Abstract
Significance: Heme oxygenase-1 (HO-1) is a potential therapeutic target in many diseases, especially those mediated by oxidative stress and inflammation. HO-1 expression appears to regulate the homeostatic activity and distribution of mononuclear phagocytes (MP) in lymphoid tissue under physiological conditions. It also regulates the ability of MP to modulate the inflammatory response to tissue injury. Recent Advances: The induction of HO-1 within MP—particularly macrophages and dendritic cells—modulates the effector functions that they acquire after activation. These effector functions include cytokine production, surface receptor expression, maturation state, and polarization toward a pro- or anti-inflammatory phenotype. The importance of HO-1 in MP is emphasized by their expression of specific receptors that primarily function to ingest heme-containing substrate and deliver it to HO-1. Critical Issues: MP are the first immunological responders to tissue damage. They critically affect the outcome of injury to many organ systems, yet few therapies are currently available to specifically target MP during disease pathogenesis. Elucidation of the role of HO-1 expression in MP may help to direct broadly applicable therapies to clinical use that are based on the immunomodulatory capabilities of HO-1. Future Directions: Unraveling the complexities of HO-1 expression specifically within MP will more completely define how HO-1 provides cytoprotection in vivo. The use of models in which HO-1 expression is specifically modulated in bone marrow-derived cells will allow for a more complete characterization of its immunoregulatory properties. Antioxid. Redox Signal. 20, 1770–1788.
Introduction
Heme oxygenase-1 (HO-1) is an inducible enzyme that degrades pro-oxidant heme into equimolar quantities of carbon monoxide (CO), iron, and biliverdin (170, 192). Biliverdin is immediately converted to bilirubin by biliverdin reductase (193). The byproducts of HO-1 enzymatic activity are cytoprotective because of their antioxidant and anti-inflammatory properties (83, 122, 130). The well-established ability of HO-1 expression to prevent injury in a number of disease models has been extensively reviewed elsewhere (13, 127, 130, 141, 159, 186). This review presents current evidence and concepts on the involvement of HO-1 and heme on mononuclear phagocytes (MP) with a particular emphasis on macrophages and dendritic cells (DC). The interest in these cell types stems from their central role in heme recycling, innate immunity, and antigen presentation. We examine evidence suggesting that HO-1 modulates the immune system during homeostasis and disease by regulating the function of MP.
The Mononuclear Phagocyte System
The mononuclear phagocyte system (MPS) consists of cells derived from myeloid progenitors during hematopoiesis. These cells include circulating monocytes in the blood, in addition to tissue macrophages and DC, which are MP (69). MP have specialized properties including high motility, phagocytosis, and the ability to generate a remarkable array of soluble factors and surface molecules (156). They are present throughout the body as resident populations in many organs including the brain, skin, liver, lung, kidney, and heart. They can also be recruited from the circulation to sites of local injury. Tissue-resident MP are sentinels for disease, serving as first responders to tissue insult (145). The phenotypes of MP are heterogeneous and dependent on the microenvironment that they inhabit (15, 44, 62). There is evidence that tissue homeostasis is subject to regulation by tissue resident MP, and that multiple modes of communication exist between parenchymal cells and resident MP (64, 81, 82, 146, 181, 191). In the heart, for example, resident macrophages express high levels of HO-1 and CD163 and directly interact with endothelial cells and cardiac myocytes (134). Tissue-resident MP may integrate signals from the surrounding tissue as part of a global network (37, 98).
The primary functions of MP arise from their ability to ingest and process material from the extracellular environment: (i) antigen presentation to cells of the adaptive immune system and (ii) homeostatic or injury-induced clearance of senescent or damaged cells, cellular debris, and pathogens. The former is generally considered a functional hallmark of DC, which are more motile than macrophages, and thus more commonly migrate to regional lymphoid tissue where they present processed antigen to the adaptive immune system (7, 185). Macrophages, on the other hand, are less efficient at antigen presentation, but are well suited for phagocytic clearance of cellular debris due to their catabolic phenotype (117). Thus, although macrophages and DC are both MP, and therefore unified in their ability to ingest extracellular material, their role in the immune system diverges thereafter. In accordance with the distinct functions of the cells that comprise the MPS, the result of HO-1 expression in these cells is complex and multifaceted.
Cellular damage or death results in the generation of damage/danger-associated molecular patterns (DAMPs) (17, 78, 108). DAMPs bind to pattern recognition receptors (PRR) on MP, resulting in the release of chemical mediators, such as cytokines and chemokines (96, 106, 107). These mediators activate resident inflammatory cells and attract circulating MP to the site of damage (66). They also stimulate accelerated hematopoietic generation of MP in the bone marrow. At the site of injury, the resident and infiltrating MP remove dead or dying cells and cellular debris. The composition of the material ingested by MP is essential in determining the downstream inflammatory response (22, 108). For example, infection activates MP when microbial peptides or polysaccharide (also called pathogen-associated molecular patterns [PAMPs]) are recognized by PRR on their surface. These PRR include the toll-like receptor (TLR) family of innate immune receptors (4). In organ transplantation, DAMPs formed as a result of ischemia/reperfusion injury activate the innate immune system (86, 94, 135). Thus, infection and transplantation generate an inflammatory response by activating the MPS, resulting in the production of cytokines and chemokines and priming of the adaptive immune system (42). Recognition of phosphatidylserines on apoptotic cells by MP PRRs is also important during the process of development, but does not result in an inflammatory response (28, 38).
Sterile inflammation is principally regulated at the level of the MPS because MP integrate a variety of signals that shape the evolution of an immune response (12). They express a diverse repertoire of receptors on their cell surface. These receptors have broad ligand specificity (unlike antigen-specific receptors on lymphocytes) and are linked to downstream signaling pathways that regulate the differentiation and effector function(s) of MP (76). An immune response to sterile tissue injury is dependent upon (i) the distribution of MP in the injured organ, (ii) the nature of the injury stimulus and the cell-associated antigens (i.e., MP receptor ligands) that are generated by it, (iii) the ligand-receptor interaction on the MP cell surface, and (iv) ligand processing and the activation of downstream effector pathways in MP. In a number of disease states, these events dictate whether the response to tissue injury favors injury resolution or tolerance (an anti-inflammatory response) or chronic inflammation and exaggerated tissue injury (a pro-inflammatory response) (44, 156).
MP are remarkably heterogeneous. Macrophages, the most broadly distributed and numerous members of the MPS, respond to an extremely wide range of environmental cues that can result in dramatic changes in their morphology and gene expression (44). Macrophages orchestrate both the initiation and resolution of inflammation and therefore can mediate either pro- or anti-inflammatory immune responses. For simplicity, we will refer to a classification scheme for macrophages with two broad categories, with the caveat that, in fact, it is a simplified framework placed on a continuum of possible functional states (116).
Classically activated macrophages, called M1, are important immune effector cells involved in pro-inflammatory responses (60, 61). They are typically activated by two stimuli. The first, IFN-γ, is produced by a number of cell types including NK cells and TH1 cells. The second stimulus typically comes from ligation of one of the TLRs by molecules bearing patterns associated with microbes (PAMPs; e.g., TLR4) or damage (DAMPs; e.g., TLR3 and TLR9) (60). Alternatively activated macrophages, also called M2, can be subdivided into M2a-M2c based on their induction stimulus (M2a, interleukin [IL]-4; M2b, immune complexes; M2c, IL-10/TGF-β) and expression of a relatively distinct array of intracellular and surface markers (60, 116, 196). M2 macrophages are considered anti-inflammatory, with pro-angiogenic and tissue remodeling properties that are associated with the resolution of inflammation (56). The notion that the polarization of macrophages extends beyond a simple M1 versus M2 classification system is supported by the recent identification of several novel macrophage subsets. These include hemorrhage-specialist macrophages (Mhem)/hemorrhage-associated macrophages (HA-mac), macrophages generated with oxPAPC (Mox), and M4 macrophages, which are discussed in detail below. It is likely that additional macrophage subtypes will continue to be identified.
Investigations of MP biology in the context of heme recycling and HO-1 activity provide compelling evidence that protection from oxidative damage is only one of the modes by which HO-1 exerts functional effects on the immune response (20, 24, 90, 174). The literature on the role of HO-1 in immune signaling pathways is complex because of the variety of experimental systems used, the ability of nearly all cells to make HO-1 under certain conditions, the dependence of certain observations on substrate availability, and because pharmacologic modulators of HO-1 expression are not entirely specific (105). However, consistent themes have emerged indicating a role for HO-1 in inflammatory responses.
MP HO-1 in Tissue Homeostasis
A role for HO-1 in immunity became apparent during the characterization of an HO-1-deficient patient (187) and HO-1-deficient mice (8). Kapturczak and colleagues showed that the proportions of T cells, B cells, and macrophages were relatively normal in young HO-1-deficient mice, but the mice produced abnormally high levels of immunoglobulin in the circulation. Additionally, stimulation of HO-1−/− splenocytes with LPS resulted in significantly elevated levels of tumor necrosis factor-alpha (TNF-α), IL-6, IL-12, and IL-1 in comparison to splenocytes from wild-type mice (83). These results suggest that HO-1 deficiency potentiates the pro-inflammatory activation of stimulated MP. In both humans (187) and mice (90), the cytoarchitecture of the secondary lymphoid tissues is markedly abnormal. The two published reports of human HO-1 deficiency indicated that the patients were essentially asplenic (61, 187). Hmox1−/− mice first exhibit splenomegaly, but as they age the relative spleen size diminishes and the red pulp is progressively replaced by fibrotic tissue resulting from progressive depletion of splenic macrophages. Therefore, the asplenia observed in the HO-1-deficient human could have been secondary to heme-toxicity mediated by ROS in the splenic microenvironment and depletion of intra-splenic macrophages that recycle heme after phagocytosis of senescent red blood cells (90). Collectively, these findings suggest that both the innate and adaptive immunity are strongly affected by HO-1 deficiency.
The anti-inflammatory properties of HO-1 have been described in many disease models (6, 10, 123, 129). HO-1 can dampen the inflammatory response indirectly (i.e., extrinsic to the MPS) by preventing tissue injury and necrotic cell death (i.e., DAMP production). However, recent evidence has shown that HO-1 expression within cells of the immune system (i.e., intrinsic to the MPS) modulates the inflammatory response directly by affecting differentiation pathways and effector functions (11, 174, 183). Induction of HO-1 in human peripheral blood monocytes inhibits their chemotactic activity (115). MP infiltrating sites of tissue injury robustly express HO-1 (48, 97). Further, HO-1 deficiency in vivo leads to chronic inflammation, perturbed distribution and function of the MPS, and exaggerated inflammatory responses in disease models (65, 83, 90, 131).
The IL-10/HO-1 Axis in MP
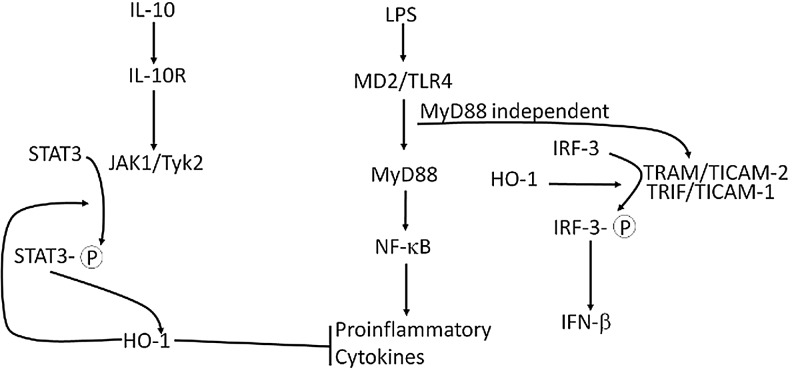
Several lines of evidence suggest that some immunoregulatory properties of HO-1 are related to IL-10 and vice-versa (Fig. 1). Chen and colleagues found that a single intramuscular injection of an adeno-associated viral vector encoding IL-10 in recipient rats bearing abdominal aortic allografts protected the grafts from neointimal proliferation. Inhibition of HO activity with zinc protoporphyrin IX (ZnPP) abrogated the protection (40). Blocking HO activity with tin protoporphyrin IX (SnPP) also abrogates or reduces the protective effects of IL-10 in septic shock (95) and ethanol toxicity (50). These findings are consistent with an earlier report by Lee and Chau that IL-10 administration protected mice treated with a lethal dose of lipopolysaccharide (LPS; endotoxin), while pretreatment with ZnPP abrogated the protection. Notably, they showed that IL-10 is a potent inducer of HO-1 in primary macrophages and in the J774.1 macrophage cell line. IL-10 treatment suppressed LPS-activated production of TNF-α, and treatment with anti-sense oligonucleotides complementary to HO-1 attenuated the inhibitory effect of IL-10. These effects appeared to be related to CO generation (95). Thus, IL-10 can suppress LPS-induced immune responses in an HO-1-dependent manner. Although IL-10 does appear to regulate HO-1 expression, the effect on the downstream anti-inflammatory activity of IL-10 remains somewhat controversial and therefore warrants further elucidation (137).
FIG. 1.
The HO-1/IL-10 axis. Evidence supports the involvement of HO-1 in both IL-10 receptor signaling and signals mediated by the MyD88-independent TLR4 pathway. The main control points appear to be STAT-3 and IRF-3, respectively, although the precise mechanisms of control remain to be addressed experimentally. See text for a discussion of these pathways. HO-1, heme oxygenase-1; IL, interleukin; IRF-3, interferon regulatory factor-3; MyD88, myeloid differentiation factor 88; TLR, toll-like receptor; STAT-3, signal transducer and activator of transcription-3.
