Abstract
Objective
A substantial proportion of children with attention-deficit/hyperactivity disorder (ADHD) also display emotion regulation deficits manifesting as chronic irritability, severe temper outbursts, and aggression. The amygdala is implicated in emotion regulation, but its connectivity and relation to emotion regulation in ADHD has yet to be explored. The purpose of this study was to examine the relationship between intrinsic functional connectivity (iFC) of amygdala circuits and emotion regulation deficits in youth with ADHD.
Method
Bilateral amygdala iFC was examined using functional magnetic resonance imaging in 63 children with ADHD, aged 6 to 13 years. First, we examined the relationship between amygdala IFC and parent ratings of emotional lability (EL) in children with ADHD. Second, we compared amygdala iFC across subgroups of children with ADHD and high EL (n = 18), ADHD and low EL (n = 20), and typically developing children (TDC), all with low EL (n = 19).
Results
Higher EL ratings were associated with greater positive iFC between the amygdala and rostral anterior cingulate cortex in youth with ADHD. EL scores were also negatively associated with iFC between bilateral amygdala and posterior insula/superior temporal gyrus. Patterns of amygdala-cortical iFC in ADHD participants with low EL were not different from the comparison group, and the effect sizes for these comparisons were smaller than those for the trend-level differences observed between the high-EL and TDC groups.
Conclusions
In children with ADHD and a range of EL, deficits in emotion regulation were associated with altered amygdala–cortical iFC. When comparing groups that differed on ADHD status but not EL, differences in amygdala iFC were small and nonsignificant, highlighting the specificity of this finding to emotional deficits, independent of other ADHD symptoms.
Keywords: attention-deficit/hyperactivity disorder, amygdala, emotional lability, functional magnetic resonance imaging (fMRI)
Although attention-deficit/hyperactivity disorder (ADHD) is diagnosed solely on the basis of deficits in attention, hyper-activity, and impulsivity, clinicians and parents have long observed that difficulties with emotion self-regulation represent key associated features.1–3 A substantial proportion of children with ADHD exhibit frequent temper outbursts or “rage attacks,” low frustration tolerance, and chronic irritability,4,5 which carry different labels across research groups (e.g., emotional impulsivity,6 emotional lability,7 deficient emotional self-regulation,8,9 negative emotionality,10 or emotional dysregulation11–13). Although these behaviors occur in a number of psychiatric conditions,14 a substantial proportion of children with ADHD exhibit difficulties regulating negative affect.5,7,11,15,16 In 1 sample of 358 children, nearly half of those with ADHD exhibited significantly impairing levels of parent-rated emotional lability.15 Conversely, in a sample of 5- to 9-year-olds recruited solely for concerns regarding severe tantrums, 75% were diagnosed with ADHD.17 Such childhood deficits in emotion regulation appear to persist and predict later-life difficulties.6 Specifically, 1 longitudinal study found that emotional impulsivity uniquely predicted impairment in 7 of 10 major adult life domains in children with ADHD followed into adulthood.6 Furthermore, recent work supports the notion of a familial subtype of ADHD that is particularly characterized by emotion dysregulation.8
Current neurobiological models of ADHD suggest deficits in regulatory processes ascribed to brain areas within prefrontal cortex (PFC) and spanning network nodes throughout sensory and limbic cortex as well as striatal and cerebellar regions.18–20 Such defects in prefrontal regulation have been hypothesized to account for the range of behavioral, cognitive, and emotional symptoms of ADHD.21 This theory is supported by evidence of a thinner cortex in children with ADHD, most prominently in the medial and superior prefrontal and precentral regions involved in cognitive and emotional control.22 Still, most neuroimaging studies have focused on the association between prefrontal control mechanisms and non-affective aspects of ADHD, such as deficits in working memory,23 attention,24,25 cognitive control,26 and response inhibition.27 By contrast, relatively little is known about emotion-related neural mechanisms in ADHD, especially early in development.
The amygdala is essential to the processing and regulation of emotional information and has received considerable experimental attention, both in animal and in human neuroimaging studies. Studies of individuals with ADHD are limited, although they do suggest alteration in amygdala structure and function. For example, children28 and adults29 with ADHD show behavioral deficits in specific functions typically associated with the amygdala, such as facial and contextual emotion processing. Structural neuro-imaging studies provide evidence of ADHD-related differences in amygdala morphometry,30 whereas functional studies in children with ADHD using negative emotional stimuli (i.e., fearful, anxious, angry faces) yield mixed results, with studies demonstrating amygdala hyper-activation,31–33 hypoactivation,34,35 or no differences31,36 relative to healthy comparisons. A recent study found that children characterized as having severe mood dysregulation (SMD), a syndrome with particularly pronounced emotion regulation deficits and high rates of comorbid ADHD, exhibit hypoactivation of the left amygdala in response to neutral faces, relative to control and ADHD groups.32 Of note, 83% of the SMD group were diagnosed with ADHD, which suggests that observed differences in amygdala response were related specifically to emotion dysregulation. A recent study of effective connectivity during a task probing negative emotions showed greater connectivity between amygdala and lateral PFC in unmedicated adolescents with ADHD compared to healthy comparisons, suggesting disruption of amygdala-based circuits.37 Notably, stimulants normalized this connectivity. Thus, although evidence for ADHD-related disruptions in amygdala function and functional connections with PFC is building, the relationship with specific symptoms of emotion dysregulation has received little attention.
Intrinsic, or resting state, functional connectivity (iFC), a measure of the functional synchrony between brain regions independent of a specific task, is being increasingly used to examine functional circuits in ADHD.38,39 Findings of increased orbitofrontal connectivity with striatum and anterior cingulate cortex (ACC) in ADHD relative to comparisons are particularly relevant to emotion regulation.40 However, iFC linked specifically to emotion regulation deficits or to amygdala-based circuitry in ADHD has not been previously examined.
