Abstract
This study was performed to investigate the hypolipidemic, antiobese, and antiatherogenic effects of resveratrol in apoE-deficient mice fed an atherogenic diet (20% fat and 1% cholesterol). These animals were fed an atherogenic diet containing 0.02% lovastatin (w/w) or 0.02% resveratrol (w/w) for 12 weeks. Resveratrol and lovastatin supplementation significantly reduced either the body weight or epididymal fat weight without altering the food intake and food efficiency ratio. Resveratrol significantly decreased the plasma total cholesterol (total-C), low-density lipoprotein cholesterol (LDL-C), non-high-density lipoprotein cholesterol (non-HDL-C) concentrations, apoB/apoA-I ratio, hepatic cholesterol, and triglyceride (TG) contents, whereas significantly it increased the plasma HDL-C concentration compared with the control and lovastatin groups. Plasma and hepatic TG and plasma apoB levels were significantly lower in both the lovastatin and resveratrol groups than in the control group without altering the plasma apoA-I concentration. Both resveratrol and lovastatin significantly decreased hepatic fatty acid and TG synthesis, whereas they increased fatty acid oxidation (β-oxidation) except for the carnitine palmitoyltransferase activity compared with the control group. However, there was no difference in hepatic 3-hydroxyl-3-methylglutaryl-CoA reductase activity among the groups, although hepatic acyl-CoA: cholesterol acyltransferase activity was significantly lower in the lovastatin groups than in the control group. In epididymal adipose tissue, resveratrol supplementation led to an increase in β-oxidation and decrease in TG synthesis, compared with the control group. Tissue morphology revealed that there were dramatic decreases in hepatic lipid droplets and aortic fatty streaks by resveratrol and lovastatin supplementation. This study demonstrates that resveratrol exerts not only antiobesity and hypolipidemic effects, but also protective effects for the liver and aorta through the modulation of lipid metabolism in both the liver and white adipose tissues.
Key Words: : apoE-deficient mice, body fat, lipid metabolism, resveratrol
Introduction
Cardiovascular disease (CVD) is the single largest cause of mortality and morbidity in both developed and developing countries. The elevation of low-density lipoprotein cholesterol (LDL-C) concentration is strongly associated with the development and progression of CVD.1 For that reason, plasma total-C- and LDL-C-lowering therapy is very important for treating and preventing CVD and its complications. It has been more recently discovered that other lipoproteins besides LDL are also closely involved in atherogenesis, including very low-density lipoprotein (VLDL), intermediate density lipoprotein (IDL), and high-density lipoprotein (HDL). Each lipoprotein also has distinct apolipoproteins, which play essential roles in the regulation of lipid metabolism as well as the stabilization of lipoprotein structure. ApoA-I is a major apolipoprotein of HDL and plays a crucial role in reverse cholesterol transport.2 ApoB is located in VLDL, LDL, and IDL, which are all atherogenic lipoproteins.2 These apoA-I and apoB concentrations in plasma are also important in predicting atherosclerosis and CVD2,3 using the concentrations of plasma total-C and LDL-C.
Many studies have searched for phytochemicals possessing hypolipidemic and antiobesity capacities.4,5 Most of all, resveratrol is a naturally occurring phytochemical in grapes, peanuts, and other plants, and it has been demonstrated to improve CVD,6 obesity,7 diabetes,8 cancer,9 and to regulate angiogenesis10 as well as to ameliorate the aging process11 since its bioactivity was first elucidated in the 1990's. Lovastatin, a 3-hydroxyl-3-methylglutaryl-CoA (HMG-CoA) reductase inhibitor, is a member of the statin drug class used for lowering cholesterol in hypercholesterolemic patients.12 Among the experimental animal models, apoE-deficient mice exhibit severe hypercholesterolemia and atherosclerotic lesions because apoE is an important apolipoprotein with antiatherosclerotic functions. Thus, this animal model has been widely used in studies on experimental atherosclerosis as an ideal model of human atherosclerosis.13,14 This study was performed to assess the hypolipidemic, antiobese, and antiatherosclerotic effects of resveratrol compared with lovastatin in apoE-deficient mice.
Materials And Methods
Animals and diets
Four-week-old male homozygous apoE-deficient mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). After a 1-week adaptation period, thirty apoE-deficient mice were randomly divided into three groups. The control group was provided with an atherogenic diet (20% fat and 1% cholesterol) based on an AIN-76 semisynthetic diet, while the two experimental groups received an atherogenic control diet supplemented with 0.02% (w/w) lovastatin (Chong Kun Dang Pharm., Seoul, Korea) or 0.02% (w/w) resveratrol (Sigma Chemical Co., St Louis, MO, USA) for 12 weeks (Table 1). Body weight and food intake were measured every week and every day, respectively. After 12 weeks, blood was collected from the inferior vena cava to determine the plasma biomarkers. Plasma was prepared by centrifugation at 1500 g, 4°C for 10 min. The liver, fat pads (epididymal and perirenal fat), and aorta were removed, rinsed, and weighed. All samples were stored at −70°C until analysis. The current study protocol was approved by the Ethics Committee at Kyungpook National University for animal studies.
