Abstract
Aims: Nuclear factor E2-related factor 2 (Nrf2), a key cytoprotective transcription factor, regulates also proangiogenic mediators, interleukin-8 and heme oxygenase-1 (HO-1). However, hitherto its role in blood vessel formation was modestly examined. Particularly, although Nrf2 was shown to affect hematopoietic stem cells, it was not tested in bone marrow-derived proangiogenic cells (PACs). Here we investigated angiogenic properties of Nrf2 in PACs, endothelial cells, and inflammation-related revascularization. Results: Treatment of endothelial cells with angiogenic cytokines increased nuclear localization of Nrf2 and induced expression of HO-1. Nrf2 activation stimulated a tube network formation, while its inhibition decreased angiogenic response of human endothelial cells, the latter effect reversed by overexpression of HO-1. Moreover, lack of Nrf2 attenuated survival, proliferation, migration, and angiogenic potential of murine PACs and affected angiogenic transcriptome in vitro. Additionally, angiogenic capacity of PAC Nrf2−/− in in vivo Matrigel assay and PAC mobilization in response to hind limb ischemia of Nrf2−/− mice were impaired. Despite that, restoration of blood flow in Nrf2-deficient ischemic muscles was better and accompanied by increased oxidative stress and inflammatory response. Accordingly, the anti-inflammatory agent etodolac tended to diminish blood flow in the Nrf2−/− mice. Innovation: Identification of a novel role of Nrf2 in angiogenic signaling of endothelial cells and PACs. Conclusion: Nrf2 contributes to angiogenic potential of both endothelial cells and PACs; however, its deficiency increases muscle blood flow under tissue ischemia. This might suggest a proangiogenic role of inflammation in the absence of Nrf2 in vivo, concomitantly undermining the role of PACs in such conditions. Antioxid. Redox Signal. 20, 1693–1708.
Introduction
The discovery of endothelial progenitor cells, broadly defined as proangiogenic cells (PACs), pointed out a new direction in the treatment of cardiovascular disorders (CVDs), since they were recognized as responsible for postnatal vasculogenesis in physiological and regenerative neovascularization after, for example, hind limb ischemia (HLI) (5, 6). Following the action of cytokines and growth factors, such as vascular endothelial growth factor (VEGF) and stromal cell-derived factor-1 (SDF-1), progenitors of bone marrow (BM) origin circulate in peripheral blood (PB) and might contribute to the formation of blood vessels in damaged/ischemic tissue (7, 10, 46). Whether PACs act through paracrine effects and/or direct incorporation into foci of neovascularization are still being questioned (5, 21, 37, 44, 52).
It is crucial for PACs to survive in conditions of the increased production of reactive oxygen species (ROS) that accompany ischemia and/or inflammatory response and be able to participate in tissue repair. Such a resistance may be achieved by enhanced expression of several antioxidant enzymes, such as glutathione peroxidase-1 (Gpx-1) (12, 20). Although low levels of ROS are required for proper function of adult cells and differentiation of progenitors, the conditions of severe oxidative stress, being an essential mechanism underlying the pathogenesis of CVDs, may lead to PAC damage decreasing their antioxidative and proangiogenic functions (24, 26, 54). Accordingly, impaired angiogenesis in Gpx-1-deficient mice associated with PAC dysfunction was reported (19).
Innovation.
This work identifies a novel, direct role of nuclear factor E2-related factor 2 (Nrf2) in angiogenic properties of both bone-marrow-derived proangiogenic progenitor cells and mature endothelial cells. Moreover, by showing that lack of Nrf2 did not impair revascularization after hind limb ischemia, despite decreased mobilization and angiogenic potential of proangiogenic cells in vivo, the data indicate the limited significance of those cells in inflammatory angiogenesis. The revealed involvement of Nrf2 in neovascularization processes, in addition to its cytoprotective effects, might open a new direction in research on therapeutic neovascularization in cardiovascular disorders.
It has been suggested that genetic modifications of PACs could overcome detrimental effects of excessive ROS production and increase the efficiency of cell therapy in CVDs. An approach of improving not only antioxidative but also angiogenic potential of PACs by transferring genes encoding hypoxia-inducible factor-1α (HIF-1α) (28) or heme oxygenase-1 (HO-1) [reviewed in Jazwa et al. (26)] has created great expectations of PAC application. Nonetheless, hitherto the effects of therapeutic neovascularization for CVD treatment have not been sufficient to enter common clinical schedules (41). It is therefore important to study the biology of PACs further and clarify the mechanism of their therapeutic action.
A cytoprotective role of HO-1 against oxidative stress and vascular inflammation is well known. Moreover, its importance in blood vessel formation, vascular repair, and functioning of PACs was indicated in different animal models, such as wire-induced carotid artery injury model, HLI, or retinal ischemia (13, 33–35, 47). In the latter, HO-1-deficient PACs were impaired in their ability to migrate into ischemic areas and repair the acellular capillaries (13). Importantly, it seems that proangiogenic activity of HO-1 may be closely related to VEGF and SDF-1 since HO-1 not only induces their production (15, 31) but also can be involved in VEGF- (29) and SDF-1- (13) dependent neovascularization.
So far nuclear factor E2-related factor 2 (Nrf2) transcription factor has been known mostly as a regulator of detoxifying, antioxidative, anti-inflammatory, as well as antiapoptotic gene [reviewed in Baird and Dinkova-Kostova (8)]. Under basal conditions Nrf2-mediated transcription is blocked because of inhibitory effect of cytoplasmic protein Keap1, which facilitates Nrf2 proteasomal degradation (25). Under chemical and oxidative stresses the disruption of Keap1-Nrf2 complex and subsequent Nrf2 activation by electrophiles, ROS and/or the action of different kinases like MAPK (55) or phosphoinositide-3-OH kinase (PI3K) (32), are crucial for induction of protective cellular mechanisms. Accordingly, genetic deletion of Nrf2 renders cells and animals more sensitive to detrimental effects of oxidants and inflammatory agents, while activation of the Keap1-Nrf2 pathway allows survival and protection in stressful conditions [reviewed in Baird and Dinkova-Kostova (8) and Motohashi and Yamamoto (40)]. It seems, however, that Nrf2 action may be much broader since it also regulates crucial proangiogenic factors, interleukin (IL)-8 (56) and HO-1 (4). We confirmed those interactions in human microvascular endothelial cells (HMEC-1), proving also that IL-8 is regulated by Nrf2 independently of HO-1 (17, 36).
Hitherto no direct experimental evidence is available on the role of Nrf2 in PACs. The importance of Nrf2 was shown, however, in survival and differentiation of hematopoietic stem progenitor cells (HSPCs) (39). In the light of those data and the fact that HO-1 deficiency may cause PAC dysfunction (13, 34), we hypothesize an instrumental role of Nrf2 in PACs.
Only a few articles suggested a link between Nrf2 and neovascularization or angiogenic potential of endothelial cells. It was shown, for example, that retinal vascular development is delayed in Nrf2−/− mice under non-inflammatory conditions (48), whereas in response to ischemia the Nrf2 deficiency increased blood flow restoration following left pulmonary artery ligation (42) or femoral artery ligation (FAL) (23). Thus, to examine proangiogenic function of Nrf2 we investigated its role in angiogenic signal transduction in endothelial cells and in regulation of angiogenic potential of both endothelial cells and BM-derived PACs. Then, we determined the influence of Nrf2 on revascularization in the murine model of HLI.
Results
Nrf2 is activated in response to proangiogenic stimulation of HMEC-1
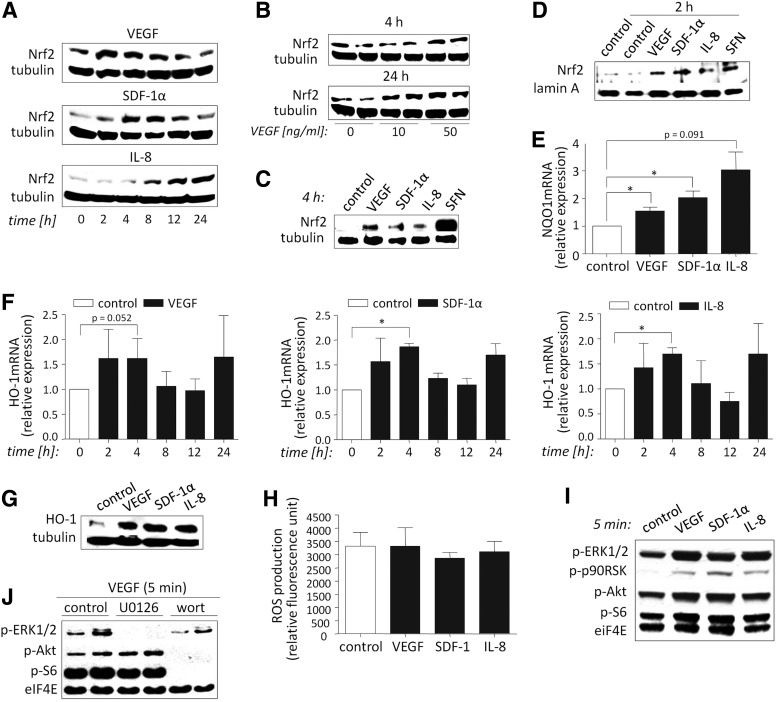
First, we investigated an involvement of Nrf2 in response to angiogenic mediators. As shown in Figure 1A treatment of HMEC-1 with VEGF, SDF-1α, and IL-8 increased Nrf2 protein, while no changes were observed at mRNA level (not shown). The action of VEGF tended to be concentration-dependent especially after 24 h (Fig. 1B). Additionally we excluded the effect of prestimulation by showing similar increase in Nrf2 protein after starvation of cells for 24 h before treatment with cytokines in 2% FBS-containing medium (Fig. 1C). Moreover, we reported nuclear localization of Nrf2 in response to proangiogenic factors (Fig. 1D), while no significant changes in Nrf2 level were detected in the cytoplasmic fraction (not shown). Accordingly, the expression of Nrf2 activity targets antioxidant NAD(P)H:quinone oxidoreductase 1 (NQO1) and HO-1 increased in response to VEGF, SDF-1, and IL-8 stimulation (Fig. 1E–G).
