Abstract
Background
Latin America has among the highest gastric cancer incidence rates in the world, for reasons that are still unknown. In order to identify region-specific risk factors for gastric cancer, we conducted a meta-analysis summarizing published literature.
Methods
Searches of PubMed and regional databases for relevant studies published up to December 2011 yielded a total of 29 independent case-control studies. We calculated summary odds ratios (OR) for risk factors reported in at least five studies, including socioeconomic status (education), lifestyle habits (smoking and alcohol use), dietary factors (consumption of fruits, total vegetables, green vegetables, chili pepper, total meat, processed meat, red meat, fish and salt) and host genetic variants (IL1B-511T, IL1B-31C, IL1RN*2, TNFA-308A, TP53 codon 72 Arg and GSTM1 null). Study-specific ORs were extracted and summarized using random-effects models.
Results
Chili pepper was the only region-specific factor reported in at least five studies. Consistent with multifactorial pathogenesis, smoking, alcohol use, high consumption of red meat or processed meat, excessive salt intake and carriage of IL1RN*2 were each associated with a moderate increase in gastric cancer risk. Conversely, higher levels of education, fruit consumption, and total vegetable consumption were each associated with a moderately decreased risk. The other exposures were not significantly associated. No prospective study data were identified.
Conclusion
Risk factor associations for gastric cancer in Latin America are based on case-control comparisons that have uncertain reliability, particularly with regard to diet; the specific factors identified and their magnitudes of association are largely similar to those globally recognized. Future studies should emphasize prospective data collection and focus on region-specific exposures that may explain high gastric cancer risk.
Keywords: epidemiology, gastric cancer, Latin-America, meta-analysis, risk factors
BACKGROUND
Gastric cancer represents the second leading cause of cancer death worldwide [1]. This neoplasia arises primarily as a consequence of chronic Helicobacter pylori infection [2] which is typically acquired in childhood and, if untreated, usually lifelong [3]. The great variation in gastric cancer incidence across populations may be related to differences in prevalence of H. pylori infection and/or environmental and host cofactors that modify gastric cancer risk [4].
Latin American countries have high prevalence of H. pylori infection [5, 6] and some of the highest gastric cancer incidence rates in the world [1]. In order to identify region-specific risk factors that could be amenable to tailored interventions, we summarized the published literature on gastric cancer risk in Latin American populations. We contrasted our findings with global meta-analyses, which generally overlook regional studies included in local databases.
MATERIALS AND METHODS
Search strategy and selection criteria
The literature databases PubMed® (U.S. National Library of Medicine, Bethesda, MD), LILACS® (Latin America and the Caribbean Literature on Health Sciences; http://lilacs.bvsalud.org/en), and SciELO® (Scientific Electronic Library Online; http://scielo.org) were searched for observational studies evaluating gastric cancer risk factors in the 20 countries comprising Latin America as defined by the United Nations Educational Scientific and Cultural Organization [7], published in any language up to December 31, 2011.
To identify studies in PubMed, the following search strategy was used: (gastric cancer OR stomach cancer) AND (risk OR risk factors OR risk assessment OR epidemiologic factors OR diet OR food habits OR fruit OR vegetables OR sodium, dietary OR salts OR table salt OR sodium chloride, dietary OR nitrites OR meat OR chili pepper OR tobacco use OR smoking OR alcohol OR alcoholic beverages OR alcohol drinking OR polymorphism, genetic OR polymorphism, single nucleotide OR SNPs) AND (case-control studies OR cohort studies OR cohort OR case-control) AND (Latin America OR Central America OR South America OR Argentina OR Aruba OR Bolivia OR Brazil OR Colombia OR Costa Rica OR Cuba OR Chile OR Dominican Republic OR Ecuador OR El Salvador OR Guatemala OR Honduras OR Mexico OR Nicaragua OR Panama OR Paraguay OR Peru OR Uruguay OR Venezuela). Analogous strategies were used to search the other two databases.
Two investigators (PB and MCC) independently reviewed titles and abstracts for selection of potentially relevant articles; any disagreement was resolved by consulting a third reviewer (FMG). Full-text articles were retrieved for potential inclusion if at least one risk factor was mentioned. Citations of retrieved articles were reviewed for studies that may have been missed or absent from our searches.
The following information was abstracted from each selected article: year of publication, first author, recruitment period, study location (country), numbers of cases and controls, source of controls, participant age range (or mean), proportion of males, tumor distribution by histologic type and anatomic subsite, risk factors, and odds ratios (OR) for gastric cancer. From each study, we extracted fully adjusted ORs with corresponding 95% confidence intervals (CIs), and the adjustment variables. For studies reporting association with genetic polymorphisms, genotype frequencies in cases and controls were also extracted. In addition, we obtained national incidence rates of gastric cancer from GLOBOCAN 2008 estimates for the countries where these studies were conducted [8].
Risk factors
ORs were summarized for risk factors that were reported in at least five studies, including socioeconomic status (SES; level of education), lifestyle habits (smoking and alcohol use), dietary factors (consumption of total fruits, total vegetables, green vegetables, chili pepper, total meat, processed or salted meat, red meat, fish, and table salt use), and human genetic variants IL1B-31C, IL1B-511T, IL1RN*2, TNFA-308A, TP53 codon 72 Arg and GSTM1 null.
Other risk factors reported in fewer than five studies and therefore not summarized included sociodemographic and geographic characteristics (occupation, rural vs. urban residence, refrigerator use, drinking water source and altitude of residence), personal characteristics (height, weight, body mass index, ethnicity, birth order, family history of gastric cancer and ABO blood group), specific types of alcohol consumed, dietary components (consumption of carbohydrates, fats, oils, legumes, tubers, grains, cereals, dairy products, desserts, salty snacks, beverages, micronutrients, trace elements and specific types of fruits, vegetables or meat), cooking methods, and genetic variants (in IL6, IL8, IL10, TLR2, TLR3, TLR4, NOS2, XRCC1, XRCC3, hOGG1, CYP1A1, GSTP1, GSTT1, MCP1, CYP2E1, CDH1 and MTHFR). Regarding region-specific dietary factors other than chili pepper, four of these studies reported on mate, four on beans (including black, fava or kidney beans), two on sweet potatoes or other root tubers and two on maize preparations.
Statistical analysis
Since the categories of the dietary factors varied across studies, our meta-analysis summarized the ORs for the highest vs. the lowest consumption category for each study. For studies only reporting subgroups of vegetable consumption (e.g., yellow, green and other), we summarized type-specific ORs by random effects models to obtain an average effect for total vegetables.
In our primary analysis, smoking and alcohol use were analyzed as binary variables (i.e., ever vs. never smokers and ever vs. never drinkers). Because some studies reported stratum-specific associations each compared to the same referent (e.g., current or former smokers vs. never smokers), we averaged those risk estimates using random effects models to estimate the overall effects. As a secondary analytic approach, the effects of current and former smoking were considered in separate meta-analyses. Also, dose-response was evaluated for lifetime exposure to cigarette smoking.
Host genetic polymorphisms were analyzed as binary variables mainly assuming a dominant genetic model; ORs were calculated if not provided in the original report. Given the almost complete linkage disequilibrium between the two reviewed IL1B polymorphisms [9], we performed a meta-analysis combining the study-specific OR for either IL1B-511 or IL1B-31. For studies that reported both associations, we averaged those risk estimates by random effects models.
For each risk factor, a summary OR with corresponding 95% CI was obtained using the random effects method of DerSimonian and Laird [10]. We calculated the standard error for the ln(OR) using the reported 95% CI of the OR [11]. Between-study heterogeneity was assessed for statistical significance using the Q test and quantified with the I2 statistic as low (<25%), moderate (25%–50%) or high (>50%) [12, 13]. If moderate or high heterogeneity was identified for a given risk factor, meta-regression models were used to examine the extent to which one or more of the following study-related quality or other characteristics might be explanatory: type of controls (gastroenterology patients, non-gastroenterology patients, healthy volunteers or population-based sample), sample size (≤200, 201–400, 401–600, or >600 subjects), adjustment for SES-related variables such as education and/or income (present vs. absent), and national gastric cancer incidence rate (<16 vs. ≥16 cases/100,000 population). Galbraith plots were also used to visually identify studies which were major contributors to moderate or high heterogeneity [14]. Data points positioned over or below the 95% CI of the regression line were defined to be outliers. Sensitivity analyses excluding such studies were performed to assess the influence on the summary OR.
For each of the studied risk factors, publication bias was investigated by visual inspection of Begg’s funnel plots and formally tested using Egger’s regression asymmetry method [15, 16].
Meta-analyses were performed with Stata version 11 (StataCorp, College Station, TX) using a combination of published macros, including metan, metareg, galbr, metafunnel and metabias [17]. A p-value less than 0.05 was considered statistically significant for all tests except the heterogeneity and Egger tests, for which p<0.10 was considered significant. All statistical tests were two-sided.
RESULTS
Literature search and description of studies
The literature searches identified a total of 417 articles: 204 from PubMed, 120 from LILACS, and 93 from SciELO (Figure 1). After excluding 354 irrelevant, insufficiently documented or duplicate publications, 63 full text articles were retrieved for further evaluation; 12 additional publications were identified from citations of these articles. Thus, we evaluated 75 articles reporting risk factor associations with gastric cancer.
Figure 1.
Flow diagram of the literature search.
Twenty-four publications reported exposure factors for which less than five articles were identified. Six articles were excluded [18–23] because the authors had other publications involving the same risk factors in larger, but overlapping samples. Thus, a total of 45 articles (33 written in English and 12 in Spanish) [24–68] published between 1990 and 2011 were included in this meta-analysis (Supplementary Table 1).