These findings raise the question of how IL-10 signaling and HO-1 are related. We can simplify the logic by initially assuming that signaling by LPS through TLR4 is not directly interconnected to IL-10 and that the suppressive effects of IL-10 are exerted at the endpoint of the TLR4 pathway (Fig. 1). Also, while treatment with LPS induces HO-1, it appears to be via a separate pathway, since LPS can induce HO-1 in the presence of IL-10 neutralizing antibodies (137). IL-10 binds to a receptor tetramer complex that activates phosphorylation of Janus tyrosine kinases, JAK1 and Tyk2, which then phosphorylate two specific tyrosine residues that form part of a docking site for signal transducer and activator of transcription-3 (STAT-3). STAT-3 is tyrosine phosphorylated by the receptor associated JAKs and then translocates to the nucleus where it binds to STAT binding elements in the promoters of various genes (49). Ricchetti and colleagues found that IL-10-induced expression of HO-1 requires STAT-3 in macrophage cell lines. Cells transfected with a STAT-3 dominant-negative-encoding adenovirus did not express HO-1 when stimulated with IL-10 (137). This interconnection was recently demonstrated in vivo in a liver ischemia-reperfusion injury model, in which liver damage is largely the result of Kupffer cell-mediated injury (85). Mice treated with adenovirus coding for HO-1 were protected against liver injury, but the protection was essentially abrogated when the mice were also treated with STAT-3 small interfering RNA (siRNA). Further, CD68+ macrophages co-stained for HO-1 and phosphorylated STAT-3, and STAT-3 knockdown decreased the frequency of such cells.
HO-1 in TLR4 Signaling
LPS binds to MD-2 associated with TLR4, which induces a homotypic interaction of TLR4, resulting in two possible downstream signals. One is the myeloid differentiation factor 88 (MyD88)-dependent pathway, which results in activation of nuclear factor-kappa B (NF-κB) and the production of pro-inflammatory cytokines (e.g., TNF-α and IL-6). The other is MyD88-independent, leading to the activation of IFN-β transcription via interferon regulatory factor-3 (IRF-3) (144) (Fig. 1). Tzima and colleagues conditionally ablated HO-1 in MP and showed that HO-1 deficiency in these cells impairs IFN-β production induced by TLR-4 or TLR-3 agonists (174). These TLRs share a common adaptor molecule, TRIF (TIR-domain containing adaptor reducing interferon-β), which forms a complex with other molecules and results in phosphorylation of IRF-3 (3). IRF-3 nuclear accumulation was significantly reduced in HO-1-deficient macrophages and immunoprecipitation of IRF-3 indicated that it directly interacts with HO-1 (174). Therefore, HO-1 may directly affect TLR-mediated signaling.
Pathways for HO-1 Substrate Acquisition in the MPS
The MPS is principally responsible for heme-iron uptake and recycling. The pool of free heme is often dramatically increased following tissue injury because heme is an integral component of many intracellular enzymes (e.g., cytochromes, peroxidases, and nitric oxide [NO] synthase). Additionally, damaged myocytes and erythrocytes are significant sources of heme release during injury because they contain high concentrations of myoglobin (Mb) and hemoglobin (Hb), respectively (18). Heme and Hb mediate tissue injury by generating free radicals via Fenton chemistry and by scavenging NO, respectively. NO plays an important role in regulation of smooth muscle tone, platelet aggregation, and the expression of endothelial adhesion molecules (20, 107, 108). Free heme also binds to TLR4 and causes the secretion of pro-inflammatory cytokines (52). Therefore, mechanisms exist to scavenge heme/Hb and funnel it to the HO system for degradation. Receptor-mediated mechanisms prevent the cytotoxicity associated with heme, which can generate ROS in the extracellular space, or in the cell membrane and cytosol because it is lipid-soluble (17, 78). Receptor-mediated uptake ensures that heme is segregated to the lysosomal pathway where it is specifically delivered to the HO enzyme system for degradation (149). Thus, HO converts pro-oxidant heme into cytoprotective byproducts and Fe2+, which is quickly bound to ferritin.
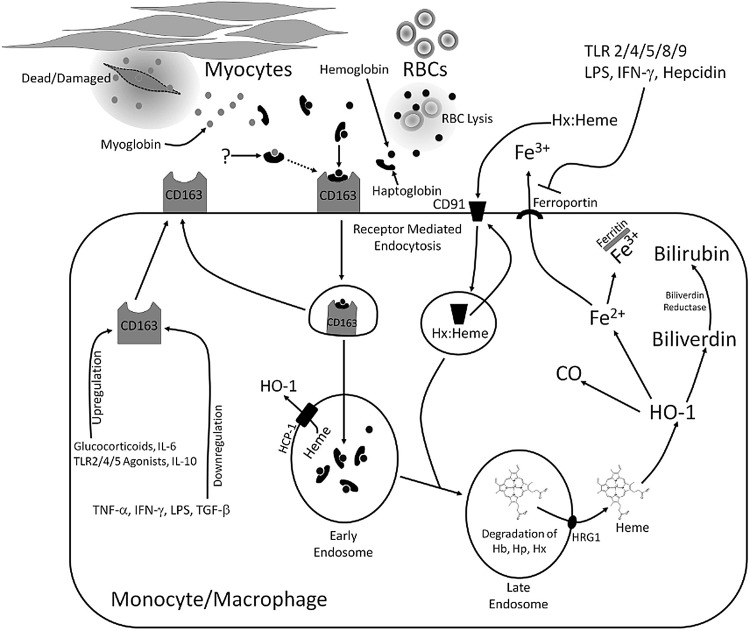
HO-1 is upregulated after injury because its induction is coupled to the increased bioavailability of heme and its delivery to the enzyme (2, 24). Macrophages have at least two specific cell-surface receptor systems for heme uptake, which couple tissue injury to HO-1 induction and modulation of macrophage function. Scavenging of heme contained in Hb is mediated by CD163, a receptor that is expressed only on the surface of monocytes and macrophages (149, 175). CD163-dependent clearance and degradation of Hb involves several steps (Fig. 2). Hb released by intravascular hemolysis immediately forms a complex with haptoglobin (Hp), an acute phase reactant produced in the liver (91). Hp expression is stimulated by the proinflammatory cytokine, IL-6 (124); so, circulating Hp levels can be used as a clinical marker of infection (high Hp) (9). On the other hand, severe hemolysis rapidly depletes Hp levels (140, 169). On the surface of circulating monocytes or tissue macrophages, CD163 binds to Hb complexed with Hp at high affinity or, when Hp is depleted, to free Hb with low affinity (151). Ligation of the CD163 receptor by Hp:Hb leads to receptor-mediated endocytosis and clearance of Hb. In the early endosome, the CD163 receptor is recycled to the cell surface, while the globin chain of Hb is degraded in the late endosome. Heme from Hb is then transported from the lumen of the phagolysosome to microsomal HO-1 via the lysosomal transporters HCP-1 or HRG-1 where it is degraded to iron, CO, and biliverdin (150, 184). CO is an anti-inflammatory and cytoprotective gas (129, 160). Iron is quickly sequestered by ferritin, which is co-induced with HO-1 (16, 19), consistent with the finding that CD163+ macrophages stain strongly positive for iron in in vivo models (53). Regulation of iron homeostasis in macrophages plays an important role in their response to inflammatory stimuli and production of TNF-α and IL-6 (179, 197). Biliverdin and bilirubin are potent antioxidants, but also have inhibitory effects on complement activation, T-cell proliferation, and cytokine production (63, 121, 167, 188).
FIG. 2.
Mechanisms of substrate acquisition for HO-1 in macrophages and monocytes. Hemoglobin (Hb) released by erythrocyte lysis is rapidly bound by the acute-phase protein, haptoglobin (Hp). Free extracellular heme is bound by the acute-phase protein, hemopexin (Hx). The Hp:Hb and Hx:heme complexes bind to the CD163 and CD91 surface receptors, respectively, and are internalized by receptor-mediated endocytosis. The mechanism of myoglobin (Mb) clearance is currently unknown and may involve either a cell surface receptor such as CD163 or Hx-mediated clearance of heme after it is liberated from Mb by oxidation. In the early endosome, the CD163 or CD91 receptor is recycled to the cell membrane. In the late endosome, Hp and Hx and the globin chain of Hb are degraded, liberating the heme molecule. Heme is transported to the cytosol by HRG1 (or HCP-1 if it is liberated in the early endosome) for degradation by HO-1, producing equimolar quantities of CO, Fe2+, and biliverdin. CO has anti-inflammatory properties. Due to its ferroxidase activity, ferritin oxidizes ferrous iron (Fe2+) to ferric iron (Fe3+) for safe storage. Iron can also be exported from the cell by ferroportin. Biliverdin is rapidly converted to bilirubin, a potent antioxidant, by the enzyme biliverdin reductase. As depicted, this pathway is subject to control by immunological mediators such as cytokines and TLR receptor agonists (see text for discussion). CO, carbon monoxide; Hx:heme, hemopexin complexed with heme.
CD163-mediated uptake of Hb by macrophages is an anti-inflammatory response to tissue injury, which is attributed to the clearance of toxic Hb and to the downstream effects that Hb uptake and degradation exerts on macrophage differentiation and effector function (111). Classically activated pro-inflammatory macrophages are CD163lo/−, while alternatively activated M2 macrophages, known for their ability to dampen the immune response, express high levels of CD163 (35). While it seems likely that heme-containing Mb, which is toxic when released after myocardial injury or rhabdomyolysis, is cleared by CD163 or a similar mechanism, it remains to be confirmed experimentally.
The uptake of Hb by CD163 on monocyte/macrophage (MM) is tightly regulated by cytokines and TLR agonists (Fig. 2) (36, 67, 176, 182). In cultured macrophages, Hp:Hb increases CD163 expression, IL-10 secretion, and HO-1 expression. The latter is suppressed with blocking antibodies for the CD163 receptor or soluble antibodies to IL-10. This finding, which was recapitulated in macrophages isolated from skin blisters and patients on cardiopulmonary bypass, suggests that IL-10 represents a link between increased ability for clearance of Hb during inflammation and the induction of HO-1 in MM (133).
The anti-inflammatory effect of CD163 is likely HO-1 dependent. Both HO-1 and CD163 expression is upregulated by the functionally divergent cytokines IL-6 (an early pro-inflammatory marker) and IL-10 (anti-inflammatory) (35, 95, 109, 168, 175), suggesting that these cytokines form a positive feedback system to increase the capacity for Hb clearance (CD163) and metabolism (HO-1) during acute inflammation and then again during resolution of the inflammatory response. Collectively, these data support an anti-inflammatory function whereby CD163 clears toxic free Hb and delivers it to HO-1, stimulating the secretion of IL-10 and the generation of anti-inflammatory CO and antioxidant bilirubin. Tissue-resident macrophages and monocytes infiltrating sites of injury strongly express CD163 on their surface. Since the anti-inflammatory mediators IL-10 and the glucocorticoids enhance CD163 and HO-1 expression in these cells, CD163+ macrophages have been implicated in the resolution of inflammation and in wound healing (133, 189).
The CD163 scavenger receptor (SR) appears to be specific for Hb or Hp:Hb complexes (151). However, free heme is highly toxic and can be released at the site of injury by heme proteins including Mb, enzymes such as the cytochromes or catalase, or by oxidation of Hb. In these scenarios, the circulating acute phase protein, hemopexin (Hx) binds to heme and serves as a means for its clearance (68, 70, 78). In 2005, Hvidberg and colleagues identified the low-density lipoprotein (LDL) receptor-related protein, CD91, as the hemopexin complexed with heme (Hx:heme) complex receptor (70). Hx:heme complexes (but not heme alone) undergo receptor-mediated endocytosis upon binding to CD91, which is followed by recycling of CD91 to the cell membrane, degradation of Hx in the endosome, and delivery of heme to HO-1 (Fig. 2). Current evidence supports overlapping functions for the CD91/Hx:Heme and CD163/Hp:Hb systems. Mice deficient for the genes encoding Hp and Hx are more sensitive to hemolytic stress than mice that lack either gene alone (171, 172). Additionally, both receptors are expressed on anti-inflammatory macrophages and co-regulated by glucocorticoids (103, 111). However, unlike CD163, the CD91 receptor is expressed on hepatocytes, fibroblasts, and syncytiotrophoblasts in addition to macrophages (110, 158). The downstream consequences of heme ingestion in macrophages are not dependent on the mechanism(s) of its delivery to HO-1 (33). Therefore, although the downstream effects of CD91-mediated heme clearance have not been as well characterized as the CD163-mediated system, by increasing the pool of substrate available to HO-1, the CD91 system may have overlapping effects on the inflammatory response.
HO-1 is a Heme-Dependent Integrator of the MPS Anti-Inflammatory Response
Schaer and colleagues were the first to demonstrate that Hb-mediated induction of HO-1 through the CD163 receptor is a unique and defining transcriptional response in macrophages (148). In contrast to previous reports that used Hb contaminated with endotoxin (138, 176), these macrophages are characterized by an anti-inflammatory gene expression profile and a unique three-gene signature of increased hmox-1 and glutamate-cysteine ligase modified (gclm) and suppressed [δ]-aminolevulinate synthase (alas1), the rate-limiting enzyme in heme synthesis (142). This gene expression profile clearly distinguishes heme-induced CD163+ macrophages from the M1 or M2 macrophage subsets. Interestingly, the unique Hb-CD163 genetic signature is a direct result of increased intracellular heme, as treatment of macrophages with free heme (which enters the cell independent of the CD163 receptor) results in an identical transcriptional profile as compared to CD163-dependent ingestion of Hb (148). The CD163+ HO-1+ macrophage population is significantly expanded in the bone marrow and liver of patients with sepsis and in atherosclerotic plaques in heme-rich regions of neovascularization or intraplaque hemorrhage (IPH) (31, 152). Thus, this macrophage subset is functional in vivo and HO-1 may be the operative enzyme system through which ingested heme-containing material directly influences the inflammatory response.
