Abstract
Accumulating research has shown that chronic D-galactose (D-gal) exposure induces symptoms similar to natural aging in animals. Therefore, rodents chronically exposed to D-gal are increasingly used as a model for aging and delay-of-aging pharmacological research. Mitochondrial dysfunction is thought to play a vital role in aging and age-related diseases; however, whether mitochondrial dysfunction plays a significant role in mice exposed to D-gal remains unknown. In the present study, we investigated cognitive dysfunction, locomotor activity, and mitochondrial dysfunction involved in D-gal exposure in mice. We found that D-gal exposure (125 mg/kg/day, 8 weeks) resulted in a serious impairment in grip strength in mice, whereas spatial memory and locomotor coordination remained intact. Interestingly, muscular mitochondrial complex I deficiency occurred in the skeletal muscle of mice exposed to D-gal. Mitochondrial ultrastructure abnormality was implicated as a contributing factor in D-gal-induced muscular impairment. Moreover, three combinations (A, B, and C) of nutrients applied in this study effectively reversed D-gal-induced muscular impairment. Nutrient formulas B and C were especially effective in reversing complex I dysfunction in both skeletal muscle and heart muscle. These findings suggest the following: (1) chronic exposure to D-gal first results in specific muscular impairment in mice, rather than causing general, premature aging; (2) poor skeletal muscle strength induced by D-gal might be due to the mitochondrial dysfunction caused by complex I deficiency; and (3) the nutrient complexes applied in the study attenuated the skeletal muscle impairment, most likely by improving mitochondrial function.
Key Words: : aging, dietary supplement, mitochondria
Introduction
D-galactose (D-gal), a physiological nutrient derived from lactose in milk,1 is metabolized in animals by D-galactokinase and galactose-1-phosphate uridyltransferase (GALT). Although some researchers have challenged the D-gal-induced aging model,2,3 some other researchers indicate that D-gal-treated animals showed some hallmarks of aging, such as a shortened lifespan,4 cognitive dysfunction,5 presbycusis,6 increased oxidative stress,4,7 decreased antioxidant enzyme activity,8 diminished immune responses,9 increased advanced glycation endproducts,10 accumulation of mitochondrial DNA mutations,11 and mitochondrial dysfunction.5,12
Mitochondria are not only the major sites of intracellular reactive oxygen species (ROS) production, but also targets of ROS. This ROS-induced oxidative damage contributes to mitochondrial dysfunction, which in turn produces more ROS.13 This vicious cycle of ROS production and oxidative damage in the mitochondria suggests that mitochondrial dysfunction plays an important role in the aging process.14 However, there is little substantial evidence to demonstrate exactly how mitochondria act in D-gal-treated mice.
As described in previous publications,13,15,16 we have identified a group of mitochondria targeting antioxidants and cofactors, which are referred to as “mitochondrial nutrients.” With these mitochondrial nutrients, we aim to reverse the mitochondrial dysfunction that occurs during the aging process. In addition, the synergetic beneficial effects of nutrient combinations were demonstrated to be even more powerful than single nutrient supplements alone in promoting mitochondrial function.17,18 In the present study, three nutrient formulas were designed to rescue mitochondrial dysfunction based on compounds whose mitochondrial beneficial effects were well documented by both our laboratory and other laboratories. Additionally, each formula was designed to relieve one of the disorders during the aging process. Formula A was designed to inhibit tumorigenesis and was composed of resveratrol, grape seed extract, quercetin, pterostilbene, fisetin, and black tea (theaflavins). Formula B targeted mitochondrial metabolic deregulation and was composed of ubiquinol CoQ10, R-lipoic acid, acetyl-l-carnitine, pyrroloquinoline quinine (PQQ), tocopherols, and tocotrienols (mixed). Finally, formula C targeted mitochondrial-related steroid degeneration and was composed of vitamin D3 (cholecalciferol), green tea extract (98% polyphenols, 45% epigallocatechin gallate [EGCG]), and dehydroepiandrosterone (DHEA).
In this study, we investigated the cognitive function and locomotor activity in D-gal-exposed mice. Furthermore, we revealed mitochondrial impairment and evaluated the effects of mitochondrial nutrient formulas on the mitochondrial dysfunction induced by D-gal.
Materials and Methods
Animals and treatment procedure
Eight-week-old adult male C57BL/6J mice (Laboratory Animal Center, the Fourth Military Medical University, Xi'an, China) were randomly divided into the following 5 groups, each consisting of 12 animals: normal control, D-gal administration, and D-gal administration with one of the 3 mitochondrial nutrient formulas (A, B, or C). The three D-gal treatment groups supplemented with nutrients were fed AIN93G chows that also contained formula A, B, or C for 10 weeks.
Formula A: trans-resveratrol (51.4 mg/kg), grape seed extract (30.8 mg/kg), quercetin (12.5 mg/kg), pterostilbene (4 mg/kg), fisetin (9.9 mg/kg), and black tea (theaflavins) (90.4 mg/kg). Formula B: ubiquinol CoQ10 (20.6 mg/kg), R-lipoic acid (30.8 mg/kg), acetyl-l-carnitine (150 mg/kg), PQQ (3 mg/kg), tocopherols (mixed) (73.8 mg/kg), and tocotrienols (mixed) (29.8 mg/kg). Formula C: vitamin D3 (cholecalciferol) (400 IU/kg), green tea extract (98% polyphenols, 45% EGCG) (149 mg/kg), and DHEA (10 mg/kg).
