Abstract
Background:
The primary aim of this prospective non-randomized study was to evaluate the effect of hemi-body irradiation (HBI) on pain and quality of life in cancer patients with extensive bone metastases. The secondary aim was to evaluate side-effects and cost-effectiveness of the treatment.
Materials and Methods:
Between March 2008 and December 2010, a total of 23 (male = 14, female = 9, median age = 60 years) diagnosed cases of metastatic cancer patients (prostate = 11, breast = 6, and lung = 6) received HBI, which was delivered as lower (n = 7) (dose = 8 Gy), upper (n = 8) (dose = 6 Gy), or sequential HBI (n = 8) with a Telecobalt unit (Theratron 780C). Among them, one lung cancer patient died at 2 months and one prostate cancer patient defaulted after the second follow-up. Thus, 21 patients (male = 13, female = 8, median age = 65 years) (prostatic cancer = 10, breast cancer = 6, and lung cancer = 5) were followed up for a minimum of 6 months. Evaluations were performed before and at 2, 4, 8, 16, and 24 weeks after treatment. Pain evaluation was done by Visual Analogue Scale (VAS), Verbal Rating Scale (VRS), Percentage of Pain Relief (PRR), and Global Pain Score (GPS). Toxicity was assessed by CTC v-3 toxicity scores in the medical record. Assessment of oral morphine consumption was done before and after radiation using paired t-test, and correlation analysis was also done with decrease of morphine consumption and reduction of pain score using statistical analysis.
Results:
Response (control of pain) was partial (PR) in 67% and complete (CR) in 22% of patients. For most patients, the pain control lasted throughout the follow-up period (6 months). From 66.66% patients requiring 13 or more Morphine (10 mg) tablets per day prior to HBI, none of the patients required to consume 13 or more Morphine (10 mg) tablets per day following HBI, which was correlated with significant reduction in various pain scores (P < 0.05). One way ANOVA with Dunnett's Multiple Comparison Test (P < 0.05) was significant in VAS score changes, VRS score changes, PPR score changes, and GPS score changes. Along with the decrease in morphine tablets, the Linear Correlation of various scales for pain reduction like VAS, VRS, PPR, and GPS were significant. As such, the quality of life was better due to decreased pain and also, a decrease in the dose of analgesics. Grade 1 and 2 hematological toxicity and grade 1 diarrhea were observed as common side-effects. The average total cost of treatment including hospital stay, medicines, and radiation charges was around INR 400.00.
Conclusion:
This study shows that hemibody irradiation is not only an effective modality for palliation of severe bone pain in advanced cancer cases but also economical, involves short hospital stay, with acceptable side-effects, utilizes the simple Telecobalt machine, and is less cumbersome in comparison to other currently available pain palliation methods like oral morphine and radiopharmaceuticals.
Keywords: Bone metastasis, hemibody irradiation, palliation, pain
Introduction
Bone metastases are a major complication of many solid tumors like prostate, lung, and breast cancers.[1,2] Although bone metastases are often clinically silent to start with, they may lead to serious sequelae such as pain, fractures, and hypercalcemia.[3] Most patients experiencing bone pain eventually require opiates, which can significantly alter the patient's quality of life (QoL). The treatment options for painful bone metastases include analgesics, bisphosphonates, surgical intervention and radiotherapeutic treatments, including external beam radiation therapy (EBRT), hemibody irradiation (HBI), and radiopharmaceuticals.[4,5] For uncomplicated bone metastases, a non-surgical approach like EBRT is arguably the most commonly used therapeutic modality. The goal of palliative radiotherapy for bone metastases is to rapidly improve the QoL of patients. The most desirable treatment should be clinically efficacious, minimally toxic, time-efficient, and cost-effective. Different radiotherapy regimens, including single-fraction and multiple-fraction regimens, are used.[6,7,8,9] Multiple randomized trials have demonstrated the equivalence of single-fraction and multiple-fraction palliative radiotherapy.
The present study was undertaken to prospectively evaluate the usefulness of wide field radiotherapy (hemi body) for pain palliation in wide-spread diffuse bone metastases and to find out its relative usefulness over other measures currently available for pain management in terms of logistics, patient comfort, and cost-effectiveness in a resource-poor set up.