The present study examines how amygdala iFC relates to emotional lability (EL) in children with combined-type ADHD (ADHD-CT). Here, we define EL as the tendency to display rapidly changing emotions. We test the hypothesis that high parent ratings of EL in a large sample of children with ADHD are dimensionally associated with altered iFC between the amygdala and PFC, even after controlling for levels of behavioral dyscontrol, as assessed by ratings of hyperactive-impulsive symptoms. We control for hyperactivity given the high correlation with emotional impulsivity (r = 0.62) in youth with ADHD.6 Furthermore, unlike inattention, hyper-activity, and emotion dysregulation have been found to decline in parallel from preschool to early school age,41 suggesting a shared developmental trajectory. As a secondary means to disentangle the relationship between amygdala iFC and EL, we then establish subgroups of ADHD-CT youth selected for high versus low EL, and compare each to a group of typically developing comparisons (TDC) who have low EL, by definition. We predict that TDCs will differ from the high-EL ADHD subgroup, but not the low-EL ADHD subgroup. We also predict that differences in amygdala iFC will be larger between groups that differ in EL (high-EL ADHD versus TDC) compared to those that differ only on ADHD status (low-EL ADHD versus TDC), further supporting the specific link between amygdala iFC and EL. We note the theoretical advantage of obtaining a high-EL comparison group without ADHD for analyzing the interaction between diagnosis and EL severity. However, we were unable to recruit such children, as ADHD comorbidity is common in children with high EL, and we could not detect high EL in children without psychiatric comorbidities.
METHOD
Participants
We included 63 children with a diagnosis of ADHD-CT (aged 9.2 ± 2.0 years, 50 male and 13 female) who successfully completed both the behavioral and MRI protocols of a larger ongoing study of ADHD (Table 1). In all, 85 participants completed scans and 17 were excluded, before group assignment, from further analyses based on maximum head displacement (>3 mm). An additional 5 were excluded because of excessive mean framewise displacement (>0.25 mm), resulting in the final sample. Participants were recruited on the basis of ADHD symptoms, unrelated to emotional disturbances: thus, the sample had widely distributed EL scores. Children were recruited from those seeking or receiving clinical services at the NYU Child Study Center and through Web and community postings to the general public. All children were right-handed and free of MRI contraindications. Children meeting diagnostic criteria for current major depression, or pervasive developmental disorders, psychosis, substance use disorders, bipolar disorder, or Full Scale IQ <80 were excluded. Current comorbid DSM-IV-TR diagnoses were identified in 17 individuals: oppositional defiant disorder (ODD) (n = 14), anxiety disorder NOS (n = 2), generalized anxiety disorder (n = 1), obsessive compulsive disorder (n = 1), dysthymic disorder (n = 1), enuresis (n = 3), encopresis (n = 2), and tic disorders (n = 1).
TABLE 1.
Clinical and Demographic Characteristics of the Attention-Deficit/Hyperactivity Disorder (ADHD) Group Used for the Dimensional and Subgroup Analysis
| ADHD-CT (n = 63) | High EL (n = 18) | Low EL (n = 20) | TDC (n = 19) | |
|---|---|---|---|---|
| Male, n (%) | 50 (79) | 16 (89) | 14 (70) | 15 (79) |
| Age, y | 9.4 (2.0) | 9.9 (1.6) | 9.5 (1.9) | 10.5 (1.9) |
| Eyes, n open (%) | 28 (44) | 8 (44) | 13 (65) | 11 (58) |
| Race, n (%) | ||||
| White | 29 (46) | 7 (39) | 8 (40) | 12 (63) |
| African American | 10 (16) | 5 (28) | 5 (25) | 4 (21) |
| Asian Pacific Islander | 4 (6) | 0 (0) | 2 (10) | 1 (5) |
| American Indian | 0 | 0 | 0 | 0 |
| Other | 14 (22) | 5 (27) | 5 (25) | 2 (11) |
| Mixed | 6 (10) | 1 (6) | 0 (0) | 0 |
| Ethnicity, n (%) | ||||
| Hispanic | 23 (37) | 9 (50) | 6 (30) | 3 (16) |
| SES, n (%) | ||||
| 1 (low) | 4 (6) | 1 (6) | 1 (5) | 2 (11) |
| 2 | 7 (11) | 4 (22) | 2 (10) | 0 |
| 3 | 7 (11) | 3 (16) | 3 (15) | 3 (16) |
| 4 | 21 (34) | 5 (28) | 5 (25) | 6 (32) |
| 5 (high) | 24 (38) | 5 (28) | 9 (45) | 8 (41) |
| Full Scale IQ | 105.5 (14.6) | 98.9 (16.8) | 109.3 (13.5) | 109.8 (13.7) |
| Maximum movement (mm) | 1.33 (1.5) | 1.15 (2.0) | 1.88 (1.4) | 1.88 (2.0) |
| Mean framewise displacement (mm) | 0.13 (0.05) | 0.13 (0.06) | 0.14 (0.05) | 0.17 (0.02) |
| CPRS-R: Emotional Lability Scalea | 58 (14.6) | 77.9 (8.5) | 44.5 (4.2) | 44.5 (4.3) |
| (range) | (41–90) | (65–90) | (41–54) | (41–55) |
| CPRS-R: Hyperactivity Scaleb | 72 (10.8) | 76.5 (8.4) | 71.8 (11.6) | 45.9 (5.5) |
| (range) | (54–90) | (56–90) | (54–90) | (43–67) |
| Psychiatric comorbidity (%) | 27 | 22 | 15 | 0 |
Note: Means and standard deviations (in parentheses) are presented for each group, unless noted otherwise. ADHD-CT = attention-deficit/hyperactivity disorder, combined type; CPRS-R = Connors Parent Rating Scale–Revised; EL = emotional lability; SES = socioeconomic status.
CPRS-R Emotional Lability (EL) Scale parent ratings (t scores) differed between high-EL and low-EL groups (t36= 14.7, p < .0001) and between typically developing comparisons (TDC) and the high-EL group (t35= −14.8, p < .0001). Skewness = 0.82.
Hyperactivity-impulsivity (t scores) ratings differed between each of the ADHD groups and the comparison group (High EL vs. comparisons: t35 = −13.0, p < .0001 and Low EL vs. comparisons: t36= −8.8, p < .0001). No differences on hyperactivity scores were found between the high- and low-EL groups.
