Abstract
Significance: Heme degradation, which was described more than 30 years ago, is still very actively explored with many novel discoveries on its role in various disease models every year. Recent Advances: The heme oxygenases (HO) are metabolic enzymes that utilize NADPH and oxygen to break apart the heme moiety liberating biliverdin (BV), carbon monoxide (CO), and iron. Heme that is derived from hemoproteins can be toxic to the cells and if not removed immediately, it causes cell apoptosis and local inflammation. Elimination of heme from the milieu enables generation of three products that influences numerous metabolic changes in the cell. Critical Issues: CO has profound effects on mitochondria and cellular respiration and other hemoproteins to which it can bind and affect their function, while BV and bilirubin (BR), the substrate and product of BV, reductase, respectively, are potent antioxidants. Sequestration of iron into ferritin and its recycling in the tissues is a part of the homeodynamic processes that control oxidation-reduction in cellular metabolism. Further, heme is an important component of a number of metabolic enzymes, and, therefore, HO-1 plays an important role in the modulation of cellular bioenergetics. Future Directions: In this review, we describe the cross-talk between heme oxygenase-1 (HO-1) and its products with other metabolic pathways. HO-1, which we have labeled Nike, the goddess who personified victory, dictates triumph over pathophysiologic conditions, including diabetes, ischemia, and cancer. Antioxid. Redox Signal. 20, 1709–1722.
Heme Synthesis and Degradation Pathways in Cell Metabolism
The heme degradation pathway is a critical and the only catalytic process that enables removal of toxic heme in cells and tissues. Heme in complex with proteins such as hemoglobin, myoglobin, cytochrome c, cytochrome p450, nitric oxide synthases (NOS), or guanylate cyclase is not dangerous for cells. The heme moiety is critical for protein function and in most, if not all, cases, oxygen and NADPH are required for their activity (1, 91). Free heme can cause oxidative injury when the iron is rapidly lost from the heme porphyrin ring and contributes in the ferrous state toward the generation of reactive oxygen species (ROS). Heme is essential for all aerobic organisms and is synthesized from protoporphyrin IX and ferrous ion (Fig. 1). Heme regulates the expression of many genes, in addition to HO-1. Heme can bind to Bach1, a repressor protein that acts through an Maf recognition element site in the promoter of target gene which inhibits expression (120).
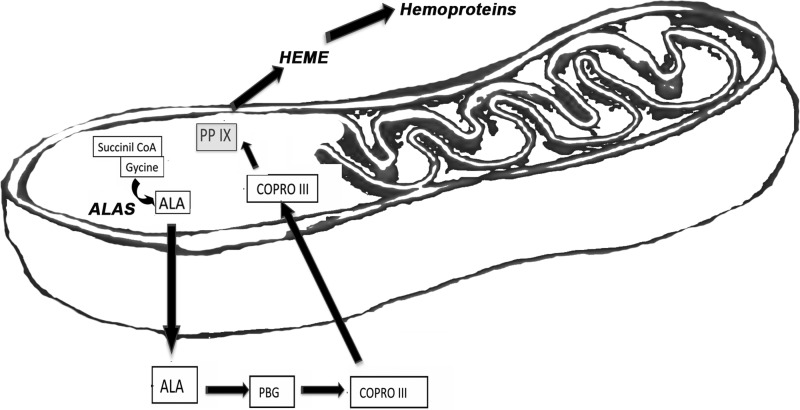
FIG. 1.
Heme synthesis pathway. Heme biosynthesis is catalyzed by 5-aminolevulonate synthase (ALAS). The first step of condensation of glycine and succinyl CoA to 5-aminolevulinic acid (ALA) occurs in mitochondria. Coproporphyrinogen III (COPRO III) is formed as a first porphyrin and transported back to mitochondria from the cytosol, where is gets converted to protoporphyrinogen IX. The final conversion of protoporphyrinogen IX to protoporphyrin IX (PPIX) and insertion of the iron atom into the ring system generates heme b.
The first step of heme biosynthesis in eukaryotic cells is catalyzed by 5-aminolevulonate synthase (ALAS). The ALAS1 isoform is expressed in all tissues and is sensitive to the presence of heme as a negative feedback loop, while ALAS2 is restricted to erythrocytes. These enzymes catalyze the condensation step of glycine and succinyl CoA to 5-aminolevulinic acid (ALA). Heme biosynthesis is initiated in mitochondria from where ALA is transported into the cytosol where the first porphyrin ring is formed, coproporphyrinogen III. ABCB6 then transports this metabolite back into mitochondria for the final steps of synthesis, including decarboxylation of two proprionate residues to protoporphyrinogen IX (Fig. 1). The final conversion of protoporphyrinogen IX to protoporphyrin IX requires oxygen and is followed by the insertion of the iron atom into the porphyrin ring generating heme b. The enzyme catalyzing this reaction is known as ferrochelatase.
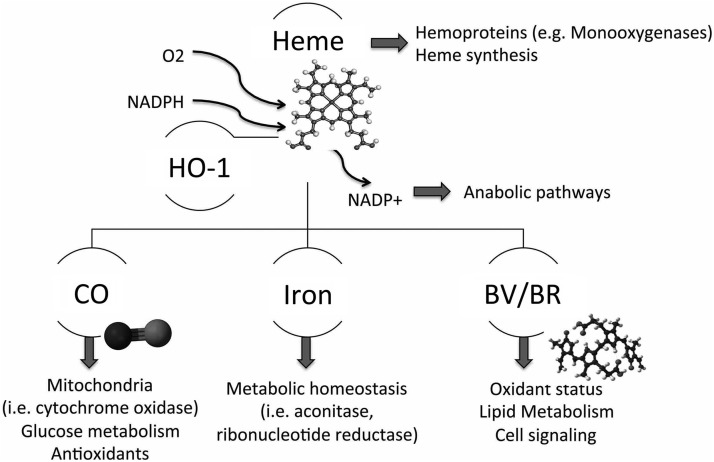
There are two HOs that convert heme to biliverdin (BV) (69). In addition to heme, the HO-1 isoform is induced by oxidative stress, cytokines, heavy metals, and bacterial endotoxins. The second isoform, HO-2, is constitutively expressed in multiple organs with highest expression in the brain and testes (27, 86). Liberation of carbon monoxide (CO) uses one molecule of NADPH, while conversion of BV to bilirubin (BR) consumes an additional two NADPH molecules. NADP+ fuels, in turn, the pentose phosphate pathway and other anabolic processes that enable the regeneration of proton force and generate important molecules for the biosynthesis of nucleic acids and proteins (Fig. 2). HO-1 is a critical member of cellular metabolism, and its activity may influence other NADPH- and oxygen-consuming pathways, including fatty acid synthesis, oxidative metabolism of cytochrome p450, or modulation of ROS generation in phagocytes. All these pathways use a common pool of NADPH, and, therefore, acceleration of one may influence the activity of the others. Another enzyme, HO-2 contains two heme binding sites that enable its action as oxygen sensor (114).
FIG. 2.
Cross-talk of heme degradation pathway and cell metabolism. The heme degradation pathway is the only catalytic process that enables removal of toxic heme in cells and tissues. Heme is a part of hemoproteins and regulates its own synthesis. Generation of carbon monoxide (CO), biliverdin/bilirubin (BV/BR), and iron influences several cellular processes, including glucose lipid and nucleotide metabolism.
One of the main regulators of HO-1 expression is the oxidant responsive transcription factor, nuclear factor (erythroid-derived 2)-like 2 (Nrf2). Nrf2 is critical in rapidly proliferating cells and in the presence of a highly active PI3K-Akt pathway, it redirects glucose and glutamine toward anabolic pathways (75). Among the HO-1 products, CO has been best characterized and shown to promote the shuttling of glucose toward the pentose phosphate pathway rather than the tricarboxylic acid (TCA) cycle (102). However, it is likely that the other heme degradation products, including a 4th product, NADP+, also influence cellular metabolism.
Crosstalk between heme and anabolic pathways
Anabolic pathways that are responsible for generating proteins and nucleic acids require energy from ATP. ATP is generated primarily in mitochondria via oxidative phosphorylation and the electron transport chain and in the cytosol during glycolysis. Both processes can be modulated by CO (23). CO targets mitochondria and modulates the heme-containing cytochrome oxidases, resulting in an increase in ROS production and thereby enabling cytoprotective conditioning of the cell (23, 85). CO is also a competitive inhibitor of the terminal cytochrome oxidase, binding selectively to the reduced binuclear heme a3/CuB center (51). Inhibition of cytochrome oxidase results in suppression of oxidative phosphorylation and lower ATP production that corresponds to enhanced mitochondrial redox signaling. CO suppresses oxidative respiration by 12% under normoxic conditions and at a much higher rate of 70% at 1% oxygen (23, 51). In macrophages, CO-induced mitochondrial-dependent ROS stabilize p38 MAPK and peroxisome proliferator-activated receptor γ (PPARγ) to elicit anti-inflammatory and cytoprotective effects (10, 11, 124). Such transient signaling is beneficial for cellular preconditioning and mitochondria biogenesis where cellular energy demands are increased.
Importantly, CO has been shown to increase the oxygen consumption rate (OCR) in the brain, resulting in a decrease in glucose utilization and decreased lactate production with improved oxidative phosphorylation (5). It is well established that this metabolic switch from glycolysis to anabolic synthesis and increased respiration may be an important preconditioning mechanism leading to long-term protection of the cells in response to secondary insults. Similar to neurons, we have recently found that CO increases the OCR and ROS in cancer cells that correlated with cell cycle inhibition and a concomitant increase in cell death in response to chemotherapeutics (112a). An improvement in respiration in response to CO in astrocytes was accompanied by an increase in OCR, a decrease in lactate production, and a reduction in glucose utilization (5), similar to our observation in cancer cell cultures. Overall, CO improves cellular metabolism with high ATP/ADP ratios and decreases lactate production, suggesting that even when glucose is less utilized, there is still effective ATP production (3).
