Abstract
Background
Treatment for primary prostate cancer (CaP) is the withdrawal of androgens. However, CaP eventually progresses to grow in a castration-resistant state. The mechanisms involved in the development and progression of castration-resistant prostate cancer (CRPC) remain unknown. We have previously generated LNCaP-IL6+ cells by treating LNCaP cells chronically with interleukin-6 (IL-6), which have acquired the ability to grow in androgen-deprived conditions.
Methods
We compared the protein expression profile of LNCaP and LNCaP-IL6+ cells using two-dimensional gel electrophoresis. The gels were then silver stained in order to visualize proteins and the differentially expressed spots were identified and characterized by micro sequencing using MALDI-PMF mass spectrometry.
Results
In this study, we have identified RhoGDIα (GDIα) as a suppressor of prostate cancer growth. Expression of GDIα was reduced in LNCaP-IL6+ cells and was downregulated in more aggressive prostate cancer cells compared to LNCaP cells. Over expression of GDIα inhibited the growth of prostate cancer cells and caused LNCaP-IL6+ cells reversal to androgen-sensitive state, while down regulation of GDIα enhanced growth of androgen-sensitive LNCaP prostate cancer cells in androgen-deprived conditions. In addition, GDIα suppressed the tumorigenic ability of prostate tumor xenografts in vivo.
Conclusions
These results demonstrate that loss of GDIα expression promotes the development and progression of prostate cancer.
Keywords: Prostate cancer, RhoGDIα, IL-6
Introduction
Prostate cancer (CaP) is the most common type of cancer in American men and ranks second to lung cancer in cancer-related deaths. One of the important challenges facing CaP is its evolution to castration resistance, for which no effective treatment has been developed. Understanding the molecular mechanisms leading to castration resistance is the key to developing successful therapies to combat this lethal response. IL-6 has been implicated in the modulation of growth and differentiation in many cancers and is associated with poor prognosis in renal cell carcinoma, ovarian cancer, lymphoma and melanoma (1). Elevated expression of IL-6 and its receptor have been consistently demonstrated in human prostate cancer cell lines and clinical specimens of prostate cancer and benign prostate hyperplasia (2–4). Multiple studies have demonstrated that IL-6 is elevated in the sera of patients with metastatic prostate cancer and the levels of IL-6 correlate with tumor burden, serum PSA and clinically evident metastases (5,6). In addition, serum IL-6 levels are elevated in men with castration-resistant prostate cancer compared to normal controls, benign prostatic hyperplasia, prostatitis and localized prostate cancer (5). Collectively, these data suggest that elevated IL-6 levels are associated with the lethal phenotype of prostate cancer.
IL-6 functions as a paracrine growth factor for the human LNCaP androgen-sensitive prostate cancer cells and as an autocrine growth factor for the human DU145 and PC3 androgen-insensitive prostate cancer cells (7). It has also been reported that IL-6 mediates LNCaP cell growth arrest and induces neuroendocrine differentiation (8–10).Targeting IL-6 signaling using an anti-IL-6 monoclonal antibody induces regression of human prostate cancer xenografts in nude mice (11), while inhibition of IL-6 with CNT0328, an anti-IL-6 monoclonal antibody inhibits the conversion of an androgen –dependent to independent phenotype in a prostate cancer xenograft in vivo model (12). These studies suggest that IL-6 promotes CRPC progression.
RhoGDI (GDI) is a cellular regulatory protein that acts primarily by controlling the cellular distribution and activity of Rho GTPases (13). GDI family comprises three mammalian members: GDIα, which is ubiquitously expressed; GDIβ which has hematopoietic tissue-specific expression, and GDIγ which is membrane-anchored through an amphipathic helix and is preferentially expressed in brain, pancreas, lung, kidney and testis (14). GDIα binds to and negatively regulates most Rho GTPases including RhoA, Rac1 and Cdc42 (14). It has been shown that overexpression of GDI in various cell lines induces disruption of the actin cytoskeleton and loss of substratum adherence and microinjection of GDIα into fibroblasts inhibits cell motility (15,16). GDIα mRNA level was found to be lower in the metastatic lineage (T24T) of a human bladder cancer cell line (T24) suggesting that Rho activation plays a role in the control of progression to metastasis (17). Although GDIα is aberrantly expressed in several tumor tissues, its role in cancer progression remains to be unraveled. In this study we show that GDIα suppresses prostate cancer cell growth, and down regulation of GDIα promotes the progression of androgen-sensitive cells to a castration-resistant state.
Materials and Methods
Cell culture and transfections
LNCaP, LAPC-4, PC3, C4-2, and DU145 prostate cancer cells were cultured in RPMI-1640 medium containing either 10% complete fetal bovine serum (FBS) or 10% charcoal-dextran–stripped FBS and penicillin/streptomycin as described previously (29). LNCaP passage numbers <30 were used throughout the study. IL-6–overexpressing LNCaP-IL6+ cells were cultured in RPMI 1640 containing 10% FBS as described previously (18). For transfection studies, cells were transiently transfected with expressing plasmids using Lipofectamine 2000 (Invitrogen).