Forty-four children (69%) with ADHD-CT were naive to psychotropic medications. Sixteen children (25%) were taking psychotropic medications during the study period; 14 were taking stimulants, which were suspended for at least 24 hours before the scan, and 1 was taking a nonstimulant plus an antidepressant and another took only a non-stimulant treatment for ADHD. Two participants taking stimulants were also taking combinations of nonstimulant ADHD treatments (n = 1) or antidepressants (n = 1), at the time of the scan. All parents or guardians provided written informed consent as approved by the New York University (NYU) School of Medicine Institutional Review Board. Children provided written assent. Families received financial compensation for participation.
To further assess the specificity of amygdala iFC deficits to EL, in a secondary analysis, functional magnetic resonance imaging (fMRI) data were obtained from 26 TDC children with no DSM-IV-TR Axis I diagnosis. Seven of these children were excluded because of excessive movement (mean framewise displacement >0.25), resulting in a final sample of 19 children. Study exclusions were the same as for the children with ADHD, and the groups were matched for sex and age.
Assessments
For all participants, the presence or absence of ADHD and comorbid diagnoses was determined by licensed psychologists/psychiatrists or by supervised postdoctoral fellows based on the results of diagnostic evaluations conducted with the Kiddie SADS–Present and Lifetime Version42 semistructured interview of parents and children. Cases were regularly presented at weekly case conferences at which diagnostic consensus was achieved. Diagnostic procedures integrated K-SADS parent and child reports, school reports, prior mental health records, as well as teacher feedback on the Conners’ Teacher Rating Scale–Revised, Long Version (CTRS-R:L43), which was available for 89% of the sample. Estimates of intelligence were obtained using an abbreviated IQ screener.44 A parent also completed the Conners’ Parent Rating Scale–Revised, Long Version (CPRS-R:L43). Age- and sex-adjusted t scores were calculated for each subscale (possible scores = 38–90; mean = 50).
For the purposes of this study, we indexed EL using the Emotional Lability (J Scale) of the CPRS-R-L Global Index. This scale comprises the items: “Temper outbursts,” “Cries often and easily,” and “Mood changes quickly and drastically.” This scale has been normed in nearly 2,000 male and female individuals aged 6 to 17 years, with high internal consistency coefficients (0.7) and 6- to 8-week test–retest reliability (0.7).43 High discriminant validity was established between youth with ADHD, those with clinically derived “emotional problems” and a nonclinical sample.43 The scale has been used extensively in recent research as a measure of emotional problems.15, 45–47 The CPRS-R-L Hyper-activity subscale (C Scale) was used as a covariate to control for symptoms of behavioral dysregulation. Although EL is typically less highly correlated with inattention, supplementary findings used both inattention and hyperactivity as covariates, as indexed by the CPRS-R-L DSM Scale (N Scale).
MRI Data Acquisition
Imaging data were collected on a 3.0 Tesla Siemens Allegra at the NYU Center for Brain Imaging. Each participant completed at least one 6.5-minute resting-state fMRI scan within a 45-minute session to collect iFC data. These functional scans were collected using a customized multi-echo echo planar imaging (EPI) sequence (repetition time [TR] = 2,000 ms; effective echo time [TE] = 33 ms; flip angle = 90°, 33 slices, matrix = 64 × 64; field of view [FOV] = 240 × 192 mm; acquisition voxel size = 3 × 3 × 4 mm; number of volumes = 197). For spatial normalization and localization, high-resolution T1-weighted anatomical scans were acquired using a magnetization prepared gradient-echo sequence (TR = 2,530 ms, TE = 4.35 ms, inversion time = 1100 ms, flip angle = 7°, 128 slices, field of view = 256 mm, voxel size = 1.3 × 1.3 × 1 mm). Because of the ongoing study design, 2 sets of instructions were used: some participants were asked to keep their eyes open during the scans (viewing a black screen with a centered fixation cross), whereas others were asked to keep their eyes closed (Table 1). Given its potential impact on iFC indices, eye status was included as a covariate in all group-level analyses.
Image Preprocessing
For participants with more than 1 iFC scan within a session, the scan with the smallest amount of maximum calculated motion was selected for analysis. For the majority of subjects (96%), the first scan was used. Only scans with less than 3 mm of maximum head displacement in any axis were analyzed. As micromovements have been shown to potentially introduce artifactual correlations, mean framewise displacement (FD) was also computed, per recently described methods.48 Subjects with mean FD >0.25 mm were excluded from further analyses and each subject mean FD was included as a covariate in group-level analyses. Groups did not differ on mean FD (Table 1), and mean FD was not correlated with EL scores for ADHD or typically developing comparisons.
Preprocessing techniques were completed using AFNI (http://afni.nimh.nih.gov) and FSL software (www.fmrib.ox.ac.uk). Preprocessing consisted of the following; slice time correction for interleaved acquisitions using Fourier interpolation; 3-dimensional motion correction using least-squares alignment of each volume to the mean image using Fourier interpolation; despiking of extreme time series outliers using a continuous transformation function; temporal band-pass filtering between 0.009 and 0.1 Hz using Fourier transformation; spatial smoothing (Gaussian kernel full width at half maximum = 6 mm); mean-based intensity normalization of all volumes by the same factor; and linear and quadratic detrending. Nuisance signals from 6 motion parameters, white matter, and cerebrospinal fluid (derived from appropriate masks), and the global signal (mean across all voxels in the brain) were removed to control for movement and physiological processes (e.g., fluctuations related to cardiac and respiratory cycles and large-scale neural signals). Linear registration of the high-resolution structural images to the MNI152 template was carried out using the FSL tool FLIRT with 12 degrees of freedom (df) and refined using FNIRT nonlinear registration. Linear registration of each participant’s functional time series to the high-resolution structural image was performed using FSL’s FLIRT (6 df). Functional-to-anatomical co-registration was improved by intermediate registration to a low-resolution image and b0 unwarping. These preprocessing steps resulted in a 4-dimensional (4D) residual functional volume in native functional space, for each participant. For group comparisons, each participant’s 4D residual volume was spatially normalized by applying the calculated transformation to MNI152 standard space (2 × 2 × 2-mm resolution).