FIG. 1.
Nrf2 is activated in response to proangiogenic stimulation of HMEC-1. HMEC-1 were stimulated with growth factors VEGF (50 ng/ml), SDF-1α (100 ng/ml), and IL-8 (200 ng/ml) for indicated time (2, 4, 8, 12, or 24 h). (A) The level of Nrf2 protein is increased after stimulation with growth factors. (B) The effect of VEGF on Nrf2 protein tends to be dose dependent after 24 h of treatment. HMEC-1 were treated with 10 or 50 ng/ml VEGF for 4 or 24 h. (C) The level of Nrf2 protein is increased after starvation of cells in 2% FBS-containing medium for 24 h and further growth factor stimulation. (A–C) Western blot. Tubulin: control constitutive protein. SFN (2.5 μM): positive control. (D) Growth factors induce Nrf2 localization in the nucleus. Western blot. SFN: positive control. Lamin A: control constitutive protein of nuclear fraction. (E–G) The expression of Nrf2 target genes, NQO1 and HO-1, is increased in response to stimulation with growth factors. (E) 24 h, (F) different time points. RT-PCR; (G) 4 h. Western blot. Tubulin: control constitutive protein. (H) Incubation with growth factors for 2 h does not change the ROS production. DCF fluorescence measurement. (I) Akt and ERK1/2 are activated and their downstream targets S6 and p90RSK tend to be phosphorylated after 5 min of stimulation with growth factors. (J) pAkt/pS6 and pERK1/2 are decreased after incubation of VEGF-stimulated cells with wortmannin and U0126, respectively. Western blot. eIF4E: control constitutive protein; Western blot: representative experiments (nJ=experiment in duplicate); each bar represents the mean±SEM of three to four independent experiments (nH=2) performed in duplicates. *p<0.05, control versus growth factor stimulation. ERK-1/2, extracellular signal-regulated kinase-1/2; HMEC-1, human microvascular endothelial cells; HO-1, heme oxygenase-1; IL-8, interleukin-8; NQO1, NAD(P)H:quinone oxidoreductase 1; Nrf2, nuclear factor E2-related factor 2; ROS, reactive oxygen species; SDF-1, stromal cell-derived factor-1; SFN, sulforaphane; VEGF, vascular endothelial growth factor.
Examining the mechanism potentially responsible for Nrf2 activation, we did not observe any differences in ROS production after 2 h of stimulation with growth factors (Fig. 1H). However, activation (phosphorylation) of extracellular signal-regulated kinase-1/2 (ERK1/2) and Akt (acting downstream of MEK1/2 and PI3K, respectively) increased in response to cytokines tested (Fig. 1I). We noticed also a slight activation of the respective downstream kinase targets, p90RSK and ribosomal protein S6 (Fig. 1I). Moreover, pAkt/pS6 and pERK1/2 were decreased after incubation of VEGF-stimulated cells with kinase inhibitors, wortmannin and U0126 (Fig. 1J), indicating the involvement of PI3K and MEK1/2 signaling, respectively.
Silencing of Nrf2 causes diminished angiogenic potential of HMEC-1
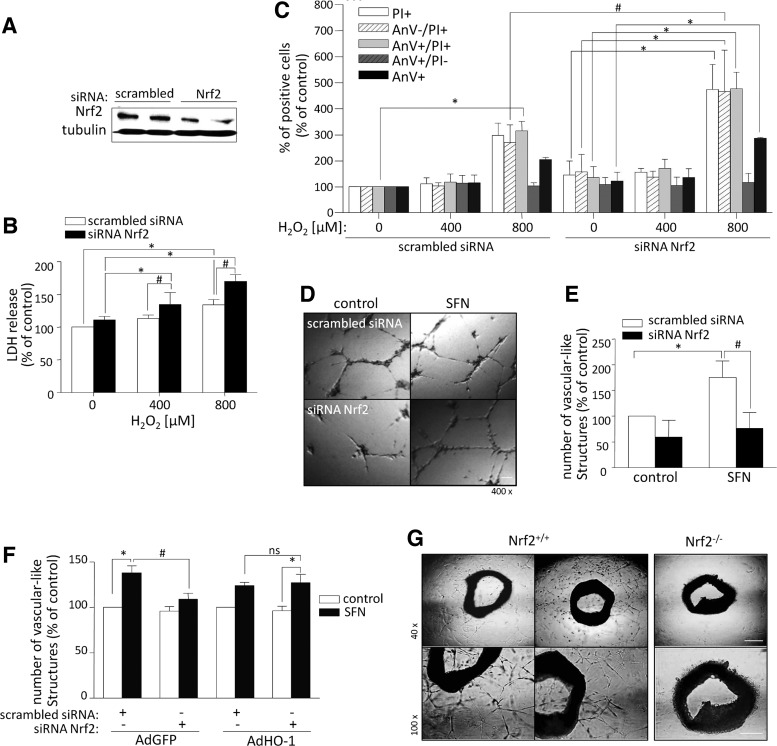
Since the data suggested Nrf2 involvement in angiogenic signal transduction, we further analyzed its influence on angiogenic properties of HMEC-1. Nrf2 expression was decreased using siRNA against human Nrf2 mRNA (Fig. 2A). First, confirming cytoprotective function of Nrf2 (8), we showed H2O2-dependent increase in mortality of Nrf2-silenced cells in comparison to control (Fig. 2B). Accordingly, flow cytometry analysis revealed higher percentage of late-apoptotic and necrotic cells when Nrf2 is silenced (Fig. 2C). Further, activation of Nrf2 by its known inducer sulforaphane (SFN) stimulated vascular-like network formation on Matrigel, while its inhibition attenuated angiogenic response of HMEC-1 (Fig. 2D, E). Importantly, similar effects on viability and vascular-like network formation were observed when we used two other sequences of siRNA against Nrf2 (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars). Moreover, confirming the role of Nrf2 downstream targets, overexpression of HO-1, but not GFP, reversed the effect evoked by Nrf2 silencing (Fig. 2F). Overall, Nrf2 deficiency not only increased the sensitivity of HMEC-1 to oxidative stress but also impaired its angiogenic potential.
FIG. 2.
Silencing of Nrf2 decreases angiogenic potential of HMEC-1 and diminishes angiogenic response of murine aortic endothelium. (A) Nrf2 protein level is decreased after transfection of HMEC-1 for 64 h with siRNA against human Nrf2 mRNA (50 nM). Western blot. Tubulin: control constitutive protein. Representative experiment. (B–E) HMEC-1 were transfected for 48 h with siRNA against human Nrf2 mRNA (50 nM) or scrambled siRNA (50 nM). Nrf2 deficiency increases mortality and the rate of apoptosis/necrosis of HMEC-1 under oxidative stress conditions. (B) LDH release after stimulation with H2O2 for 24 h. (C) The percentage of apoptotic/necrotic cells after stimulation with H2O2 for 24 h. Staining, respectively, for annexin V (AnV) and propidium iodide (PI). Flow cytometry. (D, E) The number of vascular-like structures formed by SFN-treated Nrf2-silenced HMEC-1 is reduced. (D) Representative pictures of vascular-like structures formed by HMEC-1 with silenced Nrf2 and/or after stimulation with 2.5 μM SFN. Culture on Matrigel for 16 h in 2% FBS-containing medium. Magnification 400×(scale bar: 20 μm). (E) Quantitative analysis. (F) HO-1 overexpression reverses the inhibitory effect of siRNA Nrf2 on network formation on Matrigel. Cells were transfected with siRNA Nrf2 for 24 h and then transduced with AdHO-1 or AdGFP as a control for the next 24 h and seeded on Matrigel in the presence of SFN for 16 h. Quantitative analysis. (G) Nrf2 deficiency diminishes capillary formation from murine aortic rings. Aortic rings were isolated from Nrf2+/+ and Nrf2−/− mice and incubated on Matrigel in 2% FBS-containing medium for 12 days. Representative images of vascular structures. Magnification 40×(top panel, scale bar: 200 μm), 100×(bottom panel, scale bar: 100 μm); each bar represents the mean±SEM of three to five independent experiments performed in duplicates or triplicates. *p<0.05, control versus H2O2/SFN; #p<0.05, scrambled siRNA versus siRNA Nrf2.
Lack of Nrf2 decreases angiogenic response of murine aortic endothelium
To verify whether the influence of Nrf2 on angiogenic potential of HMEC-1 is extended to other types of endothelium, we performed aortic ring assay using vessels isolated from Nrf2+/+ and Nrf2−/− mice. We observed significant capillary sprouting from aortic cross segments of wild-type mice, while very modest effect was reported when Nrf2 was absent, pointing at the importance of Nrf2 for angiogenic response of murine aortic endothelium (Fig. 2F). Endothelial phenotype of the sprouts was confirmed by CD31 staining (Supplementary Fig. S2).
PACs lacking Nrf2 reveal higher mortality and defective angiogenic properties
Since our studies indicate the importance of Nrf2 in angiogenic capacity of mature endothelial cells, we also examined its role in potential endothelial progenitors.
BM-derived mononuclear cells (MNCs) were isolated from Nrf2+/+ and Nrf2−/− mice. At day 3 of culture, colonies were formed, which in the absence of Nrf2 had lower growth rate and were smaller than the wild-type counterparts (microscopic observations). Starting at day 7, independently of genotype, majority of cells showed the cobblestone morphology typical for endothelial cells (Supplementary Fig. S3, left).
