Abstract
Aim:
To assess the nutritional status of gynecological cancer patients using scored Patient Generated Subjective Global Assessment (PG-SGA) then compare it with the body mass index (BMI), hemoglobin, serum albumin, and approximate percentage weight lost in last 1 month so as to find any one parameter that can be used in place of the comprehensive assessment tool.
Materials and Methods:
Sixty gynecological cancer patients were assessed for their nutritional status using BMI, serum albumin, hemoglobin, percentage weight lost in last 1 month, and scored PG-SGA. Correlation, sensitivity, specificity, and predictive values of the former four parameters compared to scored PG-SGA were calculated.
Results:
88.33% of cases were at risk of or had some degree of malnutrition according to scored PG-SGA. Serum albumin level ≤ 2 g/dl had highest specificity and positive predictive value at 1, whereas percentage weight lost in last month had better overall sensitivity, specificity, and positive and negative predictive values of 0.5833, 0.9444, 0.875, and 0.7727, respectively. The Pearson's correlation coefficient between scored PG-SGA and percentage weight lost in last 1 month was 0.784, highest among all the parameters.
Conclusion:
88.33% of gynecologic cancer cases had some degree of malnutrition or were at risk of malnutrition. Approximate percentage weight lost in last 1 month, that is, ≥ 5% may be used in place of the comprehensive scored PG-SGA to triage the patients in case the latter is not used for some reason. Severe hypoalbuminemia ≤ 2 g/dl is an indicator of severe malnutrition in gynecologic cancer cases.
Keywords: BMI, gynecologic cancer, hemoglobin, nutrition, scored PG-SGA, serum albumin
Introduction
Malnutrition is a common and underrecognized problem in cancer patients, correlated to a number of physical, psychological, and clinically relevant adverse effects; including impaired tolerance to anticancer therapy, adverse reactions, and reduced quality of life.[1] So cancer patients should be screened for malnutrition at frequent intervals and managed accordingly.[2,3] Actual physician practice, however, is often inadequate in addressing the nutrition aspects of cancer patients.[4] The various nutrition assessment methods may be arbitrarily divided into subjective (dietetic history), objective (serum albumin, hemoglobin, body mass index (BMI), weight lost in 1-6 months), or comprehensive nutrition assessment tools (scored Patient Generated Subjective Global Assessment (PG-SGA), Malnutrition Universal Screening Tool, and Mini Nutritional Assessment). A screening tool should be easy, standardized, rapid, noninvasive, and cost-effective to identify cancer patients at nutritional risk in daily clinical practice. To date, the scored PG-SGA and the Malnutrition Screening Tool (MST) are the best validated tools for use in oncology patients. Scored PG-SGA has been accepted by the Oncology Nutrition Dietetic Practice Group of the American Dietetic Association as the standard nutrition assessment tool for patients with cancer.[3] In India instead of any comprehensive nutrition assessment tool, the nutrition level of a cancer patient is mostly judged from his/her BMI, hemoglobin, or serum albumin level. This problem of underestimating the importance of proper nutritional assessment in cancer patients is prevalent in more developed nations too because of cumbersome and detailed nature of these nutrition assessment tools.[5,6]
The aim of this study was to evaluate the prevalence of malnutrition using the scored PG-SGA as the nutrition assessment tool in gynecological cancer patients, then compare the performance of BMI, hemoglobin, serum albumin, and approximate percentage weight lost in last 1 month with scored PG-SGA; so as to find any one parameter that can be used in place of the comprehensive assessment tool if required or refute the usefulness of the former parameters.
Materials and Methods
This is a small observational, cross-sectional study conducted in the department of gynecologic oncology in a tertiary cancer care center in India. The study was conducted over a period of two months from August 2012 to September 2012. During this period Sixty random cases attending the gynecologic outpatient in Gujarat Cancer Research Institute (GCRI) in a specific time interval between 2 pm to 5 pm, were assessed for their nutritional status. This time interval was chosen because of less patient load there by providing ample time to explain the procedure and fill out the PG-SGA form. Verbal consent was taken from the patients after explaining the procedure and purpose of the study. Nutritional assessment was done by scored PG-SGA, hemoglobin, serum albumin, BMI, and percentage weight lost in last month in all 60 cases. The inclusion criterion was any case with histologically proven gynecologic cancer visiting the hospital for the first time. The exclusion criteria were any form of anticancer treatment received prior to the assessment and physical, cognitive, or emotional problems of the patients that prevented them from completing the scored PG-SGA form. This study was approved by Institutional Review Board.