In 2009, Boyle and colleagues clarified the importance of HO-1 expression in macrophage responses to IPH (31). They showed that monocytes infiltrating atherosclerotic plaques with IPH are “adaptively modeled” by Hb ingestion and develop into a unique population of CD163hi HLA-DRlo macrophages, which were originally referred to as HA-mac. HA-mac generated in culture with Hp:Hb robustly express HO-1 and IL-10, and exhibit suppressed intracellular oxidative stress. Neutralizing IL-10 antibodies blocked the Hp:Hb-mediated differentiation of HA-mac, implicating autocrine IL-10 production in the differentiation process (31). Boyle and colleagues also showed that HO-1 is required for the development of HA-mac based on four observations (33). First, inhibition of lysosomal processing prevented HA-mac development in vitro, suggesting a requirement for heme delivery to HO-1 from the endosomal compartment. Second, phagocytosis of oxidatively damaged erythrocytes, a source of heme that is ingested via the macrophage scavenger receptor CD204, drove HA-mac differentiation to the same extent as heme or CD163-Hb. Thus, the macrophage response to heme is not dependent on the CD163 receptor, but instead, a downstream mediator such as HO-1. This finding supports the notion that upregulation of CD163 expression is a response to increased free heme in the external environment, while the differentiation and functional characteristics of HA-mac are dependent upon heme processing by HO-1. Third, when macrophages were treated with heme, the resulting upregulation of CD163, suppression of HLA-DR, and secretion of IL-10 is blocked by inhibition of HO-1 with ZnPP or siRNA. Fourth, HO-1 induction by heme in HA-mac is mediated by the transcription factor NF-E2-related factor 2 (Nrf2), a master regulator of the antioxidant response that induces HO-1 upon binding to the antioxidant response element in the hmox-1 promoter/enhancer after release from its negative regulator Kelch-like ECH-associated protein 1 (Keap-1) (5, 72, 74, 75, 100). HO-1 induction in heme-treated macrophages is blocked by Nrf2 siRNA, while IL-10 secretion and HA-mac differentiation is potentiated by the pharmacological activation of Nrf2. Therefore, activation of HO-1 is an important regulatory step in the development of HA-mac, a process that is facilitated by, but not dependent upon expression of surface CD163. Whether other transcription factors, such as the AP-1 family, are involved in HA-mac differentiation remains to be elucidated experimentally.
HO-1 and Atheroprotective Macrophages
The general atheroprotective actions of HO-1 are well documented (114, 190). Recently, a more complete understanding of this process has implicated HO-1 expression within MP as an effector of atheroprotection (128). Microenvironmental heterogeneity in atherosclerotic plaques drives the development of distinct macrophage subpopulations that differentially effect the progression of atherosclerotic disease based on their effector functions (30). In lesions, HA-mac are iron-laden cells that are found in areas of plaque neovascularization or IPH (88). Therefore, they occupy a distinct microenvironment relative to lipid-laden foam cell macrophages. In the plaque's necrotic core, oxidized LDL (oxLDL) is bound to scavenger receptors including CD36, SR-A/B, and LOX-1 on macrophages (163). Internalized oxLDL contributes to macrophage differentiation into foam cells, which participate in plaque progression (102, 139). Spann et al. recently demonstrated that when macrophages accumulate lipid and form foam cells, their expression of pro-inflammatory mediators is suppressed (162). However, when HO-1-deficient macrophages are treated with oxLDL, generation of ROS is amplified and secretion of pro-inflammatory IL-6, MCP-1, and the IL-8 homologue, KC is increased (128). Thus, HO-1 expression in macrophages may render them resistant to foam cell formation and to production of pro-inflammatory mediators that exacerbate atherogenesis and plaque progression.
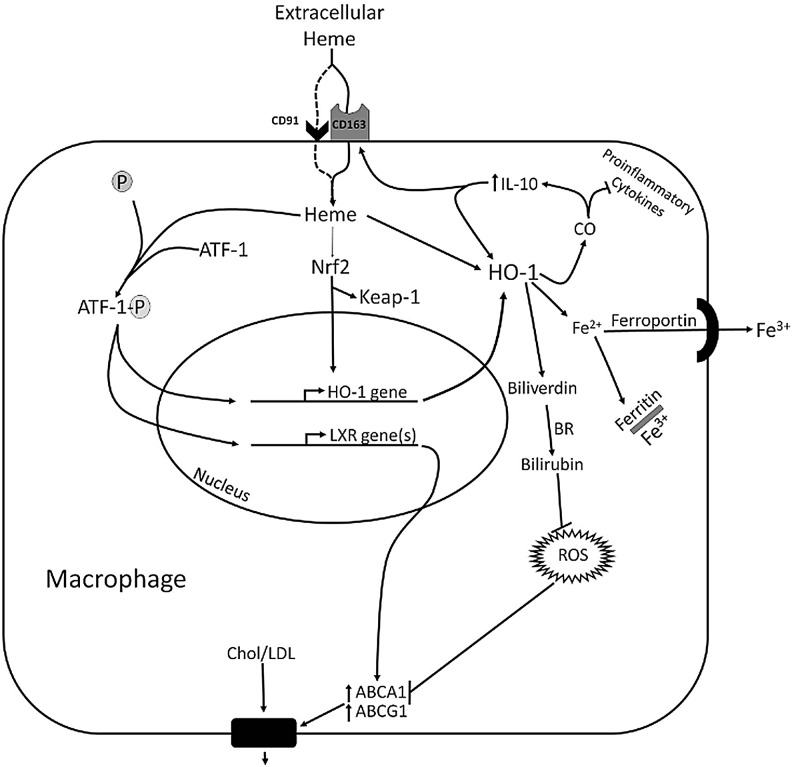
Recently, HA-mac have been renamed Mhem, and will be referred to as such hereafter (32). Mhem are co-dependent on coordinated regulation of iron homeostasis and lipid handling (Table 1). They store iron, and in contrast to foam cells, are resistant to lipid accumulation. This finding is accounted for by a coupled relationship between the redox state of the cell and the expression of lipid efflux pumps (Fig. 3). When macrophages are exposed to Hb-heme in lesions with IPH, heme uptake triggers the induction of two transcription factors, Nfr2 and activating transcription factor-1 (ATF-1) (32, 33). ATF-1 regulates two downstream pathways that may be integrated by Hb-heme-induced HO-1 activity, ultimately resulting in the atheroprotective properties of Mhem and their resistance to lipid accumulation. The first pathway is the result of ATF-1-mediated induction of HO-1 and its potent antioxidant and iron-regulatory properties. The second pathway is the result of ATF-1-mediated upregulation of lipid efflux pumps on the Mhem cell membrane by positively regulating the expression of the master lipid metabolism regulator, transcription factor liver X receptor (LXR)-β (77). LXR-β increases the expression of the ABC transporters, ABCA1 and ABCG1, which function as membrane cholesterol efflux pumps (53). However, upregulation of these pumps is blocked by reactive oxygen species (ROS), which is characteristic in foam cells. In Mhem, HO-1 induction likely prevents ROS, thus affording the cell protection from foam cell formation (53). Macrophages treated with Hp:Hb are protected against ROS formation induced by oxLDL. However, this effect is reversed when HO-1 expression is blocked with siRNA (32). Free iron is a redox active byproduct of heme degradation by HO-1. Therefore, the finding that Mhem have low intracellular ROS despite a principal role in scavenging iron-containing Hb seems counter-intuitive. However, HO-1 expression confers protection to oxidative stress in macrophages by generating antioxidants (i.e., biliverdin and bilirubin), and by promoting the sequestration and export of redox-active iron. Further, the production of IL-10 in Mhem, which appears to be at least partially dependent on HO-1 expression (32), potentiates the release of free iron from macrophages (101). In addition, the free iron exporter, ferriportin is also upregulated in Mhem (53, 194, 197). Ferritin is co-induced with HO-1 (19), resulting in sequestration of iron, thus rendering the iron biologically inactive. Accordingly, Mhem are iron-laden cells, but their intracellular free iron concentration is low and they are resistant to oxidative stress (180). The importance of iron handling in macrophages was also demonstrated in a study of chronic venous leg ulcers, which result from the inability to resolve chronic inflammation. In chronic venous leg ulcers, inflammation is driven by an iron-dependent M1 macrophage population. Although this macrophage population is CD163+ as a consequence of abundant Hb-heme, iron-overload blocks M1 to M2 polarization, and thus resolution of the inflammatory response (157).
Table 1.
Characteristics of Heme-Induced Macrophages
| Category | Heme-induced macrophages | References |
|---|---|---|
| Surface antigen expression | ↑CD163a, CD206 | (31, 33, 53) |
| ↓HLA-DRa | ||
| oxLDL scavenger receptor expression | ↓SR-A1, SR-A2, SR-B1, CD36 | (53) |
| Cytokine production | ↑IL-10a, IL-1RA | (31, 33, 53, 133) |
| ↓TNF-α | ||
| Gene expression profileb | ↑Hmox-1, Gclm | (148) |
| ↓Alas1 | ||
| Transcription factors | Nrf2a, ATF-1a, LXR-β | (32, 33) |
| Cell membrane transporters | ↑Cholesterol efflux transporters, ABCA1 and ABCG1 | (32, 53) |
| ↑Ferroportin | ||
| Intracellular characteristics | ↑Ferritin-bound iron | (31, 33, 53, 151) |
| ↓Free iron, ROSa, Lipid |
HO-1 expression is suggested to be directly involved.
CD163/Hb pathway-dependent gene expression profile.
ATF-1, activating transcription factor-1; Hb, hemoglobin; HO-1, heme oxygenase-1; IL, interleukin; LXR, transcription factor liver X receptor; Nrf2, NF-E2-related factor 2; oxLDL, oxidized low-density lipoprotein; ROS, reactive oxygen species; SR, scavenger receptor; TNF-α, tumor necrosis factor alpha.
FIG. 3.
Heme-mediated transcriptional regulation in HO-1-dependent macrophage differentiation. Ingested heme activates the transcription factors Nfr2 and ATF-1, which likely function synergistically in the induction of HO-1 expression. ATF-1 also induces the expression of LXR-β, the “master regulator” of lipid metabolism, and in turn a cascade of LXR-α and then ABCA1/ABCG1 expression. ABCA1 is a membrane channel that exports intracellular cholesterol for reverse transport via HDL. This process, which prevents foam cell formation, is blocked by intracellular ROS. Therefore, the antioxidant and anti-inflammatory effects of HO-1 expression are likely to play a role in heme-dependent anti-inflammatory macrophage differentiation. HO-1 activity results in the formation of biliverdin and bilirubin, which are potent antioxidants that scavenge ROS. HO-1 (likely through CO) dampens the production of pro-inflammatory cytokines and upregulates the production of IL-10. IL-10 positively feeds back on HO-1 and increases the expression of the CD163 Hb receptor. Thus, the downstream effects of Hb-heme-mediated macrophage differentiation are likely dependent on HO-1 expression. BR, biliverdin reductase. ATF-1, activating transcription factor-1; LXR, transcription factor liver X receptor; ROS, reactive oxygen species.
Mhem fail to develop when Nrf2 and ATF-1 are depleted by siRNA (32, 33). These transcription factors both induce HO-1 expression, suggesting that HO-1 may regulate macrophage differentiation directly and as a consequence of its ability to suppress ROS. The dependence of Mhem on HO-1 expression for development suggests that HO-1 is part of a global network of molecular signals that integrates the processing of extracellular heme with macrophage polarization and downstream effector functions (Figs. 2 and 3). A unifying mechanism that is dependent on integrated co-regulation of HO-1, IL-10, and CD163 appears to emerge (Fig. 3). Receptor-mediated internalization of heme induces HO-1 expression by activating Nfr2 and ATF-1. HO-1 expression increases IL-10 production, which increases expression of HO-1 and CD163. Therefore, the function of IL-10 and CD163 in Mhem may converge on HO-1, which appears to be necessary for not only the development of Mhem, but also their anti-inflammatory activity (31–33, 53, 133). Although these functions were determined in the context of atherosclerosis, HO-1 may also be essential in regulating macrophage functional states in broader settings because heme-release and hematoma formation are common consequences in many pathologies.
Thus far, we have focused on the mechanisms by which HO-1 regulates the MP response when its substrate, heme, is readily bioavailable (e.g., IPH). However, HO-1 may also play a role in the macrophage response to heme-independent stimuli, such as oxidized lipids (108). oxLDL and oxidized phospholipids (oxPL), formed during atherogenesis, are chemotactic to MP and mediate upregulation of HO-1 in macrophages (118, 173). Kadl and colleagues demonstrated that oxPL induce the differentiation of a novel macrophage subset, which they named Mox. Based on gene array analysis and localization studies in mouse atherosclerotic lesions, the Mox subset is distinct from M1 or M2 macrophages and characterized by expression of redox-regulatory and antioxidant genes, including robust HO-1 expression (80). However, the functional role of Mox in atherosclerosis and the involvement of HO-1 in this process has not been studied.
An interesting hypothesis arises when comparing what is known about Mhem and Mox macrophages. In Mhem, the CD163/Hb system delivers heme to the HO-1 enzyme through the endosomal system, and experimentation has demonstrated that this results in the production of the cytoprotective, immunoregulatory, and antioxidant byproducts of HO-1 activity (CO, iron, and biliverdin). Further, loss of HO-1 expression at least partially reduces the observed anti-inflammatory properties of Mhem (e.g., IL-10 production). Although it was demonstrated that oxPL induces expression of HO-1 and several other redox-regulated genes in Mox in an Nrf-2-dependent manner (80), no mechanistic interaction between HO-1 and Mox development was tested. Induction of HO-1 in Mox may not be coupled to a downstream functional effect when HO-1 enzymatic activity is restricted due to low bioavailability of heme substrate in the conditions that were used to culture Mox in vitro. This is also a possible scenario in vivo, as Mox are found in the lipid-rich region of the plaque where, relative to areas of IPH, the concentration of heme is low. In addition to HO-1, Kadl and colleagues found that numerous genes with antioxidant and redox-regulatory attributes were upregulated in Mox. Therefore, HO-1 upregulation in Mox may not be necessary for their function, but instead, a secondary consequence of Nrf-2 activation. The differences between Mox and Mhem, despite similar upregulation of HO-1, indicates that future research in this area is warranted, with particular attention to the association between heme substrate availability and the immunobiological effects of HO-1. In addition, the observation that foam cells form despite HO-1 upregulation when murine macrophages are treated with oxLDL could also be explained by the relatively low bioavailability of heme substrate in the in vitro conditions in which these studies were conducted (71). When macrophages are cultured from human monocytes treated with heme, they are resistant to lipid accumulation because the expression of the oxLDL SRs, including CD36, is significantly suppressed (53). However, oxLDL-mediated activation of Nrf2 in monocyte-derived macrophages appears to play a promiscuous role by activating expression of both HO-1 and CD36 (21, 126). The latter is the principal SR in foam cell formation (71). The relationship between Nrf-2 and CD36 appears to be particularly important, as Nrf-2 knockout mice on an apoE−/− background are more resistant to atherosclerosis compared to their wild-type littermates, which is likely associated with a lower level of cholesterol influx in plaque macrophages due to decreased expression of CD36 (21). Assimilation of these experimental results suggests that the functional consequence of Nrf-2-dependent HO-1 induction in the context of atherosclerosis appears to change with experimental conditions and may be a function of the bioavailability of heme within the microenvironment that drives macrophages to polarize.
