miRNAs results from directly targeting the mediators critical for osteoblastogenesis: Runx2 (by miR-133 and miR-211), Cx43 (by miR-206), osteonectin (by miR-29a), and FoxO1 (by miR-182) (76, 78, 86, 97, 123). Conversely, miR-210 targets the activin receptor, thereby blocking the signaling from inhibitors of BMP pathways (149). Thus, it appears that alternations in expression of miRNAs may promote the osteoblastic differentiation, and might be potential executors of HO-1-related effects (Fig. 5).
However, induction of HO-1 in C2C12 cells also elevates the expression of miR-135, considered as a factor inhibiting osteoblastic maturation of C2C12 cells via targeting Runx2 (123). Similarly, HO-1 seems to downregulate miR-29b (103), which was shown to decrease the expression of various inhibitors of differentiation in osteoblasts from rat calvaria, such as activin receptor or β-catenin interacting protein 1 (122). In this manner, HO-1 might exert also the inhibitory effect on osteoblastogenesis (Fig. 5).
Confusing results that demonstrate both stimulatory and inhibitory impact of HO-1 on maturation of osteoblasts may be due to differences in cell models used (MSCs or dental pulp cells vs. primary osteoblasts). It should also be noticed that although pro-osteogenic activity of HO-1 has been reported in a few independent studies, HO-1 alone was insufficient to induce differentiation (14, 41, 102). Instead, it rather potentiated or decreased the effects of other factors (14, 41, 102). However, such a separation of modulatory function of HO-1 and its direct influence on osteoblastogenesis requires further experimental verification.
Adipogenesis and HO-1
Formation of adipose tissue in an adult organism includes two important stages—commitment of MSCs to adipogenic lineage followed by terminal differentiation of preadipocytes to mature adipocytes. After a lineage commitment, cultured preadipocytes have to be growth arrested, and then begin to proliferate synchronously, undergoing multiple rounds of mitosis, finished by terminal differentiation, manifested by a dramatic cell-shape change, accumulation of triglycerides, and de novo lipid synthesis (135, 204).
Several factors are engaged in regulation of the commitment process (Fig. 6). BMP2 and BMP4 have an activating role as they induce expression of cytoskeletal proteins necessary for changing the cell shape from fibroblast like to the round one. Wnt/β-catenin pathway triggers the transcription of c-myc and cyclin-D1, important for mitotic clonal expansion process. Its role, however, is not so unequivocal. High level of Wnt10b, which decreases upon the later stages of differentiation, leads to inhibition of adipogenesis through blocking the expression of major adipogenic transcription factors—peroxisomal proliferator activated receptor γ (PPARγ) and c/EBPα. In contrast, Wnt5b upregulates PPARγ and enhances adipogenesis. Finally, Hedgehog and its ligand Sonic (Shh) interfere with the adipogenic commitment, although the precise mechanism of this effect remains unknown (135, 204, 220).
FIG. 6.
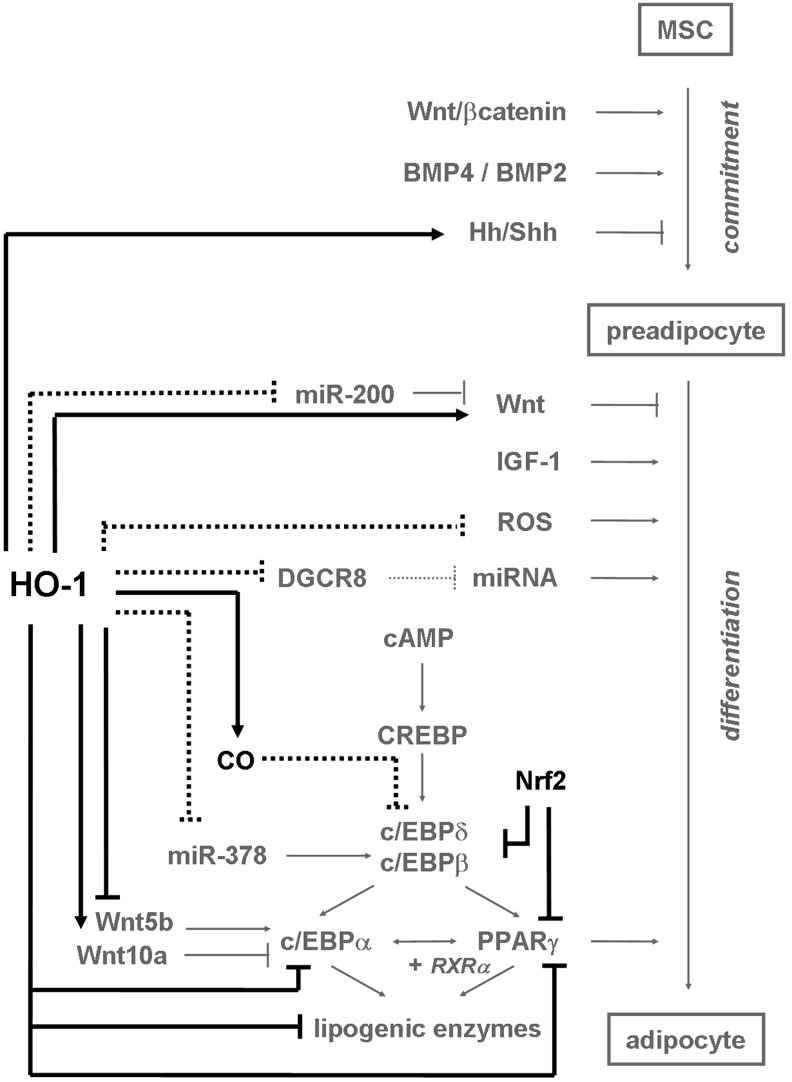
HO-1 as an inhibitor of adipogenesis. HO-1 decreases expression of c/EBPα, PPARγ, and enzymes characteristic for mature adipocytes by interaction with Wnt/β-catenin and Hh/Shh pathways. Nrf2, an HO-1 inducer, suppresses adipogenic differentiation too. HO-1 may reduce expression of miR-200c and miR-378, resulting in a decline of differentiation mediated via Wnt or c/EBPα/β, respectively. Possibly, HO-1 can also inhibit adipogenesis by decrease in ROS production, DGCR8 expression, and CO-mediated downregulation of c/EBPδ/β activities. Black—effects exerted by HO-1; gray—known pathways of adipogenesis; spots—hypothetical mechanisms regulated by HO-1 in differentiating adipocytes. (→)—induction; ( )—inhibition; (+)—in cooperation with. Hh, Hedgehog; PPARγ, peroxisomal proliferator activated receptor γ; Shh, Sonic Hedgehog.
)—inhibition; (+)—in cooperation with. Hh, Hedgehog; PPARγ, peroxisomal proliferator activated receptor γ; Shh, Sonic Hedgehog.
The committed preadipocytes are growth arrested before their clonal expansion is induced by activation of IGF and cyclic AMP (cAMP) signaling pathways (Fig. 6). First, the cAMP response element binding protein (CREBP) is activated to induce the expression of c/EBPβ. After phosphorylation and dimerisation, the c/EBPβ protein acquires the DNA-binding properties and, together with c/EBPδ, activates expression of master regulators of adipogenesis, c/EBPα and PPARγ transcription factors, binding to the c/EBP regulatory elements found in their promoters. The c/EBPα displays antimitotic activities, whereas PPARγ forms a heterodimer with retinoid-X-receptor-α to activate PPAR-response element in target genes, whose expression is typical for mature adipocytes. Both transcription factors upregulate each other and activate expression of large group of adipogenic proteins, including lipoprotein lipase, intracellular lipid binding protein, or sterol-regulatory-element-binding protein 1, which, in turn, induce production of lipogenic enzymes (135, 204).
Osteoblasts and adipocytes are believed to originate from common precursors—MSCs. The shift in a differentiation potential of MSCs from osteoblastic to adipogenic lineage is due to a cooperation of various factors, which can support bone formation concomitantly with suppressing adipogenesis, and vice versa (1, 60, 202, 203). In such a situation, HO-1 might have an opposite effect on osteoblast and adipocyte differentiation during postnatal life. And indeed, it was reported that when HO-1 in MSCs induces osteoblastogenesis, it represses adipocyte development (14, 221).
Accordingly, in vitro studies have revealed that human (14, 91, 221) and murine (121) bone-marrow-derived MSCs cultured under adipogenic conditions differentiated into mature adipocytes more reluctantly when additionally stimulated with CoPPIX. This effect was reversed if HO-1 expression was inhibited by siRNA (221). Moreover, in vivo stimulation of obese mice and rats with CoPPIX (27, 156) or stably genetic overexpression of HO-1 in mice fed a high fat diet (29) decreased the size and number of adipocytes. Again, the opposite effect was observed after SnMPIX stimulation (29).
Expression of HO-1 is lower in obese diabetic individuals as demonstrated in Zucker fatty rats (91), obese mice (121), and Zucker diabetic fatty rats (156). It can be also decreased in diabetic patients (157). HO-1 overexpression (29) or treatment with CoPPIX (91, 121, 156, 191) reduced subcutaneous and visceral in vivo fat deposits as well as decreased the elevated synthesis of lipids, what was reversed by pharmacological inhibition of HO-1 (121, 191). These effects may result from antioxidant properties of HO-1 (91, 121), as adipogenic activity of elevated H2O2 that activates c/EBPβ, c/EBPα, and PPARγ and promotes clonal expansion was recently proved in 3T3-L1 preadipocytes (113, 241) and fibroblasts (240). In muscle-derived SCs, ROS were necessary for induction of adipogenesis in hyperglycemia through activation of PKC (4). What is more, the antioxidative quercetin was able to reverse H2O2-induced adipogenesis, concomitantly with an increase in HO-1 expression (240). Accordingly, activation of Nrf2 reduced adipogenic differentiation through decreasing the ROS levels and suppressing the c/EBPβ, c/EBPα, and PPARγ (184). Since Nrf2-dependant effects are not always mediated by HO-1, and among Nrf2 mediators there is also HIF-1α (3, 247), the effect of Nrf2/HO-1/ROS axis on these transcription factors must be further experimentally proved. In addition, it must be kept in mind that mitochondrial ROS can also inhibit adipogenesis as they control expression of the adipogenic repressor CHOP-10/GADD153 (33).
HO-1 expression is downregulated during adipogenesis in parallel with increased expression of PPARγ (14, 221, 222), fatty acid synthase (FAS), and c/EBPα (222) in human MSCs differentiating in vitro. Moreover, enforced overexpression of HO-1 in vivo led to inhibition of PPARγ and c/EBPα in murine adipose tissue, the effect reversed by HO-1 activity inhibitor (29). The hindering role of HO-1 in adipogenesis has also been supported indirectly by the observation that epoxyeicosatrienoic acids (EETs), inhibitors of adipocyte differentiation, upregulate HO-1 in adipogenic cultures of human bone marrow–derived MSCs (93, 192, 222), probably due to the drop in expression of Bach1 (192, 222). Induction of HO-1 was accompanied by reduction of lipid content in mature adipocytes as well as by downregulation of FAS, c/EBPα, and PPARγ in differentiating cells (93, 222). Decrease in lipid deposits was reversed by pharmacological inhibition of HO-1, proving that suppression of adipogenesis by EETs was HO-1 dependent (93).
Effects exerted by EETs were attributed to augmented phosphorylation of Akt (93). Since CoPPIX, the HO-1 activator, increased the activity of Akt in adipocytes of obese mice (27), it was postulated that HO-1 affects development of adipose tissue in vivo in rats through modulation of Akt-dependent pathways (192). However, as Akt kinase is rather regarded as a positive stimulator of adipogenesis (39, 55, 167), the mechanism of antiadipogenic effects of HO-1 involves probably also other pathways and needs further elucidation.