Region-of-Interest Selection
We defined regions-of-interest (ROIs) representing the whole amygdala bilaterally based on the Juelich histological atlas implemented in FSL. Similar to prior work,49 we used masks that included all voxels with a minimum 50% likelihood of being correctly located in the amygdala. We considered analyzing individual subdivisions of the amygdala, as we have done previously in adults and adolescents.49, 50 However, given the young age of our sample and the lack of research on the iFC of amygdala subdivisions in typical children or those with ADHD, we chose a whole-amygdala approach.
Participant Level Analyses
Left and right amygdala time series were extracted from the 4D preprocessed resting state scan in MNI152 standard space by separately averaging across all voxels in each ROI. Next, we calculated the correlation between these time series and those of each voxel in the preprocessed resting state scan in native space. This analysis was implemented using 3dfim+ (AFNI). Individual participant-level correlation maps were converted to Z-value maps using Fisher’s r-to-z transformation and then transformed into MNI152 2mm standard space. This analysis produced subject-level maps of voxelwise correlations with the time-series of the left and right amygdala ROIs.
Group-Level Analyses
First, group-level mixed-effects analyses were carried out using FSL FEAT for the right and left amygdala ROI for all ADHD participants. Our main analysis included demeaned CPRS-R:L EL T-scores as the primary variable of interest, and sex, age, mean FD, and eye-status (open/closed) as nuisance covariates. Because the aim of the study was to examine EL iFC independent of symptoms of behavioral dysregulation, demeaned scores on the CPRS-R:L Hyperactivity scale were also included in the model. In supplementary analyses, overall DSM ADHD symptoms (inattention + hyperactivity) were used as a covariate instead of hyperactivity, to confirm the specificity of the findings to EL and not to other symptoms of ADHD. Corrections for multiple comparisons were carried out at the cluster level using Gaussian random field theory (voxel-wise: minimum Z score >2.3; cluster significance: p < 0.05, corrected). This group-level analysis produced thresholded Z-score maps indicating clusters where iFC with each ROI was significantly related to EL scores.
To further confirm the specificity of our findings, iFC in clusters showing a significant relationship between EL and amygdala iFC was then compared to that of TDCs (n = 19). To this end, we extracted 2 subgroups from the ADHD group (n = 63) used in the primary analysis: participants with EL T scores in the clinically significant range (>65) comprised the high-EL group (n = 18); and a demographically matched group (n = 20) with nonclinical EL T scores (<55) comprised the low-EL group. Because there were more participants with scores in the low than in the high-EL range, 26 were pseudorandomly excluded, blinded to EL score, to allow for age and gender matching and similarly sized groups. EL T scores were significantly different (p < .0001) between the 2 ADHD groups.
We extracted the average Fisher’s Z correlation co-efficients for significant EL-iFC clusters from the initial ADHD-only EL analyses from each participant’s preprocessed resting state scan in MNI152 standard space. These correlation coefficients were averaged across clusters such that 3 values (left amygdala positive contrasts and right and left amygdala negative contrasts) were used for group comparisons. Because the iFC measures were derived based on the EL scores in the ADHD group, univariate analyses of variance including all 3 groups (high-EL ADHD, low-EL ADHD, and low-EL TDCs) would have necessarily been significant. Thus, we conducted independent-sample t tests (SPSS 19.0; IBM, Chicago, IL) between TDCs and each of the 2 ADHD groups. Comparisons between the low- and high-EL groups were not examined for the same reason that an analysis of variance was not used. To account for the 3 between-group comparisons, a Bonferroni-corrected statistical threshold was applied (p < .017). Measures of effect size were obtained from independent samples t tests comparing the TDC group to the low- and high-EL ADHD groups on the three measures of amygdala iFC (SPSS 19.0; IBM, Chicago, IL). Using previously published methods,51,52 we compared the effect sizes of these group comparisons using a z test to test the hypothesis that group differences in amygdala iFC would be less for the groups that do not vary on EL (low-EL ADHD-TDC comparison) than those that do (high-EL ADHD-TDC comparison).
Finally, to address the possibility that ODD co-morbidity was driving our EL findings, mean amygdala iFC from clusters found to be significantly associated with EL was compared between the ADHD participants with (n = 14) and without (n = 49) ODD, using an independent-samples t test.
RESULTS
Characteristics for the full ADHD group, the 2 ADHD subgroups and the TDC group included in the secondary analyses are presented in Table 1. As designed, EL scores differed between ADHD groups and between the high-EL ADHD and the TDC group. TDCs also showed significantly lower hyperactivity scores than either ADHD subgroup, although the high- and low-EL ADHD subgroups did not differ on hyperactivity scores. The 3 groups did not differ in regard to age (F2,54 =1.5, p = .24), sex (F2,54 = 0.99, p = .038), IQ (F[2,53] = 3.18, p = .05), or movement parameters (maximum head displacement: F2,54 = 1.0, p = .37; framewise displacement: F2,54 = 1.4, p = .25) and the 2 ADHD groups did not differ in diagnostic comorbidity (15% versus 22%; p = .62)
Amygdala IFC and Emotional Lability in ADHD
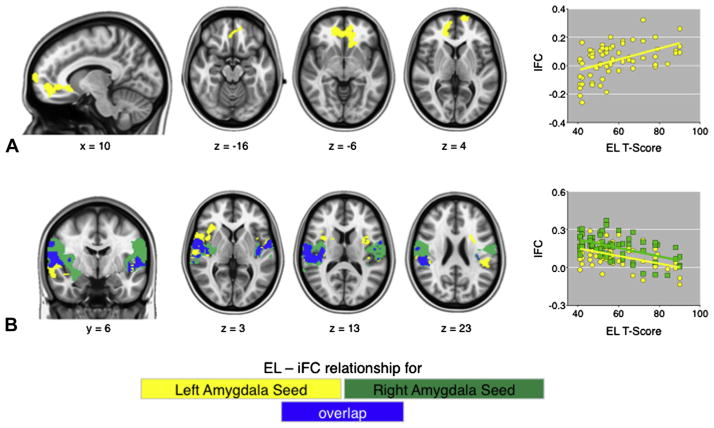
While controlling for hyperactivity, EL scores were positively associated with iFC between left amygdala and medial PFC regions including rostral anterior cingulate cortex (rACC) and frontal pole (Table 2 and Figure 1) in children with ADHD. EL scores were also negatively associated with left amygdala–bilateral insula/superior temporal gyrus (STG) iFC as well as right amygdala–bilateral posterior insula iFC (Table 2 and Figure 1). In this case, high EL was associated with decreased positive iFC between both amygdala seeds and the bilateral insula/STG clusters (Figure 1). When hyperactivity and inattention were both used as covariates, findings were nearly identical to those found when hyperactivity was the only covariate (Figure S1). When divided into groups based on the presence or absence of comorbid ODD, iFC did not differ between the groups for any of the clusters (all p > .05).