A CXCL4-dependent macrophage subset was recently identified and named M4 (58). CXCL4 is a chemokine derived from the α-granules of activated platelets and is encoded by the Pf4 gene (34). Pf4 knockout mice on an apoe−/− background are resistant to atherosclerosis (143). Therefore, M4 macrophages could be atherogenic (58). Interestingly, CD163 gene expression is significantly downregulated in CXCL4-induced macrophages, and HO-1 is not upregulated when they are treated with Hp:Hb complexes (59).
The functional effects of HO-1 expression in atherosclerotic disease as they relate to the MPS are complex. However, consistent themes have emerged. In areas of IPH, where heme-Hb is abundant, HO-1 expression prevents the formation of foam cells by directing Mhem differentiation and lipid export. Foam cells produce a microenvironment abundant with ROS, which amplifies the retention of oxidized lipids such as oxLDL in the vascular wall. HO-1 expression within foam cells attenuates their production of pro-inflammatory cytokines and downregulates the expression of lipid SRs (73, 128).
HO-1 and DC
DC are a heterogeneous group of MP that are distributed throughout the body, and like macrophages, have functional properties that are related to their microenvironmental niche (54, 165). In general, DC serve as a bridge between the adaptive and innate immune systems because their efficiency in antigen capture, processing, and presentation enables them to activate T-cell-mediated immunity, induce tolerance, and integrate signals arising from innate immune activation (166). The term “DC” encompasses four distinct cell types: classical DC (cDC), monocyte-derived DC, plasmacytoid DC, and Langerhans cells (45, 155). The functional and developmental differences between these DC subpopulations have been reviewed elsewhere (147, 155). This section will focus on aspects of DC immunobiology including maturation and interactions with the adaptive immune system, specifically as they relate to HO-1.
In vivo, the majority of DC exist in an immature state (185), expressing low levels of co-stimulatory molecules (i.e., CD80 and CD86) and MHCII. Immature cDC are highly phagocytic, and they reside within the parenchymal tissues and lymphoid organs where they constantly internalize cell-associated material from the environment around them. In the absence of inflammatory stimuli, highly motile immature DC from the peripheral tissue migrate to draining lymph nodes where they induce tolerance to phagocytized cell-associated self-antigens (166). DC activated by tissue injury or infection undergo phenotypic maturation, whereby they become less phagocytic and upregulate surface molecules that participate in antigen presentation, such as CD80, CD86, and MHCII (7). These changes enable mature DC to activate antigen-specific naïve T cells to differentiate into adaptive immune effector cells (29). The expression of HO-1 in DC correlates with their maturity, suggesting that HO-1 may regulate DC maturation, and therefore, downstream adaptive immune responses (Table 2) (39).
Table 2.
Summary of Conflicting Reports on the Role of HO-1 in Dendritic Cell Maturation
| Culture conditions | Maturation stimulusb | Key findings | References |
|---|---|---|---|
| Rat BMDC: | High HO-1 expression in immature DC | (39) | |
| GM-CSF (1.5 ng/ml)a | TNF-a | ||
| IL-4 (4 ng/ml)a | Poly(I:C) | Stimulus-induced maturation (MHCII, CD80, CD86 and IL-12, 1L-6, TNF-α secretion) inhibits HO-1 expression | |
| LPS | |||
| Human MDDC: | CpG | ||
| GM-CSF (500 UL/ml) | CD40L | HO-1 induction with CoPP or IL-10 blocks DC maturation, but preserves IL-10 secretion | |
| IL-4 (40 ng/ml) | |||
| Murine BMDC: | LPS | No reduction in HO-1 expression after stimulation with LPS [as in Ref. (39)] at low GM-CSF concentration | (131) |
| 100 U/ml GM-CSF | |||
| 800 U/ml GM-CSF | Increased GM-CSF concentration or addition of IL-4 generated results similar to those previously reported (39) | ||
| 100 U/ml GM-CSF + 10 ng/ml IL-4 | IL-4 significantly increases LPS-induced maturation (i.e., MHCII and CD86) | ||
| Human MDDC: | LPS blocks, but CoPP or CORM2 rescues, HO-1 expression in mature DC | (136) | |
| GM-CSF (1000 U/ml) | CO (CORM2 or CoPP) blocks TLR3 and TLR4 induced: | ||
| IL-4 (40 ng/ml) | Phenotypic maturation (CD80, CD86) | ||
| LPS | Pro-inflammatory cytokine section (IL-12p70, IL-12p40, IL-23) | ||
| Murine BMDC: | Poly(I:C) | T-cell proliferation in MLR | |
| GM-CSF (10 ng/ml from COS cells)a | Biliverdin, bilirubin, deferoxamine, or heme have no affect on DC maturation | ||
| CO preserves IL-10 secretion in mature DC | |||
| Rat BMDC: | LPS | LPS induces HO-1 expression and DC maturation (MHCII, CD80, CD86) | (89) |
| IL-4 (4 ng/ml) | |||
| GM-CSF (1.5 ng/ml) | CoPP pretreatment prevents LPS-induced DC maturation | ||
| Murine MDDC: | LPS | CoPP prevents DC differentiation from monocytes and prevents DC phenotypic maturation (MHCII, CD86, CD83), T-cell proliferation in MLR, IL-12p40, IL-12p70, and TNF-α (but not IL-10) secretion | (97) |
| GM-CSF (10 ng/ml) | |||
| IL-4 (10 ng/ml) | CoPP inhibits LPS-induced maturation of BMDC independent of HO-1 expression (in HO-1−/− and HO-1+/+ BMDC) by directly activating STAT-3 (132, 137) | ||
| HO-1+/+ and HO-1−/− murine BMDC: | LPS | IL-10-mediated induction of HO-1 expression in BMDC is STAT-3 dependent | (105) |
| GM-CSF (10 ng/ml) | No reduction in HO-1 expression after stimulation with LPS [as in Ref. (39)] |
Differences in the expression of HO-1 with respect to maturation stimuli may be a result of different culture conditions. Evaluating this possibility is problematic because many authors do not indicate the biological activities of the cytokines used to propagate the BMDC. Murine BMDC propagated with GM-CSF±IL-4 represent the CD8− DC subpopulation in the mouse spleen (155), while those propagated with Flt3-ligand more closely resemble CD8+ DC based on phenotype and the ability to cross-present antigens (46, 120).
From the culture supernatant of COS cells transfected with rat IL-4 or GM-CSF cDNA
Stimulus and specificity: TNF-α, Th1 inflammatory cytokine; Poly(I:C), polyinosinic-polycytidylic acid, TLR3; LPS, TLR4; CpG, cytosine phosphate guanine, TLR9; CD40L, lymphocyte stimulus.
BMDC, bone marrow-derived dendritic cell; CO, carbon monoxide; CORM2, CO releasing molecule 2; tricarbonyldichlororuthenium II; DC, dendritic cell; GM-CSF, granulocyte macrophage colony-stimulating factor; LPS, lipopolysaccharide; MDDC, monocyte-derived dendritic cell; MHC, major histocompatibility complex; STAT-3, signal transducer and activator of transcription-3; TLR, toll-like receptor.
The study of DC in vivo is hampered by their low frequency in tissues and peripheral blood, the tendency of some DC subpopulations to form cellular conjugates, and variable expression of lineage-specific surface markers (155, 178). Therefore, information concerning HO-1 expression in primary DC isolates is generally limited to flow cytometry or immunohistology. To generate substantial numbers of DC to study, they can be propagated in culture from bone marrow (bone marrow-derived DC [BMDC]), blood monocytes (monocyte derived DC), or cord blood progenitors. Our experience and reports in the literature indicate that culture conditions used for this purpose can strongly affect HO-1 expression levels, and therefore, the reported effect of HO-1 expression in DC (Table 2).
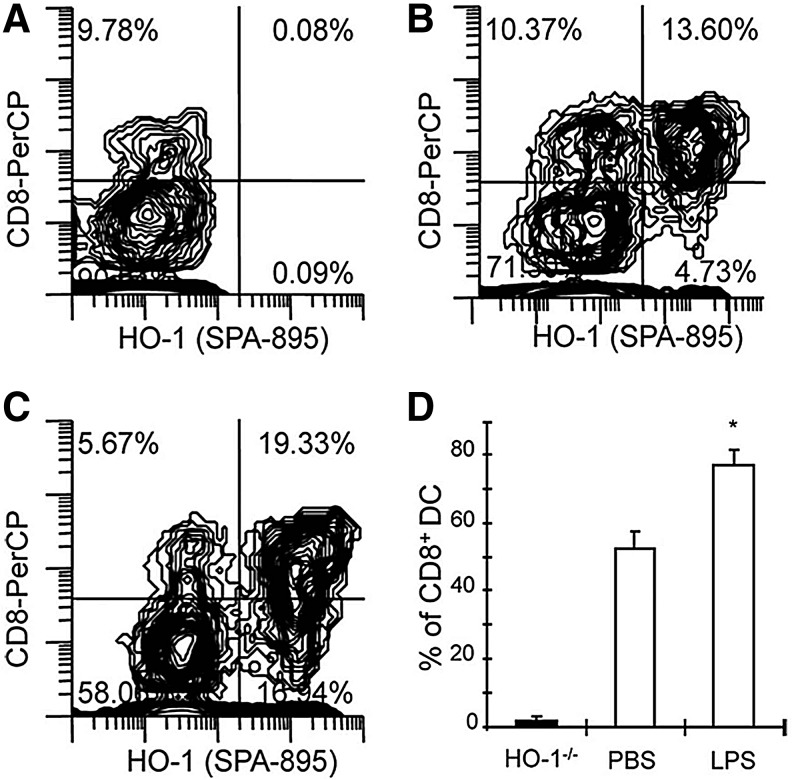
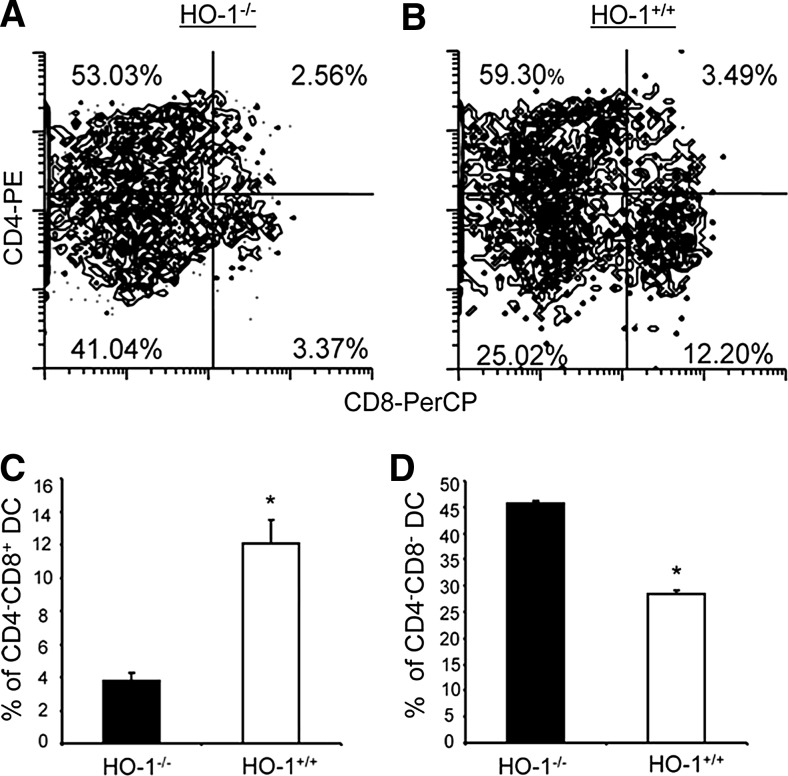
In the mouse spleen, three distinct cDC subsets have been identified based on the expression of CD4 and CD8: CD4−CD8−, CD4+CD8−, and CD4−CD8+ (119). Based on the expression of transcription factors and functional characteristics, CD8+ splenic DC equivalents are found in most peripheral tissues and characterized by a CD103+ CD11b− phenotype (51, 57). Homologues of many murine DC subsets have been found in humans, although the effect of HO-1 expression in human DC subsets has not been fully determined experimentally (155, 177). HO-1 expression appears to be particularly important in vivo in CD8+ splenic DC, which express the highest level of HO-1 at baseline and after LPS treatment, relative to the CD8− splenic DC subset (Fig. 4). Differential expression of HO-1 among splenic DC subsets has also been confirmed in the rat (39). The CD8+ DC population is depleted in HO-1 deficient mice, while the CD4+CD8− subset is not affected (Fig. 5) (131). Further, deficiency of CD8+ DC is prevented in humanized HO-1 transgenic mice that are deficient for endogenous HO-1 but express human hmox-1 from a bacterial artificial chromosome (87).
FIG. 4.
CD8+ splenic DC express HO-1 protein in vivo. Coexpression of CD8 and HO-1 in splenic DC from HO-1−/− mice (A), PBS-injected wild-type mice (B), and LPS-treated wild-type mice (C). Figures are representative of three separate experiments. Numbers within graph quadrants are the percentage of CD11c+MHC II+ positive cells. The histogram (D) shows the average of three experiments expressed as the proportion of CD8+ cells expressing HO-1 divided by total CD8+ cells in HO-1 wild-type mice 24 h after PBS and LPS administration (n=3 in each group; *p<0.05). Reprinted by permission from Park et al. (131). DC, dendritic cell; LPS, lipopolysaccharide; MHC, major histocompatibility complex.
