Abstract
Neutrophil-mediated tissue injury is a shared pathogenesis of both chronic pulmonary diseases and acute responses to pathogens, allergens, and airborne pollutants. Interventions to minimize toxic effects of neutrophil-derived oxidants and proteases are usually limited to corticosteroids, which can have adverse side effects. We used a rodent model of endotoxin-induced lung injury to test the hypothesis that the dietary supplement γ-tocopherol (γT), a natural form of vitamin E with antioxidant and novel anti-inflammatory properties, will protect from adverse nasal and pulmonary inflammatory responses induced by endotoxin (lipopolysaccharide; LPS). Male Fisher F344 rats were intranasally (i.n.) instilled with LPS for 2 consecutive days. Beginning 2 days before i.n. LPS, the rats were gavaged daily with 30 mg/kg γT. Twenty-four hours after the last i.n. LPS, bronchoalveolar lavage fluid (BALF) was collected, and pulmonary and nasal tissues were analyzed for gene expression and morphometric analyses of neutrophils and intraepithelial mucosubstances (IM). LPS caused increased BALF total cells (70% increase), neutrophils (300%), protein (35%), PGE2 (500%), and secreted mucins (75%). Robust increases in neutrophils and IM were detected in conducting airways. Pulmonary expression of MUC5AC, MIP-2, CINC-1, and MCP-1 was elevated three- to eightfold by LPS. Treatment with γT inhibited LPS-induced increases in BALF total cells, neutrophils, protein, PGE2, and secreted mucins, as well as IM and tissue neutrophil influx. Furthermore γT induced the expression of the regulatory cytokines IL-10 and IFN-γ while decreasing MUC5AC, MIP-2, CINC-1, and MCP-1. These data demonstrate novel therapeutic effects of the dietary vitamin E γT promoting anti-inflammatory pathways to protect from neutrophil-mediated lung injury.
Keywords: Neutrophil, Endotoxin, γ-Tocopherol, Prostaglandin E2, Intraepithelial mucosubstances, Free radicals
Chronic neutrophilic lung diseases such chronic obstructive pulmonary disease (COPD) and cystic fibrosis are the second leading contributor to disability-adjusted life years in North America [1]. Polymorphonuclear leukocytes (neutrophils; PMNs) are also the primary airway granulocytes in severe asthma, as well as asthma diagnoses associated with the obese and the elderly [2,3]. Furthermore, airway infiltration and activation of PMNs are critical features in acute lung injury in response to inhalation exposure to airborne toxicants such as ozone, particulate matter, and biogenic toxicants [4–6]. As such, interventions that control the recruitment or activation of airway PMNs would have broad applications in the mitigation of the morbidity associated with a number of acute and chronic airway diseases.
Recruitment of PMNs into lung airspaces occurs in response to a variety of airway stimuli and involves the stepwise process of adhesion molecule expression on PMNs, epithelial cells, and endothelial cells in both the pulmonary capillaries and the bronchial venules [7]. Reactive oxygen species (ROS) and elastase released from emigrated neutrophils are primary factors in the pathogenesis of neutrophil-mediated airway injury and disease [8,9]. Interventions to protect from oxidant-induced tissue injury mediated by PMNs include, among others, glucocorticosteroids [10]; inhibitors of NADPH oxidase, which is responsible PMN oxidative burst [11]; and antioxidants that interact directly with and neutralize ROS. Included in the last category are micronutrients with antioxidant properties such as α-tocopherol (αT) and ascorbate (vitamin C), both of which have been used to reverse oxidant-mediated pulmonary injury with varying degrees of success [12,13].
We have previously shown that γ-tocopherol (γT), the major form of vitamin E in U.S. diets, can prevent eosinophilic inflammation and airway remodeling in experimental asthma [14] and in ozone exacerbation of allergic rhinitis in laboratory rodents [15]. In subsequent studies in vitro we found that γT inhibits IL-13-induced production of eotaxin from airway epithelial cells [16], an effect that would target eosinophil recruitment and explain the antiallergic effects we documented in rodent studies. Recently members of our group described the γT-mediated interruption of neutrophil signal transduction pathways and γT-caused decreased production of inflammatory leukotrienes [17]. This led us to hypothesize that, like the inhibition of airway eosinophil recruitment, γT would block the recruitment and activation of neutrophils into lung and thereby inhibit tissue injury associated with neutrophil mediator production. In the current study we used a well-characterized rodent model of endotoxin-induced airway injury that is dependent on neutrophils [18] to test the effects of dietary supplementation with γT on the treatment-related changes in histological, morphological, biochemical, and molecular endpoints. Herein we describe attenuation by γT of endotoxin-induced inflammatory cell recruitment, cytokine gene expression, prostanoid production, and overproduction of mucus in both nasal and pulmonary airways.
Materials and methods
Animals
Male F344/N rats, 9–10 weeks of age, were obtained from Harlan Sprague–Dawley (Indianapolis, IN, USA) and randomly assigned to one of six experimental groups (n = 7/group). Rats were used in accordance with guidelines set forth by the All-University Committee on Animal Use and Care at Michigan State University. Animals were housed two per cage in polycarbonate boxes on Aspen chip bedding, covered with filter lids, and had free access to tap water and food (Tek Lad 22/5 Rodent Diet W 8649; Harlan).
Treatment protocols
Rats were instilled intranasally (i.n.) with 150-µl volumes of endotoxin (lipopolysaccharide (LPS) from Pseudomonas aeruginosa; Sigma Chemical Co., St. Louis, MO, USA) in pyrogen-free saline into each nasal passage (300 µl total volume), for total doses of 0, 5, or 20 µg (equivalent to 2500 and 10,000 endotoxin units (EU), respectively). For instillations, rats were lightly anesthetized with 4% isoflurane in oxygen. A second instillation was given 24 h later. Intranasal challenge procedures were conducted between 10:00 and 11:00 AM each day.
