Summary
Objective
The study sought to document the experience of immunological improvement among Ghanaian PLHIV on HAART comparing different categories of patients.
Setting
Serology Unit, Komfo Anokye Teaching Hospital, Kumasi, Ghana.
Participants
The study comprised a convenient sample of 303 treatment naïve HIV patients due to start HAART.
Methods
Questionnaires were used to collect patient demographic and clinical data. Four CD4 counts were measured at six-monthly intervals to determine rates of CD4 change. These were pre-therapy, 1st post-therapy, 2nd post-therapy, and 3rd post-therapy counts. The rates of CD4 change among the different categories of patients were also compared.
Results
At baseline, women had higher CD4 count (mean of 77.4cells/°l), and mean age of participants was 40 years. The CD4 count increased from a mean baseline of 70.2 cells/°l to 229.2, 270.0, and 297.6 cells/°l at 6, 12, and 18 months of treatment respectively (P <0.0001 at each time point). There were no gender (P=0.46) and age (P=0.96) differences in treatment response. There was no difference (P=0.18) in treatment response comparing those with CD4 <250 cells/°l and those whose CD4 count was between 250 and 350 cells/°l at baseline although patients with baseline CD4 count <250 cells/°l showed larger increases after 12 months of treatment. Out of 282 patients with pre-therapy CD4 count "250 cells/°l, 241 (85.5%) and 41 (14.5%) were adherents and nonadherents respectively. Mean rate of increase was 15.2 and 8.4 cells/°l/month in adherent and non-adherent patients respectively (p=0.2).
Conclusion
The study suggests that a sustained CD4 increase could be achieved in adherent patients commencing therapy with baseline CD4 count ≤250 cells/°l, and that these patients have greater ability for immunological recovery during 12 months of treatment The study, therefore, concludes that significant immunological improvement is possible among Ghanaian PLHIV on HAART as long as a high level of treatment adherence is observed.
Keywords: Immunological, Response, HIV, HAART, Ghana
Introduction
In recent years, global commitment, action and resources to combat the human immunodeficiency virus (HIV) pandemic have increased markedly1. Despite recent progress, at the end of 2009, an estimated 33.3 million people globally were living with HIV, and more than 2.6 million new HIV infections occurred in that year with 1.8 million AIDS (acquired immune deficiency syndrome) related deaths2. Sub-Saharan Africa remains the hardest hit region, with two-thirds of the global burden.2,3 The burden of HIV/AIDS in resource-limited settings is extensive.3 In Ghana, the pandemic is well documented and has gone beyond a health problem, and now encompasses all socio-economic aspects of life.4
Effective care for people living with HIV/AIDS (PLHIV) requires highly active antiretroviral therapy (HAART) for those who are eligible for treatment5. Since its introduction in 1996, mortality and morbidity rates in HIV-infected individuals in countries with widespread access to HAART have plummeted.6,7 The main effect of HAART is to suppress viral replication, allowing the individual's immune system to recover and protecting him/her from the development of AIDS and death. In most sub-Saharan African countries, decisions to initiate and monitor ART rely on clinical and immunological assessment, with viral load not part of the management protocol due to resource limitations.8
In Ghana, hitherto, the national CD4 cell count value for patients initiating treatment was ≤250 cells/µl and ≤350 cells/µl for pregnant women.9 However, the national policy, in improving better response to therapy in accordance with World Health Organization (WHO) guidelines, has since 2008, increased the CD4 cell count to ≤350 cells/µl and below for all patients.10 The immunological response of PLHIV initiated on HAART in Ghana is however not well documented although elsewhere there is documentary evidence on immunological response to HAART.6,7 The study objective, therefore, was to document the experience of immunological improvement among Ghanaian PLHIV on HAART; comparing different categories of patients.