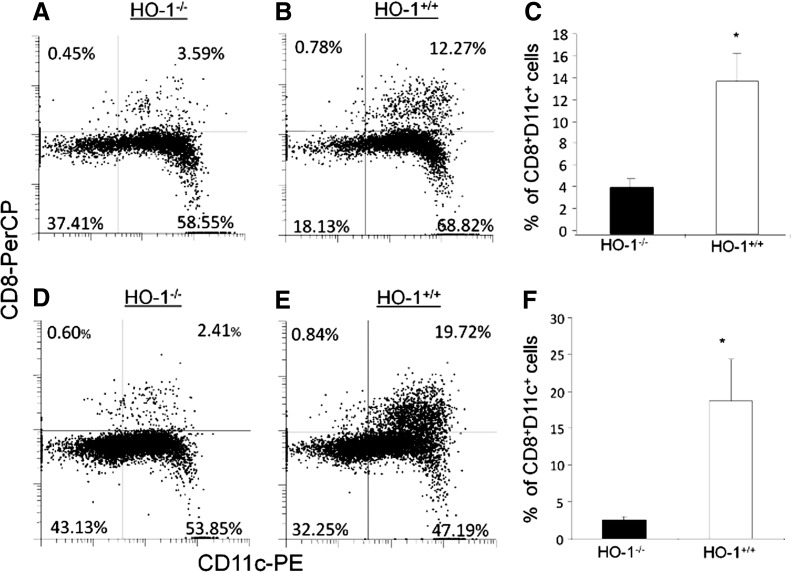
FIG. 5.
DC from HO-1 knockout mice are deficient in the CD8+ DC subset. Splenic cells were harvested from both wild-type mice and HO-1 knockout mice. CD8 and CD4 expression is shown on CD11c+ and MHCII+-gated cells from knockout mice (A) and wild-type mice (B). Figures are representative of three separate experiments. Numbers within graph quadrants depict the percentage of CD11c+ MHC II+ positive cells. The histograms shown depict the average from three animals in the percentage of CD4− CD8+ DC (C) and CD4− CD8− cells (D) in wild- type mice and knockout mice (n=3; *p<0.05). Reprinted by permission from Park et al. (131).
Several important physiological functions are uniquely attributable to CD8+ DC (154). First, CD8+ splenic DC [and, presumably, their peripheral equivalents (23, 47)] express SRs [CD36 (26), DEC 205 (92, 93), and Clec9A (195)] that are necessary for the recognition, phagocytic clearance, and tolerant response to self-moieties that originate from apoptotic, necrotic, or senescent cells during homeostasis and tissue injury (25). In addition, these DC are uniquely capable of antigen cross presentation on MHC class I molecules to CD8+ T-cells, which results in protective immunity to certain pathogens and tolerance to self-tissue (1, 14, 23). Therefore, loss of CD8+ DC could contribute to the chronic inflammation observed in HO-1-deficient mice and humans. These observations also suggest that HO-1 expression is necessary for the development and/or peripheral distribution of CD8+ DC in the lymphoid organs (Fig. 6 and Table 3) (131).
FIG. 6.
Development of late DC precursors (pre-DC) into CD8+ DC in HO-1-deficient mice. Splenocytes (1×107) from GFP+ B6 mice and GFP+ HO-1−/− mice were i.v. injected into HO-1−/− and HO-1+/+ mice. Spleens from these mice were harvested 5 days after injection, and cells were used for analysis of data. Cells shown were gated on GFP+ and MHC II+ events. Plots show the appearance of CD11c+ CD8+ DC in the spleens of HO-1−/− (A, D) and HO-1+/+ (B, E) mice receiving adoptively transferred splenocytes from GFP+ C57BL/6J (A–C) and GFP+ HO-1−/− (D–F) mice. Numbers within each quadrant are the percentage of gated cells. (C, F) show the mean and SEM of CD11c+CD8+ splenocytes detected in recipient mice (genotype indicated on the x-axis) in three separate experiments (n=3/group; *p=0.05). Table 3 was added to this review for clarity. Reprinted by permission from Park et al. (131).
Table 3.
Experimental Approach Used in Figure 6
| Panel | Pre-DC | Host | Result |
|---|---|---|---|
| B | HO-1+/+ | HO-1+/+ | Control: generation of CD8+ DC from transferred pre-DC |
| A | HO-1+/+ | HO-1−/− | HO-1+/+ CD8+ DC fail to develop in the HO-1−/− spleen |
| E | HO-1−/− | HO-1+/+ | HO-1−/− CD8+ DC develop normally the in HO-1+/+ spleen |
| D | HO-1−/− | HO-1−/− | HO-1−/− CD8+ fail to develop from the in HO-1−/− spleen |
A role for CD8+ DC in antigen cross presentation to CD8+ T cells is further supported by the work of Remy and colleagues using a mouse model of DC-induced diabetes in Ins-hemagglutinin (HA) mice (136), which express the HA antigen on islet cells of the pancreas. In these mice, co-adoptive transfer of naïve anti-HA CD8+ T cells with HA peptide-loaded, LPS-matured DC results in islet cell destruction and diabetes development in 6–9 days (113). However, when DC are co-treated with either cobalt protoporphyrin (CoPP; to induce HO-1 overexpression) or CO releasing molecule 2 (CORM2; tricarbonyldichlororuthenium II), the induction of diabetes in Ins-HA mice is prevented indefinitely. Thus, HO-1 and its byproduct, CO, inhibit antigen cross-presentation-mediated immunogenicity of DC in vivo, which appears to result from HO-1/CO-mediated inhibition of DC maturation and IRF-3-dependent cytokine production (39, 136). Although HO-1 induction blocks LPS-induced phenotypic maturation, pro-inflammatory cytokine secretion, and alloreactive T-cell proliferation, the ability to produce anti-inflammatory IL-10 is retained (39). IL-10 has been shown to promote the maintenance of an immature DC phenotype (39, 125), to potently induce tolerance by inhibiting pro-inflammatory cytokine secretion (164), and to be significantly upregulated in rat and human BMDC treated with the HO-1 inducer CoPP or CoPP and LPS (39). Therefore, the ability of HO-1 expression to attenuate autoimmune disease (153, 174) and allograft rejection (84) could be related to a selective induction of tolerogenic DC through an IL-10-dependent mechanism. HO-1/CO also appears to influence DC migration, in vivo. The treatment of rat kidney donors with CO before graft procurement prevents donor-derived DC from trafficking into secondary lymphoid organs and the peripheral circulation, where they stimulate alloreactive T cells (89, 104).
In many of the studies that have examined the correlation between DC maturation and HO-1 expression, CoPP was used to pharmacologically upregulate HO-1 activity (Table 2). Mashreghi and colleagues showed that when BMDC are treated with CoPP, inhibition of DC maturation is not dependent on HO-1 expression (105). BMDC generated from HO-1-deficient mice or treated with HO-1-specific siRNA were prevented from LPS-induced maturation when treated with CoPP. This was due to a direct activating effect on STAT3 by CoPP. However, in other studies, treatment with CORM2 (136) or methylene chloride (89) prevented LPS-induced DC maturation, lending support to the notion that HO-1 is involved in DC immunobiology. Jung and colleagues showed that HO-1 expression promoted the phenotypic maturation of BMDC in response to TLR stimulation by positively regulating the expression of indoleamine 2,3-dioxygenase. Moreover, inhibition of HO-1 with ZnPP or siRNA blocked DC maturation and pro-inflammatory cytokine secretion after stimulation with LPS (79).
Collectively, these data support the notion that HO-1 expression is involved in the process of DC maturation. Conflicting reports may arise as a function of different culture conditions, the use of nonspecific pharmacological agents for manipulation of HO-1 expression, and an inability to recapitulate DC heterogenity and functional characteristics in vitro.
HO-1 and Antigen Presentation
Studies of HO-1 expression in T cells show that they are capable of expressing HO-1 under specific conditions (27, 43, 161). But the functional consequence of HO-1 expression in T cells is somewhat controversial. Several lines of evidence have appeared to support the concept that HO-1 expression in antigen-presenting cells (APCs) strongly influences the T-cell responses in which those APC participate. The use of HO-1-deficient mice and HO-1 siRNA has also served to substantiate evidence that, in some cases, was generated using pharmacologic inhibition. Using an in vitro system, we showed that HO-1 expression in DC was required to support regulatory T cell-mediated suppression of polyclonal T-cell proliferation. Notably, a lack of HO-1 in the regulatory T cells or in the responder T cells did not affect their ability to suppress or proliferate, respectively. But a lack of HO-1 in the APC abrogated suppression and appeared to enhance proliferation. The same effect was observed when BMDC were used as APCs (55). These observations were consistent with an earlier report that induction of HO-1 in DC inhibits their ability to stimulate alloreactive T cells (39, 112) and supported the concept that HO-1 has an importantly regulatory role in APC function. Cheng et al. confirmed these observations indirectly in vivo by adoptive transfer of BMDC from HO-1−/− mice or HO-1+/+ mice into mice receiving allogeneic abdominal aorta transplants 10 days later. Administration of HO-1-deficient BMDC resulted in worse intimal and medial hyperplasia and increased cellular infiltrate. The authors explored this phenomenon further and convincingly showed that a lack of HO-1 in DC, whether because of HO-1 deficiency or due to siRNA knockdown, favors proliferation of CD4+ T cells in vivo and in vitro (41). This is also a potential explanation for the accumulation of CD4+ cells observed in the HO-1-deficient mice as they age (8). There is some evidence that this effect could be due to a linkage between MHC class II expression and HO-1. A lack of HO-1 appears to favor increased expression of MHC class II in DC, which appears to be related to inhibition of CIITA (MHC class II gene) by HO-1 (41, 43).
Conclusions
The immunomodulatory function of HO-1 in MP appears to be directly coupled to upstream receptors such as CD163, CD91, or TLR4 that sense tissue injury by detecting the presence of intracellular material (i.e., Hb) or PAMPs in the extracellular environment. These upstream signals are relayed to HO-1, which appears to modulate the downstream effector functions of MP. The role played by HO-1 in MP is complex, and likely due to the sum of degradation of pro-oxidant heme in addition to the individual actions of CO, biliverdin/bilirubin, and iron/ferritin. Relative to other cell types, HO-1 is expressed at high levels in MP, which is likely necessary given their ability to ingest potentially noxious extracellular material. The level of HO-1 expression in these cells is closely correlated to their effector function. Despite their similarities, macrophages and DC play divergent roles in the MPS during both homeostasis and disease. Therefore, commonalities and differences emerge when examining the role of HO-1 expression in macrophages versus DC. Macrophages express receptors that sense injury as an increase in the bioavailability of heme. Subsequent HO-1 induction favors the differentiation of anti-inflammatory macrophages, which may help to dampen the inflammatory response and favor healing. HO-1 expression among DC subsets seems to be heterogeneous and related to unique functional characteristics of the various DC subsets in vivo. Given their efficacy at antigen presentation, HO-1 expression in DC appears to dampen the immune response by modulating stimulus-induced phenotypic maturation, and thus, the stimulation of adaptive immune effector cells. In general, HO-1 induction favors an anti-inflammatory MP profile, which appears to be tightly coupled to positive feedback to and from IL-10. Our understanding of the ability of HO-1 to modulate the immune system due to the cell-intrinsic effects of its expression in MP is relatively new in comparison to the well-described anti-inflammatory function of HO-1 after damage to tissue. The latter is likely linked to the cytoprotective effect of HO-1, and its ability to prevent inflammation secondary to cell death. The former offers promise as a potential therapeutic approach to immunomodulation in wide range of important human diseases, and therefore warrants further investigation.
Abbreviations Used
- APC
antigen-presenting cell
- ATF-1
activating transcription factor-1
- BMDC
bone marrow-derived dendritic cell
- BR
biliverdin reductase
- cDC
classical dendritic cell
- CO
carbon monoxide
- CoPP
cobalt protoporphyrin IX
- CORM2
CO releasing molecule 2; tricarbonyldichlororuthenium II
- CVU
chronic venous leg ulcer
- DAMP
damage/danger-associated molecular pattern
- DC
dendritic cell
- Fe2+
ferrous iron
- Fe3+
ferric iron
- GM-CSF
granulocyte macrophage colony-stimulating factor
- HA
hemagglutinin
- HA-mac
hemorrhage-associated macrophage
- Hb
hemoglobin
- HLA-DR
human leukocyte antigen (MHC class II)
- hmox-1
heme oxygenase-1 gene
- HO-1
heme oxygenase-1
- Hp
haptoglobin
- Hp:Hb
haptoglobin complexed with hemoglobin
- Hx
hemopexin
- Hx:heme
hemopexin complexed with heme
- IL
interleukin
- INFγ
interferon gamma
- IPH
intraplaque hemorrhage
- IRF-3
interferon regulatory factor-3
- Keap-1
Kelch-like ECH-associated protein 1
- LDL
low-density lipoprotein
- LPS
lipopolysaccharide
- LXR
transcription factor liver X receptor
- M1
classically activated macrophage
- M2
alternative activated macrophage
- Mb
myoglobin
- MDDC
monocyte-derived dendritic cell
- MHC
major histocompatibility complex
- Mhem
hemorrhage-specialist macrophage
- MM
monocyte/macrophage
- Mox
macrophages generated with oxPAPC
- MP
mononuclear phagocyte(s)
- MPS
mononuclear phagocyte system
- MyD88
myeloid differentiation factor 88
- NF-κB
nuclear factor-kappa B
- NO
nitric oxide
- Nrf2
NF-E2-related factor 2
- oxLDL
oxidized low-density lipoprotein
- oxPL
oxidized phospholipids
- PAMP
pathogen-associated molecular pattern
- PRR
pattern recognition receptor
- ROS
reactive oxygen species
- siRNA
small interfering RNA
- SnPP
tin protoporphyrin IX
- SR
scavenger receptor
- STAT-3
signal transducer and activator of transcription-3
- TLR
toll-like receptor
- TNFα
tumor necrosis factor alpha
- ZnPP
zinc protoporphyrin IX
Acknowledgments
This review was supported by the NIH grants R01 DK 083390 (to J.F.G. and A.A.), the core resource of the UAB-UCSD O'Brien Center (P30 DK079337; to A.A.), AHA grant 0655318B (to J.F.G.), and T32 HL007918 Training Program in Cardiovascular Pathophysiology (to T.D.H.).