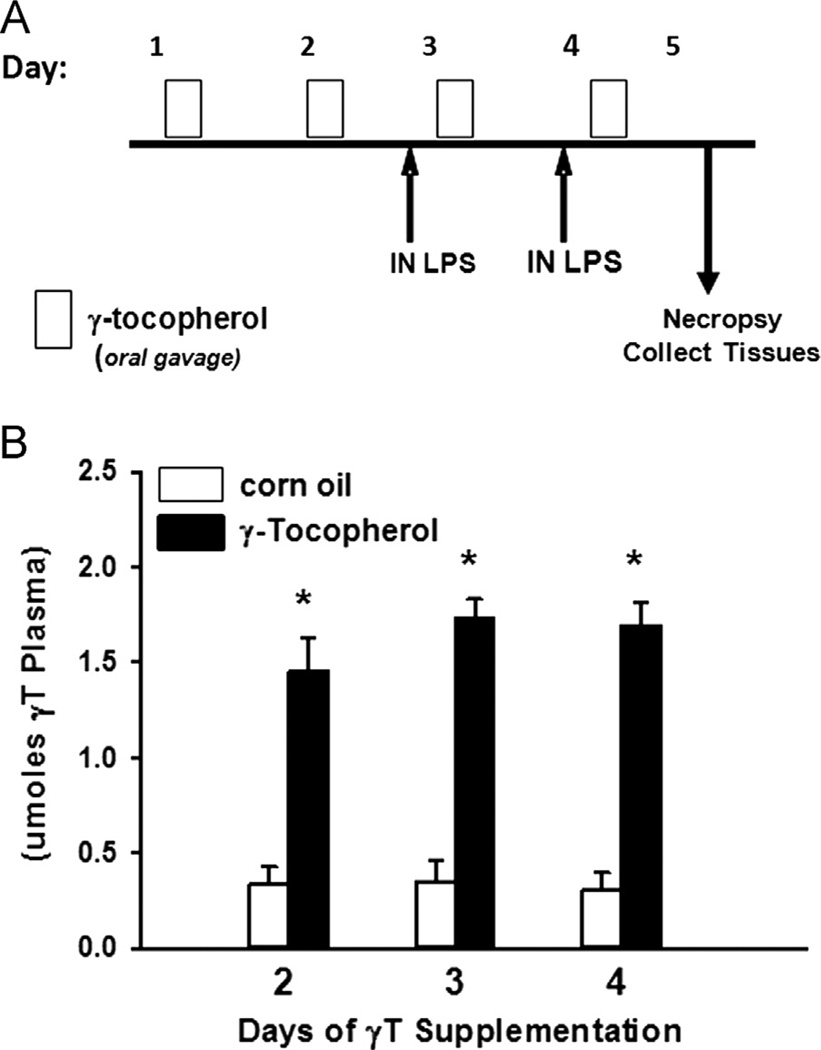
Two days before i.n. LPS procedures, rats were begun on a daily supplementation regimen of 30 mg/kg body wt of d-γ-tocopherol (highly purified R,R,R isomer, naturally occurring 96% pure; Yasoo Health, Johnson City, TN, USA) diluted in 0.5 ml tocopherol-stripped corn oil (Dyets, Bethlehem, PA, USA). Stock dilutions were kept under nitrogen at 4 °C and contained no other tocopherols, tocotrienols, or oxidation products. γT was administered by oral gavage each day at 6:00 PM for 4 consecutive days, such that two administrations of γT were given before the first i.n. LPS dose. Animals were euthanized and tissues were collected 24 h after the last dose of i.n. LPS. The treatment and supplementation protocol is depicted in Fig. 1A. Separate animals (n = 3/group) were used to determine circulating γT levels 24 h after 2, 3, and 4 days of supplementation.
Fig. 1.
Experimental protocol. (A) Rats were given 0 or 30 mg/kg γT in corn oil by oral gavage for 4 consecutive days and on the 3rd and 4th days were instilled with endotoxin (LPS; 0 or 20 µg) by intranasal instillation (i.n.), and tissues were collected as described under Materials and methods on day 5. (B) Plasma concentration of γ-tocopherol was determined 24 h after 2, 3, and 4 days of supplementation in rats not given LPS. *p < 0.05, significantly different from respective group given corn oil.
Necropsy and tissue preparation
Animals were anesthetized with sodium pentobarbital (50 mg/kg), a midline laparotomy was performed, 4 ml of blood was drawn into a Vacutainer for separation of plasma, and the animals were exsanguinated by cutting the abdominal aorta. Immediately after death, the trachea was exposed and cannulated, and the heart and lung were excised en bloc. The bronchus to the left lung was temporarily closed with a hemostatic clamp, and 5 ml of sterile saline was instilled through the tracheal cannula and withdrawn to recover bronchoalveolar lavage fluid (BALF) from the right lung lobes. A second saline lavage was performed and combined with the first.
After lavage, the right lung lobes were ligated and removed. The right cranial lobe was also excised and placed in RNAlater (Qiagen, Valencia, CA, USA) and held at 4 °C until further processing for RNA isolation. The clamp was removed from the left bronchus, and the left lobe was inflated under constant pressure (30 cm H2O) with neutral-buffered formalin for 2 h while immersed in a large volume of fixative. Heads were removed and placed in fixative. Twenty-four hours later, two sections were excised from the left lung lobe, at the levels of the 5th and 11th airway generation, to sample proximal and distal airways, respectively [26]. Heads were decalcified in 13% formic acid for 5 days. Nasal sections were taken from the anterior nasal cavity by making a perpendicular cut to the hard palate at the level immediately posterior to the upper incisors. Tissue blocks were then embedded in paraffin, and 5- to 6-µm-thick sections were cut from the anterior surfaces. Lung sections were stained with hematoxylin and eosin for routine histology and with Alcian blue (pH 2.5)/periodic acid–Schiff (AB/PAS) to detect intraepithelial mucosubstances (IM). PMNs within the lung and nasal sections were immunohistochemically identified by using a rabbit-derived antibody to rat PMNs (gift from Robert A. Roth, Michigan State University). Briefly, formalin-fixed tissue sections were digested for 10 min in a pepsin:HCl solution before incubation overnight with rat PMN antiserum. These immunohistochemical sections were then incubated with rabbit IgG conjugated to alkaline phosphatase, which reacted with a substrate (Vector Red Substrate Kit; Vector Laboratories, Burlingame, CA, USA) to form a red stain as imaged under a light microscope. The samples were counterstained with hematoxylin. Positively stained PMNs were identified not only by the red color but by other distinct morphological features, including cell size and a multilobular nucleus. Site-specific morphometry of IM and PMNs was conducted in the respiratory epithelium and submucosa, respectively, of the proximal and distal axial pulmonary axial airways and of the proximal nasal septum.
Plasma γ-tocopherol
Concentrations of plasma γT were determined as described previously [14]. Briefly, plasma was extracted and eluted by HPLC approaches and concentrations of tocopherols were determined via electrochemical detection by comparison to external standards.
Bronchoalveolar lavage
Total leukocytes in BALF were enumerated with a hemocytometer, and fractions of eosinophils, PMNs, macrophages, and lymphocytes were determined in a cytospin sample stained with Diff-Quik (Dade Behring, Newark, DE, USA). Total protein was determined by the bicinchoninic acid method (BCA Kit 23225; Thermo Fisher, Rockford, IL, USA). Secretion of mucosubstances into airways was estimated by an ELISA for mucin glycoprotein 5AC using a mouse monoclonal antibody to the human MUC5AC protein (Mucin 5AC Ab-1; Neomarkers, Fremont, CA, USA) that has reactivity to the rat rMuc5AC core protein. Bound primary antibody was detected with a biotinylated rabbit anti-mouse secondary antibody and quantitated using horseradish peroxidase-conjugated avidin/biotin complex (ABC Reagent; Vector Laboratories) and a fluorescent substrate (QuantaBlue; Pierce Chemical, Rockford, IL, USA). Prostaglandin E2 (PGE2) was determined by commercial EIA using a Prostaglandin E Metabolite Kit (Cayman Chemical, Ann Arbor, MI, USA).
