Abstract
Purpose:
The purpose was to determine the correlation of clinical factors and lung dose volume parameters with significant radiation pneumonitis (RP) in non-small cell lung cancer patients treated with combined modality therapy.
Materials and Methods:
Between January 2008 and December 2010, 52 patients of non-small cell lung cancer were treated with combined modality therapy with radical intent. Radiation pneumonitis was correlated with ipsilateral (V20 ipsi, V5 ipsi and MLD ipsi) and whole lung (V20, V5, and MLD) dose volume parameters. Clinical factors like pulmonary function tests (PFT), site of tumor, planning target volume, and type of treatment were also correlated with incidence of significant pneumonitis.
Results:
Out of 52 patients, 35.3% developed grade 2 or more pneumonitis. On univariate analysis, factors significantly correlating with radiation pneumonitis were V5 (P = 0.002), V5 ipsi (P = 0.000), V20 (P = 0.019), V20 ipsi (P = 0.004), MLD (P = 0.008) and MLD ipsi (P = 0.008). On multivariate analysis, V5 ipsi was retained as the most significant factor. Concurrent chemoradiation caused significantly more RP than neoadjuvant chemoradiation (P = 0.004). A cutoff of 65% for V5 ipsi had a sensitivity of 65% and a specificity of 91%.
Conclusion:
The correlation between pneumonitis and dosimetric constraints has been validated. Adding ipsilateral V20, V5, and MLD to the classical total lung constraints identifies patients likely to develop pulmonary toxicity in patients undergoing chemoradiation.
Keywords: Chemoradiation, ipsilateral dose volume parameters, locally advanced non small cell lung cancers, radiation pneumonitis
Introduction
Chemoradiotherapy is the standard treatment of locally advanced lung cancer which is associated with significant dose limiting toxicities like radiation pneumonitis (RP) and esophagitis.[1,2] In a dose escalation study of 109 patients, grade 2 to 3 pneumonitis was seen in 14.6% and this was associated with lung-dosimetric parameters such as the mean lung dose (MLD), volume of lung that received at least 20 Gy (V20), and the normal-tissue complication probability (NTCP) of the lung. With a cutoff of 30% for V20 and 20 Gy for MLD the positive and negative predictive values were 50% to 71% and 85% to 89% respectively.[3] Paradoxically, the Vdose and MLD referring to both lungs as a single functional unit do not take into account the possible chances of an imbalance between the doses to the lung with the primary tumor and the contralateral lung; substantially, the final value of each dosimetric parameter is just a mean value for the total lung parenchyma, so an extreme case could be characterized by no dose to the contralateral lung by concentrating the radiation beams on one lung. The focus of this analysis is to assess the relevance of ipsilateral dosimetric parameters on RP.
Materials and Methods
A total of 52 patients of NSCLC treated with radical radiotherapy from January 2008 to December 2010 were included in this analysis. The clinical stage was IIB in 4 patients, IIIA in 16 patients, and IIIB in 32 patients. No patients with stage IV were included. The primary tumor was located in the upper lobe in 73%, middle lobe in 10%, and lower lobe in 17% patients. A total of 37 were treated with concurrent chemoradiotherapy and 21 with neoadjuvant chemotherapy followed by radiotherapy alone. Concurrent chemotherapy included 50 mg/m2 of cisplatin on first and eighth day and etoposide 50 mg/m2 on days 1-5 of the first and last week of radiation therapy. Neoadjuvant chemotherapy consisted of a combination of cisplatin 75 mg/m2 on day 1 and etoposide 100 mg/m2 on days 1-3 administered three weekly for four cycles which was followed by radical radiotherapy.