Preparation of whole cell extracts
Cells were lysed in a high-salt buffer containing 10 mM Hepes (pH 7.9), 0.25 M NaCl, 1% Nonidet P-40, and 1 mM EDTA with protease inhibitors, and total protein in the lysates was determined with the Coomassie Plus Protein Assay Reagent (Pierce).
Cytosolic and Nuclear Protein Preparation
Cells were harvested, washed with PBS twice, and resuspended in a hypotonic buffer [10 mmol/L HEPES-KOH (pH 7.9), 1.5 mmol/L MgCl2, 10 mmol/L KCl, and 0.1% NP40] and incubated on ice for 10 minutes. Nuclei were precipitated by 3,000xg centrifugation at 4°C for 10 minutes. The supernatant was collected as the cytosolic fraction. After washing once with the hypotonic buffer, the nuclei were lysed in a lysis buffer [50 mmol/L Tris-HCl (pH 8), 150 mmol/L NaCl, 1% TritonX-100] by mechanical disruption for 30 minutes at 4°C. The nuclear lysate was precleared by centrifugation at 4°C for 15 minutes. Protein concentration was determined using the Coomassie Plus protein assay kit (Pierce, Rockford,IL).
Proteomic analysis using two-dimensional electrophoresis
Prior to two-dimensional electrophoresis, the protein samples were purified using a 2D Clean-Up kit (GE health care) according to the manufacturer’s instructions. Differentially expressed proteins were identified using two-dimensional gel electrophoresis and mass spectrometry. Two-dimensional gel electrophoresis was performed using immobiline strips (pI range, 3–10; GE Healthcare, piscataway, NJ, USA) with proteins being separated according to charge and subsequently molecular weight. The gels were then silver stained in order to visualize proteins and the differentially expressed spots were identified by MALDI-PMF mass spectrometry.
Western blot analysis
Equal amounts of protein were loaded on 10% SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat milk in 1× PBS + 0.1% Tween 20 and probed with the indicated primary antibodies. The chemiluminescent signal was detected by enhanced chemiluminescence kit (Amersham) after incubation with the appropriate horseradish peroxidase–conjugated secondary antibodies
Measurement of PSA
PSA levels were measured in the culture supernatants using ELISA (United Biotech, Inc.) according to the manufacturer's instructions and as described previously (19).
In vitro cell proliferation
Cells (104 cells/well) were plated in 12 well plates in RPMI containing 10% FBS. After two or three days in regular culture medium with 10% FBS, cells were switched into phenol red-free RPMI containing either 10% FBS or 10% charcoal-stripped FBS (Hyclone, UT). Two days later, cells number were counted using Coulter counter.
Apoptosis Assays and Cell Death Detection ELISA
Cells were cultured under androgen-depleted conditions (10% charcoal-stripped serum) for 3–7 days after transfection with the indicated plasmids. The degree of apoptosis was measured by cell death detection ELISA according to the manufacturer's instructions. Briefly, floating and attached cells were collected and homogenized in 400 μl of incubation buffer. Five microliters of the supernatant diluted in 95 μl of incubation buffer was used in the ELISA. The wells were coated with anti-histone antibodies and then incubated with the lysates, horseradish peroxidase-conjugated anti-DNA antibodies, and the substrate subsequently, and absorbance was read at 620 nm.
In vivo tumor growth
Four-to six-week-old athymic male nude mice (Harlan, Indianapolis, IN) were injected s.c. in both the flanks with 2x106 cells (LNCaP-IL6+ /neo and LNCaP-IL6+/GDI) resuspended 1:1 in Matrigel (BD Biosciences, Bedford, MA) and complete culture medium. The volume of the growing tumors was estimated by measuring their three dimensions (Length×Width×Depth) with calipers (23).
Statistical analysis
All data are presented as means ± standard deviation of the mean (SD). Statistical analyses were performed with Microsoft Excel analysis tools, differences between individual groups were analyzed by paired t test. P < 0.05 was considered statistically significant.