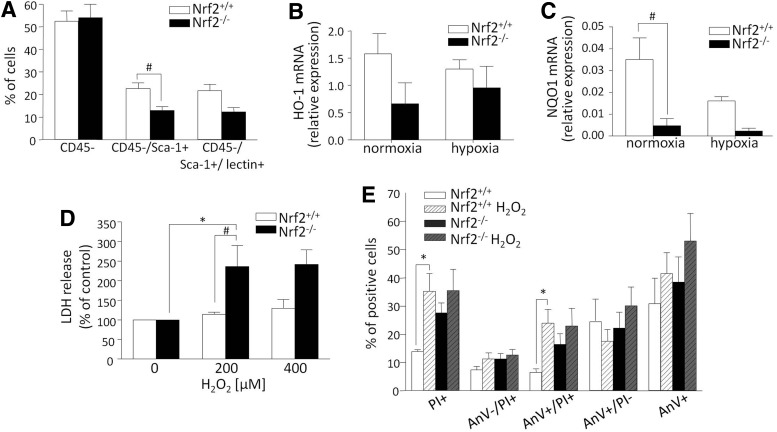
One of the most extensively confirmed characteristics of PACs is their ability to uptake AcLDL and lectin BS-1 binding (6). As shown in Supplementary Figure S3 (right), independently of genotype, majority of in vitro-cultured BM cells were positive in both tests. Further, to examine the percentage of cells of nonhematopoietic/progenitor/endothelial phenotype, using flow cytometry we analyzed surface markers: CD45, Sca-1, and lectin BS-1 binding (Fig. 3A). We gated cells of forward/side scatter characteristics and distinguished three populations: CD45−, CD45−/Sca-1+, and CD45−/Sca-1+/lectin+. CD45-negative cells accounted for about 50% of the whole cultured population. While CD45−/Sca-1+ population was more abundant in case of Nrf2+/+ cells (vs. Nrf2−/−), no genotype-dependent differences were observed concerning other populations (Fig. 3A). Importantly, in our model, cells of endothelial progenitor phenotype (positive for Sca-1 and lectin) represent only a small percentage of all cultured BM-derived cells, implying the heterogeneity of such population, but still, as shown further in this section (Fig. 4C, D), of high angiogenic potential. Therefore, in accordance with the nomenclature used currently more often in the literature (45, 51), the cells are called PACs.
FIG. 3.
PACs lacking Nrf2 reveal lower expression of antioxidant genes and higher mortality under oxidative stress conditions. Mononuclear cells were isolated from bone marrow of Nrf2+/+ and Nrf2−/− mice and cultured for 7–10 days in vitro. (A) Analysis of surface marker expression. Flow cytometry (nNrf2+/+=3, nNrf2−/−=3); Nrf2 deficiency in PACs affects the mRNA level of (B) HO-1 and (C) NQO1. PACs were cultured for 24 h under normoxia or hypoxia (0.5% O2). RT-PCR (nNrf2+/+=3, nNrf2−/−=3). (D, E) PAC survival under conditions of oxidative stress is impaired in the absence of Nrf2. (D) LDH release after stimulation with H2O2 for 24 h (nNrf2+/+=3–9, nNrf2−/−=2–8). (E) The percentage of apoptotic/necrotic cells after stimulation with 200 μM H2O2 for 24 h. Staining, respectively, for annexin V (AnV) and propidium iodide (PI). Flow cytometry (nNrf2+/+=6, nNrf2−/−=6); each bar represents the mean±SEM. *p<0.05, control versus H2O2; #p<0.05, Nrf2+/+ versus Nrf2−/−. PACs, proangiogenic cells.
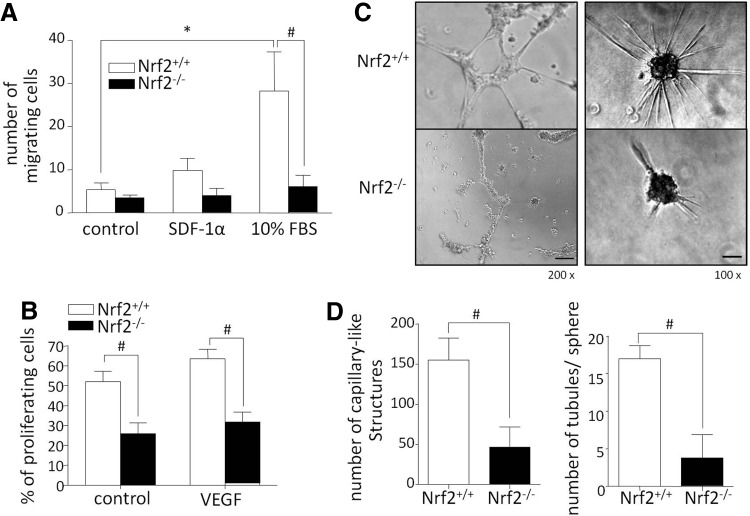
FIG. 4.
PACs lacking Nrf2 reveal defective angiogenic properties. (A) PAC migration is inhibited in the absence of Nrf2. Cells were cultured for 24 h in Boyden chambers in 2% FBS-containing medium. As a chemoattractant, in the bottom of the chamber, 100 ng/ml of SDF-1α or 10% FBS was used. Quantitative analysis of migrating cells. Staining with crystal violet (nNrf2+/+=7, nNrf2−/−=6–7). (B) Proliferation of PAC Nrf2−/− is inhibited. Cells were cultured for 24 h in 2% FBS-containing medium and 50 ng/ml VEGF. Quantitative analysis of proliferating cells (PCNA+) (nNrf2+/+=6, nNrf2−/−=5). (C) The number of vascular-like structures formed by PAC Nrf2−/− is reduced. Representative pictures of networks after 12 h of culture on Matrigel (left) and capillaries after 48 h of spheroidal culture in collagen gel (right) in medium containing 2% FBS. Magnification 200×(left panel, scale bar: 50 μm), 100×(right panel, scale bar: 100 μm). (D) Quantitative analysis of vascular-like structures formed on Matrigel (left) (nNrf2+/+=10, nNrf2−/−=7) and in collagen gel (right) (nNrf2+/+=3, nNrf2−/−=3); each bar represents the mean±SEM. *p<0.05, control versus 10% FBS; #p<0.05, Nrf2+/+ versus Nrf2−/−.
We found that HO-1 tends to be downregulated in the PAC Nrf2−/− both in standard conditions (normoxia) and under hypoxia (Fig. 3B). In such cells we observed also significant decrease in NQO1 under normoxia and a similar tendency under hypoxia (Fig. 3C). Inhibition of antioxidant genes (14, 35) suggests that Nrf2-deficient PACs may be more sensitive to oxidative stress (vs. PAC Nrf2+/+). Indeed, we noted higher mortality of PAC Nrf2−/− treated with H2O2 (Fig. 3D) and a tendency to increase the ROS production both in basal conditions and under oxidative stress (Supplementary Fig. S4). Noteworthy, other reports suggest that PACs are more resistant to oxidative stress than mature endothelial cells (20). However, in our study PAC Nrf2−/− were more sensitive (nearly two times at 400 μM H2O2) than HMEC-1 with silenced Nrf2, suggesting considerable importance of Nrf2 in progenitor cells. One need to remember, however, that not fully silenced Nrf2 (∼90%) could still confer partial protection of HMEC-1. Moreover, the percentage of late-apoptotic and necrotic PACs tended to increase when Nrf2 was absent, but in contrast to PAC Nrf2+/+ no significant further influence of 200 μM H2O2 was noted (Fig. 3E).
Further analysis revealed also the influence of Nrf2 on angiogenic properties of PACs. As shown in Figure 4A and Supplementary Figure S5A the lack of Nrf2 resulted in inhibition of PAC migration in response to 10% FBS and a similar tendency was observed when SDF-1α was used as chemoattractant. Moreover, either basal or VEGF-induced proliferation was diminished in PAC Nrf2−/− (Fig. 4B and Supplementary Fig. S5B). Further PAC Nrf2−/− formed less vascular-like structures on Matrigel in comparison to wild-type cells, which maintained high angiogenic potential (Fig. 4C, D-left). Similarly, using three-dimensional spheroid assay that imitates in vivo conditions, we observed diminished capillary sprouting from spheres formed by Nrf2-deficient PACs in collagen gel (Fig. 4C, D-right). Taking together, these results indicate not only the survival defect of PAC Nrf2−/− but also its impaired angiogenic properties.
Nrf2 deficiency influences angiogenic gene profile in PACs
Differences in angiogenic response of PAC Nrf2+/+ and Nrf2−/− in vitro could imply changes in the functioning of those cells in vivo and influence the processes of recruitment to the damaged/hypoxic tissue.
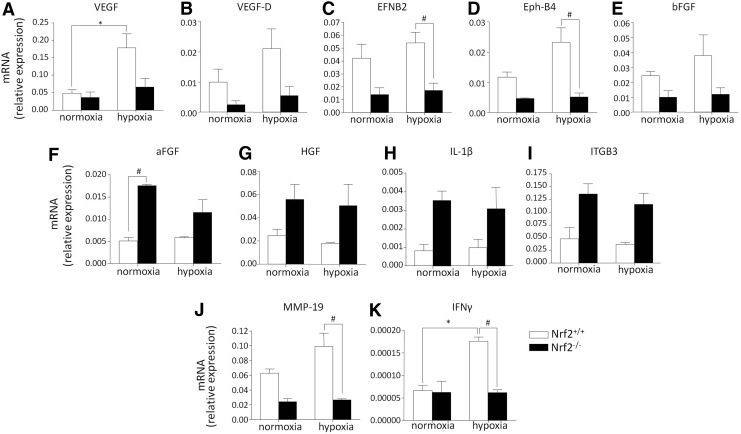
First, to investigate whether PAC Nrf2−/− response to hypoxia is impaired, we performed analysis of angiogenic transcriptome of PACs cultured under normoxia or hypoxia. Among 84 analyzed genes related to neovascularization, Nrf2 deficiency tended to influence the expression of 26 genes in normoxia (9 upregulated, 17 downregulated vs. Nrf2+/+) and 26 in hypoxia (6 upregulated, 20 downregulated vs. Nrf2+/+), decreasing 37, while increasing 15 genes in total (Table 1). Particularly, we revealed that deficiency of Nrf2 in hypoxia might decrease proangiogenic factors (VEGF, VEGF-D, EFNB2, Eph-B4, and bFGF) but, on the other hand, increase other stimulators of neovascularization (aFGF, HGF, IL-1β, and ITGB3) or inhibit anti-angiogenic compounds (MMP-19 and IFNγ) (Fig. 5A–K). Statistically significant genotype-dependent effect was evident for EFNB2, Eph-B4, aFGF, IFNγ, and MMP-19, while the effect of hypoxia for VEGF and IFNγ. Moreover, the lack of Nrf2 convincingly impaired hypoxic response of PACs; hypoxia upregulated (at least twofold) the expression of 14 genes related to neovascularization in wild-type cells while only 4 in PAC Nrf2−/− (Table 1). Overall, the lack of Nrf2 was associated with a decrease in some proangiogenic mediators—what may be related to the impaired angiogenic response in vitro demonstrated earlier—while numerous other agents were upregulated, raising the concerns about the final outcome of the absence of Nrf2 for revascularization processes in vivo.
