Abstract
Aim: The aim was to investigate somatostatin receptor (sstr) expression in normal prostate by determining the maximum standardized uptake value (SUVmax) of 68Ga-DOTATOC PET/CT in neuroendocrine tumor (NET) patients, without NET involvement of the prostate gland, for establishing the reference standard.
Methods: Sixty-four NET patients underwent 68Ga-DOTATOC PET/CT. SUVmax of the prostate gland, normal liver, testes, and gluteus muscles were evaluated. The prostate gland size was measured. Statistical analysis was performed using dedicated software (SPSS13).
Results: Mean/median 68Ga-DOTATOC SUVmax values were as follows: normal prostate 2.6±0.0, slightly enlarged prostate 4.2±1.6, prostatic hypertrophy 4.9±1.6, prostatic hyperplasia 5.0±1.5, prostate cancer 9.5±2.1, normal liver 7.3±1.8, testes 1.8±0.5, and gluteus 1.0±0.2. The normal prostate gland had three times less sstr expression than normal liver tissue. Strong correlation was found between patient age and sstr expression in prostate/prostate size. No significant difference existed in sstr expression between prostatic hypertrophy and hyperplasia. Much higher sstr expression was found in prostatic cancer compared with normal prostate.
Conclusion: 68Ga-DOTATOC PET/CT defines the baseline sstr uptake in prostate not affected by NET (significantly lower than in the liver). Higher values were established in prostatic hyperplasia and hypertrophy. Only concomitant prostate cancer was associated with higher SUVmax in comparison with non-neoplastic liver.
Key words: : 68Ga-DOTATOC PET/CT, neuroendocrine tumor, prostate gland, prostate size, somatostatin receptor expression
Introduction
Somatostatin (sst) is a small, cyclic neuropeptide formed of 14 or 28 amino acids, present in neurons and endocrine cells. It inhibits the secretion of a wide range of hormones.1 The antiproliferative action has the ability to control cell growth with the potential for therapeutic application. Somatostatin actions are mediated by transmembrane domain G-protein-coupled receptors. Five distinct subtypes of somatostatin receptors (sstr) are identified (sstr 1 to 5) and multiple subtypes may frequently coexist in the same cell.
Naturally occurring sst has a very low metabolic stability in vivo with a half-life less than 2 minutes. More stable synthetic sst analogues have been developed2,3 and for in vivo diagnostic purposes labeled with gamma-emitters: Tc-99m,4–6 In-111,7,8 Ga-67,9 and with positron emitters: C-11,10 F-18,11 Ga-68,9 and Cu-64.12 PET technology has shown dramatic improvement in diagnostic performance compared with sstr gamma scintigraphy.13
Current sst analogues for PET/CT diagnostics include 68Ga-DOTATOC, 68Ga-DOTANOC, and 68Ga-DOTATATE all of which bind to sstr2, predominantly expressed in neuroendocrine tumors (NET).14 The SUVmax of 68Ga-DOTATOC PET/CT scans tended to be higher than in 68Ga-DOTATATE counterparts for detection of NET.15,16 Beside NET, overexpression of sstr occurs in prostate cancer17 where 30% of the prostate cancer cells express sstr.18 The degree of sstr expression is correlated with Gleason score and tumor stage and appears to have a negative influence on patient outcome.19
Normal prostate is composed of repeating cellular units that contain stromal and epithelial components. The epithelial compartment contains luminal epithelial (secretory) cells, basal cells, and a minor component of neuroendocrine cells, whose function may be to regulate the growth, differentiation, and secretion of the prostate gland. The number of neuroendocrine cells increases in high grade and high stage prostate cancers, particularly in hormonally treated and hormone-refractory (androgen-independent) prostate cancer.20 Neuroendocrine cells are androgen-insensitive, and do not express the nuclear androgen receptor.20,21 Neuroendocrine differentiation of prostate cancer is associated with tumor progression and the androgen-independent state, for which there is currently no successful therapy. There may be a potential role for somatostatin agonists in the treatment of prostate cancer,22 which could be explored in theranostics23 where the same peptide is used (sst analogue), labeled either with a positron emitter (68Ga) for diagnostic localization, or with a beta emitter (177Lu) subsequently targeted for therapy.
To differentiate normal prostate from pathological uptake of 68Ga-DOTATOC PET/CT study, it is necessary to establish normal reference values. A recent study24 demonstrates the values for maximum standardized uptake value (SUVmax) of 68Ga-DOTATOC in normal human tissues, but data for prostate are lacking.
The aim of this study was to establish the 68Ga-DOTATOC PET/CT SUVmax values in prostate glands of NET patients, without NET involvement of the prostate.
Methods
Sixty-four patients (mean 60±14 years; age range 24–97 years) with histologically confirmed metastatic NET, without prostate involvement, were studied. Patients had not been on octreotide therapy nor had octreotide therapy for 6 weeks before the date of 68Ga-DOTATOC PET/CT. Urologic examination (including rectal examination and transrectal ultrasonography) was performed. Prostate hyperplasia was defined as increase in number (proliferation) of cells (enlargement usually affecting the middle prostate lobe), and prostate hypertrophy as increase in cell size.
