Abstract
Background: Aging impairs mesenteric lymph flow, which is crucial for fluid and macromolecule homeostasis, fat absorption, and immune function. Previously, we demonstrated that mast cells (MCs) line mesenteric lymphatic vessels (MLVs) with a greater degree of basal activation of MCs in aged mesentery. The number of intact MCs available to react acutely to inflammatory stimuli was decreased with age. However, the role of mast cells in recruiting other immune cells towards MLVs and its aging-associated alterations has not been explored before in great detail.
Methods and Results: In this study we treated live mesenteric tissue isolated from Sprague Dawley (SD) rats, as well as adult 9-mo and aged 24-mo Fischer-344 (F-344) rats for 2 hours with MC activators (48/80 and Substance P) and performed whole mount IHC and vital dye staining of the mesenteric segments containing MLVs to identify immune cell recruitment towards MLVs after mast cell (MC) activation. Number of major histocompatibility complex (MHC) class II positive APCs and eosinophils near MLVs was counted and compared between treatments and ages.
Conclusions: With greater density of MCs near MLVs, we for the first time demonstrated that mesenteric MC activation by compound 48/80 and Substance P resulted in recruitment of MHC class II positive cells and eosinophils towards MLVs. This effect was reduced in cromolyn-injected rats, thus confirming that MCs are necessary for such recruitment. The immune cell presence near MLVs after MC activation was reduced in aged tissues. We link these findings to our previous report of lesser number of intact MCs available for initiating an acute immune response in aged mesentery. Cumulatively, these findings serve as the first step in study of the aging-associated mechanisms that link MCs, lymphatic vessels, and disordered immune function in the elderly.
Introduction
The lymphatic system is crucial for fluid and macromolecule homeostasis, fat absorption, and immune function; deeper understanding of the lymphatic-related components of all of these functions has attracted more researchers in to this field during the last decades.1 Aging creates several challenges to the lymphatic system by altering microenvironment2 and ultrastructure3 of lymphatic vasculature, which may create additional challenges to lymph flow and related functions mentioned above during various disease states.4,5 Aging is associated with impaired lymphatic pumping with a significant decrease in frequency of spontaneous contractions and therefore a consequent decrease in minute productivity of lymphatic vessels.6,7 Aging causes “immunosenescence” that contributes to increased mortality and morbidity in the aged population. Immunosenescence is characterized by a decreased ability of the immune system to recognize and combat foreign antigens, as well as a decreased ability to maintain tolerance to self-antigens. This results in an increased susceptibility to infection, cancer, and reduced responses to vaccination in the elderly.8–12 Research linking decreased lymphatic function with impaired immunity in aging is very sparse. Recently we demonstrated that mast cells (MCs) existing near mesenteric lymphatic vessels (MLVs) exhibit aging-associated increased levels of basal activation. We linked this pre-existing activation of MCs in aged mesentery with previously observed changes in lymphatic contractility in aged MLVs.6,7 Furthermore, we proposed that such high levels of pre-existing activation of MCs would alter the initial phases of acute inflammation and immune response in elderly.13 While considering potential interactions between MLVs and MCs in aged mesenteric tissues during initial phases of acute inflammation and immune response, we attempted to answer the question on general importance of mesenteric MCs located near MLVs for these processes.
It is well established that cells of the immune system are required to communicate between each other through secretion of soluble mediators and direct cell–cell interaction to develop an effective immune response. Among the cells of the immune system, MCs appears to be one of the most versatile in terms of ability to respond to multiple stimuli and to selectively release different types and amounts of mediators. Depending on the type of activation and the combination of stimuli they receive, MCs secrete a diverse range of vasoactive mediators that can trigger, direct, or suppress an immune response. In brief, MC-derived soluble products can be divided into two categories: (a) pre-formed mediators, such as histamine, proteoglycans, and neutral proteases and certain cytokines, in particular tumor necrosis factor-alpha (TNF-α), that are rapidly and instantaneously released upon MC activation; (b) newly synthesized mediators, such as cytokines, chemokines, lipid mediators, growth and angiogenic factors that start to be synthesized after MC activation.14,15 Subsequently, the important MC-dependent initial steps of immune response require the recruitment of other cell types to the site of the pathogen invasion. In particular, the MC-related mediators like histamine, TNF alpha, and PGD2 have been shown to direct dendritic cell migration to lymph nodes, cause activation and maturation of dendritic cells, and modulate dendritic cell capacity to promote T cell responses.16–19 TNF alpha, a preformed cytokine has been shown to increase neutrophil influx, which helps in clearing infectious agents and reducing morbidity and mortality.20,21 Furthermore, pro-inflammatory mediators released by MCs have been found to attract eosinophils to the site of inflammation.22 Eosinophils are known to secrete an array of cytotoxic granules that activate MCs to release histamine, lipid mediators, eicosanoids, and TNF alpha.23,24 Thus MCs and eosinophils together are implicated in the pathogenesis of many allergic diseases.
MLVs have a well-developed population of MCs located in close proximity to them.13 Being located at the border between the biologically aggressive environment of the gut lumen and inner compartments of the abdomen, the inner surface of these vessels provide much greater surface area for initial contact and interaction with inflammatory mediators, products of tissue metabolism, and various foreign and self-antigens than the inner surface of downstream lymph nodes located far from the gut wall. MCs as sentinel cells being localized by MLVs may be considered as ideal candidates to provide an important functional link between MLVs and other immune cells located in the mesenteric tissue. It is already demonstrated that collecting lymphatic vessels play an important role in transportation of antigen-presenting cells (APCs) to lymph nodes where they present antigen to T cells. This process is dependent on factors released by initial lymphatics such as lymphatic endothelium-expressed CCL21–Leu, which serve as a ligand for CCR7 positive dendritic cells (DCs) and helps in migration of APCs from tissue to the initial lymphatic vessels.25,26 However, the functional role of the activation of MCs located by collecting MLVs have not been investigated with respect to the recruitment and activation of major histocompatibility complex (MHC) class II positive APCs and the localization of eosinophils towards collecting lymph vessels. Moreover, the aging-associated changes of these potentially MC-dependent processes that likely occur nearby MLVs have not been studied before.
Research devoted to the role of MCs in aging is limited. There is evidence of increased number of MCs in dermis of skin,27 lung vessels,28 and mesentery with increased degree of basal activation of aged MCs in the mesentery.13 MCs were shown to undergo aging-induced reprogramming, and aged dermal MCs in mice were prone to degranulation with PGE2 unlike that in young mice.29 We hypothesized that acute activation of MCs located near MLVs induce activation and recruitment of MHC class II positive APCs, as well as recruitment of eosinophils towards these collecting lymph vessels, and that there is an aging-related decrease in such acute MC-dependent immune cell mobilization near MLVs in aged mesenteric tissues due to the pre-existing sustained activation of MCs confirmed by us earlier.13
Methods
Animals, surgery, and experimental protocols
For our experiments we used male Sprague Dawley (SD) rats weighing 200–250 g obtained from the Charles River Laboratories International, Inc., and male Fischer-344 (F-344) rats of ages 9 months and 24 months, corresponding to adult age and elderly rats weighing 400–420 g, respectively, obtained from the NIH National Institute of Aging. The rats were anesthetized by 0.3 mL/kg fentanyl/droperidol solution IM and 2.5 mg/kg diazepam IM. All tissue dissections were performed as described below for various subsets of experiments as nonsurvival surgical procedures. All animal procedures for the current studies were reviewed and approved by the Texas A&M Health Science Center Institutional Animal Care and Use Committee.