Radiotherapy was provided by use of a linear accelerator (Clinac C 600; Varian Medical Systems, Palo Alto, CA) with 6 MV photon beam. All patients underwent three-dimensional treatment planning by use of Eclipse computer software (version 8.0; Varian Medical Systems). Treatment planning was based on CT scans with 5 mm section thickness and 5 mm intervals obtained in the treatment position on a wing board. Tissue heterogeneity correction was routinely performed. Radiotherapy was administered with an angled field technique (coplanar) to include the entire target volume (planning target volume (PTV) in the isodose 100% (range, 95-107%) area. The total prescribed dose was 45-55 Gy in 36%, 56-66 Gy in 74% patients. Many patients receiving 56-66 Gy underwent treatment in two phases with a repeat CT simulation after 40 Gy. Those who received 45-50 Gy had large tumors which were lying adjacent to spinal cord where dose escalation was not possible even in two phases. The gross tumor volume (GTV) was defined as tumor extension and metastatic lymph nodes. The clinical target volume was a 5 mm expansion beyond GTV, and PTV consisted of 1 cm margin around CTV (1.5 cm craniocaudal margin in middle and lower lobe tumors). Elective nodal irradiation was never administered. The primary GTV was contoured on the CT parenchyma window, whereas the nodal GTV was contoured on the mediastinum only. The right lung and the left lung were contoured separately and then taken into consideration as a single structure called “total lung,” which was defined as Right lung + Left lung – PTV[4]. Accordingly, the ipsilateral lung was defined as Right lung/Left lung – PTV. All treatment plans were approved if conventional dosimetric lung constraints were respected: V20 of 37% or lower, and MLD of 20 Gy or lower; moreover, the dose delivered to the spinal cord was limited to less than 45 Gy. V20, V5, and MLD were also evaluated on the ipsilateral lung (which is the lung on the side containing >50% of the PTV), and they were registered as V20 ipsi, V5 ipsi, and MLD ipsi, respectively.[4,5] During and after treatment completion, patients were followed up every 4 weeks usually for up to 6 months and every 2 months on an outpatient basis. RP was graded according to the National Cancer Institute Common Toxicity Criteria, version 3.0.[6] Apart from dose volume constraints, PTV, lung lobe, pulmonary function test (PFT), and type of treatment (concurrent chemoradiotherapy vs. neoadjuvant chemotherapy followed by radiotherapy) were also correlated with the risk of pneumonitis.
Statistics
Univariate comparisons between subjects with and without RP were done using independent samples t-test. For multivariate analysis, forward stepwise logistic regression analysis was performed using SPSS. Receiver operator characteristics (ROC) curve was plotted and the area under the curve was estimated. All statistical tests were two sided, and P = 0.05 was considered statistically significant.[7]
Results
After a median follow-up period of 24 months (range, 4-48 months), 44.5% had no RP, 21.2% had grade 1 RP, and 35.3% had RP of grade 2 or higher. Grade 3 pneumonitis was reported in only one patient. Radiotherapy and chemotherapy compliance was 80%. The FeV1 was more than 2 l in 30%, 1-2 l in 35%, less than 1 l in 15% and FeV1 status was unknown in 20% patients. For the entire cohort, the lung injury arose after a median period of 49 days from the beginning of radiotherapy. A total of 27.7% patients on neoadjuvant treatment developed RP as compared to 42.9% patients with concurrent chemoradiotherapy and this difference was significant (P = 0.004). The median follow-up of these patients was 14 months.
The mean V20, MLD, V5 ipsi, and MLD ipsi for the entire cohort was 30.2%, 16.6 Gy, 63.9, and 28.8 Gy. On univariate analysis, factors significantly correlating with radiation pneumonitis were V5 (P = 0.002), V5 ipsi (P = 0.000), V20 (P = 0.019), V20 ipsi (P = 0.004), MLD (P = 0.008), MLD ipsi (P = 0.008) [Table 1]. If we analyze the no-RP group and the RP group, mean values of V5 were 35.6 vs. 50.3, V5 ipsi 57 vs. 71, V20 25.9 vs. 33.6, V20 ipsi 46.5 vs. 60, MLD 13.8 vs. 18.8, MLD ipsi 24 vs. 32.6 [Table 1]. On multivariate analysis for the development of pneumonitis of grade 2 or higher, V5 ipsi was retained as the most significant factor (P = 0.02). PTV, PFT, site of tumor were not significantly different between the two groups but all lower lobe patients developed RP (n = 10). A higher incidence of RP was observed in patients receiving concurrent chemoradiation than in neoadjuvant chemotherapy followed by the radiotherapy group and this difference was significant (P = 0.004). On ROC curve analysis, with 65 as cutoff for V5 ipsi the specificity is 91.3% and sensitivity is 62% [ROC 0.83 (CI 71.4-94.1)] [Figure 1]. The area under the ROC curve for V5 ipsi was 0.83 (CI 71.4 to 94.1; Figure 1). Using the ROC curve, a cutoff of 65 was selected for V5 ipsi, which gave a specificity of 91.3% and sensitivity of 62%.