Morphometry of stored intraepithelial mucosubstances
To estimate the amount of the intraepithelial mucosubstances in lining maxilloturbinates and the respiratory epithelium lining the axial pulmonary airways, the volume density of AB/PAS-stained mucosubstances was quantified using computerized image analysis and standard morphometric techniques. Briefly, the area of AB/PAS-stained mucosubstance was estimated by setting colorized thresholds to highlight only IM, and these areas were then automatically calculated using the public domain NIH Image program (written by Wayne Rasband, U.S. National Institutes of Health, and available at http//rsb.info.nih.gov/nih-image/). The length of the basal lamina underlying the surface epithelium was calculated from the contour length of the digitized image of the basal lamina. The volume of stored mucosubstances per unit of surface area of epithelial basal lamina was estimated using the method described in detail by Harkema and Hotchkiss [19] and was expressed as nanoliters of intraepithelial mucosubstances per square millimeter of basal lamina (i.e., volume density).
Morphometry of submucosal PMNs
The numeric density of PMNs in the mucosa underlying the respiratory epithelium of conducting pulmonary airways was determined by counting PMN-positive stained cells with distinct neutrophil morphology. The length of the basal lamina was calculated from its contour length in a digitized image as described above, and the data were expressed as the number of PMNs divided by the length of the underlying basal lamina.
Mucin gene expression
Total RNA was extracted using the RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen). The evaluation of relative mRNA expression levels of the chemoattractant cytokines MIP-2, CINC-1, and MCP-1 and of the mucin glycoprotein 5AC was performed by two-step RT-PCR using manufacturer protocols (Applied Biosystems, Foster City, CA, USA). Briefly, reverse transcription was performed using High Capacity cDNA Archive Kit reagents. Quantitative mRNA expression analysis was performed using an ABI PRISM 7900 HT sequence detection system at Michigan State University’s Research Technology Support Facility using TaqMan Gene Expression Assay reagents (Applied Biosystems). Relative gene expression was normalized to 18S. Data were expressed as fold increase in RNA expression compared to control animals, which were set at a value of 1.
Statistical analysis
Data are expressed as the mean ± SE. Grubb’s outlier test was used to determine statistical outliers. Data were analyzed using a completely randomized analysis of variance (SigmaStat version 12; Jandel Scientific). Multiple comparisons were made by Student–Newman–Keuls post hoc test. Criterion for significance was set at p ≤ 0.05.
Results
Circulating γT
Daily oral gavage with 30 mg/kg γT led to a four- to fivefold increase in plasma vitamin E to approximately 1.5 µM within 2 days (Fig. 1B). Repeated supplementation did not induce further increase in plasma γT.
Bronchoalveolar lavage fluid
Instillation with 5 or 20 µg LPS induced robust airway inflammation as indicated by increases in BALF PMNs (Table 1). Increased BALF macrophages were elicited by 20, but not 5 µg LPS. Supplementation with γT attenuated LPS-induced inflammation to control levels for both PMNs and macrophages.
Table 1.
Effects of γT supplementation on LPS-induced BALF cellularity.
| Experimental group |
Total cells (× 104) |
Neutrophils (× 105) |
Macrophages (× 105) |
Lymphocytes (× 105) |
|---|---|---|---|---|
| Saline/corn oil | 8.5 ± 0.3 | 0.9 ± 1.3 | 7.4 ± 0.3 | 0.1 ± 0.03 |
| Saline/γ-tocopherol | 8.3 ± 0.7 | 1.5 ± 0.7 | 6.5 ± 1.0 | 0.2 ± 0.1 |
| 5 µg LPS/corn oil | 13.7 ± 2.0* | 6.1 ± 1.7* | 7.1 ± 0.7 | 0.8 ± 0.1 |
| 5 µg LPS/γ-tocopherol | 12.1 ± 1.2* | 2.9 ± 1.1** | 8.3 ± 0.8 | 0.7 ± 0.2 |
| 20 µg LPS/corn oil | 20.6 ± 2.5*,*** | 9.4 ± 3.0*,*** | 10.3 ± 1.5*,*** | 0.9 ± 0.3 |
| 20 µg LPS/γ-tocopherol | 11.4 ± 1.4** | 2.0 ± 0.9** | 8.9 ± 1.1 | 1.0 ± 0.4 |
Data are expressed as group mean ± SEM.
p < 0.05, significantly different from respective group given 0 µg endotoxin.
p < 0.05, significantly different from respective group given corn oil.
p < 0.05, significantly different from respective group given 5 µg endotoxin.
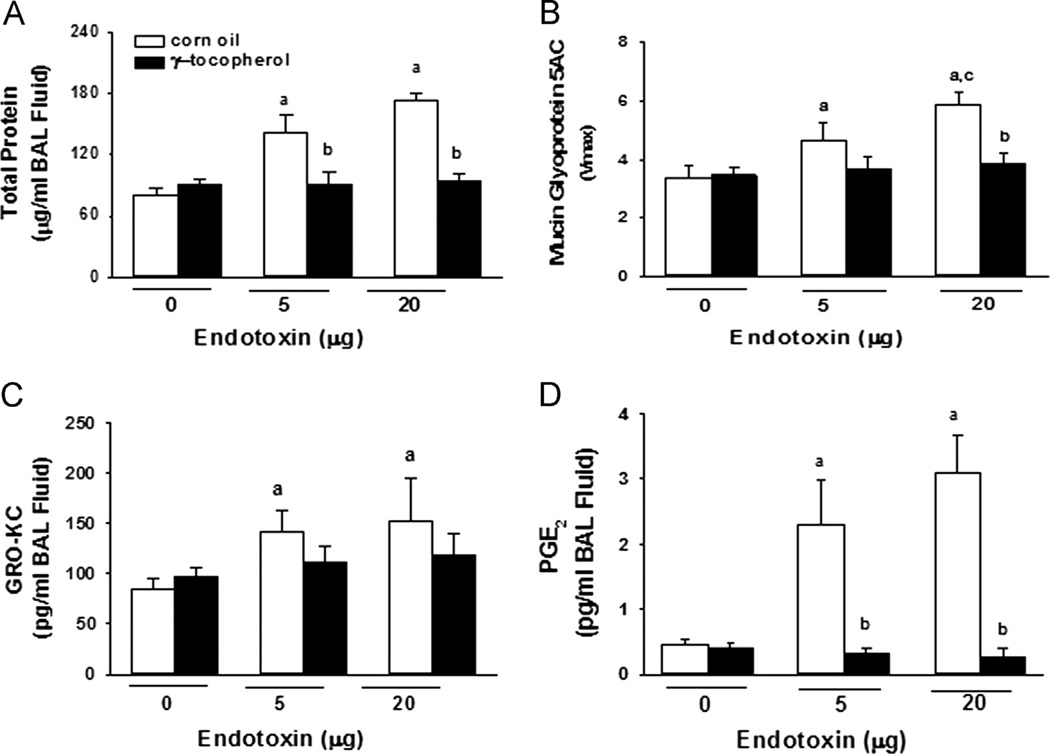
Total protein (Fig. 2A) and mucin glycoprotein 5AC (Fig. 2B; an estimate of mucus secretion) were significantly increased in BALF after instillation with either 5 or 20 µg LPS. Endotoxin-induced increases in total protein were completely blocked by γT supplementation. Increases in mucus secretion were not significant in γT-supplemented rats given 5 µg LPS and were significantly inhibited in rats given 20 µg LPS. Instillation with 5 or 20 µg LPS induced accumulation of both GRO-KC and PGE2 in BALF, which was completely inhibited in rats given γT (Fig. 2C).