For the first group of experiments, we investigated the effect of MC activation on recruitment of MHC class II positive APCs, as well as on recruitment of eosinophils towards the collecting mesenteric lymphatic vessels in young healthy SD rats. We hypothesized that acute activation of MCs after treatment with compound 48/80 or Substance P will induce MHC class II positive APC and eosinophil influx towards MLVs, while MC stabilization by cromolyn sodium pretreatment will cause a reduction of such influx. This group of experiments was designed to prove that immune cell presence near MLVs after MC activation is truly a MC-dependent phenomenon. To fulfill the goals of these experiments, in one group of SD rats cromolyn sodium (Catalog #. C-0399, Sigma Aldrich, St. Louis, MO, USA) was dissolved in sterile normal saline and injected intra-peritoneally at a dose of 160 mg/kg body weight30 under aseptic conditions 1 h before the rat was anesthetized. Cromolyn had previously been reported to inhibit histamine secretion from rodent peritoneal MCs.31,32 In another group of SD rats, sterile normal saline of the same total volume as for cromolyn injected-animals was injected intraperitoneally under aseptic conditions 1 h before rats were anesthetized as described before. After anesthesia, a midline abdominal incision was made and the whole gut removed by cutting at the root of the mesentery after clamping it to avoid excess bleeding. After described procedure was completed, all animals were euthanized with an intra-cardiac injection of pentobarbital at a dose of 120 mg/kg.
Identification of MHC class II positive APCs near MLVs in normal saline and cromolyn sodium-injected Sprague Dawley rats in control conditions and after MC activation
In these experiments, we determined the number of MHC class II positive cells near MLVs in control conditions and after MC activation by compound 48/80 or Substance P. After the entire gut with mesentery was cut and removed from saline- or cromolyn-treated animals, it was washed in warm (37°C) PBS (Catalog # 6505; EMD Chemicals, Gibbstown, NJ), divided into three approximately equal size sections that were pinned down in three sylgard-coated petri dishes containing warm (37°C) DMEM F12 culture medium (Catalog # 11039-047; Life Technologies, Carlsbad, CA). They were treated in the following manner: A) the first section of the gut with mesentery in the first dish was immediately fixed with 4% paraformaldehyde (Catalog # 15711; Electron Microscopy Sciences, Hatfield, PA) for 2 h at room temperature; B) the second dish, containing the second section of the gut with mesentery, was treated with MC activator compound 48/80 (10 μg/mL) (Catalog # C2313, Sigma Aldrich), or Substance P (10−5 M) (Catalog # S6883; Sigma Aldrich), in warm DMEM F12 for 2 h at 37°C.13 After treatment for 2 h, the gut with mesentery was fixed with 4% paraformaldehyde for 2 h at room temperature. C) The third dish, containing the third section of the gut with mesentery, was treated with DMEM F12 only for 2 h at 37°C, followed by fixation with 4% paraformaldehyde at room temperature and acted as sham control. Therefore, from one animal we had immediately fixed control tissue section, one MC activator-treated tissue section (Compound 48/80 or Substance P, randomly selected for various animals), and a sham-treated tissue section. Then all fixed tissues from the three petri dishes were washed thrice with 0.3% Triton X-100 (Catalog # T8787; Sigma Aldrich) in phosphate buffered saline (PBS) for 30 min each. Next, pieces of mesentery (with gut dissected out) containing a MLV (from all three sections: control, treated, and sham) were cut and put in a 24-well plate and blocked for 1 h at room temperature by 5% goat serum (Catalog # 005-000-121; Jackson Immunoresearch, West Grove, PA, USA) in 0.1% Triton X-100 in PBS. Incubation with anti–rat MHC II (Catalog # 205401; dilution 1:200, Biolegend, San Diego, CA) was performed to detect MHC class II positive APCs near MLV and was carried out in 0.5% goat serum in 0.1% Triton X-100 overnight at 4°C, followed by washing three times with 0.1% Triton X-100 for 20 min each and incubation with Alexa Flour 488-conjugated goat anti–mouse IgG1 (Catalog # A-21121; dilution 1:200, Life Technologies) for 1 h at room temperature. For some experiments, we co-stained fluorescently mesenteric tissues for MCs to verify that MHC class II positive cells are not MCs. For this purpose we used Texas Red Avidin (Catalog # A-820; dilution 1:200, Life Technologies) for 1 h at room temperature. After washing three times for 20 min each, segments of mesentery were placed on a slide, air dried, and imaged using an Olympus DP72 fluorescent camera and Olympus CKX41 fluorescent microscope equipped with corresponding filters. The above staining provided to us information about the numbers of MHC class II positive APCs near MLVs in basal conditions and after MC activation in mesentery in normal saline and cromolyn sodium-injected SD rats.
Identification of eosinophils near MLVs in normal saline and cromolyn sodium-injected Sprague Dawley rats in control conditions and after MC activation
Afterwards, the slides used for identification of the MHC class II positive cells were stained with alkaline Sirius red for eosinophils33–35 in all groups: control, treated, and sham, as described above. This approach provided information about the presence of eosinophils near MLVs in control conditions and after MC activation by compound 48/80 or Substance P in the mesentery of normal saline and cromolyn sodium-injected SD rats.
A working solution of alkaline Sirius red was prepared by adding 0.5 mg of Direct Red 80 (Catalog # 365548; Sigma Aldrich) to 45 mL of distilled water and 50 mL of molecular grade 100% ethanol (Catalog No. E7023; Sigma Aldrich) and mixed well. One mL of 1% sodium hydroxide was added to make the solution alkaline with a pH>8. An alkaline solution of an anionic dye is attracted only to strongly basic proteins—those rich in arginine. (The guanidino side chain of arginine is a cation even at high pH). Such approach targets alkaline Sirius red staining to eosinophil granules preferentially.33–35 Previously fixed and dried slides were stained in alkaline Sirius red solution for 1 h, washed in tap water thoroughly and then stained with progressive Harris hematoxylin (Catalog # HHS16; Sigma Aldrich) for 3 min and washed thoroughly in tap water and dried. Slides were imaged using an Olympus DP72 fluorescent camera and Olympus CKX41 fluorescence microscope under its bright field mode, and eosinophils identified as red-colored cells against a blue/purple background.
Toluidine blue staining of MCs by MLVs
In a separate set of experiments, we used specific vital dye staining of MCs by Toluidine Blue in order to quantitatively compare the number of MCs located close to and away from MLVs. The segments of mesentery obtained from SD rats containing MLVs were fixed with 4% paraformaldehyde, washed in PBS, mounted on slides and air dried. Dry slides were then stained with an acidic solution of Toluidine Blue (Catalog # 198161, Sigma Aldrich, pH 2.2–2.3) for 5 min, washed with distilled water for 10 min; air dried and imaged using an Olympus DP72 fluorescent camera and Olympus CKX41 fluorescent microscope under its bright field mode. MCs were identified by violet/purple staining of mast cell granules labeled by toluidine blue.13 The number of MCs within one diameter on each side of MLVs was counted and considered as close to MLVs. MCs away from vessel in the clear field of mesentery were counted for the same length of vessel visible in rest of the whole image.