Heme is a part of numerous metabolic enzymes as a core catalytic unit or as a cofactor in enzymatic processes (e.g., cytochrome p450, iNOS, and guanylate cyclase) (Table 1). Several studies show that HO-2 is important in part, in the control of both gluconeogenesis and glycolysis. HO-2-deficient mice are hyperglycemic and insulin resistant (97). A recent report suggested that HO-1 and HO-2 can interact with the glycolytic enzyme 6-phosphorfructo-2-kinase/fructose-2,6-biphosphates 4 (PFKFB4) (62). Overexpression of HO-2 increased expression of PFKFB4, which was inhibited in responses to glucose deprivation (≤2.5 mM) and occurred concurrently with induction of HO-1 (62).
Table 1.
Heme Types in Hemoproteins and Their Function
| Type of heme | Function of hemoprotein | Hemoprotein |
|---|---|---|
| Heme a | Multiheme enzyme | Cytochrome c oxidase (subunit) |
| Heme b | Electron transfer | Cytochrome b562 |
| Gas transportation/storage | Hemoglobin | |
| Gas transportation/storage and binding of small molecules | Myoglobin | |
| Binding of small molecules | CO-sensing proteins | |
| Enzyme | Cytochrome c peroxidase | |
| Enzyme | Catalase I | |
| Enzyme | Cytochrome P450 | |
| Mitochondrial cytochrome c | ||
| Heme d1 | Enzyme | Cytochrome cd1 nitrite reductase |
| Heme P460 | Multiheme enzyme (c+P460) | Hydroxylamine oxidoreductase |
| SiroHeme | Enzyme | Sulfite reductase |
| Unusual heme | Enzyme | Myeloperoxidase |
Cyclooxygenase (COX) is a heme protein that regulates vascular function and inflammation through the generation of prostaglandin H2 from arachidonic acid. The cross-talk between COX and heme degradation is well established (38). Induction of HO-1 by cobalt protoporphyrin (CoPP) or adenoviral transfer of HO-1 blocks inflammation, including COX-2 expression in response to endotoxin in endothelial cells (94). Overexpression of HO-1 in endothelial cells resulted in a marked decrease in PGE2 and 6-keto PGF1α levels in response to tumor necrosis factor α (TNFα) that correlated with an increase in G1 arrest (57). In the same experiments, the authors confirmed that COX activity increased in cells as measured by the levels of PGI2 and PGE2 in the presence of HO-1 antisense (57). CO induces COX-2 expression and PGE2 production in macrophages in response to endotoxin (66).
Cross-talk between HO-1 and catabolic pathways
Heme degradation belongs to the category of catabolic pathways that generates small molecules and modulates signal transduction. The main catabolic pathway in cells is glucose metabolism. Carbohydrates, including glucose, are the major short-term fuel found in cells, as these are simpler to metabolize than proteins and fat when there is a need for an immediate energy source. Therefore, the control of glucose metabolism is critical for cell and tissue homeostasis (Fig. 3). Induction of HO-1 or application of CO or BR leads to inhibition of high blood glucose as well as to all diabetic-associated complications, including nephropathy, endothelial cell dysfunction, and insulin resistance (2). CO potentiates glucose-stimulated insulin secretion, which is accompanied by an increase in the acid α-glucoside hydrolases and partially dependent on iNOS and cGMP signaling (77). Similarly, application of BV or BR inhibits NADPH-dependent superoxide production and decreases glucose levels in a db/db diabetic mouse model, (30) which could be explained, in part, as an ROS scavenging mechanism or via direct effects on cell metabolism.
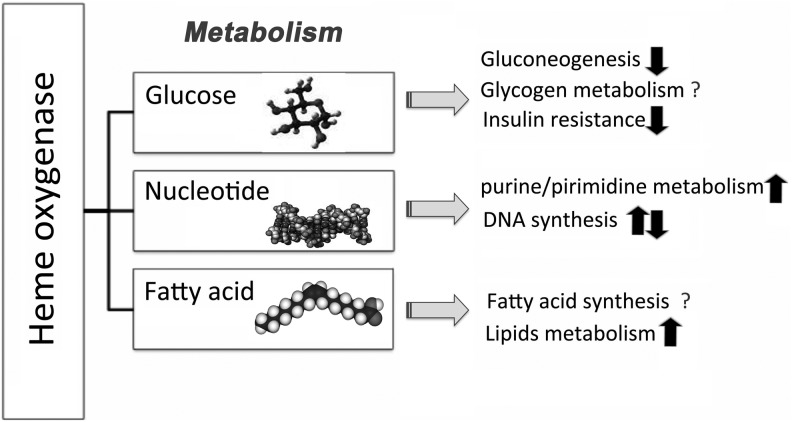
FIG. 3.
Heme oxygenase-1 (HO-1) and metabolic effects in cells. Overall activity of metabolic pathways, both anabolic and catabolic, in the presence of active HO-1. HO-1 activity may influence other NADPH- and oxygen-consuming pathways, including fatty acid synthesis, oxidative metabolism of cytochrome p450, or modulation of reactive oxygen species (ROS) generation in phagocytes. All these pathways use a common pool of NADPH, and, therefore, acceleration or deceleration of one may influence the activity of the other.
Glucose is metabolized through glycolysis to acetyl-CoA, which is an active metabolite and can be utilized in the tricarboxylic acid cycle (TCA, Krebs cycle, and citric acid cycle) to generate NADH. NADH is then utilized during oxidative phosphorylation in the mitochondria to further fuel energy consumption by the cell. There are several important regulatory mechanisms through which HO-1 is implicated in glucose catabolism.
In addition to regulating oxidative phosphorylation, exogenous CO exposure or HO-1-derived CO can attenuate the breakdown of glucose and significantly drive the glucose conversion metabolites into the pentose phosphate pathway, supporting a role for CO in modulating glucose biotransformation (102). The pentose pathway is an alternative pathway to the TCA cycle that enables the generation of necessary metabolites for DNA biosynthesis and of aromatic amino acids for protein biosynthesis. It is plausible that part of the modulatory effects of CO on proliferation and cytoprotection can be attributed to a shift toward generation of NADPH as a result of CO-induced ROS generation. CO targeting of mitochondrial complexes influences the generation of critical mediators during anabolic processes such as generation of ribose-5-phosphate and erythrose-4-phosphate. Similarly, the second step of heme degradation that is catalyzed by biliverdin BV reductase generates additional NAD+, which can also influence the pentose pathway and the TCA cycle. The data supporting this hypothesis are missing in the literature; therefore, the interaction between heme degradation and other metabolic pathways needs to be further investigated. Inhibition of the TCA cycle by blocking fumarase expression with either a selective inhibitor or in mice lacking fumarase leads to strong induction of HO-1 (29). Lack of fumarase, which is responsible of conversion of fumarate to malate, is typical in hereditary leiomyomatosis and renal cell cancer and leads to inhibition of the TCA cycle. However, even with deletion of fumarase, cells can survive, in part, due to the compensatory induction of hypoxia-inducible factors (HIF). Stabilization of HIF in cells lacking fumarase occurs even under normoxia primarily due to induction of HO-1 and heme degradation. Frezza et al. found that inhibition of HO-1 in the absence of fumarase renders cells synthetically lethal (29). Treatment with the HO-1 inhibitor, ZnPP, or knockdown of HO-1 by shRNA in kidney cells enabled selective apoptosis of fumarase-negative cell clones. These studies provide the first link as to how heme degradation is fueled by heme synthesis resulting from inhibition of the TCA cycle.
Targeting Mitochondria and Cellular Respiration by HO-1-Derived CO
Energy balance and CO
CO is a therapeutic molecule that is currently being evaluated in several clinical trials (www.clinicaltrials.gov). When produced endogenously or administered exogenously in cells, it can modulate inflammatory processes, vascular homeostasis, and cellular responses to various stimuli (87). A balance between mitochondrial metabolism and cytoprotective danger-like signaling enables improvement in the majority of disease conditions after short treatment with low, nontoxic doses of CO (Fig. 4). One of the main functions of endogenous CO is to act as a diffusible messenger in cells to regulate signal transduction corresponding to the cellular need at the time. CO is a nonreactive and nonmetabolized molecule; however, by binding to hemoproteins, it can modulate cellular function, including metabolism. CO has a larger selectivity as compared with nitric oxide (NO), which unlike CO can interact with Fe2+ within the protophorphirin ring as well as with nitrosylate thiol groups that can alter protein functionality (41).
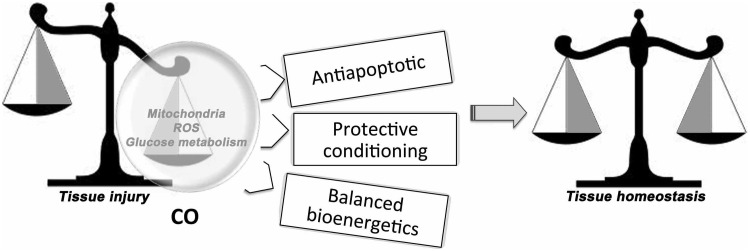
FIG. 4.
Metabolic pathways modulated by CO in response to tissue injury. Protective conditioning of cells after treatment with CO. CO targets mitochondrial respiration and carbohydrate metabolism to prevent damage in response to danger signals such as mechanical or pathogen-induced injury.
One of the most well-described targets for CO is cytochrome c oxidase as described above. There are, however, multiple hemoproteins, including sGC and iNOS that can also act as targets for CO (107, 123). Recently, CO was found to increase cytochrome c oxidase activity and cellular OCR (5, 85) in astrocytes and stimulated mitochondrial biogenesis in neurons and cardiomyocytes (101) via the nuclear receptor PPARγ and its coactivator 1α (PGC 1α) as well as Nrf2. CO effects on mitochondria biogenesis, ATP production, and regulation of COX activity are dependent on early induction of Bcl-2 protein in astrocytes. Bcl-2 is also one of the potential targets for CO (5). CO enhanced the interaction between cytochrome c oxidase and Bcl-2, which can facilitate antiapoptotic and cytoprotective effects that are seen with CO. This effect is synergistic with CO-mediated inhibition of proapoptotic Bcl-2-related proteins in response to different types of stress (78, 122).