References
- 1.Ackerman AL. and Cresswell P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat Immunol 5: 678–684, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A. and Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 gene ablation and expression. J Am Soc Nephrol 11: 965–973, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Akira S. and Takeda K. Toll-like receptor signalling. Nat Rev Immunol 4: 499–511, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Akira S, Takeda K, and Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol 2: 675–680, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, and Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 274: 26071–26078, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Alcaraz MJ, Fernandez P, and Guillen MI. Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr Pharm Des 9: 2541–2551, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, and Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity 25: 153–162, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Ambrose CT. The Osler slide, a demonstration of phagocytosis from 1876 Reports of phagocytosis before Metchnikoff's 1880 paper. Cell Immunol 240: 1–4, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Anderton R. Hemolysis and haptoglobin levels. N Engl J Med 284: 1044, 1971 [DOI] [PubMed] [Google Scholar]
- 10.Araujo JA, Meng L, Tward AD, Hancock WW, Zhai Y, Lee A, Ishikawa K, Iyer S, Buelow R, Busuttil RW, Shih DM, Lusis AJ, and Kupiec-Weglinski JW. Systemic rather than local heme oxygenase-1 overexpression improves cardiac allograft outcomes in a new transgenic mouse. J Immunol 171: 1572–1580, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Araujo JA, Zhang M, and Yin F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front Pharmacol 3: 119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ariel A. and Timor O. Hanging in the balance: endogenous anti-inflammatory mechanisms in tissue repair and fibrosis. J Pathol 229: 250–263, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Bach FH. Heme oxygenase-1 and transplantation tolerance. Hum Immunol 67: 430–432, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, Henn V, Kloetzel PM, Gurka S, and Kroczek RA. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med 207: 1273–1281, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bain CC. and Mowat AM. Intestinal macrophages—specialised adaptation to a unique environment. Eur J Immunol 41: 2494–2498, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, Eaton JW, and Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem 267: 18148–18153, 1992 [PubMed] [Google Scholar]
- 17.Balla G, Vercellotti GM, Muller-Eberhard U, Eaton J, and Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest 64: 648–655, 1991 [PubMed] [Google Scholar]
- 18.Balla J, Balla G, Lakatos B, Jeney V, and Szentmihalyi K. [Heme-iron in the human body]. Orv Hetil 148: 1699–1706, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Balla J, Jacob HS, Balla G, Nath K, and Vercellotti GM. Endothelial cell heme oxygenase and ferritin induction by heme proteins: a possible mechanism limiting shock damage. Trans Assoc Am Physicians 105: 1–6, 1992 [PubMed] [Google Scholar]
- 20.Balla J, Vercellotti GM, Jeney V, Yachie A, Varga Z, Eaton JW, and Balla G. Heme, heme oxygenase and ferritin in vascular endothelial cell injury. Mol Nutr Food Res 49: 1030–1043, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Barajas B, Che N, Yin F, Rowshanrad A, Orozco LD, Gong KW, Wang X, Castellani LW, Reue K, Lusis AJ, and Araujo JA. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler Thromb Vasc Biol 31: 58–66, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bauer S, Muller T, and Hamm S. Pattern recognition by Toll-like receptors. Adv Exp Med Biol 653: 15–34, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Bedoui S, Whitney PG, Waithman J, Eidsmo L, Wakim L, Caminschi I, Allan RS, Wojtasiak M, Shortman K, Carbone FR, Brooks AG, and Heath WR. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat Immunol 10: 488–495, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Belcher JD, Beckman JD, Balla G, Balla J, and Vercellotti G. Heme degradation and vascular injury. Antioxid Redox Signal 12: 233–248, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belz GT, Behrens GM, Smith CM, Miller JF, Jones C, Lejon K, Fathman CG, Mueller SN, Shortman K, Carbone FR, and Heath WR. The CD8alpha(+) dendritic cell is responsible for inducing peripheral self-tolerance to tissue-associated antigens. J Exp Med 196: 1099–1104, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belz GT, Vremec D, Febbraio M, Corcoran L, Shortman K, Carbone FR, and Heath WR. CD36 is differentially expressed by CD8+ splenic dendritic cells but is not required for cross-presentation in vivo. J Immunol 168: 6066–6070, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Biburger M, Theiner G, Schadle M, Schuler G, and Tiegs G. Pivotal Advance: Heme oxygenase 1 expression by human CD4+ T cells is not sufficient for their development of immunoregulatory capacity. J Leukoc Biol 87: 193–202, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Bird DA, Gillotte KL, Horkko S, Friedman P, Dennis EA, Witztum JL, and Steinberg D. Receptors for oxidized low-density lipoprotein on elicited mouse peritoneal macrophages can recognize both the modified lipid moieties and the modified protein moieties: implications with respect to macrophage recognition of apoptotic cells. Proc Natl Acad Sci U S A 96: 6347–6352, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bousso P. T-cell activation by dendritic cells in the lymph node: lessons from the movies. Nat Rev Immunol 8: 675–684, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Box LC, Angiolillo DJ, Suzuki N, Box LA, Jiang J, Guzman L, Zenni MA, Bass TA, and Costa MA. Heterogeneity of atherosclerotic plaque characteristics in human coronary artery disease: a three-dimensional intravascular ultrasound study. Catheter Cardiovasc Interv 70: 349–356, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Boyle JJ, Harrington HA, Piper E, Elderfield K, Stark J, Landis RC, and Haskard DO. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol 174: 1097–1108, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boyle JJ, Johns M, Kampfer T, Nguyen AT, Game L, Schaer DJ, Mason JC, and Haskard DO. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ Res 110: 20–33, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Boyle JJ, Johns M, Lo J, Chiodini A, Ambrose N, Evans PC, Mason JC, and Haskard DO. Heme induces heme oxygenase 1 via Nrf2: role in the homeostatic macrophage response to intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 31: 2685–2691, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Brandt E, Petersen F, Ludwig A, Ehlert JE, Bock L, and Flad HD. The beta-thromboglobulins and platelet factor 4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J Leukoc Biol 67: 471–478, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Buechler C, Ritter M, Orso E, Langmann T, Klucken J, and Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol 67: 97–103, 2000 [PubMed] [Google Scholar]
- 36.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med 337: 762–769, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Butcher MJ. and Galkina EV. Phenotypic and functional heterogeneity of macrophages and dendritic cell subsets in the healthy and atherosclerosis-prone aorta. Front Physiol 3: 44, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang MK, Binder CJ, Miller YI, Subbanagounder G, Silverman GJ, Berliner JA, and Witztum JL. Apoptotic cells with oxidation-specific epitopes are immunogenic and proinflammatory. J Exp Med 200: 1359–1370, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chauveau C, Remy S, Royer PJ, Hill M, Tanguy-Royer S, Hubert FX, Tesson L, Brion R, Beriou G, Gregoire M, Josien R, Cuturi MC, and Anegon I. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood 106: 1694–1702, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Chen S, Kapturczak MH, Wasserfall C, Glushakova OY, Campbell-Thompson M, Deshane JS, Joseph R, Cruz PE, Hauswirth WW, Madsen KM, Croker BP, Berns KI, Atkinson MA, Flotte TR, Tisher CC, and Agarwal A. Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc Natl Acad Sci U S A 102: 7251–7256, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng C, Noorderloos M, van Deel ED, Tempel D, den Dekker W, Wagtmans K, Duncker DJ, Soares MP, Laman JD, and Duckers HJ. Dendritic cell function in transplantation arteriosclerosis is regulated by heme oxygenase 1. Circ Res 106: 1656–1666, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Chong AS. and Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol 12: 459–471, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chora AA, Fontoura P, Cunha A, Pais TF, Cardoso S, Ho PP, Lee LY, Sobel RA, Steinman L, and Soares MP. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest 117: 438–447, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chow A, Brown BD, and Merad M. Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol 11: 788–798, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Crozat K, Guiton R, Guilliams M, Henri S, Baranek T, Schwartz-Cornil I, Malissen B, and Dalod M. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunol Rev 234: 177–198, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Daro E, Pulendran B, Brasel K, Teepe M, Pettit D, Lynch DH, Vremec D, Robb L, Shortman K, McKenna HJ, Maliszewski CR, and Maraskovsky E. Polyethylene glycol-modified GM-CSF expands CD11b(high)CD11c(high) but notCD11b(low)CD11c(high) murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J Immunol 165: 49–58, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Desch AN, Randolph GJ, Murphy K, Gautier EL, Kedl RM, Lahoud MH, Caminschi I, Shortman K, Henson PM, and Jakubzick CV. CD103+ pulmonary dendritic cells preferentially acquire and present apoptotic cell-associated antigen. J Exp Med 208: 1789–1797, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devey L, Ferenbach D, Mohr E, Sangster K, Bellamy CO, Hughes J, and Wigmore SJ. Tissue-resident macrophages protect the liver from ischemia reperfusion injury via a heme oxygenase-1-dependent mechanism. Mol Ther 17: 65–72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donnelly RP, Dickensheets H, and Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. J Interferon Cytokine Res 19: 563–573, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Drechsler Y, Dolganiuc A, Norkina O, Romics L, Li W, Kodys K, Bach FH, Mandrekar P, and Szabo G. Heme oxygenase-1 mediates the anti-inflammatory effects of acute alcohol on IL-10 induction involving p38 MAPK activation in monocytes. J Immunol 177: 2592–2600, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, Bhattacharya D, Stappenbeck TS, Holtzman MJ, Sung SS, Murphy TL, Hildner K, and Murphy KM. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med 207: 823–836, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, Oliveira MF, Oliveira PL, Graca-Souza AV, and Bozza MT. Characterization of heme as activator of Toll-like receptor 4. J Biol Chem 282: 20221–20229, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Finn AV, Nakano M, Polavarapu R, Karmali V, Saeed O, Zhao X, Yazdani S, Otsuka F, Davis T, Habib A, Narula J, Kolodgie FD, and Virmani R. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol 59: 166–177, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Geissmann F, Gordon S, Hume DA, Mowat AM, and Randolph GJ. Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 10: 453–460, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.George JF, Braun A, Brusko TM, Joseph R, Bolisetty S, Wasserfall CH, Atkinson MA, Agarwal A, and Kapturczak MH. Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am J Pathol 173: 154–160, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghassabeh GH, De Baetselier P, Brys L, Noel W, Van Ginderachter JA, Meerschaut S, Beschin A, Brombacher F, and Raes G. Identification of a common gene signature for type II cytokine-associated myeloid cells elicited in vivo in different pathologic conditions. Blood 108: 575–583, 2006 [DOI] [PubMed] [Google Scholar]
- 57.Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, Price J, Yin N, Bromberg J, Lira SA, Stanley ER, Nussenzweig M, and Merad M. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med 206: 3115–3130, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gleissner CA. Macrophage Phenotype Modulation by CXCL4 in Atherosclerosis. Front Physiol 3: 1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gleissner CA, Shaked I, Erbel C, Bockler D, Katus HA, and Ley K. CXCL4 downregulates the atheroprotective hemoglobin receptor CD163 in human macrophages. Circ Res 106: 203–211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon S. Alternative activation of macrophages. Nat Rev Immunol 3: 23–35, 2003 [DOI] [PubMed] [Google Scholar]
- 61.Gordon S. The macrophage: past, present and future. Eur J Immunol 37Suppl 1: S9–S17, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Gordon S. and Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Haga Y, Tempero MA, Kay D, and Zetterman RK. Intracellular accumulation of unconjugated bilirubin inhibits phytohemagglutin-induced proliferation and interleukin-2 production of human lymphocytes. Dig Dis Sci 41: 1468–1474, 1996 [DOI] [PubMed] [Google Scholar]
- 64.Hayashidani S, Tsutsui H, Shiomi T, Ikeuchi M, Matsusaka H, Suematsu N, Wen J, Egashira K, and Takeshita A. Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 108: 2134–2140, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Hill-Kapturczak N, Chang SH, and Agarwal A. Heme oxygenase and the kidney. DNA Cell Biol 21: 307–321, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Hirsiger S, Simmen HP, Werner CM, Wanner GA, and Rittirsch D. Danger signals activating the immune response after trauma. Mediators Inflamm 2012: 315941, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hogger P, Dreier J, Droste A, Buck F, and Sorg C. Identification of the integral membrane protein RM3/1 on human monocytes as a glucocorticoid-inducible member of the scavenger receptor cysteine-rich family (CD163). J Immunol 161: 1883–1890, 1998 [PubMed] [Google Scholar]