Table 1.
Compositions of Experimental Diets (%)
| Component | Control | Lovastatin | Resveratrol |
|---|---|---|---|
| Casein | 20.0 | 20.0 | 20.0 |
| D,L-methionine | 0.3 | 0.3 | 0.3 |
| Sucrose | 50.0 | 49.8 | 48.57 |
| Cellulose powder | 5.0 | 5.0 | 5.0 |
| Corn Oil | 10.0 | 10.0 | 10.0 |
| Lard | 10.0 | 10.0 | 10.0 |
| Choline bitartrate | 0.2 | 0.2 | 0.2 |
| AIN-mineral | 3.5 | 3.5 | 3.5 |
| AIN-vitamin | 1.0 | 1.0 | 1.0 |
| Lovastatin | — | 0.02 | — |
| Resveratrol | — | — | 0.02 |
| Total | 100 | 100 | 100 |
AIN-mineral, AIN-76 mineral mixture (Harlan Teklad Co. Madison, WI, USA); AIN-vitamin, AIN-76 vitamin mixture (Harlan Teklad Co.).
Plasma and hepatic lipid profiles
The plasma triglyceride (TG), total-C and HDL-C concentrations were quantitatively determined using the respective commercial kits (Asan Pharm a Company, Hwasung, Republic of Korea) based on the enzymatic reactions. Plasma apoA-I and apoB levels were also measured using the respective enzymatic kits (Eiken, Tokyo, Japan). The lipid fraction in the liver was extracted with a procedure developed by Folch et al.,15 and the hepatic cholesterol and TG contents were analyzed with the same enzymatic kits used in the plasma analyses.
Lipid metabolic enzyme activities in the liver and epididymal fat pad
To measure the lipid-regulating enzyme activities in the liver and epididymal white adipose tissue (WAT), cytosolic, mitochondrial, and microsomal fractions were prepared according to the method by Hulcher and Oleson.16 The protein concentrations in each fraction were measured according to Bradford's method17 using bovine serum albumin as the standard. The cytosolic fatty acid synthase (FAS) activity was measured according to Nepokroeff's method18 by monitoring the malonyl-CoA-dependent oxidation of the NADPH at 340 nm, and malic enzyme (ME) activity was measured according to Ochoa's method19 by monitoring the production of NADPH at 340 nm. The glucose-6-phosphate dehydrogenase (G6PD) activity was assayed by spectrophotometric methods according to the procedures described by Pitkanen et al.,20 and the phosphatidate phosphohydrolase (PAP) activity was determined using Walton's method.21 The mitochondrial carnitine palmitoyltransferase (CPT) activity was analyzed using Markwell's method,22 and fatty acid β-oxidation was determined using Lazarow's method23 by monitoring the reduction of NAD to NADH at 340 nm.
Hepatic HMG-CoA reductase and acyl-CoA:cholesterol acyltransferase (ACAT) activities were determined as described by Shapiro et al.24 and by Gillies et al.,25 respectively.
Histopathological analysis in the liver and aorta
The liver and aorta were fixed in 10% paraformaldehyde/PBS, embedded in paraffin, and stained with hematoxylin and eosin (H&E). To determine the presence of lipids, an adjacent section of the aortic arch was cryosectioned and stained with the Oil Red O (ORO) solution. The stained area was then viewed using a microscope at a magnification of 200×.
Statistical analysis
The data are expressed as the mean±standard error (SE). Significant differences among the groups were determined by one-way ANOVA using the SPSS program (SPSS, Inc., Chicago, IL). The differences between the means were assessed using the Duncan's multiple range test and statistical significances were considered at P<.05.
Results
Body weight gain, food intake, and organ weight
Body weight gain was significantly lower in both the resveratrol and lovastatin groups compared with the control group, whereas the food intake and food efficiency ratio were not different between the groups (Table 2). When comparing the organ weights, resveratrol and lovastatin supplementation resulted in significant reductions in epididymal WAT weight without the differences in the liver and perirenal WAT weights (Table 2).
Table 2.