Methods
Study design
The study was conducted between October 2006 and July 2009. Baseline CD4 cell counts before initiation of therapy for the study population was ≤250 cells/µl for patients who enrolled in 2006/2007. However, in the course of the study, baseline CD4 cell count was changed from ≤250 to ≤350 cells/µl in 2008 as a national policy for the start of ART10. The change was, therefore, captured into the study, and few patients with baseline CD4 cell counts of ≤350 cells/µl were enrolled in the early part of 2008. These patients were stratified into two CD4 categories: those starting treatment with CD4 cell count <250 cells/µl and those starting treatment with CD4 cell count between 250–350 cells/µl. Antiretroviral therapy was based on tripledrugs regimens consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitors (non-NRTIs)10. ‘Adherent’ was defined as patients without treatment interruptions while ‘non-adherent’ was defined as patients who defaulted in treatment during the study period. Adherence was monitored using selfreports from patients.9,10
Study population and sample collection
The study population composed of HIV-positive patients, 15 years and above, who were treatment naïve. These patients had enrolled at the KATH HIV Clinic and were due to start HAART treatment. Patients' demographic data was collected in the form of questionnaires, and their clinical data extracted from their clinic files. The total sample size was 303. This sample size followed the convenient sampling approach11 where every patient who met the eligibility criteria for initiating HAART and consented to take part in the study, was selected until the study period was over. About 3ml of whole blood was collected from each patient using sterile Becton Dickinson (BD) vacutainer tubes containing ethylene-diamine-tetra-acetic acid (EDTA) to prevent coagulation.
CD4+ lymphocyte measurement by flow cytometry
The laboratory analyses were carried out at the Serology Unit of the Komfo Anokye Teaching Hospital (KATH) in Kumasi. CD4 cell count was determined by immune-labelling and fluorescence-activated cell sorter (FACS) analysis using BD FACSCount automated reader (Becton Dickinson, San Jose, CA, USA) according to the manufacturer's instruction. The test used fluorochrome-labelled anti-CD3, CD4 and CD8 monoclonal antibody detection system. In all, four CD4 count measurements were taken at six monthly intervals. These were pre-therapy, 1st post- therapy, 2nd post- therapy, and 3rd post-therapy counts. Quality control was done on a daily basis following the manufacturer's instructions12, to validate patient results and to ensure system linearity.
Statistical analysis
Patient demographic data and CD4 counts were entered into Microsoft Excel 2007, and later imported to GraphPad Prism version 5 (GraphPad Software, Inc., USA) for statistical analysis. To normalize the results, the logarithm (log10) of each CD4 count value was obtained. The mean of the logarithmic values for each group (pre-therapy, 1st, 2nd and 3rd post-therapy counts) was converted back to a base 10 number using the antilogarithmic formula (=10⋀ [mean value]) to obtain the geometric mean.13 Paired sample t-test was used to determine the rate of CD4 increases, gender response to treatment, and treatment response by baseline CD4 count. One-way analysis of variance was used to determine the association between treatment response and age of patient. For all comparisons, Pvalue was set at P<0.05 for establishing statistical significance. A two-tailed P-value was used.
Ethical approval
The Committee on Research, Publication and Ethics of the KNUST Medical School and Komfo Anokye Teaching Hospital, gave approval for the study to be undertaken. Written informed consent form was obtained from each participant.
Results
Baseline characteristics of patients
CD4 count values were expressed in geometric mean. Of the 303 patients who were eligible and consented to take part in the study, 262 (86.5%) were adherents. Of this, 200 (76.3%) patients with baseline CD4 cell count of <250 cells/µl were enrolled in the years 2006/2007 to evaluate their immune response to HAART.
Sixty-two patients who commenced therapy with baseline CD4 cell count of ≤350 cells/µl were also enrolled in the early part of year 2008 to compare treatment response by baseline categories.
Forty-one (13.5%) pa tients out of the 303 patients were non-adherents. The main baseline characteristics of patients are shown in Tables 1 and 2.
Table 1.