Table 1.
Angiogenic Transcriptome of Proangiogenic Cell Nrf2+/+ and Nrf2−/−
| Nrf2+/+ | Nrf2−/− | Normoxia | Hypoxia | ||||
|---|---|---|---|---|---|---|---|
| Gene | Hypoxia vs. normoxia | Gene | Hypoxia vs. normoxia | Gene | Nrf2−/− vs. Nrf2+/+ | Gene | Nrf2−/− vs. Nrf2+/+ |
| Increased ratio of mRNA level | |||||||
| Anpep | 2.27 | Fgfr3 | 2.01 | Cxcl1 | 2.42 | Cxcl2 | 2.35 |
| Bai1 | 2.90 | Figf | 2.20 | Cxcl2 | 4.33 | Fgfr3 | 2.15 |
| Cxcl1 | 2.53 | Tgfb3 | 2.62 | Fgf1 | 3.41 | Hgf | 2.76 |
| Cxcl2 | 2.75 | Thbs2 | 2.18 | Fgfr3 | 2.78 | Il1b | 3.14 |
| Tymp | 2.46 | Hgf | 2.27 | Itgb3 | 3.14 | ||
| Fgfr3 | 2.60 | Il1b | 4.34 | ||||
| Figf | 2.11 | Itgb3 | 2.87 | ||||
| Ifng | 2.67 | Lep | 2.52 | ||||
| Pecam1 | 2.25 | Pgf | 2.94 | ||||
| Pgf | 2.62 | ||||||
| Sphk1 | 2.23 | ||||||
| Tbx4 | 2.27 | ||||||
| Tgfb3 | 2.24 | ||||||
| Vegfa | 3.69 | ||||||
| Decreased ratio of mRNA level | |||||||
| Csf3 | 0.30 | Ccl2 | 0.38 | Ccl11 | 0.42 | ||
| Lep | 0.38 | Cxcl5 | 0.30 | Ccl2 | 0.45 | ||
| Efnb2 | 0.33 | Csf3 | 0.24 | ||||
| Ephb4 | 0.40 | Tymp | 0.49 | ||||
| Fgf2 | 0.41 | Efnb2 | 0.32 | ||||
| Figf | 0.25 | Epas | 0.5 | ||||
| Il6 | 0.47 | Ephb4 | 0.22 | ||||
| Mmp19 | 0.39 | Ereg | 0.25 | ||||
| Mmp2 | 0.46 | Fgf2 | 0.31 | ||||
| Mmp9 | 0.20 | Figf | 0.26 | ||||
| Serpinf1 | 0.44 | Ifng | 0.35 | ||||
| Stab1 | 0.47 | Il6 | 0.37 | ||||
| Tgfb2 | 0.36 | Lama5 | 0.44 | ||||
| Tgfb3 | 0.25 | Mmp19 | 0.27 | ||||
| Thbs2 | 0.14 | Sphk1 | 0.41 | ||||
| Timp1 | 0.39 | Tbx4 | 0.34 | ||||
| Timp2 | 0.49 | Tgfb3 | 0.30 | ||||
| Thbs2 | 0.15 | ||||||
| Timp1 | 0.49 | ||||||
| Vegfa | 0.37 | ||||||
PACs were cultured for 24 h under normoxia or hypoxia. The ratios between mRNA level in normoxic and hypoxic conditions or in Nrf2+/+ and Nrf2−/− mice were shown. Changes higher than two times were listed. Transcriptome analysis, PCR array (nNrf2+/+=2–3; nNrf2−/−=2–3).
Nrf2, nuclear factor E2-related factor 2; PACs, proangiogenic cells.
FIG. 5.
Nrf2 level influences the expression of genes involved in neovascularization. PACs were cultured for 24 h under normoxia or hypoxia (0.5% O2). The mRNA level of several genes differentially regulated in PAC Nrf2+/+ and Nrf2−/− is shown (A–K). Transcriptome analysis, PCR array. Each bar represents the mean±SEM (nNrf2+/+=2–3; nNrf2−/−=2–3). *p<0.05, normoxia versus hypoxia; #p<0.05, Nrf2+/+ versus Nrf2−/−.
Proangiogenic capacity of PACs and PAC mobilization in response to ischemia is disrupted in the absence of Nrf2
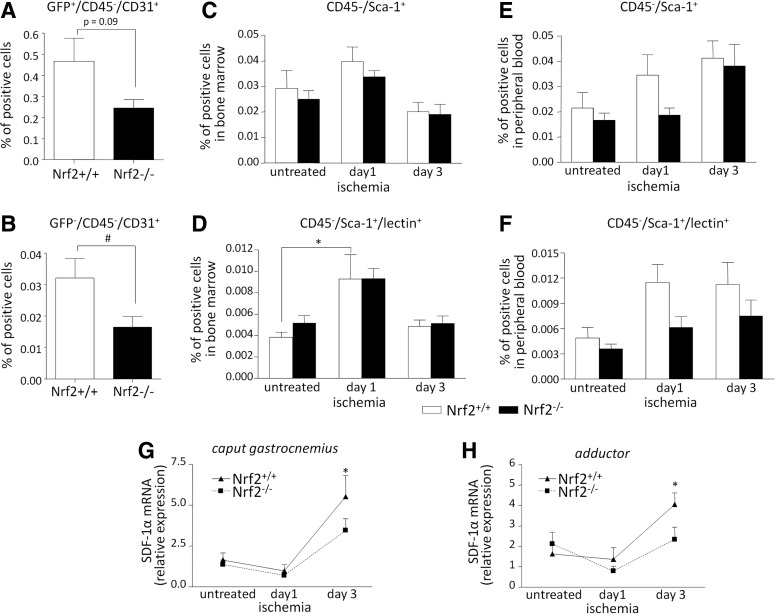
To underscore the relevance of Nrf2 in functioning of PACs in vivo, we further analyzed proangiogenic capacity of PAC Nrf2−/− using Matrigel plug assay. In GFP transgenic mice Nrf2-deficient PACs injected subcutaneously with Matrigel recruited less GFP-positive endothelial cells than that of wild-type PACs (p=0.09; Fig. 6A). Also we found a decreased number of GFP-negative endothelial cells, suggesting inhibited differentiation of injected PAC Nrf2−/− (Fig. 6B).
FIG. 6.
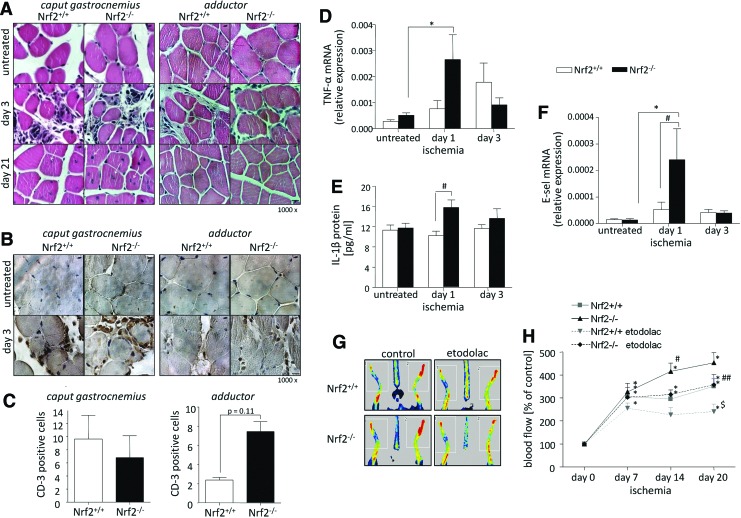
Proangiogenic response and mobilization of PACs are disrupted in Nrf2−/− mice. (A, B) 500,000 of PACs (Nrf2+/+ or Nrf2−/−) were mixed with Matrigel and injected subcutaneously at the ventral side of GFP transgenic mice. At day 15 the percentage of endothelial cells defined as (A) GFP+/CD45−/CD31+ and (B) GFP−/CD45−/CD31+ was determined in the Matrigel plugs. Flow cytometry (nNrf2+/+=8; nNrf2−/−=10). (C–F) Nrf2+/+ and Nrf2−/− mice were subjected to FAL. Preoperatively (untreated) and at days 1 and 3 after surgery, the percentage of the progenitors defined as CD45−/Sca-1+ and CD45−/Sca-1+/lectin+ was determined in the bone marrow (C, D) (nNrf2+/+=8; nNrf2−/−=6–8) and peripheral blood (E, F) (nNrf2+/+=10–12; nNrf2−/−=10–11). Flow cytometry. (G, H) SDF-1α gradient in response to FAL is impaired in Nrf2−/− mice. The ratio of SDF-1α expression at the mRNA level (RT-PCR) in caput gastrocnemius (G) (nNrf2+/+=8–11; nNrf2−/−=9–10) and adductor (H) (nNrf2+/+=5–8; nNrf2−/−=6–8) muscles to the mRNA level of SDF-1α in the bone marrow; each bar represents the mean±SEM. *p<0.05, untreated versus ischemia (appropriate time point), #p<0.05, Nrf2+/+ versus Nrf2−/−. FAL, femoral artery ligation.