Effects of Resveratrol Supplementation for 12 Weeks on Body Weight Gain, Food Intake, Food Efficiency Ratio, and Organ Weights in Apolipoprotein E-Deficient Mice Fed an Atherogenic Diet
| Control | Lovastatin | Resveratrol | |
|---|---|---|---|
| Body weight gain (g/day) | 0.099±0.004a | 0.084±0.002b | 0.085±0.004b |
| Food intake (g/day) | 3.45±0.05 | 3.43±0.09 | 3.42±0.12 |
| FER | 0.028±0.001 | 0.026±0.001 | 0.027±0.002 |
| Organ weights (g) | |||
| Liver | 4.59±0.07 | 4.82±0.06 | 4.73±0.09 |
| Epididymal WAT | 0.79±0.08a | 0.55±0.06b | 0.57±0.04b |
| Perirenal WAT | 0.25±0.02 | 0.22±0.02 | 0.23±0.02 |
Data are the mean±SE, n=10.
Means in the same row not sharing a common superscript are significantly different between the groups at P<.05.
FER, food efficiency ratio=body weight gain/food intake; WAT, white adipose tissue.
Plasma and hepatic lipid profiles
Both total-C and LDL-C concentrations in plasma were significantly lower in the resveratrol group than in the control group after 12 weeks, whereas plasma TG concentration was significantly lower in both the lovastatin and resveratrol groups than in the control group (Table 3). Resveratrol supplementation resulted in significantly higher plasma HDL-C concentrations compared with the control and lovastatin groups without any differences in the plasma apoA-I concentrations (Table 3). The plasma apoB concentration and apoB/apoA-I ratio were significantly lower in both lovastatin and resveratrol groups than in the control group (Table 3). Consequently, the resveratrol group exhibited lower levels of all atherogenic factors, including plasma total-C, LDL-C, TG, and apoB concentrations and the apoB/apoA-I ratio (Table 3).
Table 3.
Effects of Resveratrol Supplementation for 12 Weeks on Plasma and Hepatic Lipid Profile in Apolipoprotein E-Deficient Mice Fed an Atherogenic Diet
| Control | Lovastatin | Resveratrol | |
|---|---|---|---|
| Plasma (mM) | |||
| Total cholesterol | 38.85±1.36a | 37.23±1.14a | 29.44±0.75b |
| TG | 1.07±0.11a | 0.84±0.08b | 0.82±0.07b |
| HDL-cholesterol | 0.15±0.03b | 0.16±0.01b | 0.28±0.03a |
| LDL-cholesterol | 34.01±1.02a | 29.83±1.88ab | 28.99±0.62b |
| nonHDL-cholesterol | 37.85±1.45a | 35.84±1.29a | 28.72±0.84b |
| ApoA-I | 10.04±0.85 | 10.57±0.78 | 10.48±0.51 |
| ApoB | 41.17±0.79a | 33.09±1.99b | 36.37±1.14b |
| ApoB/apoA-I ratio | 4.10±0.18a | 3.13±0.26b | 3.47±0.12b |
| Liver (mmol/g) | |||
| Cholesterol | 0.10±0.001b | 0.08±0.001b | 0.07±0.001a |
| TG | 0.44±0.03a | 0.33±0.02b | 0.31±0.03b |
Data are the mean±SE. LDL-cholesterol=(Total-cholesterol)−(HDL-cholesterol)−(Triglycerides/5).
Means not sharing a common letter are significantly different among groups at P<.05.
ApoA-I, apolipoprotein A-I; apoB, apolipoprotein B.
Hepatic lipid analyses revealed that the resveratrol group had significantly lower levels of both cholesterol and TG, whereas the lovastatin group only had lower TG content, compared with the control group (Table 3).
Lipid metabolism-related enzyme activities in the liver and epididymal WAT
The results of the lipid metabolism-related enzyme activities in the liver and epididymal fat pad are presented in Table 4. In the liver, the activities of adipogenic enzymes, including G6PD, FAS, ME, and PAP were all significantly lower in both the lovastatin and resveratrol groups than in the control group. When compared with the control group, the hepatic mitochondrial fatty acid β-oxidation was significantly elevated by lovastatin and resveratrol supplementation without differences in the activity of CPT, the enzyme acting at the first step of fatty acid oxidation. Hepatic HMG-CoA reductase activity was not significantly different among the groups, while the activity of ACAT, an esterification enzyme of free cholesterol, was significantly lower in the lovastatin group than in the control group (Table 4).
Table 4.