Baseline characteristics of study participants before the start of HAART for adherent patients (262)
| Characteristics | n | % | Characteristics | n | % |
| Gender | Religion | ||||
| Women | 180 | 68.7 | Christianity | 193 | 73.6 |
| Men | 82 | 31.3 | Moslem | 19 | 7.3 |
| Other | 1 | 0.4 | |||
| Missing Data | 49 | 18.7 | |||
| Employment Status | Marital Status | ||||
| Unemployed | 40 | 15.3 | Single | 57 | 21.8 |
| Self-employed | 144 | 54.9 | Married | 91 | 34.7 |
| Workers | 29 | 11.1 | Divorced/Widowed | 77 | 29.4 |
| Missing Data | 49 | 18.7 | Missing Data | 37 | 14.1 |
| Level of Education | WHO Staging | ||||
| Non-Educated | 36 | 13.7 | I | 35 | 13.4 |
| Primary/JSS | 127 | 48.5 | II | 70 | 26.7 |
| Secondary | 36 | 13.7 | III | 59 | 22.5 |
| Tertiary | 15 | 5.8 | IV | 7 | 2.7 |
| Missing Data | 48 | 18.3 | Missing Data | 91 | 34.7 |
| Age group (year)* | Baseline CD4 (cells/µl)† | (n=200) | |||
| 15–34 | 83 | 31.7 | <100 | 92 | 46 |
| 35–54 | 156 | 59.5 | 100–199 | 72 | 36 |
| 55–74 | 23 | 8.8 | 200–250 | 36 | 18 |
Age (year), mean (40), minimum (15), maximum (70)
Baseline CD4 (cells/µl), geometric mean (70.2), minimum (2), maximum (248)
Table 2.
mean baselines CD4 between various characteristics at start of therapy
| Characteristics | Baseline CD4 (cells/µl) |
| Age (year) | |
| 15–34 | 56.8 |
| 35–54 | 78.3 |
| 55–74 | 75.8 |
| Sex | |
| Women | 77.4 |
| Men | 57.4 |
Treatment outcome
Treatment outcome of two hundred (200) adherent patients who started therapy with baseline CD4 cell count of <250 cells/µl were enrolled in 2006 and 2007 to evaluate their immune response to HAART after 6, 12, and 18 months of treatment.
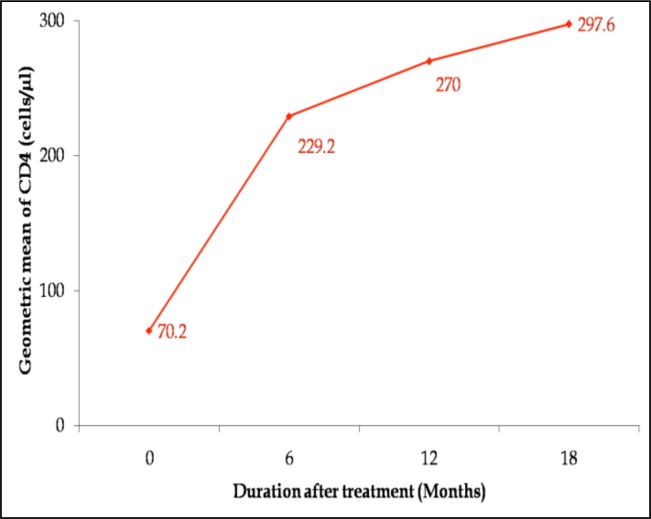
The mean pre-therapy CD4 cell count was 70.2 cells/µl. The most significant immunological response to treatment occurred after 6 months of therapy with a mean CD4 increase of 229.2 cells/µl (p<0.0001). Subsequently, there were slower steady increases in CD4 counts as seen in figure 1. Increases were, however, significant at each time point: from first to second post-therapy (p=0.002) and from second to third post-therapy (p=0.0036) counts. Overall, the immunological response to HAART was very significant after 18 months of treatment (from 70.2 to 297.6 cells/µl) with p<0.0001.
Figure 1.
Rate of CD4 increases showing immunological response of 200 hundred patients who commenced therapy with baseline count of <250 cells/µl in 2006/2007.
Adherence and treatment response
In all, there were two hundred and eighty two (282) patients who started therapy with baseline CD4 count of <250 cells/µl during the study period (2006–2008). Of this, 241 (85.5%) and 41 (14.5%) were adherents and non-adherents respectively.