These 45 articles represented 29 independent studies assessing different risk factors among overlapping samples. No prospective study data were identified. All 29 studies correspond to case-control comparisons, including three with population-based controls, eight with other healthy controls, and 18 with hospital-based controls. Eight studies were conducted in Brazil, seven in Colombia, four in Mexico, three in Uruguay, two in Costa Rica, two in Venezuela, one in Chile, one in Peru, and one in Honduras. In terms of the total sample size (cases and controls combined), seven studies included less than 200 subjects, eleven studies between 201 and 400, two between 401 and 600 subjects, and nine were based on more than 600 subjects.
Adjustment variables differed among the studies. Twenty-three studies adjusted for age and sex, eleven for sociodemographic characteristics (e.g., SES, urban vs. rural residence, or level of education), ten for diet-related variables, seven for both smoking and alcohol use, five for H. pylori infection, and four for other personal characteristics (i.e., body mass index, race/ethnicity, country of birth, or family history of gastric cancer).
Regarding characteristics of cases, 12 studies provided information about the anatomic location of the tumor and 18 studies had histological subtype. Among the classified tumors, the proportion localized to noncardia sites ranged from 75 to 100% and the proportion of tumors classified as intestinal type ranged from 35 to 100%. Nine articles presented analyses stratified by these variables, with less than five studies for any given risk factor [36, 43, 48–51, 53, 56, 59].
Associations with SES
Education
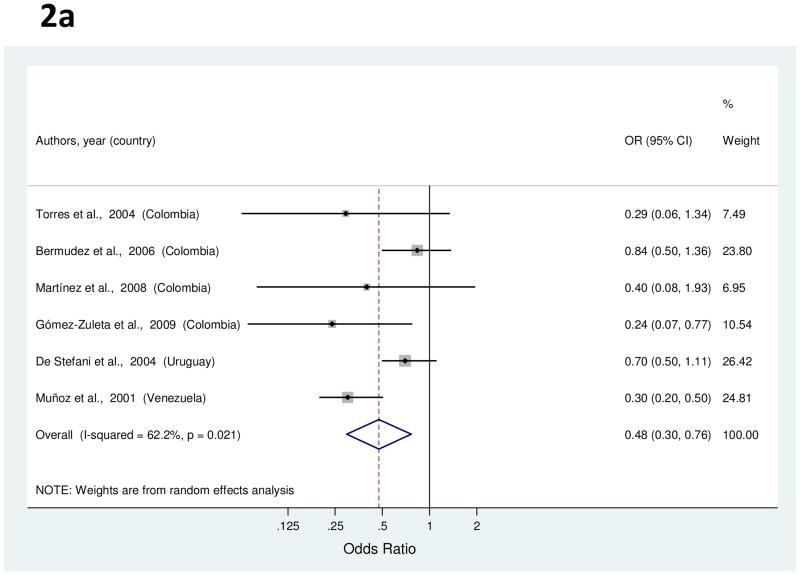
Six studies examined the association between education and gastric cancer [35, 37, 38, 40, 62, 66]. Study-specific OR for the highest level of education as compared to the lowest ranged from 0.24 to 0.84 (Figure 2a). The summary OR indicated a significant inverse association with a 52% decreased risk of gastric cancer (Table 1). High heterogeneity was detected among the studies, but meta-regression analysis of potential explanatory factors failed to explain the variability. The association was attenuated when an outlier study [66] was excluded (38%; 95% CI, 9–57%).
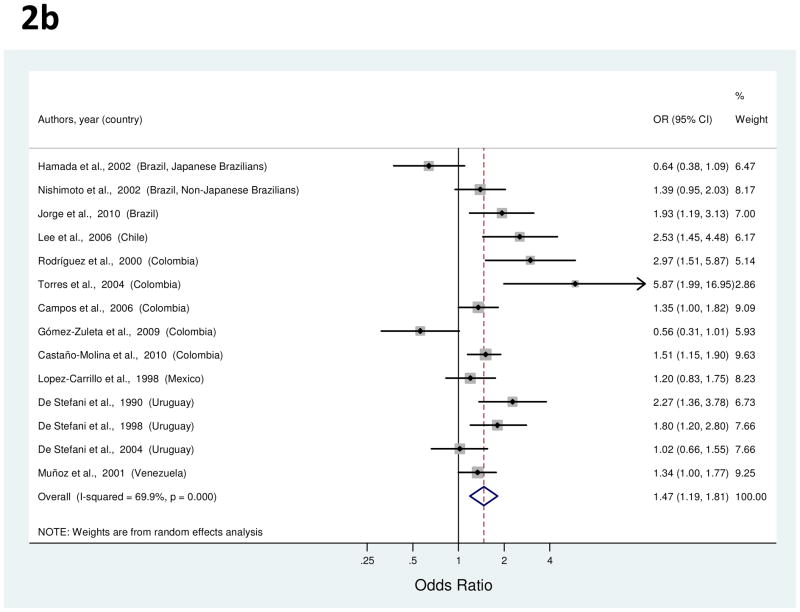
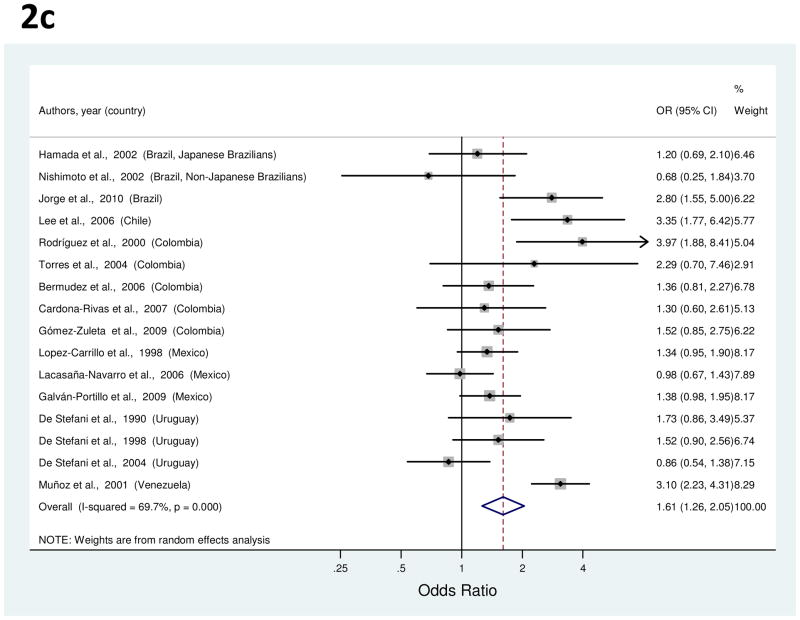
Figure 2.
a to c, random-effects estimates and 95% CIs of gastric cancer odds ratio (OR) associated with (A) education (highest vs. lowest level), (B) cigarette smoking (smokers vs. nonsmokers) and (C) alcohol use (drinkers vs. nondrinkers). Study-specific RRs are shown as squares, with the size of the symbol inversely proportional to the study-specific variance. Summary ORs are shown as diamonds, with the middle corresponding to the point estimate and the width representing the 95% CI.
Table 1.
Summary of risk factor associations with gastric cancer
| Risk Factor | Exposure categories | Number of studies by type of controls | Summary OR for gastric cancer (95% CI) | PQ for heterogeneity | I2 for heterogeneity,% | PEgger’s for publication bias | |||
|---|---|---|---|---|---|---|---|---|---|
| Highesta (min to max) | Referentb (min to max) | Hospital- basedc | Healthy volunteers | Population- based | |||||
| Education | some to secondary/superior | none to 0–2 years | 5 | 1 | 0 | 0.48 (0.30 –0.76) | 0.02 | 62.2 | 0.47 |
| Smoking | smokers | never smokers | 10 | 3 | 1 | 1.47 (1.19 –1.81) | <0.001 | 69.9 | 0.38 |
| current smokers | never smokers | 5 | 1 | 0 | 1.60 (1.13 –2.27) | 0.01 | 69.2 | 0.49 | |
| former-smokers | never smokers | 5 | 1 | 0 | 1.23 (0.95 –1.60) | 0.25 | 24.1 | 0.46 | |
| Alcohol use | drinkers | never drinkers | 11 | 3 | 2 | 1.61 (1.26 –2.05) | <0.001 | 69.7 | 0.84 |
| Total fruit consumption | frequent consumption to daily | infrequent consumption to <2 times/day | 9 | 1 | 1 | 0.68 (0.49 –0.94) | <0.001 | 75.7 | 0.67 |
| Total vegetable consumption | frequent consumption to daily | infrequent consumption to <3.05 portions/day | 9 | 1 | 2 | 0.58 (0.43 –0.77) | <0.001 | 74.2 | 0.57 |
| Green vegetable consumption | >4 times/week to daily | < 1 times/week to<5 times/week | 3 | 1 | 1 | 0.87 (0.65 –1.16) | 0.57 | 0 | 0.10 |
| Chili pepper consumption | often to >9 jalapeños/day | never to < 3 jalapeños/day | 3 | 1 | 2 | 2.30 (0.94 –5.64) | <0.001 | 90.1 | 0.28 |
| Total meat consumption | >5 times/week to >8 times/week | <3 to <4 times/week | 3 | 1 | 1 | 1.14 (0.47 –2.73) | <0.001 | 89.6 | 0.85 |
| Processed or salted meat consumption | frequent consumption to >5times/week | no consumption to infrequent | 5 | 0 | 1 | 1.64 (1.08 –2.48) | 0.02 | 64.5 | 0.30 |
| Red meat consumption | frequent consumption to daily | infrequent to less than 2 times/week | 5 | 0 | 0 | 1.73 (1.20 –2.51) | 0.02 | 64.5 | 0.24 |
| Fish consumption | 3–4 times/week to daily | infrequent consumption to <1 times/week | 4 | 1 | 1 | 0.86 (0.45 –1.67) | <0.001 | 80.7 | 0.72 |
| Table salt use | frequently to always | infrequent | 4 | 1 | 2 | 2.24 (1.53 –3.29) | 0.03 | 57.2 | 0.22 |
| IL1B-511(rs16944) or IL1B-31(rs1143627) | T or C carrier, respectively | C/C or T/T, respectively | 7 | 3 | 1 | 1.07 (0.78 –1.47) | 0.04 | 47.2 | 0.26 |
| IL1RN VNTR | *2 carrier (homozygous or heterozygous) | *2 non-carrier | 7 | 3 | 1 | 1.51 (1.15 –1.99) | 0.04 | 48.6 | 0.41 |
| TNFA-308(rs1800629) | A carrier | G/G | 3 | 2 | 1 | 0.96 (0.70 –1.31) | 0.63 | 0 | 0.15 |
| TP53 codon 72(rs1042522) | Pro carrier | Arg/Arg | 3 | 3 d | 0 | 0.87(0.66 –1.15) | 0.52 | 0 | 0.99 |
| GSTM1 deletion | null/null genotype | present/null or present/present | 3 | 2 | 0 | 1.36 (0.83 –2.23) | 0.07 | 53.2 | 0.41 |
Abbreviations: OR, odds ratio; CI, confidence interval; VNTR, variable number tandem repeat.
