Abstract
Psychiatric disturbance is common and disabling after traumatic brain injury (TBI). Few studies have investigated the trajectory of psychiatric symptoms in the first 6 months postinjury, when monitoring and early treatment might prevent persistent difficulties. The aim of this study was to examine the trajectory of psychiatric symptoms 1–6 months post-TBI, the patient/injury characteristics associated with changes, and characteristics predictive of persisting symptoms. A secondary analysis was performed on data from a clinical trial with three data collection points. Across eight centers, 872 participants with complicated mild to severe TBI were administered the Brief Symptom Inventory (BSI) at 30, 90, and 180 days postinjury. Mixed-effects models were used to assess longitudinal changes in the BSI Global Severity Index (GSI). Multi-variate logistic regression was used to assess predictors of clinically significant GSI elevations persisting to 6 months post-TBI. In general, GSI scores improved over time. Women improved faster than men; race/ethnicity was also significantly associated with rate of change, with Hispanics showing the most and African Americans the least improvement. Clinically significant psychiatric symptoms (caseness) occurred in 42% of the sample at 6 months, and more than one type of symptom was common. Significant predictors of caseness included African American race, age from 30 to 60 years, longer post-traumatic amnesia (PTA) duration, pre-TBI unemployment, and pre-TBI risky alcohol use. Findings indicate that psychiatric symptoms are common in the first 6 months post-TBI and frequently extend beyond the depression and anxiety symptoms that may be most commonly screened. Patients with longer PTA and preinjury alcohol misuse may need more intensive monitoring for symptom persistence.
Key words: : longitudinal studies, neurobehavioral signs and symptoms, psychological outcome, traumatic brain injury
Introduction
Psychiatric disturbance is a significant problem after traumatic brain injury (TBI). In addition to the effect of disorders such as anxiety and depression on quality of life, psychiatric difficulties negatively affect many social outcomes as well as functional independence.1–3 Depression is among the most common and well-studied of the psychiatric sequelae, affecting people with TBI at about 8 times the rate in the general population during the first year postinjury.3 Also common are anxiety disorders4 and anger and irritability.5,6 Other frequently reported symptoms include lability, apathy, and delusional thinking.7–9
To prevent and treat these difficulties, it is important to understand both the time course of emotional disturbance and the risk factors predictive of longer-term problems. Of the few studies examining the longitudinal trajectory of psychiatric symptoms after TBI, some report improvement over the first 1–2 years.8,10 However, in more than 1000 cases followed 2 years from the point of injury, Hart and colleagues found substantial persistence of depression symptoms from year 1 to year 2, and more than 25% of persons who were not depressed at year 1 had developed clinically significant symptoms by year 2.11 That study and another longitudinal examination of depression after TBI found that averaged trends masked highly varied patterns of change, including worsening, improvement, and stability.12 Evidence on the longitudinal trajectory of symptoms other than depression is lacking. In addition, there are few data bearing on the trajectory of psychiatric symptoms in the first 6 months after injury, when prevention efforts might be most relevant.
Earlier studies have found that preinjury psychiatric problems, including alcohol abuse, predict psychiatric difficulties after injury.2,13 However, new-onset psychiatric disturbances affect significant numbers of people with TBI who have no psychiatric history.3,13 A recent systematic review found compelling evidence of an association between TBI and both depression and aggression, controlling for premorbid status, and suggestive evidence for an association between TBI and psychosis as well as completed suicide.14
Other demographic risk factors for post-traumatic depression and/or anxiety have included female gender, younger age, lower education, and preinjury unemployment.2,10,15 The effects of severity of TBI are uncertain. Studies using the Glasgow Coma Scale (GCS) score to estimate severity have generally not found an association to later psychiatric symptoms.3,16 Dikmen and colleagues10 found an inverse relationship between length of coma, or time to follow commands, and severity of depression within a few months of injury, but no relationship at 12- or 18-month follow-up. Injury severity may be related to psychiatric outcomes through the effect of brain damage on cognitive function, because cognitive deficits have been associated with psychiatric sequelae.8,15,16
In the current study, participants with TBI were assessed on a wide range of psychiatric symptoms at three time points during the first 6 months postinjury. Two main questions were addressed:
1. What is the trajectory of psychiatric symptoms from 1 to 6 months post-TBI, and what patient factors are associated with change within this early time frame?
2. What factors predict clinically significant psychiatric disturbance persisting to 6 months post-TBI?
Methods
Participants
Participants comprised a subset of the 1213 participants enrolled in the National Institutes of Health (NIH)-funded Citicoline for Brain Injury Treatment (COBRIT) trial.17 This was a double-blind, placebo-controlled, phase III trial conducted in eight clinical centers in which patients with complicated mild, moderate, and severe TBI were evaluated at 30, 90, and 180 days after injury. COBRIT participants were screened from consecutive emergency department admissions for TBI. Of 2738 eligible patients, 1213 (44%) entered the trial; the others refused or were unable to provide consent directly or by proxy within the stipulated time frame. Enrolled participants were randomly assigned to receive a 90-day regimen of 2000 mg/day of either citicoline or placebo started within 24 h of injury. The primary analysis revealed that citicoline and placebo groups did not differ on cognitive or functional status at any assessment interval.17 Further analysis revealed no significant group differences on the outcomes examined in the present study; therefore, the drug and placebo groups were collapsed into one participant pool.