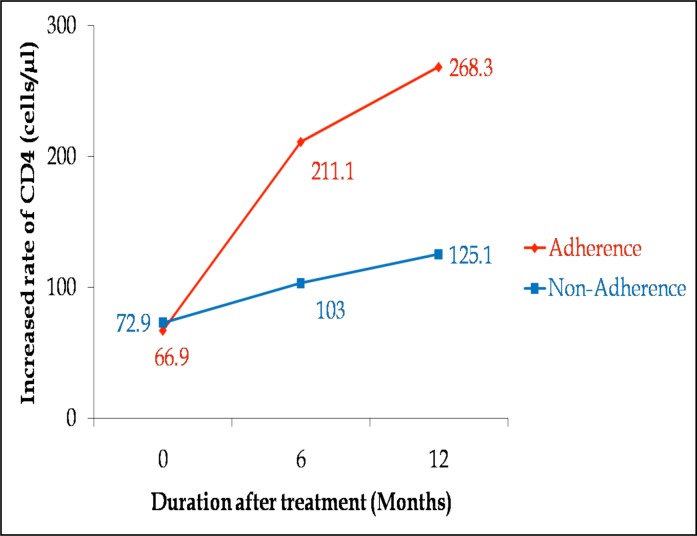
The immune responses of these two categories (both of which had baseline CD4 count <250 cells/µl) were compared. Adherence was monitored using self-reports from patients.9,10 The mean pre-therapy CD4 count for non-adherent patients was 72.9 cells/µl which was slightly higher than adherent patients (66.9 cells/µl) as shown in figure 2. After 6 months of treatment, CD4 count had increased to 211.1 (p<0.0001) and 103 cells/µl (p=0.21) for adherents and non-adherents respectively.
Figure 2.
Comparing immune recovery in adherent and non-adherent patients.
Thereafter, a steady rise of 57.2 cells/µl (P<0.0001) and 22.1 cells/µl (P=0.48) was observed for adherents and non-adherents respectively.
Overall, CD4 cell count increases after 12 months of treatment were 201.4 and 52.2 cells/µl for adherents and non-adherents respectively. The study, however, could not establish a difference in treatment response between adherents and non-adherents after 12 months of therapy (p=0.2).
Gender response to HAART
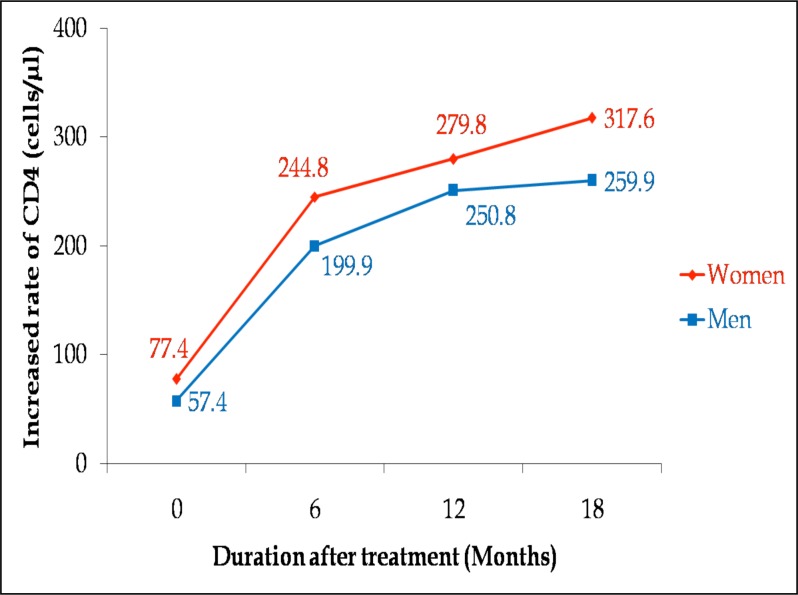
Among the adherent patients in the study, there was a higher proportion of women (68.7%) than men (31.3%). Women exhibited higher CD4 count values (77.4 cells/µl) than men (57.4 cells/µl) before the start of therapy. The respective CD4 increases for men and women were 199.9 and 244.8 cells/µl at 6 months, and 250.8 and 279.8 cells/µl at 12 months (Figure 3). After 18 months of treatment, CD4 count had increased from baseline of 57.4 cells/µl to 259.9 cells/µl in men (P<0.0001), and from baseline of 77.4 cells/µl to 317.6 cells/µl in women (P<0.0001). A one-way analysis of variance of individual patient's counts showed significant CD4 increases for both men and women respectively. The study, however, found no gender difference in treatment response (P=0.46) after 18 months.
Figure 3.
Rate of CD4 increases showing gender response to treatment for 262 patients who commenced therapy with baseline count of ≤350 cells/µl and were adherent.