Nutritional assessment
The scored PG-SGA is a validated nutritional assessment tool for cancer patients.[7]
The form has two sections: A medical history section that is completed by the patient, and a physical assessment section that is completed by nursing, medical, or dietetic staff. The medical history section includes history of weight change; dietary intake change; oncology nutrition impact symptoms like nausea, pain abdomen, etc., that have persisted for greater than 2 weeks; and functional capacity. The healthcare professional section includes an evaluation of metabolic demand, diagnosis, and comorbidities in relation to nutrition requirements and elements of the physical examination. Features are subjectively graded according to severity and combined into a global assessment in which patients are classified as being well-nourished (category-A), moderately or at risk of being malnourished (category-B), or severely malnourished (category-C). For each component of the scored PG-SGA, a point (0-4) is awarded depending on the impact of the component on nutritional status. The total score is then summed and this provides a guideline as to the level of nutrition intervention required.[8] Higher score indicates greater level of malnutrition. Score 0-1 means no intervention is required, 2-3 indicates the need of patient education with symptomatic treatment, and score 4-8 requires intervention by a dietitian in conjunction with physician as indicated by symptoms. A score ≥ 9 indicates severe malnutrition in critical need for nutritional intervention and symptom management.[8] The muscle status, fat store, and fluid accumulation were assessed clinically. The presence of ascites was established radiologically and any associated condition that is expected to increase the metabolic demand was abstracted from the clinical notes. The initial values of hemoglobin and serum albumin were extracted from the case file. The values of percentage weight lost in last month were collected from the PG-SGA forms. Criteria for normal hemoglobin level and mild, moderate, and severe anemia was defined according to World Health Organization (WHO) at ≥12 g/dl, 11-11.9 g/dl, 8-10.9 g/dl, and ≤8 g/dl, respectively.[9] The values for normal level of serum albumin and mild, moderate, and severe hypoalbuminemia were taken as >3.5 g/dl, 3-3.5 g/dl, 2.1-2.9 g/dl, and ≤2 g/dl, respectively.[10,11] Weight loss of ≥5% of previous weight in last 1 month was considered significant.[12] The remaining subcategories of percentage weight loss were derived from the scored PG-SGA form.[8] Height of the patients was measured with a wall mounted scale and weight by a bathroom scale. Height and weight were used to determine BMI (weight (kg)/height (m2)), which was further classified according to the World Health Organization's age-and sex-adjusted criteria as undernourished if ≤18.5 kg/m2, normal weight if 18.5-24.9 kg/m2, overweight if 25-29.9 kg/m2, and obese if ≥30 kg/m2.[13]
Statistical analysis
PG-SGA score, BMI, percentage weight loss, hemoglobin, and serum albumin levels were converted to categorical variables as described above. Descriptive analyses were based on standard statistics such as relative frequencies for categorical variables. Statistical analysis was carried out using Statistical Package for Social Sciences (SPSS)-20 (SPSS Inc., Chicago, IL). The cases were divided into various groups for comparison purpose and to look for any effect of age, site of cancer, or stage of cancer on the nutritional level. One-way analysis of variance (ANOVA) was used to look for the difference between the groups in relation to their mean PG-SGA score and to find out any significant difference in the mean values of BMI, hemoglobin, serum albumin, and percentage weight loss in various PG-SGA score category. Pearson's correlation coefficient of BMI, hemoglobin, serum albumin, and percentage weight lost in last month with scored PG-SGA was calculated with all the parameters as continuous measure. Assuming scored PG-SGA the standard nutrition assessment tool, the sensitivity, specificity, and positive and negative predictive values were calculated. Statistical significance was reported at the conventional P ≤ 0.05 level. For calculation of sensitivity, specificity and predictive values PG-SGA score ≥ 9, BMI ≤ 18.5, hemoglobin ≤ 8 g/dl, serum albumin level ≤ 2 g/dl, and percentage weight loss ≥ 5% was considered as these are the ones who need most immediate management because of their severe nature.[8,9,10,12,13]
Results
The average age of the study group was 46.65 years (range: 13-74 years). The cases were randomly divided into three age groups to assess the effect of age on nutrition. Twelve patients belonged to 13-35 years age group, 32 belonged to 36-55 years group, and 16 belonged to 56-75 years age group. Twenty-three patients had cancer cervix, 24 had cancer ovary, eight patients had cancer endometrium, three had cancer vulva, one had choriocarcinoma, and one had vaginal rhabdomyosarcoma. The stage wise distribution of patient population irrespective of cancer site was like this, 23 cases in stage I, seven in stage II, 22 cases in stage III, and eight in stage IV.