- 68.Hrkal Z, Vodrazka Z, and Kalousek I. Transfer of heme from ferrihemoglobin and ferrihemoglobin isolated chains to hemopexin. Eur J Biochem 43: 73–78, 1974 [DOI] [PubMed] [Google Scholar]
- 69.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol 18: 49–53, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, and Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood 106: 2572–2579, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Ishii T, Itoh K, Ruiz E, Leake DS, Unoki H, Yamamoto M, and Mann GE. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ Res 94: 609–616, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, and Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 275: 16023–16029, 2000 [DOI] [PubMed] [Google Scholar]
- 73.Ishikawa K, Sugawara D, Wang X, Suzuki K, Itabe H, Maruyama Y, and Lusis AJ. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ Res 88: 506–512, 2001 [DOI] [PubMed] [Google Scholar]
- 74.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, and Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13: 76–86, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Itoh K, Wakabayashi N, Katoh Y, Ishii T, O'Connor T, and Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 8: 379–391, 2003 [DOI] [PubMed] [Google Scholar]
- 76.Janeway CA., Jr.Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol 54Pt 1: 1–13, 1989 [DOI] [PubMed] [Google Scholar]
- 77.Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, and Mangelsdorf DJ. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc Natl Acad Sci U S A 96: 266–271, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, and Balla G. Pro-oxidant and cytotoxic effects of circulating heme. Blood 100: 879–887, 2002 [DOI] [PubMed] [Google Scholar]
- 79.Jung ID, Lee JS, Lee CM, Noh KT, Jeong YI, Park WS, Chun SH, Jeong SK, Park JW, Son KH, Heo DR, Lee MG, Shin YK, Kim HW, Yun CH, and Park YM. Induction of indoleamine 2,3-dioxygenase expression via heme oxygenase-1-dependant pathway during murine dendritic cell maturation. Biochem Pharmacol 80: 491–505, 2010 [DOI] [PubMed] [Google Scholar]
- 80.Kadl A, Meher AK, Sharma PR, Lee MY, Doran AC, Johnstone SR, Elliott MR, Gruber F, Han J, Chen W, Kensler T, Ravichandran KS, Isakson BE, Wamhoff BR, and Leitinger N. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ Res 107: 737–746, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kai H, Kuwahara F, Tokuda K, and Imaizumi T. Diastolic dysfunction in hypertensive hearts: roles of perivascular inflammation and reactive myocardial fibrosis. Hypertens Res 28: 483–490, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Kanzaki Y, Terasaki F, Okabe M, Hayashi T, Toko H, Shimomura H, Fujioka S, Kitaura Y, Kawamura K, Horii Y, Isomura T, and Suma H. Myocardial inflammatory cell infiltrates in cases of dilated cardiomyopathy as a determinant of outcome following partial left ventriculectomy. Jpn Circ J 65: 797–802, 2001 [DOI] [PubMed] [Google Scholar]
- 83.Kapturczak MH, Wasserfall C, Brusko T, Campbell-Thompson M, Ellis TM, Atkinson MA, and Agarwal A. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am J Pathol 165: 1045–1053, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katori M, Busuttil RW, and Kupiec-Weglinski JW. Heme oxygenase-1 system in organ transplantation. Transplantation 74: 905–912, 2002 [DOI] [PubMed] [Google Scholar]
- 85.Ke B, Shen XD, Ji H, Kamo N, Gao F, Freitas MC, Busuttil RW, and Kupiec-Weglinski JW. HO-1-STAT3 axis in mouse liver ischemia/reperfusion injury: regulation of TLR4 innate responses through PI3K/PTEN signaling. J Hepatol 56: 359–366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kim BS, Lim SW, Li C, Kim JS, Sun BK, Ahn KO, Han SW, Kim J, and Yang CW. Ischemia-reperfusion injury activates innate immunity in rat kidneys. Transplantation 79: 1370–1377, 2005 [DOI] [PubMed] [Google Scholar]
- 87.Kim J, Zarjou A, Traylor AM, Bolisetty S, Jaimes EA, Hull TD, George JF, Mikhail FM, and Agarwal A. In vivo regulation of the heme oxygenase-1 gene in humanized transgenic mice. Kidney Int 82: 278–291, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, and Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 349: 2316–2325, 2003 [DOI] [PubMed] [Google Scholar]
- 89.Kotsch K, Martins PN, Klemz R, Janssen U, Gerstmayer B, Dernier A, Reutzel-Selke A, Kuckelkorn U, Tullius SG, and Volk HD. Heme oxygenase-1 ameliorates ischemia/reperfusion injury by targeting dendritic cell maturation and migration. Antioxid Redox Signal 9: 2049–2063, 2007 [DOI] [PubMed] [Google Scholar]
- 90.Kovtunovych G, Eckhaus MA, Ghosh MC, Ollivierre-Wilson H, and Rouault TA. Dysfunction of the heme recycling system in heme oxygenase 1-deficient mice: effects on macrophage viability and tissue iron distribution. Blood 116: 6054–6062, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, and Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature 409: 198–201, 2001 [DOI] [PubMed] [Google Scholar]
- 92.Kronin V, Wu L, Gong S, Nussenzweig MC, and Shortman K. DEC-205 as a marker of dendritic cells with regulatory effects on CD8 T cell responses. Int Immunol 12: 731–735, 2000 [DOI] [PubMed] [Google Scholar]
- 93.Lahoud MH, Ahmet F, Zhang JG, Meuter S, Policheni AN, Kitsoulis S, Lee CN, O'Keeffe M, Sullivan LC, Brooks AG, Berry R, Rossjohn J, Mintern JD, Vega-Ramos J, Villadangos JA, Nicola NA, Nussenzweig MC, Stacey KJ, Shortman K, Heath WR, and Caminschi I. DEC-205 is a cell surface receptor for CpG oligonucleotides. Proc Natl Acad Sci U S A 109: 16270–16275, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Land WG. The role of postischemic reperfusion injury and other nonantigen-dependent inflammatory pathways in transplantation. Transplantation 79: 505–514, 2005 [DOI] [PubMed] [Google Scholar]
- 95.Lee TS. and Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med 8: 240–246, 2002 [DOI] [PubMed] [Google Scholar]
- 96.Leitinger N, Tyner TR, Oslund L, Rizza C, Subbanagounder G, Lee H, Shih PT, Mackman N, Tigyi G, Territo MC, Berliner JA, and Vora DK. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc Natl Acad Sci U S A 96: 12010–12015, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Listopad J, Asadullah K, Sievers C, Ritter T, Meisel C, Sabat R, and Docke WD. Heme oxygenase-1 inhibits T cell-dependent skin inflammation and differentiation and function of antigen-presenting cells. Exp Dermatol 16: 661–670, 2007 [DOI] [PubMed] [Google Scholar]
- 98.Liu G. and Yang H. Modulation of macrophage activation and programming in immunity. J Cell Physiol 228: 502–512, 2013 [DOI] [PubMed] [Google Scholar]
- 99.Lo D, Freedman J, Hesse S, Palmiter RD, Brinster RL, and Sherman LA. Peripheral tolerance to an islet cell-specific hemagglutinin transgene affects both CD4+ and CD8+ T cells. Eur J Immunol 22: 1013–1022, 1992 [DOI] [PubMed] [Google Scholar]
- 100.Lou H, Du S, Ji Q, and Stolz A. Induction of AKR1C2 by phase II inducers: identification of a distal consensus antioxidant response element regulated by NRF2. Mol Pharmacol 69: 1662–1672, 2006 [DOI] [PubMed] [Google Scholar]
- 101.Ludwiczek S, Aigner E, Theurl I, and Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood 101: 4148–4154, 2003 [DOI] [PubMed] [Google Scholar]
- 102.Lusis AJ. Atherosclerosis. Nature 407: 233–241, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maniecki MB, Moller HJ, Moestrup SK, and Moller BK. CD163 positive subsets of blood dendritic cells: the scavenging macrophage receptors CD163 and CD91 are coexpressed on human dendritic cells and monocytes. Immunobiology 211: 407–417, 2006 [DOI] [PubMed] [Google Scholar]
- 104.Martins PN, Reutzel-Selke A, Jurisch A, Denecke C, Attrot K, Pascher A, Kotsch K, Pratschke J, Neuhaus P, Volk HD, and Tullius SG. Induction of carbon monoxide in donor animals prior to organ procurement reduces graft immunogenicity and inhibits chronic allograft dysfunction. Transplantation 82: 938–944, 2006 [DOI] [PubMed] [Google Scholar]
- 105.Mashreghi MF, Klemz R, Knosalla IS, Gerstmayer B, Janssen U, Buelow R, Jozkowicz A, Dulak J, Volk HD, and Kotsch K. Inhibition of dendritic cell maturation and function is independent of heme oxygenase 1 but requires the activation of STAT3. J Immunol 180: 7919–7930, 2008 [DOI] [PubMed] [Google Scholar]
- 106.Matzinger P. The danger model: a renewed sense of self. Science 296: 301–305, 2002 [DOI] [PubMed] [Google Scholar]
- 107.Matzinger P. An innate sense of danger. Ann N Y Acad Sci 961: 341–342, 2002 [DOI] [PubMed] [Google Scholar]
- 108.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, and Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res 108: 235–248, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mitani K, Fujita H, Kappas A, and Sassa S. Heme oxygenase is a positive acute-phase reactant in human Hep3B hepatoma cells. Blood 79: 1255–1259, 1992 [PubMed] [Google Scholar]
- 110.Moestrup SK, Gliemann J, and Pallesen G. Distribution of the alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res 269: 375–382, 1992 [DOI] [PubMed] [Google Scholar]
- 111.Moestrup SK. and Moller HJ. CD163: a regulated hemoglobin scavenger receptor with a role in the anti-inflammatory response. Ann Med 36: 347–354, 2004 [DOI] [PubMed] [Google Scholar]
- 112.Moreau A, Hill M, Thebault P, Deschamps JY, Chiffoleau E, Chauveau C, Moullier P, Anegon I, Alliot-Licht B, and Cuturi MC. Tolerogenic dendritic cells actively inhibit T cells through heme oxygenase-1 in rodents and in nonhuman primates. FASEB J 23: 3070–3077, 2009 [DOI] [PubMed] [Google Scholar]
- 113.Morgan DJ, Liblau R, Scott B, Fleck S, McDevitt HO, Sarvetnick N, Lo D, and Sherman LA. CD8(+) T cell-mediated spontaneous diabetes in neonatal mice. J Immunol 157: 978–983, 1996 [PubMed] [Google Scholar]
- 114.Morita T. Heme oxygenase and atherosclerosis. Arterioscler Thromb Vasc Biol 25: 1786–1795, 2005 [DOI] [PubMed] [Google Scholar]
- 115.Morita T, Imai T, Yamaguchi T, Sugiyama T, Katayama S, and Yoshino G. Induction of heme oxygenase-1 in monocytes suppresses angiotensin II-elicited chemotactic activity through inhibition of CCR2: role of bilirubin and carbon monoxide generated by the enzyme. Antioxid Redox Signal 5: 439–447, 2003 [DOI] [PubMed] [Google Scholar]
- 116.Mosser DM. and Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Murray PJ. and Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11: 723–737, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nagy L, Tontonoz P, Alvarez JG, Chen H, and Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell 93: 229–240, 1998 [DOI] [PubMed] [Google Scholar]
- 119.Naik S, Vremec D, Wu L, O'Keeffe M, and Shortman K. CD8alpha+ mouse spleen dendritic cells do not originate from the CD8alpha- dendritic cell subset. Blood 102: 601–604, 2003 [DOI] [PubMed] [Google Scholar]
- 120.Naik SH, O'Keeffe M, Proietto A, Shortman HH, and Wu L. CD8+, CD8-, and plasmacytoid dendritic cell generation in vitro using flt3 ligand. Methods Mol Biol 595: 167–176, 2010 [DOI] [PubMed] [Google Scholar]
- 121.Nakagami T, Toyomura K, Kinoshita T, and Morisawa S. A beneficial role of bile pigments as an endogenous tissue protector: anti-complement effects of biliverdin and conjugated bilirubin. Biochim Biophys Acta 1158: 189–193, 1993 [DOI] [PubMed] [Google Scholar]
- 122.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int 70: 432–443, 2006 [DOI] [PubMed] [Google Scholar]
- 123.Nath KA, Vercellotti GM, Grande JP, Miyoshi H, Paya CV, Manivel JC, Haggard JJ, Croatt AJ, Payne WD, and Alam J. Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int 59: 106–117, 2001 [DOI] [PubMed] [Google Scholar]
- 124.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, and Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113: 1271–1276, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nolan KF, Strong V, Soler D, Fairchild PJ, Cobbold SP, Croxton R, Gonzalo JA, Rubio A, Wells M, and Waldmann H. IL-10-conditioned dendritic cells, decommissioned for recruitment of adaptive immunity, elicit innate inflammatory gene products in response to danger signals. J Immunol 172: 2201–2209, 2004 [DOI] [PubMed] [Google Scholar]
- 126.Olagnier D, Lavergne RA, Meunier E, Lefevre L, Dardenne C, Aubouy A, Benoit-Vical F, Ryffel B, Coste A, Berry A, and Pipy B. Nrf2, a PPARgamma alternative pathway to promote CD36 expression on inflammatory macrophages: implication for malaria. PLoS Pathog 7: e1002254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ollinger R, Wang H, Yamashita K, Wegiel B, Thomas M, Margreiter R, and Bach FH. Therapeutic applications of bilirubin and biliverdin in transplantation. Antioxid Redox Signal 9: 2175–2185, 2007 [DOI] [PubMed] [Google Scholar]
- 128.Orozco LD, Kapturczak MH, Barajas B, Wang X, Weinstein MM, Wong J, Deshane J, Bolisetty S, Shaposhnik Z, Shih DM, Agarwal A, Lusis AJ, and Araujo JA. Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circ Res 100: 1703–1711, 2007 [DOI] [PubMed] [Google Scholar]
- 129.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, and Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med 6: 422–428, 2000 [DOI] [PubMed] [Google Scholar]
- 130.Otterbein LE, Soares MP, Yamashita K, and Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol 24: 449–455, 2003 [DOI] [PubMed] [Google Scholar]
- 131.Park DJ, Agarwal A, and George JF. Heme oxygenase-1 expression in murine dendritic cell subpopulations: effect on CD8+ dendritic cell differentiation in vivo. Am J Pathol 176: 2831–2839, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, Murakami M, and Hirano T. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol 173: 3844–3854, 2004 [DOI] [PubMed] [Google Scholar]
- 133.Philippidis P, Mason JC, Evans BJ, Nadra I, Taylor KM, Haskard DO, and Landis RC. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res 94: 119–126, 2004 [DOI] [PubMed] [Google Scholar]