According to COBRIT eligibility criteria, all participants were 18–70 years of age and had acute nonpenetrating TBI with GCS score off paralytics 3–12 or 13–15 with trauma-related computed tomography abnormality meeting a specific criterion for lesion size.17 Participants were excluded for bilaterally fixed and dilated pupils, nonfluency in English, or preinjury disease likely to affect outcomes (e.g., dementia or schizophrenia). For the current study, we selected COBRIT participants who had provided data on the dependent measure described below, the Brief Symptom Inventory (BSI),18 and for whom data were complete on all predictor variables. For Question 1, participants had completed at least one BSI at any of the three outcome intervals (N=872). For Question 2, participants had completed the BSI at 180 days (N=711). We compared participants included in each analysis with those who were excluded as a result of missing data on predictor variables (8% of the sample for Question 1 and 4% for Question 2) or missing/incomplete data on the BSI (20% of the sample for Question 1 and 37% for Question 2). Cognitive inability to complete self-report measures accounted for 43% (n=203) of the missing data at 30 days, 14% (n=57) at 90 days, and 7% (n=31) at 180 days; the remainder of missing data points was the result of loss to follow-up. In both analyses, participants who were not included were significantly less educated, more likely to be male, and more likely to have sustained moderate/severe versus complicated mild TBI.
Measures
Demographic variables included age, sex, race/ethnicity, preinjury employment status, and education. Injury variables included mechanism and severity of TBI. Severity was estimated using GCS score at time of randomization to COBRIT groups (13–15, complicated mild vs. <13, moderate/severe) and duration of post-traumatic amnesia (PTA). PTA was measured retrospectively using a structured interview19 in which a trained data collector assisted the participant in estimating the number of days or, if <1 day, the number of hours, between the TBI and the resumption of continuous recall of events. PTA duration was categorized as follows: <24 h, 1–6 days, 7–13 days, 14–29 days, and ≥30 days. Alcohol use in the year before injury was assessed with the Alcohol Use Disorders Identification Test (AUDIT),20 with the score dichotomized at the cutoff for problematic drinking (<8, ≥8).
The COBRIT trial included a battery of measures administered at 30, 90, and 180 days after injury.21 The BSI was selected to measure psychiatric status, because it has sound psychometric properties, including excellent stability over time,22 and predicts real-world outcomes, such as rehospitalization.23 The original scale and its short form have been well validated in studies of TBI.24,25 The BSI includes 53 items describing psychiatric symptoms for which the respondent is asked to rate “how much each problem has distressed or bothered you during the past 7 days, including today,” from 0 (not at all) to 4 (extremely). Clinical scales provide summary scores on eight symptom dimensions: Obsessive-Compulsive; Interpersonal Sensitivity; Depression; Anxiety; Hostility; Phobic Anxiety; Paranoid Ideation; and Psychoticism. Raw scores are converted to normalized T scores with a mean of 50 and standard deviation (SD) of 10, with separate norms for men and women. The Global Severity Index (GSI) represents an average of the clinical scales and is the BSI's most sensitive, specific single indicator of positive psychiatric diagnosis.18 For Question 1, concerning patterns of longitudinal change in psychiatric distress, GSI T scores from the 30-, 90-, and 180-day assessments were used. For Question 2, concerning predictors of psychiatric disturbance at 6 months post-TBI, BSI scores at the 180-day assessment were dichotomized using criteria for “caseness,” defined as a GSI T score ≥63 or any two clinical scales ≥63.18
Procedures
The protocol was approved by the institutional review boards of all participating sites, and written informed consent was obtained from either participants or their legally authorized representatives (LARs). For those who used a LAR originally, consent for continued involvement was acquired directly from the participant once capacity had improved. Independent oversight was provided by an external data safety monitoring board. All data were stored and analyzed by a data coordinating center at Columbia University (New York, NY).
Demographic variables, GCS, and mechanism of injury were recorded during acute hospitalization. Outcome assessments were performed in person or by phone at 30±7, 90±10, and 180±10 days. PTA interview and AUDIT were administered at 30 days, or 90 days if the participant was cognitively unable to respond reliably at 30 days. All self-report instruments, including the BSI, were administered by interview, with study personnel recording each response. This was done to prevent missing items and ensure comparability of data collected by phone and in person. Instruments assessing social or emotional status, including the BSI, were not given to participants who were disoriented to time, place, or circumstances. All data were double scored by an investigator at each site before being entered into the study database. Computerized scoring was used to generate BSI T scores and flag unusual response patterns (e.g., providing the most extreme answer for each item) so that such data could be discarded as invalid.
Statistical analysis
For Question 1, mixed-effects models with random intercepts were used to measure longitudinal change in GSI T scores between 30-, 90-, and 180-day assessments. A missing at random (MAR) mechanism was assumed for measurements that were missing at any of the three time points. Mixed-effects models provide unbiased estimates under the MAR mechanism. We examined whether any predictor modified the rate of change in GSI after adjustment for other statistically significant predictors. Effect modification was evident when a statistically significant interaction, predictor x time, was observed in the multi-variate mixed-effects model. Akaike's Information Criterion (AIC) was used to evaluate the fit of the multi-variate models. A spatial power covariance structure was applied to account for the decreasing correlation between repeated measures of GSI with increasing time while taking into consideration the unequal spacing between time points.
For Question 2, preliminary analyses were conducted to test for associations between each predictor and caseness at 180 days using Fisher's exact test or Cochran-Armitage's trend test. Predictors significantly associated with caseness (at α=0.05) were further assessed using multi-variate logistic regression. A multiple logistic regression model was built and evaluated for goodness of fit based on the likelihood ratio statistic. All statistical analyses were conducted in SAS 9.2 statistical software (SAS Institute Inc., Cary, NC).