Labeling technique and quality control of the radiopharmaceutical was performed according to the method described in a previous publication.25 68Ga-DOTATOC was prepared in the radiopharmacy of the Zentralklinik Bad Berka under GMP conditions and used in patients in agreement with specific German regulations for the use of in-house prepared radiopharmaceuticals (Arzneimittelgesetz § 4a) and in accordance with regulations by the Federal Office for Radiation Protection (BfC). The informed consent was obtained from all patients in accordance with the German regulations concerning the administration of radiolabeled substances to humans, and documentation of the data in a database was approved by the patients and the local institutional ethics committee.
Patients' weight and height were measured and recorded immediately prior to 68Ga-DOTATOC PET/CT. Patients were drinking 1.5 L of water-equivalent oral contrast dispersion Gastrografin 1 hour before the start of acquisition. To increase renal washout and decrease radiation exposure to the urinary bladder, 20 mg of i.v. furosemide was given after injection of 68Ga-DOTATOC. All patients were examined on a dual-modality PET/CT tomograph (Biograph duo; Siemens Medical Solutions).
Acquisition started 45–90 minutes (mean 70±14 minutes) after injection of 108–135 MBq (mean 121±7 MBq) of 68Ga-DOTATOC. First, a topogram was acquired. The patients were given 100 mL of intravenous contrast (by an automated injection pump), followed by computed tomography (CT) scanning in the craniocaudal direction with 30 second delay, and PET scanning in caudocranial direction.
After scatter and attenuation correction, PET emission data were reconstructed using an attenuation-weighted ordered-subsets maximization expectation approach with two iterations and eight subsets on 128×128 matrices and with a 5-mm Gaussian postreconstruction filtering.
The PET/CT images were assessed using E.soft (syngo-based nuclear medicine software).
In each of 64 patient studies SUVmax was calculated for the following organs: prostate gland, right testis, left testis, right gluteus maximus, left gluteus maximus, and normal liver.
For placing the region of interest (ROI) for different organs/structures SUVmax measurement, the PET/CT fusion images were used. The whole prostate was outlined, with special attention to exclude the urinary bladder from the ROI over the prostate gland. The ROI positioned over the prostate was carefully verified in all three planes (transversal, coronal, and sagital). The whole testes were included in the ROI over testes. For liver ROI the normal tissue of the liver was chosen for ROI, with care not to include possible metastases, present in the liver in some NET patients. Gluteus ROIs were symmetrically placed on both sides (right and left) inside the gluteus maximus muscle.
Prostate gland dimensions were measured on transverse CT slices. Transverse and anteroposterior diameters were obtained.
Statistical analysis was performed (Anova; Pearson Correlations) using dedicated statistical software (SPSS13).
Results
The intravenous injection of 68Ga-DOTATOC was well tolerated. No local or systemic side effects were evident during the time of observation (up to 180 minutes post injection). The results of data reconstruction and image analysis are displayed in figures (Figs. 1–3).
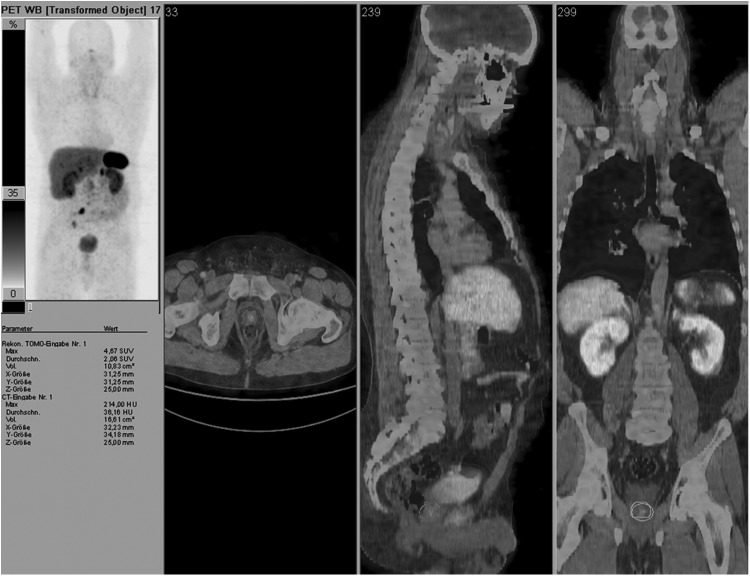
FIG. 1.
Prostate gland region of interest (ROI) on the fused 68Ga-DOTATOC PET/CT images. Maximum intensity projection (MIP) of the same NET patient is in the upper left corner.
FIG. 2.
Right testicle ROI in the fused 68Ga-DOTATOC PET/CT images.
FIG. 3.
Right gluteus maximus muscle ROI in the fused 68Ga-DOTATOC PET/CT images.
The SUVmax values (mean value, standard deviation, range and coefficient of variation) obtained for prostate gland, left and right testes, liver, left and right gluteus maximus (as background radioactivity) are tabulated (see Table 1).