To check an impact of Nrf2 on the recruitment of BM-derived progenitors in response to tissue hypoxia, we used HLI model in Nrf2+/+ and Nrf2−/− mice. Preoperatively (untreated) and at days 1 and 3 after FAL we assessed the percentage of PACs defined as CD45−/Sca-1+ and CD45−/Sca-1+/lectin+, in BM and PB (Fig. 6C–F).
In the BM the level of both populations at day 1 after FAL (vs. untreated) was increased either in Nrf2−/− or in Nrf2+/+ mice, with more pronounced differences in case of the latter (p<0.05 for CD45−/Sca-1+/lectin+) (Fig. 6C, D). Here, the weaker influence of ischemia was observed for more generally characterized CD45−/Sca-1+ population (Fig. 6C).
Examining the mobilization of progenitors to PB we observed greater influence of Nrf2 expression than in case of BM (Fig. 6E, F). In both populations tested the number of Nrf2−/− cells (vs. Nrf2+/+) at day 1 after FAL tended to be smaller. Importantly, based on the analysis of blood morphology in control conditions, one could infer that observed changes of the number of progenitors do not arise from changes in immune cell fractions, which were similar regardless of genotype (Supplementary Fig. S6A–E).
It is suggested that mobilization and recruitment of progenitors, including PACs, to the damaged/ischemic tissue may be mediated by SDF-1, which expression increases under conditions of oxygen deficiency (10, 53). Indeed, in accordance with observed tendency of defective mobilization of Nrf2-deficient progenitors, the gradient of SDF-1α produced between the hypoxic hind limb muscles, thigh (adductor) or calf (caput gastrocnemius) muscle, and BM of Nrf2−/− mice after FAL was disrupted in comparison to Nrf2+/+ mice (Fig. 6G, H).
Lack of Nrf2 modulates revascularization after FAL
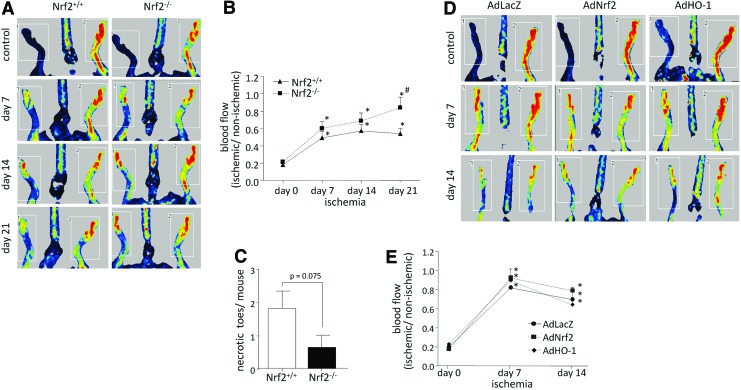
Surprisingly, contrary to expectations, despite some impairment of PAC mobilization, the degree of revascularization of ischemic tissue assessed by blood flow measurement after FAL was better in Nrf2−/− mice (Fig. 7A, B). Although we observed gradually increased perfusion from day 0 to 21 independently of the genotype, such an effect was faster in Nrf2−/− mice (Fig. 7A, B). Importantly, our results confirmed recent report of Ichihara et al. that shows that the ablation of Nrf2 promotes ischemia-induced neovascularization (23). In addition, we noted increased number of necrotic toes of Nrf2+/+ mice (p=0.075 vs. Nrf2−/−) (Fig. 7C), the evaluation of which provides an additional method of estimating the rate of recovery after ischemia (38). However, we did not observe any significant differences in the number of blood vessels (capillaries and small arteries) in the ischemic muscles of Nrf2+/+ and Nrf2−/− mice at day 21 after FAL (not shown).
FIG. 7.
Nrf2 deficiency increases revascularization after FAL. Nrf2+/+ and Nrf2−/− mice were subjected to FAL. (A, B) The ratio of blood flow in the left limb (ischemic) to right limb (non-ischemic) is higher in Nrf2−/− mice. Blood flow was measured 30 min after surgery (day 0) and at days 7, 14, and 21. Laser Doppler perfusion imaging. (A) Representative pictures. (B) Quantitative analysis (nNrf2+/+=15, nNrf2−/−=11). (C) The number of necrotic toes at day 21 after FAL is slightly higher in Nrf2+/+ mice (nNrf2+/+=15, nNrf2−/−=11). (D, E) The rate of perfusion did not change significantly in Nrf2−/− mice after AdNrf2 or AdHO-1 transduction compared with control vectors (AdLacZ). Blood flow was measured 30 min after surgery and injection of adenoviral vectors (day 0) and at days 7 and 14. Laser Doppler perfusion imaging. (D) Representative pictures. (E) Quantitative analysis (n=5–6); each bar/point represents the mean±SEM. *p<0.05, day 0 versus appropriate time point; #p<0.05, Nrf2+/+ versus Nrf2−/−. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
To check whether the enforced expression of Nrf2 or HO-1 would reverse effects of Nrf2 knockout, we injected adenoviral vectors harboring those genes (AdNrf2, AdHO-1 or control, and AdLacZ vectors) to the ischemic muscles of Nrf2−/− mice. However, no significant influence of Nrf2 or HO-1 overexpression was observed (Fig. 7D, E). At day 7, blood flow in the treated limb was already close to control values in the healthy limb. Fourteen days after FAL perfusion, values remained at similar levels (Fig. 7D, E).
Antioxidant capacity is diminished while the pro-inflammatory response is increased in ischemic muscles of Nrf2−/− mice
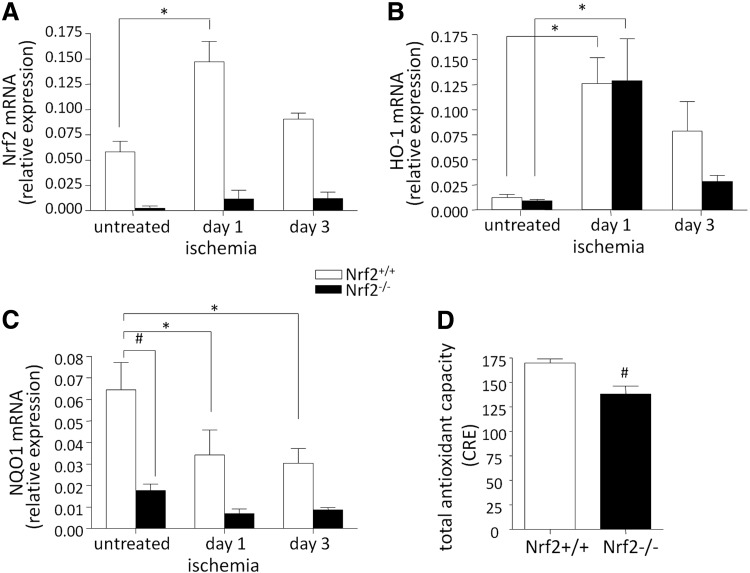
To examine the mechanisms underlying better regeneration of damaged tissue of Nrf2−/− mice, we tested the hypothesis of the impact of inflammatory response, actually suggested earlier by Ichihara et al. (23). Due to the close dependency of inflammation and oxidative stress, first we examined the level of Nrf2 and its antioxidative targets, HO-1 and NQO1, in ischemic muscles (caput gastrocnemius) of Nrf2+/+ and Nrf2−/− mice preoperatively (untreated) and at days 1 and 3 after FAL. We showed that Nrf2 is induced in response to tissue hypoxia at day 1 after surgery in wild-type mice (Fig. 8A), while confirmed an absence of Nrf2 in Nrf2−/− mice (Fig. 8A). The level of HO-1 was increased in Nrf2+/+ muscles at day 1 after FAL (vs. untreated) (Fig. 8B). Interestingly in Nrf2−/− mice, despite the lack of Nrf2, HO-1 expression was also elevated in response to hypoxia (Fig. 8B). On the other hand, irrespective of the genotype, NQO1 tended to be downregulated after FAL, but its basal and ischemic levels were lower in Nrf2−/− mice (Fig. 8C). In the latter also the total antioxidant capacity in ischemic muscles was lower in comparison to Nrf2+/+ mice (Fig. 8D).
FIG. 8.
Lack of Nrf2 changes its target gene expression and antioxidant capacity in ischemic muscles after FAL. Nrf2+/+ and Nrf2−/− mice were subjected to FAL. (A–C) Preoperatively (untreated) and at days 1 and 3 after surgery the mRNA level of, respectively, Nrf2, HO-1, and NQO1 was checked in caput gastrocnemius. RT-PCR (nNrf2+/+=10–12, nNrf2−/−=10–12). (D) Total antioxidant capacity (copper reducing equivalents [CREs]) is lower in Nrf2−/− ischemic muscles (nNrf2+/+=4, nNrf2−/−=4); each bar represents the mean±SEM. *p<0.05, untreated versus ischemia (appropriate time point); #p<0.05, Nrf2+/+ versus Nrf2−/−.
Nonetheless, the overall level of the inflammatory response in ischemic muscles after FAL, assessed by hematoxylin/eosin staining, was similar independently of genotype (Fig. 9A). Inflammatory cell infiltration was evident at day 3 after FAL and higher in caput gastrocnemius than in adductor muscle (Fig. 9A, middle), indicating that the former is more vulnerable to ischemia. Importantly, at day 21 the regeneration of caput gastrocnemius that lacks Nrf2 seemed to be more intensive than its wild-type counterpart (Fig. 9A, bottom), as assessed by centrally located nuclei in muscle fibers. At that time, the adductor appeared completely regenerated, regardless of genotype (Fig. 9A, bottom).
FIG. 9.