Results
Question 1: Longitudinal changes in psychiatric symptoms
Participants included in Question 1 (N=872) were 74% male with a mean age of 40.2±15.9 years. They were 82% white, 14% African American, and 4% Hispanic. There were 71% with GCS 13–15 with positive neuroimaging, 3% with GCS 9–12, and 26% with GCS ≤8. Of these participants, 548 (63%) completed all three BSI assessments, 236 (27%) completed two, and 88 (10%) completed one.
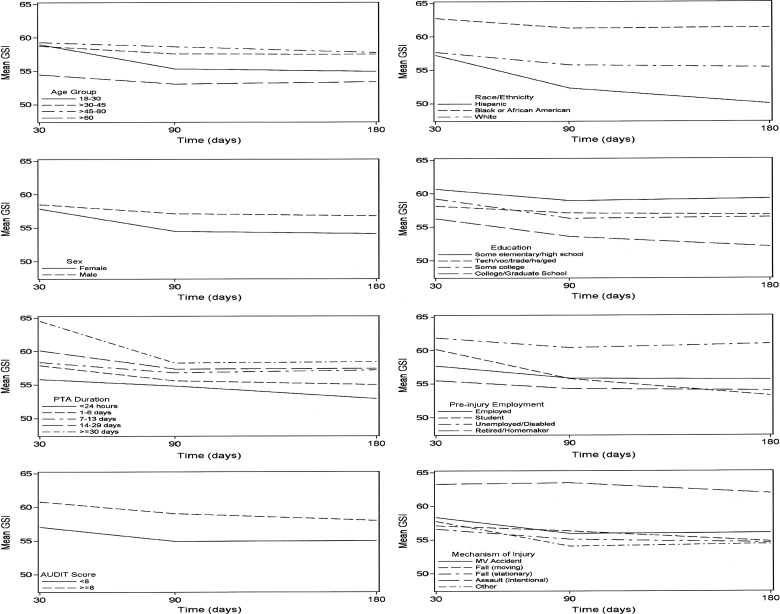
Univariate analyses showed that the average value of GSI was 58.59 (95% confidence interval [CI], 57.75–59.42) at 30 days and improved by about 2.5 points (β=−2.31; 95% CI, −2.95 to −1.67) by 180 days, with more than 90% of the observed improvement occurring between 30 and 90 days (β=−2.51; 95% CI, −3.32 to −1.70). Figure 1 displays the unadjusted results for each predictor variable. Results of the multi-variate model are presented in Table 1. Significant modification of the rate of change in GSI was observed with respect to race/ethnicity and sex. Although racial/ethnic groups did not differ on GSI at 30 days, Hispanics improved significantly faster from 30 to 180 days and African Americans improved significantly less, both compared to whites. Males and females were similar in GSI at 30 days, yet the rate of improvement was faster for females from 30 to 180 days.
FIG. 1.
Longitudinal effects of patient and injury characteristics (unadjusted) on Brief Symptom Inventory (BSI) General Severity Index (GSI) from 30 to 180 days post TBI. PTA, post-traumatic amnesia; AUDIT, Alcohol Use Disorders Identification Test; MV, motor vehicle.
Table 1.
Best-Fit Multi-Variate Mixed-Effects Model of Longitudinal Change in GSI between 30 and 180 Daysa
| Variable | β (95% CI) |
|---|---|
| Intercept | 51.94 (48.68–55.19) |
| Time (days) | |
| 30 | 0.00 (ref) |
| 90 | −1.95 (−2.76 to −1.14) |
| 180 | −2.20 (−3.22 to −1.18) |
| Age (years) | |
| 18–30 | 0.00 (ref) |
| >30–45 | 2.19 (0.25–4.13) |
| >45–60 | 3.79 (1.90–5.67) |
| >60 | 0.72 (−1.95–3.39) |
| Race/ethnicity | |
| White | 0.00 (ref) |
| Black/African American | 1.73 (−0.71–4.16) |
| Hispanic | −0.16 (−4.11–3.79) |
| Race/ethnicity*time (days) | |
| White (90) | 0.00 (ref) |
| African American (90) | 1.82 (−0.05–3.70) |
| Hispanic (90) | −4.66 (−7.88 to −1.45) |
| White (180) | 0.00 (ref) |
| African American (180) | 2.61 (0.26–4.97) |
| Hispanic (180) | −5.43 (−9.64 to −1.22) |
| Sex | |
| Male | 0.00 (ref) |
| Female | 0.80 (−1.10–2.70) |
| Sex*time (days) | |
| Male (90) | 0.00 (ref) |
| Female (90) | −1.88 (−3.32 to −0.44) |
| Male (180) | 0.00 (ref) |
| Female (180) | −1.98 (−3.78 to −0.17) |
| Education | |
| <High school | 0.00 (ref) |
| High school/GED/technical | −0.62 (−2.91–1.66) |
| Some college | −0.07 (−2.59–2.45) |
| ≥College degree | −2.66 (−5.33–0.01) |
| PTA duration | |
| <24 h | 0.00 (ref) |
| 1–6 days | 2.48 (0.40–4.55) |
| 7–13 days | 3.46 (1.09–5.83) |
| 14–29 days | 4.51 (2.09–6.93) |
| ≥30 days | 5.67 (3.18–8.16) |
| Preinjury employment status | |
| Employed | 0.00 (ref) |
| Student | 0.65 (−2.09–3.40) |
| Unemployed/disabled | 3.22 (1.19–5.24) |
| Retired/homemaker | 0.52 (−2.43–3.46) |
| Alcohol use | |
| AUDIT <8 | 0.00 (ref) |
| AUDIT ≥8 | 3.30 (1.79–4.82) |
| Mechanism of injury | |
| Motor vehicle collision | 0.00 (ref) |
| Fall from moving object | 1.11 (−1.26–3.48) |
| Fall from stationary object | 0.11 (−1.75–1.97) |
| Assault | 4.52 (1.99–7.04) |
| Other | −0.82 (−4.33–2.68) |
| AICb | 16,022.9 |
n=872.