Figure 1.
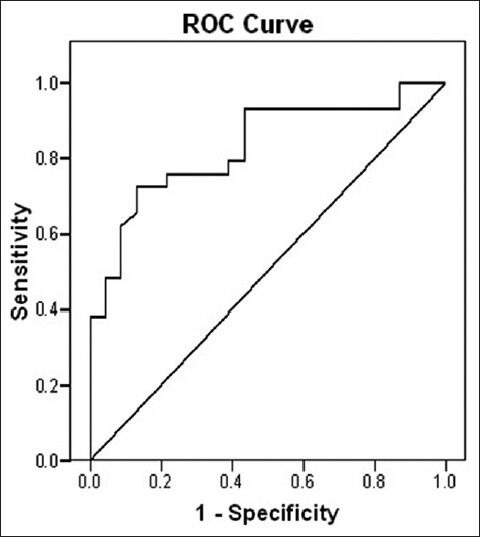
ROC curve showing specificity and sensitivity of V5 ipsi
Discussion
According to EORTC recommendations, dose volume constraints for concurrent chemoradiotherapy in non-small lung cancers should be 35% for V20 and 20 Gy for MLD for whole lung.[8] Based on these constraints we observed grade 2 or more pneumonitis in 35.3% of our patients treated with chemoradiotherapy. This is lower than 51% incidence with concurrent chemoradiotherapy reported by Tsujino.[9] The mean V20 and MLD of patients with RP was 33.6% and 18.8 Gy and these were significantly different from those in the no RP group. In spite of applying standard dose volume constraints, 55% of patients with V20 <37% and 58% with MLD <20 Gy developed RP. The constraints set for treatment planning in lung are based on studies which have derived dose volume parameters by deducting GTV from whole lung volume. These constraints if applied to treatment plans with whole lung volume minus PTV would yield higher rates of pneumonitis as is evident from our study. Our results suggest that patients with RP had mean V20 ipsi, V5 ipsi, and MLD of 60%, 71.6%, and 32.6 Gy. Since only V5 ipsi was significant on multivariate analysis, we derived a cutoff for this parameter to predict RP. A V5 ipsi more than 65% was found to be predictive of developing RP. Constraints for V20 ipsi >52%, V30 ipsi >39%, and MLD >22 Gy have been found to be predictors of radiation pneumonitis by Ramella.[10] These constraints somewhat differ from that of our study which could be because of heterogeneity in the patient population. A total of 47.4% of their patients underwent surgery after initial chemoradiation and dose volume parameters of this subset should ideally not be combined with that of chemoradiation alone. They did not separately report the dose volume parameters for the surgical and nonsurgical subset. Few authors have examined the effect of the radiation dose applied to the ipsilateral and contralateral lungs separately. In the study of Oetzel et al., lungs were analyzed as separate organs.[11] The toxicity rate was 0% if MLD ipsi was 15 Gy or lower, 13% if MLD ipsi was 17.5-20 Gy, 21% if MLD ipsi was 22.5-25 Gy, and 43% if MLD ipsi was 27.5 Gy or greater. The publications by Seppenwoolde and Yorke suggested that RP correlated with the percentage of ipsilateral lung volume exceeding 5 Gy (V5) through 30 Gy (V30), but the authors did not suggest a constraint.[12,13] The higher incidence of RP observed in patients receiving concurrent chemoradiation have also been reported by Tsujino.[9]
We did not find a correlation of PFT, PTV size, RT dose, and tumor site with RP though all patients with lower lobe developed RP. Only two thirds of patients had appropriate FeV1 according to ERS/ETS criteria for suitability for chemoradiation which explains the high rate of pneumonitis in our patients.[14] RP has been correlated with lower lobe tumors, RT dose, and PTV size earlier.[10,15,16]
The drawback of this study is the small sample size which is based on the accrual over last 5 years. Most patients present with pleural effusion and hence number of patients suitable for chemoradiotherapy is small.