Identification of MHC class II positive APCs and eosinophils near MLVs in 9-mo and 24-mo old F-344 rats in control conditions and after MC activation
In the second group of experiments, we studied the aging-related differences in MHC class II positive APC expression and eosinophil presence near MLVs after MC activation in adult (9-mo) and aged (24-mo) F-344 rats. We hypothesized that due to existence of aging-induced higher basal activation of MCs and subsequently lesser number of MCs being available for acute activation in aged mesenteric tissues;13 there will be a likely decrease in MHC II positive APC and eosinophil presence near aged MLVs after acute experimental MC activation. The average body weight of the animals used in this study was 420–440 g. It should be noted that for the F-344 National Institutes of Aging rat strain, the majority of the weight gain occurs before 7 months of age.36 Therefore, body weights in both the 9-mo and 24-mo age groups were within ranges of weight slightly above 420 g and are not significantly different between these age groups. For identification of MHC II positive APCs and eosinophils near MLVs in 9-mo and 24-mo old F-344 rats in control conditions and after MC activation we used the same experimental approaches as those described above.
Cell count and statistical analysis
All immune cells mentioned above were analyzed as follows: cells located in the field of view within a distance of one diameter on each side of the MLV were manually counted in the respective image taken and values normalized per 1 mm of vessel length. We counted only those cells which were in focus in our images where the focal depth of our optical system was 8.75 μm. Thus all our cell counts were automatically normalized to 8.75 μm of tissue thickness representing at least one half of mesenteric tissue volume, whereas average thickness of rat mesentery has been reported as 17.4 μm.37 Statistical differences were determined by one-way ANOVA and Student's unpaired t-test (JMP software version 9.0.0. for Windows) as appropriate and considered significant at p≤0.05. A minimum of four animals was used for each given type of treatment for each animal age/strain.
Results
Quantification of MC presence close to and away from MLVs
Taking into account the established ability of activated MCs to attract other immune cells,16–19 we hypothesized that higher density of MCs in mesenteric tissues located close to MLVs directs recruitment of other immune cells towards these zones with greater MC presence (i.e., towards MLVs). As described in Methods, we quantitatively compared MC numbers in these zones and found that MCs were present at a 4.5-fold greater density near MLVs than away from vessel, with this difference being statistically significant. Figure 1A demonstrates representative image of the segment of rat mesentery containing a MLV with higher density of MCs by that vessel.
FIG. 1.
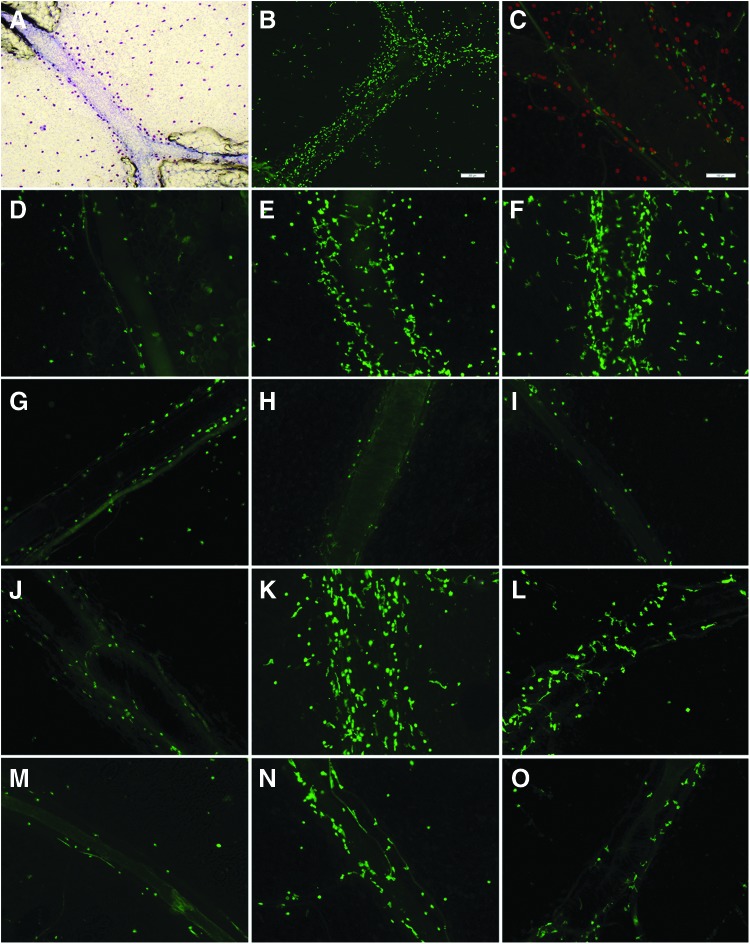
Identification of mast cell-directed increase in presence of MHC class II positive cells by mesenteric lymphatic vessels. (A) Toluidine blue staining of mast cells with higher density of mast cells by MLV. (B) Higher density of MHC class II positive cells by MLV after MC activation by compound 48/80. (C) MHC class II positive cells by MLV (green) are not mast cells (red). (D–O) MHC class II positive APCs by MLV. D–F normal saline-injected SD rat; G–I cromolyn sodium-injected SD rat; J–L normal saline-injected 9-mo F-344 rat; M–O normal saline-injected 24-mo F-344 rat. D, G, J, M: control; E, H, K, N: compound 48/80 treatment; F, I, L, O: Substance P treatment. Scale bar on B corresponds to 200 μm and applies to A and B panels, scale bar on C corresponds to 100 μm and applies to panels C–O.
Identification of MHC class II positive APCs near MLVs in normal saline injected SD rats in control conditions and after MC activation
We found an increase in number of MHC class II positive APCs near MLVs in segments treated with compound 48/80 or Substance P. Figure 1B demonstrates representative image of the segment of rat mesentery in close proximity to MLV vessel with higher density of MHC class II positive APCs by MLV after MC activation by compound 48/80. Although it has been demonstrated that in certain circumstances, a particular subset of MCs may express MHC II protein,38,39 we did not observe MCs expressing MHC II protein in our studies within all segments obtained from various animals in our studies. Staining for MHC class II positive cells along MLV never co-localized with MCs in control or in treated conditions. Figure 1C demonstrates representative image of MHC class II positive APCs and MCs in the same segment of MLV.
We determined that the number of MHC class II positive APCs near MLVs in segments treated with compound 48/80 or Substance P was significantly increased as compared to control. There was a 2.13-fold statistically significant increase in compound 48/80-treated (117±28 MHC class II positive cells/mm of MLV) and a 2.34-fold statistically significant increase in Substance P-treated segments (129±25 MHC class II positive cells/mm of MLV) compared to control in normal saline injected SD rats (55±9 MHC class II positive cells/mm of MLV). (For all experiments for identification of MHC class II positive APCs presented in this and subsequent sections as well as for identification of eosinophils presented below, there was no significant difference in number of MHCII positive APCs or eosinophils near MLVs between control and sham-treated segments. From this, we concluded that experimental conditions did not influence the outcome of the study). Figure 1D–F demonstrates representative images of MHC II positive APCs by MLV in normal saline injected rats in control (D), 48/80-treated (E), and Substance P-treated (F) conditions.