AIC for intercept only model=16,304.2.
GSI, General Severity Index; GED, General Educational Development; PTA, post-traumatic amnesia; AUDIT, Alcohol Use Disorders Identification Test; AIC, Akaike's Information Criterion; CI, confidence interval; ref, reference.
Age, PTA duration, preinjury employment status, alcohol use, mechanism of injury, and education did not modify the rate of change in GSI over time. However, GSI was worse, on average, for >30–45 and >45–60 year olds (but not those >60), compared to those 18–30. Participants who were unemployed or disabled before TBI had higher GSI than those who were employed. GSI was higher among participants with AUDIT ≥8, compared with AUDIT <8. Regarding mechanism of injury, GSI was significantly worse only among participants injured by assault (compared to the reference group, motor vehicle collision). PTA duration ≥24 h was associated with higher GSI than duration <24 h, and progressively longer PTA was associated with progressively higher GSI (Table 1).
Question 2: Psychiatric outcomes at 180 days
Participants included in Question 2 (N=711) were 73% male with a mean age of 40.6±16.0 years. They were 83% white, 13.5% African American, and 3.5% Hispanic; injury severity distribution was identical to that for Question 1.
Summary statistics for 30-, 90-, and 180-day T scores of the nine BSI clinical scales and the GSI are displayed in Table 2. Even at 180 days, clinical scale means were all elevated, relative to those of the normative population, from less than 0.3 SD (interpersonal sensitivity, anxiety) to nearly 1 SD (obsessive-compulsive). Mean GSI was elevated by slightly more than 0.5 SD.
Table 2.
Summary Statistics for T Scores and Proportions Meeting Caseness Criteria for Each BSI Clinical Scale and GSI at 30, 90, and 180 Days
| 30 days (n=713) | 90 days (n=780) | 180 days (n=711) | ||||
|---|---|---|---|---|---|---|
| Scale | Mean±SD | Proportion with score ≥63 (%) | Mean±SD | Proportion with score ≥63 (%) | Mean±SD | Proportion with score ≥63 (%) |
| Somatization | 59.9±10.9 | 41.9 | 56.3±10.8 | 26.8 | 55.3±11.0 | 24.8 |
| Obsessive-compulsive | 59.9±11.6 | 42.6 | 59.1±11.6 | 36.5 | 59.3±12.2 | 40.5 |
| Interpersonal Sensitivity | 52.4±10.4 | 22.0 | 52.3±10.4 | 21.9 | 52.6±10.9 | 24.3 |
| Depression | 55.5±10.7 | 25.1 | 54.6±10.9 | 24.4 | 54.8±11.5 | 25.0 |
| Anxiety | 54.3±12.0 | 26.9 | 52.8±12.3 | 25.8 | 52.5±12.1 | 24.5 |
| Hostility | 53.5±10.9 | 16.6 | 52.4±11.0 | 16.8 | 53.3±11.1 | 18.3 |
| Phobic anxiety | 55.8±10.7 | 30.9 | 53.9±10.0 | 24.0 | 53.8±10.2 | 25.5 |
| Paranoid ideation | 53.1±11.1 | 23.7 | 53.5±10.7 | 24.1 | 53.8±11.2 | 25.3 |
| Psychoticism | 57.4±11.0 | 30.2 | 57.0±11.4 | 28.5 | 56.6±11.2 | 27.7 |
| GSI | 58.3±11.2 | 33.4 | 56.4±11.9 | 30.0 | 56.0±12.5 | 30.7 |
BSI, Brief Symptom Inventory; GSI, General Severity Index; SD, standard deviation.
A total of 301 (42%) participants met the caseness definition on the GSI at 180 days. Of these, slightly more than half (169; 56%) had elevations on five or more of the clinical scales. Table 3 shows the proportions of participants above and below the GSI caseness cutoff relative to the predictor variables of interest. Only the GCS score classification of injury severity failed to show significant differences between caseness groups. All other variables were associated with caseness and thus included in logistic regression analyses.
Table 3.