Fig. 2.
Bronchoalveolar lavage proteins. Concentrations in BAL fluid of (A) total protein, (B) mucin glycoprotein 5AC, (C) chemoattractant cytokine GRO-KC, and (D) prostaglandin E2 were determined as described under Materials and methods. a, significantly different from respective group given 0 µg endotoxin; b, significantly different from respective group given corn oil; c, significantly different from respective group given 5 µg endotoxin; p < 0.05.
Pulmonary mucous cell metaplasia
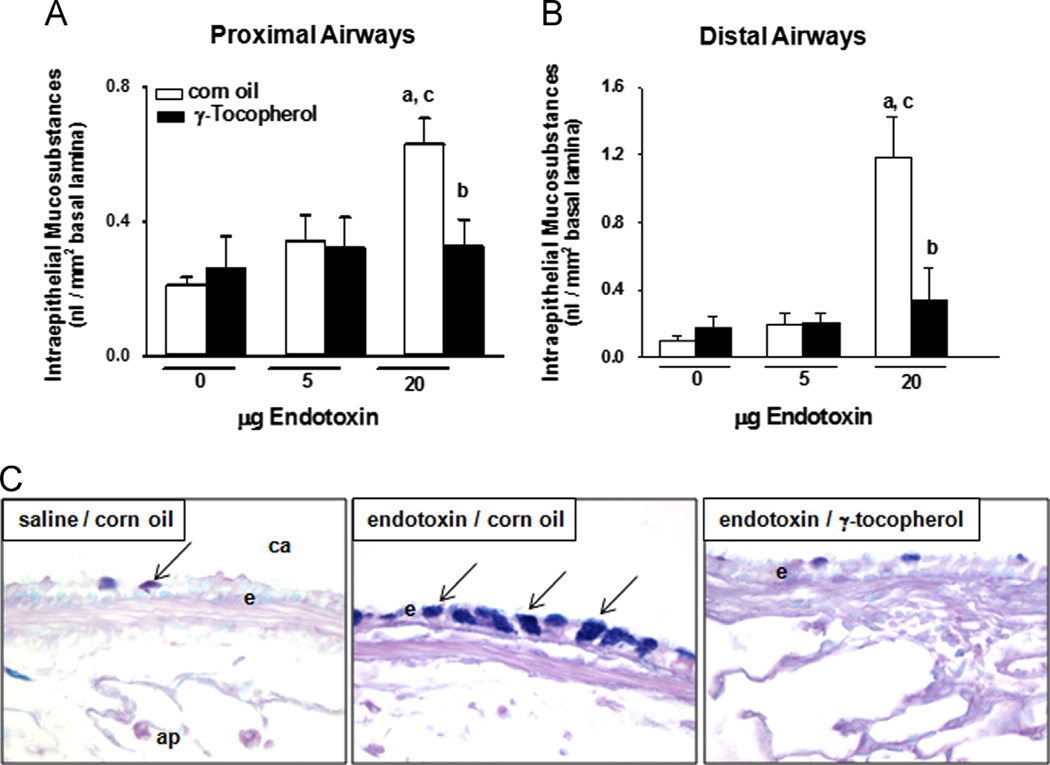
Instillation with 20, but not 5 µg LPS induced mucous cell metaplasia in proximal and distal axial airways as indicated by increased IM in proximal (230% increase) and distal (900%) conducting airways in rats (Fig. 3). Increases in IM after instillation with 5 µg LPS were not significant. Supplementation with γT reduced LPS-induced IM responses to control levels. Histochemical detection of IM was sparse in conducting airways of control rats given saline and corn oil, but robust increases in IM were induced after treatment with LPS (Fig. 3C). Supplementation with γT in LPS-treated rats resulted in minimal IM accumulation.
Fig. 3.
Mucous cell metaplasia. Intraepithelial mucosubstances in (A) proximal and (B) distal conducting airways were quantified in lung sections by morphometric approaches as described under Materials and methods. a, significantly different from respective group given 0 µg endotoxin; b, significantly different from respective group given corn oil; c, significantly different from respective group given 5 µg endotoxin; p < 0.05. (C) Mucosubstances were histochemically detected in AB/PAS-stained tissues as blue positive intracellular staining (arrows) of airway epithelium. ca, conducting airway; e, airway epithelium; ap, alveolar parenchyma. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Pulmonary neutrophils
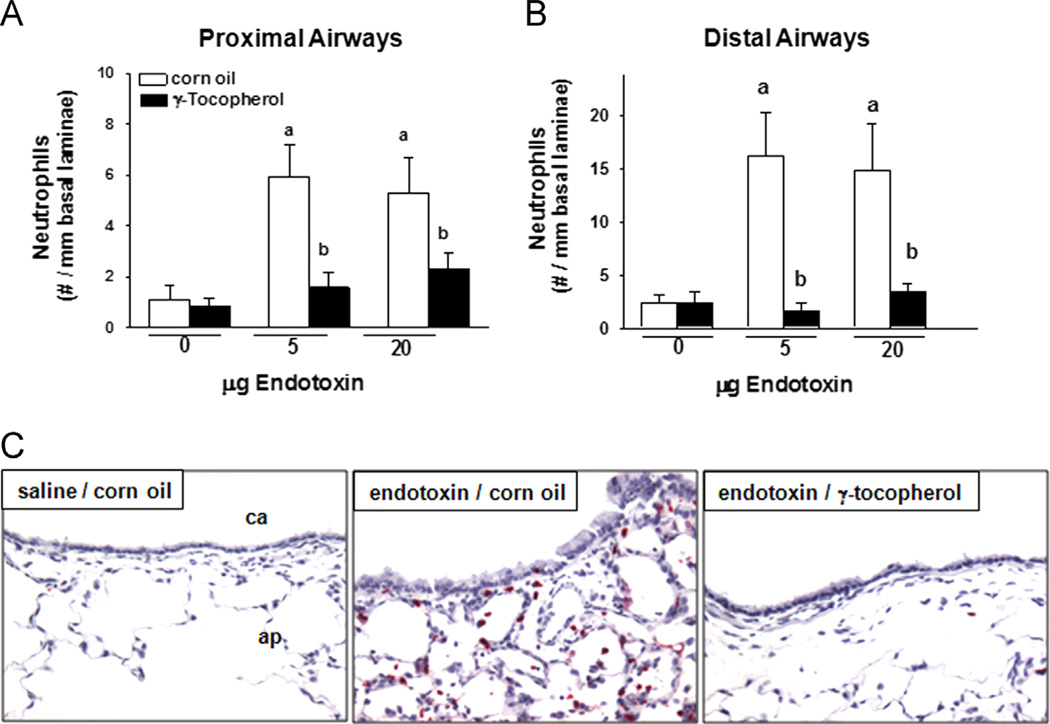
Instillation with LPS induced significant infiltration of PMNs into the submucosa of proximal (400% increase) and distal (600%) axial airways (Fig. 4A and B, respectively). Responses were equivalent in rats given either 5 or 20 µg LPS. PMN infiltration induced by both doses of LPS was reduced to control levels in rats supplemented with γT. Immunohistological detection of PMNs was minimal in conducting airways of control rats, but a marked infiltration occurred in the parenchyma and submucosa of conducting airways after treatment with LPS (Fig. 4C). LPS-induced recruitment of pulmonary PMNs was attenuated in rats supplemented with γT.