Increased OCR in response to CO treatment can be explained by mitochondrial uncoupling. This process occurs when energy-dissipating protons cross the mitochondrial inner membrane, resulting in a compensatory increase in O2 consumption that is not accompanied by production of ATP. CO induces mild mitochondrial uncoupling that is an adaptation mechanism of the cells to restore altered metabolism in diseases such as cancer and diabetes (59, 67). The uncoupling effects of CO induce mitochondria biogenesis and autophagy in vitro and in vivo (59). Such changes in mitochondria explain in part the protective effects of CO in cells as well as cardiac tissue in animal models of lethal sepsis and metabolic syndrome (58, 59, 67, 89). Low levels of exogenous CO have been shown to mimic those observed with endogenous HO-1-generated CO (23). It is, however, important to consider that application of CO in vivo leads to immediate binding of CO to hemoglobin with a half life in humans of 2–3 h and 15 min in mice unless hyperbaric oxygen exposures are employed, which will hasten CO offloading from hemoglobin. The rapid binding of CO to hemoglobin leads to an increase in the activity of the oxygen sensors in the body, and, therefore, some of the in vivo effects may be explained, in part, as a pseudohypoxia response.
Hypoxic responses and HO-1
Ischemic injury and tissue hypoxia have perhaps the most dramatic impact on cellular physiology and metabolism. The absence of oxygen requires the cell to rapidly shift to anaerobic metabolism to ensure survival, thus rendering oxygen-seeking hemoproteins less likely to be targets for O2, and this would include the HOs that require O2 for their activity. One of the major causes of cell death and organ dysfunction is ischemia or hypoxia. The reduction in inflammation after HO-1 up-regulation in response to ischemia was considered the principal protective mechanism involved in ischemia reperfusion injury (IRI) (28). For example, HO-1-overexpressing macrophages exhibit an anti-inflammatory profile and contribute to the attenuation of IRI in different organs (28). More recently, the protective effects of HO-1 during IRI have been further elucidated, and additional pathways have been defined. Transcriptome analysis of animals subjected to renal IRI where HO-1 was induced led to an up-regulation of genes belonging to the vascular endothelial growth factor receptor signaling pathway family, which may, in turn, prevent ischemia by promoting local angiogenesis (22). Accordingly, Brunt and colleagues showed that ex vivo manipulation of Akt/HO-1 genes in human endothelial progenitor cells enhanced recovery from myocardial infarction by permitting better adaptation to tissue hypoxia (15). Corroborating these findings, Vallabhaneni showed that HO-1 adenoviral gene transfer prevented hemorrhagic shock-induced liver injury in vivo and decreased cellular respiration under hypoxic conditions, resulting in increased intracellular oxygen levels in the setting of low oxygen tensions (106). Moreover, HO-1 up-regulation during renal IRI enhanced the expression of genes involved in amino-acid and nitrogen metabolism, indicating a mechanism of tolerance in response to ischemia by means of a metabolic adaptation through increased amino-acid metabolism (22).
In response to tissue hypoxia, there is a dramatic increase in HIF-1α stabilization as O2 tensions in the cell decrease. HIF signaling seems to be a key step for HO-1 and CO-mediated cytoprotection, as it relates to modulation of the inflammatory response (10, 21). Unpublished data from our lab showed that during an ischemic episode, treatment with CO increased HIF-1α in the kidney and correlated with improved renal function and survival after IRI (Fig. 5). Similar protection against IRI was observed in kidney allografts in pigs (40) and also in the presence of HO-1 overexpression in the infarcted porcine heart (115). These results confirm a work by Chin et al., which described that CO gas increases HIF-α translational activation in macrophages (43). Perhaps the most intriguing findings in the field of IRI and tissue hypoxia were those of Mishra et al. (74) and Zuckerbraun et al. that independently showed that HO-1 and CO exposure paradoxically reduced tissue hypoxia in the lung and liver by increasing O2 availability. In the lung, the reduction of hypoxia-induced Egr-1 signaling by CO was mediated by the blockade of ERK in a cGMP-dependent manner (74); while in the liver, HO-1 overexpression protected against hemorrhage-induced hypoxia by decreasing OCR and increasing oxygen tensions, thus preventing bioenergetic failure (106).
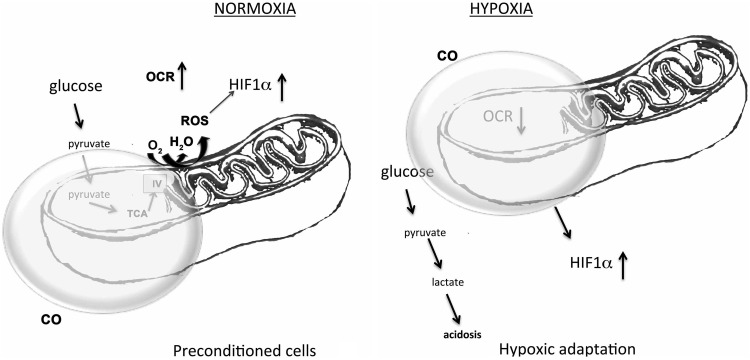
FIG. 5.
Effects of CO on cellular bioenergetics under normoxic and hypoxic conditions. CO differentially influences the oxygen consumption rate (OCR) under normoxic conditions, inducing protective preconditioning. In the presence of low oxygen tension, CO inhibits OCR further to sustain energy requirements, and fuel hypoxic metabolism.
CO has also been shown to potentiate lung fibrinolysis through a mechanism dependent on sGC activation; while in the liver, CO acts via an ROS-NFκB pathway to prevent hepatocyte apoptosis (31, 125). Together, these data suggest that HO-1/CO have pleiotropic effects during an ischemic episode, reducing cellular inflammation and apoptosis and enhancing oxygen availability. Metabolically, this is quite astonishing given that CO has long been viewed as a molecule that would reduce O2 availability. Quite the opposite was found when the concentrations of CO, equal to those generated by HO-1, permitted better tissue oxygenation, likely reducing the need for anaerobic metabolism and enabling the energy-requiring survival mechanisms to maintain cell and tissue stability (Fig. 5). These data meld well with the findings in the heart and muscle showing that CO increases mitochondrial biogenesis (84, 101).
BR as a Metabolite in Cells
BR as an antioxidant and its role in cell metabolism
BR, the terminal product of heme catabolism, demonstrates clear antioxidant capabilities in vitro (98). BR inhibits the oxidation of phosphatidyl choline in multilamellar liposomes more effectively than equimolar concentrations of vitamin E (100). BR also inhibits protein oxidation in vitro in the presence of a variety of oxidants, including peroxynitrite (73), superoxide, and hydroxyl radicals (79). Despite these well-characterized effects in vitro, the degree to which BR protects from oxidative stress in vivo is comparatively under-studied. Animal (25) and human (12) models of hyperbilirubinemia currently indicate a physiological antioxidant effect for BR. For example, in humans with Gilbert's syndrome (GS), a benign condition of hyperbilirubinemia, serum lipids and low-density lipoprotein (LDL) (16, 117) are resistant to ex vivo copper oxidation. Interestingly, when serum from GS individuals is assessed for oxidized LDL in the absence of ex vivo oxidation, GS individuals also possess reduced absolute oxLDL concentrations (70, 104). These data support the capacity of elevated BR to inhibit LDL oxidation. However, a recent study demonstrated that oxLDL and LDL concentrations are reduced in hyperbilirubinemic individuals. When oxLDL was expressed relative to LDL, LDL appeared more oxidized in GS compared with controls (12). Recent reports suggest that BR might not prevent physiological LDL oxidation in vivo, although absolute oxLDL levels are clearly reduced in hyperbilirubinemic individuals (12). Accumulation of oxidized LDL within the vascular wall and resident macrophages represents one hypothesis (of many) that might explain the development of atherosclerosis (99). Therefore, altered BR metabolism might lead to protection from atherosclerosis by preventing LDL oxidation; however, more research is necessary to verify these findings. Irrespective of whether or not BR protects from LDL oxidation, it is obvious that elevated BR (or BV) is associated with protection from multiple pathologic sequelae which are associated with oxidative stress. For example, BR or BV treatment protects against neointima formation in animals undergoing experimental angioplasty (82). In human studies, mildly elevated BR is consistently associated with preserved vascular structure (14, 42, 108), improved vascular function (70), and a reduced risk of cardio/cerebrovascular disease (92), diabetes (39), and cancer (43, 72, 105). In addition, hyperbilirubinemic individuals have reduced concentrations of advanced glycation end products (52), indicating that BR might protect against oxidative glucose, protein, and DNA damage (76, 109).
BR clearly protects from physiological protein and thiol oxidation. In GS subjects, reduced thiol concentrations and the GSH:GSSG are greater (12, 37). Therefore, elevated BR may, in part, prevent sulfhydryl oxidation or potentially increase glutathione synthesis (33). These data are of potential interest, because BR accumulates differentially within organs (119) and could, therefore, influence cellular redox status and cell signaling in vivo.
BR modulates metabolic responses during inflammation
It has become evident in recent years that BR modulates the immune response. BR potently inhibits complement activation at the C1 step of the complement cascade (Bulmer et al; unpublished data) as well as proinflammatory cytokine production (TNFα and IL-6) and nitric oxide synthesis in experimental endotoxemia models (60, 90). Specifically, BR increases complement degradation via increased decay accelerating factor expression in endothelial cells (56). Elevated BR is associated with increased circulating CO and iron concentrations in vivo (110). These data indicate that elevated BR might stimulate a positive feedback loop of HO activation that is induced by a mild hemolytic effect (110). Elevated BR is also associated with increased circulating IL-1β and reduced interleukin-6 concentrations, indicating novel immune-modulatory effects of BR (110). These data are supported by increased IL-1β expression in neutrophils that are incubated with BR (113). Interleukin-6 is an activator of hepatic C-reactive protein (CRP), linking elevated BR to reduced CRP, as demonstrated in many studies (46, 118). BR is also negatively related to adiposity and is associated with reduced circulating cholesterol and triacylglycerol concentrations in humans (12, 18, 111). More recently, elevated BR in mutant hyperbilirubinemic Gunn rats was shown to induce a >50% reduction in cholesterol concentrations and reduced body mass in young female animals, providing the first evidence of a role for BR in modulating whole body metabolism (12, 111). One potential mechanism of the effects of BR on lipid lowering might depend on aryl-hydrocarbon receptor activation (83). Absence of the aryl hydrocarbon receptor (AhR) resulted in increased expression of genes regulating cholesterol biosynthesis (103). Furthermore, administration of an AhR ligand (2,3,7,8-tetrachlorodibenzo-p-dioxin) decreases the expression of cholesterol biosynthetic genes (103). Whether BR per se inhibits cholesterol synthesis in an AhR-dependent manner has not yet been determined. BR also inhibits triacylglycerol lipase activity and stimulates glucose oxidation in adipoctytes, independent of adenylate cyclase activity and cAMP concentrations (93). These findings curiously appear related to a growing body of literature showing that elevated BR is related to reduced HBA1c (81), improved insulin sensitivity, and reduced prevalence of metabolic syndrome in healthy and diseased individuals (36, 118). BR concentrations are lower in overweight individuals and negatively associated with fasting glucose, insulin, and abdominal obesity (49). Furthermore, BR concentrations increase with acute weight loss (6), suggesting that metabolism is intricately related to HO and BR metabolism (Fig. 6).