Materials and Methods
The study was conducted between March 2007 and December 2010 at the Department of Radiotherapy, Medical College, Kolkata. The study was prospective in nature, but no randomization was done. Clearance from institutional ethics committee was obtained. All patients were diagnosed cases of cancer with multiple skeletal metastases and had received all systemic forms of therapy including chemotherapy and/or hormone therapy and/or biologic therapy. All cases were on analgesic treatment according to the WHO analgesic ladder and were already on oral morphine. Patients were selected in all diagnosed cases of primary cancer having histopathological and/or radiological suggestion of widespread bone metastases, with (Inclusion criteria) Karnofsky Performance Status ≥60, pain not controlled by analgesic therapy, and baseline hemogram within normal limits (Hb > 10 g/dl; TLC > 3500/μl; Platelet count > 100,000/μl). Exclusion criteria included unconscious, uncooperative or very poor performance status patients with or without presence of bed sore, ulceration at the intended area of interest of radiation, compromised liver and renal function, history of recent prior radiation within 6 months to the intended site of radiation, and presence of any cord compression, neuropathy, Superior Vena Cava Obstruction (SVCO), pathological fracture or brain metastasis. Liver function test, urea, creatinine, BUN, and complete blood count (CBC) were checked at every visit. Any form of chemotherapy, hormone therapy, or biologic therapy was withheld prior to radiation. Informed consent was taken after verbally explaining about the procedure to patients and their family members. No explanation was given about the possible benefits so as not to involve any bias during assessment of pain post-treatment.
All patients were admitted, and they received anti-emetic premedication prior to radiation. After radiation, they also received anti-emetics (ondansetron tablets/injections 4-8 mg for two to three times/day) and hydration.
All patients were treated with HBI by Co 60 machine (Theratron 780 C, Theratronics International, Ontario, Canada). They received either upper HBI (UHBI) or lower HBI (LHBI) or both. The field borders were kept as follows- for UHBI, the superior border was kept at the angles of mandible extending over the chin, the inferior border was kept at the midpoint of umbilicus. For LHBI, the superior border corresponded to match the lower border of the UHBI and the inferior border was kept just below the knee. The lateral borders were kept 2 cm away from the lateral margin of the body. All cases were treated by anterior – posterior portals by extended SSD method. CBC was checked at 4 days, 2 weeks, and then at 4 weekly intervals for 3 months post-radiation. CBC was done outside, and the reports were presented to us by the patient's family.
For non-ambulatory patients who were unable to come back to the center for follow-up, we obtained information from the patient's family. We made them understand the Visual Analog Scale (VAS), and they got it recorded from the patients at their residence. Ambulatory patients staying near and with good transportation facilities were asked to attend the hospital for pain assessment and supply of analgesics. Pain assessment scoring was done as per the 11 point VAS noted on a 10 cm Scale[10,11] or divided one rupee in to 10 equal parts by coins for better understanding locally. All patients were asked to give their assessment of respective individual pain level VAS prior to irradiation, and then at 2, 4, 8, 16, and 24 weeks post-radiation. Patients were also asked to rate pain intensity on a Verbal Rating Scale (VRS), which is a scale of 0 to 4 (none to excruciating).[12] At the second visit (after 2 weeks), the patient was clinically examined and compliance was determined from the study diary. Patient's pain perception was noted on the Visual Analog Scale (VAS) and pain intensity on Verbal Rating Scale (VRS). The patient's new pain perception noted on VAS scale was compared with the previous one. Values of 0, 1, or 2 were assigned if the score decreased, remain unchanged, or increased. Percentage of Pain Relief (PPR) during current treatment was noted on a scale of 0 to 4: 0 (100%), 1 (80 to 100%), 2 (50 to < 80%), 3 (30 to < 50%), 4 (0 to < 30%). A Global Pain Score was calculated as the sum of the change in VAS, VRS, and the % of Pain Relief (PPR). The Global Pain Score ranged from 0 (no pain at all) to 10 (maximum, increasing pain).[12,13,14] Adverse events if any were recorded.
The same procedures were repeated at each of the subsequent visits till stoppage of study after 6 months. Change in VAS pain score, VRS score, and PPR score was assessed at each visit and Global Pain Score was calculated.