Characteristics of Participants by Caseness Level at 180 Daysa
| Characteristics | GSI <63 and ≤1 elevated symptom (%) (n=410) | GSI ≥63 or ≥2 elevated symptoms (%) (n=301) | p value* |
|---|---|---|---|
| Age (years) | 0.014 | ||
| 18–30 | 151 (60.9) | 97 (39.1) | |
| >30–45 | 78 (51.3) | 74 (48.7) | |
| >45–60 | 109 (52.9) | 97 (47.1) | |
| >60 | 72 (68.6) | 33 (31.4) | |
| Sex | 0.008 | ||
| Female | 127 (65.8) | 66 (34.2) | |
| Male | 283 (54.6) | 235 (45.4) | |
| Race/ethnicity | <0.001 | ||
| White | 356 (60.2) | 235 (39.8) | |
| African American | 37 (38.5) | 59 (61.5) | |
| Hispanic | 17 (70.8) | 7 (29.2) | |
| Education | <0.001† | ||
| <High school | 36 (43.4) | 47 (56.6) | |
| High school/GED/technical | 165 (55.2) | 134 (44.8) | |
| Some college | 109 (59.2) | 75 (40.8) | |
| ≥College degree | 100 (69.0) | 45 (31.0) | |
| GCS severity | 0.50 | ||
| Moderate and severe | 125 (59.8) | 84 (40.2) | |
| Complicated mild | 285 (56.8) | 217 (43.2) | |
| PTA duration | 0.001† | ||
| <24 h | 79 (67.0) | 39 (33.0) | |
| 1–6 days | 135 (61.4) | 85 (38.6) | |
| 7–13 days | 72 (55.8) | 57 (44.2) | |
| 14–29 days | 63 (54.8) | 52 (45.2) | |
| ≥30 days | 61 (47.3) | 68 (52.7) | |
| Preinjury employment status | <0.001 | ||
| Employed | 287 (58.8) | 201 (41.2) | |
| Student | 42 (65.6) | 22 (34.4) | |
| Unemployed/disabled | 40 (40.0) | 60 (60.0) | |
| Retired/homemaker | 41 (69.5) | 18 (30.5) | |
| Alcohol use | 0.003 | ||
| AUDIT <8 | 285 (61.7) | 177 (38.3) | |
| AUDIT ≥8 | 125 (50.2) | 124 (49.8) | |
| Mechanism of injury | 0.023 | ||
| Motor vehicle collision | 218 (57.5) | 161 (42.5) | |
| Fall from moving object | 50 (61.0) | 32 (39.0) | |
| Fall from stationary object | 100 (64.1) | 56 (35.9) | |
| Assault | 25 (39.7) | 38 (60.3) | |
| Other | 17 (54.8) | 14 (45.2) |
n=711.
Fisher's exact test p value, unless otherwise specified.
Cochran-Armitage's trend test p value.
GED, General Educational Development; GCS, Glasgow Coma Scale; PTA, post-traumatic amnesia; AUDIT, Alcohol Use Disorders Identification Test; GSI, General Severity Index.
After adjustment for all predictors, sex and mechanism of injury were no longer associated with caseness in the multi-variate analysis (Table 4). Participants with ages >30–45 and >45–60 years, but not those >60, had increased odds of caseness, compared to participants in the youngest age group. African Americans had 1.81-fold higher odds of caseness, compared to whites. A lower odds of caseness was observed among Hispanics, compared to whites, but this did not reach significance. The odds of caseness were significantly lower for college graduates, compared to those with less than a high school education.
Table 4.
Multi-Variate Logistic Regression Predicting GSI Caseness at 180 Daysa
| Multi-variate OR (95% CI) | |
|---|---|
| Age | |
| 18–30 | 1.00 (ref) |
| >30–45 | 1.66 (1.05–2.61) |
| >45–60 | 1.69 (1.10–2.61) |
| >60 | 1.27 (0.68–2.36) |
| Sex | |
| Female | 1.00 (ref) |
| Male | 1.35 (0.93–1.97) |
| Race/ethnicity | |
| White | 1.00 (ref) |
| African American | 1.81 (1.11–2.96) |
| Hispanic | 0.54 (0.21–1.36) |
| Education | |
| <High school | 1.00 (ref) |
| High school/GED/technical | 0.77 (0.45–1.31) |
| Some college | 0.76 (0.42–1.36) |
| ≥College degree | 0.49 (0.26–0.91) |
| PTA duration | |
| <24 h | 1.00 (ref) |
| 1–6 days | 1.36 (0.82–2.25) |
| 7–13 days | 1.64 (0.93–2.89) |
| 14–29 days | 1.88 (1.06–3.34) |
| ≥30 days | 2.38 (1.35–4.23) |
| Preinjury employment status | |
| Employed | 1.00 (ref) |
| Student | 0.85 (0.46–1.57) |
| Unemployed/disabled | 1.80 (1.11–2.91) |
| Retired/homemaker | 0.82 (0.41–1.64) |
| Alcohol use | |
| AUDIT <8 | 1.00 (ref) |
| AUDIT ≥8 | 1.45 (1.02–2.05) |
| Mechanism of injury | |
| Motor vehicle collision | 1.00 (ref) |
| Fall from moving object | 1.28 (0.76–2.17) |
| Fall from stationary object | 0.96 (0.62–1.47) |
| Assault | 1.62 (0.87–3.01) |
n=711.
GED, General Educational Development; PTA, post-traumatic amnesia; AUDIT, Alcohol Use Disorders Identification Test; OR, odds ratio; CI, confidence interval; ref, reference.
Compared to participants with PTA duration <24 h, the odds of caseness increased as PTA duration increased, yet statistically significant differences were only observed for the two highest PTA duration groups (14–29 and ≥30 days). Participants who were unemployed or disabled before their TBI had 1.80-fold higher odds of caseness, compared to those who had been employed. Last, participants with AUDIT ≥8 had 1.45-fold higher odds, compared with those with AUDIT <8.
Discussion
We examined the trajectory of psychiatric symptoms over the first 6 months after TBI, and the predictors of persisting symptoms at a clinically significant level (caseness) at 6 months, in a large sample ranging from complicated mild to severe injury. There was overall improvement in psychiatric symptoms, with most change occurring early (from 30 to 90 days). However, over 40% of the sample met criteria for caseness at 6 months, confirming previous work showing a high rate of psychiatric disturbance within the first year post-TBI.3,13 Importantly, when distress was evident, it occurred across multiple symptom domains, consistent with previous work showing 44% comorbidity across psychiatric diagnoses in TBI cases interviewed at least 1 year after injury.26 The present study suggests that psychiatric comorbidity may surface early in the course of recovery and reinforces the importance of evaluating multiple symptoms beyond the commonly used screens for depression and anxiety.