Treatment response in different age categories
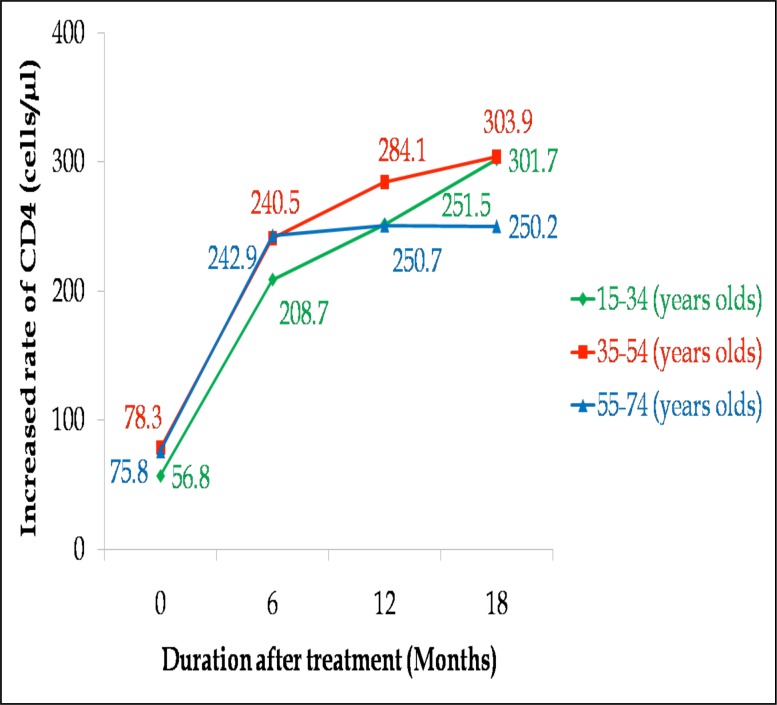
The age of the patients was stratified into three categories namely: 15–34 years (category A), 35–54 years (category B) and 55–74 years (category C) for adherent patients. Baseline CD4 was 56.8, 78.3, 75.8 cells/µl for categories A, B and C respectively. There were rapid initial CD4 increases at 6 months for all categories (category A: 208.7, category B: 240.5, category C: 242.9 cells/µl). This was followed by slower steady increases afterwards as shown in Figure 4. After 18 months of treatment, CD4 cell count rose from 56.8, 78.3 and 75.8 cells/µl pre-therapy count to 301.7, 303.9 and 250.2 cells/µl in category A, category B and category C respectively (p<0.0001 for all categories). There was no CD4 increase between second and third post-therapy counts (250.7 and 250.2 cells/µl) in category C.
Figure 4.
Immune recovery among different age categories of patients on HAART.
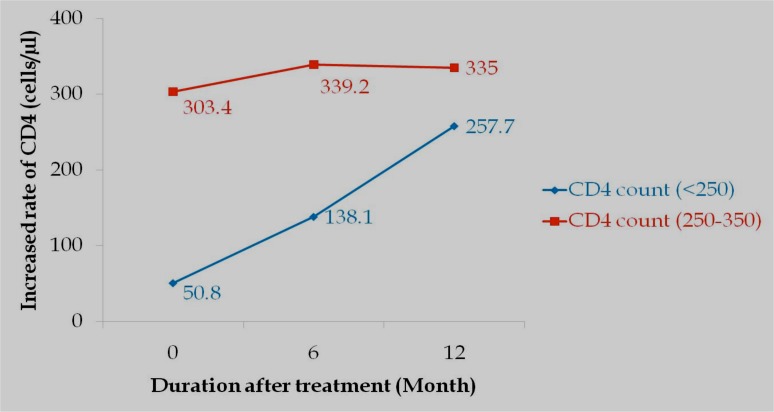
Baseline CD4 and treatment response
Among those who were adherent, patients were categorized into two CD4 strata: category A (baseline CD4 count of <250 cells/µl) and category B (baseline CD4 count between 250–350 cells/µl) for patients who enrolled in 2008. These were sixty-two (62) in total. Mean pre-therapy CD4 count was 50.8 and 303.4 cells/µl for categories A and B respectively. CD4 increases from baseline were 87.3 and 206.9 cells/µl at 6 and 12 months, respectively for category A, and 35.8 and 31.6 cells/µl for category B (shown in figure 5).
Figure 5.