Range of highest categories of exposure.
Range of lowest categories of exposure.
Includes gastroenterology and other medical services.
Includes one study [27] for which genotype frequencies among controls were estimated from allele frequencies under the assumption of Hardy-Weinberg equilibrium.
Associations with lifestyle habits
Smoking
Fourteen studies examined the association of smoking and gastric cancer [24, 25, 32–36, 40, 42, 49, 58, 59, 62, 66]. Study-specific ORs for ever smokers vs. never smokers ranged from 0.56 to 5.87 (Figure 2b). The summary OR associated smoking with a 47% increased risk of gastric cancer (Table 1). High between-study heterogeneity was detected among the studies, but there were no significant explanatory variables in meta-regression analysis. A Galbraith plot indicated that four outlier studies [24, 34, 35, 40] contributed to heterogeneity; the summary OR estimated after their exclusion was 1.49 (95% CI, 1.29–1.73).
Six of the 14 studies [24, 25, 36, 58, 59, 66] evaluated the association of smoking and gastric cancer separately for current and for former smokers. Study-specific ORs for current smokers vs. never smokers ranged from 0.70 to 2.69. The summary OR showed a 60% increased risk of gastric cancer among current smokers (Table 1). Between-study heterogeneity was high, but meta-regression analysis failed to explain the variability. The summary OR was slightly modified (OR, 1.41; 95% CI, 1.05–1.89) after excluding an outlier study [59]. On the other hand, study-specific ORs for former-smokers vs. never smokers ranged from 0.60 to 1.90. The summary OR was 1.23 with low heterogeneity across studies (Table 1).
Five studies reported the association between lifetime exposure to cigarette smoking, measured in pack-years, and gastric cancer risk [24, 25, 42, 59, 62]. In a dose response meta-analysis, the increase in gastric cancer risk per 10 pack-years was 12% (95% CI, 6–18%).
Alcohol
Risk estimates for alcohol use were reported in 16 studies [24, 25, 32–35, 37, 40, 41, 49, 52, 56, 58, 59, 62, 66]. For six of these studies [24, 25, 58, 59, 62, 66], dose-specific ORs for ever drinkers were averaged since overall associations were not reported. Study-specific ORs comparing ever drinkers vs. never drinkers ranged from 0.68 to 3.97 (Figure 2c). The summary OR showed ever drinkers had a significant 61% increased risk of gastric cancer (Table 1). Between-study heterogeneity was high, but meta-regression analysis failed to explain this variability. In a sensitivity analysis excluding five outlier studies [33, 34, 52, 62, 66], the summary OR was slightly attenuated (OR, 1.45; 95% CI, 1.24–1.70).
Associations with dietary factors
Total fruits
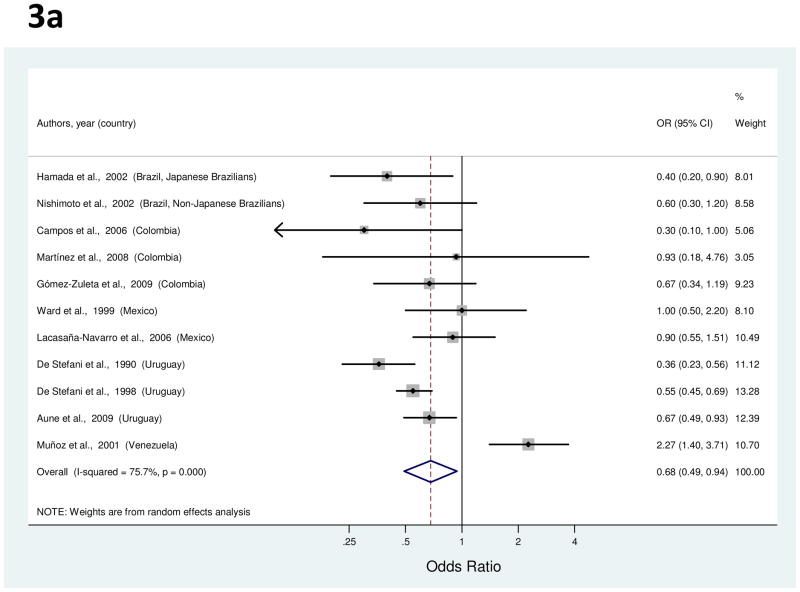
Eleven studies examined the association between fruit consumption and gastric cancer risk [24, 25, 36, 38, 40, 50, 52, 58, 60, 63, 66]. Study-specific OR for the highest consumption as compared to the lowest ranged from 0.30 to 2.27 (Figure 3a). The summary OR indicated a significant inverse association with a 32% decreased risk of gastric cancer (Table 1). High heterogeneity was detected among the studies, but the decrease was similar (39%; 95% CI, 28–47%) after the exclusion of two outlier studies [58, 66] which reported extreme opposite effects. Meta-regression analysis identified nominal significance of SES adjustment (p=0.03), reflecting such adjustment in one of the outlier studies and not in the other.
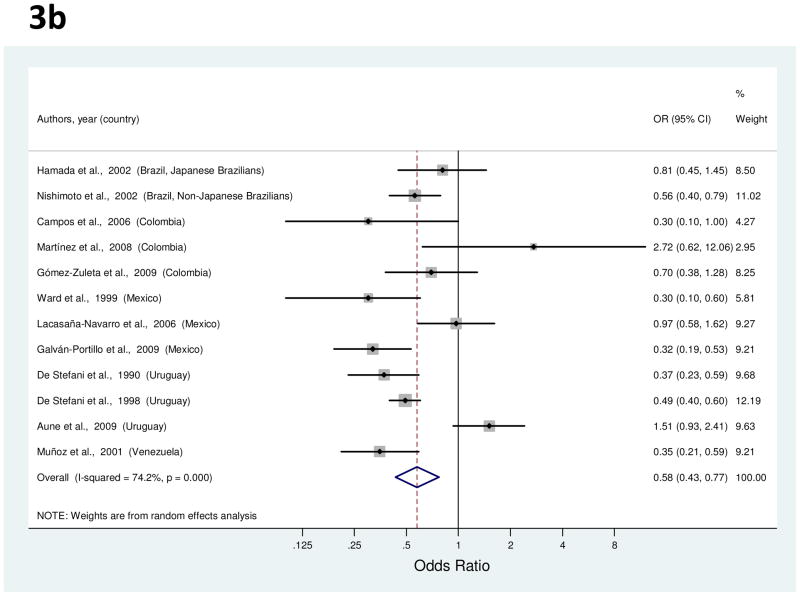
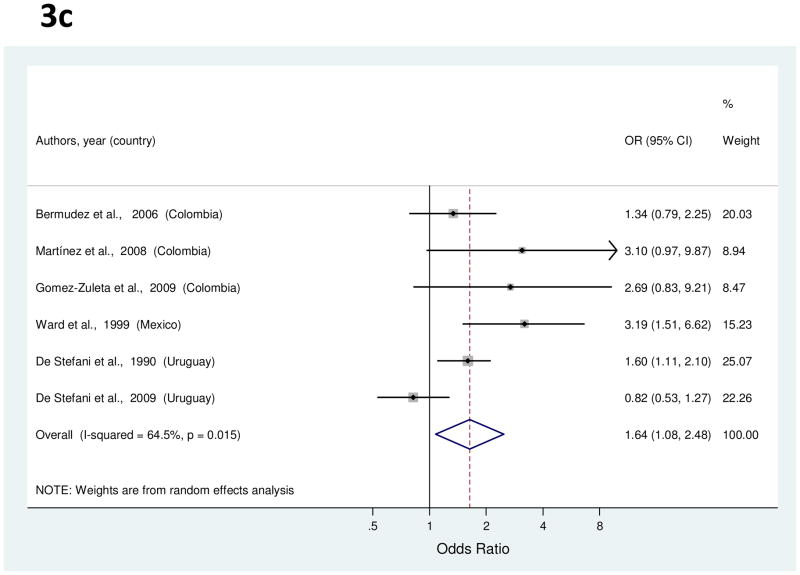
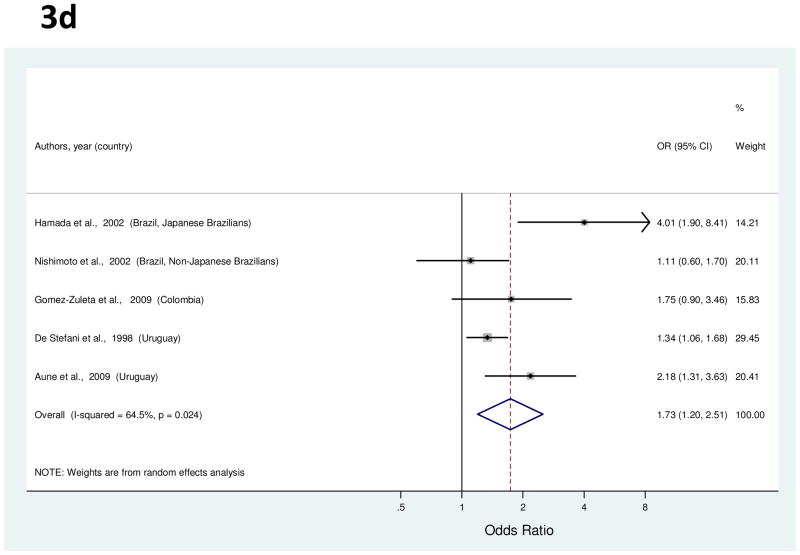
Figure 3.