Effects of Resveratrol Supplementation for 12 Weeks on Lipid-Regulating Enzyme Activities in the Liver and Epididymal White Adipose Tissue in Apolipoprotein E-Deficient Mice Fed an Atherogenic Diet
| Control | Lovastatin | Resveratrol | |
|---|---|---|---|
| Liver | |||
| Fatty acid synthesis (nmol/min/mg protein) | |||
| G6PD | 38.41±2.00a | 20.62±1.59b | 21.98±2.35b |
| ME | 12.97±0.10a | 9.12±0.40b | 10.22±0.24b |
| FAS | 35.59±5.28a | 21.08±2.55b | 19.24±1.21b |
| TG synthesis (nmol/min/mg protein) | |||
| PAP | 15.55±0.70a | 12.11±0.13b | 10.92±0.14c |
| Fatty acid oxidation (nmol/min/mg protein) | |||
| β-oxidation | 12.97±1.28b | 19.60±2.68a | 20.14±2.04a |
| CPT | 20.29±1.23 | 24.11±1.26 | 20.30±2.11 |
| Cholesterol biosynthesis and esterification (pmol/min/mg protein) | |||
| HMG-CoA reductase | 231.45±0.72 | 225.81±2.69 | 230.07±1.94 |
| ACAT | 195.88±26.83a | 102.29±29.41b | 165.77±10.51ab |
| Epididymal WAT | |||
| Fatty acid synthesis (nmol/min/mg protein) | |||
| G6PD | 69.92±6.60 | 67.45±6.44 | 67.06±4.05 |
| ME | 22.92±1.87 | 21.92±0.73 | 20.14±0.83 |
| FAS | 4.91±0.74 | 4.11±0.16 | 3.77±0.62 |
| TG synthesis (nmol/min/mg protein) | |||
| PAP | 34±9.31a | 29±9.56ab | 27±10.36b |
| Fatty acid oxidation (nmol/min/mg protein) | |||
| β-oxidation | 116.01±9.31a | 120.17±9.56a | 158.11±10.36b |
| CPT | 203.86±30.54 | 166.78±28.67 | 220.24±33.56 |
Data are the mean±SE.
Means not sharing a common letter are significantly different among groups at P<.05.
G6PD, glucose-6-phosphate dehydrogenase; ME, malic enzyme; FAS, fatty acid synthase; CPT, carnitine palmitoyl transferase; PAP, phosphatidate phosphohydrolase; HMG-CoA reductase, 3-hydroxyl-3-methylglutaryl coenzyme A reductase; ACAT, acyl-CoA: cholesterol acyltransferase.
Unlike the results of the hepatic lipid-regulating enzyme activities, there were no significant differences in the epididymal enzyme activities of G6PD, ME, FAS, and CPT among the groups. However, resveratrol supplementation resulted in significantly higher epididymal β-oxidation and lower PAP activities, respectively, compared with the control group.
Histopathological changes in the liver and aorta
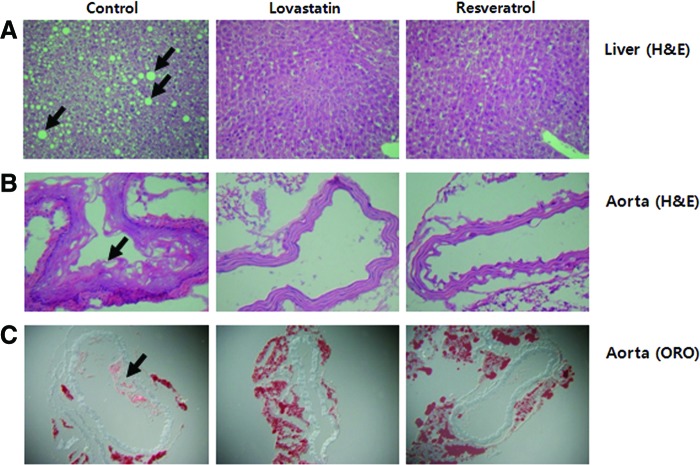
The effects of resveratrol on the pathogenesis of steatosis and atherosclerosis were evaluated in apoE-deficient mice. As shown in Figure 1, both lipid droplet numbers and their sizes were higher due to the accumulation of hepatic lipids in the control group, whereas the lovastatin and resveratrol groups showed much less and smaller lipid droplets (Fig. 1A). Figure 1B and C show the representative H&E- and ORO-stained histological differences of the aortas from the experimental groups. Dietary resveratrol and lovastatin exposure remarkably inhibited the endothelial damage and fatty streak formation shown by the H&E staining of the aortic arch. ORO staining revealed that resveratrol and lovastatin significantly inhibited fatty plaques and lesion formation by lipid deposition in the aortic arch when compared with the control group, which was fed an atherogenic diet.
FIG. 1.
Effects of resveratrol supplementation for 12 weeks on the liver (A) and aorta (B, C) morphologies in apoE-deficient mice fed an atherogenic diet. (A) Hematoxylin and eosin (H&E)-stained transverse section of the liver; (B) H&E-stained transverse section of aortic arch; (C) Oil Red O-stained cryosection of aortic arch; advanced fatty plaques containing lipid-rich components were present in the aortic arch, as well as hepatic lipid droplets in the control group, but not in the lovastatin and resveratrol groups (arrows: hepatic lipid droplets and aortic fatty streak. Magnification 200×). Color images available online at www.liebertpub.com/jmf
Discussion
The ApoE-deficient mice used in this experiment are one of the hypercholesterolemic animal models that have also been shown to mimic the initiation and progression of human atherosclerosis.26 This study demonstrated that dietary resveratrol in apoE-deficient mice resulted in hypolipidemic, antiobese, and antiatherosclerotic effects by improving lipid metabolism in the plasma, liver, and WAT. Several studies have provided evidence regarding the potential antiobesity effects of resveratrol, which reduced the body weight, liver weight, and/or adiposity in animals fed a high-fat diet or in obese Zucker rats fed a normal diet.27–29 The present results showed a similar body weight and adiposity-lowering effects of resveratrol without any differences in the liver weight.