11.67% of all cases were well-nourished (PG-SGA category-A). 48.33% cases were at risk of malnutrition or had moderate malnutrition (PG-SGA category-B), whereas 40% of the study population were severely malnourished (PG-SGA category-C) using scored PG-SGA as the nutrition assessment tool. There was no significant difference between the PG-SGA scores of the three age groups (P = 0.609). Analysis of the effect of site of cancer on PG-SGA score was done after excluding the five cases of cancer vulva, choriocarcinoma, and vaginal sarcoma. No significant difference was found between the rest cancer sites in relation to PG-SGA score (P = 0.712), whereas the scores varied significantly between different cancer stages (P = 0.002) as shown in Figure 1. When the effects of cancer site and stage on nutrition level were combined to see where more of severe malnutrition exists, the result is shown in Figure 2.
Figure 1.
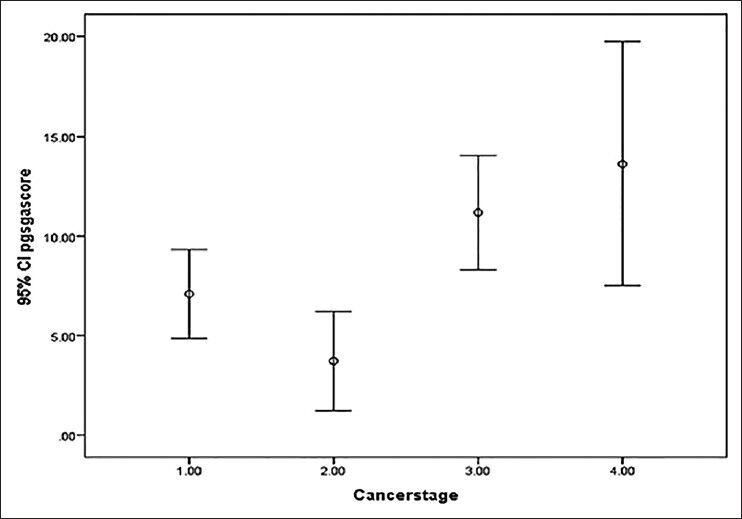
One-way analysis of variance between cancer stage and Patient Generated Subjective Global Assessment score, P = 0.002* (1.00 = stage I, 2.00 = stage II, 3.00 = stage III, 4.00 = stage IV)
Figure 2.