Identification of MHC class II positive APCs near MLVs in cromolyn sodium injected SD rats in control conditions and after MC activation
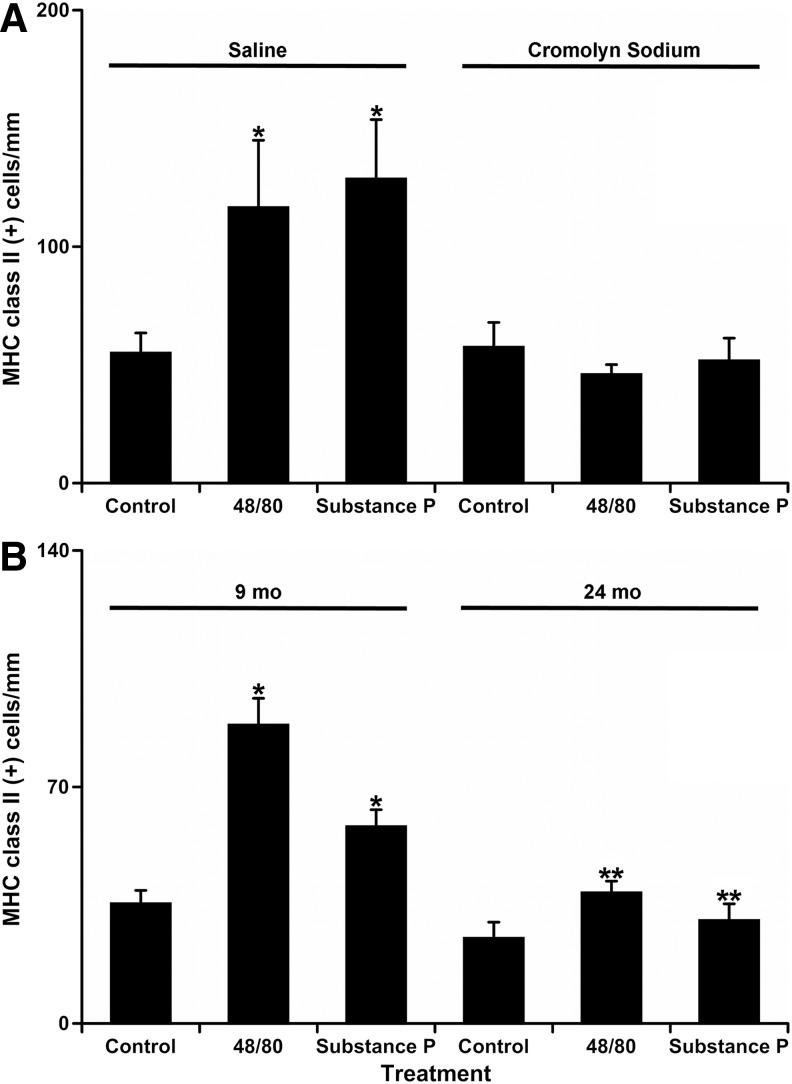
We found that unlike in normal saline injected rats, there was no significant difference in number of MHC class II positive APCs near MLVs between control segments (57±10 MHC class II positive cells/mm of MLV) and segments treated with compound 48/80 (46±4 MHC class II positive cells/mm of MLV) or Substance P (52±10 MHC class II positive cells/mm of MLV) in cromolyn pre-treated rats. In addition, there was no statistically significant difference in cell numbers between normal saline-injected control and cromolyn-injected control segments, thus proving that cromolyn sodium by itself did not influence the results of the study. Figure 1G–I demonstrates representative images of MHC class II positive APCs by MLV in cromolyn injected rats in control (G), 48/80-treated (H), and Substance P-treated (I) conditions. Figure 2A demonstrates the graphical representation of the quantitative analysis of number of MHC class II positive APCs near MLV normalized per 1 mm of MLV length in normal saline and cromolyn sodium-injected SD rats in control conditions and after treatment by compound 48/80 and Substance P.
FIG. 2.
Quantitative analysis of number of MHC class II positive APCs near mesenteric lymphatic vessels in control conditions and after mast cell activation by compound 48/80 and Substance P. (A) Comparison of mesenteric segments from saline and cromolyn sodium-treated SD rats. (B) Comparison of mesenteric segments from 9-mo and 24-mo old F-344 rats. Values are means±SE; * indicates significant differences (p<0.05) before and after treatment in saline-injected SD rats (A) or within same age group; ** indicates significant differences between 9-mo and 24-mo age groups within same treatment.
Identification of MHC class II positive APCs near MLVs in 9-mo and 24-mo old F-344 rats in control conditions and after MC activation
Taking into account our own data presented above, which confirmed that recruitment of MHC II positive APCs towards MLV is mast cell-directed process, we hypothesized that observed aging-associated increase in basal activation of MCs near MLV in 24-mo old animals, together with corresponding decrease in fraction of mast cells available for acute activation,13 will likely lead to decrease in MHC class II positive APC presence near aged MLV after acute experimental MC activation.
We found that the number of MHC class II positive APCs near MLV in control segments in 24-mo old F-344 rats (25±5 MHC class II positive cells/mm of MLV) was 29% lower than that of 9-mo old F-344 rats (35±4 MHC class II positive cells/mm of MLV), but this difference was not statistically significant. In 9-mo old mesenteric segments, MC activation induced a 2.5-fold statistically significant increase in compound 48/80-treated (88±8 MHC class II positive cells/mm of MLV) and a 1.66-fold statistically significant increase in Substance P-treated segments (58±5 MHC class II positive cells/mm of MLV) compared to control segments of 9-mo-old F-344 rats. On the other hand, we discovered that the increase in number of the MHC class II positive APCs was small and not statistically significant in 24-mo-old segments after MC activation by compound 48/80 (39±4 MHC class II positive cells/mm of MLV) as well as by Substance P (30±5 MHC class II positive cells/mm of MLV) when compared to control segments of 24-old F-344 rats. Importantly, we found that the difference in numbers of MHC class II positive APCs near MLV between ages became significantly different after acute treatment with compound 48/80 and Substance P: 56% and 48% lower in 24-mo-old F-344 rats than that of 9-mo-old F-344 rats, respectively. Figure 1J–L demonstrates representative images of MHC class II positive APCs by MLVs in 9-mo-old F-344 rats in control (J), 48/80-treated (K), and Substance P-treated (L) conditions. Figure 1M–O demonstrates representative images of MHC II positive APCs by MLVs in 24-mo-old F-344 rats in control (M), 48/80-treated (N), and Substance P-treated (O) conditions. Figure 2B demonstrates the graphical representation of the quantitative analysis of number of MHC class II positive APCs near MLVs normalized per 1 mm of MLV length in 9-mo and 24-mo F-344 rats in control conditions and after treatment by compound 48/80 and Substance P.