- 134.Pinto AR, Paolicelli R, Salimova E, Gospocic J, Slonimsky E, Bilbao-Cortes D, Godwin JW, and Rosenthal NA. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One 7: e36814, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pratschke J, Paz D, Wilhelm MJ, Laskowski I, Kofla G, Vergopoulos A, MacKenzie HJ, Tullius SG, Neuhaus P, Hancock WW, Volk HD, and Tilney NL. Donor hypertension increases graft immunogenicity and intensifies chronic changes in long-surviving renal allografts. Transplantation 77: 43–48, 2004 [DOI] [PubMed] [Google Scholar]
- 136.Remy S, Blancou P, Tesson L, Tardif V, Brion R, Royer PJ, Motterlini R, Foresti R, Painchaut M, Pogu S, Gregoire M, Bach JM, Anegon I, and Chauveau C. Carbon monoxide inhibits TLR-induced dendritic cell immunogenicity. J Immunol 182: 1877–1884, 2009 [DOI] [PubMed] [Google Scholar]
- 137.Ricchetti GA, Williams LM, and Foxwell BM. Heme oxygenase 1 expression induced by IL-10 requires STAT-3 and phosphoinositol-3 kinase and is inhibited by lipopolysaccharide. J Leukoc Biol 76: 719–726, 2004 [DOI] [PubMed] [Google Scholar]
- 138.Ritter M, Buechler C, Kapinsky M, and Schmitz G. Interaction of CD163 with the regulatory subunit of casein kinase II (CKII) and dependence of CD163 signaling on CKII and protein kinase C. Eur J Immunol 31: 999–1009, 2001 [DOI] [PubMed] [Google Scholar]
- 139.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med 340: 115–126, 1999 [DOI] [PubMed] [Google Scholar]
- 140.Rougemont A, Dumbo O, Bouvier M, Soula G, Perrin L, Tamoura B, Yerly S, Dolo A, Brenner E, Kodio B, et al. Hypohaptoglobinaemia as an epidemiological and clinical indicator for malaria. Results of two studies in a hyperendemic region in West Africa. Lancet 2: 709–712, 1988 [DOI] [PubMed] [Google Scholar]
- 141.Ryter SW. and Choi AM. Cytoprotective and anti-inflammatory actions of carbon monoxide in organ injury and sepsis models. Novartis Found Symp 280: 165–175; discussion 175–181, 2007 [PubMed] [Google Scholar]
- 142.Ryter SW. and Tyrrell RM. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radic Biol Med 28: 289–309, 2000 [DOI] [PubMed] [Google Scholar]
- 143.Sachais BS, Turrentine T, Dawicki McKenna JM, Rux AH, Rader D, and Kowalska MA. Elimination of platelet factor 4 (PF4) from platelets reduces atherosclerosis in C57Bl/6 and apoE−/− mice. Thromb Haemost 98: 1108–1113, 2007 [PubMed] [Google Scholar]
- 144.Saitoh S. and Miyake K. Mechanism regulating cell surface expression and activation of Toll-like receptor 4. Chem Rec 6: 311–319, 2006 [DOI] [PubMed] [Google Scholar]
- 145.Salomao R, Brunialti MK, Rapozo MM, Baggio-Zappia GL, Galanos C, and Freudenberg M. Bacterial sensing, cell signaling, and modulation of the immune response during sepsis. Shock 38: 227–242, 2012 [DOI] [PubMed] [Google Scholar]
- 146.Sanders DB, Larson DF, Hunter K, Gorman M, and Yang B. Comparison of tumor necrosis factor-alpha effect on the expression of iNOS in macrophage and cardiac myocytes. Perfusion 16: 67–74, 2001 [DOI] [PubMed] [Google Scholar]
- 147.Satpathy AT, Wu X, Albring JC, and Murphy KM. Re(de)fining the dendritic cell lineage. Nat Immunol 13: 1145–1154, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schaer CA, Schoedon G, Imhof A, Kurrer MO, and Schaer DJ. Constitutive endocytosis of CD163 mediates hemoglobin-heme uptake and determines the noninflammatory and protective transcriptional response of macrophages to hemoglobin. Circ Res 99: 943–950, 2006 [DOI] [PubMed] [Google Scholar]
- 149.Schaer CA, Vallelian F, Imhof A, Schoedon G, and Schaer DJ. CD163-expressing monocytes constitute an endotoxin-sensitive Hb clearance compartment within the vascular system. J Leukoc Biol 82: 106–110, 2007 [DOI] [PubMed] [Google Scholar]
- 150.Schaer CA, Vallelian F, Imhof A, Schoedon G, and Schaer DJ. Heme carrier protein (HCP-1) spatially interacts with the CD163 hemoglobin uptake pathway and is a target of inflammatory macrophage activation. J Leukoc Biol 83: 325–333, 2008 [DOI] [PubMed] [Google Scholar]
- 151.Schaer DJ, Schaer CA, Buehler PW, Boykins RA, Schoedon G, Alayash AI, and Schaffner A. CD163 is the macrophage scavenger receptor for native and chemically modified hemoglobins in the absence of haptoglobin. Blood 107: 373–380, 2006 [DOI] [PubMed] [Google Scholar]
- 152.Schaer DJ, Schaer CA, Schoedon G, Imhof A, and Kurrer MO. Hemophagocytic macrophages constitute a major compartment of heme oxygenase expression in sepsis. Eur J Haematol 77: 432–436, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sheikh SZ, Hegazi RA, Kobayashi T, Onyiah JC, Russo SM, Matsuoka K, Sepulveda AR, Li F, Otterbein LE, and Plevy SE. An anti-inflammatory role for carbon monoxide and heme oxygenase-1 in chronic Th2-mediated murine colitis. J Immunol 186: 5506–5513, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shortman K. and Heath WR. The CD8+ dendritic cell subset. Immunol Rev 234: 18–31, 2010 [DOI] [PubMed] [Google Scholar]
- 155.Shortman K. and Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol 2: 151–161, 2002 [DOI] [PubMed] [Google Scholar]
- 156.Sica A. and Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H, Hainzl A, Schatz S, Qi Y, Schlecht A, Weiss JM, Wlaschek M, Sunderkotter C, and Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest 121: 985–997, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Smith A. and Morgan WT. Hemopexin-mediated heme transport to the liver. Evidence for a heme-binding protein in liver plasma membranes. J Biol Chem 260: 8325–8329, 1985 [PubMed] [Google Scholar]
- 159.Soares MP. and Bach FH. Heme oxygenase-1 in organ transplantation. Front Biosci 12: 4932–4945, 2007 [DOI] [PubMed] [Google Scholar]
- 160.Soares MP, Brouard S, Smith RN, and Bach FH. Heme oxygenase-1, a protective gene that prevents the rejection of transplanted organs. Immunol Rev 184: 275–285, 2001 [DOI] [PubMed] [Google Scholar]
- 161.Song R, Mahidhara RS, Zhou Z, Hoffman RA, Seol DW, Flavell RA, Billiar TR, Otterbein LE, and Choi AM. Carbon monoxide inhibits T lymphocyte proliferation via caspase-dependent pathway. J Immunol 172: 1220–1226, 2004 [DOI] [PubMed] [Google Scholar]
- 162.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, Raetz CR, Wang EW, Kelly SL, Sullards MC, Murphy RC, Merrill AH, Jr., Brown HA, Dennis EA, Li AC, Ley K, Tsimikas S, Fahy E, Subramaniam S, Quehenberger O, Russell DW, and Glass CK. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 151: 138–152, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Steinbrecher UP. Receptors for oxidized low density lipoprotein. Biochim Biophys Acta 1436: 279–298, 1999 [DOI] [PubMed] [Google Scholar]
- 164.Steinbrink K, Wolfl M, Jonuleit H, Knop J, and Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol 159: 4772–4780, 1997 [PubMed] [Google Scholar]
- 165.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol 30: 1–22, 2012 [DOI] [PubMed] [Google Scholar]
- 166.Steinman RM, Hawiger D, and Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol 21: 685–711, 2003 [DOI] [PubMed] [Google Scholar]
- 167.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, and Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 235: 1043–1046, 1987 [DOI] [PubMed] [Google Scholar]
- 168.Sulahian TH, Hogger P, Wahner AE, Wardwell K, Goulding NJ, Sorg C, Droste A, Stehling M, Wallace PK, Morganelli PM, and Guyre PM. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine 12: 1312–1321, 2000 [DOI] [PubMed] [Google Scholar]
- 169.Tabbara IA. Hemolytic anemias. Diagnosis and management. Med Clin North Am 76: 649–668, 1992 [DOI] [PubMed] [Google Scholar]
- 170.Tenhunen R, Marver HS, and Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem 244: 6388–6394, 1969 [PubMed] [Google Scholar]
- 171.Tolosano E, Fagoonee S, Hirsch E, Berger FG, Baumann H, Silengo L, and Altruda F. Enhanced splenomegaly and severe liver inflammation in haptoglobin/hemopexin double-null mice after acute hemolysis. Blood 100: 4201–4208, 2002 [DOI] [PubMed] [Google Scholar]
- 172.Tolosano E, Hirsch E, Patrucco E, Camaschella C, Navone R, Silengo L, and Altruda F. Defective recovery and severe renal damage after acute hemolysis in hemopexin-deficient mice. Blood 94: 3906–3914, 1999 [PubMed] [Google Scholar]
- 173.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, and Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell 93: 241–252, 1998 [DOI] [PubMed] [Google Scholar]
- 174.Tzima S, Victoratos P, Kranidioti K, Alexiou M, and Kollias G. Myeloid heme oxygenase-1 regulates innate immunity and autoimmunity by modulating IFN-beta production. J Exp Med 206: 1167–1179, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ugocsai P, Barlage S, Dada A, and Schmitz G. Regulation of surface CD163 expression and cellular effects of receptor mediated hemoglobin-haptoglobin uptake on human monocytes and macrophages. Cytometry A 69: 203–205, 2006 [DOI] [PubMed] [Google Scholar]
- 176.Van den Heuvel MM, Tensen CP, van As JH, Van den Berg TK, Fluitsma DM, Dijkstra CD, Dopp EA, Droste A, Van Gaalen FA, Sorg C, Hogger P, and Beelen RH. Regulation of CD 163 on human macrophages: cross-linking of CD163 induces signaling and activation. J Leukoc Biol 66: 858–866, 1999 [DOI] [PubMed] [Google Scholar]
- 177.Villadangos JA. and Shortman K. Found in translation: the human equivalent of mouse CD8+ dendritic cells. J Exp Med 207: 1131–1134, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vremec D. and Shortman K. The isolation and identification of murine dendritic cell populations from lymphoid tissues and their production in culture. Methods Mol Biol 415: 163–178, 2008 [DOI] [PubMed] [Google Scholar]
- 179.Wang L, Harrington L, Trebicka E, Shi HN, Kagan JC, Hong CC, Lin HY, Babitt JL, and Cherayil BJ. Selective modulation of TLR4-activated inflammatory responses by altered iron homeostasis in mice. J Clin Invest 119: 3322–3328, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang WGD, Woo J, Ward M, Sui G, Torti SV, Torti FM, and Beaty MW. Ferritin H is a novel marker of early erythroid precursors and macrophages. Histopathology 62: 931–940, 2013 [DOI] [PubMed] [Google Scholar]
- 181.Watts JA, Zagorski J, Gellar MA, Stevinson BG, and Kline JA. Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J Mol Cell Cardiol 41: 296–307, 2006 [DOI] [PubMed] [Google Scholar]
- 182.Weaver LK, Pioli PA, Wardwell K, Vogel SN, and Guyre PM. Up-regulation of human monocyte CD163 upon activation of cell-surface Toll-like receptors. J Leukoc Biol 81: 663–671, 2007 [DOI] [PubMed] [Google Scholar]
- 183.Weis N, Weigert A, von Knethen A, and Brune B. Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Mol Biol Cell 20: 1280–1288, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.White C, Yuan X, Schmidt PJ, Bresciani E, Samuel TK, Campagna D, Hall C, Bishop K, Calicchio ML, Lapierre A, Ward DM, Liu P, Fleming MD, and Hamza I. HRG1 Is essential for heme transport from the phagolysosome of macrophages during erythrophagocytosis. Cell Metab 17: 261–270, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Wilson NS, El-Sukkari D, Belz GT, Smith CM, Steptoe RJ, Heath WR, Shortman K, and Villadangos JA. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood 102: 2187–2194, 2003 [DOI] [PubMed] [Google Scholar]
- 186.Wu ML, Ho YC, and Yet SF. A central role of heme oxygenase-1 in cardiovascular protection. Antioxid Redox Signal 15: 1835–1846, 2011 [DOI] [PubMed] [Google Scholar]
- 187.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, and Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest 103: 129–135, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yamashita K, McDaid J, Ollinger R, Tsui TY, Berberat PO, Usheva A, Csizmadia E, Smith RN, Soares MP, and Bach FH. Biliverdin, a natural product of heme catabolism, induces tolerance to cardiac allografts. FASEB J 18: 765–767, 2004 [DOI] [PubMed] [Google Scholar]
- 189.Yamazaki H, Ohta K, Tsukiji H, Toma T, Hashida Y, Ishizaki A, Saito T, Arai S, Koizumi S, and Yachie A. Corticosteroid enhances heme oxygenase-1 production by circulating monocytes by up-regulating hemoglobin scavenger receptor and amplifying the receptor-mediated uptake of hemoglobin-haptoglobin complex. Biochem Biophys Res Commun 358: 506–512, 2007 [DOI] [PubMed] [Google Scholar]
- 190.Yet SF, Layne MD, Liu X, Chen YH, Ith B, Sibinga NE, and Perrella MA. Absence of heme oxygenase-1 exacerbates atherosclerotic lesion formation and vascular remodeling. FASEB J 17: 1759–1761, 2003 [DOI] [PubMed] [Google Scholar]
- 191.Yndestad A, Damas JK, Oie E, Ueland T, Gullestad L, and Aukrust P. Systemic inflammation in heart failure—the whys and wherefores. Heart Fail Rev 11: 83–92, 2006 [DOI] [PubMed] [Google Scholar]
- 192.Yoshida T. and Kikuchi G. Purification and properties of heme oxygenase from pig spleen microsomes. J Biol Chem 253: 4224–4229, 1978 [PubMed] [Google Scholar]
- 193.Yoshinaga T, Sassa S, and Kappas A. The occurrence of molecular interactions among NADPH-cytochrome c reductase, heme oxygenase, and biliverdin reductase in heme degradation. J Biol Chem 257: 7786–7793, 1982 [PubMed] [Google Scholar]
- 194.Zhang DL, Senecal T, Ghosh MC, Ollivierre-Wilson H, Tu T, and Rouault TA. Hepcidin regulates ferroportin expression and intracellular iron homeostasis of erythroblasts. Blood 118: 2868–2877, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang JG, Czabotar PE, Policheni AN, Caminschi I, Wan SS, Kitsoulis S, Tullett KM, Robin AY, Brammananth R, van Delft MF, Lu J, O'Reilly LA, Josefsson EC, Kile BT, Chin WJ, Mintern JD, Olshina MA, Wong W, Baum J, Wright MD, Huang DC, Mohandas N, Coppel RL, Colman PM, Nicola NA, Shortman K, and Lahoud MH. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity 36: 646–657, 2012 [DOI] [PubMed] [Google Scholar]
- 196.Zhang X, Goncalves R, and Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol Chapter 14: Unit 141, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhang Z, Zhang F, An P, Guo X, Shen Y, Tao Y, Wu Q, Zhang Y, Yu Y, Ning B, Nie G, Knutson MD, Anderson GJ, and Wang F. Ferroportin1 deficiency in mouse macrophages impairs iron homeostasis and inflammatory responses. Blood 118: 1912–1922, 2011 [DOI] [PubMed] [Google Scholar]