The dose of each ingredient was described as mg/kg or IU/kg body weight per day.
From the third week, D-gal (125 mg/kg) was administered daily for 8 weeks through subcutaneous injection. Mice in the control group were administered saline. Each mouse was kept in an individual cage with free access to food and water. The body weight and food intake of the animals were measured weekly. We did not find any differences in the body weight among groups; however, the food intake in groups receiving formula A and C was significantly lower compared with other groups as measured at the sixth week after starting treatment (data not shown). All animal protocols were approved by the animal ethics committee of Xi'an Jiaotong University School of Life Science and Technology.
Morris water maze
The Morris water maze (MWM) has been widely used to measure cognitive deficits in brain-damaged animals and cognitive loss during aging.8 At the eighth week following the start of D-gal administration, the animals' spatial memory was assayed by the MWM.18,19
Rotarod test
There is a decline in motor coordination with advancing age.20 In the present study, impairment of motor coordination was evaluated by using a Rotarod test (47600-Mouse Rota-Rod; Ugo Basile, Varese, Italy). The procedure was performed as previously described.2
Grip strength test
The impairment of neuromuscular function was evaluated by a grip strength meter (Cat. 47106 Grip Strength Meter for Mouse; Ugo Basile). The procedure was performed as previously reported.21
Tissue collection
The animals were sacrificed by cervical dislocation. The gastrocnemius muscle, brain, liver, and heart were harvested. The gastrocnemius muscle from one leg was rapidly placed in paraformaldehyde/glutaradehyde fixative as a sample for electron microscopy assessment.
Electron microscopy
As described in a previous publication,22 the fixed gastrocnemius was embedded in the Spurr's embedding medium. Ultrathin sections were double stained with uranyl acetate, examined, and photographed with transmission electron microscopy (JEM-100SX; NEC, Tokyo, Japan). Images for analysis were taken at a magnification of 15,000× and 40,000×.
Mitochondria isolation
Mitochondria in muscle tissues were isolated according to the method published by Birch-Machin et al.23
Mitochondrial complex and dehydrogenase activity estimation