Fig. 4.
Pulmonary neutrophils. Neutrophils surrounding (A) proximal and (B) distal conducting airways were enumerated in sections of lung tissues by morphometric approaches as described under Materials and methods. a, significantly different from respective group given 0 µg endotoxin; b, significantly different from respective group given corn oil; p < 0.05. (C) Neutrophils were identified by their staining positive (red cells) for anti-rat neutrophil antibody in immunohistochemically processed tissues. ca, conducting airway; ap, alveolar parenchyma.
Pulmonary gene expression
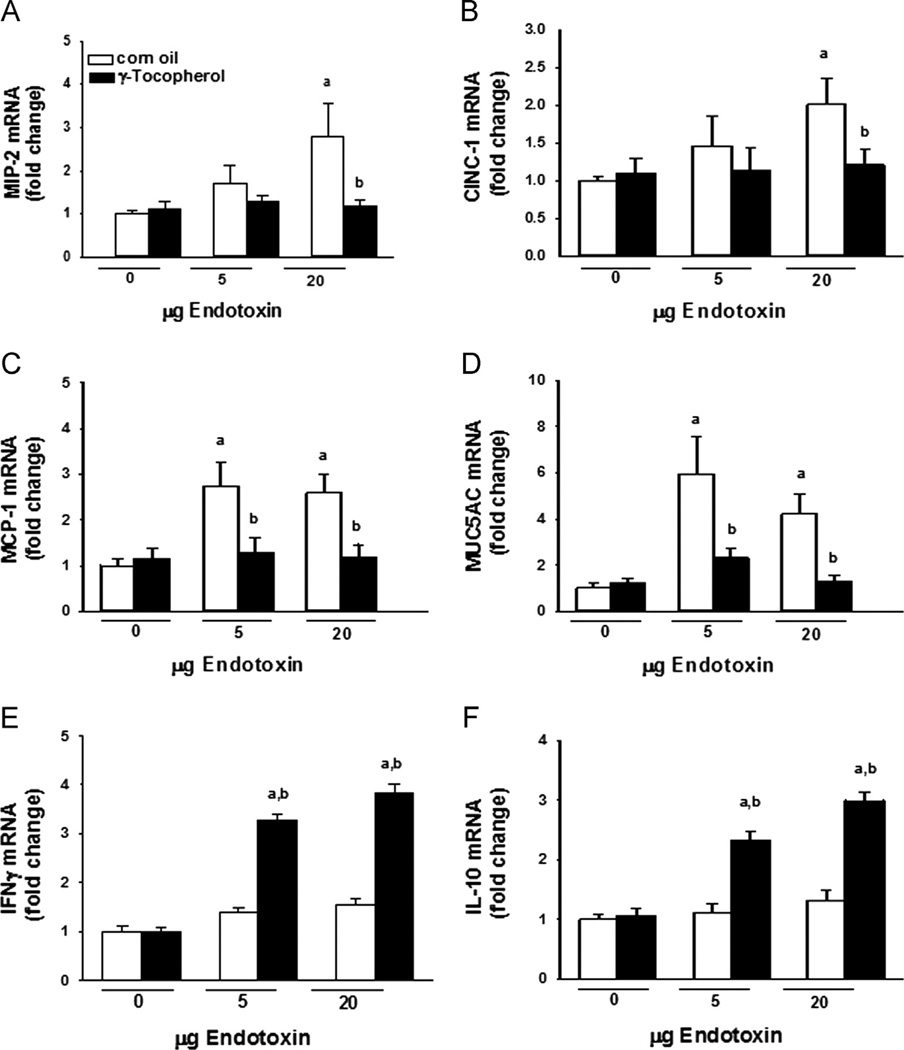
Expression of the genes for the chemotactic and inflammatory proteins MIP-2, CINC-1, and MCP-1 was significantly elevated (two- to threefold) only after instillation with 20 µg LPS (Fig. 5). Elevations in the mucin gene MUC5AC expression were evident in rats given 5 or 20 µg LPS. Supplementation with γT blocked all LPS-induced increases in pulmonary gene expression. Pulmonary expression of IFN-γ and IL-10 was significantly increased only in rats given endotoxin and supplemented with γT.
Fig. 5.
Pulmonary cytokines. Expression in lung tissues of (A) MIP-2, (B) CINC-1, (C) MCP-1, (D) MUC5AC, (E) IFN-γ, and (F) IL-10 was determined by RT-PCR as described under Materials and methods. a, significantly different from respective group given 0 µg endotoxin; b, significantly different from respective group given corn oil; p < 0.05.
Nasal inflammatory and mucous cell responses
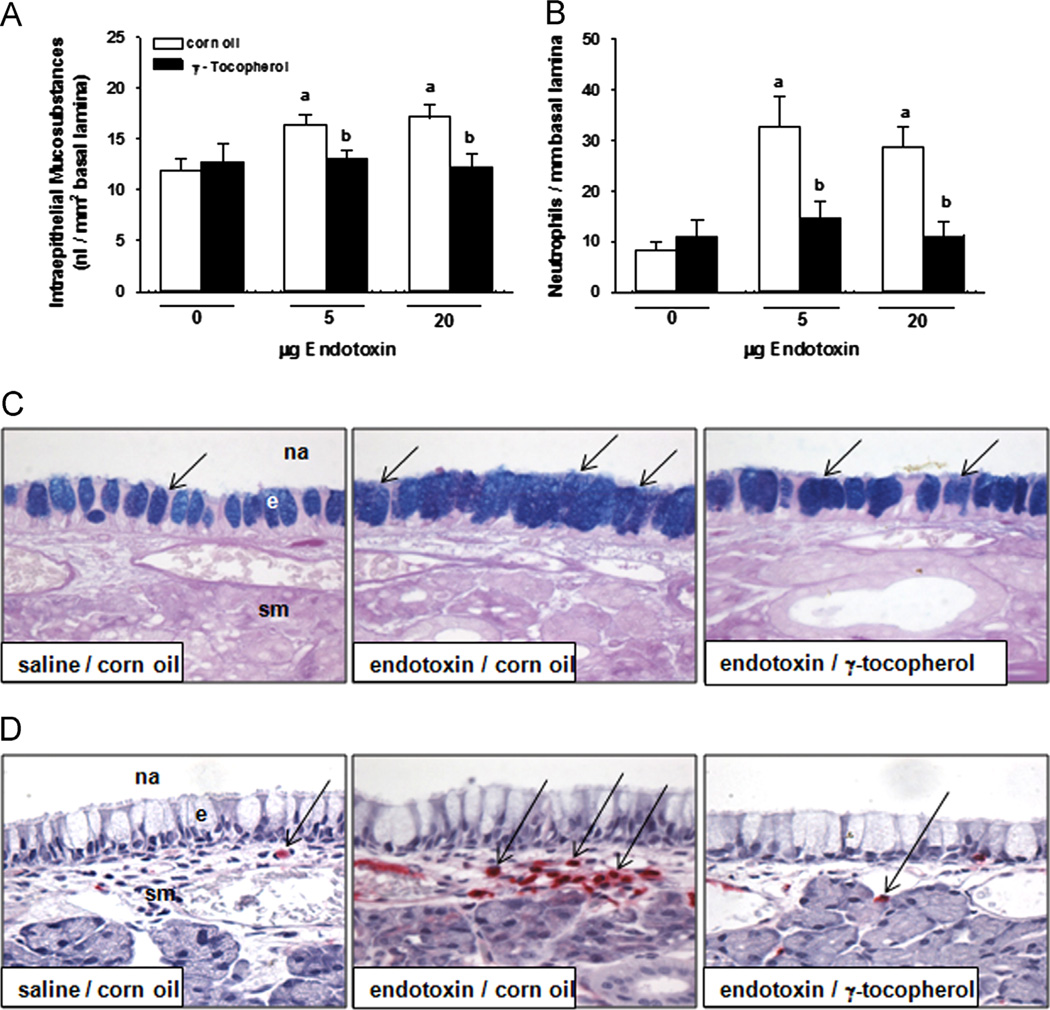
Both 5 and 20 µg LPS caused increased IM (38% increase) and accumulation of submucosal PMNs (400%) in the nasal septum that were attenuated by γT supplementation (Fig. 6A and B, respectively). Mucous goblet cells in the respiratory epithelium lining the midseptum appeared enlarged and more numerous in rats instilled with LPS (Fig. 6C). Few PMNs were detected in the submucosa underlying the epithelium in midseptum in control rats, but LPS instillation caused a marked infiltration of PMNs in the subepithelial compartment (Fig. 6D). Supplementation with γT prevented both the mucous cell metaplasia and the neutrophilic inflammation induced by LPS.