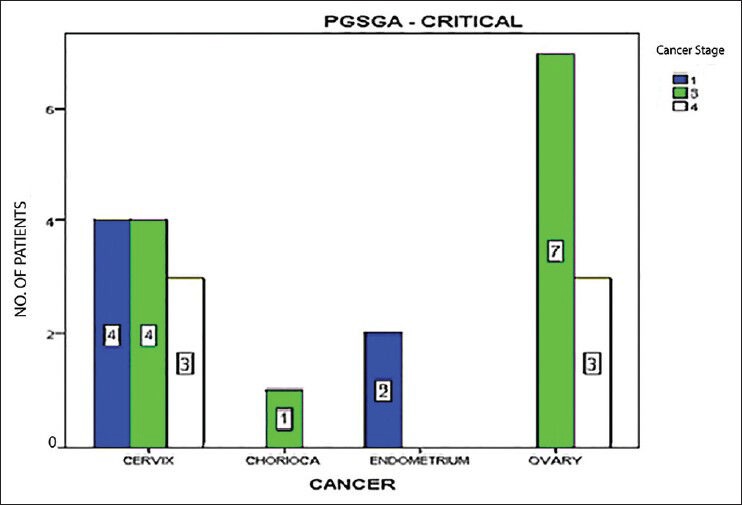
Distribution of severe malnutrition across cancer sites and stages. The numbers inside the bars are the exact number of cases in each group, color code for stage of cancer is shown in upper right corner. CHORICA: Choriocarcinoma
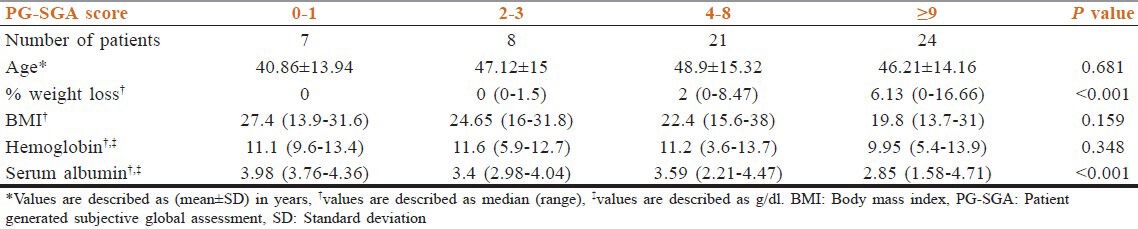
BMI identified 26.66% patients as below normal, 41.67% normal, and 31.67% as overweight or obese. Five percent cases had severe hypoalbuminemia of ≤2 g/dl and 95% had normal albumin level or mild to moderate deficiency. 13.33% cases had severe anemia with hemoglobin level ≤8 g/dl, 41.67% had moderate anemia, 13.33% had mild anemia, and 31.67% had normal hemoglobin level. 26.67% of 60 patients had lost ≥5% of their previous weight in last 1 month. Table 1 shows the age and nutritional characteristics of the study population.
Table 1.
Summary of age, percentage (%) weight lost in last 1 month, BMI, hemoglobin, and serum albumin distribution in various PG-SGA score category
Assuming scored version of PG-SGA to be gold standard for nutritional assessment in cancer patients; the sensitivity, specificity, and positive and negative predictive values of other parameters are shown in Table 2 and their Pearson's correlation coefficients with PG-SGA score is shown in Table 3.
Table 2.
Sensitivity, specificity, and positive and negative predictive values of percentage (%) weight lost in last 1 month, body mass index, hemoglobin, and serum albumin compared to scored PG-SGA
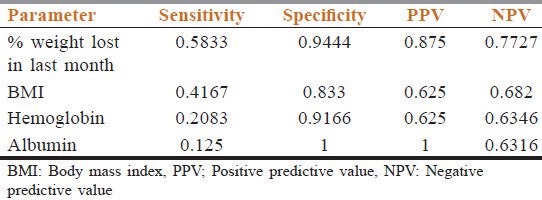
Table 3.
The values of pearson's correlation coefficient between PG-SGA score and other parameters

Only the severe categories are taken into account for calculation purpose.