Regarding the hypolipidemic effects of resveratrol, Cho et al.30 observed decreases in the amount of both plasma and hepatic lipids with decreased hepatic HMG-CoA reductase activity and mRNA expression in hamsters fed a high-fat diet. Both Soleas et al.31 and Goldberg et al.32 indicated that trans-resveratrol treatments significantly decreased the intracellular concentration of apoB, resulting in less VLDL and LDL production in a HepG2 cell line. Daily consumption of resveratrol (10 mg/kg of body weight) significantly decreased the TG and cholesterol concentrations in both the plasma and liver of hyperlipidemic obese Zucker rats when compared with the control group.28 In the present study, the amount of daily resveratrol consumed by the apo E-deficient mice was approximately 28.5 mg/kg body weight based on food intake, that is equivalent to 85.5 mg resveratrol/60 kg man based on the conversion of the mice dose to a human equivalent dose using the body surface area normalization method.33 As previously discussed, our study also showed a significant decrease in the plasma total cholesterol, LDL-C, TG, and apoB concentrations as well as hepatic cholesterol and TG accumulation by resveratrol supplementation. The plasma HDL-C concentration was also increased by resveratrol in the present study, which corresponds with the report by Penumathsa et al.34 The National Cholesterol Education Program (NCEP) update recommended the use of non-HDL-C as a target for individuals with high TG (≥200 mg/dL). However, some patients who achieve a significantly lowered LDL-C or are within the recommended LDL-C range still develop CV events.35 Some epidemiological studies have indicated that higher the apoB/apoA-I ratio, the higher the CV risk is because high TG and low HDL-C are also part of the atherogenic lipid profile.35,36 A report by Do et al.37 showed the spontaneous HDL-C-raising and TG-lowering effects of resveratrol in normal diet fed apoE-deficient mice. In general, there is an inverse relationship between plasma TG and HDL-C,38 and the results of this study show a substantial decrease in plasma TG coupled with an increase in HDL-C as well as an increase in the apoB/apoA-I ratio. On the other hand, several studies suggested that resveratrol administration did not alter the lipid profile. This includes the plasma total-C, LDL-C, and TG as well as HDL-C in rats receiving either a standard or high-fat diet.39,40 Interestingly, lovastatin, used as a positive control, was not effective in lowering the plasma and hepatic cholesterol levels in this particular atherogenic animal model. However, it significantly decreased plasma and hepatic TG levels, although it is one of the most frequently used cholesterol-lowering agents. A study by Xu et al.41 suggested that simvastatin supplementation with a high cholesterol diet significantly lowered plasma TG, but not the LDL-C. Steinmetz et al.42 also suggested that treatment with simvastatin had no effect on the serum cholesterol levels in either normal or hypercholesterolemic mice.
The antiadipogenic activity of resveratrol has been elucidated in several experiments. Studies on isolated cells revealed the inhibition of adipogenesis in the presence of resveratrol.43,44 Resveratrol (10–100 μM) was found to inhibit fatty acid synthesis from acetate in isolated rat hepatocytes. This effect was accompanied by the lessened activity of acetyl-CoA carboxylase (ACC) and decreased incorporation into TG, but the FAS and HMG-CoA reductase activities were unchanged.45 The biological benefits of resveratrol have also been suggested to involve AMPK, which prevents the development of hyperlipidemia, atherosclerosis, and diabetes by increasing glucose uptake46 and by inhibiting fatty acid and/or TG synthesis45,47 as well as cholesterol synthesis.48 In addition to the results discussed above, the present study also showed evidence for not only decreased hepatic FA and TG synthesis, but also for increased β-oxidation from resveratrol. Some reports confirmed that resveratrol increases the mitochondrial content of the liver.40,49 It seems that the extra mitochondria content induced by resveratrol may affect the improvement in hepatic lipid metabolism, especially mitochondrial β-oxidation, although the mitochondrial contents were not measured directly in this study. Results suggest that resveratrol consumption is effective in enhancing FA oxidation in both the liver and WAT, and a significant lowering of hepatic TG content was partially due to the improvement in overall lipid-regulating enzyme activities. A high-fat and cholesterol-containing diet induces the accumulation of hepatic cholesterol and TG as well as an increase in liver weight.50 In this study, the ingestion of resveratrol with an atherogenic diet significantly lowered the hepatic cholesterol and TG contents and hepatic lipid droplets without altering the liver weight in apoE-deficient mice.