Identification of eosinophils in normal saline-injected SD rats in control conditions and after MC activation
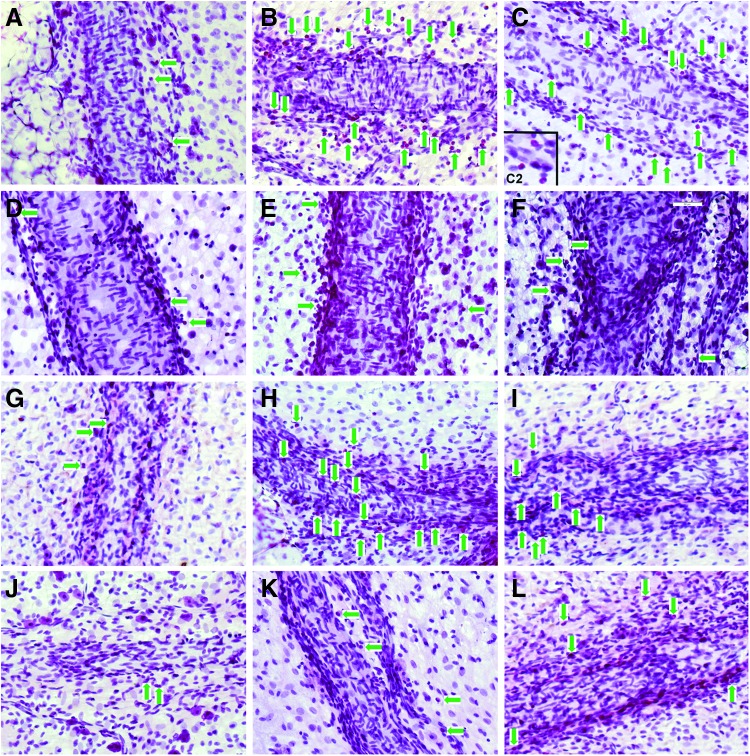
We determined that the increase in number of eosinophils near MLV in segments treated with compound 48/80 or Substance P was significant as compared to control. There was a 5.5-fold statistically significant increase in compound 48/80-treated (44±18 eosinophils/mm of MLV) and a 5.0-fold statistically significant increase in Substance P-treated segments (40±5 eosinophils/mm of MLV) compared to control in normal saline injected SD rats (8±1 eosinophils/mm of MLV). Figure 3A–C demonstrates representative images of eosinophils by MLV in normal saline-injected rats in control (A), 48/80-treated (B), and Substance P-treated (C) conditions.
FIG. 3.
Identification of mast cell-directed increase in the presence of eosinophils by mesenteric lymphatic vessels. Eosinophils marked by green arrows, (not all of these cells pointed by arrows). (A–C) normal saline-injected SD rats; (D–F) cromolyn sodium-injected SD rats; (G–I) 9-mo F-344 rats; (J–L) 24-mo F-344 rats. A, D, G, J: control; B, E, H, K: compound 48/80 treatment; C, F, I, L: Substance P treatment. C2: zoomed image of portion of panel C to demonstrate in closer view the color and shape of eosinophils. Scale bar on F corresponds to 50 μm and applies to all panels.
Identification of eosinophils near MLV in cromolyn sodium-injected SD rats in control conditions and after MC activation
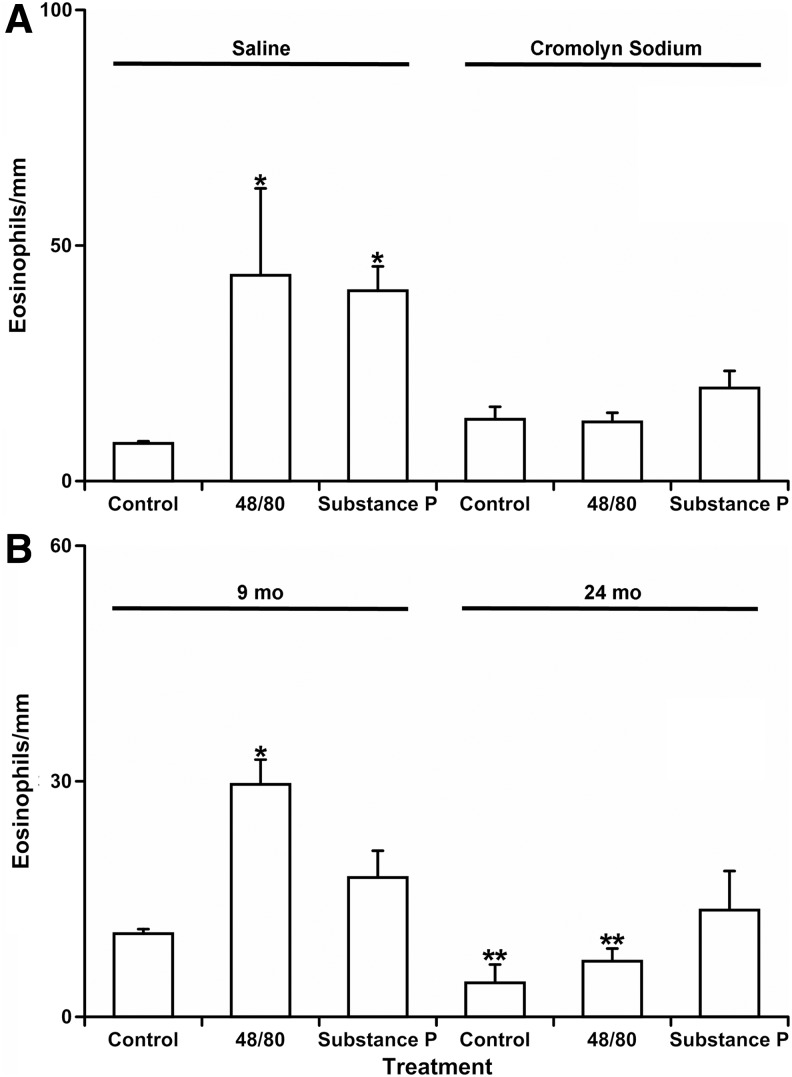
We found that unlike in normal saline-injected rats, there was no significant difference in number of eosinophils near MLV between control segments (13±3 eosinophils/mm of MLV) and segments treated with compound 48/80 (13±2 eosinophils/mm of MLV) or Substance P (20±4 eosinophils/mm of MLV) in cromolyn pre-treated rats. In addition, there was no statistically significant difference in cell numbers between normal saline injected-control and cromolyn-injected control segments. Figure 3D–F demonstrates representative images of eosinophils by MLV in cromolyn injected rats in control (D), 48/80-treated (E), and Substance P-treated (F) conditions. Figure 4A demonstrates the graphical representation of the quantitative analysis of number of eosinophils near MLV normalized per 1 mm of MLV length in normal saline and cromolyn sodium-injected SD rats in control conditions and after treatment by compound 48/80 and Substance P.
FIG. 4.
Quantitative analysis of number of eosinophils near mesenteric lymphatic vessels in control conditions and after mast cell activation by compound 48/80 and Substance P. (A) Comparison of mesenteric segments from saline and cromolyn sodium treated SD rats. (B) Comparison of mesenteric segments from 9-mo and 24-mo old F-344 rats. Values are means±SE; * indicates significant differences (p<0.05) before and after treatment in saline-injected SD rats (A) or within same age group; ** indicates significant differences between 9-mo and 24-mo age groups within same treatment.
Identification of eosinophils near MLV in 9-mo and 24-mo old F-344 rats in control conditions and after MC activation
Taking into account our own data presented above, which confirmed that recruitment of eosinophils towards MLV is mast cell-directed process, we hypothesized that observed aging-associated increase in basal activation of MCs near MLV in 24-mo-old animals, together with corresponding decrease in fraction of mast cells available for acute activation,13 will likely lead to decrease in eosinophil presence near aged MLV after acute experimental MC activation. In these experiments, we treated live mesenteric tissue segments isolated from 9-mo and 24-mo-old F-344 rats with the chemical MCs activator- compound 48/80 and the inflammatory neuropeptide Substance P for 2 h at 37°C.