TABLE 2.
Clusters With Significant Associations With Emotional Lability (EL) Scores
| Right amygdala ROI | Cluster Size | X | Y | Z | Max Z | p |
|---|---|---|---|---|---|---|
| Negative relationship with EL | ||||||
| Cluster 1: Posterior insula (L) | 2,245 | 340 | 312 | 6 | 4.47 | .0003 |
| Parietal operculum | 356 | 324 | 16 | 3.64 | ||
| Precentral gyrus | 352 | 38 | 30 | 3.42 | ||
| 358 | 0 | 12 | 3.35 | |||
| Insula | 334 | 322 | 24 | 3.06 | ||
| Cluster 2: Posterior insula (R) | 4,400 | 62 | 38 | 32 | 4.91 | .0001 |
| 56 | 328 | 14 | 4.85 | |||
| 40 | 38 | 10 | 4.64 | |||
| 26 | 0 | 310 | 3.74 | |||
|
| ||||||
| Left amygdala ROI | ||||||
|
| ||||||
| Positive relationship with EL | ||||||
| Rostral ACC | 1,329 | 18 | 48 | 2 | 4.00 | .0043 |
| 36 | 44 | 310 | 3.28 | |||
| Frontal Pole | 314 | 72 | 10 | 3.60 | ||
| Negative relationship with EL | ||||||
| Cluster 1: Superior temporal gyrus (L) | 1,675 | 352 | 6 | 36 | 3.85 | .0009 |
| 324 | 6 | 28 | 3.74 | |||
| Insula | 342 | 312 | 4 | 3.58 | ||
| 344 | 12 | 328 | 3.53 | |||
| 350 | 328 | 20 | 3.78 | |||
| Insula | 338 | 8 | 10 | 3.02 | ||
| Cluster 2: Superior temporal gyrus (R) | 3,238 | 66 | 320 | 8 | 4.64 | .0001 |
| Insula | 38 | 318 | 10 | 4.11 | ||
| 42 | 32 | 310 | 3.88 | |||
| 34 | 8 | 14 | 3.52 | |||
| Post-central gyrus | 66 | 38 | 34 | 3.34 | ||
| 60 | 4 | 4 | 3.34 | |||
| 36 | 26 | 6 | 3.23 | |||
Note: Cluster size is represented by the number of voxels present in the cluster. X, Y, Z represent Montreal Neurological Institute (MNI) Coordinates for the cluster peak maximum Z values. ACC = anterior cingulate cortex; L = left; Max = maximum; R = right; ROI = region of interest.
FIGURE 1.
Direction of the relationship between dimensional parent emotional lability (EL) ratings, co-varying for hyperactivity, and extracted time-series values for the significant clusters are shown in the scatter plots. Note: Images in row A demonstrate that increased EL is associated with increased amygdala connectivity (“iFC”) with the highlighted prefrontal regions, whereas row B shows that increased EL (“EL T-score”) is associated with decreased amygdala–insula/STG connectivity (“iFC”). Scatter plots address EL T-scores (x-axis) versus amygdala correlations (y-axis).
Group Amygdala iFC Comparisons
To further test the specificity of altered amygdala iFC to EL, we conducted t tests to examine differences between low-EL ADHD and TDC and between high-EL ADHD and TDC and compared their effect sizes. Amygdala iFC did not differ between the low-EL and TDC groups for any of the clusters assessed (Figure 2). We found a trend toward significantly greater positive left amygdala–ACC iFC for the high-EL group compared to the TDCs (t35 = −2.30, p = .027; Figure 2). The high-EL group also exhibited a trend toward decreased iFC relative to the TDC group between the left amygdala and insula/STG (t[35] = 2.30, p = .028) and between the right amygdala and the insula/STG clusters (t[35] = 2.02, p = .051; Figure 2). Effect sizes for the low-EL versus TDC were significantly smaller than those for the high-EL versus TDC for each of the 3 clusters (left amygdala–ACC: z = 4.12, p < .001; left amygdala–insula/STG: z = 4.72, p < .0001; right amygdala–insula/STG: z = 2.63, p < .0001).
FIGURE 2.
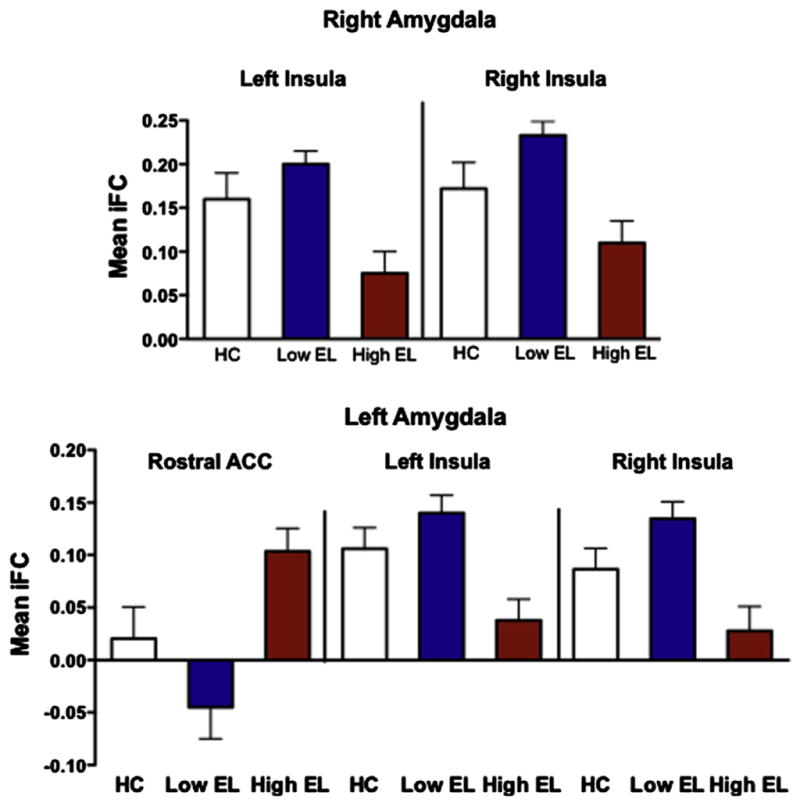
Mean correlation coefficients between each of the amygdala seeds and the significant cortical regions (derived from the initial attention-deficit/hyperactivity disorder [ADHD]–only analyses) displayed for each of the groups. Note: Error bars indicate SD. IFC = intrinsic functional connectivity; rACC = rostral anterior cingulate cortex; SFG = superior frontal gyrus; STG = superior temporal gyrus.