The study medications were dispensed to the subjects according to their pain. A study diary was asked to be maintained by each patient regarding the intensity of pain relief. Daily consumption of tablets (10 mg morphine tablets) was according to their pain. The analgesic intake was evaluated according to the dose of morphine used before and at 6 months after the treatment for calculation. During follow-up, patients were assessed for pain at the outpatients department (OPD) office room and if they could not come individually, then the assessment was taken from their family members who could either tell in terms of the rupee scale or bring a letter from the patient explaining his/her pain level. Fentanyl transdermal patch was selected as rescue analgesic in case of inadequate pain relief using morphine tablets. The physician's Global Clinical Assessment (GCA) of efficacy and safety was graded on a four-point scale as poor (0), satisfactory (1), good (2), and excellent (4).[14]
Statistical methods
Measures of central tendency including median and standard deviation were calculated for the patient characteristics including age, sex, primary tumor, and for pain scores before and after treatment. Radiation therapy details were noted, and the median and range were also noted. One way ANOVA with Dunnett's Multiple Comparison Test was used to compare the pain scores before and after radiation. Correlation between pain relief and analgesic consumption was done, and degree of association was assessed by Pearson's correlation coefficient.
Results
A total of 23 patients were included. Among them, one lung cancer patient died at 2 months and one prostate cancer patient defaulted after the second follow-up. So, 21 patients (male = 12, female = 9, median age = 65 years) (prostate cancer = 10, breast cancer = 6, and lung cancer = 5) were followed up for a minimum of 6 months. All the patients were in an advanced stage of cancer with multiple bone metastases. Patients’ characteristics are depicted in Table 1. All patients had completed treatment. Out of 21 cases, 10 cases had sequential both UHBI and LHBI, 5 cases had LHBI, and 6 cases had UHBI [Table 2]. For all patients, the pain control lasted throughout the follow-up period (6 months). The number of patients requiring ≥13 morphine tablets decreased from 66.66% at the beginning of the study to nil after the study, which correlated with reduction in pain score significantly [Tables 3 and 4]. One way ANOVA with Dunnett's Multiple Comparison Test (P < 0.05) were significant in VAS score changes, VRS score changes, PPR score changes, and GPS score changes. The Linear Correlation of various scales for pain reduction like VAS, VRS, PPR, and GPS were significant [Figures 1 and 2]. Response (control of pain) was partial (PR) in 67% and complete (CR) in 22% of patients. None of the patients had any grade 3 or 4 toxicity. Only grade 1 or 2 nausea and vomiting was seen in 10 patients, and grade 1 diarrhea was seen in 5 patients. There was no grade 3 or grade 4 hematological toxicity. Five patients had grade 1 and 2 leukopenia but did not require antibiotics or G-CSF support. Two patients had grade 2 thrombocytopenia, which was self-limiting on both occasions. Grade 1 and 2 hematological toxicities recovered mostly by 2 weeks without any intervention. The average total cost of treatment including hospital stay, medicines, and radiation charges was around INR 400.00 per patient.
Table 1.
Pre-treatment patient characteristics
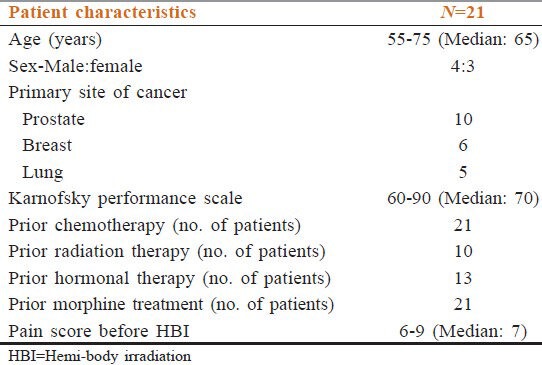
Table 2.
Treatment characteristics

Table 3.
Reduction of Morphine tablets in response to therapy (Morphine 10 mg tab/daily)
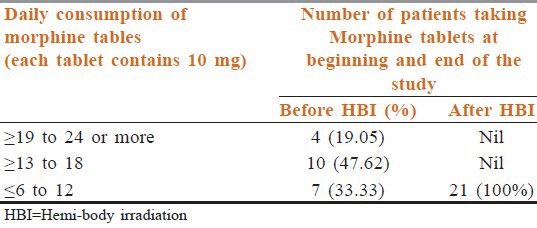
Table 4.