Longitudinal modeling of symptom trajectory revealed a monotonic “dose-response” relationship between psychiatric symptoms and TBI severity, as measured by PTA duration, but not GCS score. To our knowledge, this is the first study to show such a consistent relationship of early psychiatric symptom severity to the extent of diffuse brain injury, as gauged by length of PTA. PTA duration of at least 2 weeks yielded the highest risk of psychiatric morbidity at 6 months. Further work is needed to investigate whether this relationship may be mediated by other factors that could lead to emotional distress, such as severity of cognitive dysfunction or level of functional disability.
The findings for race and ethnicity were striking. Compared to white participants, African Americans showed less recovery of psychiatric symptoms and higher rates of caseness at 6 months, whereas Hispanics showed better recovery and lower rates of caseness (although the latter finding did not reach statistical significance). Our finding of less psychological distress among Hispanics is congruent with studies in the general population showing better health outcomes among this group, despite greater poverty, a finding that has been termed the Hispanic health paradox.27 The paradox is not fully explained, but may result, in part, from greater ethnic identification, religious participation, and strong family and social support.28 With regard to African Americans with TBI, the finding of higher levels of psychiatric symptom reporting has been reported previously.29 Studies have also found worse self-reported functional outcomes among this racial group, even after adjusting for other demographic factors and injury severity.30,31 These perplexing findings may relate to unmeasured sources of vulnerability to stress that are not “factored out” with statistical adjustment, cultural differences in interpretation of symptoms, or both. Future research might benefit from mixed-methods approaches to explore how people from different backgrounds perceive different symptoms and levels of functional capacity. Our findings should also be replicated in future work because of the relatively low numbers of nonwhite participants contributing to the observed interactions.
Previous research on TBI has suggested that a cluster of demographic factors additional to, but partly correlated with, race, including low level of education, preinjury unemployment, and assault as the mechanism of injury, are associated with less-favorable outcomes.1,10,32,33 Our results are generally consistent with these findings and reinforce that the presence of such characteristics should prompt clinicians to closely monitor levels of emotional distress. This is even more important when there is evidence of preinjury alcohol misuse, which consistently predicts unfavorable psychiatric outcomes, such as depression.3,10,29 Alcohol and TBI may interact in a “vicious cycle” in which hazardous use may predispose to depression, and depression may lead to alcohol relapse.34 Given the high rate of pre- and postinjury alcohol misuse in the TBI population, it is important to prevent relapse after TBI and monitor for psychiatric sequelae with greater vigilance in that subgroup.
In contrast to the well-recognized adverse effects of increasing age on functional outcomes of TBI,35 emotional distress peaked in middle age in this sample and older age was associated with less symptom report. This is consistent with previous findings of fewer post-traumatic symptoms reported by older, compared to younger, persons with TBI5 and is also consistent with lower prevalence rates of mood disorders in older age in the general population.36 However, our finding of better emotional outcomes in females was not consistent with national findings nor with most previous TBI literature.3,32,37,38 One explanation for this discrepancy may be our use of a gender-corrected instrument to measure psychiatric symptoms. It may be that males are actually more distressed after TBI than females, relative to their normative counterparts. If so, it reinforces the importance of using appropriate controls or corrections for demographic factors when drawing conclusions about effects of TBI.
Several limitations of this study should be noted. Aside from preinjury alcohol use, we did not assess for premorbid psychiatric disorders, a known risk factor for post-TBI symtpoms.13 However, many symptoms of depression and anxiety, in particular, arise de novo after TBI and show poor resolution,39 emphasizing the importance of screening for these disorders regardless of premorbid history. Another limitation is that there were no data available on treatment received (rehabilitation or psychiatric treatment) during the study interval. Clearly, improvement or lack thereof over a 6-month period could be affected by treatment. We also lacked a control group of people who had sustained traumatic injury without brain injury; thus, whereas we can draw conclusions based on the norms for the BSI, we cannot fully separate the effects of injury to the brain from those of general trauma. It should be noted that, with the BSI, we were measuring symptom severity, rather than specific psychiatric diagnoses. Future research should also consider the issue of symptom validity and possible over-reporting, which was not measured here. Finally, whereas we included participants with a wide range of TBI severity, those at both ends of the spectrum were excluded: The COBRIT trial did not enroll participants with uncomplicated mild TBI, and participants with severe limitations were unable to complete a self-report measure of emotional distress.
Despite these limitations, our findings confirm that there is a high rate of clinically significant psychiatric disturbance in the first 6 months after TBI, and that symptoms occur both within and outside the typically screened depression and anxiety clusters. People with longer PTA duration and preinjury alcohol misuse should be monitored closely for symptom development. Further research should investigate the reasons for disparities in symptom report among persons with TBI from different racial/ethnic groups. In future, our understanding of symptom development and expression will undoubtedly be strengthened by inclusion of additional predictive factors, such as genomic data and information from sensitive neuroimaging methods.