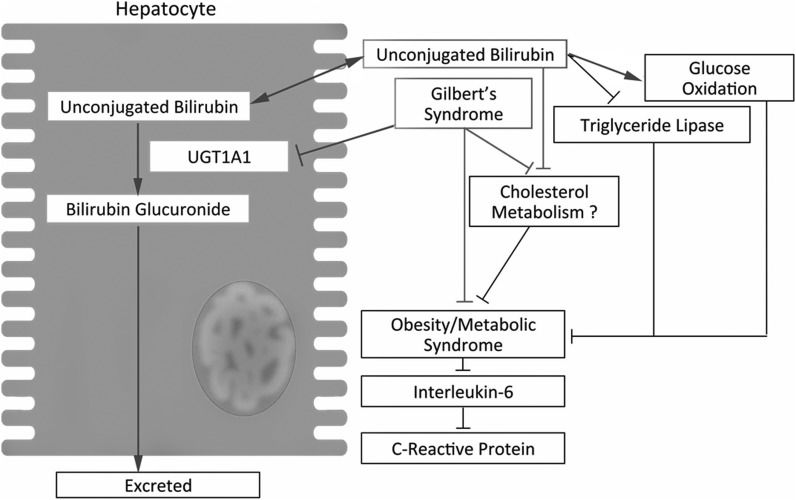
FIG. 6.
Interaction between bilirubin and lipid/glucose metabolism. Unconjugated BR originating from heme catabolism primarily in the spleen is glucuronidated by hepatic uridine glucuronosyltransferase 1A1 (UGT1A1) forming bilruibin glucuronides, which are excreted in the bile and involved in the digestion of fat in the intestine. Gilbert's syndrome (GS) is characterized by reduced UGT1A1 inducibility/expression and increased in circulating unconjugated BR. In adipocytes, increased unconjugated BR inhibits triacylglycerol lipase activity and increases glucose oxidation. The increase in unconjugated BR also reduces circulating cholesterol concentrations (possibly mediated by impaired cholesterol biosynthesis), and contributes to the reduced prevalence of obesity and metabolic syndrome in hyperbilirubinemic individuals.
Iron and Metabolism
Free iron is the third product of heme degradation and one that induces transcription of the heavy chain of ferritin to accelerate immediate sequestration of this potential oxidant. Iron accumulation leads to liver disease and diabetes; however, iron is critical in numerous biological processes as a key component of the oxidation-reduction reactions in the mitochondrial respiratory chain as well as oxygen binding to hemoglobin. Free iron is toxic because of its participation in Fenton chemistry; reduced iron reacts with hydrogen peroxide or lipid peroxides and produces highly reactive radicals. Interestingly, a diet high in iron induces AMP kinase (AMPK) signaling and improves glucose tolerance in mice (45). Increased glucose uptake in mice fed a high iron diet was dependent on activated liver kinase B1 (LKB1) that phosphorylated AMPK. A mechanism for LKB1 activation involved changes in redox status, which resulted in a decrease in acetylation of LKB1. Importantly, glucose levels in the serum correlated with low lactate levels and decreased expression of gluoconeogenic genes in the liver in animals fed a high iron diet. Similar to high iron levels in the serum in mice, serum ferritin levels in humans correlated with a decrease in the incidence of type 2 diabetic and obese subjects as compared with healthy subjects (32, 65). Increased iron or ferritin stores are positively correlated with the development of glucose intolerance and type 2 diabetes in humans. Type 2 diabetes is common in individuals with the genetic disorder hemochromatosis in which mutation of the hemochromatosis gene (HFE), which is involved in iron absorption, results in accumulation of iron in the tissues.
Proper deposition of iron in complex with ferritin is critical for homeostasis and iron metabolism. Adiponectin is one of the cytokines that regulates iron storage through induction of HO-1 in an AMPK-PPAR-α-dependent mechanism (65) and is, thus, critical for iron metabolism. Mice fed a diet high in iron or cultured adipocytes treated with iron exhibited decreased adiponectin mRNA and protein (32), which might define a negative feedback loop that regulates iron overload (65).
Heme Metabolism in the Metabolic Syndrome, Diabetes, and Cancer
Diabetes is a prototypical metabolic syndrome, while cancer can also be associated with abnormal metabolism. Diabetes and obesity are associated with elevated oxidative and inflammatory activities (enchanced level of cytokines, such as TNF, IL-6, IL-1β, and resistin, which lead to c-Jun-N-terminal kinase [JNK] and NFκB pathway activation). The HO system inhibits inflammation through suppression of macrophage infiltration and decreases in pro-oxidant and proinflammatory transcription factors. A common feature of metabolic syndromes is abnormal glycolysis and/or alterations in mitochondrial respiration. Otto Warburg defined what has come to be considered a common feature of cancer cells: a metabolic switch from mitochondrial respiration to anaerobic glycolysis that leads to generation of lactate from pyruvate instead of pyruvate entering the TCA cycle. Hypoxic tumors strive to survive in an environment with insufficient oxygen and nutrition while producing the majority of ATP utilizing the less efficient glycolytic pathway. Reprogramming to carbohydrate metabolism and anabolic pathways in cancer serves as an adaptation to the high proliferative rate of cancer cells and is controlled, in part, by HIF and downstream oncogenes (34). Since HO-1-derived CO is a potent regulator of HIF-1α, it is plausible that heme metabolism can influence and participate in cancer progression (Fig. 7).
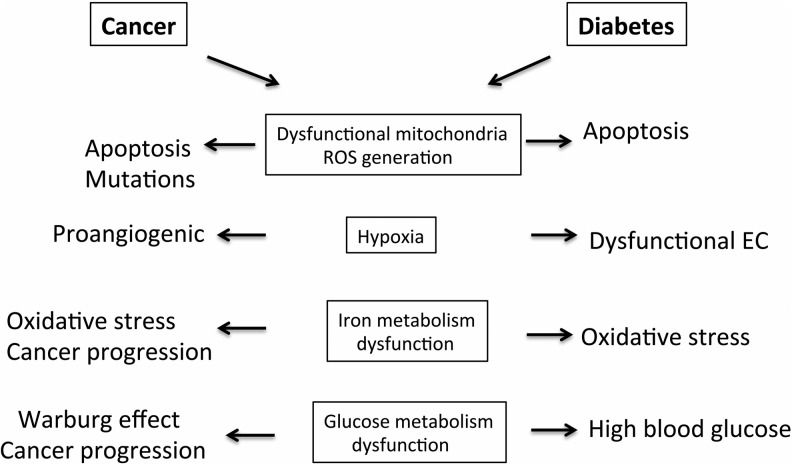
FIG. 7.
Similarities in metabolic dysfunction in cancer and diabetes. Changes in metabolism that occur in cancer and diabetes that contribute to disease progression. The major dysfunctional metabolic pathways in cancer cells and diabetic milieu are presented.
The most striking representation of the role of HO-1 and its products in metabolic disorders are through the analysis of HO-1 knockout mice and human HO-1 deficiency. Lack of HO-1 in humans is associated with growth retardation, increased iron deposition, and anemia among abnormalities in coagulation and early death (53, 116). All the disorders associated with HO-1 deficiency are due to oxidative stress and are strongly associated with metabolic syndrome. It is likely that if HO-1-deficient humans survived longer, they would develop metabolic complications such as diabetes and cancer. Similar to human HO-1 deficiency, HO-1 knockout mice that survive (survival rate ∼1%–5% of litters) show high levels of oxidative stress, enlarged organs and are severely sensitive to stress and injury with a shortened life span. These null mice do not develop cancer or diabetes without additional stimuli. It is likely, however, that those mice which survive compensate with HO-2.
Metabolic syndrome is a complex clinical problem, including abnormal body fat distribution, insulin resistance, atherogenic dyslipidaemia, elevated blood pressure, as well as a proinflammatory and a prothrombotic state in the tissues (4). Each of these changes in the function of cells and tissues can be regulated by the heme degradation pathway (26) (Fig. 7). CoPP, a pharmacologic inducer of HO-1, resulted in increased adiponectin levels and improved insulin sensitivity via increased AMPK phosphorylation in the diabetic obese rat model (80). Further, induction of HO-1 selectively enhanced polarization toward an anti-inflammatory M2 macrophage phenotype and reduced pericardial adiposity and cardiac injury in diabetic cardiomyopathy in obese rats (48). Similar results were reported in spontanously hypertensive rats after treatment with hemin (61). Hemin therapy lowered blood pressure, decreased glycemia, reduced insulin resistance as well as proteinuria/albuminuria, and enhanced glucose transport. However, the direct role of HO-1 in regulating metabolism of a single cell in this scenario has not been tested.