Discussion
In this study group, 88.33% had some degree of malnutrition or were at risk of malnutrition. Various studies done previously also show corresponding figure between 40 and 80% depending upon the type of assessment tool used, site and stage of cancer, etc.[14] One study on gynecological cancer patients in Australia found only 20% in the PG-SGA category B, 80% in category A, and none in category C.[15] The high prevalence of malnutrition in India compared to the more developed nations may be because of high prevalence of preexisting malnutrition and also the late stages at diagnosis. In this study there was no statistically significant effect of age or cancer site on nutritional status of cases. According to a study by Bozzetti et al., age was not related with nutritional status.[16] The usual belief however is that elderly patients are more prone to malnutrition in cancer because of preexisting problems of dietary intake apart from the effects of ageing per se.[17] Some studies regarding the effect of cancer site on nutrition do show significant predisposition to malnutrition in some cancers compared to others.[18,19] Maximum number of severe malnutrition cases had advanced stages of cancer (stage III/IV), which of course is expected. When we combined the effects of cancer site and stage on nutrition the result implied that while cancer ovary patients are at risk of severe malnutrition towards the later stages, cancer cervix cases are at risk even in stage I. Other studies however inferred the opposite, that is, cancer ovary cases are at risk in early stage; while cancer cervix and endometrium cases were at less risk in initial stage.[15,20]
Hemoglobin, BMI, serum albumin, and percentage weight loss are important prognostic factors; but with some drawbacks for using them as sole nutritional indicators.[21,22] The disadvantages of the parameters are as follows: Malnourished cancer patients may have a BMI within the healthy or overweight range, with body fat masking loss of lean body mass so this is best useful to assess chronic malnutrition, and as alterations in body composition occur later during the malnutrition process.[23] Serum albumin level is affected by non-nutritional factors also, such as hydration state and other disease processes, which can obscure the effects of actual nutrient deprivation so it should not be considered as nutritional marker, but inflammatory response marker.[23] Hemoglobin level is affected by many factors apart from malnutrition, like menorrhagia, hookworm infestation, etc., Unlike scored PG-SGA none of the objective parameters takes into account increased metabolic demand of the cancer patient and nutrition impact symptoms. Unless symptoms of relevance to the patients such as decreased appetite, pain, nausea, constipation, vomiting, and diarrhea are adequately addressed, then it is unlikely that improvements will be made in the patients’ nutritional status. Hence, though these parameters are useful and low levels of them should not be overlooked by any means, malnutrition associated and caused by cancer needs special management.
How well the objective parameters may predict nutrition status can be judged by estimating the sensitivity, specificity, and predictive values; which are shown in Table 2. Serum albumin level has a specificity of 1 meaning, a patient with albumin level ≤2 g/dl is a strong indicator of severe malnutrition as is inferred by a positive predictive value of 1. Studies have shown that hypoalbuminemia occurs towards the later stages of cancer with established malnutrition and is an independent poor prognostic factor.[24] BMI and hemoglobin levels also have considerably high specificity, but their use as sole indicators of malnutrition in cancer is limited by rather low predictive values. For any given tumor type, survival is shorter in patients who experienced pretreatment weight loss.[25,26] Hence, in this study we tried to evaluate its usefulness compared to scored PG-SGA. The value of percentage weight lost in 1 month had better overall sensitivity, specificity, and predictive values compared to other parameters. Percentage weight loss has the ability to detect mild to extreme nutritional changes and its high sensitivity and specificity in cancer has been recently confirmed and has been suggested to be used alone if not using any other tool of comprehensive nutrition assessment.[27] The hemoglobin, serum albumin, and BMI were negatively correlated and approximate percentage weight lost in last 1 month was positively correlated with PG-SGA score as evident from Table 3. According to a Malaysian study, PG-SGA score is significantly correlated with anthropometric measurements.[28] Though all the correlations were statistically significant and in the expected direction, only the coefficient between percentage weight lost and PG-SGA score was >0.7, meaning, proportionate change in PG-SGA score will result in proportionate change only in the value of percentage weight loss.
Limitation of this study is the selection bias introduced involuntarily by the exclusion of patients with physical, cognitive, or emotional problems that prevented them from completing the scored PG-SGA form. Though this small observational study has insufficient power to conclusively detect any correlations between PG-SGA and the other diagnostic tools here analyzed (BMI, hemoglobin, albumin, and weight loss), however the results of our study should be of interest to the vast majority of the oncology’ community, as malnutrition is a common and underrecognized problem in cancer patients.