The activities of mitochondrial complex I, II, IV, α-ketoglutarate dehydrogenase (α-KGDH), and pyruvate dehydrogenase (PDH) were measured spectrophotometrically using conventional assays as previously described.24
Statistical analysis
Values are expressed as the mean±SEM. Differences among groups were analyzed by using one-way analysis of variance (ANOVA) followed by the LSD post hoc tests or an unpaired t-test in SPSS 17.0 software. (IBM, Armonk, NY, USA). A value of P<.05 was considered statistically significant.
Results
Behavioral tests in D-gal-exposed mice
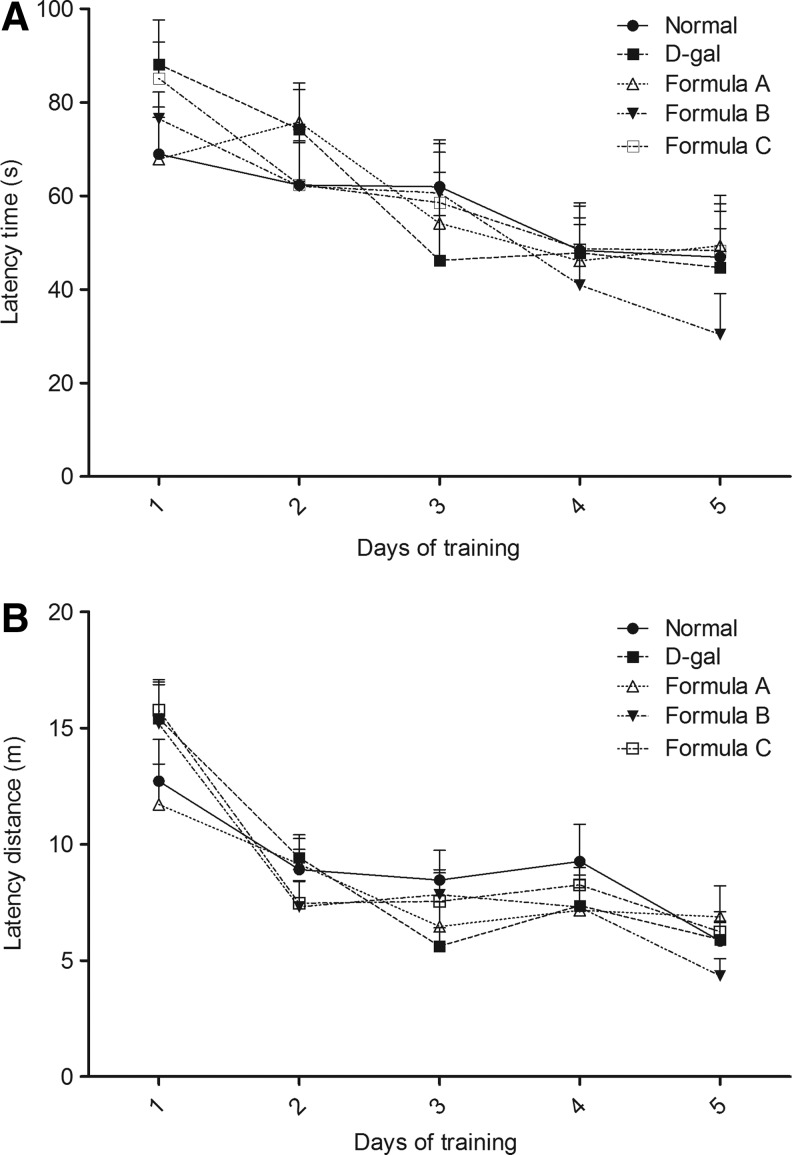
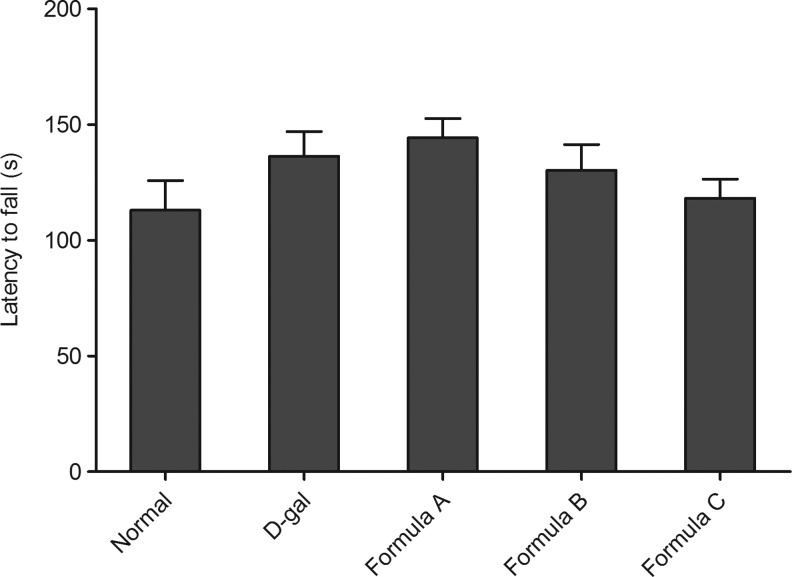
The MWM was applied to measure cognitive deficiencies in D-gal-exposed mice. We found that 8 weeks of D-gal exposure did not affect the escape latency (Fig. 1A) or swimming distance (Fig. 1B) of mice in the MWM compared with the normal control. Additionally, the administration of nutrient combinations in the D-gal mice did not cause any alteration in the spatial memory compared with the D-gal group. Similarly, the motor coordination detected with the rotarod test showed no changes between groups (Fig. 2).
FIG. 1.
Spatial memory test with the Morris water maze. (A) Latency time and (B) latency distance for finding the platform were tested. Values are mean±SEM of 12 mice. No difference of latency time and distance was found between control and D-galactose (D-gal) exposure mice.
FIG. 2.
Coordination test with the rotarod. Latency to fall was tested. Values are mean±SEM of 12 mice. No difference of latency time was found between control and D-gal exposure mice.
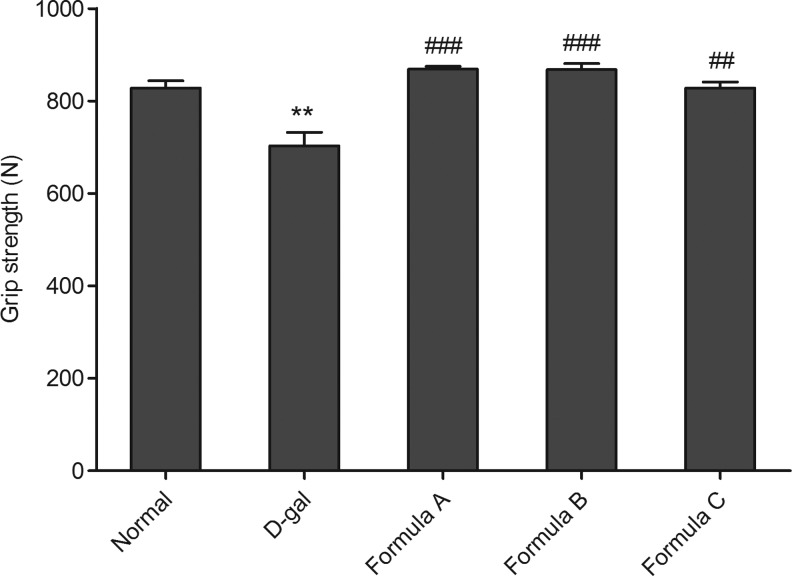
A decrease in strength is a reliable indicator of the aging process. The rodent grip strength test is a putative measure of muscular strength and has been previously used to screen for neuromuscular function. In the present study, D-gal-treated mice exhibited significantly weaker grip strength than mice in the control group (t-test, n=12; P<.01, Fig. 3), suggesting that D-gal induces skeletal muscle strength impairment in mice. Nutrient formula A, B, and C efficiently restored muscle strength.
FIG. 3.
Grip strength of forelimb was tested. Values are mean±SEM of 12 mice. **P<.01 versus normal group, ##P<.01 versus D-gal group, ###P<.001 versus D-gal group.