CoPP induced HO-1 up-regulation in nonobese diabetic mice and led to a decrease in blood glucose, increased β cell survival, and decreased inflitration of CD11c+ dendritic cells (44, 63). CoPP also exerted significant HO-1-independent effects, including induction of STAT3 (71). Importantly, there is a mild association between microsatellite polymorphisms in the HO-1 gene promoter region in type 2 diabetes syndrome manifestation (7, 20). Bao et al. found that elevated HO-1 plasma levels were increased in newly diagnosed type 2 diabetic patients which was associated with impaired glucose tolerance (8). This study, however, did not clarify whether HO-1 is a functional protein that mediates the protection or serves as a marker of oxidative stress. Validation in HO-1 knockout mice is waranted (71).
In contrast to the diabetic milieu, the role of HO-1 in tumor metabolism and growth is much less understood (9a, 32a, 50, 80a, 95, 112, 117a). HO-1 levels are elevated in the majority of cancers; however, the activity of HO-1 varies, and, therefore, heme metabolism can be dramatically altered in the tumors. Consumption of heme contained in red meat is one of the risk factors for colcorectal cancer. Heme induces cytotoxicity and ROS generation in the colon that is compensated for by hyperproliferation and hyperplasia of crypt cells (47).
HO-1 is expressed in both infiltrating leukocytes as well as in cancer cells depending on the type of tumor (Table 2). In PCa patients, nuclear and enzymatically inactive HO-1 correlated with cancer progression (88, 112a), while better survival rates were observed in colorectal cancer patients where colonic HO-1 expression correlated with lower rates of lymphatic tumor invasion (9). Overexpression of HO-1 in androgen-sensitive and androgen-insensitive PCa cell lines led to a marked reduction in cell proliferation and migration that was associated with lower metalloproteinase-9 expression (35). In a study by Li et al., a combination of HO-1 overexpression in the presence of low expression levels of phosphatase and tensin homolog (PTEN) correlated with disease progression in prostate cancer patients (64).
Table 2.
Heme Oxygenase-1 Expression and Its Role in Tumors
| Type of cancer | HO-1 localization | Role |
|---|---|---|
| Lung cancer | Cytoplasmic | Associated with higher stage (24) |
| Prostate cancer | Nuclear | Associated with disease progression (64) |
| Pancreatic cancer | Cytoplasmic | Proangiogenic and protumorigenic (9a) |
| Head and neck cancer | Nuclear | Associated with malignant progression (32a) |
| Glioma | High in macrophages | Proangiogenenic (80a) |
| Colon cancer | Cytoplasmic and in macrophages | Associated with better prognosis (9) |
| Gastric cancer | Cytoplasmic and in macrophages | Associated with better prognosis (117a) |
There is further evidence that HO-1 expression plays a role in cancer incidence in humans with the discovery of a GT length polymorphism of the promoter for HO-1 which has been shown to be highly correlative with disease severity, including cancer (19, 54). Individuals with the long (GT)n repeat and associated low expression of HO-1 showed an association with a higher frequency of gastric or lung adenocarcinoma and oral squamous cancer than those with short GT repeats and higher HO-1 expression (68). HO-1 expression in nonsmall cell lung cancer was associated with more advanced and metastatic disease (24), while others reported no correlation or decreased expression of HO-1 in macrophages in cancer specimens as compared with controls (13). Inhibition of HO-1 using an epidermal growth factor receptor inhibitor and cisplatin decreased proliferation of the lung cancer cell line A549 (24). Further, knockdown of HO-1 in A549 induced apoptosis and activation of caspase-3 as well as sensitized cells to cisplatin treatment (55) and irradiation (121). In contrast, overexpression of HO-1 was shown to inhibit lung cancer growth in vivo and in vitro through regulation of several oncomirs and angiomirs (96). HO-1, in part, acts in lung cancer through inhibition of mir-378 and reduces tumor growth, metastases, and angiogenesis (96). There are multiple reports suggesting that overexpression of HO-1 can block or accelerate tumor growth depending on the cancer cell type. It is likely that the metabolic status of cancer cells influences how the heme degradation enzymes modulate tumor growth.
Innovation
This review provides a comprehensive discussion about the influence of heme catabolism on cellular metabolic signaling pathways. Heme is a critical component of multiple hemoproteins that are implicated in glucose, lipid, and protein metabolism. Heme turnover is tightly regulated by the heme oxygenases (HO) with the products of degradation eliciting remarkable biologic effects, including modulating signal transduction and biochemical activity. We describe the consequences of the absence of HO-1, the isoform that is critical in the stress response, and how inappropriate heme metabolism contributes to the development of pathology, including increased susceptibility to cancer, diabetes, and ischemia-reperfusion injury.
Concluding Remarks
The ability of the cell to modulate metabolic processes and adapt to a continuum of environmental cues should be dynamic. The idea of stasis is inherently flawed, as the milieu in which the organism finds itself is always and continuously challenged by the environment. We have focused this review on the role of heme, a fundamental and necessary asset of all cells, in controlling metabolism and overall cellular function. We have summarized the properties of the HO catalytic pathway and the bioactive products it generates that are intimately intertwined with cellular metabolism. It has become apparent that cellular bioenergetics, including glucose and lipid metabolism, are strongly influenced by both CO and BR. Their role in metabolic syndromes dictates the therapeutic application of both molecules in such settings. Nike was the goddess of victory and success and clearly, the success of the cell requires the elegant synchrony of heme biology and metabolism to adapt to the environment and ensure continued survival.
Abbreviations Used
- AhR
aryl hydrocarbon receptor
- ALA
5-aminolevulinic acid
- ALAS
5-aminolevulonate synthase
- AMPK
AMP kinase
- BR
bilirubin
- BV
biliverdin
- CO
carbon monoxide
- CoPP
cobalt protoporphyrin
- COPRO
coproporphyrinogen
- COX
cyclooxygenase
- CRP
C-reactive protein
- Egr-1
early growth response protein
- ERK
extracellular signal regulated kinase
- GS
Gilbert's syndrome
- GSH
glutathione
- HIF
hypoxia-inducible factor
- HO
heme oxygenase
- IRI
ischemia reperfusion injury
- JNK
c-jun N-terminal kinase
- LDL
low-density lipoprotein
- LKB1
liver kinase B1
- NOS
nitric oxide synthase
- Nrf2
nuclear factor 2
- OCR
oxygen consumption rate
- PFKFB
6-phosphorfructo-2-kinase/fructose-2,6-biphosphates 4
- PGE
prostaglandin
- PGI
prostacyclin
- PPARγ
peroxisome proliferator-activated receptor γ
- ROS
reactive oxygen species
- STAT
signal transducer and activator of transcription
- TCA
tricarboxylic acid
- TNF
tumor necrosis factor
- UGT1A1
uridine glucuronosyltransferase 1A1
References
- 1.Abraham NG, Lutton JD, and Levere RD. Heme metabolism and erythropoiesis in abnormal iron states: role of delta-aminolevulinic acid synthase and heme oxygenase. Exp Hematol 13: 838–843, 1985 [PubMed] [Google Scholar]
- 2.Abraham NG, Tsenovoy PL, McClung J, and Drummond GS. Heme oxygenase: a target gene for anti-diabetic and obesity. Curr Pharm Des 14: 412–421, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Ahlstrom K, Biber B, Aberg A, Waldenstrom A, Ronquist G, Abrahamsson P, Stranden P, Johansson G, and Haney MF. Metabolic responses in ischemic myocardium after inhalation of carbon monoxide. Acta Anaesthesiol Scand 53: 1036–1042, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Alberti L, Girola A, Gilardini L, Conti A, Cattaldo S, Micheletto G, and Invitti C. Type 2 diabetes and metabolic syndrome are associated with increased expression of 11beta-hydroxysteroid dehydrogenase 1 in obese subjects. Int J Obes (Lond) 31: 1826–1831, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Almeida AS, Queiroga CS, Sousa MF, Alves PM, and Vieira HL. Carbon monoxide modulates apoptosis by reinforcing oxidative metabolism in astrocytes: role of Bcl-2. J Biol Chem 287: 10761–10770, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson C, Weeke P, Fosbol EL, Brendorp B, Kober L, Coutinho W, Sharma AM, Van Gaal L, Finer N, James WP, Caterson ID, Rode RA, and Torp-Pedersen C. Acute effect of weight loss on levels of total bilirubin in obese, cardiovascular high-risk patients: an analysis from the lead-in period of the Sibutramine Cardiovascular Outcome trial. Metabolism 58: 1109–1115, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Bao W, Song F, Li X, Rong S, Yang W, Wang D, Xu J, Fu J, Zhao Y, and Liu L. Association between heme oxygenase-1 gene promoter polymorphisms and type 2 diabetes mellitus: a HuGE review and meta-analysis. Am J Epidemiol 172: 631–636, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Bao W, Song F, Li X, Rong S, Yang W, Zhang M, Yao P, Hao L, Yang N, Hu FB, and Liu L. Plasma heme oxygenase-1 concentration is elevated in individuals with type 2 diabetes mellitus. PLoS One 5: e12371, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker JC, Fukui H, Imai Y, Sekikawa A, Kimura T, Yamagishi H, Yoshitake N, Pohle T, Domschke W, and Fujimori T. Colonic expression of heme oxygenase-1 is associated with a better long-term survival in patients with colorectal cancer. Scand J Gastroenterol 42: 852–858, 2007 [DOI] [PubMed] [Google Scholar]
- 9a.Berberat PO, Dambrauskas Z, Gulbinas A, Giese T, Giese N, Künzli B, Autschbach F, Meuer S, Büchler MW, Friess H. Inhibition of heme oxygenase-1 increases responsiveness of pancreatic cancer cells to anticancer treatment. Clin Cancer Res 11: 3790-3798, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Bilban M, Bach FH, Otterbein SL, Ifedigbo E, de Costa d'Avila J, Esterbauer H, Chin BY, Usheva A, Robson SC, Wagner O, and Otterbein LE. Carbon monoxide orchestrates a protective response through PPARgamma. Immunity 24: 601–610, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Bilban M, Haschemi A, Wegiel B, Chin BY, Wagner O, and Otterbein LE. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med 86: 267–279, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Boon A-C, Hawkins CL, Bisht K, Bakrania B, Coombes JS, Wagner K-H, and Bulmer AC. Reduced circulating oxidized LDL is associated with hypocholesterolemia and enhanced thiol status in Gilbert's syndrome. Free Radic Biol Med 52: 2120–2127, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boschetto P, Zeni E, Mazzetti L, Miotto D, Lo Cascio N, Maestrelli P, Marian E, Querzoli P, Pedriali M, Murer B, De Rosa E, Fabbri LM, and Mapp CE. Decreased heme-oxygenase (HO)-1 in the macrophages of non-small cell lung cancer. Lung Cancer 59: 192–197, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Breimer LH, Wannamethee G, Ebrahim S, and Shaper AG. Serum bilirubin and risk of ischemic heart disease in middle-aged British men. Clin Chem 41: 1504–1508, 1995 [PubMed] [Google Scholar]
- 15.Brunt KR, Wu J, Chen Z, Poeckel D, Dercho RA, Melo LG, Funk CD, Ward CA, and Li RK. Ex vivo Akt/HO-1 gene therapy to human endothelial progenitor cells enhances myocardial infarction recovery. Cell Transplant 21: 1443–1461, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Bulmer AC, Blanchfield JT, Toth I, Fassett RG, and Coombes JS. Improved resistance to serum oxidation in Gilbert's syndrome: a mechanism for cardiovascular protection. Atherosclerosis 199: 390–396, 2008 [DOI] [PubMed] [Google Scholar]
- 17.This reference has been deleted.