Acknowledgments
This work was funded by Cooperative Agreement #U01 HD042738 from the NIH (National Institute of Child Health and Human Development/National Center for Medical Rehabilitation Research).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Whelan-Goodinson R., Ponsford J.L., Schonberger M., and Johnston L. (2010). Predictors of psychiatric disorders following traumatic brain injury. J. Head Trauma Rehabil. 25, 320–329 [DOI] [PubMed] [Google Scholar]
- 2.Hart T., Brenner L., Clark A.N., Bogner J.A., Novack T.A., Chervoneva I., Nakase-Richardson R., and Arango-Lasprilla J.C. (2011). Major and minor depression after traumatic brain injury. Arch. Phys. Med. Rehabil. 92, 1211–1219 [DOI] [PubMed] [Google Scholar]
- 3.Bombardier C.H., Fann J.R., Temkin N.R., Esselman P.C., Barber J., and Dikmen S.S. (2010). Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 303, 1938–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashman T.A., Spielman L.A., Hibbard M.R., Silver J.M., Chandna T., and Gordon W.A. (2004). Psychiatric challenges in the first 6 years after traumatic brain injury: cross-sequential analyses of axis I disorders. Arch. Phys. Med. Rehabil. 85, 4 Suppl. 2, S36–S42 [DOI] [PubMed] [Google Scholar]
- 5.Dikmen S., Machamer J., Fann J.R., and Temkin N.R. (2010). Rates of symptom reporting following traumatic brain injury. J. Int. Neuropsychol. Soc. 16, 401–411 [DOI] [PubMed] [Google Scholar]
- 6.Hanks R.A., Temkin N., Machamer J., and Dikmen S.S. (1999). Emotional and behavioral adjustment after traumatic brain injury. Arch. Phys. Med. Rehabil. 80, 991–997 [DOI] [PubMed] [Google Scholar]
- 7.Ciurli P., Formisano R., Bivona U., Cantagallo A., and Angelelli P. (2011). Neuropsychiatric disorders in persons with severe traumatic brain injury: prevalence, phenomenology, and relationship with demographic, clinical, and functional features. J. Head Trauma Rehabil. 26, 116–126 [DOI] [PubMed] [Google Scholar]
- 8.Dikmen S., and Reitan R.M. (1977). Emotional sequelae of head injury. Ann. Neurol. 2, 492–494 [DOI] [PubMed] [Google Scholar]
- 9.Rapoport M., McCauley S., Levin H., Song J., and Feinstein A. (2002). The role of injury severity in neurobehavioral outcome 3 months after traumatic brain injury. Neuropsychiatry Neuropsychol. Behav. Neurol. 15, 123–132 [PubMed] [Google Scholar]
- 10.Dikmen S.S., Bombardier C.H., Machamer J.E., Fann J.R., and Temkin N.R. (2004). Natural history of depression in traumatic brain injury. Arch. Phys. Med. Rehabil. 85, 1457–1464 [DOI] [PubMed] [Google Scholar]
- 11.Hart T., Hoffman J., Pretz C., Kennedy R., Clark A.N., and Brenner L. (2012). A longitudinal study of major and minor depression following traumatic brain injury. Arch. Phys. Med. Rehabil. 93, 1343–1349 [DOI] [PubMed] [Google Scholar]
- 12.Pagulayan K.F., Hoffman J.M., Temkin N.R., Machamer J.E., and Dikmen S.S. (2008). Functional limitations and depression after traumatic brain injury: examination of the temporal relationship. Arch. Phys. Med. Rehabil. 89, 1887–1892 [DOI] [PubMed] [Google Scholar]
- 13.Gould K.R., Ponsford J.L., Johnston L., and Schonberger M. (2011). The nature, frequency and course of psychiatric disorders in the first year after traumatic brain injury: a prospective study. Psychol. Med. 41, 2099–2109 [DOI] [PubMed] [Google Scholar]
- 14.Hesdorffer D.C., Rauch S.L., and Tamminga C.L. (2009). Long-term psychiatric outcomes following traumatic brain injury: a review of the literature. J. Head Trauma Rehabil. 24, 452–459 [DOI] [PubMed] [Google Scholar]
- 15.Demakis G.J., Hammond F.M., and Knotts A. (2010). Prediction of depression and anxiety 1 year after moderate-severe traumatic brain injury. Appl. Neuropsychol. 17, 183–189 [DOI] [PubMed] [Google Scholar]
- 16.Rapoport M., McCauley S., Levin H., Song J., and Feinstein A. (2002). The role of injury severity in neurobehavioral outcome 3 months after traumatic brain injury. Neuropsychiatry Neuropsychol. Behav. Neurol. 15, 123–132 [PubMed] [Google Scholar]
- 17.Zafonte R.D., Bagiella E., Ansel B.M., Novack T.A., Friedewald W.T., Hesdorffer D.C., Timmons S.D., Jallo J., Eisenberg H., Hart T., Ricker J.H., Diaz-Arrastia R., Merchant R.E., Temkin N.R., Melton S., and Dikmen S.S. (2012). Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT). JAMA 308, 1993–2000 [DOI] [PubMed] [Google Scholar]
- 18.Derogatis L. (1993). Brief Symptom Inventory (BSI): Administration, Scoring and Procedures Manual (4th ed.). NCS Pearson: Minneapolis, MN [Google Scholar]
- 19.Hart T., Vaccaro M., Hays C., and Maiuro R. (2012). Anger self-management training for people with traumatic brain injury: a preliminary investigation. J. Head Trauma Rehabil. 27, 113–122 [DOI] [PubMed] [Google Scholar]
- 20.Saunders J.B., Aasland O.G., Babor T.F., de la Fuente J.R., and Grant M. (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction 88, 791–804 [DOI] [PubMed] [Google Scholar]