Ultrastructure of the mitochondria in muscle
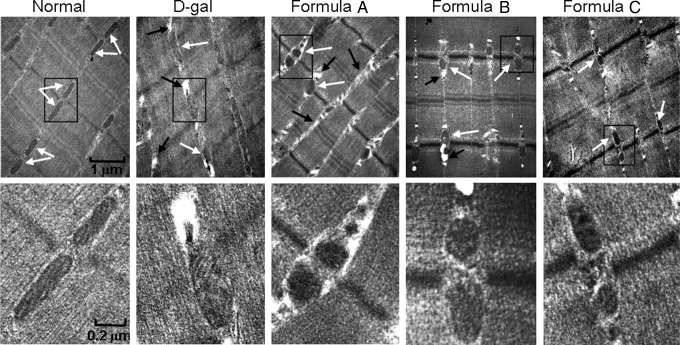
To understand the grip strength loss caused by D-gal exposure, we further examined the morphology of mice gastrocnemius muscles with transmission electron microscopy (Fig. 4). In the normal group, long rows of mitochondria with similar rod-like shape and size were regularly aligned in pairs between myofibrils. In contrast, swollen and elongated mitochondria with obvious cristae disruption appeared in D-gal-treated mice. In addition, more vacuoles, along with the distorted mitochondria, were observed in mice treated with D-gal compared with control mice. There were varied effects on the morphology of the mitochondria of mice treated with nutrient formula. Mitochondria of mice treated with formula A or formula B remained broken and contained vacuoles, although the size of the mitochondria was smaller than in mice treated with D-gal alone. The aberrant morphology of mitochondria following D-gal exposure was greatly ameliorated for mice treated with formula C; mitochondria were nearly intact with less vacuoles present after the treatment (Fig. 4).
FIG. 4.
Electron microscopy of gastrocnemius. D-gal-treated mice had a broader range of mitochondrial sizes than the normal control group, and an increased number of vacuoles and swollen and/or poorly formed mitochondria presented in the D-gal-treated group compared with the normal control group. All the formulas partially reversed the aberrant mitochondria induced by D-gal, and formula C additionally reduced the formation of vacuoles. White arrows indicate mitochondria and black arrows indicate vacuoles. The boxed sections in the upper panels are shown enlarged in the lower panels.
These results suggest that D-gal exposure results in a remarkable mitochondrial morphology distortion in skeletal muscle. Nutrient formulas, especially formula C, effectively ameliorated the mitochondrial morphology impairment in gastrocnemius muscle induced by D-gal.
Mitochondrial complex and dehydrogenase activities
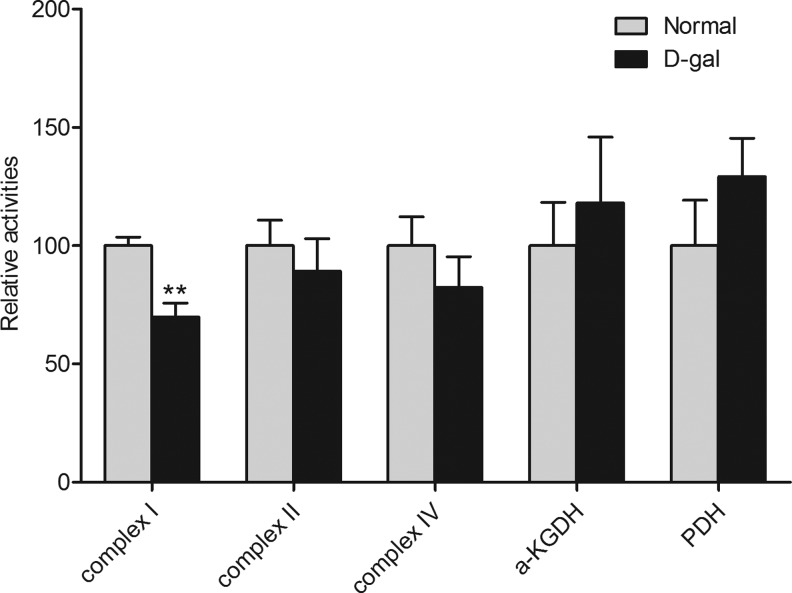
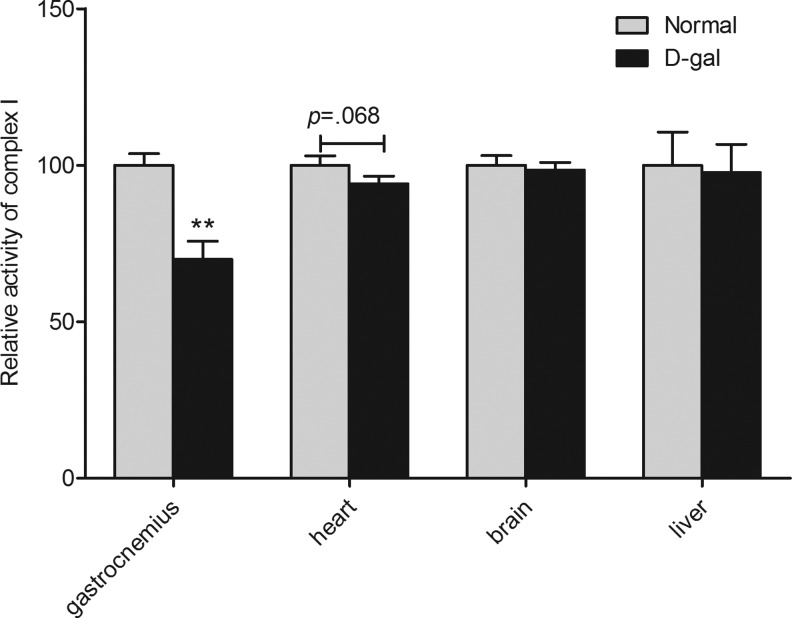
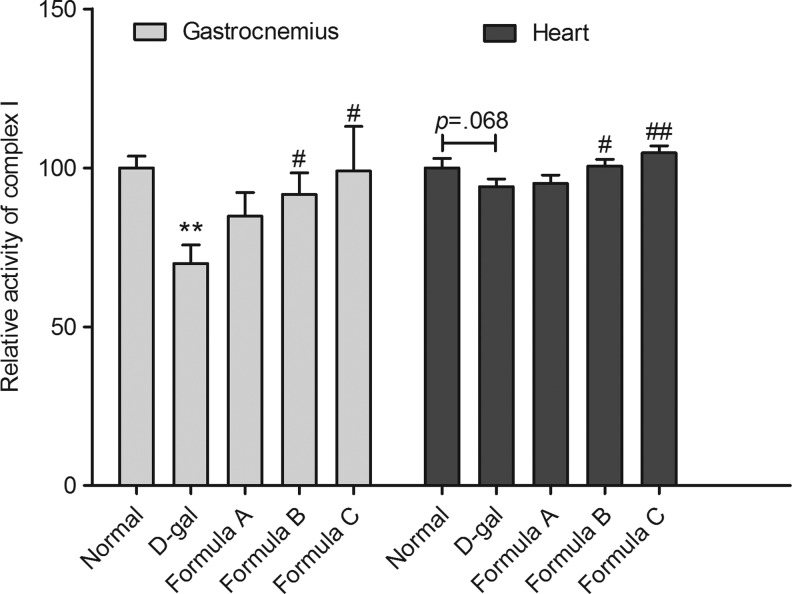
In view of the abnormal mitochondrial morphology in the skeletal muscle of the D-gal-treated mice, we sought to determine the activities of some key enzymes in the electron transport chain (ETC) and the tricarboxylic acid (TCA) cycle. Among ETC complexes I, II, and IV, α-KGDH and PDH, we found that only complex I activity was impaired in the gastrocnemius muscle of D-gal-treated mice (n=7; P<.05, Fig. 5). The activity of complex I was also assayed in several other tissues, such as the liver, heart, and brain. The results demonstrated that the complex I activity was decreased specifically in the skeletal muscle, with a marginal decrease in heart muscle, compared with the control mice (Fig. 6). Interestingly, we found that formula B and C successfully promoted complex I activity following D-gal treatment in the skeletal muscle and in heart muscle (Fig. 7).