Lack of Nrf2 increases inflammatory response after FAL. Nrf2+/+ and Nrf2−/− mice were subjected to FAL. (A) Representative pictures of the morphology of muscles (caput gastrocnemius—left panel, adductor—right panel), untreated and ischemic (days 3 and 21 after FAL). Hematoxylin/eosin staining. Arrows indicate centrally located nuclei of muscle fibers, which suggest intensive regeneration of tissue. Magnification 1000×(scale bar: 10 μm). (B, C) The lack of Nrf2 tends to increase the degree of T cell infiltration into ischemic muscle. (B) Representative pictures of CD3 antigen staining at day 3 after FAL (caput gastrocnemius—left panel, adductor—right panel). Magnification 1000×(scale bar: 10 μm). Immunohistochemistry. (C) Quantitative analysis of CD-3-positive cells (nNrf2+/+=2–3, nNrf2−/−=2). (D–F) Preoperatively (untreated) and at days 1 and 3 after FAL the expression of TNF-α and E-sel (D, F respectively, RT-PCR) was examined in caput gastrocnemius (nNrf2+/+=10–12; nNrf2−/−=10–11), while the protein level of IL-1β was tested in the plasma (E) (Luminex) (nNrf2+/+=11; nNrf2−/−=10–12). (G, H) Nrf2+/+ and Nrf2−/− mice were subjected to FAL and etodolac treatment [10 mg/(kg bw·day−1)]. The ratio of blood flow in the left limb (ischemic) to right limb (non-ischemic) is higher in Nrf2−/− mice, but tends to be reversed after etodolac treatment. Blood flow was measured 30 min after surgery (day 0) and at days 7, 14, and 20. Laser Doppler perfusion imaging. (G) Representative pictures. (H) Quantitative analysis (nNrf2+/+=6, nNrf2−/−=5–6); each bar/point represents the mean±SEM. *p<0.05, untreated versus ischemia (appropriate time point) or day 0 versus appropriate time point; $p<0.05, control (olive oil) versus etodolac; #p<0.05, Nrf2+/+ versus Nrf2−/−; ##p<0.05, Nrf2+/+ etodolac versus Nrf2−/− etodolac. E-sel, E-selectin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Further analysis revealed comparable expression of CD3, a T cell surface marker, among cells infiltrating hypoxic caput gastrocnemius, in Nrf2−/− and Nrf2+/+ mice (Fig. 9B, C-left). However, in adductor, we noticed a tendency toward increased number of CD3-positive cells in Nrf2−/− mice (p=0.11 vs. Nrf2+/+) (Fig. 9B, C-right). Accordingly, we observed augmented expression of proinflammatory cytokine, TNF-α, in ischemic calf muscle, caput gastrocnemius, lacking Nrf2 at day 1 after FAL (Fig. 9D), while IL-1β level increased in the plasma of Nrf2−/− mice (Fig. 9E). Finally, we found stronger hypoxia-dependent induction of the expression of adhesion molecule E-selectin (E-sel) in Nrf2-deficient calf muscle (Fig. 9F).
The study of Ichihara et al. showed an increased cyclooxygenase-2 (COX-2) expression in Nrf2−/− mice (23), suggesting the mechanism of enhancement of inflammatory response in such animals. Therefore, in the next experiment, we treated mice with etodolac [10 mg/(kg bw·day−1)], a non-steroidal anti-inflammatory COX-2 selective inhibitor. Upon such treatment we observed a tendency to diminished blood flow in the Nrf2−/− mice subjected to FAL to the level noted in wild-type counterparts, and decreased revascularisation in ischemic limbs of the latter (Fig. 9G, H). Decreased levels of prostaglandin E2 detected in the skeletal muscle homogenates at the end of the experiment (day 20) confirmed the proper inhibition of COX activity both in Nrf2+/+ (p<0.05) and Nrf2−/− mice (Supplementary Fig. S7). Interestingly, no significant genotype-dependent effects on either COX-1 or COX-2 expression were noted at day 1 or 3 upon ischemic conditions (not shown), what does not confirm the results of Ichihara et al. (23).
Discussion
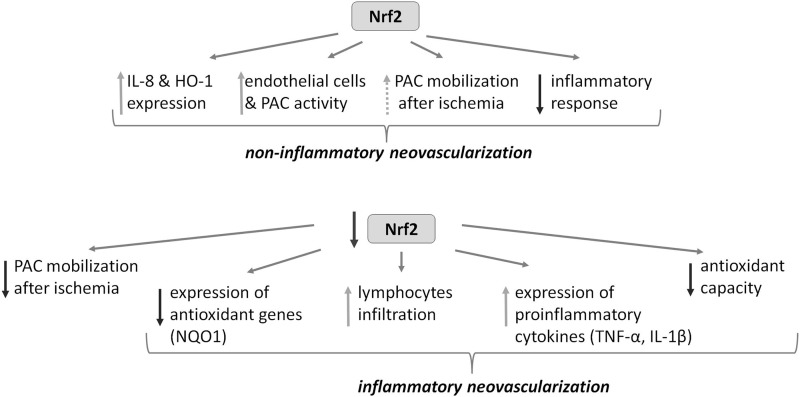
The salient finding of this study is the demonstration of involvement of Nrf2 in angiogenic signal transduction and angiogenic potential of endothelial cells and BM-derived PACs. Treatment of endothelial cells with VEGF, SDF-1, and IL-8 stabilized Nrf2 and induced HO-1 expression. Nrf2 activation stimulated vascular-like network formation, while its inhibition decreased angiogenic response of human endothelial cells, the latter effect reversed by HO-1 overexpression. Moreover, the lack of Nrf2 diminished angiogenic potential of PACs both in vitro and in vivo and inhibited PAC response to hypoxia. Surprisingly, the rate of blood flow recovery in Nrf2-deficient ischemic muscles was better than in wild-type counterparts. It might suggest an involvement of compensatory mechanisms in the absence of Nrf2 in vivo and advantage of neovascularization induced by inflammation, the latter indicated by increased T cell infiltration, expression of adhesion molecules and TNF-α in ischemic muscles, and enhanced oxidative stress (Fig. 10). In accordance, treatment with nonsteroidal anti-inflammatory drug etodolac tended to diminish blood flow in the Nrf2-deficient mice to the level observed in wild-type counterparts.
FIG. 10.
Simplified diagram of Nrf2 participation in the processes of neovascularization and the effects of its deficiency. Nrf2 regulating proangiogenic genes, taking part in the functioning of endothelial cells and BM-derived PACs, and regulating the expression of cytoprotective genes may be an important mediator of neovascularization. However, in the absence of Nrf2 in vivo, following ischemia, angiogenesis associated with oxidative stress and inflammatory response could occur, despite an impaired function and mobilization of PACs. BM, bone marrow.
Our recent results confirmed that Nrf2 induced HO-1 expression in HMEC-1 (36). In addition, reports of both our and other groups indicated that HO-1 may be a mediator of proangiogenic effects of VEGF and SDF-1 (13, 29). We demonstrated that in HMEC-1 treated with VEGF, but also SDF-1 and IL-8, the level of Nrf2 protein and its nuclear localization are increased (with no changes in cytoplasmic fraction), probably through a mechanism involving the activity of ERK1/2 and Akt. In such conditions the level of ROS was not changed. The increase of Nrf2 protein level might be explained by the inhibition of proteasomal degradation of Nrf2 and/or by Akt-dependent activation of ribosomal protein S6, which may correlate with increased translation (43). Then dissociated from Keap1 or newly translated Nrf2 might localize in the nucleus.
Importantly, in accordance with postulated role of Nrf2 in angiogenic signaling pathways, we revealed that its deficiency not only increases the sensitivity of HMEC-1 to oxidative stress but also impairs the formation of vascular-like structures in response to SFN. The latter effect was reversed by overexpression of HO-1, suggesting the involvement of HO-1 in proangiogenic activity of Nrf2. Taking together, it is possible that Nrf2 which regulates HO-1 might take part in growth factor-dependent neovascularization.
In addition to HMEC-1, Nrf2 affected also a proper angiogenic response of murine aortic endothelium since Nrf2 deficiency potently impaired formation of capillaries from the aortic rings.
Although proangiogenic effect of Nrf2 was very modestly tested so far, Valcarcel-Ares et al. reported that in VEGF-treated human coronary artery endothelial cells, silencing of Nrf2 leads to the inhibition of cell proliferation, migration, and vascular-like structure formation on Matrigel (49). Moreover, knockdown of Nrf2 inhibited oxidized phospholipid-induced elevation of VEGF mRNA and endothelial cell sprout formation (2). Here we extend the knowledge on proangiogenic role of Nrf2 by showing its involvement in the response of endothelial cells to cytokines and reporting for the first time its significance in BM-derived PACs. Importantly, we demonstrated that Nrf2-deficient PACs have impaired angiogenic properties in vitro and in vivo.
Decreased survival and slight increase in ROS production of PAC Nrf2−/− under oxidative stress was shown, what could be related to observed decrease in NQO1 and HO-1 expression. Although so far no data have been published concerning the role of Nrf2 in PAC resistance to oxidative stress, it was shown to be protective, for example, in HSPCs (39). Moreover, Nrf2−/− BM demonstrated defective stem cell function, since reduced chimerism after transplantation was observed that was not rescued by antioxidant N-acetylcysteine treatment. Such findings suggest a crucial ROS-independent function of Nrf2 in the hematopoiesis and stem cell survival (39).
Transcriptome analysis revealed a variety of changes in angiogenic gene profile between PAC Nrf2−/− and PAC Nrf2+/+. It could be hypothesized that the participation of PACs in neovascularization in vivo may be determined by the resultant effect of Nrf2 deficiency on the mediators of neovascularization. However, the response of PAC Nrf2−/− to hypoxia is impaired as reported, for example, for VEGF, EFNB2, and Eph-B4. This is consistent with results of Kim et al. that show that Nrf2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1α and in turn VEGF expression (30). Our analyses also indicate for inhibition of HIF-1α activation in hypoxic Nrf2−/− cells (in preparation).