Results
GDIα was identified by downregulated expression in LNCaP-IL-6+ cells compared to LNCaP cells
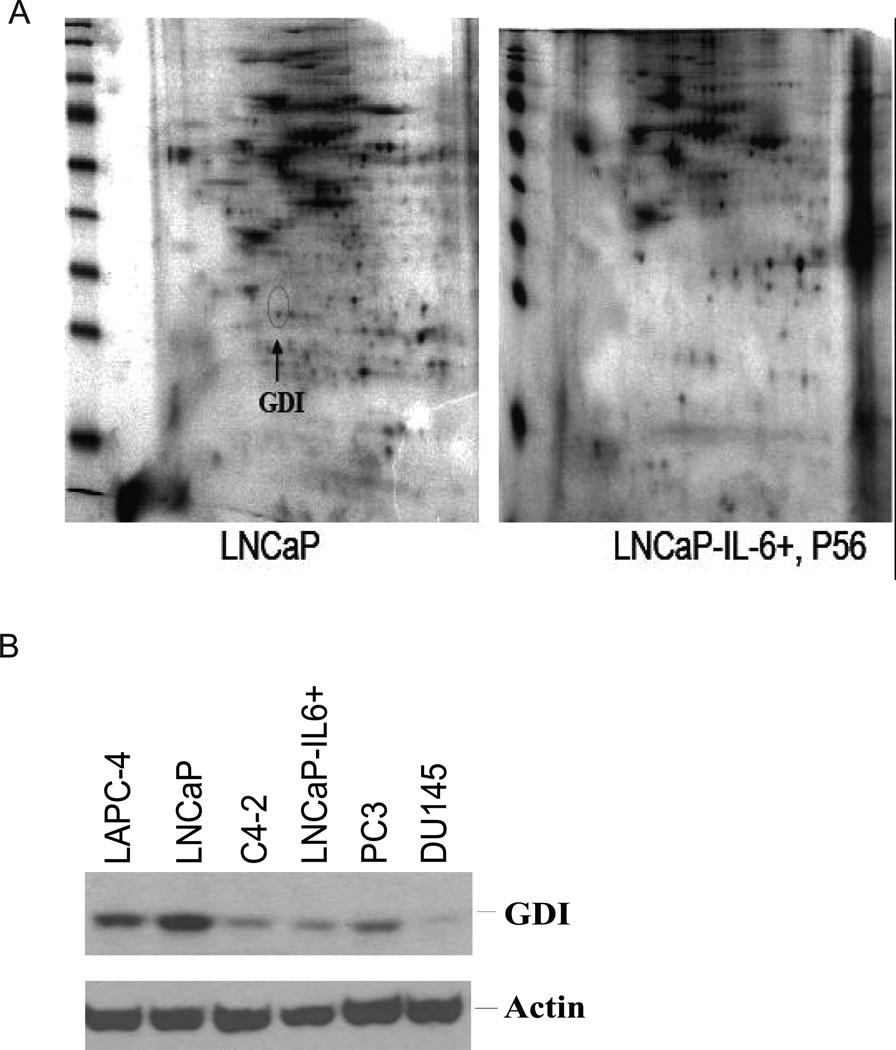
We previously generated a subline of LNCaP cells, LNCaP-IL6+, by chronically treating LNCaP cells with 5 ng/ml IL-6 (18). LNCaP-IL6+ cells were found to have acquired the ability to secrete IL-6 and to grow in castration resistant conditions in vitro and in vivo (18). To identify factors that potentially mediate prostate cancer cell growth induced by IL-6, the protein expression profile in LNCaP and LNCaP-IL-6+ cells was analyzed by 2-D gel electrophoresis (Fig 1A). The differentially expressed spots were isolated from the 2-D gels and micro sequenced by MALDI-PMF. One of the spots that were present in parental LNCaP cells was lost in LNCaP-IL-6+ cells. The spot was identified as GDIα by MALDI-PMF micro sequencing mass spectrometry.
Fig 1. Identification and characterization of GDIα.
A. Identification of GDIα protein that is down-regulated in LNCaP-IL6+ cells compared to LNCaP cells. 2-D gel analysis of LNCaP and LNCaP-IL-6+ cells. Arrow indicates GDIα. B. GDIα expression is decreased in androgen-insensitive cells vs androgen sensitive cells. GDIα expression was analyzed by Western blot using whole cell lysates of androgen-sensitive LNCaP, LAPC-4 cells and androgen-insenistive C4-2, LNCap-IL6+, PC3 and DU145 cells using antibodies specifically against GDIα. Actin was used as loading control.
GDIα expression is decreased in androgen-insensitive cells vs. androgen sensitive cells
To test whether down regulation of GDIα expression is associated with the progression of CRPC, we analyzed the expression levels of GDIα in androgen sensitive LNCaP, LAPC-4 cells and androgen–insensitive C4-2, LNCaP-IL-6+, PC-3 and DU145 cells by western blot analysis using antibodies against GDIα. The levels of GDIα protein were decreased in the androgen–insensitive cells compared to those in androgen-sensitive cells. These results suggest that androgen–insensitive growth is associated with decreased levels of GDIα protein (Fig 1B).