DISCUSSION
In a large sample of 6- to 13-year-old children with ADHD, iFC of a corticoamygdalar network was related to parent ratings of emotional lability. Controlling for hyperactivity, high levels of EL were specifically associated with increased positive iFC between bilateral amygdala and medial prefrontal regions and less positive iFC between amygdala and bilateral insula/STG, suggesting a disruption in emotional control networks in a subset of children with ADHD. Accounting for inattention as well as hyperactivity yielded very similar results to hyperactivity alone, further highlighting the specificity of these results EL and not to other symptoms of ADHD. The relationship between amygdala iFC and EL was further supported by a subgroup analysis that showed that amygdala iFC in the low-EL group did not differ from that of the comparison group, as would be expected, as the groups did not differ on EL. Thus, the group differences on hyperactivity scores appeared to have no effect on amygdala iFC. Larger effects were observed when comparing the high-EL and TDC groups, which would be predicted because of group differences in EL. These large effect sizes observed between high-EL and TDC iFC comparisons (range = 0.66–0.76), suggest that these subgroup analyses did not meet statistical significance because they were underpowered to detect group differences. Because ODD diagnoses were equivalent between the high- and low-EL groups, and because our results did not differ between groups with and without ODD, this co-morbidity is unlikely to have driven our findings. Subthreshold mood and anxiety symptoms recorded as “NOS” cases were rare and therefore also unlikely to account for these findings.
Study hypotheses regarding altered iFC between the amygdala and cortical regions were supported. Our results suggest that elevated positive amygdala-PFC iFC is associated with difficulty in regulating the expression of negative emotions. These findings are consistent with theories of emotional perception where amygdala and rostral ACC are involved in the identification of emotional significance and generation of affective responses.53 More recent task-based studies in adults suggest that the interactions between medial PFC/rostral ACC and amygdala specifically play a role in affect regulation, particularly in relation to negative emotions.54
In addition, regions in bilateral posterior insula extending into temporal cortex emerged as having significantly decreased positive iFC with the amygdala in ADHD youth with higher EL. Although we did not specifically predict findings in the insula, this region is structurally connected with the amygdala and with other limbic and cortical association areas. Intrinsic functional connectivity and activation of the posterior insula have been associated with emotion, interoception, and perception,55,56 particularly perception of one’s own emotions. Strokes localized to the posterior insula have also been reported to result in impaired processing of valence and intensity of negative facial expressions and words.57 The emerging evidence of the posterior insula’s involvement in emotional processing raises the possibility that a failure in emotional perception and related action may partly underlie observed emotion dysregulation in ADHD.
Our findings should be interpreted in light of several limitations. First, we were unable to recruit and to study typically developing children with high EL. Despite the theoretical interest, recruiting such children is not practical. ADHD comorbidity is overwhelmingly present in children with high EL,17 and we could not detect high EL in children lacking other psychiatric disorders. Accordingly, we were unable to stratify controls according to high- and low-EL status, because of the lack of variability in their EL scores. Second, we were not able to test whether EL-related alterations in amygdala circuits are unique to children with ADHD or are also observed in other disorders characterized by high EL such as ODD or depression. Future studies comparing EL and amygdala iFC between diagnoses are needed. Third, to confirm this circuit’s involvement in emotion regulation in childhood, additional work involving self-report and active experimental manipulation of emotion regulation will be required. Finally, although most children were drug treatment naive, and nearly all of those currently treated were off medications when scanned, we cannot completely exclude the possibility that prior pharmacotherapy may have affected iFC findings.
In summary, children with ADHD who are impaired by high EL exhibited aberrant iFC in regions associated with emotion regulation when examined dimensionally. These findings are unlikely to be ascribable to hyperactivity as evidenced by greater differences between groups differing on EL and hyperactivity (high-EL versus TDC) than between groups differing only on hyperactivity, and not EL (low-EL versus TDC). This suggests that a subset of youth with ADHD have specific disruptions in amygdala networks that underlie emotion regulation impairments. Furthermore, resting-state functional connectivity appears to be suitable for detecting emotion-relevant differences in iFC in youth with ADHD. Our findings validate the need for further research focused on emotion regulation difficulties in a subgroup of children with ADHD.
Supplementary Material
Scatter plots show the direction of the relationship between dimensional parent emotional lability (EL) ratings, co-varying for hyperactivity and impulsivity, and extracted time-series values for the significant clusters. Images in row A demonstrate that increased EL is associated with increased amygdala connectivity (“iFC”) with the highlighted prefrontal regions, whereas row B shows increased EL (“EL T-score”) is associated with decreased amygdala-insula/STG connectivity (“iFC”). Note: Scatter plots address EL T-scores (x-axis) versus amygdala correlations (y-axis).
Acknowledgments
This research was partially supported by grants from the National Institute of Mental Health (R01MH081218 to F.X.C., K23 MH074821 to A.K.R., and K23MH087770 to A.D.M.), the National Institutes of Drug Abuse (K12 DA000357 for L.H.’s effort), the National Institute of Child Health and Human Development (R01HD065282 to F.X.C.), the Stavros Niarchos Foundation (to F.X.C.), the Leon Levy Foundation (to M.P.M., A.D.M.), and the endowment provided by Phyllis Green and Randolph Cowen. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors thank all participants for their cooperation.