Fig. 6.
Nasal responses. Mucous cell metaplasia was determined morphometrically as increases in (A) intraepithelial mucosubstances in (C) the respiratory epithelium lining the nasal septum stained with AB/PAS, which appear as intracellular blue-stained material (arrows). (B) Submucosal neutrophils were enumerated by morphometric approaches in nasal septum in immunohistochemically treated tissue to detect (D) cells staining positive (arrows, red cells) for anti-rat neutrophil antibody, as described under Materials and methods. a, significantly different from respective group given 0 µg endotoxin; b, significantly different from respective group given corn oil; p < 0.05. na, nasal airway; e, epithelium, sm, submucosa. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article).
Discussion
Inhalation or airway instillation of endotoxin in laboratory rodents is well known to elicit acute inflammatory responses that include neutrophil emigration and mucous cell metaplasia [19–21]. In this study we demonstrate that dietary supplementation of rats with γT attenuates endotoxin-induced airway cytokine production, inflammatory cell infiltration, and mucous overproduction and hypersecretion. These findings are notable for the comprehensive ameliorative effects in both nasal and pulmonary airways by γT against a potent airway inflammagen after a relatively brief, 3-day pretreatment. Our results suggest that γT might be an effective adjunct or alternative medicine to inhibit adverse airway responses during exacerbation of bronchitis or chronic obstructive pulmonary diseases as well as nonallergic rhinitis, which are characterized by neutrophilic inflammation and hypersecretion.
We have previously described how γT supplementation can attenuate allergic airway inflammation during experimental asthma and rhinitis [14]. In that study, allergen-induced eosinophil influx, mucus production, and TH2 cytokine expression were inhibited by γT pretreatment. Specifically, nasal expression of interleukin-4, -5, and -13 was blocked, while at the same time pulmonary expression of IFN-γ was increased in allergic rats. Those results suggested to us that γT may activate TH1 pathways that might counter the allergen-induced induction of TH2 mediators. However, our present results with a nonallergenic stimulus such as endotoxin demonstrate a broader inflammation-modulating profile for γT. Interestingly in this study we again detected a γT-associated increase in IFN-γ expression in lungs with the highest dose of endotoxin (i.e., the most inflamed group), in addition to expression of IL-10, which has an anti-inflammatory role in endotoxin-induced lung injury [22]. These increases in regulatory cytokines were paralleled by decreases in LPS-induced signals for PMN chemoattraction (MIP-2, GRO-KC) and mucus production (MUC5AC). Taken together with our previous results, γT seems to have comparable therapeutic effects against both acquired (TH2) and innate (TH1) immune stimuli in part by promoting the production of regulatory cytokines such as IFN-γ and IL-10.
This demonstration of the general anti-inflammatory properties of γT in both endotoxin- and allergen-induced airway injury models suggests that γT may act at a common upstream pathway or pathways, similar to corticosteroids or nonsteroidal anti-inflammatory drugs. Members of our group have previously described the high-affinity inhibitory capacity of a γT metabolite for cyclooxygenase [23], which effectively outcompetes arachidonic acid for binding to the enzyme and blocking prostanoid synthesis. Here we demonstrate the prevention of endotoxin-induced accumulation of PGE2 in BALF from rats pretreated with γT. We have previously reported similar effects of γT to reduce PGE2 in allergic rat lungs, where γT pretreatment also depressed production in airways of the cysteinyl leukotrienes LTC4 and LTB4 [14]. However, we did not assess the role of leukotrienes or other prostanoids in the current study.
Protection from endotoxin-induced inflammation by γT may be mediated by the inhibition of atypical protein kinase C (aPKC), an enzyme critical to NF-κB activation by LPS in macrophages [24]. Unlike classic PKC isoforms, aPKC’s such as PKCζ are insensitive to regulation by calcium or diacyl glycerol and rather are modulated by intracellular adaptor and regulator proteins. Our group recently used another γT isoform, γ-tocotrienol, to inhibit PKCζ in human airway epithelial cells by the induction of prostate-apoptosis-response 4, an endogenous negative regulator of aPKC [16]. Functional PKCζ is required for LPS-induced expression of adhesion molecules and PMN chemoattractants in airway epithelium in vitro [25] and the recruitment of PMN to the lungs in response to airway endotoxin in laboratory rodents [26]. Furthermore its activation regulates appropriate adhesion molecule expression on both endothelial cell and PMNs [27,28]. Thus, PKCζ is necessary in multiple cell types involved in chemoattractant production and the subsequent transmigration of airway PMNs in response to endotoxin, providing γT isoforms with several cellular targets for inhibition in our model.
We have previously demonstrated that endotoxin-induced mucous cell metaplasia in rats is dependent, in part, on airway emigration of PMNs [29]. Neutrophil-derived elastase, reactive oxygen species, and matrix metalloproteinases have been implicated in mucin-gene expression in airway cells [30,31]. In the present study low-dose endotoxin (5 µg) induced airway and tissue PMN infiltration as well as expression of MUC5AC, but histological detection of mucous cell metaplasia was evident only with 20 µg endotoxin. The difference in gene expression and accumulation of intraepithelial mucous may be due to hypersecretion of mucous, which may have surpassed mucin production at the lower endotoxin dose. It should be noted that endotoxin can induce MUC5AC production in cultured airway epithelium in the absence of PMNs, in part by PKCζ-mediated pathways that involve PGE2, EGFR, and MMP-9 [32,33]. As such the inhibition by γT of airway remodeling to a hypersecretory epithelium may be due to its effects on both neutrophil-dependent and neutrophil-independent pathways.