GDIα inhibits cell growth and induces apoptotic cell death
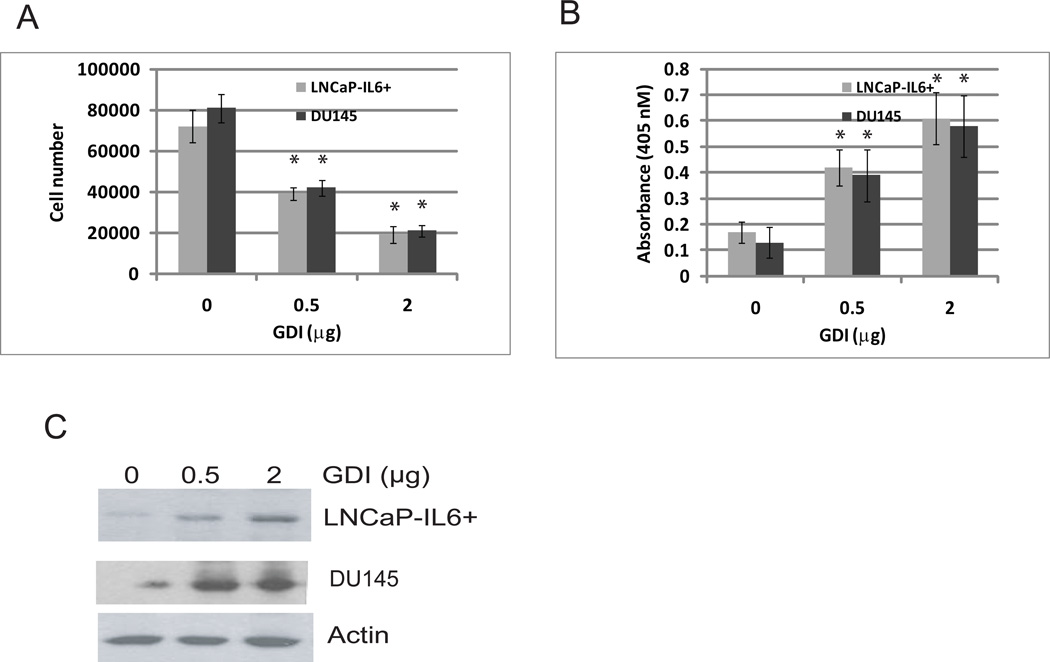
To examine the effects of GDIα on cell growth in vitro, LNCaP-IL-6+ and DU145 cells that express low levels of GDIα protein were transfected with different concentrations of expression plasmids encoding GDIα and cell numbers were determined. Overexpresion of GDIα inhibited the growth of LNCaP-IL-6+ and DU145 cells in vitro (Fig 2A). Apoptosis was measured by analyzing the degree of DNA fragmentation with the Cell Death Detection ELISA kit (Roche). Over expression of GDIα induced significant levels of apoptotic cell death compared to the vector control (p< 0.01) (Fig 2B). These data suggest that overexpression of GDIα inhibits the growth of prostate cancer cells via induction of apoptotic cell death.
Fig 2. Expression of GDIα inhibited growth and induced apoptotic cell death in vitro.
A. Over expression of GDIα inhibits LNCaP-IL6+ and DU145 cells growth in vitro. LNCaP-IL6+ and DU145 cells were transfected with different doses of plasmids containing GDIα cDNA. The cell number was determined 3 days after transfection. B. Overexpression of GDIα induces apoptotic cell death. LNCaP-IL6+ and DU145 cells were transfected with different doses of expression plasmids containing GDIα cDNA. Apoptotic cell death was determined 3 days after transfection. C. GDIα expression by Western blot analysis using antibody specific against GDIα. * indicates statistical significance compared to controls.
RhoGDIα inhibits LNCaP-IL-6+ cell growth in androgen-deprived conditions
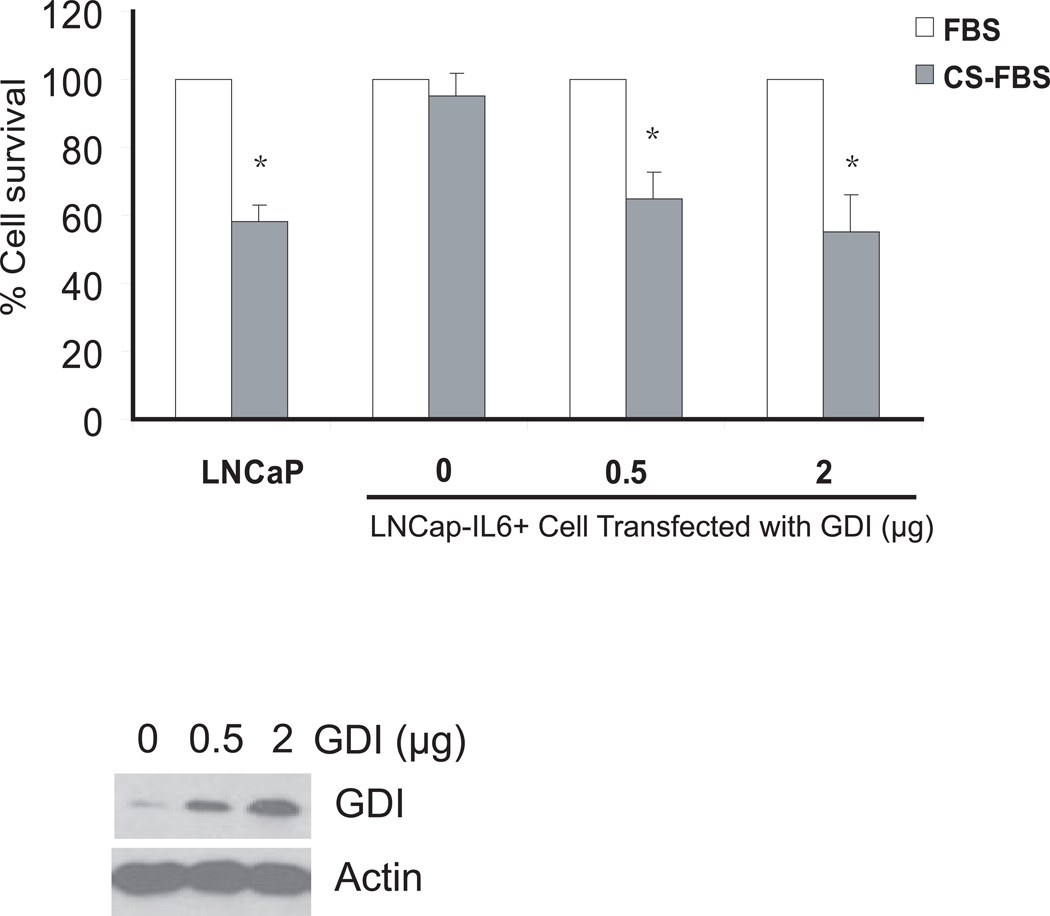
To determine the potential significance of over expression of GDIα in prostate cancer cells, LNCaP-IL-6+ were transfected with plasmids expressing control or GDIα. After transfection, cells were switched to media containing either FBS or charcoal-stripped FBS (CS-FBS) and allowed to grow for 3 more days and cell numbers were determined. The growth of LNCaP-IL-6+ cells transfected with vector control grown in CS-FBS was reduced ~5–10% compared to those grown in FBS. The growth of LNCaP-IL-6+ cells transfected with GDIα grown in similar conditions showed reduction by 40 to 50% (Fig 3). These results suggest that over expression of GDIα can reduce the growth of LNCaP-IL-6+ cells in androgen–deprived conditions in vitro.