FIG. 5.
Activities of complex I, II, IV, α-ketoglutarate dehydrogenase (α-KGDH) and pyruvate dehydrogenase (PDH) in gastrocnemius mitochondria. Chronic D-gal treatment induced complex I-specific impairment in gastrocnemius mitochondria. Values are mean±SEM of 12 mice. **P<.01 versus normal group.
FIG. 6.
Activities of complex I in mitochondria from multiple tissues. D-gal administration impairs the activity of complex I in gastrocnemius. Values are mean±SEM of 12 mice in gastrocnemius, 11 mice in heart, and 12 mice in brain and liver. **P<.01 versus normal group.
FIG. 7.
Effects of nutrient formula on the activity of complex I in gastrocnemius and heart mitochondria. Activities of complex I of gastrocnemius (n=12) and heart (n=11) in all groups were assayed. All values are expressed as mean±SEM. **P<.01 versus normal group, #P<.05 versus D-gal group, ##P<.01 versus D-gal group.
Discussion
Mice exposed to D-gal exhibited controversial behavior, especially with regard to cognitive impairment
In mice exposed to D-gal, a decline in coordination skills has been reported as an increased falling rate on the rotarod test,9 deficient spatial memory as measured by extended escape latency time on the MWM test,8 and reduced locomotor activity as determined by the locomotor activity test.25,26
Although the doses and duration of administration of D-gal required to produce a model for aging in mice vary over a wide range in published literature,27–29 it has been reported that 8 weeks of continuous subcutaneous injection of D-gal at a dose more than 100 mg/kg can induce significant behavioral impairment by MWM in C57BL/6J mice.26 Administration of D-gal at 40 mg/kg for 10 weeks can also induce an increased falling rate on the rotarod test.9 However, in the present study, 8 weeks of continuous D-gal exposure in C57BL/6J mice at a dose of 125 mg/kg resulted in neither a spatial memory loss nor impaired locomotor coordination. Although several mouse strains have been used in the D-gal-induced aging model, most of the studies that have reported deficiencies in learning and memory, as measured with the MWM test, were performed with the Kunming mouse strain (Table 1). Therefore, it is possible that D-gal-induced cognitive impairment may be strain specific. In the C57BL/6J strain, only a few studies have reported memory loss induced by D-gal and the mice were all aged more than 12 weeks. In our study, 8-week-old mice demonstrated no memory loss, which is consistent with a recent study.2 This suggests that the age of the mice may also be a key factor in learning and memory, especially in the premature aging model.
Table 1.