Our results showing the switch from inflammatory cytokine and chemokine production to anti-inflammatory mediators (IL-10, IFN-γ) extend our recent observations in an early phase clinical study in which a γT-enriched vitamin E supplement diminished sputum neutrophil numbers after inhaled endotoxin of similar potency (20,000 EU) in rats [34]. Interestingly, although supplementation strategies differed between our animal and human studies with regard to frequency and dosage, each regimen boosted circulating γT by similar 4- to 5-fold increases. This is notable because the baseline and boosted circulating levels of γT were approximately 10-fold greater in humans compared to rats in our respective studies. Despite these differences, each intervention was efficacious against airway endotoxin responses. In a separate animal study that assessed γT pharmacokinetics in rats (33 mg/kg/day), we demonstrated 4-fold increases in γT content in both blood and pulmonary compartments, with no significant effect on circulating αT [14]. Similar evaluations were not conducted in the clinical study, but instead metabolism of γT was evaluated in serum as the appearance of 3-carboxychromanol (γ-CEHC), the terminal oxidation metabolite of γT [35]. Long-chain β-oxidation metabolites of γT, including 9- and 13-carboxychromanols, inhibit COX-2 [23]. Further studies are needed to describe the appearance of bioactive metabolites in both the circulation and the pulmonary tissues of γT-supplemented rats.
Frequent systemic administration of γT has been reported to enhance eosinophilic inflammation in mice sensitized and challenged to ovalbumin [36]. These studies, however, used higher doses (100 mg/kg/day, for 9 days) than the current study (30 mg/kg/day, for 4 days) that resulted in up to sixfold higher concentrations of circulating γT than our dosing regimen. Furthermore we provided γT by oral delivery rather than the subcutaneous route in mice, which would bypass liver metabolism. This is an important distinction considering that the anti-inflammatory metabolites of γT are known to be more potent than its precursor in inhibiting COX-2- and 5-LOX-mediated eicosanoids [17,23]. Thus, the pharmacokinetics and metabolism of γT may be critical for determining a therapeutic index that modulates airway inflammation in response to different stimuli.
Our analyses focused solely on the inflammatory responses in the pulmonary compartment, so effects of γT supplementation on circulating neutrophil numbers or cytokine production are unknown. A second limitation of this study is the absence of analyses for bioactive γT metabolites (e.g., CEHC), which we believe are the primary mediators that modulate inflammatory responses. Also, our assessment of arachidonic acid (AA)-derived products was limited to PGE2, rather than evaluation of other specific products of COX-2 and 5-LOX as we have previously shown. Future studies to assess metabolism of both γT and AA will yield important insights into the mechanism by which γT protects from airway inflammation. Last, intervention with γT after LPS treatment would lend insight into the therapeutic efficacy of targeting preexisting neutrophilia that occurs with COPD and other chronic airway diseases.
In conclusion, dietary supplementation with γT had comprehensive anti-inflammatory effects in an airway injury model of endotoxin-induced neutrophil inflammation and airway remodeling. We have previously described similar ameliorative effects of γT in experimental asthma, suggesting that common or proximal pathways of inflammatory cascades are targeted by γT or its metabolites. In this regard inhibition of COX2 and PKCζ, and production of regulatory cytokines such as IFN-γ and IL-10, may underlie the broad anti-inflammatory effects associated with a relatively brief supplementation with γT. Importantly, these results in animals provide a mechanistic foundation for our recent observations in an early phase clinical study showing efficacy of γT to inhibit airway PMNs induced by inhaled endotoxin. Taken together, γT might be an effective adjunct therapy for adverse airway conditions characterized by neutrophilic inflammation and airway remodeling, including bronchitis or chronic obstructive pulmonary diseases.
Acknowledgment
This work was supported by NIH:NCCAM P01 AT002620.
References
- 1.Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 2.Brooks CR, Gibson PG, Douwes J, Van Dalen CJ, Simpson JL. Relationship between airway neutrophilia and aging in asthmatics and non-asthmatics. Respirology. 2013;18:857–865. doi: 10.1111/resp.12079. [DOI] [PubMed] [Google Scholar]
- 3.Fitzpatrick S, Joks R, Silverberg JI. Obesity is associated with increased asthma severity and exacerbations, and increased serum immunoglobulin E in inner-city adults. Clin. Exp. Allergy. 2012;42:747–759. doi: 10.1111/j.1365-2222.2011.03863.x. [DOI] [PubMed] [Google Scholar]
- 4.Dillon MA, Harris B, Hernandez ML, Zou B, Reed W, Bromberg PA, Devlin RB, Diaz-Sanchez D, et al. Enhancement of systemic and sputum granulocyte response to inhaled endotoxin in people with the GSTM1 null genotype. Occup. Environ. Med. 2011;68:783–785. doi: 10.1136/oem.2010.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, Devlin RB, Becker S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J. Allergy Clin. Immunol. 2006;117:1396–1403. doi: 10.1016/j.jaci.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 6.Bosson J, Barath S, Pourazar J, Behndig AF, Sandstrom T, Blomberg A, Adelroth E. Diesel exhaust exposure enhances the ozone-induced airway inflammation in healthy humans. Eur. Respir. J. 2008;31:1234–1240. doi: 10.1183/09031936.00078407. [DOI] [PubMed] [Google Scholar]
- 7.Wagner JG, Roth RA. Neutrophil migration mechanisms, with an emphasis on the pulmonary vasculature. Pharmacol. Rev. 2000;52:349–374. [PubMed] [Google Scholar]
- 8.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moraes TJ, Zurawska JH, Downey GP. Neutrophil granule contents in the pathogenesis of lung injury. Curr. Opin. Hematol. 2006;13:21–27. doi: 10.1097/01.moh.0000190113.31027.d5. [DOI] [PubMed] [Google Scholar]
- 10.Condino-Neto A, Whitney C, Newburger PE. Dexamethasone but not indomethacin inhibits human phagocyte nicotinamide adenine dinucleotide phosphate oxidase activity by down-regulating expression of genes encoding oxidase components. J. Immunol. 1998;161:4960–4967. [PubMed] [Google Scholar]
- 11.El-Benna J, Dang PM, Perianin A. Towards specific NADPH oxidase inhibition by small synthetic peptides. Cell. Mol. Life Sci. 2012;69:2307–2314. doi: 10.1007/s00018-012-1008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samet JM, Hatch GE, Horstman D, Steck-Scott S, Arab L, Bromberg PA, Levine M, McDonnell WF, et al. Effect of antioxidant supplementation on ozone-induced lung injury in human subjects. Am. Respir. Crit. Care Med. 2001;164:819–825. doi: 10.1164/ajrccm.164.5.2008003. [DOI] [PubMed] [Google Scholar]
