Abstract
Background:
Improved survival after childhood cancer is attributed to intensive, aggressive therapy, adverse sequelae of which can manifest months to years after completion of treatment. There is little information about the late adverse effects of both childhood cancer and its therapy in survivors in India.
Aim:
To determine the long-term sequelae associated with therapy in childhood cancer survivors attending a tertiary cancer center in India.
Materials and Methods:
We studied 155 consecutive survivors of childhood cancer who were ≤14 years at the time of diagnosis and had completed 3 years of follow-up. The study included a complete history and clinical examination, with specific investigations to detect organ toxicity. Quality of life (QOL) was assessed from responses to a standardized questionnaire. Neurocognitive assessment was carried out in 20 survivors with an adaptation of the revised Wechsler adult intelligence scale for adults and the Malins intelligence scale for children.
Results:
The late effects included impaired fertility in 38 patients (24.5%), impaired growth pattern in 7 (4.5%), endocrine dysfunction in 7 (4.5%) and second malignancy in 2 (1.2%). Three of the 20 patients assessed had severe neurocognitive impairment. A high QOL was reported by 60% of survivors and an “average” QOL by 38%.
Conclusion:
Our study showed that most survivors had a good QOL and our results will help clinicians to better monitor childhood cancer survivors in countries with limited resources.
Keywords: Childhood cancer, follow-up, late effects, survivors
Introduction
An increase in the number of survivors of childhood cancer has heightened the appreciation of the late complications caused by the disease and its treatment. In the USA, the 5-year survival rate from all types of childhood cancer increased from 51% in 1973 to 79% in 1997 respectively.[1] The incidence of childhood cancer in India is 9 per million.[2]. However the latest cumulative risk for childhood cancer in the Madras metropolitan Tumor Registry is 1 in 426 for boys and 1 in 585 for girls[3].
It is now recognized that the therapy responsible for improved survival can also produce adverse long-term, health-related outcomes that manifest months to years after completion of treatment. These late effects include organ dysfunction, second malignancies and adverse psychosocial sequelae.[4]
Monitoring after childhood cancer should have two main goals: To confirm continued remission and to monitor for late effects of cancer or its therapy. Although anecdotal information indicates that survival rates from childhood cancer have increased steadily in India, there is a paucity of data regarding long-term follow-up of survivors. The purpose of this study was to determine the long-term sequelae associated with therapy in childhood cancer survivors attending a tertiary cancer center in India.
Materials and Methods
Materials