D-Galactose-Induced Memory Loss in Different Mice Strains
| D-gal treatment | ||||||
|---|---|---|---|---|---|---|
| Strain | Sex | Age | Dose (mg/kg) | Duration | Memory loss (MWM) | Reference |
| ICR | M | 5 week | 50 | 60 days | Yes | 30 |
| Laca | M | 2–3 month | 100 | 6 weeks | Yes | 5 |
| Swiss albino | M | 2–3 month | 100 | 6 weeks | Yes | 31 |
| Kunming | M | 6 week | 50 | 8 weeks | Yes | 32 |
| Kunming | M | 8 week | 500 | 8 weeks | Yes | 33 |
| Kunming | M | 10 week | 50 | 8 weeks | Yes | 34 |
| Kunming | M | 10 week | 50 | 8 weeks | Yes | 35 |
| Kunming | M | 10 week | 500 | 8 weeks | Yes | 36 |
| Kunming | M and F | 3 month | 150 | 6 weeks | Yes | 37 |
| Kunming | M | 15 month | 500 | 8 weeks | Yes | 19 |
| Kunming | – | 1.5 month | 500 | 45 days | No | 38 |
| C57BL/6J | F | 12 week | 100/200 | 8 weeks | Yes | 26 |
| C57BL/6J | F | 5 month | 50 | 8 weeks | Yes | 39 |
| C57BL/6J | F | 8 week | 100 | 6 weeks | No | 2 |
D-gal, D-galactose; MWM, Morris water maze.
D-gal induced a muscle-specific impairment and mitochondrial complex I deficiency
Aging is associated with a progressive loss of muscle mass and strength, a condition known as sarcopenia. Poor muscular strength is highly predictive of disability40 and mortality,41 and often results in the loss of independent living, thereby affecting an individual's quality of life.42 As a putative and direct measurement of muscular strength, the grip strength test has been used to screen for neuromuscular function.43 In the grip strength test, we found that D-gal-treated mice possessed significantly weaker grip strength when compared with the control group (Fig. 3).
It has been shown that aging is associated with a significant decline in mitochondrial function. Furthermore, as a key regulator of apoptosis and ATP production, mitochondrial dysfunction has emerged as a critical player in the onset and progression of sarcopenia.44 We found that the mitochondria in the gastrocnemius muscle exhibited a markedly abnormal morphology characterized by an increase in the heterogeneity of mitochondrial size, an impairment in mitochondrial integrity, an increase in the number of vacuoles, and a swelling of the mitochondria in mice treated with D-gal (Fig. 4). The abnormal morphology suggested that poor muscular strength induced by D-gal might be tightly associated with mitochondrial decay.
It is well known that energy production through oxidative phosphorylation occurs in mitochondria and is catalyzed by successive enzyme complexes. PDH and α-KGDH in the TCA cycle catalyze reactions producing reduced nicotinamide adenine dinucleotide (NADH), the most important electron donor for the ETC. The electron transfer chain consists of mitochondrial complexes I, II, and IV as the initial and terminal electron acceptors in electron transfer chain. We measured the activity of these key mitochondrial enzymes and found that complex I activity was significantly depressed in D-gal mice (Fig. 5). Among the tissues we investigated, the complex I activity was depressed in skeletal muscle and marginally depressed in the heart, but not in the liver and brain (Fig. 6). In GALT-deficient mice, dietary galactose is abnormally metabolized to galactitol, which is a similar condition as in D-gal overloaded mice. Galactitol was found to accumulate in the heart and skeletal muscle other than the liver, brain, and kidney in GALT-deficient mice,45 whereas accumulated galactitol can lead to osmotic stress and ROS production.5 Therefore, in our results, the muscle-specific damage is probably caused by galactitol accumulation.
As complex I is the major site of physiological and pathological ROS production,46,47 we inferred that decreased complex I activity enhanced ROS production, which in turn induced mitochondrial fragmentation or other morphology distortion as seen in the present study.
Nutrient formulas effectively rescued mitochondrial dysfunction induced by D-gal
As mitochondrial dysfunction is considered to play a major role in the aging process, three different nutrient formulas were designed to ameliorate mitochondrial dysfunction. The beneficial effects on mitochondrial function for all the components of each formula have been studied in our laboratory or in other laboratories. We assigned these compounds to different groups of mitochondrial nutrients as previously described.16 Previous studies in rodents have shown that combinations of nutrients have synergistic effects in various metabolic pathways and are more potent than treating the animals with individual nutrients.48 For example, supplementing mice with lipoic acid, carnitine, and CoQ10 had a more significant beneficial effect on age-related oxidative stress than individual treatment with these compounds.15,18 Therefore, in the present study, we designed nutrient combinations and assessed the effects these combinations had on mitochondrial impairment induced by D-gal exposure in mice.
All three nutrient formulas partially rescued the D-gal-induced muscle impairment. Formula B and C successfully rescued mitochondrial complex I deficiency after treatment with D-gal. These results suggested that D-gal-induced mitochondrial dysfunction in both skeletal muscle and heart muscle could be ameliorated by treatment with several compounds targeting mitochondrial metabolic pathways. These compounds consist of free radical scavengers, key cofactors of various enzymes in the mitochondria and steroid metabolites synthesized in mitochondria.
In conclusion, the present study demonstrated that chronic systemic exposure to D-gal causes muscle impairment and mitochondrial complex I deficiency. Interestingly, the mitochondrial nutrient formulas partially restored mitochondrial morphology and complex I activity, and rescued the loss in grip strength induced by D-gal. Further studies are required to elucidate the underlying mechanisms of muscle-specific mitochondrial dysfunction under D-gal exposure.