Fig 3. Effect of over expression of GDIα on LNCaP-IL6+ cell growth in the presence and absence of androgen in vitro.
LNCaP-IL6+ Cells were cultured in RPMI-1640 supplemented with 10% FBS or 10% charcoal-stripped FBS (CS-FBS) and cultured for 72 h. MTT values for the complete FBS were expressed as 100% and MTT values for charcoal–stripped FBS were expressed as % relative to complete FBS. * indicates statistical significance compared to the value of FBS conditions. The bottom panel shows GDIα protein expression by Western blot analysis using antibody against GDIα.
Downregulation of GDIα promotes growth of LNCaP cells in androgen-deprived conditions
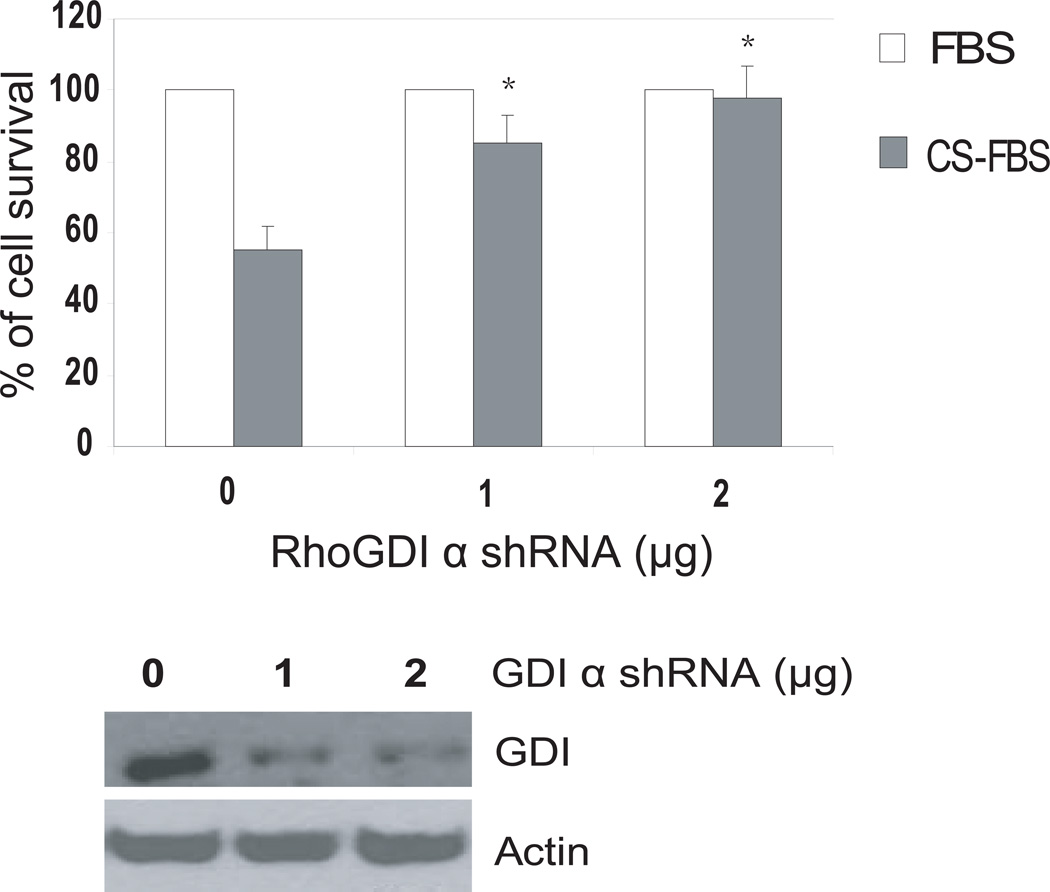
LNCaP cells express higher levels of GDIα protein and do not grow well in CS-FBS condition. To test whether knockdown of GDIα expression stimulates androgen- independent growth of androgen-sensitive LNCaP cells, LNCaP cells were transfected with shRNA specifically for GDIα and GFP shRNA as control. Cells were cultured in the presence and absence of androgen and cell growth was determined. The growth of androgen-sensitive LNCaP transfected with GFP control was reduced by approximately 50% after 72 hr in CS-FBS compared to that in regular FBS. In cells transfected with GDIα shRNA there was only 5–15% reduction in growth in CS-FBS compared to FBS indicating that knockdown of GDIα protein expression can enhance the growth of LNCaP cells in androgen deprived conditions in vitro ( Fig 4).