Table 1.
Biodistribution of 68Ga-DOTATOC on the Basis of SUVmax Values
| SUVmax values for 68Ga-DOTATOC | ||||||
|---|---|---|---|---|---|---|
| Organ | Patient number | Mean | Median | SD | Range | CV [%] |
| Prostate (including cancer) | 64 | 4.5 | 1.8 | 2.0–10.9 | 40.0 | |
| Prostate (without cancer) | 62 | 4.5 | 1.6 | 2.0–8.7 | 35.6 | |
| Right testicle | 64 | 1.8 | 0.5 | 0.7–3.3 | 27.8 | |
| Left testicle | 64 | 1.8 | 0.5 | 0.8–3.1 | 27.8 | |
| Right gluteus muscle | 64 | 1.0 | 0.2 | 0.5–1.8 | 20.0 | |
| Left gluteus muscle | 64 | 1.0 | 0.2 | 0.6–1.6 | 20.0 | |
| Liver | 64 | 7.3 | 1.8 | 3.2–12.1 | 24.6 | |
SUVmax, maximum standardized uptake value; SD, standard deviation; CV, coefficient of variation.
Prostate had median SUVmax value of 4.5 with the range from 2–10.9 in the whole group of NET patients. When 2 NET patients with prostate cancer were excluded the median was still the same, but the range of values was lower (2.0–8.7).
Testes showed very low mean uptake values (SUVmax=1.8) being identical bilaterally and slightly above the background radioactivity (represented by gluteus maximus SUVmax value of 1.0) with remarkably low variability of 27.8%.
Our whole group of 64 NET patients had mean age of 60±14 years, ranging from 24 to 97 years. Strong correlation was found between patient age and sstr expression, determined by SUVmax value on 68Ga-DOTATOC PET/CT (Pearson's correlation coefficient was 0.325 and significance was 0.009; p<0.01). The sstr expression was significantly higher in patients older than 55 years than those below 55 years (p<0.05) (see Table 2).
Table 2.
Prostate SUVmax Values in the 2 Age Groups of NET Patients (2 Patients with Prostate Cancer Were Excluded)
| Patient age [years] | Patient number | Median prostate SUVmax | SD | Range | CV [%] |
|---|---|---|---|---|---|
| >55 (mean:67.8) | 39 | 4.7 | 1.7 | 2.1–8.7 | 36.2 |
| <55 (mean:45.1) | 23 | 4.0 | 1.3 | 2.0–6.5 | 32.5 |
In the whole group of 62 NET patients (without 2 NET patients with prostate cancer) there was a strong correlation between patient age and prostate size (Pearson's correlation coefficient was 0.448; two-tailed significance was 0.000; p<0.01) (see Table 3).
Table 3.
Prostate Size and SUVmax (Mean, SD, and Range) Values in Our Subgroups of NET Patients
| Prostate dimensions [cm] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NET patients | Transversal diameter | AP diameter | Prostate SUVmax | |||||||
| Group | No | Mean | SD | Range | Mean | SD | Range | Mean/median | SD | Range |
| Whole | 64 | 5.0 | 0.9 | 3.2–7.3 | 3.9 | 0.8 | 2.5–6.1 | 4.5 | 1.8 | 2.0–10.9 |
| Whole without cancer | 62 | 5.0 | 0.9 | 3.2–7.3 | 3.9 | 0.7 | 2.5–6.1 | 4.5 | 1.6 | 2.0–8.7 |
| Prostate cancer | 2 | 6.0 | 0.8 | 5.4–6.6 | 5.8 | 0.5 | 5.4–6.1 | 9.5 | 2.1 | 8.0–10.9 |
| Normal prostate dimensions | 2 | 3.4 | 0.3 | 3.2–3.6 | 2.6 | 0.1 | 2.5–2.6 | 2.6 | 0.0 | 2.6–2.6 |
| Slightly enlarged prostate | 33 | 4.6 | 0.4 | 3.4–5.3 | 3.5 | 0.3 | 3.0–4.1 | 4.2 | 1.6 | 2.1–8.4 |
| P. hypertrophy | 17 | 5.8 | 0.8 | 4.6–7.3 | 4.5 | 0.7 | 3.7–5.8 | 4.9 | 1.6 | 2.0–8.5 |
| P. hyperplasia | 10 | 5.5 | 0.8 | 3.7–6.3 | 4.2 | 0.9 | 2.8–6.1 | 5.0 | 1.5 | 3.4–8.7 |
AP, anteroposterior.
There were only 2 patients in our NET group, with normal prostate dimensions according to Shaaban's criteria.26 All other 62 patients (including patients with concomitant prostate cancer) had enlarged prostate.