Conclusion
88.33% of gynecologic cancer cases had some degree of malnutrition or were at risk of malnutrition before starting any anticancer treatment and 40% had severe malnutrition in critical need of nutritional intervention. Advanced stage cancer cases are at more risk of malnutrition. Approximate percentage weight lost in last 1 month, that is, ≥5% can sometimes be used in place of the comprehensive scored PG-SGA to triage the patients in case the latter is not used for some reason. Severe hypoalbuminemia ≤2 g/dl is a highly specific indicator for severe malnutrition with high positive predictive value.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Antoun S, Baracos V. Malnutrition in cancer patient: When to have a specialized consultation? Bull Cancer. 2009;96:615–23. doi: 10.1684/bdc.2009.0860. [DOI] [PubMed] [Google Scholar]
- 2.Kaikani W, Bachmann P. Consequences of a comorbidity often neglected in oncology: Malnutrition. Bull Cancer. 2009;96:659–64. doi: 10.1684/bdc.2009.0875. [DOI] [PubMed] [Google Scholar]
- 3.Huhmann MB, August DA. Review of American society for parenteral and enteral nutrition (ASPEN) clinical guidelines for nutrition support in cancer patients: Nutrition screening and assessment. Nutr Clin Pract. 2008;23:182–8. doi: 10.1177/0884533608314530. [DOI] [PubMed] [Google Scholar]
- 4.Adams KM, Kohlmeier M, Powell M, Zeisel SH. Nutrition in medicine: Nutrition education for medical students and residents. Nutr Clin Pract. 2010;25:471–80. doi: 10.1177/0884533610379606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiro A, Baldwin C, Patterson A, Thomas J, Andreyev HJ. The views and practice of oncologists towards nutritional support in patients receiving chemotherapy. Br J Cancer. 2006;95:431–4. doi: 10.1038/sj.bjc.6603280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeCicco PV, Wunderlich SM, Emmolo JS. Determination of malnourishment in the head and neck cancer patient: Assessment tools and nutrition education of radiation oncologists. Support Care Cancer. 2011;19:123–30. doi: 10.1007/s00520-009-0796-y. [DOI] [PubMed] [Google Scholar]
- 7.Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. 2002;56:779–85. doi: 10.1038/sj.ejcn.1601412. [DOI] [PubMed] [Google Scholar]
- 8.Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12:S15–9. doi: 10.1016/0899-9007(96)90011-8. [DOI] [PubMed] [Google Scholar]
- 9.WHO, Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2001. [Last accessed on 2013 Aug 30]. Available from: http://www.WHO/NMH/NHD/MNM/11.1 .
- 10.Sama SK, Bhargava S, Nath NG, Talwar JR, Nayak NC, Tandon BN, et al. Noncirrhotic portal fibrosis. Am J Med. 1971;51:160–9. doi: 10.1016/0002-9343(71)90234-8. [DOI] [PubMed] [Google Scholar]
- 11.Lai CC, You JF, Yeh CY, Chen JS, Tang R, Wang JY, et al. Low preoperative serum albumin in colon cancer: A risk factor for poor outcome. Int J Colorectal Dis. 2011;26:473–81. doi: 10.1007/s00384-010-1113-4. [DOI] [PubMed] [Google Scholar]
- 12.Beghetto MG, Luft VC, Mello ED, Polanczyk CA. Accuracy of nutritional assessment tools for predicting adverse hospital outcomes. Nutr Hosp. 2009;24:56–62. [PubMed] [Google Scholar]
- 13.Ali SM, Lindstrom M. Socioeconomic, psychosocial, behavioural, and psychological determinants of BMI among young women: Differing patterns for underweight and overweight/obesity. Eur J Public Health. 2006;16:325–31. doi: 10.1093/eurpub/cki187. [DOI] [PubMed] [Google Scholar]
- 14.Gomez Candela C, Olivar Roldán J, García M, Marín M, Madero R, Pérez-Portabella C, et al. Assessment of a malnutrition screening tool in cancer patients. Nutr Hosp. 2010;25:400–5. [PubMed] [Google Scholar]