We did not observe, however, significant differences in the level of HGF, IL-1β, or aFGF (upregulated in PAC Nrf2−/−) in the ischemic muscles of Nrf2−/− and Nrf2+/+ mice (not shown). Nonetheless, in vivo Matrigel plug assay revealed that Nrf2 deficiency decreases differentiation potential and angiogenic capacity of PACs. We also observed a tendency to inhibited mobilization of PAC Nrf2−/− to the PB at day 1 after FAL in comparison to PAC Nrf2+/+, without affecting the BM PACs. It could be associated with decreased expression of MMP-9 in the Nrf2-deficient PACs, an enzyme important in the initiation of progenitor mobilization (3, 22) (Table 1). On the other hand, impaired SDF-1 gradient in vivo could be partially responsible for observed defective mobilization of PAC Nrf2−/−.
Surprisingly, but in accordance with previous report of Ichihara et al. (23), we showed that the regeneration of ischemic muscles and restoration of blood flow after FAL is better in Nrf2−/− mice. However, we were not able to detect, observed by the same group, significant augmentation in the number of capillaries and arterioles in the ischemic muscles of Nrf2−/− mice, probably because of different time points analyzed [e.g., 1 week (23) vs. 3 weeks after FAL (this study) in case of arterioles].
Since Nrf2 exerts antioxidative and anti-inflammatory effects (8) and taking into account that in the ischemic tissue the expression of proinflammatory cytokines and related induction of oxidative stress occur (16), the lack of Nrf2 could induce inflammatory revascularization. Indeed, in the ischemic muscles of Nrf2−/− mice, we observed decreased level of antioxidant NQO1 and diminished total antioxidant capacity. Importantly, HO-1 level was elevated both in Nrf2+/+ and Nrf2−/− mice. This, together with an increased T cell infiltration in ischemic muscles (adductor) of Nrf2−/− mice (vs. Nrf2+/+), might promote inflammatory revascularization due to elevated production of angiogenic factors in injured tissues (18). Moreover, we found augmented expression of TNF-α as well as E-sel in ischemic calf muscle and increased IL-1β in the plasma of Nrf2−/− mice what might contribute to neovascularization processes (9, 50). Further analyses indicate that the global production of inflammation-related proangiogenic mediators can be increased in Nrf2−/− mice (not shown). The detailed mechanism is the subject of ongoing study.
Supporting the hypothesis of enhanced inflammatory revascularization and following the data of Ichihara et al. that show increased COX-2 expression in Nrf2−/− mice (23), we demonstrated that administration of etodolac, a COX-2 inhibitor, tends to reduce blood flow recovery after FAL. Since etodolac decreased the revascularization also in wild-type mice, one could assume an advantageous effect of inflammation even in basal conditions. Noteworthy, adenoviral overexpression of neither Nrf2 nor HO-1 changed the perfusion of ischemic limb of Nrf2−/− mice. At the basis of observed differences might lie the systemic effect of etodolac while only local effect of transgenes. Moreover, adenoviral vectors could additionally enhance inflammatory response. Further investigation of Nrf2 gene therapy with other carriers will be the subject of other study.
Ichihara et al. additionally observed reduced expression of thioredoxin 1 and total glutathione concentration, while increased proinflammatory mediators (MCP-1, TNF-α, and COX-2), proangiogenic factors (VEGF and Ang-1), and adhesion molecules in Nrf2−/− thigh muscles after FAL. Accordingly the infiltration of T lymphocytes, macrophages, and CD31-positive cells was noticed (23). In contrast, we did not observe genotype-dependent differences in COX-2 expression. The reason for such variability is not known and this justifies further investigations on the role of Nrf2 in revascularization.
Interestingly, recent studies indicate that TNF-α may inhibit proangiogenic properties of PACs (11). However, because of better tissue revascularization in Nrf2−/− mice, we postulate a minor role of PACs in this experimental setting, especially that PAC Nrf2−/− seemed to be even more sensitive to oxidative stress than mature endothelial cells with silenced Nrf2. Thus, it appears that increased inflammatory response and oxidative stress stimulates tissue repair and may overcome the effects of PAC impairment, resulting in the stronger revascularization in Nrf2−/− mice (Fig. 10). However, the work of Uno et al., showing that the formation of retinal secondary capillary network is delayed in Nrf2−/− mice under non-inflammatory conditions (48), together with our results that indicate proangiogenic activity of Nrf2 provide grounds to assume that Nrf2 could be an important player in physiological, non-inflammatory neovascularization.
Materials and Methods
Cell culture in vitro and incubation experiments
HMEC-1, obtained from Dr. Francisco Candal [Centers for Disease Control and Prevention, Atlanta, (1)], were cultured in MCDB 131 medium (Gibco) under control (normoxic) or hypoxic conditions as described previously (17). Where indicated, 20 μM wortmannin (PI3K inhibitor) and 1 μM U0126 (MEK1/2 inhibitor) were used 1 h before stimulation with VEGF.
Animals and care
Nrf2-deficient C57BL/6 mice (Nrf2−/−) were generated by Prof. Masayuki Yamamoto as described previously (25) and kindly provided by Prof. Antonio Cuadrado (Universidad Autonoma de Madrid) together with control, Nrf2+/+ mice. Mice aged 13–28 weeks (males and females) were used in experiments. Genotypes were verified by PCR. All animal work was approved by the Local Ethical Committee for Animal Research at the Jagiellonian University.
Isolation and in vitro culture of PACs
BM was isolated from tibial and femoral bones of Nrf2+/+ and Nrf2−/− mice, overlaid onto Ficoll Type 400 (Sigma), and centrifuged at 400 g for 45 min. Buffy coat MNCs were collected and resuspended in EBM-2 (Lonza) with EGM-2 MV supplement (Lonza) and 10% FBS. MNCs were seeded onto dishes precoated with 20 mg/ml fibronectin (Sigma)/0.25% gelatin (Sigma). Medium was changed after 3 days. Cells were used for experiments 7–10 days after isolation (5 days for proliferation assessment) and were called PACs. To define phenotype the cells were subjected to flow cytometry to simultaneously detect CD45, Sca-1, and lectin binding (antibodies—see Analysis of BM and PB fractions) or incubated with 10 μg/ml DiI-AcLDL (Biomedical Technologies) or 5 μg/ml FITC-labeled Griffonia (Bandeiraea) simplicifolia lectin BS-1 (Vector Laboratories).
Hind limb ischemia
Ischemic injury was produced by unilateral left FAL in Nrf2+/+ and Nrf2−/− mice. Before surgery, mice were anesthetized by Aerrane and the limb was shaved. Skin incision was made in the middle portion of the left thigh, and after careful dissection of the nerve, the proximal end of the femoral artery and next the distal portion of the saphenous artery were each ligated (27, 38). Immediately after surgery (day 0) and at indicated time points blood flow was measured using Laser Doppler Perfusion Imager (LDPI) System (PIM II; Perimed) over the same region of interest (leg and foot) of both hind limbs. Analyses were performed by calculating the average perfusion for each ischemic and non-ischemic foot.
Where indicated, etodolac (10 mg/kg body weight in 100 μl of olive oil) (cat. No. E0516; Sigma) or control, olive oil, was administered to mice by oral gavage starting 2 days before FAL.
Analysis of BM and PB fractions
Murine BM and PB cells were isolated preoperatively (untreated) and 1 or 3 days after FAL. Cells were gated according to their forward/side scatter characteristics and CD45−/Sca-1+ and CD45−/Sca-1+/lectin+ subsets were assessed by standardized flow cytometric protocol. All blood samples were collected with syringe with heparin. Red blood cell lysis was performed using lysis buffer (BD Pharmalyse). Flow cytometry analysis was performed for Sca-1 (fluorochrome PE; BD Pharminogen), CD45 (fluorochrome APC/Cy7; BioLegend), and lectin (fluorochrome FITC; Vector Labs) (1: 500) using BD LSR II cytometer.
Statistical analysis
Data were analyzed with Graphpad Prism 5 using unpaired Student's t-test when comparing two groups, or a one-way ANOVA followed by a Bonferroni post hoc test for multiple comparisons. All experiments in vitro were performed in duplicate or triplicate and most were repeated at least three times. The number of animals per group was highlighted below appropriate figures. All data are presented as mean±standard error (SEM). Differences were accepted as statistically significant at p<0.05.