Change in CD4 count for two CD4 categories: baseline of <250 and 250–350 cells/µl respectively.
There was a decrease of 4.2 cells/µl at 12 months from the previous count. Statistically, however, the study found no significant difference in (P=0.18) treatment response between the two baseline CD4 categories.
Discussion
This study showed a rapid first phase of immunological recovery within six (6) months of therapy with a mean of 229.2 cells/µl. This was followed by more gradual second and third phases of immunological recovery with a mean of 270 cells/µl at 12 months and 297.6 cells/µl at 18 months of treatment. The large early increases in CD4 counts within the first six months after administration of HAART may be due to immune reconstitution and redistribution of CD4 T cells that were sequestered from the lymphoid tissues into the circulating blood14. Touloumi et al. (2008) 14 explain that, it takes a longer period for new CD4 cells to be formed, and this might have accounted for the subsequent slower rate of CD4 count increases. After 18 months of treatment, immunological response of patients was significant (p<0.0001), with an average rate of 12.6 cells/µl/month. This is consistent with other studies15,16 where a sustained immunological response was reported after a successful initiation of HAART.
Although adherence to therapy is a major challenge especially in developing countries, the study observed a high level of adherence in most patients. This is consistent with a study in Cambodia where as high as 95% of patients enrolled in a comprehensive care programme were adherents.17 Non-adherent patients commenced treatment with a slightly higher baseline CD4 count than adherent patients. However, adherent patients chalked up significant CD4 increases at each time point (p<0.0001), whereas in non-adherent patients, CD4 increases were non-significant (p=0.21 and 0.48 at 6 and 12 months respectively). As in other studies18,19, the study observed that adherent patients had better immune recovery compared to baseline CD4 levels than non-adherent patients. Rougemont et al.20 reports adherence to be a major key to attaining good immunological recovery. The inability of the study to detect a significant difference (P=0.2) may have been due to the large disparity in numbers between adherent and non- adherent patients.
There was higher proportion of women than men in the study with women demonstrating higher CD4 count (77.4cells/µl) than men (57.4cells/µl) at baseline. Perhaps this may be a reflection of women seeking treatment earlier than their male counterparts. Good immunological recovery was observed during therapy in both sexes. Although women had more favourable responses than men during therapy, the study found no gender differences in treatment response (p=0.46).
The age of the patients was stratified to ascertain the immune recovery among different age categories of patients starting HAART. The different age categories had similar for baseline CD4 counts.
The study showed that younger and middle aged patients responded to treatment better than older patients. This may be due to the fact that younger age is associated with more rapid CD4 recovery due to preserved thymic function.21,22 The slower immune response in older patients may be due to the gradual deterioration of the immune system as a result of functional decline of T cells with age. It is reported that age of patient at start of HAART could influence CD4 cell recovery.22
The study observed that patients in the lower CD4 category showed larger and significant increases at 6 and 12 months respectively. In line with the findings of this study, many studies have23–26 reported greater CD4 increases among highly advanced adherent patients. A study27 also reported significant increases after four (4) weeks of treatment in patients with <200 cells/µl in a study in Botswana. A number of studies28, 29 have also shown that a good immunological response to HAART can be achieved regardless of the CD4 count at initiation of therapy.
A major limitation of the study was the small number of patients who participated. This was partly due to patients' unwillingness to participate and partly due to time constraints on the part of the authors. Thus, the study results may not be generalized. The study suggests that more studies on treatment adherence and immune response among patients on HAART may be required. This will help strengthen National HIV/AIDS Control Program's establishment of an early warning system for HIV drug resistance and monitoring effectiveness of treatment, using adherence as one of the indicators.
Conclusion
The study can conclude that significant immunological improvement is possible among Ghanaian PLHIV on HAART as long as a high level of treatment adherence is observed. The data has shown that significant CD4 gains are possible after 18 months of treatment in patients commencing therapy with baseline CD4 count ≤250 cells/µl.
Acknowledgement
The authors would like to acknowledge Mr Kwabena Adjei-Asante and the entire staff of Serology unit for their support and assistance. We wish to thank Madam Aisha Yussif and all staff of HIV clinic of Komfo Anokye Teaching Hospital for their assistance. We express our gratitude to all participants who consented to take part in the study. Sincere thanks go to Sharon Dynne and Rafiq Okine formally of Noguchi Memorial Institute for Medical Research for providing support during data analysis.