- 18.Bulmer AC, Verkade HJ, and Wagner KH. Bilirubin and beyond: a review of lipid status in Gilbert's syndrome and its relevance to cardiovascular disease protection. Prog Lipid Res 52: 193–205, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Chang KW, Lee TC, Yeh WI, Chung MY, Liu CJ, Chi LY, and Lin SC. Polymorphism in heme oxygenase-1 (HO-1) promoter is related to the risk of oral squamous cell carcinoma occurring on male areca chewers. Br J Cancer 91: 1551–1555, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA, Pan WH, Jou YS, and Chau LY. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet 111: 1–8, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Chin BY, Jiang G, Wegiel B, Wang HJ, Macdonald T, Zhang XC, Gallo D, Cszimadia E, Bach FH, Lee PJ, and Otterbein LE. Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci U S A 104: 5109–5114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correa-Costa M, Azevedo H, Amano MT, Goncalves GM, Hyane MI, Cenedeze MA, Renesto PG, Pacheco-Silva A, Moreira-Filho CA, and Camara NO. Transcriptome analysis of renal ischemia/reperfusion injury and its modulation by ischemic pre-conditioning or hemin treatment. PLoS One 7: e49569, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Amico G, Lam F, Hagen T, and Moncada S. Inhibition of cellular respiration by endogenously produced carbon monoxide. J Cell Sci 119: 2291–2298, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Degese MS, Mendizabal JE, Gandini NA, Gutkind JS, Molinolo A, Hewitt SM, Curino AC, Coso OA, and Facchinetti MM. Expression of heme oxygenase-1 in non-small cell lung cancer (NSCLC) and its correlation with clinical data. Lung Cancer 77: 168–175, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennery PA, McDonagh AF, Spitz DR, Rodgers PA, and Stevenson DK. Hyperbilirubinemia results in reduced oxidative injury in neonatal Gunn rats exposed to hyperoxia. Free Radic Biol Med 19: 395–404, 1995 [DOI] [PubMed] [Google Scholar]
- 26.Durante W. Protective role of heme oxygenase-1 against inflammation in atherosclerosis. Front Biosci (Landmark Ed) 16: 2372–2388, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ewing JF. and Maines MD. In situ hybridization and immunohistochemical localization of heme oxygenase-2 mRNA and protein in normal rat brain: Differential distribution of isozyme 1 and 2. Mol Cell Neurosci 3: 559–570, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Ferenbach DA, Kluth DC, and Hughes J. Hemeoxygenase-1 and renal ischaemia-reperfusion injury. Nephron Exp Nephrol 115: e33–e37, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Frezza C, Zheng L, Folger O, Rajagopalan KN, MacKenzie ED, Jerby L, Micaroni M, Chaneton B, Adam J, Hedley A, Kalna G, Tomlinson IP, Pollard PJ, Watson DG, Deberardinis RJ, Shlomi T, Ruppin E, and Gottlieb E. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature 477: 225–228, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Fujii M, Inoguchi T, Sasaki S, Maeda Y, Zheng J, Kobayashi K, and Takayanagi R. Bilirubin and biliverdin protect rodents against diabetic nephropathy by downregulating NAD(P)H oxidase. Kidney Int 78: 905–919, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Fujita T, Toda K, Karimova A, Yan SF, Naka Y, Yet SF, and Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med 7: 598–604, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Gabrielsen JS, Gao Y, Simcox JA, Huang J, Thorup D, Jones D, Cooksey RC, Gabrielsen D, Adams TD, Hunt SC, Hopkins PN, Cefalu WT, and McClain DA. Adipocyte iron regulates adiponectin and insulin sensitivity. J Clin Invest 122: 3529–3540, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Gandini NA, Fermento ME, Salomón DG, Blasco J, Patel V, Gutkind JS, Molinolo AA, Facchinetti MM, Curino AC. Nuclear localization of heme oxygenase-1 is associated with tumor progression of head and neck squamous cell carcinomas. Exp Mol Pathol 93: 237-245, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Giraudi PJ, Bellarosa C, Coda-Zabetta CD, Peruzzo P, and Tiribelli C. Functional Induction of the Cystine-Glutamate Exchanger System X(c) Activity in SH-SY5Y Cells by Unconjugated Bilirubin. PLoS One 6: e29078, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goda N. and Kanai M. Hypoxia-inducible factors and their roles in energy metabolism. Int J Hematol 95: 457–463, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Gueron G, De Siervi A, Ferrando M, Salierno M, De Luca P, Elguero B, Meiss R, Navone N, and Vazquez ES. Critical role of endogenous heme oxygenase 1 as a tuner of the invasive potential of prostate cancer cells. Mol Cancer Res 7: 1745–1755, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Guzek M, Jakubowski Z, Bandosz P, Wyrzykowski B, Smoczynski M, Jabloiska A, and Zdrojewski T. Inverse association of serum bilirubin with metabolic syndrome and insulin resistance in Polish population. Przegl Epidemiol 66: 495–501, 2012 [PubMed] [Google Scholar]
- 37.Hagymasi K, Kocsis I, Lengyel G, Sipos P, Ferer J, and Blazovics A. Further evidence of altered redox status of hyperbilirubinaemic patients: role of bilirubin in Gilbert syndrome. Acta Biol Szegediensis 47: 131–134, 2003 [Google Scholar]
- 38.Haider A, Olszanecki R, Gryglewski R, Schwartzman ML, Lianos E, Kappas A, Nasjletti A, and Abraham NG. Regulation of cyclooxygenase by the heme-heme oxygenase system in microvessel endothelial cells. J Pharmacol Exp Ther 300: 188–194, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Han SS, Na KY, Chae DW, Kim YS, Kim S, and Chin HJ. High serum bilirubin is associated with the reduced risk of diabetes mellitus and diabetic nephropathy. Tohoku J Exp Med 221: 133–140, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Hanto DW, Maki T, Yoon MH, Csizmadia E, Chin BY, Gallo D, Konduru B, Kuramitsu K, Smith NR, Berssenbrugge A, Attanasio C, Thomas M, Wegiel B, and Otterbein LE. Intraoperative administration of inhaled carbon monoxide reduces delayed graft function in kidney allografts in Swine. Am J Transplant 10: 2421–2430, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Ho K, Klapper MH, and Dorfman LM. Kinetics of carbon monoxide binding to singly reduced human methemoglobin. J Biol Chem 253: 238–241, 1978 [PubMed] [Google Scholar]
- 42.Hopkins PN, Wu LL, Hunt SC, James BC, Vincent GM, and Williams RR. Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease. Arterioscler Thromb Vasc Biol 16: 250–255, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Horsfall LJ, Rait G, Walters K, Swallow DM, Pereira SP, Nazareth I, and Petersen I. Serum bilirubin and risk of respiratory disease and death. JAMA 305: 691–697, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Hu CM, Lin HH, Chiang MT, Chang PF, and Chau LY. Systemic expression of heme oxygenase-1 ameliorates type 1 diabetes in NOD mice. Diabetes 56: 1240–1247, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Huang J, Simcox J, Mitchell TC, Jones D, Cox J, Luo B, Cooksey RC, Boros LG, and McClain DA. Iron regulates glucose homeostasis in liver and muscle via AMP-activated protein kinase in mice. FASEB J 27: 2845–2854, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang HJ, Lee SW, and Kim SH. Relationship between bilirubin and C-reactive protein. Clin Chem Lab Med 49: 1823–1828, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Ijssennagger N, Rijnierse A, de Wit NJ, Boekschoten MV, Dekker J, Schonewille A, Muller M, and van der Meer R. Dietary heme induces acute oxidative stress, but delayed cytotoxicity and compensatory hyperproliferation in mouse colon. Carcinogenesis 34: 1628–1635, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Jadhav A, Tiwari S, Lee P, and Ndisang JF. The heme oxygenase system selectively enhances the anti-inflammatory macrophage-M2 phenotype, reduces pericardial adiposity and ameliorated cardiac injury in diabetic cardiomyopathy in Zucker diabetic fatty rats. J Pharmacol Exp Ther 345: 239–249, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Jenko-Praznikar Z, Petelin A, Jurdana M, and Ziberna L. Serum bilirubin levels are lower in overweight asymptomatic middle-aged adults: an early indicator of metabolic syndrome? Metabolism 62: 976–985, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Jozkowicz A, Was H, and Dulak J. Heme oxygenase-1 in tumors: is it a false friend? Antioxid Redox Signal 9: 2099–2117, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kajimura M, Fukuda R, Bateman RM, Yamamoto T, and Suematsu M. Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid Redox Signal 13: 157–192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalousova M, Novotny L, Zima T, Braun M, and Vitek L. Decreased levels of advanced glycation end-products in patients with Gilbert syndrome. Cell Mol Biol (Noisy-le-grand) 51: 387–392, 2005 [PubMed] [Google Scholar]
- 53.Kawashima A, Oda Y, Yachie A, Koizumi S, and Nakanishi I. Heme oxygenase-1 deficiency: the first autopsy case. Hum Pathol 33: 125–130, 2002 [DOI] [PubMed] [Google Scholar]