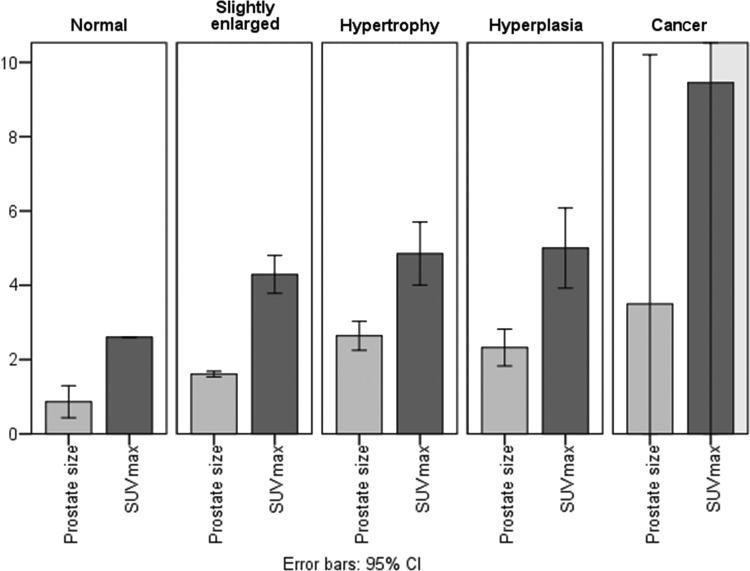
Prostate size (calculated as the mean transverse diameter multiplied by the mean anteroposterior (AP) diameter of prostate gland, and divided by 10) and the SUVmax value in the prostate gland, for subgroups of NET patients with a normal-sized, slightly enlarged prostate gland, prostate hypertrophy, and prostate hyperplasia were compared (Fig. 4). The relationship between prostate size, patient age, and prostate SUVmax was also analyzed (see Table 4) (Fig. 5).
FIG. 4.
Prostate SUVmax and size (calculated as the mean transverse diameter multiplied by the mean AP diameter of prostate gland, and divided by 10) in subgroups of NET patients.
Table 4.
Patient Age (Mean, SD), Prostate Size, and SUVmax in Subgroups of NET Patients
| NET patients | Prostate size [cm] | Prostate SUVmax | |||||
|---|---|---|---|---|---|---|---|
| Group | No | Age Mean±SD | Transv. | AP | Transv. x AP | Mean | Median |
| Whole | 64 | 60.0±14.0 | 5.0 | 3.9 | 18.5 | 4.5 | |
| Whole without cancer | 62 | 59.5±13.9 | 5.0 | 3.9 | 18.0 | 4.5 | |
| With cancer | 2 | 76.5±3.5 | 6.0 | 5.8 | 35.0 | 9.5 | |
| Normal P. dimensions | 2 | 65.5±0.7 | 3.4 | 2.6 | 8.7 | 2.6 | |
| Slightly enlarged P. | 33 | 54.2±14.6 | 4.6 | 3.5 | 16.1 | 4.2 | |
| P. hypertrophy | 17 | 67.4±10.3 | 5.8 | 4.5 | 26.4 | 4.9 | |
| P. hyperplasia | 10 | 62.1±12.2 | 5.5 | 4.2 | 23.2 | 5.0 | |
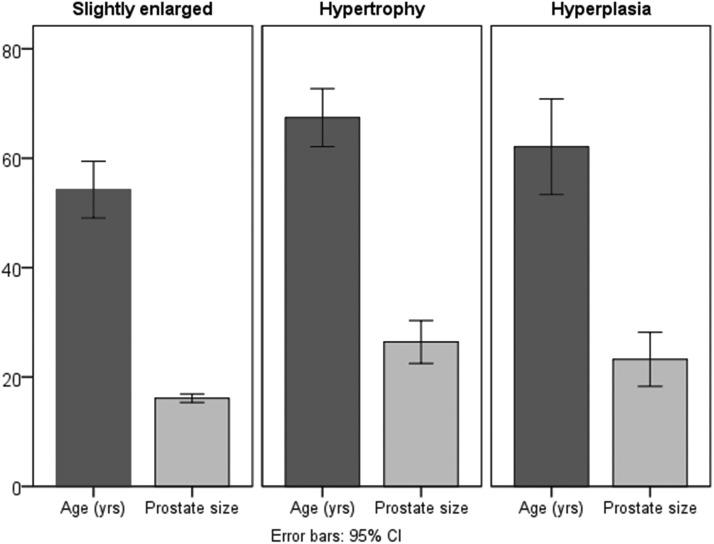
FIG. 5.
Patients' age related to the prostate size: slightly enlarged, hypertrophy, hyperplasia.
Patient age in the group with slightly enlarged prostate gland (54.2±14.6 years) was significantly lower (p<0.01) than in the group with more pronounced prostate hypertrophy (67.4±10.3 years). There was no significant difference between the age of NET patients with prostate hypertrophy and prostate hyperplasia (62.1±12.2 years), nor between the age of NET patients with slightly enlarged prostate and those with prostate hyperplasia.