- 13.Rocksen D, Ekstrand-Hammarstrom B, Johansson L, Bucht A. Vitamin E reduces transendothelial migration of neutrophils and prevents lung injury in endotoxin-induced airway inflammation. Am. J. Respir. Cell Mol. Biol. 2003;28:199–207. doi: 10.1165/rcmb.4899. [DOI] [PubMed] [Google Scholar]
- 14.Wagner JG, Jiang Q, Harkema JR, Ames BN, Illek B, Roubey RA, Peden DB. gamma-Tocopherol prevents airway eosinophilia and mucous cell hyperplasia in experimentally induced allergic rhinitis and asthma. Clin. Exp. Allergy. 2008;38:501–511. doi: 10.1111/j.1365-2222.2007.02855.x. [DOI] [PubMed] [Google Scholar]
- 15.Wagner JG, Harkema JR, Jiang Q, Illek B, Ames BN, Peden DB. Gamma-tocopherol attenuates ozone-induced exacerbation of allergic rhino-sinusitis in rats. Toxicol. Pathol. 2009;37:481–491. doi: 10.1177/0192623309335630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Moreland M, Wagner JG, Ames BN, Illek B, Peden DB, Jiang Q. Vitamin E forms inhibit IL-13/STAT6-induced eotaxin-3 secretion by up-regulation of PAR4, an endogenous inhibitor of atypical PKC in human lung epithelial cells. J. Nutr. Biochem. 2012;23:602–608. doi: 10.1016/j.jnutbio.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Z, Yin X, Jiang Q. Natural forms of vitamin E and 13′-carboxychromanol, a long-chain vitamin E metabolite, inhibit leukotriene generation from stimulated neutrophils by blocking calcium influx and suppressing 5-lipoxygenase activity, respectively. J. Immunol. 2011;186:1173–1179. doi: 10.4049/jimmunol.1002342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wagner JG, Harkema JR, Roth RA. Pulmonary leukostasis and the inhibition of airway neutrophil recruitment are early events in the endotoxemic rat. Shock. 2002;17:151–158. doi: 10.1097/00024382-200202000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Harkema JR, Hotchkiss JA. In vivo effects of endotoxin on intraepithelial mucosubstances in rat pulmonary airways: quantitative histochemistry. Am. J. Pathol. 1992;141:307–317. [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner JG, Roth RA. Neutrophil migration during endotoxemia. J. Leukocyte Biol. 1999;66:10–24. doi: 10.1002/jlb.66.1.10. [DOI] [PubMed] [Google Scholar]
- 21.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295:L379–L399. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garantziotis S, Brass DM, Savov J, Hollingsworth JW, McElvania-TeKippe E, Berman K, Walker JK, Schwartz DA. Leukocyte-derived IL-10 reduces subepithelial fibrosis associated with chronically inhaled endotoxin. Am. J. Respir. Cell Mol. Biol. 2006;35:662–667. doi: 10.1165/rcmb.2006-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc. Natl. Acad. Sci. USA. 2008;105:20464–20469. doi: 10.1073/pnas.0810962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, Chen LY, Doerner AM, Pan WW, Smith L, Huang S, Papadimos TJ, Pan ZK. An atypical protein kinase C (PKC zeta) plays a critical role in lipopolysaccharide-activated NF-kappa B in human peripheral blood monocytes and macrophages. J. Immunol. 2009;182:5810–5815. doi: 10.4049/jimmunol.0804073. [DOI] [PubMed] [Google Scholar]
- 25.Leverence JT, Medhora M, Konduri GG, Sampath V. Lipopolysaccharide-induced cytokine expression in alveolar epithelial cells: role of PKCzeta-mediated p47phox phosphorylation. Chem. Biol. Interact. 2011;189:72–81. doi: 10.1016/j.cbi.2010.09.026. [DOI] [PubMed] [Google Scholar]
- 26.Yao H, Hwang JW, Moscat J, Diaz-Meco MT, Leitges M, Kishore N, Li X, Rahman I. Protein kinase C zeta mediates cigarette smoke/aldehyde- and lipopolysaccharide-induced lung inflammation and histone modifications. J. Biol. Chem. 2010;285:5405–5416. doi: 10.1074/jbc.M109.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Javaid K, Rahman A, Anwar KN, Frey RS, Minshall RD, Malik AB. Tumor necrosis factor-alpha induces early-onset endothelial adhesivity by protein kinase Czeta-dependent activation of intercellular adhesion molecule-1. Circ. Res. 2003;92:1089–1097. doi: 10.1161/01.RES.0000072971.88704.CB. [DOI] [PubMed] [Google Scholar]
- 28.Chakrabarti S, Patel KD. The atypical zeta (zeta) isoform of protein kinase C regulates CD11b/CD18 activation in human neutrophils. Microcirculation. 2008;15:555–567. doi: 10.1080/10739680801949732. [DOI] [PubMed] [Google Scholar]
- 29.Wagner JG, Hotchkiss JA, Harkema JR. Effects of ozone and endotoxin coexposure on rat airway epithelium: potentiation of toxicant-induced alterations. Environ. Health Perspect. 2001;109(Suppl. 4):591–598. doi: 10.1289/ehp.01109s4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Lee SY, Bak SM, Suh IB, Lee SY, Shin C, Shim JJ, In KH, et al. Effects of matrix metalloproteinase inhibitor on LPS-induced goblet cell metaplasia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L127–L133. doi: 10.1152/ajplung.00047.2003. [DOI] [PubMed] [Google Scholar]
- 31.Voynow JA, Fischer BM, Malarkey DE, Burch LH, Wong T, Longphre M, Ho SB, Foster WM. Neutrophil elastase induces mucus cell metaplasia in mouse lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;287:L1293–L1302. doi: 10.1152/ajplung.00140.2004. [DOI] [PubMed] [Google Scholar]
- 32.Gray T, Nettesheim P, Loftin C, Koo JS, Bonner J, Peddada S, Langenbach R. Interleukin-1beta-induced mucin production in human airway epithelium is mediated by cyclooxygenase-2, prostaglandin E2 receptors, and cyclic AMP-protein kinase A signaling. Mol. Pharmacol. 2004;66:337–346. doi: 10.1124/mol.66.2.337. [DOI] [PubMed] [Google Scholar]
- 33.Binker MG, Binker-Cosen AA, Richards D, Oliver B, Cosen-Binker LI. LPS-stimulated MUC5AC production involves Rac1-dependent MMP-9 secretion and activation in NCI-H292 cells. Biochem. Biophys. Res. Commun. 2009;386:124–129. doi: 10.1016/j.bbrc.2009.05.136. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez ML, Wagner JG, Kala A, Mills K, Wells HB, Alexis NE, Lay JC, Jiang Q, et al. Vitamin E, γ-tocopherol, reduces airway neutrophil recruitment after inhaled endotoxin challenge in rats and in healthy volunteers. Free Radic. Biol. Med. 2013;60C:56–62. doi: 10.1016/j.freeradbiomed.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez M, Zhou H, Zhou B, Robinette C, Crissman K, Hatch G, Alexis NE, Peden D. Combination treatment with high-dose vitamin C and alpha-tocopherol does not enhance respiratory-tract lining fluid vitamin C levels in asthmatics. Inhalation Toxicol. 2009;21:173–181. doi: 10.1080/08958370802161077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills J. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. J. Immunol. 2009;182:4395–4405. doi: 10.4049/jimmunol.0803659. [DOI] [PMC free article] [PubMed] [Google Scholar]