References
- 1.WHO/UNAIDS, author. AIDS epidemic update: December 2006. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization; 2006. [Google Scholar]
- 2.UNAIDS, author. Report on the global AIDS epidemic. Geneva: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2010. [Google Scholar]
- 3.WHO/UNAIDS, author. 2009 AIDS epidemic update. Geneva: World Health Organization; 2009. [Google Scholar]
- 4.Fobil JN, Soyiri IN. An assessment of government policy response to HIV/AIDS in Ghana. Journal of Social Aspects of HIV/AIDS. 2006;3(2):457–465. doi: 10.1080/17290376.2006.9724872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jayasuriya A, Robertson C, Allan PS. Twenty-five years of HIV management. J R Soc Med. 2007;100(8):363–366. doi: 10.1258/jrsm.100.8.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palella FJ, Baker RK, Moorman AC, Chmiel JS, Wood KC, Brooks JT, Holmberg SD. Mortality in the highly active antiretroviral therapy era. Changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 7.Mocroft A, Phillips AN, Fatkenheuer G, Lundgren JD, et al. Normalisation of CD4 counts in patients with HIV-1 infection and maximum virological suppression who are taking combination antiretroviral therapy: an observational cohort study. Lancet. 2007;370:407–413. doi: 10.1016/S0140-6736(07)60948-9. [DOI] [PubMed] [Google Scholar]
- 8.Schwab U, Collini P, Obeng-Baah J, Sarfo FS, Norman B, Appiah L, Chadwick D, Bedu-Addo G. A sustained increase in CD4 count and high proportion of patients remaining on first line therapy at 36 months in a cohort of Ghanaian patients attending a public HIV treatment clinic. HIV Med. 2008;9(1):17. [Google Scholar]
- 9.National HIV/AIDS Control Programme, author. Guidelines for Anti-retroviral Therapy in Ghana. Accra-Ghana: National HIV/AIDS Control Programme, Ghana Health Service, Ministry of Health; 2005. [Google Scholar]
- 10.National HIV/AIDS Control Programme, author. Guidelines for antiretroviral therapy in Ghana. Accra-Ghana: National HIV/AIDS Control Programme, Ghana Health Service, Ministry of Health; 2008. [Google Scholar]
- 11.Duffel E, Toskin I. Guidelines of HIV surveillance among tuberculosis patients. Geneva: World Health Organisation; 2004. [Google Scholar]
- 12.Dickinson Becton. BD FACSCount system user's guide for use with BD FACSCount CD4 reagents. San Jose, CA, USA: BD Biosciences; 2007. [Google Scholar]
- 13.Costa J. Calculating geometric mean. Buzzards Bay National Estuary Program; [March 2010]. http://www.buzzardsbay.org/geomean.htm. [Google Scholar]
- 14.Touloumi G, Pantazis N, Stirnadel HA, Walker SA, Boufassa F, Vanhems P, Porter K. Rates and determinants of virologic and immunological response to HAART resumption after treatment interruption in HIV-1 clinical practice. J Acquir Immune Defic Syndr. 2008;49(5):492–498. doi: 10.1097/QAI.0b013e318186ead2. [DOI] [PubMed] [Google Scholar]
- 15.Kilaru KR, Kumar A, Sippy N, Carter AO, Roach TC. Immunological and virological responses to highly active antiretroviral therapy in a nonclinical trial setting in a developing Caribbean country. HIV Med. 2006;7(2):99–104. doi: 10.1111/j.1468-1293.2006.00347.x. [DOI] [PubMed] [Google Scholar]
- 16.Waters L, Stebbing J, Mandalia S, Bower M, Nelson M, Gazzard B, et al. A comparison of the CD4 response to antiretroviral regimens in patients commencing therapy with low CD4 counts. J Antimicrob Chemother. 2004;54(2):503–507. doi: 10.1093/jac/dkh329. [DOI] [PubMed] [Google Scholar]
- 17.Spire B, Carrieri P, Ngeth C, Delfraissy JF, Laureillard D, et al. Adherence to antiretroviral therapy in patients enrolled in a comprehensive care program in Cambodia: a 24-month follow-up assessment. Antivir Ther. 2008;13(5):697–703. [PubMed] [Google Scholar]
