Abstract
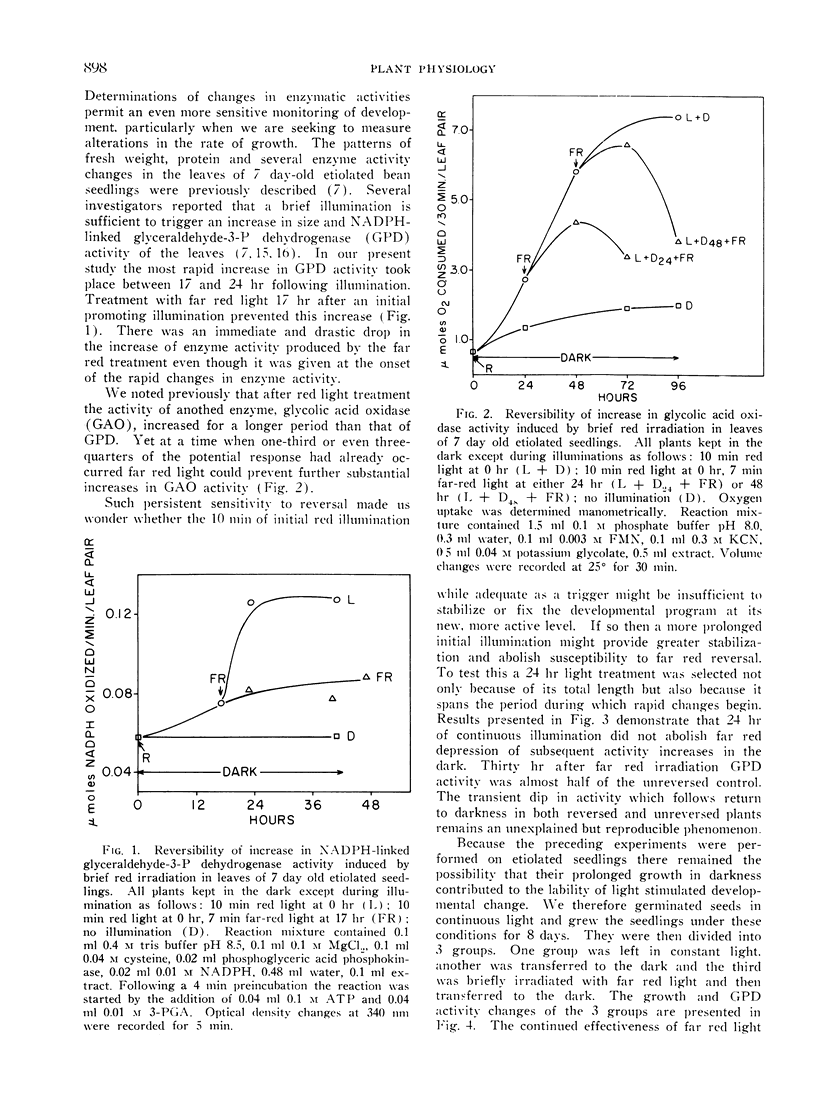
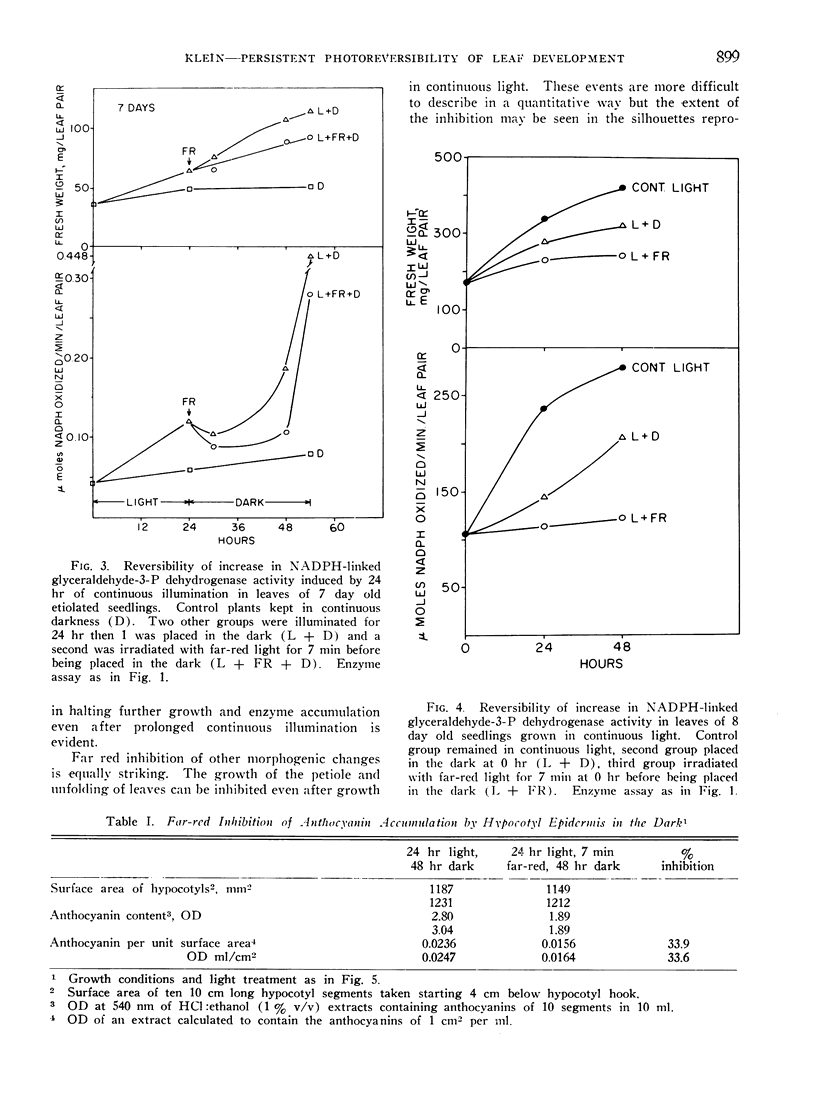
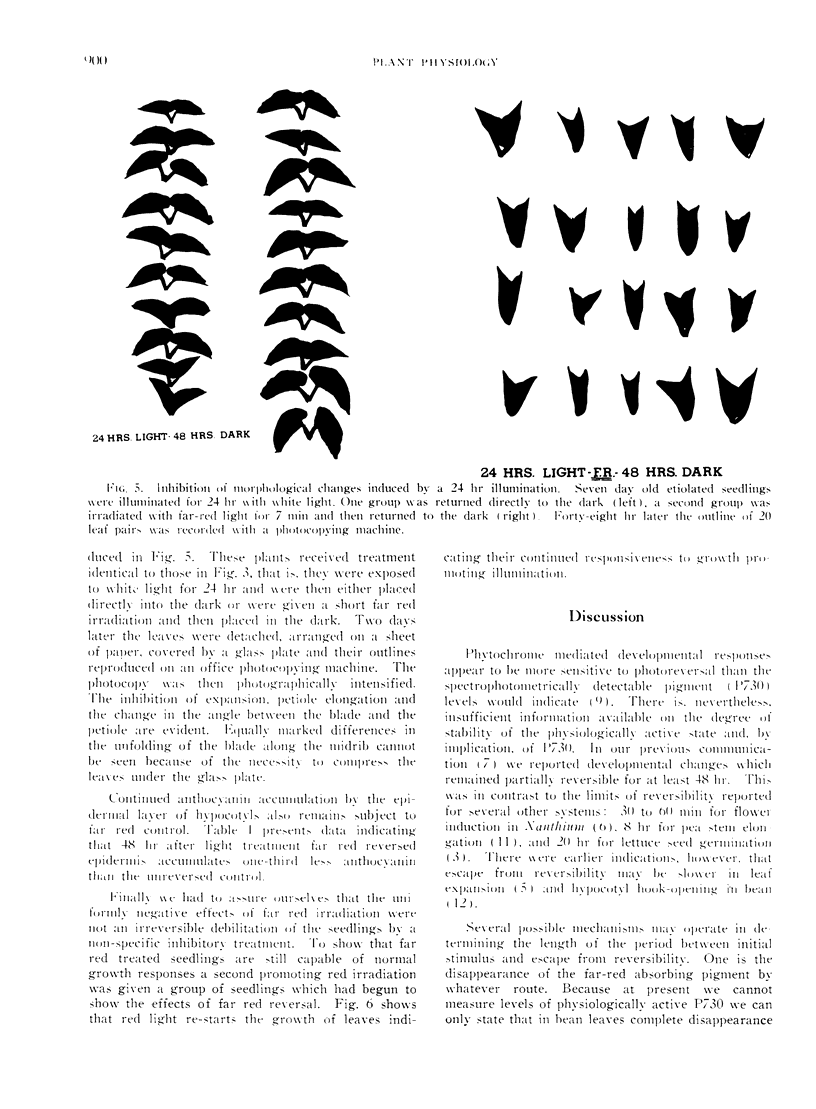
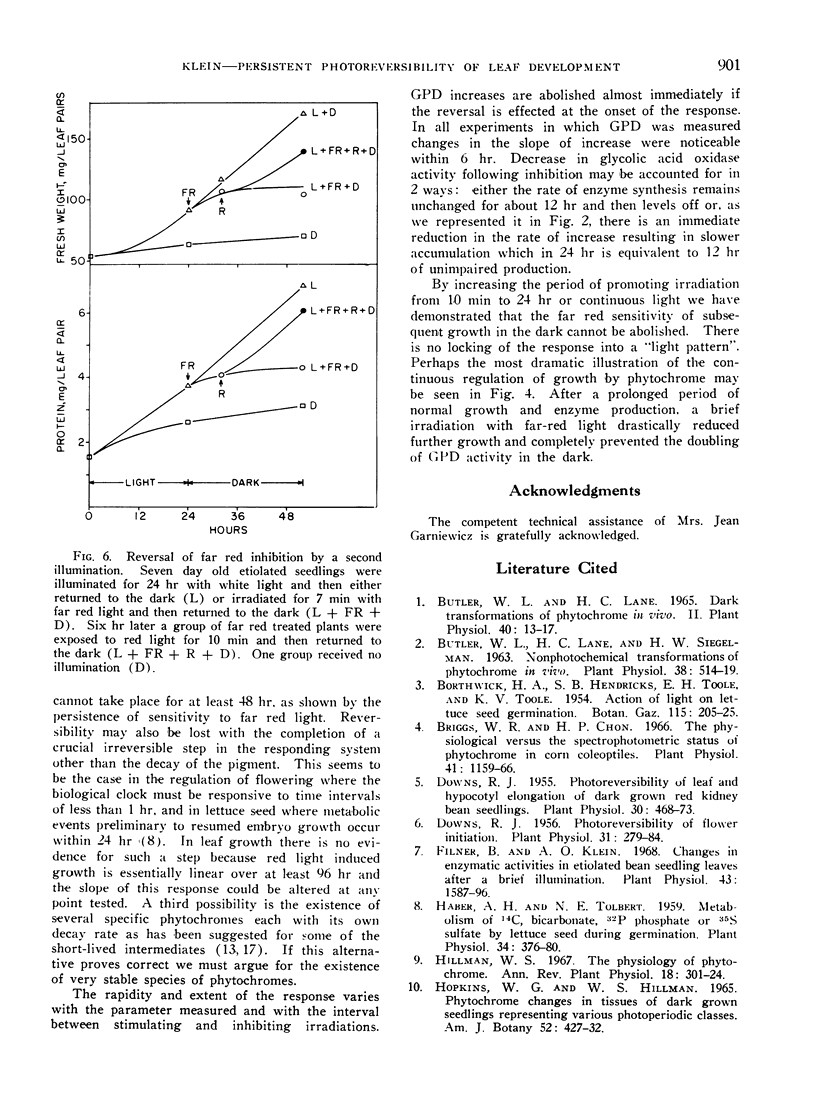
Far red light reversal of red light induced leaf expansion and enzyme changes were investigated in seedlings of Phaseolus vulgaris var. Black Valentine. In etiolated plants growth, anthocyanin accumulation and increases in glyceraldehyde-3-phosphate dehydrogenase and glycolic acid oxidase activities induced by a 10 min red irradiation were stopped by a 7 min far red irradiation given 17, 24, or 48 hr after activation. Etiolated seedlings illuminated for 24 hr with white light and seedlings grown in continuous light remained sensitive to far red reversal. This suggests that the far red sensitive receptor does not decay with time but remains associated with the site of its regulatory functions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs W. R., Chon H. P. The physiological versus the spectrophotometric status of phytochrome in corn coleoptiles. Plant Physiol. 1966 Sep;41(7):1159–1166. doi: 10.1104/pp.41.