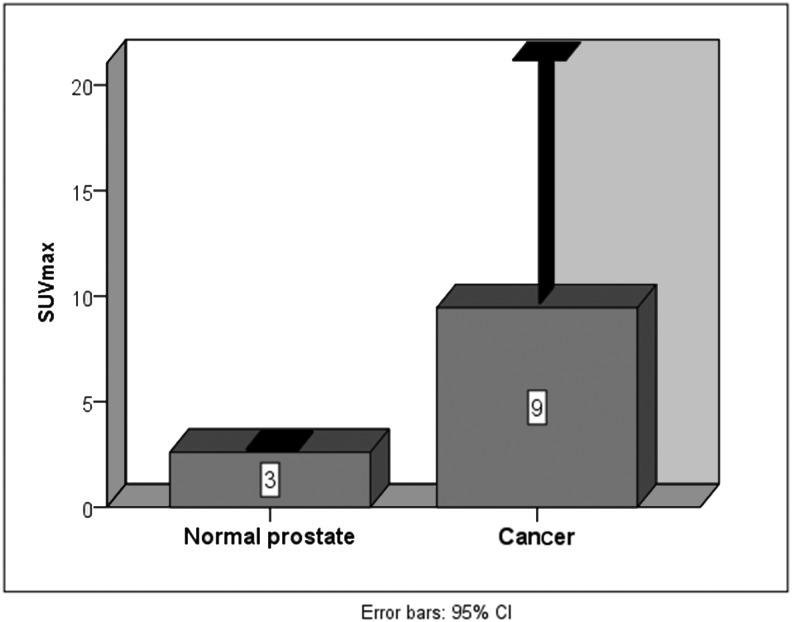
Although the SUVmax values tended to increase with the prostate dimensions (in normal prostate gland SUVmax=2.6; in slightly enlarged prostate SUVmax=4.2; in prostate hypertrophy SUVmax=4.9), Pearson's correlation coefficient for the whole group of 62 NET patients (excluding 2 prostate cancer patients) was 0.245 and the significance was slightly higher than 0.05 (p>0.05). ANOVA (one-way) showed no significant difference in SUVmax values between the groups with slight prostate enlargement, prostate hypertrophy, and prostate hyperplasia (p=0.398; p>0.05). In 2 NET patients with concomitant prostate cancer SUVmax values were the greatest (mean SUVmax=9.5) (Fig. 6).
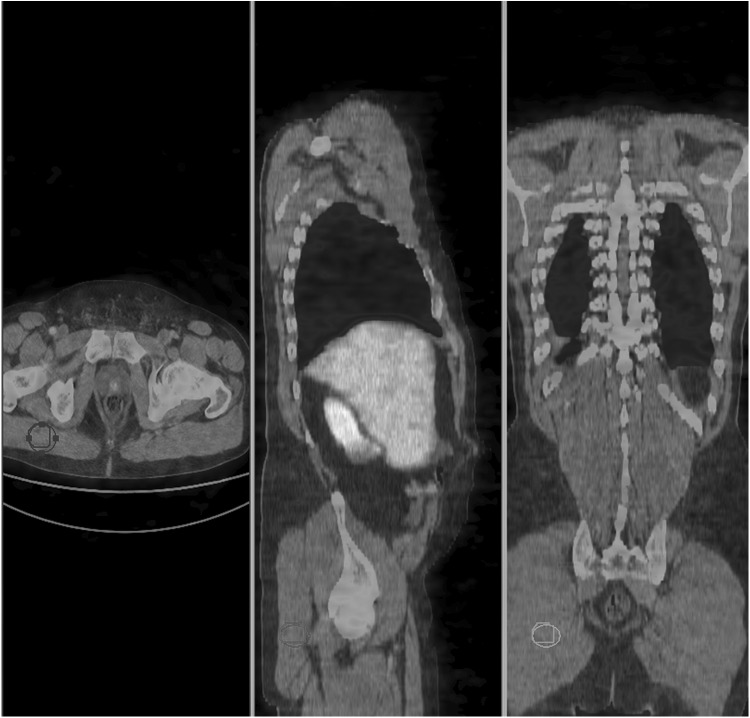
FIG. 6.
Prostate SUVmax in normal size gland and in concomitant prostate cancer.
Discussion
To differentiate normal from pathological uptake of 68Ga-DOTATOC PET/CT study, it is necessary to establish values of 68Ga-DOTATOC binding in the cancer-free and normal prostate tissue. The present study is the first approach to in vivo characterization of human sstr expression in NET-free, “apparently normal” prostate gland. It is a retrospective analysis on 64 NET patients with histologically confirmed metastatic NETs without prostate gland NET involvement referred for diagnostic whole-body 68Ga-DOTATOC PET/CT evaluation before peptide receptor radionuclide therapy.
Our population of NET patients was of relatively advanced age. The majority therefore had an enlarged prostate or documented prostate hypertrophy, which led to a strong correlation between patient age and in vivo sstr expression. (The sstr expression was significantly higher in patients older than 55 years than in those below 55 years.) If Shaaban and Woodward criteria for normal prostate diameters26 (4 cm right-left, 2 cm anteroposterior, and 3 cm craniocaudal diameter) are applied, only 2 out of 64 of our NET patients had normal prostate size. There was a significant strong correlation between patient age and prostate size in our group of NET patients. Patient age in the group with slightly enlarged prostate gland was significantly lower (p<0.01) than in the group with more pronounced prostate hypertrophy, but there was no significant difference in patient age between prostate hypertrophy and prostate hyperplasia. Two NET patients with concomitant prostate cancer (79 and 74 years old) had very enlarged prostates.