Another suggested mechanism might involve the AMPK activity (27, 192, 221). Phosphorylation of this kinase was elevated in rat adipose tissue upon CoPPIX treatment and suppressed when SnPPIX was applied in vivo (192). However, recently the pAMPK was demonstrated to induce osteoblastogenesis on the expanse of adipogenesis (94), the effect opposite to that exerted by HO-1 on adipocyte differentiation. On the other hand, HO-1 could inhibit differentiation of adipocytes via regulation of Wnt/β-catenin pathway (Fig. 6). Its activation upregulates β-catenin, shh, and Wnt10b and downregulates Wnt5b in murine adipose tissue, with specificity confirmed through applying the HO-1 inhibitor (29). Worth mentioning, the HO-1-derived CO is able to decrease expression of c/EBPβ and c/EBPδ transcriptional activities, in murine macrophages and C2C12 cells, respectively (103, 201), what could potentially inhibit transcription of adipogenic genes and mediate the antiadipogenic properties of HO-1. Finally, it should be kept in mind that apart from the reports that prove inhibition of adipogenic differentiation caused by HO-1 activity, there are a few studies that demonstrate that adenoviral HO-1 overexpression in MSCs (253) or HO-1 deficiency in MSCs isolated from HO-1−/− mice (243) does not exert any effect on adipogenesis.
As it has been recently reported, HO-1 strongly regulates expression of miRNAs in C2C12 cell line and primary satellite cells (103). Since C2C12 cells are of mesenchymal origin, capable of adipogenic differentiation under proper conditions (5, 73, 79, 115), one can suspect that HO-1-dependent changes in miRNA levels may also be relevant to adipogenesis. Regulation of adipocyte maturation by miRNAs has been widely studied and modulation of such crucial pathways as PPARγ, c/EBPα, and Wnt signaling has been reported (143, 175). Among miRNAs, the miR-200 family is regarded as an inducer of adipogenesis (Fig. 6). Thus, expression of miR-200a/b/c increases during in vitro adipogenic differentiation of murine stromal derived cells, leading to inhibition of Wnt signaling pathway (89). Interestingly, production of miR-200c was decreased in response to HO-1 overexpression in C2C12 cells (103) and, reversely, miR-200c decreased HO-1 in proximal tubular epithelial cells (195). Similar downregulation was found in case of miR-378 (103); another miRNA whose increased production, characteristic for adipogenesis, is responsible for elevated lipid synthesis in 3T3-L1 preadipocytes, via changes in c/EBPα and c/EBPβ transcriptional activities (59). Therefore, HO-1 can be suggested to influence adipogenesis also through regulation of miR-200c and miR-378. Further, it was shown that knockdown of Dicer or Drosha, resulting in decreased miRNA biogenesis, inhibits in vitro adipogenic differentiation of human bone marrow–derived stromal cells (165). Although the effect of DGCR8 knockdown has not been examined yet, one may suppose that disturbed miRNA processing resulting from HO-1-dependent inhibition of DGCR8 (103) may be also one of possible pathways that affect maturation of adipocytes.
To sum up, HO-1 seems to decrease adipogenesis by regulating the c/EBPα, PPARγ, or Wnt/β-catenin activities, and possibly by miRNA-dependent mechanisms (Fig. 6).
Myogenesis and HO-1
mSCs represent a specific type of adult SCs, with small amount of cytoplasmic organelles and dense heterochromatin. They are considered as monopotent progenitor cells committed to skeletal muscle myocytes, which are indispensable for muscle regeneration during postnatal life (173). In an adult organism, under normal conditions, mSCs remain quiescent on a periphery of muscle fibers, but upon muscle damage they rapidly leave their niche to became activated and they proliferate myoblasts, capable of differentiating and fusing into multinucleated myocytes. Within few days they form a large number of new muscle fibers or increase the size of already existing ones (12, 26, 35). Although mSCs have been supposed to differentiate also into adipocyte or osteocyte lineages (11), recent reports have not confirmed their multipotent SC characteristic (173). However, they indeed undergo the asymmetric divisions, when one daughter cell initiates differentiation while the other one self-renews and can robustly replenish quiescent cell reservoir, what is distinctive for SCs (46, 106).
Quiescent satellite cells are defined by the presence of Pax7 transcription factor, whose expression is critical for mSC development and, in consequence, for muscle growth or regeneration (173). Pax7 maintains mSCs in an undifferentiated state and its expression decreases upon differentiation of myoblasts. However, during their activation, Pax7 induces a group of key transcription factors (MyoD, myogenin, Myf5, and Myf6), so-called myogenic regulatory factors (MRFs).
Myf5 and MyoD are upregulated during activation and proliferation of mSCs. MyoD causes predominantly a cell cycle arrest and promotes a proliferation to differentiation (26, 35, 166). Since forced overexpression of MyoD in non-myogenic cells is able to convert them into fusing myoblasts (38), it is perceived as a master regulator of myogenic differentiation. Once expressed, MyoD initiates a cascade of myogenic commitment, driving the production of all MRFs. Together with myocyte enhancer factor 2 (MEF2), MyoD directly induces the expression of myogenin and Myf6 at terminal stages of differentiation. This is essential to stimulate fusion of cells into multinucleated myotubes, expressing the markers of mature muscles, such as myosin heavy chain (MHC), creatine phosphokinase (CPK), or desmin (26, 35, 107, 166).
Recently, MRFs together with MEF2 have been demonstrated to control transcription of so-called myomirs, namely, miR-1, miR-133a, miR-133b, and miR-206, a group of miRNAs specific for skeletal or cardiac muscle. Their temporal expression in differentiating myoblasts fine tunes the output of MRFs and is necessary for proper muscle development (194, 212). Both miR-1 and miR-206 inhibit proliferation and promote differentiation of myoblasts via blocking the DNA polymerase II and downregulating the MRF inhibitors (37, 95). Their upregulation coincidences with increased level of markers of differentiation (MyoD, myogenin, MHC, and CPK) and fusion of myoblasts (95). Although MRFs activate also expression of miR-133a and miR-133b, whose expression is elevated during myoblast differentiation in a similar manner as miR-1 and miR-206 (194, 212), there are inconsistent results regarding their role in this process. In some studies they were described as inhibitors of myoblast proliferation, although not as potent as miR-1 or miR-206 (95). In others they were reported to stimulate proliferation and inhibit differentiation, possibly through direct targeting the MyoD and MEF2 (37).
We have shown that HO-1 is a potent regulator of differentiation of muscle progenitors into myocytes and myotubes. This was surprising as in an earlier article lentiviral-mediated HO-1 overexpression was reported to have no effect on in vivo myoblast maturation of primary porcine myogenic cells (112). Possibly a short period of differentiation, analysis of marker connected with terminal differentiation only, and low transduction efficacy of HO-1 construct (112) could hinder the detection of potential influence. We found that either in immortalized C2C12 murine myoblast cell line retrovirally transduced for stable HO-1 overexpression or in primary mSCs isolated from mice of different HO-1 genotype (HO-1+/+, HO-1+/−, or HO-1−/−), HO-1 potently inhibits a myogenic program (Fig. 7). Specifically, HO-1 led to increased proliferation of myoblasts, whereas myotube formation as well as expression of MyoD, myogenin, and MHC were strongly reduced. All these effects were reversible by SnPPIX or HO-1-specific siRNA. Moreover, HO-1-overexpressing C2C12 cells transplanted into caput gastrocnemius muscle of NOD-SCID mice formed tumors infiltrating healthy tissue and metastasizing to the lungs, whereas their control counterparts formed normal myofibers. Further analysis revealed that inhibition of differentiation is not connected to the reduced oxidative stress, but it depends on HO-1-derived CO, which inhibits nuclear translocation of c/EBPδ and decreases its binding to MYOD promoter, thereby blocking the MyoD transcription (103).
FIG. 7.
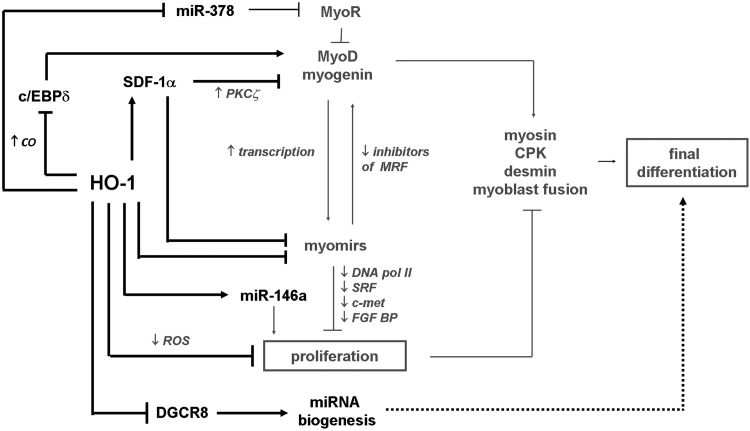
HO-1 as an inhibitor of myogenesis. HO-1 decreases myogenic differentiation by reducing MRF via c/EBPδ or SDF-1α. HO-1 and SDF-1α abolish also expression of myomirs (miR-1, miR-133a, miR-133b, and miR-206) and miR-378, whereas induces miR-146a. HO-1 inhibits DGCR8 and lowers total miRNA biogenesis, but does not affect expression of MRFs or myomirs via this pathway. Black—effect exerted by HO-1; gray—known pathways of myogenesis; spots—mechanisms regulated by HO-1 in differentiating myoblasts. (→)—induction; ( )—inhibition; (+)—in cooperation with. MRFs, myogenic regulatory factors.
)—inhibition; (+)—in cooperation with. MRFs, myogenic regulatory factors.
An additional pathway that mediates the inhibitory effect of HO-1 involves possibly the SDF-1α, whose production was strongly upregulated in C2C12 cells in response to HO-1 activation (103). This chemokine is known to decrease formation of myotubes by myoblasts, the effect mediated by activation of atypical PKCζ (163) and by regulation of MyoD expression (255). We found that stimulation of C2C12 cells with exogenous SDF-1α mimicked the influence of HO-1 overexpression either on myotube formation or on generation of MRFs (103). Moreover, HO-1 activity decreased production of miR-1, miR-133a, miR-133b, and miR-206 in C2C12 cells through a MyoD-independent pathway. The importance of this mechanism has been illustrated by experiments where enforced overexpression of miR-133b or miR-206 in HO-1-overexpressing cells partially restored the differentiating potential of myoblasts. Worth mentioning, stimulation with SDF-1α protein imitated the effects of HO-1 on myomir expression (103).
Apart from classical myomirs, HO-1 affects also the other miRNAs in differentiating muscle progenitors. Thus, production of miR-146a, which was proven to delay differentiation of myoblasts (108), was increased in C2C12 cells that overexpress HO-1 (103). On the other hand, miR-378, which negatively regulates expression of myogenic repressor of MyoD transcription (MyoR) (57), was decreased in response to HO-1 activity (103).
Interestingly, HO-1 reduces the expression of DGCR8, leading to inhibition of miRNA biogenesis in C2C12 cells and resulting in significantly lower total pools of pre-miRNA and mature miRNA (103) (Fig. 7). Enforced overexpression of DGCR8 in the HO-1-overexpressing C2C12 cells was enough to rescue the proper level of miRNA production, but did not influence differentiation of myoblasts or expression of MRFs. Accordingly, it has been reported that elimination of Dicer, another enzyme involved in miRNA processing, resulted in perturbed myogenesis, without affecting the MRFs (162). Nevertheless, despite the fact that HO-1 suppresses myogenic differentiation of myoblasts, its overall effect on skeletal muscle regeneration may also depend on the other activities, especially on enhancing the vascularization, as we have shown recently (80). Since a crosstalk between angiogenesis and myogenesis is crucial for regeneration processes (151), and HO-1 exerts proangiogenic properties (133), a decline in differentiation of myoblasts can be overwhelmed by increased exogenous signals from HO-1-potentiated blood vessel formation (80).
To conclude, HO-1 inhibits differentiation of myogenic progenitors by decreasing production of myomirs and almost complete blocking the c/EBPδ-dependent expression of MyoD. CO and SDF-1α are mediators of the observed effects, whereas total miRNA biogenesis is affected due to inhibition of DGCR8 (Fig. 7).
Miscellaneous
Involvement of HO-1 in other postnatal developmental processes has also been postulated, although not extensively characterized.
Chondrogenesis
Although HO-1 has not been examined in the context of chondrogenic differentiation of MSCs, the role of Nrf2 has been clearly demonstrated. During embryonic development, expression of Nrf2 is restricted to the proliferating and prehypertrophic chondrocytes, in contrast to the terminally differentiated cells, secreting collagen X and osteopontin (72). When prechondrogenic cell line was engineered to overexpress Nrf2, a decrease in differentiation potential was observed, evident by a declined expression of terminal chondrogenic markers (72). The underlying mechanism has not been identified, but one can suspect a role for HO-1, despite it is not the only mediator of Nrf2 action. Especially, that HO-1 is known to influence the chondrocyte viability (144) or secretion of growth factors (65), and has already been reported to counteract chondrocyte senescence induced by IL-1β (41, 43).