However, hepatic cholesterol biosynthesis and cholesterol esterification were not influenced by resveratrol, although it did cause a significant decrease in plasma total-C, LDL-C, and apoB secretions, which corresponded with the report by Gnoni.45 In addition, this result seems to be due to hepatic cholesterol homeostasis. However, in high-fat fed hamsters, resveratrol was effective in attenuating hepatic HMG-CoA reductase activity and mRNA expression.30 The endothelium plays an important role in the maintenance of vascular homeostasis. It is well known that endothelial damage disturbs vascular homeostasis, following the initiation of the atherosclerotic process.51 Several studies have suggested that resveratrol contributes to the prevention of atherosclerosis by inhibiting aortic vascular smooth muscle cell proliferation and endothelial foam cell formation.52,53 Resveratrol and lovastatin significantly attenuated the atherosclerotic plaque and endothelial lesions to which cholesterol-fed apoE-deficient mice were subjected. Resveratrol preserved the endothelial lining and exerted vasoprotective effects. Clinical trials have demonstrated that statins exert antiatherosclerotic effects that are independent of their cholesterol-lowering properties.54,55 Consistent with the results mentioned above, lovastatin treatment was also found to be effective in preventing atherosclerotic lesions without changing the plasma or hepatic cholesterol levels.
The results suggest that resveratrol supplementation improves dyslipidemia and lowers the fat pad mass and body weight, possibly by regulating the hepatic lipoprotein/apolipoprotein secretion, hepatic and adipocytic lipid metabolism, including FA synthesis and/or oxidation and TG synthesis, and attenuates steatosis and atherosclerotic lesion formation. Accordingly, it is considered that resveratrol may possess antiobese, hypolipidemic, and antiatherosclerotic capacities along with steatosis-preventing effects against diet-induced metabolic disorders in apoE-deficient mice under atherogenic conditions.
Acknowledgment
This work was funded by the Basic Science Research Program (Center for Food & Nutritional Genomics: grant number 2012-0000644, 2012M3A9C4048818) of the National Research Foundation (NRF) of Korea funded by the Ministry of Education, Science and Technology.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gylling H: Cholesterol metabolism and its implications for therapeutic interventions in patients with hypercholesterolaemia. Int J Clin Pract 2004;58:859–866 [DOI] [PubMed] [Google Scholar]
- 2.Walldius G, Jungner I, Holme I, et al. : High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet 2001;358:2026–2033 [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan SR, Berenson GS: Apolipoproteins B and A-I as predictors of risk of coronary artery disease. Lancet 2001;358:2012–2013 [DOI] [PubMed] [Google Scholar]
- 4.Jeon SM, Park YB, Choi MS: Antihypercholesterolemic property of naringin alters plasma and tissue lipids, cholesterol-regulating enzymes, fecal sterol and tissue morphology in rabbits. Clin Nutr 2004;23:1025–1034 [DOI] [PubMed] [Google Scholar]
- 5.Woo MN, Jeon SM, Kim HJ, et al. : Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem Biol Interact 2010;186:316–322 [DOI] [PubMed] [Google Scholar]
- 6.Miatello R, Vázquez M, Renna N, et al. : Chronic administration of resveratrol prevents biochemical cardiovascular changes in fructose-fed rats. Am J Hypertens 2005;18:864–870 [DOI] [PubMed] [Google Scholar]
- 7.Dal-Pan A, Blanc S, Aujard F: Resveratrol suppresses body mass gain in a seasonal non-human primate model of obesity. BMC Physiol 2010;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramadori G, Gautron L, Fujikawa T, et al. : Central administration of resveratrol improves diet-induced diabetes. Endocrinology 2009;150:5326–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi HY, Chong SA, Nam MJ: Resveratrol induces apoptosis in human SK-HEP-1 hepatic cancer cells. Cancer Genomics Proteomics 2009;6:263–268 [PubMed] [Google Scholar]
- 10.Fischer-Posovszky P, Kukulus V, Tews D, et al. : Resveratrol regulates human adipocyte number and function in a Sirt1-dependent manner. Am J Clin Nutr 2010;92:5–15 [DOI] [PubMed] [Google Scholar]