Prostate SUVmax values for 68Ga-DOTATOC in two NET patients with normal prostate size (65 and 66 years old) were identical and very low (2.6) while in two patients with concomitant prostate cancer, the values were almost four-fold higher (10.9 and 8.0). Normal prostate gland sstr expression was very low compared with prostate cancer.
The SUVmax values tended to be higher when the size of the prostate was greater (in slightly enlarged prostate 4.2 and in pronounced prostate hypertrophy 4.9) but the correlation between prostate size and SUVmax value did not reach significance (Pearson's correlation coefficient was 0.053 and p>0.05). In prostate hyperplasia (where prostate dimensions were greater than in slightly enlarged prostate group, but smaller than in the pronounced prostate hypertrophy group) mean SUVmax value was 5.0. No significant difference in SUVmax values between groups with slight prostate enlargement, pronounced prostate hypertrophy, and prostate hyperplasia could be found. Therefore, sstr expression cannot distinguish between prostate hyperplasia and prostate hypertrophy. The reason might be in the fact that these two terms prostate hypertrophy and hyperplasia are often used interchangeably, even among urologists.27
Compared to the mean value for in vivo sstr expression in normal liver tissue (SUVmax=7.3±1.8), sstr expression in our NET patients with normal prostate was almost three-fold lower (SUVmax=2.6). Normal prostate tissue sstr2 has reportedly more than six-fold greater sstr2 expression (39%) compared with liver sstr expression (5.7%),24 but in our in vivo conditions normal prostate shows three-fold lower sstr expression compared with the normal liver tissue. Higher liver uptake of 68Ga-DOTATOC than expected on the basis of sstr2 expression has been related to normal peptide metabolism in the liver.28,29
Only our patients with concomitant prostate cancer had in vivo sstr expression higher than the normal liver tissue, which could be convenient in theranostic approach (application of sst analogues both in diagnostics when labeled with 68Ga, and in therapy when labeled with Lu-177), together with the symmetrical and very low sstr expression in testes (SUVmax=1.8, just above the background level in gluteus maximus muscle with SUVmax=1.0).
The use of radiolabeled somatostatin analogues to visualize various sstr-positive tumors is widely accepted. Miederer with coworkers have detected a significant correlation of membranous sstr2 expression as determined by immunohistochemistry and 68Ga-DOTATOC PET/CT SUVmax values in patients with NETs.30 Kaemmerer has also shown highly significant correlation between immunoreactive score sstr2A and the SUVmax on the 68Ga-somatostatin analogue PET/CT study of NET patients.31 Overexpression of sstr occurs in 30% of the prostate cancer cells.17,18 The degree of sstr expression is correlated with Gleason score and tumor stage and appears to have a negative influence on patient outcome.19
However, sstr are also expressed on both normal and activated lymphocytes and macrophages. Overexpression of sstr has been found in patients with the inflammatory bowel disease, rheumatoid arthritis, Sjögren's syndrome, Graves' disease, and pulmonary diseases.32 It seems that imaging with radiolabeled sst analogues is applicable for chronic inflammation purposes, but is unsuitable for visualization of the acute infectious diseases.33 Hence, in patients with the chronic prostate inflammation augmented radiolabeled sst analogues accumulation could be expected, but there was no such case in our patient group.
Christian Boy et al. investigated sstr mRNA and SUVmax on 68Ga-DOTATOC PET/CT in normal human tissue (spleen, kidney, liver, stomach, head of pancreas, small bowel, thyroid, bone, large bowel, muscle, parotid gland, axillary lymph node, and lung), which provided the normative database of SUVmax and sstr mRNA for physiologically normal human tissues. They used real-time reverse transcriptase polymerase chain reaction to measure expression of sstr subtypes (sstr1-sstr5), and found that 68Ga-DOTATOC SUVmax values exclusively correlated with sstr2 expression, while there was no correlation of SUVmax with the expression of other four sstr subtypes.24 For the normal prostate they discovered the sstr subtypes expression (in mRNA copy numbers per microgram of total mRNA) as follows: sstr1: 54.56; sstr2: 37.23; sstr3: 25; sstr4: 155; sstr5: 3.60. Unfortunately, the data for the normal prostate 68Ga-DOTATOC SUVmax are lacking, which motivated us to conduct this study.
Although in our group of 64 NET patients with “apparently normal” prostate gland only two had normal prostate size, both had very low prostate 68Ga-DOTATOC SUVmax values (almost four-fold lower compared with the prostate SUVmax in patients with concomitant prostate Ca). Therefore, in male patients investigated by 68Ga-DOTATOC PET/CT, care should be taken not to overlook the augmented uptake in the prostate gland (especially if it is higher than in the liver tissue), which could indicate cancerous tissue.