Fig 4. Knockdown of RhoGDIa expression promotes LNCaP cell growth in androgen-deprived conditions in vitro.
Effect of knockdown of RhoGDIα expression on LNCaP cell growth in the presence and absence of androgen in vitro. LNCaP cells were cultured in RPMI-1640 supplemented with 10% FBS. After 24h, the cells were transfected with GDIα shRNA as indicated. GFP shRNA was used as control. After transfection, the cells were switched to either 10% FBS or 10% charcoal-stripped FBS (CS-FBS) and cultured for 72 h. MTT values for cell grown in complete FBS were expressed as 100% and MTT values for cell grown in charcoal stripped FBS were expressed as % relative to complete FBS. Bottom panel shows GDIα protein expression by Western blot analysis using antibody against GDIα. * indicate statistical significance compared to the value of GFP shRNA in CS-FBS conditions.
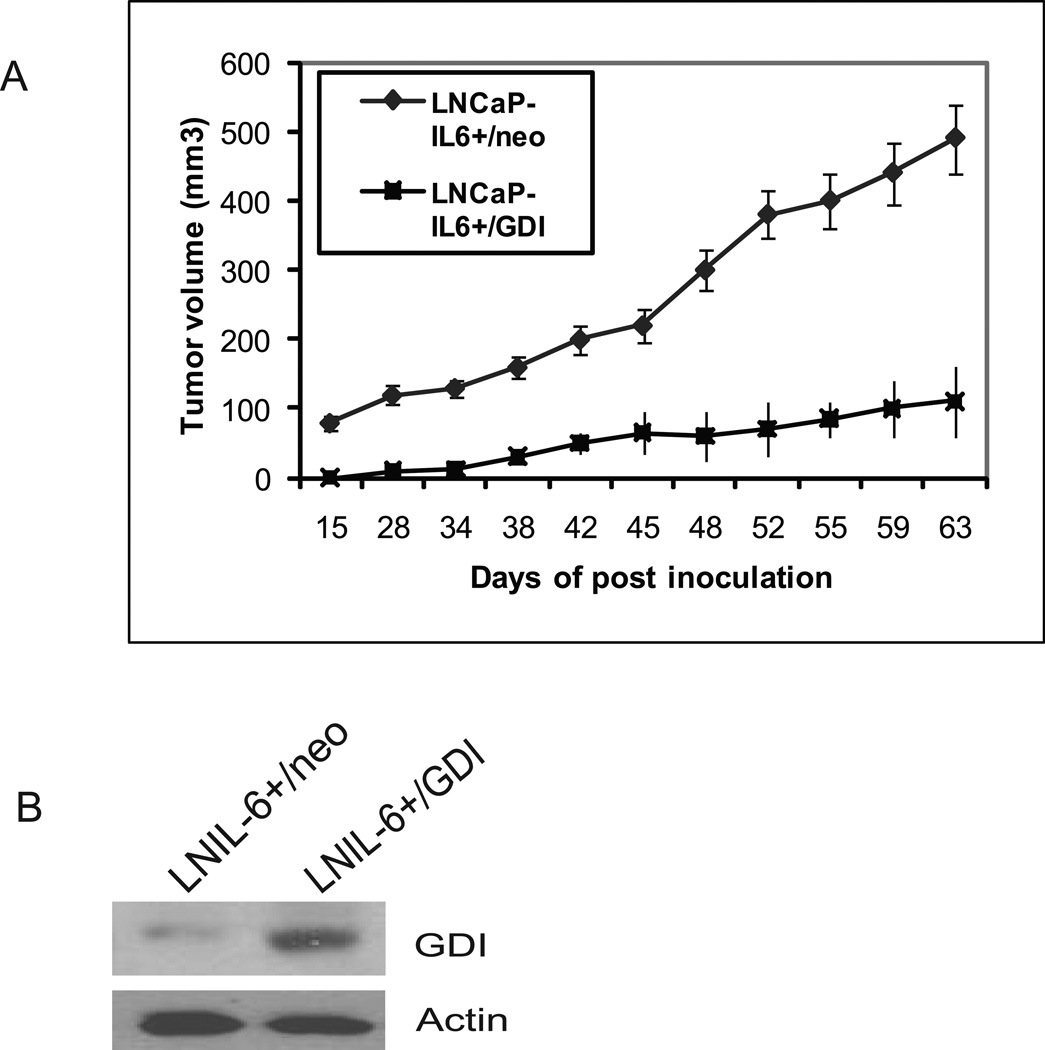
RhoGDIα suppresses LNCaP-IL-6+ tumor growth
To test the effect of GDIα on tumor formation in vivo, 8 week –old male nude mice were inoculated s.c. with 2x106 LNCaP-IL6+ cells stably transfected with GDIα or vector control. The mice developed tumors two weeks after injection with LNCaP-IL6+/neo cells, and five weeks after injection with LNCaP-IL6+/GDIα cells (Fig 5). Tumor volumes were measured twice a week. At the end of nine weeks blood and tumor tissues were collected and serum levels of PSA were determined by ELISA. The over expression of GDIα suppressed tumor growth of LNCaP-IL6+ cells. All the tumors produced PSA and the levels of PSA were 25.4± 6.5 ng/ml in mice bearing LNCaP-IL6+/neo tumors and 5.1± 2.8ng/ml in mice bearing LNCaP-Il6+/GDIα tumors. These results demonstrate that GDIα expression suppresses prostate tumor growth in vivo.