Footnotes
Disclosure: Drs. Hulvershorn, Mennes, Castellanos, Di Martino, Milham, Hummer, and Roy report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Dr. Leslie A. Hulvershorn, Indiana University School of Medicine, Riley Hospital for Children.
Dr. Maarten Mennes, Phyllis Green and Randolph Cowen Institute for Pediatric Neuroscience, New York University Child Study Center. Donders Institute for Brain, Cognition, and Behaviour, Radboud University Nijmegen Medical Centre.
Dr. F. Xavier Castellanos, Phyllis Green and Randolph Cowen Institute for Pediatric Neuroscience, New York University Child Study Center. Nathan Kline Institute for Psychiatric Research.
Dr. Adriana Di Martino, Phyllis Green and Randolph Cowen Institute for Pediatric Neuroscience, New York University Child Study Center.
Dr. Michael P. Milham, Nathan Kline Institute for Psychiatric Research. Center for the Developing Brain, Child Mind Institute.
Dr. Tom A. Hummer, Indiana University School of Medicine, Riley Hospital for Children.
Dr. Amy Krain Roy, Phyllis Green and Randolph Cowen Institute for Pediatric Neuroscience, New York University Child Study Center. Fordham University.
References
- 1.Faraone SV, Biederman J, Mennin D, Russell R, Tsuang MT. Familial subtypes of attention deficit hyperactivity disorder: a 4-year follow-up study of children from antisocial-ADHD families. J Child Psychol Psychiatry. 1998;39:1045–1053. [PubMed] [Google Scholar]
- 2.Faraone SV, Biederman J, Weber W, Russell RL. Psychiatric, neuropsychological, and psychosocial features of DSM-IV subtypes of attention-deficit/hyperactivity disorder: results from a clinically referred sample. J Am Acad Child Adolesc Psychiatry. 1998;37:185–193. doi: 10.1097/00004583-199802000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- 4.Carlson GA, Potegal M, Margulies D, Gutkovich Z, Basile J. Rages—what are they and who has them? J Child Adolesc Psychopharmacol. 2009;19:281–288. doi: 10.1089/cap.2008.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wehmeier PM, Schacht A, Barkley RA. Social and emotional impairment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health. 2010;46:209–217. doi: 10.1016/j.jadohealth.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Barkley RA, Fischer M. The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. J Am Acad Child Adolesc Psychiatry. 2010;49:503–513. doi: 10.1097/00004583-201005000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Sobanski E, Banaschewski T, Asherson P, et al. Emotional lability in children and adolescents with attention deficit/hyperactivity disorder (ADHD): clinical correlates and familial prevalence. J Child Psychol Psychiatry. 2010;51:915–923. doi: 10.1111/j.1469-7610.2010.02217.x. [DOI] [PubMed] [Google Scholar]
- 8.Surman CB, Biederman J, Spencer T, et al. Deficient emotional self-regulation and adult attention deficit hyperactivity disorder: a family risk analysis. Am J Psychiatry. 2011;168:617–623. doi: 10.1176/appi.ajp.2010.10081172. [DOI] [PubMed] [Google Scholar]
- 9.Spencer TJ, Faraone SV, Surman CB, et al. Toward defining deficient emotional self-regulation in children with attention-deficit/hyperactivity disorder using the Child Behavior Checklist: a controlled study. Postgrad Med. 2011;123:50–59. doi: 10.3810/pgm.2011.09.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Healey DM, Marks DJ, Halperin JM. Examining the interplay among negative emotionality, cognitive functioning, and attention deficit/hyperactivity disorder symptom severity. J Int Neuropsychol Soc. 2011:1–9. doi: 10.1017/S1355617711000294. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann MJ, Biehl SC, Jacob C, Deckert J. Neurobiological and psychophysiological correlates of emotional dysregulation in ADHD patients. Atten Defic Hyperact Disord. 2010;2:233–239. doi: 10.1007/s12402-010-0047-6. [DOI] [PubMed] [Google Scholar]
- 12.Musser ED, Backs RW, Schmitt CF, Ablow JC, Measelle JR, Nigg JT. Emotion regulation via the autonomic nervous system in children with attention-deficit/hyperactivity disorder (ADHD) J Abnorm Child Psychol. 2011;39:841–852. doi: 10.1007/s10802-011-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martel MM. Research review: a new perspective on attention-deficit/hyperactivity disorder: emotion dysregulation and trait models. J Child Psychol Psychiatry. 2009;50:1042–1051. doi: 10.1111/j.1469-7610.2009.02105.x. [DOI] [PubMed] [Google Scholar]
- 14.Stringaris A, Goodman R. Mood lability and psychopathology in youth. Psychol Med. 2009;39:1237–1245. doi: 10.1017/S0033291708004662. [DOI] [PubMed] [Google Scholar]
- 15.Anastopoulos AD, Smith TF, Garrett ME, et al. Self-regulation of emotion, functional impairment, and comorbidity among children with AD/HD. J Atten Disord. 2011;15:583–592. doi: 10.1177/1087054710370567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Roy AK, Bar-Haim Y, Leibenluft E, et al. Clinical features of young children referred for impairing temper outbursts. J Child Adolesc Psychopharmacol. 2013;23:588–596. doi: 10.1089/cap.2013.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tripp G, Wickens JR. Neurobiology of ADHD. Neuropharmacol. 2009;57:579–589. doi: 10.1016/j.neuropharm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 19.Biederman J. Advances in the neurobiology of ADHD. CNS Spectrums. 2007;12(4 Suppl 6):6–7. doi: 10.1017/s1092852900026006. [DOI] [PubMed] [Google Scholar]
- 20.Wickens JR, Tripp G, Gerhardt GA. Neurobiology of attention-deficit hyperactivity disorder (ADHD): from gene to therapy. J Neurosci Methods. 2007;166:293. doi: 10.1016/j.jneumeth.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Arnsten AF. The emerging neurobiology of attention deficit hyperactivity disorder: the key role of the prefrontal association cortex. J Pediatr. 2009;154:I-S43. doi: 10.1016/j.jpeds.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw P, Lerch J, Greenstein D, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- 23.Mills KL, Bathula D, Dias TG, et al. Altered cortico-striatal-thalamic connectivity in relation to spatial working memory capacity in children with ADHD. Front Psychiatry. 2012;3:2. doi: 10.3389/fpsyt.2012.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van ‘t Ent D, van Beijsterveldt CE, Derks EM, et al. Neuroimaging of response interference in twins concordant or discordant for inattention and hyperactivity symptoms. Neuroscience. 2009;164:16–29. doi: 10.1016/j.neuroscience.2009.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baving L, Laucht M, Schmidt MH. Atypical frontal brain activation in ADHD: preschool and elementary school boys and girls. J Am Acad Child Adolesc Psychiatry. 1999;38:1363–1371. doi: 10.1097/00004583-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Casey BJ, Epstein JN, Buhle J, et al. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. Am J Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- 27.Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 28.Da Fonseca D, Seguier V, Santos A, Poinso F, Deruelle C. Emotion understanding in children with ADHD. Child Psychiatry Hum Dev. 2009;40:111–121. doi: 10.1007/s10578-008-0114-9. [DOI] [PubMed] [Google Scholar]