Endothelial progenitor cells
HO-1 plays an important role in functioning of EPCs, which originate from bone-marrow-derived SCs and are suggested to postnatally develop into mature endothelium (52). Nevertheless, recently the origin and nature of EPCs has become a matter of intense debate. Their differentiation capacity is being questioned and more data indicate for rather paracrine effect of those cells, named currently proangiogenic cells (239). Nevertheless, it has been convincingly demonstrated that HO-1 improved re-endothelialization both after pharmacological (16, 232) or genetic (129) induction, the process in which EPCs could play a role. HO-1 induction by probucol and succinobucol increased both the bone-marrow-located and the released EPCs (232), which formed also more colonies, while differentiation of EPCs from knockout mice was impaired (232). Likewise, lack of HO-1 leads to reduced formation of capillaries both in vitro and in vivo. These HO-1-related properties were, however, attributed to increased migration, viability, and proliferation of EPCs, as well as elevated proangiogenic cytokine secretion (52) (Grochot-Przeczek et al., unpublished data). However, an impaired differentiation of EPCs deficient in HO-1 has been observed in the in vitro assays (Deshane et al., unpublished data) (232).
So far, the role of HO-1 in regulation of EPC differentiation through effect on angiomirs (the miRNAs involved in blood vessel formation) has not been investigated. Nevertheless, the fact that HO-1 decreases the expression of miR-378 (189) can be considered in that context. Further studies are necessary, however, to elucidate the potential interactions, which might be further complicated by the problematic characteristic of cells defined as EPCs (239).
Myofibroblasts
Decrease in Nrf2 was associated with enhanced myofibroblast phenotype in idiopathic pulmonary fibrosis, while Nrf2 activation increased myofibroblastic dedifferentiation (9). Also, HO-1 was shown to reduce the rate of fibrosis in hepatic myofibroblasts (120). Likewise, the Nrf2/HO-1 axis appears to play a protective role against renal fibrosis by inhibiting the epithelial-mesenchymal transition into myofibroblasts (90, 185). For example, activation of Nrf2 or HO-1 counteracted the profibrotic effects of ochratoxin A in renal proximal tubular cells. This effect was related to changes in miRNA levels (195).
Neurogenesis
Despite the fact that neuron formation is most prominent during prenatal life, recent studies have demonstrated its occurrence in several parts of fully developed mammalian brain. Neurogenesis in adulthood is accomplished, in a very limited range, by neural progenitor or SCs (48). It was suggested that ROS can directly influence the neuronal SC commitment promoting the switch from neurogenic to gliogenic lineage (181).
Nrf2 has already been demonstrated to augment the differentiation of neuroblastoma cell line into a neural lineage, what was revealed by increase in number of mature neuronal cells that express terminal markers, such as microtubule-associated protein 2 (248). This effect was probably mediated by ROS (181). Indeed, treatment with antioxidants reduced generation of ROS, decreased nuclear translocation of Nrf2, and halted a shift in differentiation of neural SCs toward gliogenic lineage on expense of neurogenesis (181). Interestingly, HO-1 was downregulated in MSCs during neural differentiation (13). Also in cultured astroglial cells, the relatively high expression of HO-1 gradually decreased in parallel with their terminal maturation (124). These observations may suggest a role for HO-1 in regulation of neurogenesis. Although its functions in nervous system have been extensively examined, the beneficial effects of HO-1 activity were mainly attributed to the enhanced cell survival, proliferation, and migration, or to inhibited inflammatory response (183). However, understanding a potential direct role of HO-1 in neural differentiation needs further research.
Cardiogenesis
Until recently, like a brain, the mammalian heart was regarded as a fully differentiated organ, with no regenerative capacity. It has been shown however, that cardiomyocytes can slowly divide and, even more important, the heart contains a resident progenitor cell population, what supports a possibility of myocardial renewal in adults (138).
Taking into account an important role of HO-1 in skeletal myogenesis (103), similar effects could be also suspected in cardiac myogenesis. This issue remains, however, mostly unaddressed. So far, it was found that HO-1-derived CO does enhance cardiac regeneration by facilitating the mobilization of cardiac progenitor cells, and improving their migration, proliferation, or viability (111). Similarly, induction of HO-1 in the transplanted MSCs augmented their regenerative potential in the infarcted or ischemic myocardium. These benefits were attributed to better cell viability or improved balance between production of proangiogenic growth factors and proinflammatory cytokines (82, 186, 205, 216, 246), without assessing the rate of MSC differentiation into functional cardiomyocytes.
Noteworthy, recent study has demonstrated that lentiviral overexpression of HO-1 in MSCs transplanted into the infracted myocardium significantly increased the expression of cardiomyogenic transcription factors, suggesting that HO-1 can promote the maturation of cardiomyocytes (88). Accordingly, improved differentiation of cardiac SCs was observed after stimulation of Nrf2 expression (61, 66). However, further experiments have to be conducted to elucidate a precise role of HO-1 in cardiomyocyte development and to distinguish it from the effects on cell viability or proliferation.
Conclusions and Future Perspectives
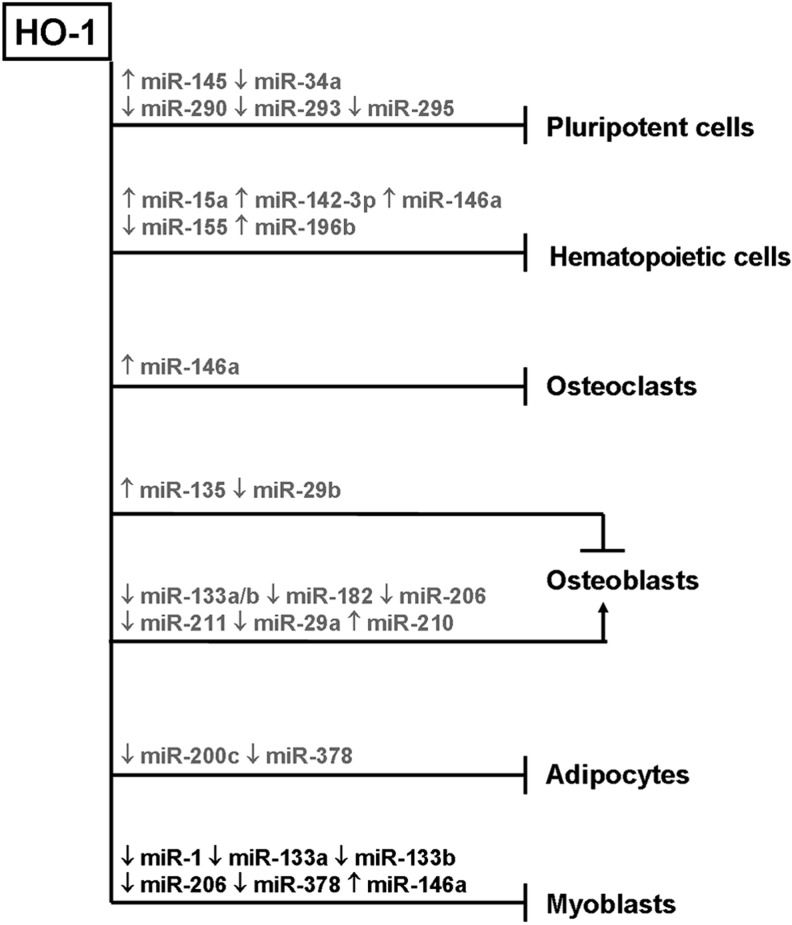
Over the last decade the novel role of HO-1 as a regulator of differentiation was demonstrated in diverse tissues (Fig. 8). It became clear that HO-1 influences major developmental processes, like hematopoietis, osteogenesis, myogenesis, or adipogenesis, and is perhaps important for maintaining pluripotency. Additionally, HO-1 can be involved in regulating the differentiation of chondrocytes, myofibroblasts, neurons, and cardiomyocytes.
FIG. 8.
Summary of HO-1-related miRNAs in differentiation of various cell types. miRNAs that mediate observed effects (black) or suggested to play a role in HO-1-regulated differentiation (gray) are shown.
The variety of tissues, where HO-1-induced effects have been observed, support a hypothesis that its role in differentiation of stem and progenitor cells may be meaningful in embryonic development and in regeneration of adult organs. However, vast majority of experiments have been conducted in vitro so far, with pharmacological regulators of HO-1 activity. This can always be associated with a risk of unspecific results. Thus, such studies should be verified by use of genetic silencing of HO-1, experiments on primary cells isolated from mice of different HO-1 genotype, and finally in vivo, in proper animal models.
The mechanism that mediates the observed effects is tissue specific and has been elucidated only in some cell types. They can be associated with influencing the oxidative status and ROS generation or, instead, they can be mediated by products of HO-1 enzymatic activity, with CO emerging as the most meaningful effector. Importantly, recent studies indicate that HO-1 may act through modification of miRNA processing efficacy and through regulation of specific miRNA expression. This opens a new possibility to understand the role of HO-1 in cell biology and elucidate why effects of HO-1 are strictly dependent on the cell type.
Abbreviations Used
- AML
acute myeloid leukemia
- ARE
antioxidant responsive element
- ATF4
activating transcription factor 4
- BMP2
bone morphogenic protein 2
- c/EBPα/β/δ
CCAAT/enhancer binding protein α/β/δ
- cAMP
cyclic AMP
- CLP
common lymphoid progenitor
- CML
chronic myeloid leukemia
- CMP
common myeloid progenitor
- CoPPIX
cobalt protoporphiryn IX
- CPK
creatine phosphokinase
- CREBP
cAMP response element binding protein
- EETs
epoxyeicosatrienoic acids
- EPC
endothelial progenitor cell
- ESC
embryonic stem cell
- FAS
fatty acid synthase
- GMP
granulocyte–macrophage progenitor
- Hh
Hedgehog
- HMGB1
high mobility group box 1
- HO-1
hemeoxygenase-1
- HSC
hematopoietic stem cell
- HSPC
hematopoietic stem and progenitor cell
- IGF
insulin-like growth factor
- IL-1β
interleukin-1β
- iPS cells
induced pluripotent stem cells
- M-CSF
macrophage colony-stimulated factor
- MEF2
myocyte enhancer factor 2
- MEP
megakaryocyte–erythroid progenitor
- MHC
myosin heavy chain
- miRNA
microRNA
- MPP
multipotent progenitor
- MRF
myogenic regulatory factor
- MSC
mesenchymal stem cell
- mSC
muscle satellite cell
- PGJ2
prostaglandin-J2
- PPARγ
peroxisomal proliferator activated receptor γ
- RAGE
receptors for advanced glycation endproducts
- RANK
receptor activator for NFκB
- RANKL
receptor activator for NFκB ligand
- ROS
reactive oxygen species
- Runx2
runt-related transcription factor 2
- SC
stem cell
- Shh
Sonic Hedgehog
- SnPPIX/SnMPIX
tin protoporphiryn IX/tin mesoporphiryn IX
- SREBP-1
sterol-regulatory-element-binding protein 1
- tBHQ
tert-butylhydroquinone
- TNFα
tumor necrosis factor α
- TRAF6
TNFα receptor associated factor 6
- TRAP
tartrate resistant acid phosphatase
- TRBP
TAR-RNA binding protein
- ZnPPIX
zinc protoporphiryn IX
Acknowledgments
This work was supported by grants POIG 01.01.02-00-109/09 and 01.01.02.069/09 from the European Union structural funds, by N N301 035240 from Ministry of Science and Higher Education and NCN 2012/06/M/NZ1/00008 from National Centre for Science. Magdalena Kozakowska is a recipient of the Iuvetus Plus grant from the Ministry of Science and Higher Education. Krzysztof Szade is a recipient of the Ventures grant from the Foundation for Polish Science. The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is also beneficiary of the structural funds from the European Union (grant Nos: POIG.02.01.00-12-064/08; POIG.02.02.00-014/08).