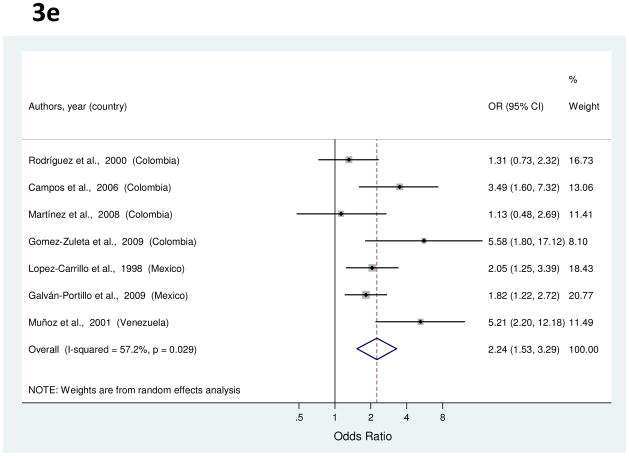
a to e, random-effects estimates and 95% CIs of gastric cancer odds ratio (OR) associated with (a) total fruit consumption (highest vs. lowest category), (b) total vegetable consumption (highest vs. lowest category), (c) processed or salted meat consumption (highest vs. lowest category), (d) red meat consumption (highest vs. lowest category), and (e) Table salt use (yes vs. no). Study-specific RRs are shown as squares, with the size of the symbol inversely proportional to the study-specific variance. Summary ORs are shown as diamonds, with the middle corresponding to the point estimate and the width representing the 95% CI.
Total vegetables
Twelve studies provided results about vegetable consumption and gastric cancer risk [24, 25, 36, 38, 40, 50, 52, 56, 58, 60, 63, 66], with study-specific ORs for highest consumption in comparison to lowest ranging from 0.30 to 2.72 (Figure 3b). For the studies by Hamada et al. [24] and Nishimoto et al. [25], average ORs derived from results for green, yellow and other vegetables were used in this meta-analysis since only type-specific associations were originally reported. The summary OR for total vegetables indicated a significant 42% risk reduction in gastric cancer risk (Table 1). Between-study heterogeneity was high, but meta-regression analysis did not identify any explanatory factors. With the exclusion of three outlier studies [38, 52, 63], the risk reduction was 53% (95% CI, 43–62%).
Green vegetables
The association of green vegetable consumption with gastric cancer was evaluated in five studies [24, 25, s38, 50, 61]. The study-specific ORs for the highest consumption compared to the lowest ranged from 0.27 to 1.00. The summary OR was 0.87, with low heterogeneity across studies.
Chili peppers
The association of chili pepper consumption was studied in six studies, including five which directly assessed chili peppers as a food item [37, 40, 48, 56, 66], and one which evaluated calculated consumption of the putative active component capsaicin [51]. The study-specific OR for the highest consumption vs. the lowest ranged from 0.50 to 2.10, except for a study [48], which had an OR of 28. The summary OR was 2.30 (Table 1). High heterogeneity was detected, but there were no significant explanatory variables in meta-regression analysis. When two outlier studies [48, 66] were excluded, the summary OR was 1.94 (95% CI, 1.40–2.68).
Total meat
Five studies provided information on total meat consumption [38, 50, 58, 65, 66], with study-specific ORs for highest consumption in comparison to lowest ranging from 0.31 to 3.10. The summary OR was 1.14 (Table 1). High heterogeneity was detected, but there were no significant explanatory variables in meta-regression analysis. Two studies [50, 66] were identified as outliers, and the summary OR derived from their exclusion was 1.53 (95% CI, 0.91–2.57).
Processed meat
Risk estimates for highest vs. lowest frequency of processed or salted meat consumption were reported in six studies [37, 38, 40, 50, 58, 64], and ranged from 0.82 to 3.19 (Figure 3c). The summary OR for processed meat indicated a significant 64% increased risk of gastric cancer (Table 1). High heterogeneity was detected, but there were no significant explanatory variables in meta-regression analysis. When two outlier studies [50, 64] were excluded, the summary OR was 1.62 (95% CI, 1.25–2.10).
Red meat
For the analysis of red meat consumption, a total of five studies were identified [24, 25, 40, 60, 65]. Study-specific ORs for the highest vs. the lowest consumption ranged from 1.11 to 4.01 (Figure 3d). The summary OR showed a significant 73% increased risk of gastric cancer (Table 1). Between-study heterogeneity was high, but meta-regression analysis failed to explain the variability. With the exclusion of an outlier study [24], the summary OR was 1.47 (95% CI, 1.13–1.90).
Fish
The association of fish consumption and gastric cancer was reported in six studies [24, 25, 40, 50, 62, 66]. The study-specific ORs for the highest consumption as compared to the lowest ranged from 0.30 to 4.76, with a summary OR of 0.86 (Table 1). Between-study heterogeneity was high, but meta-regression analysis failed to explain the variability. Two studies [50, 66] were identified as outliers, and the summary OR derived from their exclusion was 0.82 (95% CI, 0.48–1.40).
Salt
Seven studies provided information on use of table salt [34, 36, 38, 40, 49, 56, 66]. Study-specific ORs for the highest vs. the lowest intake ranged from 1.13 to 5.58 (Figure 3e). The summary OR found a significant association with a 2.24-fold increased risk of gastric cancer (Table 1). Between-study heterogeneity was high, but meta-regression analysis failed to show any significant source of heterogeneity. With the exclusion of one outlier study [66], the summary OR was 1.98 (95% CI, 1.40–2.82).
Associations with genetic variants
IL-1B polymorphisms
Eleven studies evaluated the associations of gastric cancer risk with either IL1B-511T [39, 46, 47, 57, 67], IL1B-31C [29, 53, 54] or both [26, 30, 43]. Allele frequency of the putative risk variant (i.e., IL1B-511T or IL1B-31C) among controls ranged from 45 to 80% across studies. Study-specific ORs for carriers of the risk alleles as compared to non-carriers ranged from 0.44 to 2.99, except for an outlier study [54] which had an OR of 8.0. The summary OR including all studies was 1.07, with moderate heterogeneity (Table 1). The summary OR derived from the exclusion of an outlier study was 1.0 (95% CI, 0.76–1.31).
IL-1RN variable number tandem repeat (VNTR) allele 2
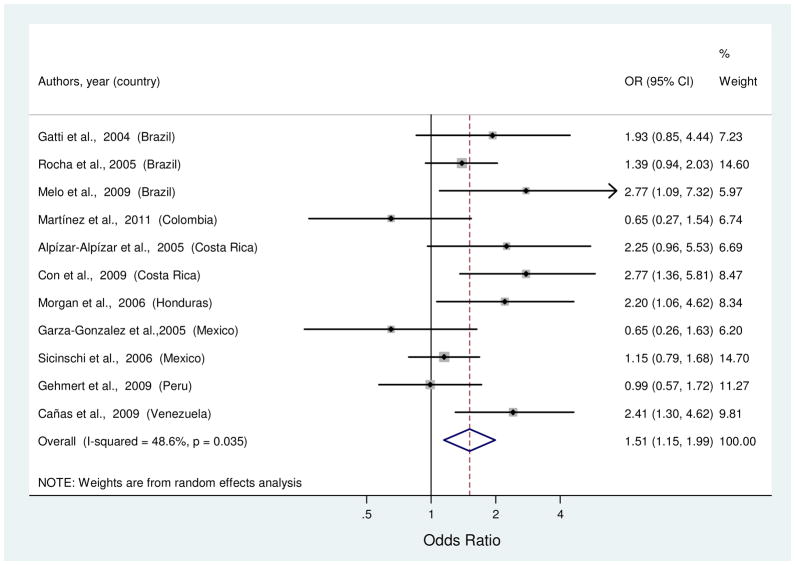
Risk estimates of the association between IL1RN*2 and gastric cancer were reported in eleven studies [26, 29, 30, 39, 43, 46, 47, 53, 54, 57, 67]. Allele frequency of the *2 VNTR among controls ranged from 17 to 38% across studies. Study-specific ORs for the comparison of *2 carriers (i.e., heterozygous or homozygous) vs. *2 non-carriers ranged from 0.65 to 2.77 (Figure 4). The summary OR found a significant 51% increased risk of gastric cancer (Table 1). Although no outliers were identified, there was moderate heterogeneity and no factors were significantly associated with this variability by meta-regression.
Figure 4.
Random-effects estimates and 95% CIs of gastric cancer odds ratio (OR) associated with IL1RN VNTR (*2 carrier vs. *2 non-carrier). Study-specific ORs are shown as squares, with the size of the symbol inversely proportional to the study-specific variance. Summary RRs are shown as diamonds, with the middle corresponding to the point estimate and the width representing the 95% CI.
TNFA-308 polymorphism
The association of TNFA-308A and gastric cancer was reported in six studies [29, 30, 35, 39, 47, 54]. Allele frequency of the A variant among controls ranged from 4 to 14% across studies. Study-specific ORs for carriers of A (i.e., heterozygous or homozygous) as compared to G/G genotype ranged from 0.41 to 1.39. The summary OR was 0.96 (Table 1), with low heterogeneity across studies.