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C. Dark Transformations of Phytochrome in vivo. II. Plant Physiol. 1965 Jan;40(1):13–17. doi: 10.1104/pp.40.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L., Lane H. C., Siegelman H. W. Nonphotochemical Transformations of Phytochrome in Vivo. Plant Physiol. 1963 Sep;38(5):514–519. doi: 10.1104/pp.38.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs R. J. Photoreversibility of Flower Initiation. Plant Physiol. 1956 Jul;31(4):279–284. doi: 10.1104/pp.31.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner B., Klein A. O. Changes in enzymatic activities in etiolated bean seedling leaves after a brief illumination. Plant Physiol. 1968 Oct;43(10):1587–1596. doi: 10.1104/pp.43.10.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber A. H., Tolbert N. E. Metabolism of C-Bicarbonate, P-Phosphate, or S-Sulfate by Lettuce Seed during Germination. Plant Physiol. 1959 Jul;34(4):376–380. doi: 10.1104/pp.34.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins W. G., Hillman W. S. Relationships between phytochrome state and photosensitive growth of Avena coleoptile segments. Plant Physiol. 1966 Apr;41(4):593–598. doi: 10.1104/pp.41.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MEGO J. L., JAGENDORF A. T. Effect of light on growth of Black Valentine bean plastids. Biochim Biophys Acta. 1961 Oct 28;53:237–254. doi: 10.1016/0006-3002(61)90437-1. [DOI] [PubMed] [Google Scholar]
- Purves W. K., Briggs W. R. Kinetically distinguishable populations of phytochrome. Plant Physiol. 1968 Aug;43(8):1259–1263. doi: 10.1104/pp.43.8.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOLBERT N. E., BURRIS R. H. Light activation of the plant enzyme which oxidizes glycolic acid. J Biol Chem. 1950 Oct;186(2):791–804. [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. III. Limiting factors in glycolysis of ascites tumor cells. J Biol Chem. 1959 May;234(5):1029–1035. [PubMed] [Google Scholar]
- ZELITCH I., OCHOA S. Oxidation and reduction of glycolic and glyoxylic acids in plants. I. Glycolic and oxidase. J Biol Chem. 1953 Apr;201(2):707–718. [PubMed] [Google Scholar]