Acknowledgments
The authors thank Dr. Yanhai Li for technical assistance and William Wilson for his editorial help. This study was supported by the National Natural Science Foundation of China (Grant No. 31070740), New Century Excellent Talents in University, National “Twelfth Five-Year” Plan for Science and Technology Support, the Fundamental Research Funds for the Central Universities, Shaanxi Province Science and Technology Research and Development Program (2013K12-01-10), BioMarker Pharmaceuticals, Inc. (No. CRM-1), and LifeExtension Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Yu SL, Lin SB, Yu YL, et al.: Isochaihulactone protects PC12 cell against H2O2 induced oxidative stress and exerts the potent anti-aging effects in D-galactose aging mouse model. Acta Pharmacol Sin 2010;31:1532–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parameshwaran K, Irwin MH, Steliou K, Pinkert CA: D-galactose effectiveness in modeling aging and therapeutic antioxidant treatment in mice. Rejuvenation Res 2010;13:729–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W, Li S, Dong HP, Lv S, Tang YY: Differential impairment of spatial and nonspatial cognition in a mouse model of brain aging. Life Sci 2009;85:127–135 [DOI] [PubMed] [Google Scholar]
- 4.Cui X, Wang L, Zuo P, et al.: D-galactose-caused life shortening in Drosophila melanogaster and Musca domestica is associated with oxidative stress. Biogerontology 2004;5:317–325 [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Prakash A, Dogra S: Naringin alleviates cognitive impairment, mitochondrial dysfunction and oxidative stress induced by D-galactose in mice. Food Chem Toxicol 2010;48:626–632 [DOI] [PubMed] [Google Scholar]
- 6.Chen B, Zhong Y, Peng W, Sun Y, Kong WJ: Age-related changes in the central auditory system: comparison of D-galactose-induced aging rats and naturally aging rats. Brain Res 2010;1344:43–53 [DOI] [PubMed] [Google Scholar]
- 7.Li WJ, Nie SP, Xie MY, Yu Q, Chen Y, He M: Ganoderma atrum polysaccharide attenuates oxidative stress induced by D-galactose in mouse brain. Life Sci 2011;88:713–718 [DOI] [PubMed] [Google Scholar]
- 8.Cui X, Zuo P, Zhang Q, et al.: Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid. J Neurosci Res 2006;83:1584–1590 [DOI] [PubMed] [Google Scholar]
- 9.Lei H, Wang B, Li WP, Yang Y, Zhou AW, Chen MZ: Anti-aging effect of astragalosides and its mechanism of action. Acta Pharmacol Sin 2003;24:230–234 [PubMed] [Google Scholar]
- 10.Tian J, Ishibashi K, Reiser K, et al.: Advanced glycation endproduct-induced aging of the retinal pigment epithelium and choroid: a comprehensive transcriptional response. Proc Natl Acad Sci USA 2005;102:11846–11851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong WJ, Wang Y, Wang Q, Hu YJ, Han YC, Liu J: The relation between D-galactose injection and mitochondrial DNA 4834 bp deletion mutation. Exp Gerontol 2006;41:628–634 [DOI] [PubMed] [Google Scholar]
- 12.Long J, Wang X, Gao H, et al.: D-galactose toxicity in mice is associated with mitochondrial dysfunction: protecting effects of mitochondrial nutrient R-alpha-lipoic acid. Biogerontology 2007;8:373–381 [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Shen W, Zhao B, et al.: Targeting mitochondrial biogenesis for preventing and treating insulin resistance in diabetes and obesity: hope from natural mitochondrial nutrients. Adv Drug Deliv Rev 2009;61:1343–1352 [DOI] [PubMed] [Google Scholar]
- 14.Harman D: The biologic clock: the mitochondria? J Am Geriatr Soc 1972;20:145. [DOI] [PubMed] [Google Scholar]
- 15.Liu J: The effects and mechanisms of mitochondrial nutrient alpha-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: an overview. Neurochem Res 2008;33:194–203 [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Ames BN: Reducing mitochondrial decay with mitochondrial nutrients to delay and treat cognitive dysfunction, Alzheimer's disease, and Parkinson's disease. Nutr Neurosci 2005;8:67–89 [DOI] [PubMed] [Google Scholar]
- 17.Aksenov V, Long J, Liu J, et al.: A complex dietary supplement augments spatial learning, brain mass, and mitochondrial electron transport chain activity in aging mice. Age 2013;35:23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Head E, Gharib AM, et al.: Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha-lipoic acid. Proc Natl Acad Sci USA 2002;99:2356–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF: Purple sweet potato color alleviates D-galactose-induced brain aging in old mice by promoting survival of neurons via PI3K pathway and inhibiting cytochrome C-mediated apoptosis. Brain Pathol 2010;20:598–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seidler RD, Bernard JA, Burutolu TB, et al.: Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 2010;34:721–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowenkamp KE, Ujhelyi L, Cline EJ, Bickford PC: Effects of intra-striatal GDNF on motor coordination and striatal electrophysiology in aged F344 rats. Neurobiol Aging 2000;21:117–124 [DOI] [PubMed] [Google Scholar]