Conclusion
Normal prostate tissue has very low (almost three-fold lower than normal liver tissue) sstr expression. Expression of sstr in hypertrophic and hyperplastic prostate is higher. Advanced age is associated with the increased prostate sstr expression. Prostate hyperplasia and hypertrophy cannot be differentiated by 68Ga/DOTATOC PET/CT. Only prostate cancer in our group of NET patients with “apparently normal” prostate gland was associated with higher sstr expression in comparison with non-neoplastic liver tissue. Care should be taken not to overlook augmented 68Ga-DOTATOC uptake in the prostate gland of male patients undergoing radiolabeled sst analogue PET/CT (especially if it is higher than in the liver tissue), which could indicate cancerous tissue.
Acknowledgments
The first author was International Atomic Energy Agency (IAEA) fellow from February to April 2010 at the Department of Nuclear Medicine and Centre for PET/CT, Zentralklinik Bad Berka, when this work was done. Vladimir Obradović was the counterpart from Serbia of IAEA TC Project SRB/6/005, which enabled this fellowship. This work was also supported by Grant No. 175018, Ministry of Education, Science and Technological Development of the Republic of Serbia.
Disclosure Statement
The authors declare that they have no conflict of interest.
References
- 1.Mazzucchelli R, Scarpelli MA, Lopez-Beltran L, et al. Immunohistochemical expression and localization of somatostatin receptors in normal prostate, high grade prostatic intraepithelial neoplasia and prostate cancer and its many faces. J Biol Regul Homeost Agents 2012;26:181. [PubMed] [Google Scholar]
- 2.Reubi JC, Waser B. Concomitant expression of several peptide receptors in neuroendocrine tumors: Molecular basis for in vivo multireceptor targeting. Eur J Nucl Med Mol Imaging 2003;30:781. [DOI] [PubMed] [Google Scholar]
- 3.Bombardieri E, Maccauro M, De Deckere E, et al. Nuclear medicine imaging of neuroendocrine tumors. Ann Oncol 2001;12(suppl2):S51. [DOI] [PubMed] [Google Scholar]
- 4.Decristoforo C, Mather SJ, Cholewinski W, et al. 99mTc-EDDA/HYNIC-TOC: A new 99mTc-labelled radiopharmaceutical for imaging somatostatin receptor-positive tumours; first clinical results and intra-patient comparison with 111In-labelled octreotide derivatives. Eur J Nucl Med 2000;27:1318. [DOI] [PubMed] [Google Scholar]
- 5.Maina T, Nock B, Nikolopoulou A, et al. [99mTc] Demotate, a new 99mTc-based [Tyr3] octreotate analogue for the detection of somatostatin receptor-positive tumours: Synthesis and preclinical results. Eur J Nucl Med Mol Imaging 2002;29:742. [DOI] [PubMed] [Google Scholar]
- 6.Storch D, Behe M, Walter MA, et al. Evaluation of [99mTc/EDDA/HYNICo]octreotide derivatives compared with [111In-DOTAo, Tyr3, Thr8]octreotide and [111In-DTPAo]octreotide: Does tumor or pancreas uptake correlate with the rate of internalization? J Nucl Med 2005;46:1561. [PubMed] [Google Scholar]
- 7.de Jong M, Bakker WH, Krenning EP, et al. Yttrium-90 and indium-111 labelling, receptor binding and biodistribution of [DOTAo,D-Phe1, Tyr3]octreotide, a promising somatostatin analogue for radionuclide therapy. Eur J Nucl Med 1997;24:368. [DOI] [PubMed] [Google Scholar]
- 8.Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]octreotide: The Rotterdam experience with more than 1000 patients. Eur J Nucl Med 1993;20:716. [DOI] [PubMed] [Google Scholar]
- 9.Smith-Jones PM, Stolz B, Bruns C, et al. Gallium-67/gallium-68-[DFO]-octreotide–a potential radiopharmaceutical for PET imaging of somatostatin receptor positive tumors: Synthesis and radiolabeling in vitro and preliminary in vivo studies. J Nucl Med 1994;35:317. [PubMed] [Google Scholar]
- 10.Henriksen G, Schottelius M, Poethko T, et al. Proof of principle for the use of 11C-labelled peptides in tumour diagnosis with PET. Eur J Nucl Med Mol Imaging 2004;31:1653. [DOI] [PubMed] [Google Scholar]
- 11.Wester H-J, Schottelius M, Scheidhauer K, et al. PET imaging of somatostatin receptors: Design, synthesis and preclinical evaluation of a novel 18F-labelled, carbohydrated analogue of octreotide. Eur J Nucl Med Mol Imaging 2002;30:117. [DOI] [PubMed] [Google Scholar]
- 12.Sprague JE, Peng Y, Sun X, et al. Preparation and biological evaluation of copper-64-labeled Tye3-octreotate using a cross-bridged macrocyclic chelator. Clin Cancer Res 2004;10:8674. [DOI] [PubMed] [Google Scholar]