- 11.Valenzano DR, Terzibasi E, Genade T, et al.: Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol 2006;16:296–300 [DOI] [PubMed] [Google Scholar]
- 12.Kervinen K, Savolainen MJ, Heikkilä JI, et al. : Lovastatin enhances hepatic uptake of low density lipoprotein in humans. J Lipid Res 1993;34:1975–1982 [PubMed] [Google Scholar]
- 13.Nakashima Y, Plump AS, Raines EW, et al. : ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb 1994;14:133–140 [DOI] [PubMed] [Google Scholar]
- 14.Kato M, Sada T, Chuma H, et al. : Severity of hyperlipidemia does not affect antiatherosclerotic effect of an angiotensin II receptor antagonist in apolipoprotein E-deficient mice. J Cardiovasc Pharmacol 2006;47:764–769 [DOI] [PubMed] [Google Scholar]
- 15.Folch J, Lees M, Sloan-Stanley GH: A simple method for isolation and purification of total lipids from animal tissues. J Biol Chem 1957;226:497–509 [PubMed] [Google Scholar]
- 16.Hulcher FH, Oleson WH: Simplified spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl CoA reductase by measurement of coenzyme A. J Lipid Res 1973;14:625–631 [PubMed] [Google Scholar]
- 17.Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254 [DOI] [PubMed] [Google Scholar]
- 18.Nepokroeff CM, Lakshmanan MR, Porter JW: Fatty-acid synthase from rat liver. Methods Enzymol 1975;35:37–44 [DOI] [PubMed] [Google Scholar]
- 19.Ochoa S: Malic enzyme: malic enzymes from pigeon and wheat germ. In: Methods in Enzymology (Colowick SP, Kaplan NO, eds.) Academic Press, New York, 1995, pp. 323–326 [Google Scholar]
- 20.Pitkanen E, Pitkanen O, Uotila L: Enzymatic determination of unbound D-mannose in serum. Eur J Clin Chem Clin Biochem 1997;35:761–766 [DOI] [PubMed] [Google Scholar]
- 21.Walton PA, Possmayer F: Mg2+-dependent phosphatidate phosphohydrolase of rat lung: development of an assay employing a defined chemical substrate which reflects the phosphohydrolase activity measured using membrane-bound substrate. Anal Biochem 1985;151:479–486 [DOI] [PubMed] [Google Scholar]
- 22.Markwell MAK, McGroarty EJ, Bieber LL, et al. : The subcellular distribution of carnitine acyltransferases in mammalian liver and kidney. J Biol Chem 1973;248:3426–3432 [PubMed] [Google Scholar]
- 23.Lazarow PB: Assay of peroxisomal β-oxidation of fatty acids. Method Enzymol 1981;72:315–319 [DOI] [PubMed] [Google Scholar]
- 24.Shapiro DJ, Nordstrom JL, Mitschelen JJ: Microassay for 3-hydroxy-3-methyl glutaryl CoA reductase in rat liver and in L-cell fibroblast. Biochim Biophys Acta 1974;370:369–377 [DOI] [PubMed] [Google Scholar]
- 25.Gillies PJ, Rathgeb KA, Robinson CS: Regulation of acyl-CoA:cholesterol acyltransferase activity in normal and atherosclerotic rabbit aortas: Role of a cholesterol substrate pool. Exp Mol Pathol 1986;44:320–339 [DOI] [PubMed] [Google Scholar]
- 26.Paigen B, Plump AS, Rubin EM: The mouse as a model for human cardiovascular disease and hyperlipidemia. Curr Opin Lipidol 1994;5:258–264 [DOI] [PubMed] [Google Scholar]
- 27.Macarulla MT, Alberdi G, Gómez S, et al.: Effects of different doses of resveratrol on body fat and serum parameters in rats fed a hypercaloric diet. J Physiol Biochem 2009;65:369–376 [DOI] [PubMed] [Google Scholar]
- 28.Rivera L, Morón R, Zarzuelo A, et al. : Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol 2009;77:1053–1063 [DOI] [PubMed] [Google Scholar]
- 29.Shang J, Chen LL, Xiao FX: Resveratrol improves high-fat induced nonalcoholic fatty liver in rats. Zhonghua Gan Zang Bing Za Zhi 2008;16:616–619 [PubMed] [Google Scholar]
- 30.Cho IJ, Ahn JY, Kim S, et al. : Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem Biophys Res Commun 2008;367:190–194 [DOI] [PubMed] [Google Scholar]
- 31.Soleas GJ, Diamandis EP, Goldberg DM: Resveratrol: a molecule whose time has come? And gone? Clin Biochem 1997;30:91–113 [DOI] [PubMed] [Google Scholar]
- 32.Goldberg DM, Hahn SE, Parkes JG: Beyond alcohol: beverage consumption and cardiovascular mortality. Clin Chem Acta 1995;237:155–187 [DOI] [PubMed] [Google Scholar]
- 33.Reagan-Shaw S, Nihal M, Ahmad N: Dose translation from animal to human studies revisited. FASEB J 2007;22:659–661 [DOI] [PubMed] [Google Scholar]