Linear correlation of different pain assessment score with reduction of morphine tablets [Pearson nonparametric correlation]
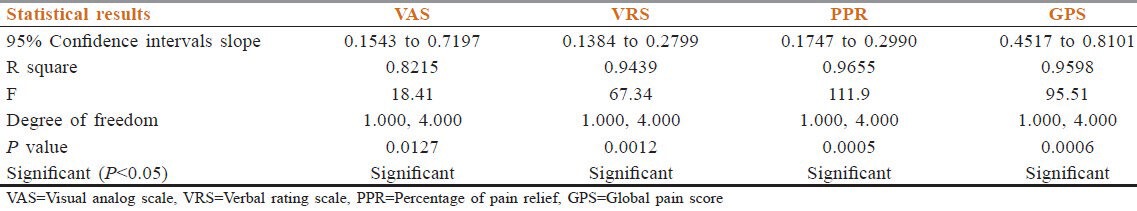
Figure 1.
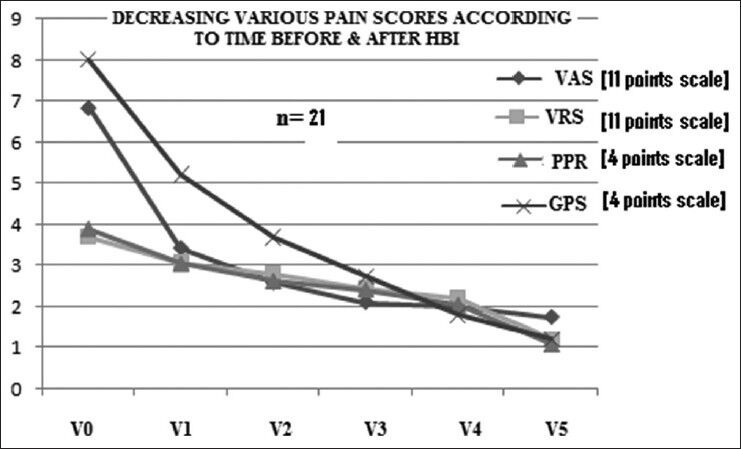
Reduction of pain significantly in all the assessment parameter. (VAS = Visual analog scale, VRS = Verbal rating scale, PPR = Percentage of pain relief, GPS = Global pain score (P > 0.05), V0: Before starting treatment, V1 through V5: 1st to 5th month after treatment)
Figure 2.
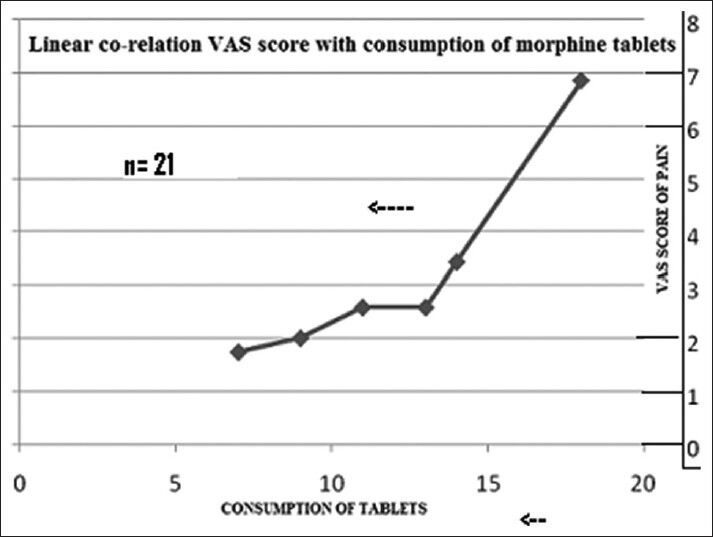
Reduction of analgesics with reduction of % of VAS score is linearly correlated (P < 0.05)
Discussion
Bone metastasis in advanced cancer often poses a difficult problem to manage. Patients can present with bone metastases at the very onset even before the primary is diagnosed or may present during or late after treatment completion. The heterogeneity of presenting symptoms and signs can often lead to a diagnostic and therapeutic dilemma. Pain due to bony involvement by metastasis leads to patient agony and hampers the QoL. The control of pain involves many agents namely- analgesics starting from NSAIDS, weak and strong opioids, and anti-depressants with or without steroids. The WHO analgesic ladder is usually followed but often misinterpreted or misunderstood by many practitioners. Moreover, the patients are often severely distressed and incapacitated or bed-ridden and, therefore, are dependent on other caregivers for getting the morphine from the hospital. This often leads to treatment defaults, which add to severe suffering from the pain. Sometimes, there are issues regarding oral morphine availability in the hospital stock owing to the complicated issues of license related to morphine- import, export, procurement, and this often leads to unavailability of the drug. Moreover, morphine supply is still not universally available at all the tertiary cancer centers, hospitals, or the peripheral district referral centers. Hence, patients often need to resort to other measures like fentanyl patches, which are costly and on an average, one has to pay at least Rs. 4000-4500 per month for such transdermal medications. Other measures like regular zolendronic acid, systemic chemotherapy, intranasal analgesic spray, anesthetic interventions like neural blocks are not cheap, require hospital stay, and also are not very efficacious in extensive widespread bone involvement.