TP53 codon 72 polymorphism
Six studies examined the association of TP53 codon 72 polymorphism and gastric cancer [27, 31, 41, 44, 55, 68]. Allele frequency of Pro among controls ranged from 27 to 38% across studies. Study-specific ORs for the comparison of Pro carriers (i.e., Arg/Pro or Pro/Pro) vs. Arg/Arg ranged from 0.51 to 1.12. The summary OR was 0.87 (Table 1), with low heterogeneity across studies.
GSTM1 polymorphism
Five studies evaluated the associations of gastric cancer risk with GSTM1 variation [28, 33, 35, 42, 45]. The frequency of null/null genotype among controls ranged from 18 to 60% across studies. Study-specific ORs for the null genotype as compared to non-null genotypes ranged from 0.81 to 5.45. The summary OR was 1.36, with high heterogeneity (Table 1); meta-regression analysis failed to show any significant source of variation. The summary OR derived from the exclusion of an outlier study [35] was 1.14 (95% CI, 0.62–1.60).
Publication bias
The p-values for Egger’s test of publication bias were greater than 0.10 for all risk factors with the exception of green vegetable consumption (p=0.10) (Table 1). A funnel plot confirmed moderately asymmetric distribution of the data points for this exposure.
DISCUSSION
Identification of risk factors may provide insight into disease etiology and suggest prevention strategies. Our meta-analysis of Latin-American studies identified increased gastric cancer risks associated with smoking, alcohol use, high consumption of red and processed meat, excessive salt intake and carriage of IL1RN*2 and decreased risks with high level of education and high consumption of fruits and vegetables. We found no significant associations with IL1B, TP53, TNFA, or GSTM1 variants, nor with high consumption of green vegetables, chili pepper, total meat, or fish. With the exception of chili pepper consumption, the factors summarized in this meta-analysis represent common exposures worldwide.
Previous meta-analyses of these gastric cancer risk factors have generally utilized international databases. By encompassing both international and regional sources, our meta-analysis aimed to summarize all available epidemiologic data from Latin American studies in order to identify exposures of particular importance in this population. In the following paragraphs we compare our regional findings with those reported by previous global studies.
In agreement with the most recent meta-analyses addressing the association between cigarette smoking and gastric cancer globally [69, 70], we found a 60% increased risk in current smokers compared to never smokers in Latin America, and a weaker association in former smokers. Although the mechanisms by which smoking increases the risk of gastric cancer are not completely understood, tobacco carcinogens may damage the gastric mucosa and smoking may adversely affect H. pylori persistence [71] as well as the efficacy of eradication therapy [72]. These effects become particularly important due to the increasing prevalence of smoking in Latin American populations [73].
The potential effect of alcohol on promotion of gastric carcinogenesis is still unclear [74]. A recent global meta-analysis of the association between alcohol drinking and gastric cancer found a significant association with heavy consumption (≥4 drinks per day), but no association with moderate consumption [75]. Our meta-analysis found that the risk of gastric cancer is increased in drinkers compared to never drinkers in Latin America, which may be related to greater alcohol consumption in the Americas as compared to other parts of the world [76].
Regarding diet, high fruit and vegetable consumption have been found to be protective by global case-control studies [77], a conclusion mirrored by our meta-analysis in Latin America. However, weak-to-null associations with fruits and vegetables have been found in prospective studies [78, 79], none of which were conducted in Latin American populations. This paradox is unexplained and whether a true association exists remains to be determined. Our regional findings on consumption of red meat, processed meat and salt do not differ with global studies of both retrospective and prospective design, supporting the hypothesis that excessive consumption of these items increases the risk of gastric cancer [80–82]. Neither our regional data nor prior global meta-analyses [83] support an association between fish consumption and gastric cancer risk.
The studies we summarized provided little or no validation data for their dietary self-report questionnaires, which generally omitted food items specific for this geographic region. There is a need for higher quality assessment instruments that cover regionally specific dietary components and preparation methods for Latin American populations. Furthermore, food frequency questionnaires may be usefully complemented by biomarker studies as a more objective method to estimate intake of specific nutrients [84].
The inverse association of education level with gastric cancer risk in our meta-analysis is in line with previous findings [85, 86]. Education captures aspects of the construct SES and may be particularly related to H. pylori infection, lifestyle habits and/or diet. Nevertheless, previous large prospective studies conducted in Europe and North America have attributed only some of the educational gradient to H. pylori infection [87] and smoking [88]. Additional mechanisms underlying the consistent protective relationship between education and gastric cancer remain to be identified.
Candidate gene and genome-wide association studies (GWAS) have implicated polymorphisms in several genes as significantly associated with gastric cancer risk, including IL1B, IL1RN, IL8, IL10, CDH1, MTHFR, PSCA, PLCE1, PTGER4, PRKAA1, and ZBTB20 [89–96]. In particular, global meta-analyses summarizing data on IL1B and IL1RN variants have suggested race-specific associations [89–92], with increased gastric cancer risk in Caucasians and weak or null associations in Asians. Caucasians have a lower prevalence than Asians of the putative risk alleles IL1B-31C and IL1B-511T. Therefore, it has been suggested that the effect is difficult to detect due to the high population frequency of the risk allele, or alternatively, that these variants do not influence gastric cancer susceptibility in Asians. Our data indicate that similar to Asians, Latin Americans have a high prevalence of IL1B risk alleles and null associations with gastric cancer. On the other hand, our results for the IL1RN*2 VNTR support its involvement in gastric carcinogenesis.
Although the association of gastric cancer with TNFA-308 and TP53 codon 72 Arg polymorphisms are not entirely consistent in global data [92, 97–99], neither of these polymorphisms appears to be associated with gastric cancer in Latin American populations. Apart from the six variants summarized by our meta-analysis, the potential of Latin American populations to identify unique risk-associated loci and/or to replicate GWAS findings has not been fully exploited. Association studies in this genetically admixed population (including Amerindian, Caucasian and African variants) offer opportunities for elucidating patterns of linkage disequilibrium, in studies of adequate sample size with proper adjustment for genetic ancestry.
Our findings, based primarily on small, convenience samples representing nine of the 20 Latin American countries, imply that most gastric cancers in this region are noncardia and intestinal-type. However there are no population-based data on subsite- and histology-specific incidence. Unfortunately, cancer registration coverage in Latin America is limited [100] and available data do not generally include these tumor characteristics.
As a meta-analysis of observational studies, our re-analysis is prone to biases inherent in the original studies. All the data for this meta-analysis were extracted from case-control comparisons, mainly using hospital-based controls, which are of uncertain validity and representativeness. Also, variation in the categorization of exposure levels may have contributed to the high heterogeneity. Although we used the reported multivariable adjusted ORs where available, there may have been residual confounding.
Assessment of H. pylori in case-control comparisons is problematic. Although essentially all gastric cancer is attributable to chronic H. pylori infection, some cases are serologically negative since the infection tends to diminish with progression of carcinogenesis [101]. The majority of studies included in this meta-analysis did not evaluate H. pylori serology, and five adjusted for infection status by multivariable regression without considering potential misclassification. A potentially better approach would be testing controls only, and comparing seropositives to gastric cancer cases regardless of serostatus. Nevertheless, our summary risk estimates should not have been substantially biased, since H. pylori infection is highly prevalent in Latin American populations and not believed to be highly correlated with most of the reviewed risk factors.
Future research efforts should be directed toward Latin American-specific exposures that may have etiologic significance, such as yerba mate consumption, locally grown fruits and vegetables, fermented and non-fermented beverages, and indoor use of wood stoves. Additional insights may be derived from the higher gastric cancer mortality in the Andes mountain range compared to adjacent coastal areas with equally high H. pylori prevalence [102]. Differences in H. pylori genotypes and its ancestral origin (European vs. African) [103], parasitic infections [104], dietary patterns, soil composition, or other environmental exposures have all been suggested as potential explanations.
About 9% of gastric carcinomas have Epstein-Barr virus (EBV) in the tumor cells [105]. EBV-positive tumors are characterized by episomal monoclonality [106], distinct clinical and genetic characteristics [107], and high anti-EBV antibody titers [108], which support viral involvement in gastric carcinogenesis. Previous studies in Latin America have found prevalence of tumor EBV positivity in gastric cancer ranging from 3.9% in Peru [109] to 16.8% in Chile [110]. The specific role of viral infection in gastric cancer development in this region, if any, may vary in magnitude across populations.
In conclusion, our meta-analysis identified risk factors for gastric cancer in Latin American countries that are similar to those identified globally. Most of our summarized risk estimates were moderate in magnitude, suggesting that additional risk factors contributing to the high incidence of gastric cancer in Latin America are yet to be recognized. Although there is insufficient evidence for dietary modifications to prevent gastric cancer, our findings further support lifestyle modifications to reduce smoking in this geographic region. In addition, the heavy burden of infection-related cancers in Latin America warrants serious consideration of a prospective epidemiologic study, which could simultaneously assess other common chronic morbidities such as diabetes, obesity, and cardiovascular disease. Solving the conundrum of the high gastric cancer incidence in Latin America would reduce mortality in this region and could improve our understanding of cancer etiology.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the United States National Institutes of Health, National Cancer Institute, and the Oak Ridge Associated Universities’Research Associates/Specialists Program.
The authors thank Nancy Terry, Biomedical Librarian at the U.S. National Institutes of Health Library, and Estela Santillán González, Librarian at the Dirección General de Epidemiologia in Mexico, for their help with reviewing the international (PubMed) and regional (LILIACS and SciELO) search strategies, respectively.