- 18.Burman WJ, Darbyshire J, Wu AW, Grund B, Roediger MP, Friedland G. for SMART Study Group. The Impact of Episodic CD4 cell countguided antiretroviral therapy on quality of life. J Acquir Immune Defic Syndr. 2008;47(2):185–193. doi: 10.1097/QAI.0b013e31815acaa4. [DOI] [PubMed] [Google Scholar]
- 19.Bansi LK UK Collaborative HIV Cohort Study, author. Are previous treatment interruptions associated with higher viral rebound rates in patients with viral suppression? AIDS. 2008;22(3):349–356. doi: 10.1097/QAD.0b013e3282f4709a. [DOI] [PubMed] [Google Scholar]
- 20.Rougemont M, Stoll BE, Elia N, Ngang P. Antiretroviral treatment adherence and its determinants in sub-Saharan Africa: a prospective study at Yaounde Central Hospital, Cameroon. AIDS Res Ther. 2009;6:21. doi: 10.1186/1742-6405-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger S, Petoumenos K, Falster K, Zhou J, Law MG, et al. Long-term patterns in CD4 response is determined by an interaction between baseline CD4 cell count, viral load and time: the Asia Pacific HIV Observational Database (APHOD) J Acquir Immune Defic Syndr. 2009;50(5):513–520. doi: 10.1097/qai.0b013e31819906d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viard JP, Mocroft A, Chiesi A, Johnson M, Lundgren JD, et al. Influence of age on CD4 cell recovery on human immunodeficiency virusinfected patients receiving highly active antiretroviral therapy: Evidence from the EuroSIDA Study. J Infect Dis. 2001;183(8):1290–1294. doi: 10.1086/319678. [DOI] [PubMed] [Google Scholar]
- 23.Smith CJ, Sabin CA, Youle MS, Cropley I, Johnson MA, Phillips AN, et al. Factors influencing increases in CD4 cell counts of HIV-positive persons receiving long-term highly active antiretroviral therapy. J Infect Dis. 2004;190(10):1860–1868. doi: 10.1086/425075. [DOI] [PubMed] [Google Scholar]
- 24.Lawn SD, Myer L, Bakker LG, Wood R. CD4 cell count recovery among HIV-infected patients with very advanced immunodeficiency commencing antiretroviral treatment in sub-Saharan Africa. BMC Infect Dis. 2006;6:59. doi: 10.1186/1471-2334-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajasekaran S, Jeyaseelan L, Krithigaipriya KA, Kuralmozhi R, et al. Increase in CD4 cell counts between 2 and 3.5 years after initiation of antiretroviral therapy and determinants of CD4 progression in India. J Postgrad Med. 2009;55(4):261–266. doi: 10.4103/0022-3859.58929. [DOI] [PubMed] [Google Scholar]
- 26.Gras L, Kesselring AM, Miedema F, Reiss P, Lange J, de Wolf, et al. CD4 cell counts of 800 cells/mm3 or greater after 7 years of highly active antiretroviral therapy are feasible in most patients starting with 350 cells/mm3 or greater. J Acquir Immune Defic Syndr. 2007;45(2):183–192. doi: 10.1097/QAI.0b013e31804d685b. [DOI] [PubMed] [Google Scholar]
- 27.Wester CW, Kim S, Bussmann H, Moffat H, Essex M, Marlink R, et al. Initial response to highly active antiretroviral therapy in HIV-1C-infected adults in a public sector treatment program in Botswana. J Acquir Immun Defic Syndr. 2005;40(3):336–343. doi: 10.1097/01.qai.0000159668.80207.5b. [DOI] [PubMed] [Google Scholar]
- 28.Staszewski S, Miller V, Sabin C, Schlecht C, Gute P, Stamm S, Leder T, Berger A, Weidemann E, Hill A, Phillips A. Determinants of sustainable CD4 lymphocyte count increases in response to antiretroviral therapy. AIDS. 1999;13(8):951–956. doi: 10.1097/00002030-199905280-00011. [DOI] [PubMed] [Google Scholar]
- 29.Douek DC, McFarland RD, Keiser PH, Zack JA, Picker LJ, Koup RA, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396(6712):690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]