References
- 1.Abdallah BM. and Kassem M.New factors controlling the balance between osteoblastogenesis and adipogenesis. Bone 50: 540–545, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Abraham NG. Molecular regulation—biological role of heme in hematopoiesis. Blood Rev 5: 19–28, 1991 [DOI] [PubMed] [Google Scholar]
- 3.Afonyushkin T, Oskolkova OV, Philippova M, Resink TJ, Erne P, Binder BR, and Bochkov VN. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol 30: 1007–1013, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, Franzin C, Cortivo R, Rossato M, Vettor R, Abatangelo G, Pozzan T, Pinton P, and Rizzuto R. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci U S A 105: 1226–1231, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akimoto T, Ushida T, Miyaki S, Akaogi H, Tsuchiya K, Yan Z, Williams RS, and Tateishi T. Mechanical stretch inhibits myoblast-to-adipocyte differentiation through Wnt signaling. Biochem Biophys Res Commun 329: 381–385, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Almeida M. Unraveling the role of FoxOs in bone—insights from mouse models. Bone 49: 319–327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alves LR, Costa ES, Sorgine MH, Nascimento-Silva MC, Teodosio C, Barcena P, Castro-Faria-Neto HC, Bozza PT, Orfao A, Oliveira PL, and Maya-Monteiro CM. Heme-oxygenases during erythropoiesis in K562 and human bone marrow cells. PLoS One 6: e21358, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambros V. and Chen X. The regulation of genes and genomes by small RNAs. Development 134: 1635–1641, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Artaud-Macari E, Goven D, Brayer S, Hamimi A, Besnard V, Marchal-Somme J, Ali ZE, Crestani B, Kerdine-Romer S, Boutten A, and Bonay M. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal 18: 66–79, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Asahara T, Kawamoto A, and Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells 29: 1650–1655, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Asakura A, Komaki M, and Rudnicki M. Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 68: 245–253, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Aziz A, Sebastian S, and Dilworth FJ. The origin and fate of muscle satellite cells. Stem Cell Rev 8: 609–622, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Barbagallo I, Tibullo D, Di Rosa M, Giallongo C, Palumbo GA, Raciti G, Campisi A, Vanella A, Green CJ, and Motterlini R. A cytoprotective role for the heme oxygenase-1/CO pathway during neural differentiation of human mesenchymal stem cells. J Neurosci Res 86: 1927–1935, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Barbagallo I, Vanella A, Peterson SJ, Kim DH, Tibullo D, Giallongo C, Vanella L, Parrinello N, Palumbo GA, Raimondo FD, Abraham NG, and Asprinio D. Overexpression of heme oxygenase-1 increases human osteoblast stem cell differentiation. J Bone Miner Metab 28: 276–288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrera LN, Rushworth SA, Bowles KM, and MacEwan DJ. Bortezomib induces heme oxygenase-1 expression in multiple myeloma. Cell Cycle 11: 2248–2252, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baruscotti I, Barchiesi F, Jackson EK, Imthurn B, Stiller R, Kim JH, Schaufelberger S, Rosselli M, Hughes CC, and Dubey RK. Estradiol stimulates capillary formation by human endothelial progenitor cells: role of estrogen receptor-{alpha}/{beta}, heme oxygenase 1, and tyrosine kinase. Hypertension 56: 397–404, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckman JD, Chen C, Nguyen J, Thayanithy V, Subramanian S, Steer CJ, and Vercellotti GM. Regulation of heme oxygenase-1 protein expression by miR-377 in combination with miR-217. J Biol Chem 286: 3194–3202, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellin M, Marchetto MC, Gage FH, and Mummery CL. Induced pluripotent stem cells: the new patient? Nat Rev Mol Cell Biol 13: 713–726, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Berardi E, Pues M, Thorrez L, and Sampaolesi M. miRNAs in ESC differentiation. Am J Physiol Heart Circ Physiol 303: H931–H939, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Bessis M. [Erythroblastic island, functional unity of bone marrow]. Rev Hematol 13: 8–11, 1958. [Article in French] [PubMed] [Google Scholar]
- 21.Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood 117: 5281–5288, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, and Wang CY. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 19: 35–42, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bissels U, Wild S, Tomiuk S, Hafner M, Scheel H, Mihailovic A, Choi YH, Tuschl T, and Bosio A. Combined characterization of microRNA and mRNA profiles delineates early differentiation pathways of CD133+ and CD34+ hematopoietic stem and progenitor cells. Stem Cells 29: 847–857, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyle WJ, Simonet WS, and Lacey DL. Osteoclast differentiation and activation. Nature 423: 337–342, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Bryder D, Rossi DJ, and Weissman IL. Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol 169: 338–346, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brzoska E, Ciemerych MA, Przewozniak M, and Zimowska M. Regulation of muscle stem cells activation: the role of growth factors and extracellular matrix. Vitam Horm 87: 239–276, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Burgess A, Li M, Vanella L, Kim DH, Rezzani R, Rodella L, Sodhi K, Canestraro M, Martasek P, Peterson SJ, Kappas A, and Abraham NG. Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice. Hypertension 56: 1124–1130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camilleri S. and McDonald F. Runx2 and dental development. Eur J Oral Sci 114: 361–373, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Cao J, Peterson SJ, Sodhi K, Vanella L, Barbagallo I, Rodella LF, Schwartzman ML, Abraham NG, and Kappas A. Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet. Hypertension 60: 467–475, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao YA, Kusy S, Luong R, Wong RJ, Stevenson DK, and Contag CH. Heme oxygenase-1 deletion affects stress erythropoiesis. PLoS One 6: e20634, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao YA, Wagers AJ, Karsunky H, Zhao H, Reeves R, Wong RJ, Stevenson DK, Weissman IL, and Contag CH. Heme oxygenase-1 deficiency leads to disrupted response to acute stress in stem cells and progenitors. Blood 112: 4494–4502, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carrer M, Liu N, Grueter CE, Williams AH, Frisard MI, Hulver MW, Bassel-Duby R, and Olson EN. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378*. Proc Natl Acad Sci U S A 109: 15330–15335, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carriere A, Carmona MC, Fernandez Y, Rigoulet M, Wenger RH, Penicaud L, and Casteilla L. Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: a mechanism for hypoxia-dependent effect. J Biol Chem 279: 40462–40469, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Chae HJ, Chin HY, Lee GY, Park HR, Yang SK, Chung HT, Pae HO, Kim HM, Chae SW, and Kim HR. Carbon monoxide and nitric oxide protect against tumor necrosis factor-alpha-induced apoptosis in osteoblasts: HO-1 is necessary to mediate the protection. Clin Chim Acta 365: 270–278, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Charge SB. and Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Chasis JA. and Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood 112: 470–478, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL, and Wang DZ. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38: 228–233, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi J, Costa ML, Mermelstein CS, Chagas C, Holtzer S, and Holtzer H. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci U S A 87: 7988–7992, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu CY, Chen CF, Rajendran RS, Shen CN, Chen TH, Yen CC, Chuang CK, Lin DS, and Hsiao CD. Overexpression of Akt1 enhances adipogenesis and leads to lipoma formation in zebrafish. PLoS One 7: e36474, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung SS, Hu W, and Park CY. The role of microRNAs in hematopoietic stem cell and leukemic stem cell function. Ther Adv Hematol 2: 317–334, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clerigues V, Guillen MI, Castejon MA, Gomar F, Mirabet V, and Alcaraz MJ. Heme oxygenase-1 mediates protective effects on inflammatory, catabolic and senescence responses induced by interleukin-1beta in osteoarthritic osteoblasts. Biochem Pharmacol 83: 395–405, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Ciesla M, Skrzypek K, Kozakowska M, Loboda A, Jozkowicz A, Dulak J. MicroRNAs as biomarkers of disease onset. Anal Bioanal Chem 401: 2051-2061, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Clerigues V, Guillen MI, Gomar F, and Alcaraz MJ. Haem oxygenase-1 counteracts the effects of interleukin-1beta on inflammatory and senescence markers in cartilage-subchondral bone explants from osteoarthritic patients. Clin Sci (Lond) 122: 239–250, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Dulak J, Deshane J, Jozkowicz A, Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation 117: 231-241, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collino F, Bruno S, Deregibus MC, Tetta C, and Camussi G. MicroRNAs and mesenchymal stem cells. Vitam Horm 87: 291–320, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Collins CA. Satellite cell self-renewal. Curr Opin Pharmacol 6: 301–306, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Curtis HJ, Sibley CR, and Wood MJ. Mirtrons, an emerging class of atypical miRNA. Wiley Interdiscip Rev RNA 3: 617–632, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Curtis MA, Low VF, and Faull RL. Neurogenesis and progenitor cells in the adult human brain: a comparison between hippocampal and subventricular progenitor proliferation. Dev Neurobiol 72: 990–1005, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Dean M, Fojo T, and Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 5: 275–284, 2005 [DOI] [PubMed] [Google Scholar]