Fig 5. Effects of overexpression of GDIα on tumor growth.
A. Over expression of GDIα suppresses LNCaP-IL6+ cell tumor growth in vivo. LNCaP-IL6+ cells/neo and LNCaP-IL6+/GDIα cells were injected into intact male nude mice (N=8). Tumor volumes were measured. B. Levels of GDIα protein in tumors originating from LNCaP-IL6+/neo and LNCaP-IL6+/GDIα cells analyzed by Western blot using antibody against GDIα.
Discussions
IL-6 has been implicated in growth and differentiation and is associated with poor prognosis in many cancers including prostate cancer. Multiple studies have demonstrated that IL-6 is elevated in the sera of patients with metastatic prostate cancer and correlates with tumor burden and clinically evident metastases (1,3–5). An interesting observation is the dynamic nature of prostate cancer cells such as LNCaP in response to IL-6. IL-6 exerts its effects in both paracrine and autocrine manner (18). Prolonged passage of LNCaP cells in the presence of IL-6 generated a subline, LNCaP-IL6+, which is adapted to IL-6 and grows in a castration resistant manner (18,20). In the present study, we analyzed protein expression profiles of parental LNCaP and LNCaP-IL-6+ cells, and identified that GDIα is down regulated in prostate cancer and its down regulation plays a critical role during prostate cancer progression to CRPC.
GDIα was identified by comparison of the protein expression profiles of LNCaP and LNCaP-IL6+ cells. GDIα is down regulated in LNCaP-IL6+ cells which exhibit higher levels of IL-6 compared to LNCaP cells. The levels of expression of GDIα are higher in androgen-sensitive LNCaP and LAPC-4 cells compared to more aggressive and androgen-insensitive C4-2, PC3, DU145 and LNCaP-IL6+ cells, suggesting that down regulation of GDIα expression may participate in the progression of prostate cancer cells to androgen-insensitive state. It should be noted that the data is obtained from prostate cancer cell lines derived from human prostate cancer. It would be interesting to examine the levels of GDIα expression in specimens directly derived from patients representing different stages of prostate cancer.
Our study shows a novel role of GDIα in prostate cancer. Overexpression of GDIα helps check uncontrolled proliferation of LNCaP-IL-6+ cells in vitro and in vivo. In addition to LNCaP-IL6+ cells, GDIα also inhibits the proliferation of DU145 prostate cancer cells at least in vitro. We have previously showed that LNCaP-IL6+ cells have the ability to grow in androgen-deprived charcoal stripped FBS conditions in cell culture (18), which was hampered by overexpression of GDIα in LNCaP-IL6+ cells. Conversely, downregulation of GDIα expression in LNCaP cells enhanced the growth of these cells in androgen-deprived charcoal-stripped FBS conditions in vitro. These results suggest that decreased expression of GDIα facilitates the progression of castration-resistance from androgen sensitive prostate cancer. The possible involvement of GDIα in castration-resistant prostate cancer progression is suggested by a recent publication in which loss of GDIα expression promotes MCF-7 breast cancer cells resistant to tamoxifen treatment (21).
In conclusion, we have identified GDIα as a suppressor of prostate cancer growth through comparison of the protein expression profiles of LNCaP and LNCaP-IL6+ cells. Overexpression of GDIα inhibits the growth of prostate cancer cells, while downregulation of GDIα enhances the growth of androgen sensitive prostate cancer cells in androgen-deprived conditions. The mechanisms of GDIα-mediated cellular signaling involved in promoting prostate cancer cell progression are currently under investigation.
Acknowledgments
This work was supported in part by grants from VA Merits I01 BX000526, NIH CA 109441 and CA 140468.