- 15.Laky B, Janda M, Bauer J, Vavra C, Cleghorn G, Obermair A. Malnutrition among gynaecological cancer patients. Eur J Clin Nutr. 2007;61:642–6. doi: 10.1038/sj.ejcn.1602540. [DOI] [PubMed] [Google Scholar]
- 16.Bozzetti F, Mariani L, Lo Vullo S, Amerio ML, Biffi R, Caccialanza G, et al. SCRINIO Working Group The nutritional risk in oncology: A study of 1,453 cancer outpatients. Support Care Cancer. 2012;20:1919–28. doi: 10.1007/s00520-012-1387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vandewoude MF. Nutritional assessment in oncogeriatrics. Tijdschr Gerontol Geriatr. 2010;41:214–20. doi: 10.1007/BF03096213. [DOI] [PubMed] [Google Scholar]
- 18.Laky B, Janda M, Kondalsamy-Chennakesavan S, Cleghorn G, Obermair A. Pretreatment malnutrition and quality of life-association with prolonged length of hospital stay among patients with gynecological cancer: A cohort study. BMC Cancer. 2010;10:232. doi: 10.1186/1471-2407-10-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zorlini R, Akemi Abe Cairo A, Salete Costa Gurgel M. Nutritional status of patients with gynecologic and breast cancer. Nutr Hosp. 2008;23:577–83. [PubMed] [Google Scholar]
- 20.Laky B, Janda M, Cleghorn G, Obermair A. Comparison of different nutritional assessments and body-composition measurements in detecting malnutrition among gynecologic cancer patients. Am J Clin Nutr. 2008;87:1678–85. doi: 10.1093/ajcn/87.6.1678. [DOI] [PubMed] [Google Scholar]
- 21.Orrevall Y, Tishelman C, Permert J, Cederholm T. Nutritional support and risk status among cancer patients in palliative home care services. Support Care Cancer. 2009;17:153–61. doi: 10.1007/s00520-008-0467-4. [DOI] [PubMed] [Google Scholar]
- 22.Pereira Borges N, D’Alegria Silva B, Cohen C, Portari Filho PE, Medeiros FJ. Comparison of the nutritional diagnosis, obtained through different methods and indicators, in patients with cancer. Nutr Hosp. 2009;24:51–5. [PubMed] [Google Scholar]
- 23.Barbosa-Silva MC. Subjective and objective nutritional assessment methods: What do they really assess? Curr Opin Clin Nutr Metab Care. 2008;11:248–54. doi: 10.1097/MCO.0b013e3282fba5d7. [DOI] [PubMed] [Google Scholar]
- 24.Penel N, Vanseymortier M, Bonneterre ME, Clisant S, Dansin E, Vendel Y, et al. Prognostic factors among cancer patients with good performance status screened for phase I trials. Invest New Drugs. 2008;26:53–8. doi: 10.1007/s10637-007-9088-x. [DOI] [PubMed] [Google Scholar]
- 25.Asp ML, Tian M, Kliewer KL, Belury MA. Rosiglitazone delayed weight loss and anorexia while attenuating adipose depletion in mice with cancer cachexia. Cancer Biol Ther. 2011;12:957–65. doi: 10.4161/cbt.12.11.18134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Utech AE, Tadros EM, Hayes TG, Garcia JM. Predicting survival in cancer patients: The role of cachexia and hormonal, nutritional and inflammatory markers. J Cachexia Sarcopenia Muscle. 2012;3:245–51. doi: 10.1007/s13539-012-0075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boleo-Tome C, Monteiro-Grillo I, Camilo M, Ravasco P. Validation of the Malnutrition Universal Screening Tool (MUST) in cancer. Br J Nutr. 2012;108:343–8. doi: 10.1017/S000711451100571X. [DOI] [PubMed] [Google Scholar]
- 28.Kwang AY, Kandiah M. Objective and subjective nutritional assessment of patients with cancer in palliative care. Am J Hosp Palliat Care. 2010;27:117–26. doi: 10.1177/1049909109353900. [DOI] [PubMed] [Google Scholar]