Supplementary Material
Abbreviations Used
- BM
bone marrow
- COX-2
cyclooxygenase-2
- CVDs
cardiovascular disorders
- ERK-1/2
extracellular signal-regulated kinase-1/2
- E-sel
E-selectin
- FAL
femoral artery ligation
- Gpx-1
glutathione peroxidase-1
- HIF-1
hypoxia-inducible factor-1
- HLI
hind limb ischemia
- HMEC-1
human microvascular endothelial cells
- HO-1
heme oxygenase-1
- HSPCs
hematopoietic stem progenitor cells
- IL-8
interleukin-8
- MNCs
mononuclear cells
- NQO1
NAD(P)H:quinone oxidoreductase 1
- Nrf2
nuclear factor E2-related factor 2
- PACs
proangiogenic cells
- PB
peripheral blood
- PI3K
phosphoinositide-3-OH kinase
- ROS
reactive oxygen species
- SDF-1
stromal cell-derived factor-1
- SFN
sulforaphane
- VEGF
vascular endothelial growth factor
Acknowledgments
This work was supported by grants N N301 314837, N N301 009639, POIG 01.01.02-00-109/09, and POIG 01.01.02-00-069/09. The Faculty of Biochemistry, Biophysics, and Biotechnology of the Jagiellonian University is a beneficiary of structural funds from European Union (Grants POIG.02.01.00-12 064/08, POIG 01.01.02-00-109/09, POIG.02.02.00-014/08, and 01.01.02-00-069/09). U.F. and A.G-P. are the recipients of the Foundation for Polish Science START Scholarship. A.J. and A.L. are the recipients of MSHE Scholarship. We thank Dr. Ewa Zuba-Surma for technical work with flow cytometry and Mrs. Agata Szade for technical help.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, and Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 99: 683–690, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Afonyushkin T, Oskolkova OV, Philippova M, Resink TJ, Erne P, Binder BR, and Bochkov VN. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol 30: 1007–1013, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, and Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 9: 1370–1376, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, and Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 274: 26071–26078, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, and Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221–228, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, and Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, Inai Y, Silver M, and Isner JM. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18: 3964–3972, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baird L. and Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol 85: 241–272, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Bradley JR. TNF-mediated inflammatory disease. J Pathol 214: 149–160, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, and Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10: 858–864, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Chen TG, Zhong ZY, Sun GF, Zhou YX, and Zhao Y. Effects of tumour necrosis factor-alpha on activity and nitric oxide synthase of endothelial progenitor cells from peripheral blood. Cell Prolif 44: 352–359, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, and Dimmeler S. Antioxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood 104: 3591–3597, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Deshane J, Chen S, Caballero S, Grochot-Przeczek A, Was H, Li Calzi S, Lach R, Hock TD, Chen B, Hill-Kapturczak N, Siegal GP, Dulak J, Jozkowicz A, Grant MB, and Agarwal A. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med 204: 605–618, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinkova-Kostova AT. and Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch Biochem Biophys 501: 116–123, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dulak J, Jozkowicz A, Foresti R, Kasza A, Frick M, Huk I, Green CJ, Pachinger O, Weidinger F, and Motterlini R. Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxid Redox Signal 4: 229–240, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Eliason JL. and Wakefield TW. Metabolic consequences of acute limb ischemia and their clinical implications. Semin Vasc Surg 22: 29–33, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Florczyk U, Czauderna S, Stachurska A, Tertil M, Nowak W, Kozakowska M, Poellinger L, Jozkowicz A, Loboda A, and Dulak J. Opposite effects of HIF-1alpha and HIF-2alpha on the regulation of IL-8 expression in endothelial cells. Free Radic Biol Med 51: 1882–1892, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman MR, Schneck FX, Gagnon ML, Corless C, Soker S, Niknejad K, Peoples GE, and Klagsbrun M. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res 55: 4140–4145, 1995 [PubMed] [Google Scholar]
- 19.Galasso G, Schiekofer S, Sato K, Shibata R, Handy DE, Ouchi N, Leopold JA, Loscalzo J, and Walsh K. Impaired angiogenesis in glutathione peroxidase-1-deficient mice is associated with endothelial progenitor cell dysfunction. Circ Res 98: 254–261, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, and Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol 24: 2021–2027, 2004 [DOI] [PubMed] [Google Scholar]
- 21.He T, Peterson TE, and Katusic ZS. Paracrine mitogenic effect of human endothelial progenitor cells: role of interleukin-8. Am J Physiol Heart Circ Physiol 289: H968–H972, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, and Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 109: 625–637, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichihara S, Yamada Y, Liu F, Murohara T, Itoh K, Yamamoto M, and Ichihara G. Ablation of the transcription factor Nrf2 promotes ischemia-induced neovascularization by enhancing the inflammatory response. Arterioscler Thromb Vasc Biol 30: 1553–1561, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Imanishi T, Tsujioka H, and Akasaka T. Endothelial progenitor cells dysfunction and senescence: contribution to oxidative stress. Curr Cardiol Rev 4: 275–286, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JD, and Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13: 76–86, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jazwa A, Jozkowicz A, and Dulak J. New vectors and strategies for cardiovascular gene therapy. Curr Gene Ther 7: 7–23, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jazwa A, Stepniewski J, Zamykal M, Jagodzinska J, Meloni M, Emanueli C, Jozkowicz A, and Dulak J. Pre-emptive hypoxia-regulated HO-1 gene therapy improves post-ischaemic limb perfusion and tissue regeneration in mice. Cardiovasc Res 97: 115–124, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang M, Wang B, Wang C, He B, Fan H, Guo TB, Shao Q, Gao L, and Liu Y. Angiogenesis by transplantation of HIF-1 alpha modified EPCs into ischemic limbs. J Cell Biochem 103: 321–334, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Jozkowicz A, Huk I, Nigisch A, Weigel G, Dietrich W, Motterlini R, and Dulak J. Heme oxygenase and angiogenic activity of endothelial cells: stimulation by carbon monoxide and inhibition by tin protoporphyrin-IX. Antioxid Redox Signal 5: 155–162, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Kim TH, Hur EG, Kang SJ, Kim JA, Thapa D, Lee YM, Ku SK, Jung Y, and Kwak MK. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1alpha. Cancer Res 71: 2260–2275, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Kozakowska M, Ciesla M, Stefanska A, Skrzypek K, Was H, Jazwa A, Grochot-Przeczek A, Kotlinowski J, Szymula A, Bartelik A, Mazan M, Yagensky O, Florczyk U, Lemke K, Zebzda A, Dyduch G, Nowak W, Szade K, Stepniewski J, Majka M, Derlacz R, Loboda A, Dulak J, and Jozkowicz A. Heme oxygenase-1 inhibits myoblast differentiation by targeting myomirs. Antioxid Redox Signal 16: 113–127, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JM, Hanson JM, Chu WA, and Johnson JA. Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J Biol Chem 276: 20011–20016, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Lin HH, Chen YH, Chang PF, Lee YT, Yet SF, and Chau LY. Heme oxygenase-1 promotes neovascularization in ischemic heart by coinduction of VEGF and SDF-1. J Mol Cell Cardiol 45: 44–55, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Lin HH, Chen YH, Yet SF, and Chau LY. After vascular injury, heme oxygenase-1/carbon monoxide enhances re-endothelialization via promoting mobilization of circulating endothelial progenitor cells. J Thromb Haemost 7: 1401–1408, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Loboda A, Jazwa A, Grochot-Przeczek A, Rutkowski AJ, Cisowski J, Agarwal A, Jozkowicz A, and Dulak J. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 10: 1767–1812, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Loboda A, Stachurska A, Florczyk U, Rudnicka D, Jazwa A, Wegrzyn J, Kozakowska M, Stalinska K, Poellinger L, Levonen AL, Yla-Herttuala S, Jozkowicz A, and Dulak J. HIF-1 induction attenuates Nrf2-dependent IL-8 expression in human endothelial cells. Antioxid Redox Signal 11: 1501–1517, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Losordo DW. and Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation 109: 2692–2697, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Madeddu P, Emanueli C, Spillmann F, Meloni M, Bouby N, Richer C, Alhenc-Gelas F, Van Weel V, Eefting D, Quax PH, Hu Y, Xu Q, Hemdahl AL, van Golde J, Huijberts M, de Lussanet Q, Struijker Boudier H, Couffinhal T, Duplaa C, Chimenti S, Staszewsky L, Latini R, Baumans V, and Levy BI. Murine models of myocardial and limb ischemia: diagnostic end-points and relevance to clinical problems. Vascul Pharmacol 45: 281–301, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Merchant AA, Singh A, Matsui W, and Biswal S. The redox-sensitive transcription factor Nrf2 regulates murine hematopoietic stem cell survival independently of ROS levels. Blood 118: 6572–6579, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Motohashi H. and Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 10: 549–557, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Napoli C, Hayashi T, Cacciatore F, Casamassimi A, Casini C, Al-Omran M, and Ignarro LJ. Endothelial progenitor cells as therapeutic agents in the microcirculation: an update. Atherosclerosis 215: 9–22, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Nijmeh J, Moldobaeva A, and Wagner EM. Role of ROS in ischemia-induced lung angiogenesis. Am J Physiol Lung Cell Mol Physiol 299: L535–L541, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peterson RT. and Schreiber SL. Translation control: connecting mitogens and the ribosome. Curr Biol 8: R248–R250, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, Alitalo K, Weissman IL, and Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A 105: 6620–6625, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spinetti G, Fortunato O, Caporali A, Shantikumar S, Marchetti M, Meloni M, Floris I, Descamps B, Sangalli E, Vono R, Faglia E, Specchia C, Pintus G, Madeddu PR, and Emanueli C. MicroRNA-15a and MicroRNA-16 impair human circulating pro-angiogenic cell (PAC) functions and are increased in the PACs and serum of patients with critical limb ischemia. Circ Res 112: 335–346, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, and Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5: 434–438, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Tongers J, Knapp JM, Korf M, Kempf T, Limbourg A, Limbourg FP, Li Z, Fraccarollo D, Bauersachs J, Han X, Drexler H, Fiedler B, and Wollert KC. Haeme oxygenase promotes progenitor cell mobilization, neovascularization, and functional recovery after critical hindlimb ischaemia in mice. Cardiovasc Res 78: 294–300, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Uno K, Prow TW, Bhutto IA, Yerrapureddy A, McLeod DS, Yamamoto M, Reddy SP, and Lutty GA. Role of Nrf2 in retinal vascular development and the vaso-obliterative phase of oxygen-induced retinopathy. Exp Eye Res 90: 493–500, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, and Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci 67:821–829, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wajant H. The role of TNF in cancer. Results Probl Cell Differ 49: 1–15, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Wara AK, Croce K, Foo S, Sun X, Icli B, Tesmenitsky Y, Esen F, Rosenzweig A, and Feinberg MW. Bone marrow-derived CMPs and GMPs represent highly functional proangiogenic cells: implications for ischemic cardiovascular disease. Blood 118: 6461–6464, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wickersheim A, Kerber M, de Miguel LS, Plate KH, and Machein MR. Endothelial progenitor cells do not contribute to tumor endothelium in primary and metastatic tumors. Int J Cancer 125: 1771–1777, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, and Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation 107: 1322–1328, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Yao EH, Yu Y, and Fukuda N. Oxidative stress on progenitor and stem cells in cardiovascular diseases. Curr Pharm Biotechnol 7: 101–108, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Yu R, Chen C, Mo YY, Hebbar V, Owuor ED, Tan TH, and Kong AN. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J Biol Chem 275: 39907–39913, 2000 [DOI] [PubMed] [Google Scholar]
- 56.Zhang X, Chen X, Song H, Chen HZ, and Rovin BH. Activation of the Nrf2/antioxidant response pathway increases IL-8 expression. Eur J Immunol 35: 3258–3267, 2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.