Footnotes
All authors declare no conflict of interest.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. Schistosomes, liver flukes and Helicobacter pylori. Vol. 61. Lyon, France: 1994. Monographs on the evaluation of carcinogenic risks to humans. [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor DN, Blaser MJ. The epidemiology of Helicobacter pylori infection. Epidemiol Rev. 1991;13:42–59. doi: 10.1093/oxfordjournals.epirev.a036078. [DOI] [PubMed] [Google Scholar]
- 4.Correa P, Piazuelo MB, Camargo MC. Etiopathogenesis of gastric cancer. Scand J Surg. 2006;95:218–24. doi: 10.1177/145749690609500402. [DOI] [PubMed] [Google Scholar]
- 5.Coelho LG, León-Barúa R, Quigley EM. Latin-American Consensus Conference on Helicobacter pylori infection. Latin-American National Gastroenterological Societies affiliated with the Inter-American Association of Gastroenterology (AIGE) Am J Gastroenterol. 2000;95:2688–91. doi: 10.1111/j.1572-0241.2000.03174.x. [DOI] [PubMed] [Google Scholar]
- 6.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 7.UNESCO. The State of Education in Latin America and the Caribbean: Guaranteeing Quality Education for All. 2008 Available at http://unesdoc.unesco.org/images/0015/001528/152895e.pdf.
- 8.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] Lyon, France: International Agency for Research on Cancer; 2010. [accessed on 30/11/2011]. Available from: http://globocan.iarc.fr. [Google Scholar]
- 9.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. Erratum in: Nature 2001;412:99. [DOI] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 11.Bradburn MJ. [accessed 20 May 2011];Updated and New Commands for Meta-Analysis in STATA. 2004 Available from: http://www.medepi.net/meta/software/Bradburn_metan_updates.pdf.
- 12.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galbraith RF. Graphical Display of Estimates Having Differing Standardv Errors. Technometrics. 1988;30:271–81. [Google Scholar]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 16.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JAC, editor. Meta-Analysis in Stata: An Updated Collection from the Stata Journal. Stata Press; 2009. [Google Scholar]
- 18.De Stefani E, Boffetta P, Ronco AL, Brennan P, Deneo-Pellegrini H, Carzoglio JC, et al. Plant sterols and risk of stomach cancer: a case-control study in Uruguay. Nutr Cancer. 2000;37:140–4. doi: 10.1207/S15327914NC372_4. [DOI] [PubMed] [Google Scholar]
- 19.De Stefani E, Ronco A, Brennan P, Boffetta P. Meat Consumption and Risk of Stomach Cancer in Uruguay: A Case-Control Study. Nutr Cancer. 2001;40:103–7. doi: 10.1207/S15327914NC402_5. [DOI] [PubMed] [Google Scholar]
- 20.Hanaoka T, Sugimura H, Nagura K, Ihara M, Li XJ, Hamada GS, et al. hOGG1 exon7 polymorphism and gastric cancer in case-control studies of Japanese Brazilians and non-Japanese Brazilians. Cancer Lett. 2001;170:53–61. doi: 10.1016/s0304-3835(01)00565-1. [DOI] [PubMed] [Google Scholar]
- 21.Garza-González E, Hold G, Pérez-Pérez GI, Bosques-Padilla FJ, Tijerina-Menchaca R, Maldonado-Garza HJ, et al. Role of polymorphism of certain cytokines in gastric cancer in Mexico. Preliminary results. Rev Gastroenterol Mex. 2003;68:107–12. Article in Spanish. [PubMed] [Google Scholar]
- 22.Castaño-Molina E, Parra-Sánchez H. Quitting smoking: a protective factor for gastric cancer. Hacia la Promoción de la Salud. 2007;12:125–132. Article in Spanish. [Google Scholar]
- 23.Martínez T, Hernández-Suárez G, Bravo MM, Trujillo E, Quiroga A, Albis R, et al. Association of interleukin-1 genetic polymorphism and CagA positive Helicobacter pylori with gastric cancer in Colombia. Rev Med Chil. 2011;139:1313–21. Article in Spanish. [PubMed] [Google Scholar]
- 24.Hamada GS, Kowalski LP, Nishimoto IN, Rodrigues JJ, Iriya K, Sasazuki S, et al. Risk factors for stomach cancer in Brazil (II): a case-control study among Japanese Brazilians in São Paulo. Jpn J Clin Oncol. 2002;32:284–90. doi: 10.1093/jjco/hyf061. [DOI] [PubMed] [Google Scholar]
- 25.Nishimoto IN, Hamada GS, Kowalski LP, Rodrigues JG, Iriya K, Sasazuki S, et al. Risk factors for stomach cancer in Brazil (I): a case-control study among non-Japanese Brazilians in São Paulo. Jpn J Clin Oncol. 2002;32:277–83. doi: 10.1093/jjco/hyf060. [DOI] [PubMed] [Google Scholar]
- 26.Gatti LL, Burbano RR, de Assumpção PP, Smith M de A, Payão SL. Interleukin-1beta polymorphisms, Helicobacter pylori infection in individuals from Northern Brazil with gastric adenocarcinoma. Clin Exp Med. 2004;4:93–8. doi: 10.1007/s10238-004-0043-2. [DOI] [PubMed] [Google Scholar]
- 27.Khayat AS, Lobo Gatti L, Moura Lima E, de Assumpção PP, Nascimento Motta FJ, Harada ML, et al. Polymorphisms of the TP53 codon 72 and WRN codon 1367 in individuals from Northern Brazil with gastric adenocarcinoma. Clin Exp Med. 2005;5:161–8. doi: 10.1007/s10238-005-0081-4. [DOI] [PubMed] [Google Scholar]
- 28.Colombo J, Rossit AR, Caetano A, Borim AA, Wornrath D, Silva AE. GSTT1, GSTM1 and CYP2E1 genetic polymorphisms in gastric cancer and chronic gastritis in a Brazilian population. World J Gastroenterol. 2004;10:1240–5. doi: 10.3748/wjg.v10.i9.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocha GA, Guerra JB, Rocha AM, Saraiva IE, da Silva DA, de Oliveira CA, et al. IL1RN polymorphic gene and cagA-positive status independently increase the risk of noncardia gastric carcinoma. Int J Cancer. 2005;115:678–83. doi: 10.1002/ijc.20935. [DOI] [PubMed] [Google Scholar]
- 30.Melo Barbosa HP, Martins LC, Dos Santos SE, Demachki S, Assumpção MB, Aragão CD, et al. Interleukin-1 and TNF-alpha polymorphisms and Helicobacter pylori in a Brazilian Amazon population. World J Gastroenterol. 2009;15:1465–71. doi: 10.3748/wjg.15.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes de Souza L, Miranda de Lima J, Dale Cotrim Guerreiro da Silva I, Manoukian Forones N. P53 Arg72Pro polymorphism in gastric cancer patients. J Gastrointest Cancer. 2009;40:41–5. doi: 10.1007/s12029-009-9078-7. [DOI] [PubMed] [Google Scholar]
- 32.Jorge YC, Duarte MC, Silva AE. Gastric cancer is associated with NOS2 -954G/C polymorphism and environmental factors in a Brazilian population. BMC Gastroenterol. 2010;10:64. doi: 10.1186/1471-230X-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee K, Cáceres A, Varela N, Csendes A, Ríos H, Quiñones L. Cytochrome P4501A1 (CYP1A1), glutathione S transferase M1 (GSTM1) polymorphisms and theirassociation with Tobacco use and alcohol consumption as gastric cancer susceptibility biomarkers. Rev Méd Chile. 2006;134:1107–15. doi: 10.4067/s0034-98872006000900004. Article in Spanish. [DOI] [PubMed] [Google Scholar]
- 34.Rodríguez A, Alvarado J, Sandler RS, Hani AC, Sanmiguel CP, Gómez G. Association between Helicobacter pylori infection and gastric cancer in Colombia. Acta Med Colomb. 2000;25:112–6. Article in Spanish. [Google Scholar]
- 35.Torres MM, Acosta CP, Sicard DM, Groot de Restrepo H. Genetic susceptibility and risk of gastric cancer in a population of Cauca. Biomédica. 2004;24:153–62. Article in Spanish. [PubMed] [Google Scholar]
- 36.Campos FI, Carrasquilla G, Koriyama C, Serra M, Carrascal E, Itoh T. Risk factors of gastric cancer specific for tumor location and histology in Cali, Colombia. World J Gastroenterol. 2006;12:5772–9. doi: 10.3748/wjg.v12.i36.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bermúdez C, Jesús Insuasty, Gamarra G. Blood group A and gastric cancer risk in the Hospital Universitario de Santander, Bucaramanga, Colombia. Acta Med Colomb. 2006;31:400–10. Article in Spanish. [Google Scholar]
- 38.Martínez T, Hernández GA, Rojas CA. Diet and Its Association with Preneoplastic Lesions and Gastric Cancer in a High-Risk Area for Gastric Cancer in Colombia I, 2000-2006. Rev Colomb Cancerol. 2008;12:74–88. Article in Spanish. [Google Scholar]
- 39.Martínez T, Hernández G, Bravo MM, Trujillo E, Quiroga A, Robayo JC, et al. Genetic Polymorphisms of IL-1B-511, IL-1RN, IL-10 Interleukins, Tumor Necrosis α-308 and Positive Helicobacter pylori CagA Infection in Gastric Cancer and Duodenal Ulcer in Different Populations in Colombia. Rev Colomb Cancerol. 2011;15:31–43. Article in Spanish. [Google Scholar]
- 40.Gómez-Zuleta M, Otero-Regino W, Ruíz-Lobo X. Risk factors for gastric cancer patients in Colombia. Rev Colomb Gastroenterol. 2009;24:134–43. Article in Spanish. [Google Scholar]
- 41.Cardona-Rivas D, Castaño-Molina E, Marín-Marmolejo JC. Gastric cancer, tobacco usage, alcohol consumption, socioeconomic stratification and polymorphism in codon 72 of gene p53 in a population of Manizales. Biosalud. 2007;6:33–44. Article in Spanish. [Google Scholar]