- 54.Kikuchi A, Yamaya M, Suzuki S, Yasuda H, Kubo H, Nakayama K, Handa M, Sasaki T, Shibahara S, Sekizawa K, and Sasaki H. Association of susceptibility to the development of lung adenocarcinoma with the heme oxygenase-1 gene promoter polymorphism. Hum Genet 116: 354–360, 2005 [DOI] [PubMed] [Google Scholar]
- 55.Kim HR, Kim S, Kim EJ, Park JH, Yang SH, Jeong ET, Park C, Youn MJ, So HS, and Park R. Suppression of Nrf2-driven heme oxygenase-1 enhances the chemosensitivity of lung cancer A549 cells toward cisplatin. Lung Cancer 60: 47–56, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Kinderlerer AR, Pombo Gregoire I, Hamdulay SS, Ali F, Steinberg R, Silva G, Ali N, Wang B, Haskard DO, Soares MP, and Mason JC. Heme oxygenase-1 expression enhances vascular endothelial resistance to complement-mediated injury through induction of decay-accelerating factor: a role for increased bilirubin and ferritin. Blood 113: 1598–1607, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Kushida T, Li Volti G, Quan S, Goodman A, and Abraham NG. Role of human heme oxygenase-1 in attenuating TNF-alpha-mediated inflammation injury in endothelial cells. J Cell Biochem 87: 377–385, 2002 [DOI] [PubMed] [Google Scholar]
- 58.Lancel S, Hassoun SM, Favory R, Decoster B, Motterlini R, and Neviere R. Carbon monoxide rescues mice from lethal sepsis by supporting mitochondrial energetic metabolism and activating mitochondrial biogenesis. J Pharmacol Exp Ther 329: 641–648, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Lancel S, Montaigne D, Marechal X, Marciniak C, Hassoun SM, Decoster B, Ballot C, Blazejewski C, Corseaux D, Lescure B, Motterlini R, and Neviere R. Carbon monoxide improves cardiac function and mitochondrial population quality in a mouse model of metabolic syndrome. PLoS One 7: e41836, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lanone S, Bloc S, Foresti R, Almolki A, Taille C, Callebert J, Conti M, Goven D, Aubier M, Dureuil B, El-Benna J, Motterlini R, and Boczkowski J. Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J 19: 1890–1892, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Levere RD, Martasek P, Escalante B, Schwartzman ML, and Abraham NG. Effect of heme arginate administration on blood pressure in spontaneously hypertensive rats. J Clin Invest 86: 213–219, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li B, Takeda K, Ishikawa K, Yoshizawa M, Sato M, Shibahara S, and Furuyama K. Coordinated expression of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 and heme oxygenase 2: evidence for a regulatory link between glycolysis and heme catabolism. Tohoku J Exp Med 228: 27–41, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Li M, Peterson S, Husney D, Inaba M, Guo K, Kappas A, Ikehara S, and Abraham NG. Long-lasting expression of HO-1 delays progression of type I diabetes in NOD mice. Cell Cycle 6: 567–571, 2007 [DOI] [PubMed] [Google Scholar]
- 64.Li Y, Su J, DingZhang X, Zhang J, Yoshimoto M, Liu S, Bijian K, Gupta A, Squire JA, Alaoui Jamali MA, and Bismar TA. PTEN deletion and heme oxygenase-1 overexpression cooperate in prostate cancer progression and are associated with adverse clinical outcome. J Pathol 224: 90–100, 2011 [DOI] [PubMed] [Google Scholar]
- 65.Lin H, Yu CH, Jen CY, Cheng CF, Chou Y, Chang CC, and Juan SH. Adiponectin-mediated heme oxygenase-1 induction protects against iron-induced liver injury via a PPARalpha dependent mechanism. Am J Pathol 177: 1697–1709, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin LC, Ho FM, Yen SJ, Wu PY, Hung LF, Huang WJ, and Liang YC. Carbon monoxide induces cyclooxygenase-2 expression through MAPKs and PKG in phagocytes. Int Immunopharmacol 10: 1520–1525, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Lo Iacono L, Boczkowski J, Zini R, Salouage I, Berdeaux A, Motterlini R, and Morin D. A carbon monoxide-releasing molecule (CORM-3) uncouples mitochondrial respiration and modulates the production of reactive oxygen species. Free Radic Biol Med 50: 1556–1564, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Lo SS, Lin SC, Wu CW, Chen JH, Yeh WI, Chung MY, and Lui WY. Heme Oxygenase-1 Gene Promoter Polymorphism is Associated with Risk of Gastric Adenocarcinoma and Lymphovascular Tumor Invasion. Ann Surg Oncol 14: 2250–2256, 2007 [DOI] [PubMed] [Google Scholar]
- 69.Maines MD. and Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A 71: 4293–4297, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, Kajikawa M, Matsumoto T, Kihara Y, Chayama K, Noma K, Nakashima A, Tomiyama H, Takase B, Yamashina A, and Higashi Y. Hyperbilirubinemia, augmentation of endothelial function, and decrease in oxidative stress in Gilbert syndrome. Circulation 126: 598–603, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Mashreghi MF, Klemz R, Knosalla IS, Gerstmayer B, Janssen U, Buelow R, Jozkowicz A, Dulak J, Volk HD, and Kotsch K. Inhibition of dendritic cell maturation and function is independent of heme oxygenase 1 but requires the activation of STAT3. J Immunol 180: 7919–7930, 2008 [DOI] [PubMed] [Google Scholar]
- 72.McCarty MF. “Iatrogenic Gilbert syndrome”—a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin. Med Hypotheses 69: 974–994, 2007 [DOI] [PubMed] [Google Scholar]
- 73.Minetti M, Mallozzi C, Stazi AMMD, and Pietraforte D. Bilirubin is an effective antioxidant of peroxynitrite-mediated protein oxidation in human blood plasma. Arch Biochem Biophys 352: 165–174, 1998 [DOI] [PubMed] [Google Scholar]
- 74.Mishra S, Fujita T, Lama VN, Nam D, Liao H, Okada M, Minamoto K, Yoshikawa Y, Harada H, and Pinsky DJ. Carbon monoxide rescues ischemic lungs by interrupting MAPK-driven expression of early growth response 1 gene and its downstream target genes. Proc Natl Acad Sci U S A 103: 5191–5196, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H, Yamamoto M, and Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22: 66–79, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Molzer C, Huber H, Steyrer A, Ziesel G, Ertl A, Plavotic A, Wallner M, Bulmer AC, and Wagner KH. In vitro antioxidant capacity and antigenotoxic properties of protoporphyrin and structurally related tetrapyrroles. Free Radic Res 46: 1369–1377, 2012 [DOI] [PubMed] [Google Scholar]
- 77.Mosen H, Salehi A, Henningsson R, and Lundquist I. Nitric oxide inhibits, and carbon monoxide activates, islet acid alpha-glucoside hydrolase activities in parallel with glucose-stimulated insulin secretion. J Endocrinol 190: 681–693, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Nakao A, Kimizuka K, Stolz DB, Neto JS, Kaizu T, Choi AM, Uchiyama T, Zuckerbraun BS, Nalesnik MA, Otterbein LE, and Murase N. Carbon monoxide inhalation protects rat intestinal grafts from ischemia/reperfusion injury. Am J Pathol 163: 1587–1598, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neuzil J, Gebicki JM, and Stocker R. Radical-induced chain oxidation of proteins and its inhibition by chain-breaking antioxidants. Biochem J 293 (Pt 3): 601–606, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nicolai A, Li M, Kim DH, Peterson SJ, Vanella L, Positano V, Gastaldelli A, Rezzani R, Rodella LF, Drummond G, Kusmic C, L'Abbate A, Kappas A, and Abraham NG. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension 53: 508–515, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80a.Nishie A, Ono M, Shono T, Fukushi J, Otsubo M, Onoue H, Ito Y, Inamura T, Ikezaki K, Fukui M, Iwaki T, Kuwano M. Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clin Cancer Res 5: 1107–1113, 1999 [PubMed] [Google Scholar]
- 81.Oda E. and Kawai R. Bilirubin is negatively associated with hemoglobin a(1c) independently of other cardiovascular risk factors in apparently healthy Japanese men and women. Circ J 75: 190–195, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Ollinger R, Bilban M, Erat A, Froio A, McDaid J, Tyagi S, Csizmadia E, Graca-Souza AV, Liloia A, Soares MP, Otterbein LE, Usheva A, Yamashita K, and Bach FH. Bilirubin: a natural inhibitor of vascular smooth muscle cell proliferation. Circulation 112: 1030–1039, 2005 [DOI] [PubMed] [Google Scholar]
- 83.Phelan D, Winter GM, Rogers WJ, Lam JC, and Denison MS. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophys 357: 155–163, 1998 [DOI] [PubMed] [Google Scholar]
- 84.Piantadosi CA, Carraway MS, Babiker A, and Suliman HB. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ Res 103: 1232–1240, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Queiroga CS, Almeida AS, and Vieira HL. Carbon monoxide targeting mitochondria. Biochem Res Int 2012: 749845, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rotenberg MO. and Maines MD. Isolation, characterization, and expression in Escherichia coli of a cDNA encoding rat heme oxygenase-2. J Biol Chem 265: 7501–7506, 1990 [PubMed] [Google Scholar]
- 87.Ryter SW. and Otterbein LE. Carbon monoxide in biology and medicine. Bioessays 26: 270–280, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Sacca P, Meiss R, Casas G, Mazza O, Calvo JC, Navone N, and Vazquez E. Nuclear translocation of haeme oxygenase-1 is associated to prostate cancer. Br J Cancer 97: 1683–1689, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sandouka A, Balogun E, Foresti R, Mann BE, Johnson TR, Tayem Y, Green CJ, Fuller B, and Motterlini R. Carbon monoxide-releasing molecules (CO-RMs) modulate respiration in isolated mitochondria. Cell Mol Biol (Noisy-le-grand) 51: 425–432, 2005 [PubMed] [Google Scholar]