We found that the number of eosinophils near MLVs in control segments in 24-mo-old F-344 rats (4±2 eosinophils/mm of MLV) was 64% lower than that of 9-mo-old F-344 rats (11±1 eosinophils/mm of MLV), and this difference was statistically significant. In 9-m-old mesenteric segments, MC activation induced a 2.73-fold statistically significant increase in compound 48/80-treated (30±3 eosinophils/mm of MLV) and a 1.64-fold but not statistically significant increase in Substance P-treated segments (18±4 eosinophils/mm of MLV) compared to control segments of 9-mo-old F-344 rats. On the other hand, we discovered that the increase in number of the eosinophils was small and not statistically significant in 24-mo-old segments after MC activation by compound 48/80 (7±2 eosinophils/mm of MLV) as well as by Substance P (14±4 eosinophils/mm of MLV) when compared to control segments of 24-mo-old F-344 rats. Lastly, we found that a difference between numbers of eosinophils near MLV after MC activation by compound 48/80 was 77% lower in 24-mo-old F-344 rats than that of 9-mo-old F-344, and this difference was statistically significant. Figure 3G–I demonstrates representative images of eosinophils by MLVs in 9-mo-old F-344 rats in control (G), 48/80-treated (H), and Substance P-treated (I) conditions. Figure 3J–L demonstrates representative images of eosinophils by MLVs in 24-mo-old F-344 rats in control (J), 48/80-treated (K), and Substance P-treated (L) conditions. Figure 4B demonstrates the graphical representation of the quantitative analysis of number of eosinophils near MLV normalized per 1 mm of MLV length in 9-mo and 24-mo F-344 rats in control conditions and after treatment by compound 48/80 and Substance P.
Discussion
Mast cells have been studied extensively in the past decade and the role of MCs in modulating the innate and adaptive immune responses has been well established.40–43 MCs are known to direct APCs, T lymphocytes, eosinophils, and neutrophils to the site of inflammation as MCs are known to store, produce, and release various vasoactive and pro-inflammatory mediators.42,44,45 In the mesentery, MCs are strategically located close to MLV; such is pre-determined by high likelihood of lymph to carry products of inflammation or microbial invasion from the gut. Such disposition of MCs may help direct immune cells towards MLVs, but careful investigation of this phenomenon has never been performed before. In this study, based on our previous findings on aging-associated decrease in number of MCs available for acute activation,13 we investigated the general effects of MC activation on APC and eosinophil presence near collecting MLVs and evaluated how this process is altered with aging.
Our findings provided for the first time the strong evidence that the increased MHC class II positive cell and eosinophil accumulation near MLVs occur as a result of MC activation. The fact that the density of MCs near MLVs is significantly higher than that away from MLVs in the clear field of mesentery can explain the increase in numbers of MHC class II positive APCs and eosinophils near MLVs. Although MCs were present all over the clear field in the mesentery, MC activation caused more influx of APCs and eosinophils near MLVs than away from them in the clear field of mesentery that also contained numerous blood vessels. It is known that the lymph carried by collecting mesenteric lymphatics is a potent source of foreign as well as auto antigens.46 Recent research has shown that collecting lymphatics are also highly permeable and have similar permeability like that of venules.47 Thus it is likely that antigens carried by lymph can diffuse out of MLVs and activate MCs located in close proximity to them. Hence, increased presence of MHC class II positive APCs near the MLVs after MC activation in acute inflammatory conditions are beneficial to the host, as APCs can sense antigens and become activated to present antigen to T cells in the neighboring lymph node and trigger a subsequent immune response or promote self-tolerance to auto antigens. Besides, activated pro–inflammatory macrophages expressing MHC class II protein are a potent source of iNOS that can release high levels of nitric oxide for a sustained period of time.48,49 High levels of nitric oxide generated by pro-inflammatory or M1 macrophages can modulate lymphatic function in aged lymphatic vessels.50 Increased and sustained release of NO by macrophages and other MHC class II positive APCs therefore may cause lymphatic relaxation, thus helping to delay pumping of lymph containing potential pathogens or inflammatory stimuli. This is likely a protective phenomenon where the host lymphatic system modulates its own pumping to localize infection or inflammation and prevent early onset of disseminated disease, especially in case of an unknown pathogen. Localization of infection also helps in efficient recruitment of innate immune cells and increase their ability and chances of successful neutralization of pathogens. Similarly, eosinophils are also a potent source of cytotoxic granules that have the ability to modulate smooth muscle function,51 and are implicated in the pathogenesis of numerous inflammatory processes, including parasitic helminthic infections and allergic diseases.52,53 Eosinophils also release pro-inflammatory cytokines to direct and promote more immune cell influx into the local tissue,54,55 and effectively present soluble antigens to CD4+ T cells, thereby promoting T cell proliferation and polarization.56 Eosinophils also are known to release major basic protein (MBP), eosinophil peroxidase, and eosinophil cationic protein that can activate MCs to release histamine, PGD2, and TNF alpha.23,55 Several studies have shown that MBP induces mast cell activation via a pathway similar to that observed with other polybasic compounds such as Substance P, compound 48/80, and bradykinin.24 Thus, we believe that the MC activation brings in other immune cells towards the MLV and these cells have the potential to influence lymphatic function and further modulate immune response.
We previously characterized the greatly decreased lymphatic contraction frequency and amplitude (however in lesser degree) of lymphatic contractions in aged mesenteric lymphatic vessels. Owing to such functional changes, the minute productivity of the aged lymphatic vessels undergoes significant decrease.6,7 Since the lymphatic system is involved in fluid balance, lipid absorption, and trafficking of immune cells, this aging-associated impairment of lymphatic function may contribute to many diseases of the aged population. In this study, we investigated for the first time the aging-associated changes in MC-directed activation and recruitment of MHC class II positive APCs and eosinophils near MLVs. We demonstrated that, although there was no significant change in the number of the MHC class II positive APCs near MLVs between adult and aged mesenteric segments in control conditions, these cell numbers increased in greater degree after MC activation by compound 48/80 or Substance P in 9-mo-old tissues and became statistically significant compared to their aged counterparts. This may indicate that basal immune function may be maintained in healthy conditions in aging, but after exposure to acute allergic or inflammatory stimuli there is decreased ability of MCs in aged mesentery to recruit or activate adequate numbers of MHC class II positive APCs towards the MLV. This may have an important consequence in understanding aging-related changes in gastrointestinal immune function. On the other hand, eosinophil numbers are significantly reduced near aged MLV in control as well after MC activated conditions, which may indicate decreased protection against parasitic infestations in the aged population. Future detailed studies will be necessary to expand our understanding of these discussed phenomena.
In spite of the fact that there was a greater degree of basal activation of MCs in the aged mesentery,13 the numbers of MHC class II positive APCs and eosinophils near MLVs was reduced in control conditions in 24-mo rats compared to 9-mo animals. We think that this discrepancy exists because the basal activation of MCs in the aged mesentery as chronic process is not able to promote increased recruitment of other immune cells towards MLVs, whereas acute activation of MCs by allergic or inflammatory stimuli is probably more efficient in directing immune cell influx towards source of these stimuli (i.e., MLVs. Stimuli). Aging is also accompanied by a functional impairment of various innate immune cells,57–61 thus aging-induced functional impairment of MCs, may be one of the reasons why we observed lesser number of MHC class II positive APCs and eosinophils near MLV in 24-mo rat.