- 21.Bagiella E., Novack T.A., Ansel B., Diaz-Arrastia R., Dikmen S., Hart T., and Temkin N. (2010). Measuring outcome in traumatic brain injury treatment trials: recommendations from the traumatic brain injury clinical trials network. J. Head Trauma Rehabil. 25, 375–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long J.D., Harring J.R., Brekke J.S., Test M.A., and Greenberg J. (2007). Longitudinal construct validity of Brief Symptom Inventory subscales in schizophrenia. Psychol. Assess. 19, 298–308 [DOI] [PubMed] [Google Scholar]
- 23.Eisen S.V., Bottonari K.A., Glickman M.E., Spiro A., 3rd, Schultz M.R., Herz L., Rosenheck R., and Rofman E.S. (2011). The incremental value of self-reported mental health measures in predicting functional outcomes of veterans. J. Behav. Health Serv. Res. 38, 170–190 [DOI] [PubMed] [Google Scholar]
- 24.Meachen S.J., Hanks R.A., Millis S.R., and Rapport L.J. (2008). The reliability and validity of the brief symptom inventory-18 in persons with traumatic brain injury. Arch. Phys. Med. Rehabil. 89, 958–965 [DOI] [PubMed] [Google Scholar]
- 25.Wilde E.A., Whiteneck G.G., Bogner J., Bushnik T., Cifu D.X., Dikmen S., French L., Giacino J.T., Hart T., Malec J.F., Millis S.R., Novack T.A., Sherer M., Tulsky D.S., Vanderploeg R.D., and von Steinbuechel N. (2010). Recommendations for the use of common outcome measures in traumatic brain injury research. Arch. Phys. Med. Rehabil. 91, 1650–1660.e1617. [DOI] [PubMed] [Google Scholar]
- 26.Hibbard M.R., Uysal S., Kepler K., Bogdany J., and Silver J. (1998). Axis I psychopathology in individuals with traumatic brain injury. J. Head Trauma Rehabil. 13, 24–39 [DOI] [PubMed] [Google Scholar]
- 27.Morales L.S., Lara M., Kington R.S., Valdez R.O., and Escarce J.J. (2002). Socioeconomic, cultural, and behavioral factors affecting Hispanic health outcomes. J. Health Care Poor Underserved 13, 477–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallo L.C., Penedo F.J., Espinosa de los Monteros K., and Arguelles W. (2009). Resiliency in the face of disadvantage: do Hispanic cultural characteristics protect health outcomes? J. Pers. 77, 1707–1746 [DOI] [PubMed] [Google Scholar]
- 29.Seel R.T., Kreutzer J.S., Rosenthal M., Hammond F.M., Corrigan J.D., and Black K. (2003). Depression after traumatic brain injury: a National Institute on Disability and Rehabilitation Research Model Systems multicenter investigation. Arch. Phys. Med. Rehabil. 84, 177–184 [DOI] [PubMed] [Google Scholar]
- 30.Hart T., O'Neil-Pirozzi T.M., Williams K.D., Rapport L.J., Hammond F., and Kreutzer J. (2007). Racial differences in caregiving patterns, caregiver emotional function and sources of emotional support following traumatic brain injury. J. Head Trauma Rehabil. 22, 122–131 [DOI] [PubMed] [Google Scholar]
- 31.Brown S.A., McCauley S.R., Levin H.S., Contant C., and Boake C. (2004). Perception of health and quality of life in minorities after mild-to-moderate traumatic brain injury. Appl. Neuropsychol. 11, 54–64 [DOI] [PubMed] [Google Scholar]
- 32.Horner M.D., Selassie A.W., Lineberry L., Ferguson P.L., and Labbate L.A. (2008). Predictors of psychological symptoms 1 year after traumatic brain injury: a population-based, epidemiological study. J. Head Trauma Rehabil. 23, 74–83 [DOI] [PubMed] [Google Scholar]
- 33.deRoon-Cassini T.A., Mancini A.D., Rusch M.D., and Bonanno G.A. (2010). Psychopathology and resilience following traumatic injury: a latent growth mixture model analysis. Rehabil. Psychol. 55, 1–11 [DOI] [PubMed] [Google Scholar]
- 34.Horner M.D., Ferguson P.L., Selassie A.W., Labbate L.A., Kniele K., and Corrigan J.D. (2005). Patterns of alcohol use 1 year after traumatic brain injury: a population-based, epidemiological study. J. Int. Neuropsychol. Soc. 11, 322–330 [DOI] [PubMed] [Google Scholar]
- 35.Marquez de la Plata C., Hart T., Hammond F.M., Frol A., Hudak A., Harper C., O'Neil-Pirozzi T., Whyte J., Carlile M., and Diaz-Arrastia R. (2008). Impact of age on long-term recovery from traumatic brain injury. Arch. Phys. Med. Rehabil. 89, 896–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Comorbidity Survey (NCS) and NCS Replication (2005). National Comorbidity Survey. Harvard Medical School. Available at: www.hcp.med.harvard.edu/ncs Accessed April22, 2013
- 37.Glenn M.B., O'Neil-Pirozzi T., Goldstein R., Burke D., and Jacob L. (2001). Depression amongst outpatients with traumatic brain injury. Brain Inj. 15, 811–818 [DOI] [PubMed] [Google Scholar]
- 38.Levin H.S., Brown S.A., Song J.X., McCauley S.R., Boake C., Contant C.F., Brundage S., Goodman H., and Kotrla K. (2001). Depression and posttraumatic stress disorder at three months after mild to moderate traumatic brain injury. J. Clin. Exp. Neuropsychol. 23, 754–769 [DOI] [PubMed] [Google Scholar]
- 39.Whelan-Goodinson R., Ponsford J., Johnston L., and Grant F. (2009). Psychiatric disorders following traumatic brain injury: their nature and frequency. J. Head Trauma Rehabil. 24, 324–332 [DOI] [PubMed] [Google Scholar]