Conclusions
These results suggest that for patients being treated by chemoradiation, ipsilateral dose volume parameters could be better predictive constraints for lung injury. This needs to be validated in a larger population.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Robinson LA, Ruckdeschel JC, Wagner H, Jr, Stevens CW American College of Chest Physicians. Treatment of non-small cell lung cancer-stage IIIA: ACCP evidence-based clinical practice guidelines (2 nd edition) Chest. 2007;132:243S–65. doi: 10.1378/chest.07-1379. [DOI] [PubMed] [Google Scholar]
- 2.Albain KS, Crowley JJ, Turrisi AT, 3rd, Gandara DR, Farrar WB, Clark JI, et al. Concurrent cisplatin, etoposide, and chest radiotherapy in pathologic stage IIIB non-small-cell lung cancer: A Southwest Oncology Group phase II study, SWOG 9019. J Clin Oncol. 2002;20:3454–60. doi: 10.1200/JCO.2002.03.055. [DOI] [PubMed] [Google Scholar]
- 3.Kong FM, Hayman JA, Griffith KA. Final toxicity results of a radiation–dose escalation study in patients with non-small-cell lung cancer (nsclc): Predictors for radiation pneumonitis and fibrosis. Int J Radiation Oncology Biol Phys. 2006;65:1075–86. doi: 10.1016/j.ijrobp.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 4.Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, et al. Clinical dose–volume histogram analysis for pneumonitis after 3d treatment for non-small cell lung cancer. Int J Radiation Oncology Biol Phys. 1999;45:323–9. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 5.Hernando ML, Marks LB, Bentel GC, Zhou SM, Hollis D, Das SK, et al. Radiation-induced pulmonary toxicity: A dose–volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:650–9. doi: 10.1016/s0360-3016(01)01685-6. [DOI] [PubMed] [Google Scholar]
- 6.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: Development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 7.Cox DR. Regression models and life-table. J R Stat Soc. 1972;334:187–220. [Google Scholar]
- 8.Senan S, De Ruysscher D, Giraud P, Mirimanoff R, Budach V Radiotherapy Group of European Organization for Research and Treatment of Cancer. Literature-based recommendations for treatment planning and execution in high-dose radiotherapy for lung cancer. Radiother Oncol. 2004;71:139–46. doi: 10.1016/j.radonc.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Tsujino K, Hirota S, Endo M, Obayashi K, Kotani Y, Satouchi M, et al. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2003;55:110–5. doi: 10.1016/s0360-3016(02)03807-5. [DOI] [PubMed] [Google Scholar]
- 10.Ramella S, Trodella L, Mineo TC, Pompeo E, Stimato G, Gaudino D, et al. Adding ipsilateral V20 and V30 to conventional dosimetric constraints predicts radiation pneumonitis in stage III a-b NSCLC treated with combined-modality therapy. Int J Radiation Oncology Biol Phys. 2010;76:110–5. doi: 10.1016/j.ijrobp.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Oetzel D, Schraube P, Hensley F, Sroka-Pérez G, Menke M, Flentje M. Estimation of pneumonitis risk in three-dimensional treatment planning using dose volume histogram analysis. Int J Radiat Oncol Biol Phys. 1995;33:455–60. doi: 10.1016/0360-3016(95)00009-N. [DOI] [PubMed] [Google Scholar]
- 12.Seppenwoolde Y, De Jaeger K, Boersma LJ, Belderbos JS, Lebesque JV. Regional differences in lung radiosensitivity after radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;60:748–58. doi: 10.1016/j.ijrobp.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 13.Yorke ED, Jackson A, Rosenzweig KE, Braban L, Leibel SA, Ling CC. Correlation of dosimetric factors and radiation pneumonitis for non-small-cell lung cancer patients in a recently completed dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:672–82. doi: 10.1016/j.ijrobp.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Brunelli A, Charloux A, Bolliger CT, Rocco G, Sculier JP, Varela G, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy) Eur Respir J. 2009;34:17–41. doi: 10.1183/09031936.00184308. [DOI] [PubMed] [Google Scholar]
- 15.Hope AJ, Lindsay PE, El Naqa I, Alaly JR, Vicic M, Bradley JD, et al. Modeling radiation pneumonitis risk with clinical, dosimetric, and spatial parameters. Int J Radiat Oncol Biol Phys. 2006;65:112–24. doi: 10.1016/j.ijrobp.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 16.Marks LB, Bentzen SM, Deasy JO, Kong FM, Bradley JD, Vogelius IS, et al. Radiation dose-volume effects in the lung. Int J Radiation Oncology Biol Phys. 2010;76:S70–6. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]