References
- 1.Simpson RJ, Hammacher A, Smith DK, Matthews JM, Ward LD. Interleukin-6: structure-function relationships. Protein Sci. 1997;6(5):929–955. doi: 10.1002/pro.5560060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegall CB, Schwab G, Nordan RP, FitzGerald DJ, Pastan I. Expression of the interleukin 6 receptor and interleukin 6 in prostate carcinoma cells. Cancer Res. 1990;50(24):7786–7788. [PubMed] [Google Scholar]
- 3.Siegsmund MJ, Yamazaki H, Pastan I. Interleukin 6 receptor mRNA in prostate carcinomas and benign prostate hyperplasia. J Urol. 1994;151(5):1396–1399. doi: 10.1016/s0022-5347(17)35267-9. [DOI] [PubMed] [Google Scholar]
- 4.Hobisch A, Rogatsch H, Hittmair A, Fuchs D, Bartsch G, Jr, Klocker H, Bartsch G, Culig Z. Immunohistochemical localization of interleukin-6 and its receptor in benign, premalignant and malignant prostate tissue. J Pathol. 2000;191(3):239–244. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH633>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 5.Drachenberg DE, Elgamal AA, Rowbotham R, Peterson M, Murphy GP. Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate. 1999;41(2):127–133. doi: 10.1002/(sici)1097-0045(19991001)41:2<127::aid-pros7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, Thompson TC. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. J Urol. 1999;161(1):182–187. [PubMed] [Google Scholar]
- 7.Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997;57(1):141–146. [PubMed] [Google Scholar]
- 8.Qiu Y, Robinson D, Pretlow TG, Kung HJ. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3'-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci U S A. 1998;95(7):3644–3649. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spiotto MT, Chung TD. STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate. 2000;42(3):186–195. doi: 10.1002/(sici)1097-0045(20000215)42:3<186::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Deeble PD, Murphy DJ, Parsons SJ, Cox ME. Interleukin-6- and cyclic AMP-mediated signaling potentiates neuroendocrine differentiation of LNCaP prostate tumor cells. Mol Cell Biol. 2001;21(24):8471–8482. doi: 10.1128/MCB.21.24.8471-8482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith PC, Keller ET. Anti-interleukin-6 monoclonal antibody induces regression of human prostate cancer xenografts in nude mice. Prostate. 2001;48(1):47–53. doi: 10.1002/pros.1080. [DOI] [PubMed] [Google Scholar]
- 12.Wallner L, Dai J, Escara-Wilke J, Zhang J, Yao Z, Lu Y, Trikha M, Nemeth JA, Zaki MH, Keller ET. Inhibition of interleukin-6 with CNTO328, an antiinterleukin-6 monoclonal antibody, inhibits conversion of androgen-dependent prostate cancer to an androgen-independent phenotype in orchiectomized mice. Cancer Res. 2006;66(6):3087–3095. doi: 10.1158/0008-5472.CAN-05-3447. [DOI] [PubMed] [Google Scholar]
- 13.Olofsson B. Rho Guanine Dissociation Inhibitors: Pivotal Molecules in Cellular Signalling. Cellular Signalling. 1999;11(8):545–554. doi: 10.1016/s0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 14.Dovas A, Couchman JR. RhoGDI: multiple functions in the regulation of Rho family GTPase activities. Biochem J. 2005;390(1):1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takaishi KKA, Kuroda S, Sasaki T, Takai Y. Involvement of rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in cell motility. Mol Cell Biol. 1999;13(1):545–554. doi: 10.1128/mcb.13.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Togawa AMJ, Ishizaki H, et al. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIαλπηα. Oncogene. 1999;18(39):5373–5380. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- 17.Seraj M, Harding M, Gildea J, Welch D, Theodorescu D. The relationship of BRMS1 and RhoGDI2 gene expression to metastatic potential in lineage related human bladder cancer cell lines. Clinical and Experimental Metastasis. 2000;18(6):519–525. doi: 10.1023/a:1011819621859. [DOI] [PubMed] [Google Scholar]
- 18.Lee SO, Chun JY, Nadiminty N, Lou W, Gao AC. Interleukin-6 undergoes transition from growth inhibitor associated with neuroendocrine differentiation to stimulator accompanied by androgen receptor activation during LNCaP prostate cancer cell progression. Prostate. 2007;67(7):764–773. doi: 10.1002/pros.20553. [DOI] [PubMed] [Google Scholar]
- 19.Lou W, Ni Z, Dyer K, Tweardy DJ, Gao AC. Interleukin-6 induces prostate cancer cell growth accompanied by activation of stat3 signaling pathway. Prostate. 2000;42(3):239–242. doi: 10.1002/(sici)1097-0045(20000215)42:3<239::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 20.Hobisch A, Ramoner R, Fuchs D, Godoy-Tundidor S, Bartsch G, Klocker H, Culig Z. Prostate cancer cells (LNCaP) generated after long-term interleukin 6 (IL-6) treatment express IL-6 and acquire an IL-6 partially resistant phenotype. Clin Cancer Res. 2001;7(9):2941–2948. [PubMed] [Google Scholar]
- 21.Barone I, Brusco L, Gu G, Selever J, Beyer A, Covington KR, Tsimelzon A, Wang T, Hilsenbeck SG, Chamness GC, Ando S, Fuqua SA. Loss of Rho GDI{alpha} and Resistance to Tamoxifen via Effects on Estrogen Receptor {alpha} J Natl Cancer Inst. 103(7):538–552. doi: 10.1093/jnci/djr058. [DOI] [PMC free article] [PubMed] [Google Scholar]