In this context, EBRT is an established modality of palliation of pain, which is neither very costly nor involves logistics of morphine supply and disposal, nor any intervention like nerve blocks or any major systemic complication. In case of widespread bone involvement, the question now is of sequencing, i.e., palliative hypofractionated radiotherapy like 30 Gy in 10 # or 20 Gy in 5 #. In this regard, HBI has been tried and has often proved efficacious in pain palliation.
In our study, all patients who had pain scores >6 did have a subjective response after HBI. The important aspect which needs to be stressed is that HBI is effective in pain control, which has not only immediate nature of effect (almost 50% reduction after only a period of 2 weeks) but also has a sustained effect throughout the 6 months follow-up period. Significant pain relief is consistent with published data of other authors’ also. Moreover, this is also correlated with the degree of decrease in analgesic consumption at follow-up. There are multiple other studies in the literature regarding analgesic usage and the efficacy of HBI.[15,16,17] Salazar et al.[18] described a significant reduction in use of strong opioids after HBI. Dearnaley DP et al.[19] described the absence of a relationship between pre- HBI analgesic requirement and likelihood of benefits in pain control. Leszek Miszczyk et al.[10] showed the reduction of percentage of patients taking strong opioids from 43.8% pre-HBI to 33.3% 5 months after HBI, and the percentage of patients who did not need analgesics increased in the same period from 6.7% to 25%.
The second question is regarding issues of compliance and logistics. For HBI, one has to come only once or twice (at a gap of 4-6 weeks in case of sequential UHBI and LHBI). Hence, it does not involve the burden of travelling often, nor any prolonged stay at the hospital ward or premises as was needed for week-long fractionated RT. Hence, this makes the patients feel more comfortable. The physician's Global Clinical Assessment (GCA) of efficacy and safety was graded on a four-point scale as good.
Normal fractionated radiotherapy is carried out from Monday to Friday; however, HBI is done usually on Saturdays, and thus, does not interfere with the usual scheduled machine treatment time. The average treatment time was 10 to 15 minutes with telecobalt machine in this study and did not hamper any other treatment of other patients as it was done on Saturdays. Hence, the logistics of man power, staff strength, or machine time never posed a hindrance to the execution of the treatment.
The last but major advantage is the cost involved. In our study, the patients had to pay once only and that too according to the standard prescribed rates of radiation exposure in West Bengal government hospital, which is very nominal. Moreover, with added advantage of minimum stay and only once or maximum travelling expenses, the overall cost of therapy is very low as compared to other options of therapy.
Conclusion
This study shows that HBI is an effective modality for palliation of severe bone pain in advanced cancer cases with acceptable side-effects. It is an economical modality of pain management, which involves short hospital stay and utilizes the simple Telecobalt machine. In comparison to other currently available pain palliation methods like oral morphine and radiopharmaceuticals, it is less cumbersome and involves the use of fewer resources.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Vakaet LA, Boterberg T. Pain Control by ionizing radiation of bone metastasis. Int J Dev Biol. 2004;48:599–606. doi: 10.1387/ijdb.041817lv. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Josef E, Shamsa F, Williams AO, Proter AT. Radiotheraputic management of osseous metastases: A survey of current patterns of care. Int J Radiat Oncol Biol Phys. 1998;40:915–21. doi: 10.1016/s0360-3016(97)00927-9. [DOI] [PubMed] [Google Scholar]
- 3.Cook RJ, Major P. Methodology for treatment Evaluation in patients with cancer metastatic to bone. J Natl Cancer Inst. 2001;93:534–8. doi: 10.1093/jnci/93.7.534. [DOI] [PubMed] [Google Scholar]