- 50.Deng Z, Du WW, Fang L, Shan SW, Qian J, Lin J, Qian W, Ma J, Rutnam ZJ, and Yang BB. The intermediate filament vimentin mediates microRNA miR-378 function in cellular self-renewal by regulating the expression of the Sox2 transcription factor. J Biol Chem 288: 319–331, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Denschlag D, Marculescu R, Unfried G, Hefler LA, Exner M, Hashemi A, Riener EK, Keck C, Tempfer CB, and Wagner O. The size of a microsatellite polymorphism of the haem oxygenase 1 gene is associated with idiopathic recurrent miscarriage. Mol Hum Reprod 10: 211–214, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Deshane J, Chen S, Caballero S, Grochot-Przeczek A, Was H, Li Calzi S, Lach R, Hock TD, Chen B, Hill-Kapturczak N, Siegal GP, Dulak J, Jozkowicz A, Grant MB, and Agarwal A. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med 204: 605–618, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faller M, Matsunaga M, Yin S, Loo JA, and Guo F. Heme is involved in microRNA processing. Nat Struct Mol Biol 14: 23–29, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature 423: 356–361, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Fischer-Posovszky P, Tews D, Horenburg S, Debatin KM, and Wabitsch M. Differential function of Akt1 and Akt2 in human adipocytes. Mol Cell Endocrinol 358: 135–143, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Fumimoto R, Sakai E, Yamaguchi Y, Sakamoto H, Fukuma Y, Nishishita K, Okamoto K, and Tsukuba T. The coffee diterpene kahweol prevents osteoclastogenesis via impairment of NFATc1 expression and blocking of Erk phosphorylation. J Pharmacol Sci 118: 479–486, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Gagan J, Dey BK, Layer R, Yan Z, and Dutta A. MicroRNA-378 targets the myogenic repressor MyoR during myoblast differentiation. J Biol Chem 286: 19431–19438, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garzon R, Garofalo M, Martelli MP, Briesewitz R, Wang L, Fernandez-Cymering C, Volinia S, Liu CG, Schnittger S, Haferlach T, Liso A, Diverio D, Mancini M, Meloni G, Foa R, Martelli MF, Mecucci C, Croce CM, and Falini B. Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci U S A 105: 3945–3950, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gerin I, Bommer GT, McCoin CS, Sousa KM, Krishnan V, and MacDougald OA. Roles for miRNA-378/378* in adipocyte gene expression and lipogenesis. Am J Physiol Endocrinol Metab 299: E198–E206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gimble JM. and Nuttall ME. The relationship between adipose tissue and bone metabolism. Clin Biochem 45: 874–879, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Gorbunov N, Petrovski G, Gurusamy N, Ray D, Kim do H, and Das DK. Regeneration of infarcted myocardium with resveratrol-modified cardiac stem cells. J Cell Mol Med 16: 174–184, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grochot-Przeczek A, Dulak J, and Jozkowicz A. Haem oxygenase-1: non-canonical roles in physiology and pathology. Clin Sci (Lond) 122: 93–103, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Gromak N. Intronic microRNAs: a crossroad in gene regulation. Biochem Soc Trans 40: 759–761, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Gu Q, Cai Y, Huang C, Shi Q, and Yang H. Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation. Pharmacogn Mag 8: 202–208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guillen M, Megias J, Gomar F, and Alcaraz M. Haem oxygenase-1 regulates catabolic and anabolic processes in osteoarthritic chondrocytes. J Pathol 214: 515–522, 2008 [DOI] [PubMed] [Google Scholar]
- 66.Gurusamy N, Ray D, Lekli I, and Das DK. Red wine antioxidant resveratrol-modified cardiac stem cells regenerate infarcted myocardium. J Cell Mol Med 14: 2235–2239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim HH, and Lee ZH. Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res 301: 119–127, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Hama M, Kirino Y, Takeno M, Takase K, Miyazaki T, Yoshimi R, Ueda A, Itoh-Nakadai A, Muto A, Igarashi K, and Ishigatsubo Y. Bach1 regulates osteoclastogenesis in a mouse model via both heme oxygenase 1-dependent and heme oxygenase 1-independent pathways. Arthritis Rheum 64: 1518–1528, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Heasman SA, Zaitseva L, Bowles KM, Rushworth SA, and Macewan DJ. Protection of acute myeloid leukaemia cells from apoptosis induced by front-line chemotherapeutics is mediated by haem oxygenase-1. Oncotarget 2: 658–668, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herrmann H, Kneidinger M, Cerny-Reiterer S, Rulicke T, Willmann M, Gleixner KV, Blatt K, Hormann G, Peter B, Samorapoompichit P, Pickl W, Bharate GY, Mayerhofer M, Sperr WR, Maeda H, and Valent P. The Hsp32 inhibitors SMA-ZnPP and PEG-ZnPP exert major growth-inhibitory effects on D34+/CD38+ and CD34+/CD38- AML progenitor cells. Curr Cancer Drug Targets 12: 51–63, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Hinoi E, Fujimori S, Wang L, Hojo H, Uno K, and Yoneda Y. Nrf2 negatively regulates osteoblast differentiation via interfering with Runx2-dependent transcriptional activation. J Biol Chem 281: 18015–18024, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Hinoi E, Takarada T, Fujimori S, Wang L, Iemata M, Uno K, and Yoneda Y. Nuclear factor E2 p45-related factor 2 negatively regulates chondrogenesis. Bone 40: 337–344, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Holst D, Luquet S, Kristiansen K, and Grimaldi PA. Roles of peroxisome proliferator-activated receptors delta and gamma in myoblast transdifferentiation. Exp Cell Res 288: 168–176, 2003 [DOI] [PubMed] [Google Scholar]
- 74.Hou W, Tian Q, Steuerwald NM, Schrum LW, and Bonkovsky HL. The let-7 microRNA enhances heme oxygenase-1 by suppressing Bach1 and attenuates oxidant injury in human hepatocytes. Biochim Biophys Acta 1819: 1113–1122, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hou W, Tian Q, Zheng J, and Bonkovsky HL. MicroRNA-196 represses Bach1 protein and hepatitis C virus gene expression in human hepatoma cells expressing hepatitis C viral proteins. Hepatology 51: 1494–1504, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hu R, Li H, Liu W, Yang L, Tan YF, and Luo XH. Targeting miRNAs in osteoblast differentiation and bone formation. Expert Opin Ther Targets 14: 1109–1120, 2010 [DOI] [PubMed] [Google Scholar]
- 77.Ibrahim NG, Lutton JD, and Levere RD. The role of haem biosynthetic and degradative enzymes in erythroid colony development: the effect of haemin. Br J Haematol 50: 17–28, 1982 [DOI] [PubMed] [Google Scholar]
- 78.Inose H, Ochi H, Kimura A, Fujita K, Xu R, Sato S, Iwasaki M, Sunamura S, Takeuchi Y, Fukumoto S, Saito K, Nakamura T, Siomi H, Ito H, Arai Y, Shinomiya K, and Takeda S. A microRNA regulatory mechanism of osteoblast differentiation. Proc Natl Acad Sci U S A 106: 20794–20799, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Itoigawa Y, Kishimoto KN, Okuno H, Sano H, Kaneko K, and Itoi E. Hypoxia induces adipogenic differentitation of myoblastic cell lines. Biochem Biophys Res Commun 399: 721–726, 2010 [DOI] [PubMed] [Google Scholar]
- 80.Jazwa A, Stepniewski J, Zamykal M, Jagodzinska J, Meloni M, Emanueli C, Jozkowicz A, and Dulak J. Pre-emptive hypoxia-regulated HO-1 gene therapy improves post-ischaemic limb perfusion and tissue regeneration in mice. Cardiovasc Res 97: 115–124, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jensen ED, Gopalakrishnan R, and Westendorf JJ. Regulation of gene expression in osteoblasts. Biofactors 36: 25–32, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang YB, Zhang XL, Tang YL, Ma GS, Shen CX, Wei Q, Zhu Q, Yao YY, and Liu NF. Effects of heme oxygenase-1 gene modulated mesenchymal stem cells on vasculogenesis in ischemic swine hearts. Chin Med J (Engl) 124: 401–407, 2011 [PubMed] [Google Scholar]
- 83.Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, and Han J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 120: 623–634, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Jozkowicz A. and Dulak J. Effects of protoporphyrins on production of nitric oxide and expression of vascular endothelial growth factor in vascular smooth muscle cells and macrophages. Acta Biochim Pol 50: 69–79, 2003 [PubMed] [Google Scholar]
- 85.Kapinas K. and Delany AM. MicroRNA biogenesis and regulation of bone remodeling. Arthritis Res Ther 13: 220, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kapinas K, Kessler CB, and Delany AM. miR-29 suppression of osteonectin in osteoblasts: regulation during differentiation and by canonical Wnt signaling. J Cell Biochem 108: 216–224, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kartikasari AE, Wagener FA, Yachie A, Wiegerinck ET, Kemna EH, and Swinkels DW. Hepcidin suppression and defective iron recycling account for dysregulation of iron homeostasis in heme oxygenase-1 deficiency. J Cell Mol Med 13: 3091–3102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kearns-Jonker M, Dai W, Gunthart M, Fuentes T, Yeh HY, Gerczuk P, Pera M, Mummery C, and Kloner RA. Genetically engineered mesenchymal stem cells influence gene expression in donor cardiomyocytes and the recipient heart. J Stem Cell Res Ther S1, pii: , 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kennell JA, Gerin I, MacDougald OA, and Cadigan KM. The microRNA miR-8 is a conserved negative regulator of Wnt signaling. Proc Natl Acad Sci U S A 105: 15417–15422, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kie JH, Kapturczak MH, Traylor A, Agarwal A, and Hill-Kapturczak N. Heme oxygenase-1 deficiency promotes epithelial-mesenchymal transition and renal fibrosis. J Am Soc Nephrol 19: 1681–1691, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim DH, Burgess AP, Li M, Tsenovoy PL, Addabbo F, McClung JA, Puri N, and Abraham NG. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J Pharmacol Exp Ther 325: 833–840, 2008 [DOI] [PubMed] [Google Scholar]
- 92.Kim DH, Liu J, Bhat S, Benedict G, Lecka-Czernik B, Peterson SJ, Ebraheim NA, and Heck BE. Peroxisome proliferator-activated receptor delta agonist attenuates nicotine suppression effect on human mesenchymal stem cell-derived osteogenesis and involves increased expression of heme oxygenase-1. J Bone Miner Metab 31: 44–52, 2012 [DOI] [PubMed] [Google Scholar]
- 93.Kim DH, Vanella L, Inoue K, Burgess A, Gotlinger K, Manthati VL, Koduru SR, Zeldin DC, Falck JR, Schwartzman ML, and Abraham NG. Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARgamma. Stem Cells Dev 19: 1863–1873, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim EK, Lim S, Park JM, Seo JK, Kim JH, Kim KT, Ryu SH, and Suh PG. Human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by AMP-activated protein kinase. J Cell Physiol 227: 1680–1687, 2012 [DOI] [PubMed] [Google Scholar]
- 95.Kim HK, Lee YS, Sivaprasad U, Malhotra A, and Dutta A. Muscle-specific microRNA miR-206 promotes muscle differentiation. J Cell Biol 174: 677–687, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim HR, Kim S, Kim EJ, Park JH, Yang SH, Jeong ET, Park C, Youn MJ, So HS, and Park R. Suppression of Nrf2-driven heme oxygenase-1 enhances the chemosensitivity of lung cancer A549 cells toward cisplatin. Lung Cancer 60: 47–56, 2008 [DOI] [PubMed] [Google Scholar]
- 97.Kim KM, Park SJ, Jung SH, Kim EJ, Jogeswar G, Ajita J, Rhee Y, Kim CH, and Lim SK. miR-182 is a negative regulator of osteoblast proliferation, differentiation, and skeletogenesis through targeting FoxO1. J Bone Miner Res 27: 1669–1679, 2012 [DOI] [PubMed] [Google Scholar]
- 98.Kim SH, Kim KH, Yoo BC, and Ku JL. Induction of LGR5 by H2O2 treatment is associated with cell proliferation via the JNK signaling pathway in colon cancer cells. Int J Oncol 41: 1744–1750, 2012 [DOI] [PubMed] [Google Scholar]
- 99.Kim SJ, Min KS, Ryu HW, Lee HJ, and Kim EC. The role of heme oxygenase-1 in the proliferation and odontoblastic differentiation of human dental pulp cells. J Endod 36: 1326–1331, 2010 [DOI] [PubMed] [Google Scholar]
- 100.Klein U, Lia M, Crespo M, Siegel R, Shen Q, Mo T, Ambesi-Impiombato A, Califano A, Migliazza A, Bhagat G, and Dalla-Favera R. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell 17: 28–40, 2010 [DOI] [PubMed] [Google Scholar]
- 101.Kongpetch S, Kukongviriyapan V, Prawan A, Senggunprai L, Kukongviriyapan U, and Buranrat B. Crucial role of heme oxygenase-1 on the sensitivity of cholangiocarcinoma cells to chemotherapeutic agents. PLoS One 7: e34994, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kook YA, Lee SK, Son DH, Kim Y, Kang KH, Cho JH, Kim SC, Kim YS, Lee HJ, Lee SK, and Kim EC. Effects of substance P on osteoblastic differentiation and heme oxygenase-1 in human periodontal ligament cells. Cell Biol Int 33: 424–428, 2009 [DOI] [PubMed] [Google Scholar]