- 42.Castaño-Molina E, Santacoloma M, Arango L, Camargo M. Gastric cancer and detoxifying genes in a colombian population. Rev Col Gastroenterol. 2010;25:252–60. [Google Scholar]
- 43.Alpízar-Alpízar W, Pérez-Pérez GI, Une C, Cuenca P, Sierra R. Association of interleukin-1B and interleukin-1RN polymorphisms with gastric cancer in a high-risk population of Costa Rica. Clin Exp Med. 2005;5:169–76. doi: 10.1007/s10238-005-0082-3. [DOI] [PubMed] [Google Scholar]
- 44.Alpízar-Alpízar W, Sierra R, Cuenca P, Une C, Mena F, Pérez Pérez GI. Association of the p53 codon 72 polymorphism to gastric cancer risk in a high risk population of Costa Rica. Rev Biol Trop. 2005;53:317–24. Article in Spanish. [PubMed] [Google Scholar]
- 45.González A, Ramírez V, Cuenca P, Sierra R. Polymorphisms in detoxification genes CYP1A1, CYP2E1, GSTT1 and GSTM1 in gastric cancer susceptibility. Rev Biol Trop. 2004;52:591–600. Article in Spanish. [PubMed] [Google Scholar]
- 46.Con SA, Takeuchi H, Con-Chin GR, Con-Chin VG, Yasuda N, Con-Wong R. Role of bacterial and genetic factors in gastric cancer in Costa Rica. World J Gastroenterol. 2009;15:211–8. doi: 10.3748/wjg.15.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morgan DR, Dominguez RL, Keku TO, Heidt PE, Martin CF, Galanko JA, et al. Gastric cancer and the high combination prevalence of host cytokine genotypes and Helicobacter pylori in Honduras. Clin Gastroenterol Hepatol. 2006;4:1103–11. doi: 10.1016/j.cgh.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 48.López-Carrillo L, Hernández Avila M, Dubrow R. Chili pepper consumption and gastric cancer in Mexico: a case-control study. Am J Epidemiol. 1994;139:263–71. doi: 10.1093/oxfordjournals.aje.a116993. [DOI] [PubMed] [Google Scholar]
- 49.López-Carrillo L, López-Cervantes M, Ramírez-Espitia A, Rueda C, Fernández-Ortega C, Orozco-Rivadeneyra S. Alcohol consumption and gastric cancer in Mexico. Cad Saúde Pública. 1998;14(Sup 3):25–32. doi: 10.1590/s0102-311x1998000700004. [DOI] [PubMed] [Google Scholar]
- 50.Ward MH, López-Carrillo L. Dietary factors and the risk of gastric cancer in Mexico City. Am J Epidemiol. 1999;149:925–32. doi: 10.1093/oxfordjournals.aje.a009736. [DOI] [PubMed] [Google Scholar]
- 51.Lopez-Carrillo L, Lopez-Cervantes M, Robles-Diaz G, Ramirez-Espitia, Mohar-betancourt E, Meneses-Garcia A. Capsaicin consumption, Helicobacter pylori positivity and gastric cancer in Mexico. Int J Cancer. 2003;106:277–82. doi: 10.1002/ijc.11195. [DOI] [PubMed] [Google Scholar]
- 52.Lacasaña-Navarro M, Galvan-Portillo M, Chen J, Lopez-Cervantes M, Lopez-Carrillo L. Methylenetetrahydrofolatereductase 677C>T polymorphism and gastric cancer susceptibility in Mexico. Eur Journal Cancer. 2006;42:528–33. doi: 10.1016/j.ejca.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 53.Sicinschi LA, Lopez-Carrillo L, Camargo MC, Correa P, Sierra RA, Henry RR, et al. Gastric cancer risk in a Mexican population: role of Helicobacter pylori CagA positive infection and polymorphisms in interleukin-1 and -10 genes. Int J Cancer. 2006;118:649–57. doi: 10.1002/ijc.21364. [DOI] [PubMed] [Google Scholar]
- 54.Garza-González E, Bosques-Padilla FJ, El-Omar E, Hold G, Tijerina-Menchaca R, Maldonado-Garza HJ, et al. Role of the polymorphic IL-1B, IL-1RN and TNF-A genes in distal gastric cancer in Mexico. Int J Cancer. 2005;114:237–41. doi: 10.1002/ijc.20718. [DOI] [PubMed] [Google Scholar]
- 55.Pérez-Pérez GI, Bosques-Padilla FJ, Crosatti ML, Tijerina-Menchaca R, Garza-González E. Role of p53 codon 72 polymorphism in the risk of development of distal gastric cancer. Scand J Gastroenterol. 2005;40:56–60. doi: 10.1080/00365520410009456. [DOI] [PubMed] [Google Scholar]
- 56.Galván-Portillo MV, Cantoral A, Oñate-Ocaña LF, Chen J, Herrera-Goepfert R, Torres-Sanchez L, et al. Gastric cancer in relation to the intake of nutrients involved in one-carbon metabolism among MTHFR 677 TT carriers. Eur J Nutr. 2009;48:269–76. doi: 10.1007/s00394-009-0010-5. [DOI] [PubMed] [Google Scholar]
- 57.Gehmert S, Velapatiño B, Herrera P, Balqui J, Santivañez L, Cok J, et al. Interleukin-1 Beta Single-Nucleotide Polymorphism’s C Allele is Associated with Elevated Risk of Gastric Cancer in Helicobacter pylori-infected Peruvians. Am J Trop Med Hyg. 2009;81:804–10. doi: 10.4269/ajtmh.2009.08-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Stefani E, Correa P, Fierro L, Carzoglio J, Deneo-Pellegrini H, Zavala D. Alcohol drinking and tobacco smoking in gastric cancer. A case-control study. Rev Epidemiol Sante Publique. 1990;38:297–307. [PubMed] [Google Scholar]
- 59.De Stefani E, Boffetta P, Carzoglio J, Mendilaharsu S, Deneo-Pellegrini H. Tobacco use and alcohol drinking as risk factor for stomach cancer: a case control in Uruguay. Cancer causes and Control. 1998;9:321–9. doi: 10.1023/a:1008829321668. [DOI] [PubMed] [Google Scholar]
- 60.De Stefani E, Boffetta P, Mendilaharsu M, Carzoglio J, Deneo-Pellegrini H. Dietary nitrosamines, heterocyclic amines, and risk of gastric cancer: A case-control study in Uruguay. Nutr Cancer. 1998;30:158–62. doi: 10.1080/01635589809514656. [DOI] [PubMed] [Google Scholar]
- 61.De Stefani E, Correa P, Boffetta P, Ronco A, Brennan P, Deneo-Pellegrini H. Plant foods and risk of gastric cancer: a case-control study in Uruguay. Eur J of Cancer Prev. 2001;10:357–64. doi: 10.1097/00008469-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 62.De Stefani E, Correa P, Boffetta P, Deneo-Pellegrini H, Ronco A, Mendilaharsu M. Dietary patterns and risk of gastric cancer: a case-control study in Uruguay. Gastric Cancer. 2004;7:211–20. doi: 10.1007/s10120-004-0295-2. [DOI] [PubMed] [Google Scholar]
- 63.Aune D, De Stefani E, Ronco A, Boffetta P, Deneo-Pellegrini H, Acosta G, et al. Fruits, vegetables and the risk of cancer: a multisite case-control study in Uruguay. Asian Pac J Cancer Prev. 2009;10:419–28. [PubMed] [Google Scholar]
- 64.De Stefani E, Aune D, Boffetta P, Deneo-Pellegrini H, Ronco AL, Acosta G, et al. Salted meat consumption and the risk of cancer: a multisite case-control study in Uruguay. Asian Pac J Cancer Prev. 2009;10:853–7. [PubMed] [Google Scholar]
- 65.Aune D, De Stefani E, Ronco A, Boffetta P, Deneo-Pellegrini H, Acosta G, et al. Meat consumption and cancer risk: a case-control study in Uruguay. Asian Pac J Cancer Prev. 2009;10:429–36. [PubMed] [Google Scholar]
- 66.Muñoz N, Plummer M, Vivas J, Moreno J, de Sanjos J, Lopez, et al. A case-control study of gastric cancer in venezuela. Int J Cancer. 2001;93:417–23. doi: 10.1002/ijc.1333. [DOI] [PubMed] [Google Scholar]
- 67.Cañas M, Morán Y, Rivero MB, Bohórquez A, Villegas V, Rendón Y, et al. Interleukin-1 genetic polymorphism: association with gastric cancer in the high-risk Central-Western population of Venezuela. Rev Med Chil. 2009;137:63–70. Article in Spanish. [PubMed] [Google Scholar]
- 68.Cañas M, Morán Y, Camargo ME, Rivero MB, Bohórquez A, Villegas V, et al. TP53 codon 72 polymorphism and gastric cancer risk: a case-control study in individuals from the central-western region of Venezuela. Invest Clin. 2009;50:153–61. Article in Spanish. [PubMed] [Google Scholar]
- 69.La Torre G, Chiaradia G, Gianfagna F, De Lauretis A, Boccia S, Mannocci A, et al. Smoking status and gastric cancer risk: an updated meta-analysis of case-control studies published in the past ten years. Tumori. 2009;95:13–22. doi: 10.1177/030089160909500103. [DOI] [PubMed] [Google Scholar]
- 70.Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 71.Cardenas VM, Graham DY. Smoking and Helicobacter pylori infection in a sample of U.S. adults. Epidemiology. 2005;16:586–90. doi: 10.1097/01.ede.0000165365.52904.4a. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki T, Matsuo K, Ito H, Sawaki A, Hirose K, Wakai K, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006;119:217–24. doi: 10.1016/j.amjmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Müller F, Wehbe L. Smoking and smoking cessation in Latin America: a review of the current situation and available treatments. Int J Chron Obstruct Pulmon Dis. 2008;3:285–93. doi: 10.2147/copd.s2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.International Agency for Research on Cancer. A Review of Human Carcinogens: Personal Habits and Indoor Combustions. 100E. Lyon, France: 2012. Monographs on the Evaluation of Carcinogenic Risks to Humans. [PMC free article] [PubMed] [Google Scholar]