- 4.Janjan N, Lutz ST, Bedwinek JM, Hartsell WF, Ng A, Pieters RS, Jr, et al. Therapeutic guidelines for the treatment of bone metastasis: A report from the American College of Radiology Appropriateness Criteria Expert Panel on Radiation Oncology. J Palliat Med. 2009;12:417–26. doi: 10.1089/jpm.2009.9633. [DOI] [PubMed] [Google Scholar]
- 5.Fairchild A, Barnes E, Ghosh S, Ben-Josef E, Roos D, Hartsell W, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: Evidence-based practice. Int J Radiat Oncol Biol Phys. 2009;75:1501–10. doi: 10.1016/j.ijrobp.2008.12.084. [DOI] [PubMed] [Google Scholar]
- 6.Sze WM, Shelley MD, Held I, Wilt TJ, Mason MD. Palliation of metastatic bone pain: Single fraction versus multiple-fraction radiotherapy: A systematic review of randomised trials. Clin Oncol (R. Coll Radiol) 2003;15:345–52. doi: 10.1016/s0936-6555(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 7.Sze WM, Shelley M, Held I, Mason M. Palliation of metastatic bone pain: Single fraction versus multiple-fraction radiotherapy: A systematic review of the randomised trials. Cochrane Database Syst Rev. 2004;2:CD004721. doi: 10.1002/14651858.CD004721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: A systematic review. J Clin Oncol. 2007;25:1423–36. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 9.Wu JS, Wong R, Johnston M, Bezjak A, Whelan T. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003;55:594–605. doi: 10.1016/s0360-3016(02)04147-0. [DOI] [PubMed] [Google Scholar]
- 10.Miszczyk L, Tukiendorf A, Gaborek A, Wydmariski J. An evaluation of half-body irradiation in the treatment of widespread, painful metastatic bone disease. Tumori. 2008;94:813–21. doi: 10.1177/030089160809400607. [DOI] [PubMed] [Google Scholar]
- 11.Emanuel EJ, Hauser J, Emanuel LL. Palliative and end of life care. In: Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison's Principles of Internal Medicine. 17th ed. Vol. 1. New York: McGraw Hill; 2008. pp. 70–1. [Google Scholar]
- 12.Serpell MG. Gabapentin in neuropathic pain syndromes: A randomized double-blind placebo-controlled trial. Pain. 2002;99:557–66. doi: 10.1016/S0304-3959(02)00255-5. [DOI] [PubMed] [Google Scholar]
- 13.Bennett M, Simpson KH. Gabapentin in treatment of neuropathic pain. Palliat Med. 2004;18:5–11. doi: 10.1191/0269216304pm845ra. [DOI] [PubMed] [Google Scholar]
- 14.Kalso E, Tasmuth T, Neuvonen PJ. Amitrytiline effectively relieves neuropathic pain following treatment of breast cancer. Pain. 1996;64:293–302. doi: 10.1016/0304-3959(95)00138-7. [DOI] [PubMed] [Google Scholar]
- 15.Nag S, Shah V. Once-a-week lower hemibody irradiation (HBI) for metastatic cancers. Int J Radiat Oncol Biol Phys. 1986;12:1003–5. doi: 10.1016/0360-3016(86)90398-6. [DOI] [PubMed] [Google Scholar]
- 16.Nseyo UO, Fontanesi J, Naftulin BN. Palliative hemibody irradiation in hormonally refractory metastatic prostate cancer. Urology. 1989;34:76–9. doi: 10.1016/0090-4295(89)90167-2. [DOI] [PubMed] [Google Scholar]
- 17.Poulter CA, Cosmatos D, Rubin P, Urtasun R, Cooper JS, Kuske RR, et al. A report of RTOG 8206: A phase III study of whether the addition of single dose hemibody irradiation to standard fractionated local field irradiation is more effective than localfield irradiation alone in the treatment of symptomatic osseous metastases. Int J Radiat Oncol Biol Phys. 1992;23:207–14. doi: 10.1016/0360-3016(92)90563-w. [DOI] [PubMed] [Google Scholar]
- 18.Salazar OM, DaMotta NW, Bridgman SM, Cardiges NM, Slawson RG. Fractionated half-body irradiation for pain palliation in widely metastatic cancers: Comparison with single dose. Int J Radiat Oncol Biol Phys. 1996;36:49–60. doi: 10.1016/s0360-3016(96)00248-9. [DOI] [PubMed] [Google Scholar]
- 19.Dearnaley DP, Bayly RJ, A’Hern RP, Gadd J, Zivanovic MM, Levington VJ. Palliation of bone metastases in prostate cancer. Hemibody irradiation or strontium-89. Clin Oncol, 1992;4:101–7. doi: 10.1016/s0936-6555(05)80975-6. [DOI] [PubMed] [Google Scholar]