- 103.Kozakowska M, Ciesla M, Stefanska A, Skrzypek K, Was H, Jazwa A, Grochot-Przeczek A, Kotlinowski J, Szymula A, Bartelik A, Mazan M, Yagensky O, Florczyk U, Lemke K, Zebzda A, Dyduch G, Nowak W, Szade K, Stepniewski J, Majka M, Derlacz R, Loboda A, Dulak J, and Jozkowicz A. Heme oxygenase-1 inhibits myoblast differentiation by targeting myomirs. Antioxid Redox Signal 16: 113–127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kreiser D, Nguyen X, Wong R, Seidman D, Stevenson D, Quan S, Abraham N, and Dennery PA. Heme oxygenase-1 modulates fetal growth in the rat. Lab Invest 82: 687–692, 2002 [DOI] [PubMed] [Google Scholar]
- 105.Krishnan V, Bryant HU, and Macdougald OA. Regulation of bone mass by Wnt signaling. J Clin Invest 116: 1202–1209, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kuang S, Gillespie MA, and Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell 2: 22–31, 2008 [DOI] [PubMed] [Google Scholar]
- 107.Kuang S. and Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med 14: 82–91, 2008 [DOI] [PubMed] [Google Scholar]
- 108.Kuang W, Tan J, Duan Y, Duan J, Wang W, Jin F, Jin Z, Yuan X, and Liu Y. Cyclic stretch induced miR-146a upregulation delays C2C12 myogenic differentiation through inhibition of Numb. Biochem Biophys Res Commun 378: 259–263, 2009 [DOI] [PubMed] [Google Scholar]
- 109.Kumar R, Halder P, Sahu SK, Kumar M, Kumari M, Jana K, Ghosh Z, Sharma P, Kundu M, and Basu J. Identification of a novel role of ESAT-6-dependent miR-155 induction during infection of macrophages with Mycobacterium tuberculosis. Cell Microbiol 14: 1620–1631, 2012 [DOI] [PubMed] [Google Scholar]
- 110.Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, and Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 134: 2895–2902, 2007 [DOI] [PubMed] [Google Scholar]
- 111.Lakkisto P, Kyto V, Forsten H, Siren JM, Segersvard H, Voipio-Pulkki LM, Laine M, Pulkki K, and Tikkanen I. Heme oxygenase-1 and carbon monoxide promote neovascularization after myocardial infarction by modulating the expression of HIF-1alpha, SDF-1alpha and VEGF-B. Eur J Pharmacol 635: 156–164, 2010 [DOI] [PubMed] [Google Scholar]
- 112.Laumonier T, Yang S, Konig S, Chauveau C, Anegon I, Hoffmeyer P, and Menetrey J. Lentivirus mediated HO-1 gene transfer enhances myogenic precursor cell survival after autologous transplantation in pig. Mol Ther 16: 404–410, 2008 [DOI] [PubMed] [Google Scholar]
- 113.Lee H, Lee YJ, Choi H, Ko EH, and Kim JW. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem 284: 10601–10609, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, and Lee SY. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106: 852–859, 2005 [DOI] [PubMed] [Google Scholar]
- 115.Lee SJ, Lee EJ, Kim SH, Choi I, Lee DM, Lee HJ, Yoon D, and Chun T. IL-17A promotes transdifferentiation of mouse myoblast cells (C2C12) into adipocytes by increasing the expression of peroxisome proliferator-activated receptor gamma through CAAT/enhancer binding protein beta signaling. Biotechnol Lett 33: 229–235, 2011 [DOI] [PubMed] [Google Scholar]
- 116.Lee SK, Lee CY, Kook YA, Lee SK, and Kim EC. Mechanical stress promotes odontoblastic differentiation via the heme oxygenase-1 pathway in human dental pulp cell line. Life Sci 86: 107–114, 2010 [DOI] [PubMed] [Google Scholar]
- 117.Lee TC. and Ho IC. Expression of heme oxygenase in arsenic-resistant human lung adenocarcinoma cells. Cancer Res 54: 1660–1664, 1994 [PubMed] [Google Scholar]
- 118.Lee YH, Lee NH, Bhattarai G, Kim GE, Lee IK, Yun BS, Hwang PH, and Yi HK. Anti-inflammatory effect of pachymic acid promotes odontoblastic differentiation via HO-1 in dental pulp cells. Oral Dis 19: 193–199, 2012 [DOI] [PubMed] [Google Scholar]
- 119.Lefebvre V. and Bhattaram P. Vertebrate skeletogenesis. Curr Top Dev Biol 90: 291–317, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li L, Julien B, Grenard P, Teixeira-Clerc F, Mallat A, and Lotersztajn S. Molecular mechanisms regulating the antifibrogenic protein heme-oxygenase-1 in human hepatic myofibroblasts. J Hepatol 41: 407–413, 2004 [DOI] [PubMed] [Google Scholar]
- 121.Li M, Kim DH, Tsenovoy PL, Peterson SJ, Rezzani R, Rodella LF, Aronow WS, Ikehara S, and Abraham NG. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes 57: 1526–1535, 2008 [DOI] [PubMed] [Google Scholar]
- 122.Li Z, Hassan MQ, Jafferji M, Aqeilan RI, Garzon R, Croce CM, van Wijnen AJ, Stein JL, Stein GS, and Lian JB. Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem 284: 15676–15684, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, and Stein GS. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci U S A 105: 13906–13911, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Volti G, Ientile R, Abraham NG, Vanella A, Cannavo G, Mazza F, Curro M, Raciti G, Avola R, and Campisi A. Immunocytochemical localization and expression of heme oxygenase-1 in primary astroglial cell cultures during differentiation: effect of glutamate. Biochem Biophys Res Commun 315: 517–524, 2004 [DOI] [PubMed] [Google Scholar]
- 125.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, and Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord 7: 1–16, 2006 [DOI] [PubMed] [Google Scholar]
- 126.Liang OD, Mitsialis SA, Chang MS, Vergadi E, Lee C, Aslam M, Fernandez-Gonzalez A, Liu X, Baveja R, and Kourembanas S. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells 29: 99–107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lichner Z, Pall E, Kerekes A, Pallinger E, Maraghechi P, Bosze Z, and Gocza E. The miR-290–295 cluster promotes pluripotency maintenance by regulating cell cycle phase distribution in mouse embryonic stem cells. Differentiation 81: 11–24, 2011 [DOI] [PubMed] [Google Scholar]
- 128.Lin CY, Peng CY, Huang TT, Wu ML, Lai YL, Peng DH, Chen PF, Chen HF, Yen BL, Wu KK, and Yet SF. Exacerbation of oxidative stress-induced cell death and differentiation in induced pluripotent stem cells lacking heme oxygenase-1. Stem Cells Dev 21: 1675–1687, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lin HH, Chen YH, Yet SF, and Chau LY. After vascular injury, heme oxygenase-1/carbon monoxide enhances re-endothelialization via promoting mobilization of circulating endothelial progenitor cells. J Thromb Haemost 7: 1401–1408, 2009 [DOI] [PubMed] [Google Scholar]
- 130.Lin Q, Weis S, Yang G, Weng YH, Helston R, Rish K, Smith A, Bordner J, Polte T, Gaunitz F, and Dennery PA. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J Biol Chem 282: 20621–20633, 2007 [DOI] [PubMed] [Google Scholar]
- 131.Lin TC, Yen JM, Gong KB, Hsu TT, and Chen LR. IGF-1/IGFBP-1 increases blastocyst formation and total blastocyst cell number in mouse embryo culture and facilitates the establishment of a stem-cell line. BMC Cell Biol 4: 14, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin TH, Tang CH, Hung SY, Liu SH, Lin YM, Fu WM, and Yang RS. Upregulation of heme oxygenase-1 inhibits the maturation and mineralization of osteoblasts. J Cell Physiol 222: 757–768, 2010 [DOI] [PubMed] [Google Scholar]
- 133.Loboda A, Jazwa A, Grochot-Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, Jozkowicz A, and Dulak J. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 10: 1767–1812, 2008 [DOI] [PubMed] [Google Scholar]
- 134.Loboda A, Was H, Jozkowicz A, and Dulak J. Janus face of Nrf2-HO-1 axis in cancer—friend in chemoprevention, foe in anticancer therapy. Lung Cancer 60: 1–3, 2008 [DOI] [PubMed] [Google Scholar]
- 135.Lowe CE, O'Rahilly S, and Rochford JJ. Adipogenesis at a glance. J Cell Sci 124: 2681–2686, 2011 [DOI] [PubMed] [Google Scholar]
- 136.Mallanna SK. and Rizzino A. Emerging roles of microRNAs in the control of embryonic stem cells and the generation of induced pluripotent stem cells. Dev Biol 344: 16–25, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marie PJ. Transcription factors controlling osteoblastogenesis. Arch Biochem Biophys 473: 98–105, 2008 [DOI] [PubMed] [Google Scholar]
- 138.Martin-Puig S, Fuster V, and Torres M. Heart repair: from natural mechanisms of cardiomyocyte production to the design of new cardiac therapies. Ann N Y Acad Sci 1254: 71–81, 2012 [DOI] [PubMed] [Google Scholar]
- 139.Mayerhofer M, Florian S, Krauth MT, Aichberger KJ, Bilban M, Marculescu R, Printz D, Fritsch G, Wagner O, Selzer E, Sperr WR, Valent P, and Sillaber C. Identification of heme oxygenase-1 as a novel BCR/ABL-dependent survival factor in chronic myeloid leukemia. Cancer Res 64: 3148–3154, 2004 [DOI] [PubMed] [Google Scholar]
- 140.Mayerhofer M, Gleixner KV, Mayerhofer J, Hoermann G, Jaeger E, Aichberger KJ, Ott RG, Greish K, Nakamura H, Derdak S, Samorapoompichit P, Pickl WF, Sexl V, Esterbauer H, Schwarzinger I, Sillaber C, Maeda H, and Valent P. Targeting of heat shock protein 32 (Hsp32)/heme oxygenase-1 (HO-1) in leukemic cells in chronic myeloid leukemia: a novel approach to overcome resistance against imatinib. Blood 111: 2200–2210, 2008 [DOI] [PubMed] [Google Scholar]
- 141.McCaig D. and Lyall F. Heme oxygenase expression in human placental villous tissue in response to exposure to in vitro ischemia-reperfusion injury. Hypertens Pregnancy 28: 256–272, 2009 [DOI] [PubMed] [Google Scholar]
- 142.McCaig D. and Lyall F. Inhibitors of heme oxygenase reduce invasion of human primary cytotrophoblast cells in vitro. Placenta 30: 536–538, 2009 [DOI] [PubMed] [Google Scholar]
- 143.McGregor RA. and Choi MS. microRNAs in the regulation of adipogenesis and obesity. Curr Mol Med 11: 304–316, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Megias J, Guillen MI, Clerigues V, Rojo AI, Cuadrado A, Castejon MA, Gomar F, and Alcaraz MJ. Heme oxygenase-1 induction modulates microsomal prostaglandin E synthase-1 expression and prostaglandin E(2) production in osteoarthritic chondrocytes. Biochem Pharmacol 77: 1806–1813, 2009 [DOI] [PubMed] [Google Scholar]
- 145.Melton C, Judson RL, and Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 463: 621–626, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Merchant AA, Singh A, Matsui W, and Biswal S. The redox-sensitive transcription factor Nrf2 regulates murine hematopoietic stem cell survival independently of ROS levels. Blood 118: 6572–6579, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Min KS, Lee YM, Hong SO, and Kim EC. Simvastatin promotes odontoblastic differentiation and expression of angiogenic factors via heme oxygenase-1 in primary cultured human dental pulp cells. J Endod 36: 447–452, 2010 [DOI] [PubMed] [Google Scholar]
- 148.Mizoguchi F, Izu Y, Hayata T, Hemmi H, Nakashima K, Nakamura T, Kato S, Miyasaka N, Ezura Y, and Noda M. Osteoclast-specific Dicer gene deficiency suppresses osteoclastic bone resorption. J Cell Biochem 109: 866–875, 2010 [DOI] [PubMed] [Google Scholar]
- 149.Mizuno Y, Tokuzawa Y, Ninomiya Y, Yagi K, Yatsuka-Kanesaki Y, Suda T, Fukuda T, Katagiri T, Kondoh Y, Amemiya T, Tashiro H, and Okazaki Y. miR-210 promotes osteoblastic differentiation through inhibition of AcvR1b. FEBS Lett 583: 2263–2268, 2009 [DOI] [PubMed] [Google Scholar]
- 150.Motohashi H, Kimura M, Fujita R, Inoue A, Pan X, Takayama M, Katsuoka F, Aburatani H, Bresnick EH, and Yamamoto M. NF-E2 domination over Nrf2 promotes ROS accumulation and megakaryocytic maturation. Blood 115: 677–686, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mounier R, Chretien F, and Chazaud B. Blood vessels and the satellite cell niche. Curr Top Dev Biol 96: 121–138, 2010 [DOI] [PubMed] [Google Scholar]
- 152.Murchison EP, Partridge JF, Tam OH, Cheloufi S, and Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc Natl Acad Sci U S A 102: 12135–12140, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Na J, Furue MK, and Andrews PW. Inhibition of ERK1/2 prevents neural and mesendodermal differentiation and promotes human embryonic stem cell self-renewal. Stem Cell Res 5: 157–169, 2010 [DOI] [PubMed] [Google Scholar]