- 29.Miller M, Hanford RB, Fassbender C, Duke M, Schweitzer JB. Affect recognition in adults with ADHD. J Atten Disord. 2011;15:452–460. doi: 10.1177/1087054710368636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frodl T, Stauber J, Schaaff N, et al. Amygdala reduction in patients with ADHD compared with major depression and healthy volunteers. Acta Psychiatr Scand. 2010;121:111–118. doi: 10.1111/j.1600-0447.2009.01489.x. [DOI] [PubMed] [Google Scholar]
- 31.Herpertz SC, Huebner T, Marx I, et al. Emotional processing in male adolescents with childhood-onset conduct disorder. J Child Psychol Psychiatry. 2008;49:781–791. doi: 10.1111/j.1469-7610.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- 32.Brotman MA, Rich BA, Guyer AE, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167:61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Posner J, Nagel BJ, Maia TV, et al. Abnormal amygdalar activation and connectivity in adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50:828–837. doi: 10.1016/j.jaac.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malisza KL, Clancy C, Shiloff D, et al. Functional magnetic resonance imaging of facial information processing in children with autistic disorder, attention deficit hyperactivity disorder and typically developing controls. Int J Adolesc Med Health. 2011;23:269–277. doi: 10.1515/ijamh.2011.055. [DOI] [PubMed] [Google Scholar]
- 35.Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Marsh R, Gerber AJ, Peterson BS. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2008;47:1233–1251. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Posner J, Maia TV, Fair D, Peterson BS, Sonuga-Barke EJ, Nagel BJ. The attenuation of dysfunctional emotional processing with stimulant medication: an fMRI study of adolescents with ADHD. Psychiatry Res. 2011;193:151–160. doi: 10.1016/j.pscychresns.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fair DA, Posner J, Nagel BJ, et al. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:1084–1091. doi: 10.1016/j.biopsych.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castellanos FX, Kelly C, Milham MP. The restless brain: attention-deficit hyperactivity disorder, resting-state functional connectivity, and intrasubject variability. Can J Psychiatry. 2009;54:665–672. doi: 10.1177/070674370905401003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomasi D, Volkow ND. Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;71:443–450. doi: 10.1016/j.biopsych.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blandon AY, Calkins SD, Keane SP, O’Brien M. Individual differences in trajectories of emotion regulation processes: the effects of maternal depressive symptomatology and children’s physiological regulation. Dev Psychol. 2008;44:1110–1123. doi: 10.1037/0012-1649.44.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 43.Conners CK, Sitarenios G, Parker JD, Epstein JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 44.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: American Psychological Association; 1999. [Google Scholar]
- 45.Gruber R, Cassoff J, Frenette S, Wiebe S, Carrier J. Impact of sleep extension and restriction on children’s emotional lability and impulsivity. Pediatrics. 2012;130:e1155–e1161. doi: 10.1542/peds.2012-0564. [DOI] [PubMed] [Google Scholar]
- 46.Childress AC, Arnold V, Adeyi B, et al. The Effects of lisdex-amfetamine dimesylate on emotional lability in children 6 to 12 years of age with ADHD in a double-blindplacebo-controlled trial. J Atten Disord. 2014;18:123–132. doi: 10.1177/1087054712448252. [DOI] [PubMed] [Google Scholar]
- 47.Banaschewski T, Jennen-Steinmetz C, Brandeis D, et al. Neuro-psychological correlates of emotional lability in children with ADHD. J Child Psychol Psychiatry. 2012;53:1139–1148. doi: 10.1111/j.1469-7610.2012.02596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roy AK, Shehzad Z, Margulies DS, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roy AK, Fudge JL, Kelly C, et al. Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2013;52:290–299. doi: 10.1016/j.jaac.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cubillo A, Smith AB, Barrett N, et al. Shared and drug-specific effects of atomoxetine and methylphenidate on inhibitory brain dysfunction in medication-naive ADHD boys. Cereb Cortex. 2014;24:174–185. doi: 10.1093/cercor/bhs296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matthews J, Altman D. Interaction 2: compare effect sizes not P values. Br Med J. 1996;313:808. doi: 10.1136/bmj.313.7060.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 54.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly C, Toro R, Di Martino A, et al. A convergent functional architecture of the insula emerges across imaging modalities. Neuroimage. 2012;61:1129–1142. doi: 10.1016/j.neuroimage.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cauda F, Costa T, Torta DM, et al. Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage. 2012;62:343–355. doi: 10.1016/j.neuroimage.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borg C, Bedoin N, Peyron R, Bogey S, Laurent B, Thomas-Anterion C. Impaired emotional processing in a patient with a left posterior insula-SII lesion. Neurocase. 2013;19:592–603. doi: 10.1080/13554794.2012.713491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter plots show the direction of the relationship between dimensional parent emotional lability (EL) ratings, co-varying for hyperactivity and impulsivity, and extracted time-series values for the significant clusters. Images in row A demonstrate that increased EL is associated with increased amygdala connectivity (“iFC”) with the highlighted prefrontal regions, whereas row B shows increased EL (“EL T-score”) is associated with decreased amygdala-insula/STG connectivity (“iFC”). Note: Scatter plots address EL T-scores (x-axis) versus amygdala correlations (y-axis).