Next, we found that aged rats had significantly less ability to bring MHC II positive APCs and eosinophils towards MLVs after experimental acute inflammatory stimulus. This was probably due to the reason that in the aged rat, more mesenteric MCs were in a degranulated state in resting conditions, and thus the number of intact MCs available to react to an acute inflammatory stimulus was decreased.13 This may be one of the causes of lesser number of immune cells seen near MLVs after MC activation. It is important to mention that, although the total number of MCs in the mesentery was ∼ 26% more in aged rat as compared to adult,13 the number of MCs lining MLVs normalized to length of vessel was not statistically different between ages. Hence the above effect could not be attributed to difference in MCs numbers by MLVs in both ages. Also, the aging-related changes in amount and relative proportion of mediators released by MCs after acute activation is unknown and such aging-related changes in mediator content may also play a contributory role in decreased immune cell presence near MLVs. It is also unknown whether levels of cytokines released by lymphatic endothelial cells in aged lymphatic are different from that of adult. Further investigations are necessary in this field of study.
However, the decreased ability of the aged body to recruit/attract MHC class II positive antigen presenting cells and eosinophils acutely to the aged MLV after inflammatory stimulus may indicate that the initial response to acute inflammation is delayed or deranged with aging. Since the acute inflammatory stimulus involved MC activation, on the basis of our current findings we can conclude that MCs located near MLVs undergo aging-related alterations in their functional status and that may contribute to delayed or impaired response in elderly.
In conclusion, in this study we treated live mesenteric tissue isolated from Sprague Dawley rats, as well as adult 9-mo and aged 24-mo F-344 rats for 2 h with MC activators (48/80 and Substance P) and performed whole mount IHC and vital dye staining of the mesenteric segments containing mesenteric lymphatic vessels to identify immune cell recruitment towards MLV after MC activation. Number of MHC class II positive APCs and eosinophils near MLV was counted and compared between treatments and ages. With greater density of MCs near MLV, we for the first time demonstrated that mesenteric MC activation by compound 48/80 and Substance P resulted in recruitment of MHC class II positive cells and eosinophils towards MLVs. This effect was reduced in cromolyn-injected rats, thus confirming that mast cells are necessary for such recruitment. The immune cell presence near MLV after MC activation was reduced in aged tissues. We link these findings to our previous report of lesser number of intact MCs available for initiating an acute immune response in aged mesentery. These findings serve as the first step in understanding the aging-associated mechanisms that link mast cells, lymphatic vessels, and disordered immune function in the elderly.
Acknowledgments
The authors thank Renee M. Lindsey (undergraduate research student, supported by NSF-STEP Grant “The Central Texas 2-STEP”) for her help in data analysis.
Author Disclosure Statement
For all authors, no competing financial interests exist.
This work was supported in parts by the National Institutes of Health (NIH RO1 AG-030578 and HL-094269) and by Texas A&M University Health Science Center College of Medicine and Department of Medical Physiology.
References
- 1.Rockson SG. Lymphatic research: Past, present, and future. Lymphat Res Biol 2009;7:183–187 [DOI] [PubMed] [Google Scholar]
- 2.Thangaswamy S, Bridenbaugh EA, Gashev AA. Evidence of increased oxidative stress in aged mesenteric lymphatic vessels. Lymphat Res Biol 2012;10:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridenbaugh EA, Nizamutdinova IT, Jupiter D, et al. Lymphatic muscle cells in rat mesenteric lymphatic vessels of various ages. Lymphat Res Biol 2013;11:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rockson SG. The aging lymphatics become more mature. Lymphat Res Biol 2013;11:1. [DOI] [PubMed] [Google Scholar]
- 5.Gashev AA, Chatterjee V. Aged lymphatic contractility: Recent answers and new questions. Lymphat Res Biol 2013;11:2–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akl TJ, Nagai T, Cote GL, Gashev AA. Mesenteric lymph flow in adult and aged rats. Am J Physiol Heart Circ Physiol 2011;301:H1828–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagai T, Bridenbaugh EA, Gashev AA. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation 2011;18:463–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nature Immunol 2004;5:133–139 [DOI] [PubMed] [Google Scholar]
- 9.Agrawal A, Agrawal S, Gupta S. Dendritic cells in human aging. Exp Gerontol 2007;42:421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sambhara S, McElhaney JE. Immunosenescence and influenza vaccine efficacy. Curr Topics Microbiol Immunol 2009;333:413–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawelec G, Derhovanessian E, Larbi A. Immunosenescence and cancer. Crit Rev Oncol/Hematol 2010;75:165–172 [DOI] [PubMed] [Google Scholar]
- 12.Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM. Aging of the innate immune system. Curr Opin Immunol 2010;22:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee V, Gashev AA. Aging-associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. Am J Physiol Heart Circ Physiol 2012;303:H693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galli S.J. S. Nakae S, M. Tsai M. Mast cells in the development of adaptive immune responses. Nature Immunology, 2005;6:135–142 [DOI] [PubMed] [Google Scholar]
- 15.Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ. Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses. Curr Opinion Immunol 2006;18:751–760 [DOI] [PubMed] [Google Scholar]
- 16.Gosset P, Bureau F, Angeli V, Pichavant M, Faveeuw C, Tonnel AB, Trottein F. Prostaglandin D2 affects the maturation of human monocyte-derived dendritic cells: Consequence on the polarization of naive Th cells. J Immunol 2003;170: 4943–4952 [DOI] [PubMed] [Google Scholar]
- 17.Pavlinkova G, Yanagawa Y, Kikuchi K, Iwabuchi K, Onoe K. Effects of histamine on functional maturation of dendritic cells. Immunobiology 2003;207:315–325 [DOI] [PubMed] [Google Scholar]
- 18.Dudeck A, Suender CA, Kostka SL, von Stebut E, Maurer M. Mast cells promote Th1 and Th17 responses by modulating dendritic cell maturation and function. Eur J Immunol 2011;41:1883–1893 [DOI] [PubMed] [Google Scholar]
- 19.Simon T, Laszlo V, Falus A. Impact of histamine on dendritic cell functions. Cell Biol Int 2011;35:997–1000 [DOI] [PubMed] [Google Scholar]
- 20.Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 1996;381:75–77 [DOI] [PubMed] [Google Scholar]
- 21.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 1996;381:77–80 [DOI] [PubMed] [Google Scholar]