- 154.Nakamura H, Fang J, Gahininath B, Tsukigawa K, and Maeda H. Intracellular uptake and behavior of two types zinc protoporphyrin (ZnPP) micelles, SMA-ZnPP and PEG-ZnPP as anticancer agents; unique intracellular disintegration of SMA micelles. J Control Release 155: 367–375, 2011 [DOI] [PubMed] [Google Scholar]
- 155.Nakasa T, Shibuya H, Nagata Y, Niimoto T, and Ochi M. The inhibitory effect of microRNA-146a expression on bone destruction in collagen-induced arthritis. Arthritis Rheum 63: 1582–1590, 2011 [DOI] [PubMed] [Google Scholar]
- 156.Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G, Kusmic C, L'Abbate A, Kappas A, and Abraham NG. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension 53: 508–515, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nowak W, Borys S, Kusinska K, Bukowaska-Strakova K, Witek P, Koblik T, Jozkowicz A, Malecki M, and Dulak J. Number of circulating pro-angiogenic cells, growth factor and antioxidative gene profiles may be altered in type 2 diabetes with and without diabetic foot syndrome. J Diabetes Invest 2013. [Epub ahead of print]; DOI: 10.111/jdi.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nowis D, Legat M, Grzela T, Niderla J, Wilczek E, Wilczynski GM, Glodkowska E, Mrowka P, Issat T, Dulak J, Jozkowicz A, Was H, Adamek M, Wrzosek A, Nazarewski S, Makowski M, Stoklosa T, Jakobisiak M, and Golab J. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene 25: 3365–3374, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.O'Brien JJ, Baglole CJ, Garcia-Bates TM, Blumberg N, Francis CW, and Phipps RP. 15-Deoxy-delta12,14 prostaglandin J2-induced heme oxygenase-1 in megakaryocytes regulates thrombopoiesis. J Thromb Haemost 7: 182–189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.O'Connell RM. and Baltimore D. MicroRNAs and hematopoietic cell development. Curr Top Dev Biol 99: 145–174, 2012 [DOI] [PubMed] [Google Scholar]
- 161.O'Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, and Baltimore D. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med 205: 585–594, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.O'Rourke JR, Georges SA, Seay HR, Tapscott SJ, McManus MT, Goldhamer DJ, Swanson MS, and Harfe BD. Essential role for Dicer during skeletal muscle development. Dev Biol 311: 359–368, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Odemis V, Boosmann K, Dieterlen MT, and Engele J. The chemokine SDF1 controls multiple steps of myogenesis through atypical PKCzeta. J Cell Sci 120: 4050–4059, 2007 [DOI] [PubMed] [Google Scholar]
- 164.Okita K. and Yamanaka S. Induced pluripotent stem cells: opportunities and challenges. Philos Trans R Soc Lond B Biol Sci 366: 2198–2207, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Oskowitz AZ, Lu J, Penfornis P, Ylostalo J, McBride J, Flemington EK, Prockop DJ, and Pochampally R. Human multipotent stromal cells from bone marrow and microRNA: regulation of differentiation and leukemia inhibitory factor expression. Proc Natl Acad Sci U S A 105: 18372–18377, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Parise G, O'Reilly CE, and Rudnicki MA. Molecular regulation of myogenic progenitor populations. Appl Physiol Nutr Metab 31: 773–781, 2006 [DOI] [PubMed] [Google Scholar]
- 167.Park HJ, Chung BY, Lee MK, Song Y, Lee SS, Chu GM, Kang SN, Song YM, Kim GS, and Cho JH. Centipede grass exerts anti-adipogenic activity through inhibition of C/EBPbeta, C/EBPalpha, and PPARgamma expression and the AKT signaling pathway in 3T3-L1 adipocytes. BMC Complement Altern Med 12: 230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pilegaard H, Ordway GA, Saltin B, and Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab 279: E806–E814, 2000 [DOI] [PubMed] [Google Scholar]
- 169.Popovic R, Riesbeck LE, Velu CS, Chaubey A, Zhang J, Achille NJ, Erfurth FE, Eaton K, Lu J, Grimes HL, Chen J, Rowley JD, and Zeleznik-Le NJ. Regulation of mir-196b by MLL and its overexpression by MLL fusions contributes to immortalization. Blood 113: 3314–3322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Poss KD. and Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci U S A 94: 10925–10930, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pulkkinen KH, Yla-Herttuala S, and Levonen AL. Heme oxygenase 1 is induced by miR-155 via reduced BACH1 translation in endothelial cells. Free Radic Biol Med 51: 2124–2131, 2011 [DOI] [PubMed] [Google Scholar]
- 172.Qiu L, Fan H, Jin W, Zhao B, Wang Y, Ju Y, Chen L, Chen Y, Duan Z, and Meng S. miR-122-induced down-regulation of HO-1 negatively affects miR-122-mediated suppression of HBV. Biochem Biophys Res Commun 398: 771–777, 2010 [DOI] [PubMed] [Google Scholar]
- 173.Relaix F. and Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 139: 2845–2856, 2012 [DOI] [PubMed] [Google Scholar]
- 174.Reya T, Morrison SJ, Clarke MF, and Weissman IL. Stem cells, cancer, and cancer stem cells. Nature 414: 105–111, 2001 [DOI] [PubMed] [Google Scholar]
- 175.Romao JM, Jin W, Dodson MV, Hausman GJ, Moore SS, and Guan LL. MicroRNA regulation in mammalian adipogenesis. Exp Biol Med (Maywood) 236: 997–1004, 2011 [DOI] [PubMed] [Google Scholar]
- 176.Rushworth SA, Bowles KM, Raninga P, and MacEwan DJ. NF-kappaB-inhibited acute myeloid leukemia cells are rescued from apoptosis by heme oxygenase-1 induction. Cancer Res 70: 2973–2983, 2010 [DOI] [PubMed] [Google Scholar]
- 177.Rushworth SA. and MacEwan DJ. HO-1 underlies resistance of AML cells to TNF-induced apoptosis. Blood 111: 3793–3801, 2008 [DOI] [PubMed] [Google Scholar]
- 178.Ryter SW, Alam J, and Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86: 583–650, 2006 [DOI] [PubMed] [Google Scholar]
- 179.Sakai E, Shimada-Sugawara M, Nishishita K, Fukuma Y, Naito M, Okamoto K, Nakayama K, and Tsukuba T. Suppression of RANKL-dependent heme oxygenase-1 is required for high mobility group box 1 release and osteoclastogenesis. J Cell Biochem 113: 486–498, 2012 [DOI] [PubMed] [Google Scholar]
- 180.Sakamoto H, Sakai E, Fumimoto R, Yamaguchi Y, Fukuma Y, Nishishita K, Okamoto K, and Tsukuba T. Deltamethrin inhibits osteoclast differentiation via regulation of heme oxygenase-1 and NFATc1. Toxicol In Vitro 26: 817–822, 2012 [DOI] [PubMed] [Google Scholar]
- 181.Santos DM, Santos MM, Moreira R, Sola S, and Rodrigues CM. Synthetic condensed 1,4-naphthoquinone derivative shifts neural stem cell differentiation by regulating redox state. Mol Neurobiol 47: 313–324, 2013 [DOI] [PubMed] [Google Scholar]
- 182.Saxena S, Shukla D, Saxena S, Khan YA, Singh M, Bansal A, Sairam M, and Jain SK. Hypoxia preconditioning by cobalt chloride enhances endurance performance and protects skeletal muscles from exercise-induced oxidative damage in rats. Acta Physiol (Oxf) 200: 249–263, 2010 [DOI] [PubMed] [Google Scholar]
- 183.Schipper HM, Song W, Zukor H, Hascalovici JR, and Zeligman D. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J Neurochem 110: 469–485, 2009 [DOI] [PubMed] [Google Scholar]
- 184.Schneider KS. and Chan JY. Emerging role of nrf2 in adipocytes and adipose biology. Adv Nutr 4: 62–66, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shin DH, Park HM, Jung KA, Choi HG, Kim JA, Kim DD, Kim SG, Kang KW, Ku SK, Kensler TW, and Kwak MK. The NRF2-heme oxygenase-1 system modulates cyclosporin A-induced epithelial-mesenchymal transition and renal fibrosis. Free Radic Biol Med 48: 1051–1063, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shu T, Zeng B, Ren X, and Li Y. HO-1 modified mesenchymal stem cells modulate MMPs/TIMPs system and adverse remodeling in infarcted myocardium. Tissue Cell 42: 217–222, 2010 [DOI] [PubMed] [Google Scholar]
- 187.Siminovitch L, McCulloch EA, and Till JE. The distribution of colony-forming cells among spleen colonies. J Cell Physiol 62: 327–336, 1963 [DOI] [PubMed] [Google Scholar]
- 188.Singh MM, Irwin ME, Gao Y, Ban K, Shi P, Arlinghaus RB, Amin HM, and Chandra J. Inhibition of the NADPH oxidase regulates heme oxygenase 1 expression in chronic myeloid leukemia. Cancer 118: 3433–3445, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Skrzypek K, Tertil M, Golda S, Ciesla M, Weglarczyk K, Collet G, Guichard A, Kozakowska M, Boczkowski J, Was H, Gil T, Kuzdzal J, Muchova L, Vitek L, Loboda A, Jozkowicz A, Kieda C, and Dulak J. Interplay between heme oxygenase-1 and miR-378 affects non-small cell lung carcinoma growth, vascularization and metastasis. Antioxid Redox Signal 19: 644–660, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.So AY, Garcia-Flores Y, Minisandram A, Martin A, Taganov K, Boldin M, and Baltimore D. Regulation of APC development, immune response, and autoimmunity by Bach1/HO-1 pathway in mice. Blood 120: 2428–2437, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwartzman ML, and Abraham NG. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther 331: 906–916, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sodhi K, Puri N, Inoue K, Falck JR, Schwartzman ML, and Abraham NG. EET agonist prevents adiposity and vascular dysfunction in rats fed a high fat diet via a decrease in Bach 1 and an increase in HO-1 levels. Prostaglandins Other Lipid Mediat 98: 133–142, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sohni A. and Verfaillie CM. Multipotent adult progenitor cells. Best Pract Res Clin Haematol 24: 3–11, 2011 [DOI] [PubMed] [Google Scholar]
- 194.Sokol NS. The role of microRNAs in muscle development. Curr Top Dev Biol 99: 59–78, 2012 [DOI] [PubMed] [Google Scholar]
- 195.Stachurska A, Ciesla M, Kozakowska M, Wolffram S, Boesch-Saadatmandi C, Rimbach G, Jozkowicz A, Dulak J, and Loboda A. Cross-talk between microRNAs, nuclear factor E2-related factor 2, and heme oxygenase-1 in ochratoxin A-induced toxic effects in renal proximal tubular epithelial cells. Mol Nutr Food Res 57: 504–515, 2013 [DOI] [PubMed] [Google Scholar]
- 196.Starczynowski DT, Kuchenbauer F, Wegrzyn J, Rouhi A, Petriv O, Hansen CL, Humphries RK, and Karsan A. MicroRNA-146a disrupts hematopoietic differentiation and survival. Exp Hematol 39: 167–178 e4, 2011 [DOI] [PubMed] [Google Scholar]
- 197.Stavridis MP, Lunn JS, Collins BJ, and Storey KG. A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development 134: 2889–2894, 2007 [DOI] [PubMed] [Google Scholar]
- 198.Stefani G. and Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol 9: 219–230, 2008 [DOI] [PubMed] [Google Scholar]
- 199.Suda T, Takubo K, and Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 9: 298–310, 2011 [DOI] [PubMed] [Google Scholar]
- 200.Sugatani T. and Hruska KA. Impaired micro-RNA pathways diminish osteoclast differentiation and function. J Biol Chem 284: 4667–4678, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Suh GY, Jin Y, Yi AK, Wang XM, and Choi AM. CCAAT/enhancer-binding protein mediates carbon monoxide-induced suppression of cyclooxygenase-2. Am J Respir Cell Mol Biol 35: 220–226, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Takada I, Kouzmenko AP, and Kato S. Molecular switching of osteoblastogenesis versus adipogenesis: implications for targeted therapies. Expert Opin Ther Targets 13: 593–603, 2009 [DOI] [PubMed] [Google Scholar]
- 203.Takada I, Kouzmenko AP, and Kato S. Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol 5: 442–447, 2009 [DOI] [PubMed] [Google Scholar]
- 204.Tang QQ. and Lane MD. Adipogenesis: from stem cell to adipocyte. Annu Rev Biochem 81: 715–736, 2012 [DOI] [PubMed] [Google Scholar]