- 13.Gabriel M, Decristoforo C, Kendler D, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J Nucl Med 2007;48:508. [DOI] [PubMed] [Google Scholar]
- 14.Antunes P, Ginj M, Yhang H, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging 2007;34:982. [DOI] [PubMed] [Google Scholar]
- 15.Poeppel TD, Binse I, Petersenn S, et al. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J Nucl Med 2011;52:1864. [DOI] [PubMed] [Google Scholar]
- 16.Todorovic-Tirnanic M, Prasad V, Baum RP, et al. Comparison of standardized uptake value (SUV) of Ga-68 DOTA-TOC and DOTA-TATE in normal organs and metastases of neuroendocrine tumor patients: Preliminary results. Eur J Nucl Med Mol Imaging 2010;37(suppl2):S302(OP588) [Google Scholar]
- 17.Reubi JC. Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 2003;24:389. [DOI] [PubMed] [Google Scholar]
- 18.Luboldt W, Zöphel K, Wunderlich G, et al. Visualization of somatostatin receptors in prostate cancer and its bone metastases with Ga-68-DOTATOC PET/CT. Mol Imaging Biol 2010;12:78. [DOI] [PubMed] [Google Scholar]
- 19.Bollito E, Berruti A, Bellina M, et al. Relationship between neuroendocrine features and prognostic parameters in human prostate adenocarcinoma. Ann Oncol 2001;12(suppl2):S159. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Niu J, Huang J. Neuroendocrine differentiation in prostate cancer. Am J Transl Res 2009;1:148. [PMC free article] [PubMed] [Google Scholar]
- 21.Bankoff H. Neuroendocrine differentiation in human prostate cancer. Morphogenesis, proliferation and androgen receptor status. Ann Oncol 2001;12(suppl2):S141. [DOI] [PubMed] [Google Scholar]
- 22.Hansson J, Abrahamsson P-A. Neuroendocrine pathogenesis in adenocarcinoma of the prostate. Ann Oncol 2001;12(suppl2):S145. [DOI] [PubMed] [Google Scholar]
- 23.Baum RP, Kulkarni HR. Theranostics: From molecular imaging using Ga-68 labelled tracers and PET/CT to personalized radionuclide therapy–The Bad Berka Experience. Theranostics 2012;2:437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boy C, Heusner TA, Poeppel TD, et al. 68Ga-DOTATOC PET/CT and somatostatin receptor (sst1-sst5) expression in normal human tissue: Correlation of sst2 mRNA and SUVmax. Eur J Nucl Med Mol Imaging 2011;38:1224. [DOI] [PubMed] [Google Scholar]
- 25.Zhernosekov KP, Filosofov DV, Baum RP, et al. Processing of generator-produced 68Ga for medical application. J Nucl Med 2007;48:1741. [DOI] [PubMed] [Google Scholar]
- 26.Shaaban AM, Woodward PJ. Prostate and Seminal Vesicles. In: Federle MP, Rosario-de Christenson ML, Woodward PJ, Abbott GF, (eds.) Shaaban AM. (Managing Ed.), Diagnostic and Surgical Imaging Anatomy – Chest-Abdomen-Pelvis. Part III Salt Lake City: Amirsys, 2007;170 [Google Scholar]
- 27.Bostwick DG. The Pathology of Benign Prostatic Hyperplasia. In: Kirby P, McCommell JD. and Fitzpatrick JM, (eds.), Textbook of Benign Prostatic Hyperplasia. London: Isis Medical Media, 2002 [Google Scholar]
- 28.Pettinato C, Sarnelli A, Di Donna M, et al. 68Ga-DOTANOC: Biodistribution and dosimetry in patients affected by neuroendocrine tumors. Eur J Nucl Med Mol Imaging 2008;51:72. [DOI] [PubMed] [Google Scholar]
- 29.Prasad V, Baum RP. Biodistribution of the Ga-68 labeled somatostatin analogue DOTA-NOC in patients with neuroendocrine tumors: Characterization of uptake in normal organs and tumor lesions. Q J Nucl Med Mol Imaging 2010;54:61. [PubMed] [Google Scholar]
- 30.Miederer M, Seidl S, Buck A, et al. Correlation of immunohistopathological expression of somatostatin receptor 2 with standardised uptake values in 68Ga-DOTATOC PET/CT. Eur J Nucl Med Mol Imaging 2009;36:48. [DOI] [PubMed] [Google Scholar]
- 31.Kaemmerer D, Peter L, Lupp A, et al. Molecular imaging with 68Ga-SSTR PET/CT and correlation to immunohistochemistry of somatostatin receptors in neuroendocrine tumors. Eur J Nucl Med Mol Imaging 2011;38:1659. [DOI] [PubMed] [Google Scholar]
- 32.Koopmans KP, Glaudemans AWJM. Rationale for the use of radiolabelled peptides in diagnosis and therapy. Eur J Nucl Med Mol Imaging 2012;39(suppl1):S4. [DOI] [PubMed] [Google Scholar]
- 33.Signore A, Mather SJ, Piaggio G, et al. Molecular imaging of inflammation/infection: Nuclear medicine and optical imaging agents and methods. Chem Rev 2010;110:3112. [DOI] [PubMed] [Google Scholar]