- 22.Shakoory B, Fitzgerald SM, Lee SA, Chi DS, Krishnaswamy G. The role of human mast cell-derived cytokines in eosinophil biology. J Interferon Cytokine Res 2004;24:271–281 [DOI] [PubMed] [Google Scholar]
- 23.Zheutlin LM, Ackerman SJ, Gleich GJ, Thomas LL. Stimulation of basophil and rat mast cell histamine release by eosinophil granule-derived cationic proteins. J Immunol 1984;133:2180–2185 [PubMed] [Google Scholar]
- 24.Piliponsky AM, Gleich GJ, Bar I, Levi-Schaffer F. Effects of eosinophils on mast cells: A new pathway for the perpetuation of allergic inflammation. Mol Immunol 2002;38:1369. [DOI] [PubMed] [Google Scholar]
- 25.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nature Rev Immunol 2005;5:617–628 [DOI] [PubMed] [Google Scholar]
- 26.Martin-Fontecha A, Lanzavecchia A, Sallusto F. Dendritic cell migration to peripheral lymph nodes. Handbook Exp Pharmacol 2009;188:31–49 [DOI] [PubMed] [Google Scholar]
- 27.Gunin AG, Kornilov NK, Vasilieva OV, Petrov VV. Age-related changes in proliferation, the numbers of mast cells, eosinophils, and cd45-positive cells in human dermis. J Gerontol Biol Sci Med Sci 2011;66:385–392 [DOI] [PubMed] [Google Scholar]
- 28.Migally NB, Tucker A, Greenlees K, Wright M, Zambernard J. Density and ultrastructure of mast cells in lung vessels of aging rats exposed to and recovering from chronic hypoxia. Cell Tissue Res 1983;232:601–608 [DOI] [PubMed] [Google Scholar]
- 29.Nguyen M, Pace AJ, Koller SH. Age-induced reprogramming of mast cell degranulation. J Immunol 2005;175:5701–5707 [DOI] [PubMed] [Google Scholar]
- 30.Dumont N, Lepage K, Cote CH, Frenette J. Mast cells can modulate leukocyte accumulation and skeletal muscle function following hindlimb unloading. J Appl Physiol 2007;103:97–104 [DOI] [PubMed] [Google Scholar]
- 31.Okayama Y, Benyon RC, Rees PH, Lowman MA, Hillier K, Church MK. Inhibition profiles of sodium cromoglycate and nedocromil sodium on mediator release from mast cells of human skin, lung, tonsil, adenoid and intestine. Clin Exp Allergy 1992;22:401–409 [DOI] [PubMed] [Google Scholar]
- 32.Theoharides TC, Patra P, Boucher W, et al. Chondroitin sulphate inhibits connective tissue mast cells. Br J Pharmacol 2000;131:1039–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reibiger I, Spanel-Borowski K. Difference in localization of eosinophils and mast cells in the bovine ovary. J Reprod Fertil 2000;118:243–249 [PubMed] [Google Scholar]
- 34.Wehrend A, Hetzel U, Huchzermeyer S, Klein C, Bostedt H. Sirius red is able to selectively stain eosinophil granulocytes in bovine, ovine and equine cervical tissue. Anatom Histol Embryol 2004;33:180–182 [DOI] [PubMed] [Google Scholar]
- 35.Meyerholz DK, Griffin MA, Castilow EM, Varga SM. Comparison of histochemical methods for murine eosinophil detection in an RSV vaccine-enhanced inflammation model. Toxicol Pathol 2009;37:249–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci 1999;54:B492–501 [DOI] [PubMed] [Google Scholar]
- 37.Barber BJ, Oppenheimer J, Zawieja DC, Zimmermann HA. Variations in rat mesenteric tissue thickness due to microvasculature. Am J Physiol 1987;253:G549–556 [DOI] [PubMed] [Google Scholar]
- 38.Kambayashi T, Allenspach EJ, Chang JT, et al. Inducible MHC class II expression by mast cells supports effector and regulatory T cell activation. J Immunol 2009;182:4686–4695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong J, Yang NS, Croft M, et al. The antigen presentation function of bone marrow-derived mast cells is spatiotemporally restricted to a subset expressing high levels of cell surface FcepsilonRI and MHC II. BMC Immunol 2010;11:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mekori YA, Metcalfe DD. Mast cells in innate immunity. Immunol Rev 2000;173:131–140 [DOI] [PubMed] [Google Scholar]
- 41.Marshall JS, King CA, McCurdy JD. Mast cell cytokine and chemokine responses to bacterial and viral infection. Curr Pharmaceut Design 2003;9:11–24 [DOI] [PubMed] [Google Scholar]
- 42.Marshall JS. Mast-cell responses to pathogens. Nature rev Immunol 2004;4:787–799 [DOI] [PubMed] [Google Scholar]
- 43.Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol 2007;19:31–38 [DOI] [PubMed] [Google Scholar]
- 44.Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol 2004;114:21–27 [DOI] [PubMed] [Google Scholar]
- 45.Dawicki W, Jawdat DW, Xu N, Marshall JS. Mast cells, histamine, and IL-6 regulate the selective influx of dendritic cell subsets into an inflamed lymph node. J Immunol 2010;184:2116–2123 [DOI] [PubMed] [Google Scholar]
- 46.Clement CC, Rotzschke O, Santambrogio L. The lymph as a pool of self-antigens. Trends Immunol 2011;32:6–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scallan JP, Huxley VH. In vivo determination of collecting lymphatic vessel permeability to albumin: A role for lymphatics in exchange. J Physiol 2010;588: 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villalta SA, Nguyen HX, Deng B, Gotoh T, Tidball JG. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Human Mol Genetics 2009;18:482–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho VW, Sly LM. Derivation and characterization of murine alternatively activated (M2) macrophages. Meth Mol Biol 2009;531:173–185 [DOI] [PubMed] [Google Scholar]
- 50.Gasheva OY, Knippa K, Nepiushchikh ZV, Muthuchamy N, Gashev AA. Age-related alterations of active pumping mechanisms in rat thoracic duct. Microcirculation 2007;14:827–839 [DOI] [PubMed] [Google Scholar]
- 51.Jacoby DB, Costello RM, Fryer AD. Eosinophil recruitment to the airway nerves. J Allergy Clin Immunol 2001;107:211–218 [DOI] [PubMed] [Google Scholar]
- 52.Gleich GJ, Loegering DA. Immunobiology of eosinophils. Ann Rev Immunol 1984;2:429–459 [DOI] [PubMed] [Google Scholar]
- 53.Rothenberg ME. Eosinophilia. New Eng J Med 1998;338:1592–1600 [DOI] [PubMed] [Google Scholar]
- 54.Rothenberg ME, Hogan SP. The eosinophil. Ann Rev Immunol 2006;24:147–174 [DOI] [PubMed] [Google Scholar]
- 55.Hogan SP, Rosenberg HF, Moqbel R, et al. Eosinophils: Biological properties and role in health and disease. Clin Exp Allergy 2008;38:709–750 [DOI] [PubMed] [Google Scholar]
- 56.MacKenzie JR, Mattes J, Dent LA, Foster PS. Eosinophils promote allergic disease of the lung by regulating CD4(+) Th2 lymphocyte function. J Immunol 2001;167:3146–3155 [DOI] [PubMed] [Google Scholar]
- 57.Pawelec G, Solana R, Remarque E, Mariani E. Impact of aging on innate immunity. J Leukocyte Biol 1998;64:703–712 [DOI] [PubMed] [Google Scholar]
- 58.Di Lorenzo G, Balistreri CR, Candore G, et al. Granulocyte and natural killer activity in the elderly. Mech Ageing Devel 1999;108:25–38 [DOI] [PubMed] [Google Scholar]
- 59.Renshaw M, Rockwell J, Engleman C, Gewirtz A, Katz J, Sambhara S. Cutting edge: iimpaired Toll-like receptor expression and function in aging. J Immunol 2002;169:4697–4701 [DOI] [PubMed] [Google Scholar]
- 60.Lloberas J, Celada A. Effect of aging on macrophage function. Exp Gerontol 2002;37:1325–1331 [DOI] [PubMed] [Google Scholar]
- 61.Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukocyte Biol 2004;76:291–299 [DOI] [PubMed] [Google Scholar]