- 22.Aliev G, Liu J, Shenk JC, et al.: Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats. J Cell Mol Med 2009;13:320–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Birch-Machin MA, Briggs HL, Saborido AA, Bindoff LA, Turnbull DM: An evaluation of the measurement of the activities of complexes I-IV in the respiratory chain of human skeletal muscle mitochondria. Biochem Med Metab Biol 1994;51:35–42 [DOI] [PubMed] [Google Scholar]
- 24.Long J, Wang X, Gao H, et al.: Malonaldehyde acts as a mitochondrial toxin: Inhibitory effects on respiratory function and enzyme activities in isolated rat liver mitochondria. Life Sci 2006;79:1466–1472 [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, Prakash A, Dogra S: Protective effect of curcumin (Curcuma longa) against D-galactose-induced senescence in mice. J Asian Nat Prod Res 2011;13:42–55 [DOI] [PubMed] [Google Scholar]
- 26.Wei H, Li L, Song Q, Ai H, Chu J, Li W: Behavioural study of the D-galactose induced aging model in C57BL/6J mice. Behav Brain Res 2005;157:245–251 [DOI] [PubMed] [Google Scholar]
- 27.Song X, Bao M, Li D, Li YM: Advanced glycation in D-galactose induced mouse aging model. Mech Ageing Dev 1999;108:239–251 [DOI] [PubMed] [Google Scholar]
- 28.Xu XH, Zhao TQ: Effects of puerarin on D-galactose-induced memory deficits in mice. Acta Pharmacol Sin 2002;23:587–590 [PubMed] [Google Scholar]
- 29.Ho SC, Liu JH, Wu RY: Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology 2003;4:15–18 [DOI] [PubMed] [Google Scholar]
- 30.Zhong SZ, Ge QH, Qu R, Li Q, Ma SP: Paeonol attenuates neurotoxicity and ameliorates cognitive impairment induced by D-galactose in ICR mice. J Neurol Sci 2009;277:58–64 [DOI] [PubMed] [Google Scholar]
- 31.Kumar A, Dogra S, Prakash A: Effect of carvedilol on behavioral, mitochondrial dysfunction, and oxidative damage against D-galactose induced senescence in mice. Naunyn Schmiedebergs Arch Pharmacol 2009;380:431–441 [DOI] [PubMed] [Google Scholar]
- 32.Lu J, Zheng YL, Luo L, Wu DM, Sun DX, Feng YJ: Quercetin reverses D-galactose induced neurotoxicity in mouse brain. Behav Brain Res 2006;171:251–260 [DOI] [PubMed] [Google Scholar]
- 33.Wu DM, Lu J, Zheng YL, Zhou Z, Shan Q, Ma DF: Purple sweet potato color repairs D-galactose-induced spatial learning and memory impairment by regulating the expression of synaptic proteins. Neurobiol Learn Mem 2008;90:19–27 [DOI] [PubMed] [Google Scholar]
- 34.Wang CQ, Yang GQ: Betacyanins from Portulaca oleracea L. ameliorate cognition deficits and attenuate oxidative damage induced by D-galactose in the brains of senescent mice. Phytomedicine 2010;17:527–532 [DOI] [PubMed] [Google Scholar]
- 35.Lu J, Zheng YL, Wu DM, Luo L, Sun DX, Shan Q: Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Biochem Pharmacol 2007;74:1078–1090 [DOI] [PubMed] [Google Scholar]
- 36.Lu J, Wu DM, Hu B, et al.: Chronic administration of troxerutin protects mouse brain against D-galactose-induced impairment of cholinergic system. Neurobiol Learn Mem 2010;93:157–164 [DOI] [PubMed] [Google Scholar]
- 37.Zhang XL, Jiang B, Li ZB, Hao S, An LJ: Catalpol ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by D-galactose. Pharmacol Biochem Behav 2007;88:64–72 [DOI] [PubMed] [Google Scholar]
- 38.Liu K, Wang C, Li G, He N: [A comparison study between D-galactose treated mice and natural aging mice]. Wei Sheng Yan Jiu 2007;36:685–688 [PubMed] [Google Scholar]
- 39.Mao GX, Deng HB, Yuan LG, Li DD, Li YY, Wang Z: Protective role of salidroside against aging in a mouse model induced by D-galactose. Biomed Environ Sci 2010;23:161–166 [DOI] [PubMed] [Google Scholar]
- 40.Rantanen T, Guralnik JM, Foley D, et al.: Midlife hand grip strength as a predictor of old age disability. JAMA 1999;281:558–560 [DOI] [PubMed] [Google Scholar]
- 41.Metter EJ, Talbot LA, Schrager M, Conwit R: Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 2002;57:B359–B365 [DOI] [PubMed] [Google Scholar]
- 42.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R: The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 2004;52:80–85 [DOI] [PubMed] [Google Scholar]
- 43.Maurissen JPJ, Marable BR, Andrus AK, Stebbins KE: Factors affecting grip strength testing. Neurotoxicol Teratol 25:543–553 [DOI] [PubMed] [Google Scholar]
- 44.Dirks AJ, Leeuwenburgh C: The role of apoptosis in age-related skeletal muscle atrophy. Sports Med 2005;35:473–483 [DOI] [PubMed] [Google Scholar]
- 45.Yager C, Ning C, Reynolds R, Leslie N, Segal S: Galactitol and galactonate accumulation in heart and skeletal muscle of mice with deficiency of galactose-1-phosphate uridyltransferase. Mol Genet Metab 2004;81:105–111 [DOI] [PubMed] [Google Scholar]
- 46.Ide T, Tsutsui H, Kinugawa S, et al.: Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res 1999;85:357. [DOI] [PubMed] [Google Scholar]
- 47.Koopman WJ, Verkaart S, Visch HJ, et al.: Human NADH:ubiquinone oxidoreductase deficiency: radical changes in mitochondrial morphology? Am J Physiol Cell Physiol 2007;293:C22–C29 [DOI] [PubMed] [Google Scholar]
- 48.Aksenov V, Long J, Liu J, et al.: A complex dietary supplement augments spatial learning, brain mass, and mitochondrial electron transport chain activity in aging mice. Age 2011;1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]