- 205.Tang YL, Tang Y, Zhang YC, Qian K, Shen L, and Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol 46: 1339–1350, 2005 [DOI] [PubMed] [Google Scholar]
- 206.Taussig DC, Miraki-Moud F, Anjos-Afonso F, Pearce DJ, Allen K, Ridler C, Lillington D, Oakervee H, Cavenagh J, Agrawal SG, Lister TA, Gribben JG, and Bonnet D. Anti-CD38 antibody-mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood 112: 568–575, 2008 [DOI] [PubMed] [Google Scholar]
- 207.Teitelbaum SL. and Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet 4: 638–649, 2003 [DOI] [PubMed] [Google Scholar]
- 208.Thomas ED, Lochte HL, Jr., Cannon JH, Sahler OD, and Ferrebee JW. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest 38: 1709–1716, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Till JE. and Mc CE. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 14: 213–222, 1961 [PubMed] [Google Scholar]
- 210.Tong AW. and Nemunaitis J. Modulation of miRNA activity in human cancer: a new paradigm for cancer gene therapy? Cancer Gene Ther 15: 341–355, 2008 [DOI] [PubMed] [Google Scholar]
- 211.Toobiak S, Shaklai M, and Shaklai N. Carbon monoxide induced erythroid differentiation of K562 cells mimics the central macrophage milieu in erythroblastic islands. PLoS One 7: e33940, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Townley-Tilson WH, Callis TE, and Wang D. MicroRNAs 1, 133, and 206: critical factors of skeletal and cardiac muscle development, function, and disease. Int J Biochem Cell Biol 42: 1252–1255, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Treiber T, Treiber N, and Meister G. Regulation of microRNA biogenesis and function. Thromb Haemost 107: 605–610, 2012 [DOI] [PubMed] [Google Scholar]
- 214.Trigona WL, Porter CM, Horvath-Arcidiacono JA, Majumdar AS, and Bloom ET. Could heme-oxygenase-1 have a role in modulating the recipient immune response to embryonic stem cells? Antioxid Redox Signal 9: 751–756, 2007 [DOI] [PubMed] [Google Scholar]
- 215.Tsai JJ, Dudakov JA, Takahashi K, Shieh JH, Velardi E, Holland AM, Singer NV, West ML, Smith OM, Young LF, Shono Y, Ghosh A, Hanash AM, Tran HT, Moore MA, and van den Brink MR. Nrf2 regulates haematopoietic stem cell function. Nat Cell Biol 15: 309–316, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tsubokawa T, Yagi K, Nakanishi C, Zuka M, Nohara A, Ino H, Fujino N, Konno T, Kawashiri MA, Ishibashi-Ueda H, Nagaya N, and Yamagishi M. Impact of anti-apoptotic and anti-oxidative effects of bone marrow mesenchymal stem cells with transient overexpression of heme oxygenase-1 on myocardial ischemia. Am J Physiol Heart Circ Physiol 298: H1320–H1329, 2010 [DOI] [PubMed] [Google Scholar]
- 217.Valencia-Sanchez MA, Liu J, Hannon GJ, and Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev 20: 515–524, 2006 [DOI] [PubMed] [Google Scholar]
- 218.van Rooij E, Quiat D, Johnson BA, Sutherland LB, Qi X, Richardson JA, Kelm RJ, Jr., and Olson EN. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell 17: 662–673, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, and Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 316: 575–579, 2007 [DOI] [PubMed] [Google Scholar]
- 220.van Tienen FH, Laeremans H, van der Kallen CJ, and Smeets HJ. Wnt5b stimulates adipogenesis by activating PPARgamma, and inhibiting the beta-catenin dependent Wnt signaling pathway together with Wnt5a. Biochem Biophys Res Commun 387: 207–211, 2009 [DOI] [PubMed] [Google Scholar]
- 221.Vanella L, Kim DH, Asprinio D, Peterson SJ, Barbagallo I, Vanella A, Goldstein D, Ikehara S, Kappas A, and Abraham NG. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone 46: 236–243, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vanella L, Kim DH, Sodhi K, Barbagallo I, Burgess AP, Falck JR, Schwartzman ML, and Abraham NG. Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes. Prostaglandins Other Lipid Mediat 96: 54–62, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang LD. and Wagers AJ. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat Rev Mol Cell Biol 12: 643–655, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang Y, Medvid R, Melton C, Jaenisch R, and Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nat Genet 39: 380–385, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Watanabe-Matsui M, Muto A, Matsui T, Itoh-Nakadai A, Nakajima O, Murayama K, Yamamoto M, Ikeda-Saito M, and Igarashi K. Heme regulates B-cell differentiation, antibody class switch, and heme oxygenase-1 expression in B cells as a ligand of Bach2. Blood 117: 5438–5448, 2011 [DOI] [PubMed] [Google Scholar]
- 226.Watanabe S, Akagi R, Mori M, Tsuchiya T, and Sassa S. Marked developmental changes in heme oxygenase-1 (HO-1) expression in the mouse placenta: correlation between HO-1 expression and placental development. Placenta 25: 387–395, 2004 [DOI] [PubMed] [Google Scholar]
- 227.Watanabe T, Hasegawa G, Yamamoto T, Hatakeyama K, Suematsu M, and Naito M. Expression of heme oxygenase-1 in rat ontogeny. Arch Histol Cytol 66: 155–162, 2003 [DOI] [PubMed] [Google Scholar]
- 228.Weissman IL. Translating stem and progenitor cell biology to the clinic: barriers and opportunities. Science 287: 1442–1446, 2000 [DOI] [PubMed] [Google Scholar]
- 229.Weng JJ. and Su Y. Nuclear matrix-targeting of the osteogenic factor Runx2 is essential for its recognition and activation of the alkaline phosphatase gene. Biochim Biophys Acta 1830, 2839–2852, 2012 [DOI] [PubMed] [Google Scholar]
- 230.Wong RJ, Zhao H, and Stevenson DK. A deficiency in haem oxygenase-1 induces foetal growth restriction by placental vasculature defects. Acta Paediatr 101: 827–834, 2012 [DOI] [PubMed] [Google Scholar]
- 231.Wruck CJ, Fragoulis A, Gurzynski A, Brandenburg LO, Kan YW, Chan K, Hassenpflug J, Freitag-Wolf S, Varoga D, Lippross S, and Pufe T. Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann Rheum Dis 70: 844–850, 2011 [DOI] [PubMed] [Google Scholar]
- 232.Wu BJ, Midwinter RG, Cassano C, Beck K, Wang Y, Changsiri D, Gamble JR, and Stocker R. Heme oxygenase-1 increases endothelial progenitor cells. Arterioscler Thromb Vasc Biol 29: 1537–1542, 2009 [DOI] [PubMed] [Google Scholar]
- 233.Xia Z, Chen C, Chen P, Xie H, and Luo X. MicroRNAs and their roles in osteoclast differentiation. Front Med 5: 414–419, 2011 [DOI] [PubMed] [Google Scholar]
- 234.Xia ZW, Zhong WW, Meyrowitz JS, and Zhang ZL. The role of heme oxygenase-1 in T cell-mediated immunity: the all encompassing enzyme. Curr Pharm Des 14: 454–464, 2008 [DOI] [PubMed] [Google Scholar]
- 235.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, and Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell 137: 647–658, 2009 [DOI] [PubMed] [Google Scholar]
- 236.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, and Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 103: 129–135, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yamaguchi Y, Sakai E, Sakamoto H, Fumimoto R, Fukuma Y, Nishishita K, Okamoto K, and Tsukuba T. Inhibitory effects of tert-butylhydroquinone on osteoclast differentiation via up-regulation of heme oxygenase-1 and down-regulation of HMGB1 release and NFATc1 expression. J Appl Toxicol [Epub ahead of print]; DOI: 10.1002/jat.2827, 2012 [DOI] [PubMed] [Google Scholar]
- 238.Yeom KH, Lee Y, Han J, Suh MR, and Kim VN. Characterization of DGCR8/Pasha, the essential cofactor for Drosha in primary miRNA processing. Nucleic Acids Res 34: 4622–4629, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yoder MC. Endothelial progenitor cell: a blood cell by many other names may serve similar functions. J Mol Med (Berl) 91: 285–295, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yoon JS, Lee HJ, Chae MK, Lee SY, and Lee EJ. Cigarette-smoke-extract-induced adipogenesis in Graves' orbital fibroblasts is inhibited by quercetin via reduction in oxidative stress. J Endocrinol 216, 145–165, 2013 [DOI] [PubMed] [Google Scholar]
- 241.Younce C. and Kolattukudy P. MCP-1 induced protein promotes adipogenesis via oxidative stress, endoplasmic reticulum stress and autophagy. Cell Physiol Biochem 30: 307–320, 2012 [DOI] [PubMed] [Google Scholar]
- 242.Zarjou A, Jeney V, Arosio P, Poli M, Antal-Szalmas P, Agarwal A, Balla G, and Balla J. Ferritin prevents calcification and osteoblastic differentiation of vascular smooth muscle cells. J Am Soc Nephrol 20: 1254–1263, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zarjou A, Kim J, Traylor AM, Sanders PW, Balla J, Agarwal A, and Curtis LM. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol Renal Physiol 300: F254–F262, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zenclussen AC, Sollwedel A, Bertoja AZ, Gerlof K, Zenclussen ML, Woiciechowsky C, and Volk HD. Heme oxygenase as a therapeutic target in immunological pregnancy complications. Int Immunopharmacol 5: 41–51, 2005 [DOI] [PubMed] [Google Scholar]
- 245.Zenclussen ML, Anegon I, Bertoja AZ, Chauveau C, Vogt K, Gerlof K, Sollwedel A, Volk HD, Ritter T, and Zenclussen AC. Over-expression of heme oxygenase-1 by adenoviral gene transfer improves pregnancy outcome in a murine model of abortion. J Reprod Immunol 69: 35–52, 2006 [DOI] [PubMed] [Google Scholar]
- 246.Zeng B, Chen H, Zhu C, Ren X, Lin G, and Cao F. Effects of combined mesenchymal stem cells and heme oxygenase-1 therapy on cardiac performance. Eur J Cardiothorac Surg 34: 850–856, 2008 [DOI] [PubMed] [Google Scholar]
- 247.Zhang Z, Wang Q, Ma J, Yi X, Zhu Y, Xi X, Feng Y, and Jin Z. Reactive oxygen species regulate FSH-induced expression of vascular endothelial growth factor via Nrf2 and HIF1alpha signaling in human epithelial ovarian cancer. Oncol Rep 29: 1429–1434, 2013 [DOI] [PubMed] [Google Scholar]
- 248.Zhao F, Wu T, Lau A, Jiang T, Huang Z, Wang XJ, Chen W, Wong PK, and Zhang DD. Nrf2 promotes neuronal cell differentiation. Free Radic Biol Med 47: 867–879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhao H, Kalota A, Jin S, and Gewirtz AM. The c-myb proto-oncogene and microRNA-15a comprise an active autoregulatory feedback loop in human hematopoietic cells. Blood 113: 505–516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhao H, Wong RJ, Doyle TC, Nayak N, Vreman HJ, Contag CH, and Stevenson DK. Regulation of maternal and fetal hemodynamics by heme oxygenase in mice. Biol Reprod 78: 744–751, 2008 [DOI] [PubMed] [Google Scholar]
- 251.Zhao H, Wong RJ, Kalish FS, Nayak NR, and Stevenson DK. Effect of heme oxygenase-1 deficiency on placental development. Placenta 30: 861–868, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Zhao Q, Wang X, Liu Y, He A, and Jia R. NFATc1: functions in osteoclasts. Int J Biochem Cell Biol 42: 576–579, 2010 [DOI] [PubMed] [Google Scholar]
- 253.Zhou H, Ramiya VK, and Visner GA. Bone marrow stem cells as a vehicle for delivery of heme oxygenase-1 gene. Stem Cells Dev 15: 79–86, 2006 [DOI] [PubMed] [Google Scholar]
- 254.Zhou Z, Han JY, Xi CX, Xie JX, Feng X, Wang CY, Mei L, and Xiong WC. HMGB1 regulates RANKL-induced osteoclastogenesis in a manner dependent on RAGE. J Bone Miner Res 23: 1084–1096, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhu W, Boachie-Adjei O, Rawlins BA, Frenkel B, Boskey AL, Ivashkiv LB, and Blobel CP. A novel regulatory role for stromal-derived factor-1 signaling in bone morphogenic protein-2 osteogenic differentiation of mesenchymal C2C12 cells. J Biol Chem 282: 18676–18685, 2007 [DOI] [PubMed] [Google Scholar]
- 256.Zwerina J, Tzima S, Hayer S, Redlich K, Hoffmann O, Hanslik-Schnabel B, Smolen JS, Kollias G, and Schett G. Heme oxygenase 1 (HO-1) regulates osteoclastogenesis and bone resorption. Faseb J 19: 2011–2013, 2005 [DOI] [PubMed] [Google Scholar]