- 34.Penumathsa SV, Thirunavukkarasu M, Koneru S, et al.: Statin and resveratrol in combination induces cardioprotection against myocardial infarction in hypercholesterolemic rat. J Mol Cell Cardiol 2007;42:508–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sacks FM, Tonkin AM, Shepherd J, et al. : Effect of pravastatin on coronary disease events in subgroups defined by coronary risk factors: the Prospective Pravastatin Pooling Project. Circulation 2000;102:1893–1900 [DOI] [PubMed] [Google Scholar]
- 36.Grundy SM, Hansen B, Smith SC Jr, et al. : American Heart Association; National Heart, Lung, and Blood Institute; American Diabetes Association. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation 2004;109:551–556 [DOI] [PubMed] [Google Scholar]
- 37.Do GM, Kwon EY, Kim HJ, et al. : Long-term effects of resveratrol supplementation on suppression of atherogenic lesion formation and cholesterol synthesis in apo E-deficient mice. Biochem Biophys Res Commun 2008;374:55–59 [DOI] [PubMed] [Google Scholar]
- 38.Ingelsson E, Massaro JM, Sutherland P, et al. : Contemporary trends in dyslipidemia in the Framingham Heart Study. Arch Intern Med 2009;169:279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aubin MC, Lajoie C, Clément R, et al.: Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia: therapeutic potential of resveratrol. J Pharmacol Exp Ther 2008;325:961–968 [DOI] [PubMed] [Google Scholar]
- 40.Baur JA, Pearson KJ, Price NL, et al. : Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006;444:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu H, Xu HE, Ryan D: A study of the comparative effects of hawthorn fruit compound and simvastatin on lowering blood lipid levels. Am J Chin Med 2009;37:903–908 [DOI] [PubMed] [Google Scholar]
- 42.Steinmetz EF, Buckley C, Shames ML, et al. : Treatment with simvastatin suppresses the development of experimental abdominal aortic aneurysms in normal and hypercholesterolemic mice. Ann Surg 2005;241:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rayalam S, Yang JY, Ambati S, et al. : Resveratrol induces apoptosis and inhibits adipogenesis in 3T3-L1 adipocytes. Phytother Res 2008;22:1367–1371 [DOI] [PubMed] [Google Scholar]
- 44.Park HJ, Yang JY, Ambati S, et al. : Combined effects of genistein, quercetin, and resveratrol in human and 3T3-L1 adipocytes. J Med Food 2008;11:773–783 [DOI] [PubMed] [Google Scholar]
- 45.Gnoni GV, Paglialonga G: Resveratrol inhibits fatty acid and triacylglycerol synthesis in rat hepatocytes. Eur J Clin Invest 2009;39:211–218 [DOI] [PubMed] [Google Scholar]
- 46.Penumathsa SV, Thirunavukkarasu M, Zhan L, et al.: Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J Cell Mol Med 2008;12:2350–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hardie DG, Pan DA: Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans 2002;30:1064–1070 [DOI] [PubMed] [Google Scholar]
- 48.Pallottini V, Montanari L, Cavallini G, et al. : Mechanisms underlying the impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in aged rat liver. Mech Ageing Dev 2004;125:633–639 [DOI] [PubMed] [Google Scholar]
- 49.Poulsen MM, Larsen JØ, Hamilton-Dutoit S, et al. : Resveratrol up-regulates hepatic uncoupling protein 2 and prevents development of nonalcoholic fatty liver disease in rats fed a high-fat diet. Nutr Res 2012;32:701–708 [DOI] [PubMed] [Google Scholar]
- 50.Seifalian AM, Piasecki C, Agarwal A, et al.: The effect of graded steatosis on flow in the hepatic parenchymal microcirculation. Transplantation 1999;68:780–784 [DOI] [PubMed] [Google Scholar]
- 51.Kinlay S, Libby P, Ganz P: Endothelial function and coronary artery disease. Curr Opin Lipidol 2001;12:383–389 [DOI] [PubMed] [Google Scholar]
- 52.Ekshyyan VP, Hebert VY, Khandelwal A, et al. : Resveratrol inhibits rat aortic vascular smooth muscle cell proliferation via estrogen receptor dependent nitric oxide production. J Cardiovasc Pharmacol 2007;50:83–93 [DOI] [PubMed] [Google Scholar]
- 53.Park DW, Baek K, Kim JR, et al. : Resveratrol inhibits foam cell formation via NADPH oxidase 1-mediated reactive oxygen species and monocyte chemotactic protein-1. Exp Mol Med 2009;41:171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ridker PM, Rifai N, Pfeffer MA, et al. : Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation 1998;98:839–844 [DOI] [PubMed] [Google Scholar]
- 55.Delanty N, Vaughan CJ: Vascular effects of statins in stroke. Stroke 1997;28:2315–2320 [DOI] [PubMed] [Google Scholar]