- 75.Tramacere I, Negri E, Pelucchi C, Bagnardi V, Rota M, Scotti L, et al. A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol. 2012;23:28–36. doi: 10.1093/annonc/mdr135. [DOI] [PubMed] [Google Scholar]
- 76.Monteiro Maristela G. A case for action. PAHO; Washington, D.C: 2007. [accessed 20 May 2012]. Alcohol and Public Health in the Americas. Available from: http://www.paho.org/Spanish/DD/PIN/A&PH.pdf. [Google Scholar]
- 77.Lunet N, Valbuena C, Vieira AL, Lopes C, Lopes C, David L, et al. Fruit and vegetable consumption and gastric cancer by location and histological type: case-control and meta-analysis. Eur J Cancer Prev. 2007;16:312–27. doi: 10.1097/01.cej.0000236255.95769.22. [DOI] [PubMed] [Google Scholar]
- 78.Lunet N, Lacerda-Vieira A, Barros H. Fruit and vegetables consumption and gastric cancer: a systematic review and meta-analysis of cohort studies. Nutr Cancer. 2005;53:1–10. doi: 10.1207/s15327914nc5301_1. [DOI] [PubMed] [Google Scholar]
- 79.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington, D.C., U.S: 2007. [Google Scholar]
- 80.Larsson SC, Orsini N, Wolk A. Processed meat consumption and stomach cancer risk: a meta-analysis. J Natl Cancer Inst. 2006;98:1078–87. doi: 10.1093/jnci/djj301. [DOI] [PubMed] [Google Scholar]
- 81.Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 82.D’Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: A meta-analysis of prospective studies. Clin Nutr. 2012;31:489–98. doi: 10.1016/j.clnu.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Wu S, Liang J, Zhang L, Zhu X, Liu X, Miao D. Fish consumption and the risk of gastric cancer: systematic review and meta-analysis. BMC Cancer. 2011;11:26. doi: 10.1186/1471-2407-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuhnle GG. Nutritional biomarkers for objective dietary assessment. J Sci Food Agric. 2012;92:1145–9. doi: 10.1002/jsfa.5631. [DOI] [PubMed] [Google Scholar]
- 85.Pukkala E, Teppo L. Socioeconomic status and education as risk determinants of gastrointestinal cancer. Prev Med. 1986;15:127–38. doi: 10.1016/0091-7435(86)90083-6. [DOI] [PubMed] [Google Scholar]
- 86.van Loon AJ, Goldbohm RA, van den Brandt PA. Socioeconomic status and stomach cancer incidence in men: results from The Netherlands Cohort Study. J Epidemiol Community Health. 1998;52:166–71. doi: 10.1136/jech.52.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nagel G, Linseisen J, Boshuizen HC, Pera G, Del Giudice G, Westert GP, et al. Socioeconomic position and the risk of gastric and oesophageal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Int J Epidemiol. 2007;36:66–76. doi: 10.1093/ije/dyl275. [DOI] [PubMed] [Google Scholar]
- 88.Mouw T, Koster A, Wright ME, Blank MM, Moore SC, Hollenbeck A, et al. Education and risk of cancer in a large cohort of men and women in the United States. PLoS One. 2008;3:e3639. doi: 10.1371/journal.pone.0003639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Camargo MC, Mera R, Correa P, Peek RM, Jr, Fontham ET, Goodman KJ, et al. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1674–87. doi: 10.1158/1055-9965.EPI-06-0189. [DOI] [PubMed] [Google Scholar]
- 90.He B, Zhang Y, Pan Y, Xu Y, Gu L, Chen L, et al. Interleukin 1 beta (IL1B) promoter polymorphism and cancer risk: evidence from 47 published studies. Mutagenesis. 2011;26:637–42. doi: 10.1093/mutage/ger025. [DOI] [PubMed] [Google Scholar]
- 91.Loh M, Koh KX, Yeo BH, Song CM, Chia KS, Zhu F, et al. Meta-analysis of genetic polymorphisms and gastric cancer risk: variability in associations according to race. Eur J Cancer. 2009;45:2562–8. doi: 10.1016/j.ejca.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 92.Persson C, Canedo P, Machado JC, El-Omar EM, Forman D. Polymorphisms in inflammatory response genes and their association with gastric cancer: A HuGE systematic review and meta-analyses. Am J Epidemiol. 2011;173:259–70. doi: 10.1093/aje/kwq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zacho J, Yazdanyar S, Bojesen SE, Tybjærg-Hansen A, Nordestgaard BG. Hyperhomocysteinemia, methylenetetrahydrofolate reductase c.677C>T polymorphism and risk of cancer: cross-sectional and prospective studies and meta-analyses of 75,000 cases and 93,000 controls. Int J Cancer. 2011;128:644–52. doi: 10.1002/ijc.25375. [DOI] [PubMed] [Google Scholar]
- 94.Shi D, Wang S, Gu D, Wu D, Wang M, Chu H, et al. The PSCA polymorphisms derived from genome-wide association study are associated with risk of gastric cancer: a meta-analysis. J Cancer Res Clin Oncol. 2012;138:1339–45. doi: 10.1007/s00432-012-1210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Abnet CC, Freedman ND, Hu N, Wang Z, Yu K, Shu XO, et al. A shared susceptibility locus in PLCE1 at 10q23 for gastric adenocarcinoma and esophageal squamous cell carcinoma. Nat Genet. 2010;42:764–7. doi: 10.1038/ng.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shi Y, Hu Z, Wu C, Dai J, Li H, Dong J, et al. A genome-wide association study identifies new susceptibility loci for non-cardia gastric cancer at 3q13.31 and 5p13.1. Nat Genet. 2011;43:1215–8. doi: 10.1038/ng.978. [DOI] [PubMed] [Google Scholar]
- 97.Gorouhi F, Islami F, Bahrami H, Kamangar F. Tumour-necrosis factor-A polymorphisms and gastric cancer risk: a meta-analysis. Br J Cancer. 2008;98:1443–51. doi: 10.1038/sj.bjc.6604277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou Y, Li N, Zhuang W, Liu GJ, Wu TX, Yao X, et al. P53 codon 72 polymorphism and gastric cancer: a meta-analysis of the literature. Int J Cancer. 2007;121:1481–6. doi: 10.1002/ijc.22833. [DOI] [PubMed] [Google Scholar]
- 99.Zhou Y, Li N, Zhuang W, Wu X. p53 Codon 72 polymorphism and gastric cancer risk in a Chinese Han population. Genet Test Mol Biomarkers. 2010;14:829–33. doi: 10.1089/gtmb.2010.0115. [DOI] [PubMed] [Google Scholar]
- 100.Valsecchi MG, Steliarova-Foucher E. Cancer registration in developing countries: luxury or necessity? Lancet Oncol. 2008;9:159–67. doi: 10.1016/S1470-2045(08)70028-7. [DOI] [PubMed] [Google Scholar]
- 101.Kokkola A, Kosunen TU, Puolakkainen P, Sipponen P, Harkonen M, Laxen F, et al. Spontaneous disappearance of Helicobacter pylori antibodies in patients with advanced atrophic corpus gastritis. APMIS. 2003;111:619–24. doi: 10.1034/j.1600-0463.2003.1110604.x. [DOI] [PubMed] [Google Scholar]
- 102.Correa P, Piazuelo MB. Natural history of Helicobacter pylori infection. Dig Liver Dis. 2008;40:490–6. doi: 10.1016/j.dld.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.de Sablet T, Piazuelo MB, Shaffer CL, Schneider BG, Asim M, Chaturvedi R, et al. Phylogeographic origin of Helicobacter pylori is a determinant of gastric cancer risk. Gut. 2011;60:1189–95. doi: 10.1136/gut.2010.234468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ek C, Whary MT, Ihrig M, Bravo LE, Correa P, Fox JG. Serologic Evidence that Ascaris and Toxoplasma Infections Impact Inflammatory Responses to Helicobacter pylori in Colombians. Helicobacter. 2012;17:107–15. doi: 10.1111/j.1523-5378.2011.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824–33. doi: 10.1053/j.gastro.2009.05.001. Erratum in: Gastroenterology 2011;140:1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ott G, Kirchner T, Müller-Hermelink HK. Monoclonal Epstein-Barr virus genomes but lack of EBV-related protein expression in different types of gastric carcinoma. Histopathology. 1994;25:323–9. doi: 10.1111/j.1365-2559.1994.tb01350.x. [DOI] [PubMed] [Google Scholar]
- 107.Akiba S, Koriyama C, Herrera-Goepfert R, Eizuru Y. Epstein-Barr virus associated gastric carcinoma: epidemiological and clinicopathological features. Cancer Sci. 2008;99:195–201. doi: 10.1111/j.1349-7006.2007.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Levine PH, Stemmermann G, Lennette ET, Hildesheim A, Shibata D, Nomura A. Elevated antibody titers to Epstein-Barr virus prior to the diagnosis of Epstein-Barr-virus-associated gastric adenocarcinoma. Int J Cancer. 1995;60:642–4. doi: 10.1002/ijc.2910600513. [DOI] [PubMed] [Google Scholar]
- 109.Yoshiwara E, Koriyama C, Akiba S, Itoh T, Minakami Y, Chirinos JL, et al. Epstein-Barr virus-associated gastric carcinoma in Lima, Peru. J Exp Clin Cancer Res. 2005;24:49–54. [PubMed] [Google Scholar]
- 110.Corvalan A, Koriyama C, Akiba S, Eizuru Y, Backhouse C, Palma M, et al. Epstein-Barr virus in gastric carcinoma is associated with location in the cardia and with a diffuse histology: a study in one area of Chile. Int J Cancer. 2001;94:527–30. doi: 10.1002/ijc.1510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.