- 90.Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger R, Choi AM, and Otterbein LE. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol 289: L1131–L1137, 2005 [DOI] [PubMed] [Google Scholar]
- 91.Schwartzman ML, Masferrer J, Dunn MW, McGiff JC, and Abraham NG. Cytochrome P450, drug metabolizing enzymes and arachidonic acid metabolism in bovine ocular tissues. Curr Eye Res 6: 623–630, 1987 [DOI] [PubMed] [Google Scholar]
- 92.Schwertner HA. and Vitek L. Gilbert syndrome, UGT1A1*28 allele, and cardiovascular disease risk: possible protective effects and therapeutic applications of bilirubin. Atherosclerosis 198: 1–11, 2008 [DOI] [PubMed] [Google Scholar]
- 93.Shepherd RE, Moreno FJ, Cashore WJ, and Fain JN. Effects of bilirubin on fat cell metabolism and lipolysis. Am J Physiol 237: E504–E508, 1979 [DOI] [PubMed] [Google Scholar]
- 94.Shih RH. and Yang CM. Induction of heme oxygenase-1 attenuates lipopolysaccharide-induced cyclooxygenase-2 expression in mouse brain endothelial cells. J Neuroinflammation 7: 86, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simon T, Anegon I, and Blancou P. Heme oxygenase and carbon monoxide as an immunotherapeutic approach in transplantation and cancer. Immunotherapy 3: 15–18, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Skrzypek K, Tertil M, Golda S, Ciesla M, Weglarczyk K, Collet G, Guichard A, Kozakowska M, Boczkowski J, Was H, Gil T, Kuzdzal J, Muchova L, Vitek L, Loboda A, Jozkowicz A, Kieda C, and Dulak J. Interplay between heme oxygenase-1 and miR-378 affects non-small cell lung carcinoma growth, vascularization, and metastasis. Antioxid Redox Signal 19: 644–660, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sodhi K, Inoue K, Gotlinger KH, Canestraro M, Vanella L, Kim DH, Manthati VL, Koduru SR, Falck JR, Schwartzman ML, and Abraham NG. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther 331: 906–916, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal 6: 841–849, 2004 [DOI] [PubMed] [Google Scholar]
- 99.Stocker R. and Keaney JF., Jr.Role of oxidative modifications in atherosclerosis. Physiol Rev 84: 1381–1478, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, and Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 235: 1043–1046, 1987 [DOI] [PubMed] [Google Scholar]
- 101.Suliman HB, Carraway MS, Ali AS, Reynolds CM, Welty-Wolf KE, and Piantadosi CA. The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest 117: 3730–3741, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takano N, Yamamoto T, Adachi T, and Suematsu M. Assessing a shift of glucose biotransformation by LC-MS/MS-based metabolome analysis in carbon monoxide-exposed cells. Adv Exp Med Biol 662: 101–107, 2010 [DOI] [PubMed] [Google Scholar]
- 103.Tanos R, Patel RD, Murray IA, Smith PB, Patterson AD, and Perdew GH. Aryl hydrocarbon receptor regulates the cholesterol biosynthetic pathway in a dioxin response element-independent manner. Hepatology 55: 1994–2004, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tapan S, Karadurmus N, Dogru T, Ercin CN, Tasci I, Bilgi C, Kurt I, and Erbil MK. Decreased small dense LDL levels in Gilbert's syndrome. Clin Biochem 44: 300–303, 2011 [DOI] [PubMed] [Google Scholar]
- 105.Temme EH, Zhang J, Schouten EG, and Kesteloot H. Serum bilirubin and 10-year mortality risk in a Belgian population. Cancer Causes Control 12: 887–894, 2001 [DOI] [PubMed] [Google Scholar]
- 106.Vallabhaneni R, Kaczorowski DJ, Yaakovian MD, Rao J, and Zuckerbraun BS. Heme oxygenase 1 protects against hepatic hypoxia and injury from hemorrhage via regulation of cellular respiration. Shock 33: 274–281, 2010 [DOI] [PubMed] [Google Scholar]
- 107.Verma A, Hirsch DJ, Glatt CE, Ronnett GV, and Snyder SH. Carbon monoxide: a putative neural messenger. Science 259: 381–384, 1993 [DOI] [PubMed] [Google Scholar]
- 108.Vitek L, Jirsa M, Brodanova M, Kalab M, Marecek Z, Danzig V, Novotny L, and Kotal P. Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels. Atherosclerosis 160: 449–456, 2002 [DOI] [PubMed] [Google Scholar]
- 109.Wallner M, Blassnigg SM, Marisch K, Pappenheim MT, Mullner E, Molzer C, Nersesyan A, Marculescu R, Doberer D, Knasmuller S, Bulmer AC, and Wagner KH. Effects of unconjugated bilirubin on chromosomal damage in individuals with Gilbert's syndrome measured with the micronucleus cytome assay. Mutagenesis 27: 731–735, 2012 [DOI] [PubMed] [Google Scholar]
- 110.Wallner M, Bulmer AC, Moelzer C, Muellner E, Marculescu R, Doberer D, Wagner O, and Wagner K-H. Haem catabolism: a novel modulator of inflammation in Gilbert's syndrome. Eur J Clin Invest 43: 912–919, 2013 [DOI] [PubMed] [Google Scholar]
- 111.Wallner M, Marculescu R, Doberer D, Wolzt M, Wagner O, Vitek L, Bulmer AC, and Wagner KH. Protection from age related increase in lipid biomarkers and inflammation contributes to cardiovascular protection in Gilbert's syndrome. Clin Sci 125: 257–264, 2013 [DOI] [PubMed] [Google Scholar]
- 112.Was H, Dulak J, and Jozkowicz A. Heme oxygenase-1 in tumor biology and therapy. Curr Drug Targets 11: 1551–1570, 2010 [DOI] [PubMed] [Google Scholar]
- 112a.Wegiel B, Gallo D, Csizmadia E, Harris C, Belcher J, Vercellotti GM, Penacho N, Seth P, Sukhatme V, Ahmed A, Pandolfi PP, Helczynski L, Biartell A, Persson JL, and Otterbein LE. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 73: 7009–7021, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Weinberger B, Archer FE, Kathiravan S, Hirsch DS, Kleinfeld AM, Vetrano AM, and Hegyi T. Effects of bilirubin on neutrophil responses in newborn infants. Neonatology 103: 105–111, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, and Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science 306: 2093–2097, 2004 [DOI] [PubMed] [Google Scholar]
- 115.Wojakowski W, Tendera M, Cybulski W, Zuba-Surma EK, Szade K, Florczyk U, Kozakowska M, Szymula A, Krzych L, Paslawska U, Paslawski R, Milewski K, Buszman PP, Nabialek E, Kuczmik W, Janiszewski A, Dziegiel P, Buszman PE, Jozkowicz A, and Dulak J. Effects of intracoronary delivery of allogenic bone marrow-derived stem cells expressing heme oxygenase-1 on myocardial reperfusion injury. Thromb Haemost 108: 464–475, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, and Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 103: 129–135, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yesilova Z, Serdar M, Ercin CN, Gunay A, Kilciler G, Hasimi A, Uygun A, Kurt I, Erbil MK, and Dagalp K. Decreased oxidation susceptibility of plasma low density lipoproteins in patients with Gilbert's syndrome. J Gastroenterol Hepatol 23: 1556–1560, 2008 [DOI] [PubMed] [Google Scholar]
- 117a.Yin Y, Liu Q, Wang B, Chen G, Xu L, Zhou H. Expression and function of heme oxygenase-1 in human gastric cancer. Exp Biol Med (Maywood) 237: 362–371, 2012 [DOI] [PubMed] [Google Scholar]
- 118.Yoshino S, Hamasaki S, Ishida S, Kataoka T, Yoshikawa A, Oketani N, Saihara K, Okui H, Shinsato T, Ichiki H, Kubozono T, Kuwahata S, Fujita S, Kanda D, Nakazaki M, Miyata M, and Tei C. Relationship between bilirubin concentration, coronary endothelial function, and inflammatory stress in overweight patients. J Atheroscler Thromb 18: 403–412, 2011 [DOI] [PubMed] [Google Scholar]
- 119.Zelenka J, Lenicek M, Muchova L, Jirsa M, Kudla M, Balaz P, Zadinova M, Ostrow JD, Wong RJ, and Vitek L. Highly sensitive method for quantitative determination of bilirubin in biological fluids and tissues. J Chromatogr B Analyt Technol Biomed Life Sci 867: 37–42, 2008 [DOI] [PubMed] [Google Scholar]
- 120.Zenke-Kawasaki Y, Dohi Y, Katoh Y, Ikura T, Ikura M, Asahara T, Tokunaga F, Iwai K, and Igarashi K. Heme induces ubiquitination and degradation of the transcription factor Bach1. Mol Cell Biol 27: 6962–6971, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang W, Qiao T, and Zha L. Inhibition of heme oxygenase-1 enhances the radiosensitivity in human nonsmall cell lung cancer a549 cells. Cancer Biother Radiopharm 26: 639–645, 2011 [DOI] [PubMed] [Google Scholar]
- 122.Zhang X, Shan P, Alam J, Davis RJ, Flavell RA, and Lee PJ. Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38alpha mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J Biol Chem 278: 22061–22070, 2003 [DOI] [PubMed] [Google Scholar]
- 123.Zuckerbraun BS, Billiar TR, Otterbein SL, Kim PK, Liu F, Choi AM, Bach FH, and Otterbein LE. Carbon monoxide protects against liver failure through nitric oxide-induced heme oxygenase 1. J Exp Med 198: 1707–1716, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zuckerbraun BS, Chin BY, Bilban M, d'Avila JC, Rao J, Billiar TR, and Otterbein LE. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. Faseb J 21: 1099–1106, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Zuckerbraun BS, McCloskey CA, Gallo D, Liu F, Ifedigbo E, Otterbein LE, and Billiar TR. Carbon monoxide prevents multiple organ injury in a model of hemorrhagic shock and resuscitation. Shock 23: 527–532, 2005 [PubMed] [Google Scholar]