We studied 155 long-term survivors of childhood cancer (114 males and 41 females) prospectively at the after-completion-of-therapy (ACT) clinic of the Medical Oncology Unit of the Cancer Institute (WIA), Chennai, India. The group comprised children in whom cancer had been diagnosed between 1968 and 2001, were ≤ 14 years at the time of diagnosis and had completed at least 3 years’ follow-up. 19 of the survivors had a family history of cancer. The pattern of diagnoses is shown in Table 1.
Table 1.
Original diagnoses of childhood cancer among survivors
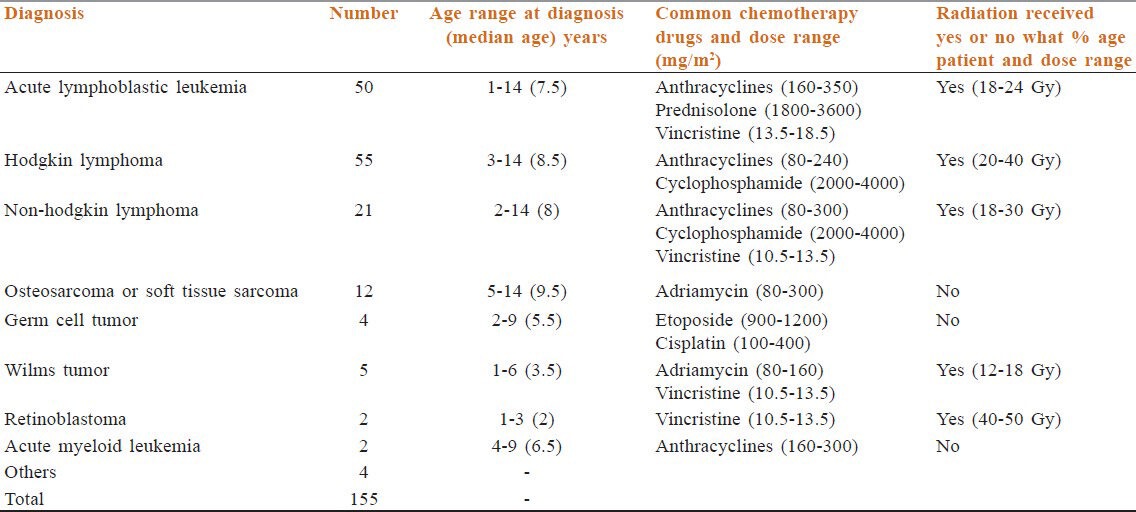
With respect to treatment, 151 persons (97%) had received chemotherapy, 85 (55%) had radiotherapy and one had high-dose chemotherapy with autologous peripheral blood stem cell support. 26 persons (17%) had undergone surgery, of which three (two with Hodgkin lymphoma and one with acute lymphoblastic leukemia [ALL]) had a splenectomy (in the lymphoma patient, splenectomy was performed as part of staging laparotomy and in the child with ALL prior splenectomy was done for trauma, two had enucleation (as part of treatment for retinoblastoma) and four had nephrectomy (as surgery for Wilms tumor treatment).
Methods
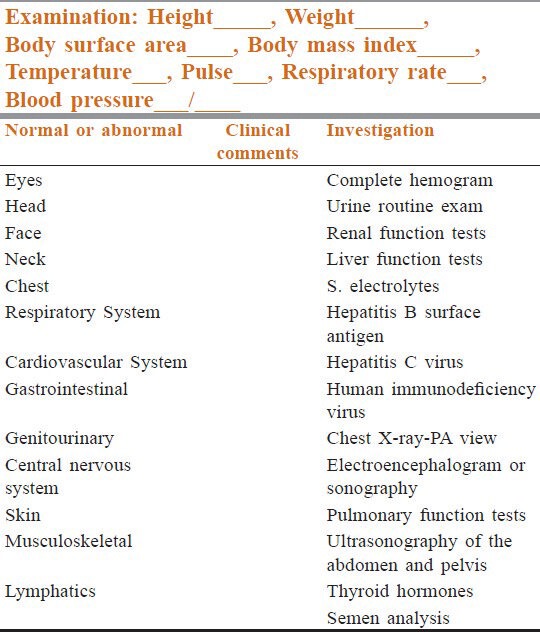
The evaluation at the ACT clinic included a complete history, anthropometric measurements and a clinical examination, with special emphasis on late toxicity and specific investigations to detect organ toxicity [Table 2] after a prior informed consent. Details of the type and doses of treatment received were noted. Semen analysis was performed for patients who received alkylating agents and radiotherapy to abdomen and pelvis. Thyroid and sex hormone evaluation were carried out in survivors who received radiotherapy to the brain and head and neck region.
Table 2.
Proforma for clinical examination and investigations at the after-completion-of-therapy clinic, Division of Medical Oncology, Cancer Institute (WIA) Chennai, India
Quality of life (QOL) was assessed in 56 childhood cancer survivors with the Cancer Institute's standardized questionnaire.[5] The dimensions of QOL included general, physical and psychological well-being, interpersonal relationships, sexual and personal ability, cognitive well-being, optimism and belief, economic well-being, informational support, patient-physician relationship and body image.
Neurocognitive assessment was performed in 20 survivors of ALL. The Malins intelligence scale for Indian children[6] was used for subjects aged 6-16 years and the revised Wechsler intelligence scale[7] for participants over 16 years of age. Abstract thinking, reasoning, attention, concentration, decision-making and memory were assessed.
All persons were given counselling and education for healthy living and referred to sub-specialists if specific toxicity was detected. The persons who had undergone splenectomy received specific counselling about the risk for severe infections.
Informed consent was obtained from all participants prior to entry to the ACT clinic and prior to QOL and neurocognitive assessments. The study was approved by the Institutional Review Board.
Results
The age range of survivors seen at the clinic is shown in Figure 1. The median age of survivors at follow-up was 24 years. 90 persons (58%) were between 15 and 25 years of age at the time of follow-up [Figure 1].
Figure 1.
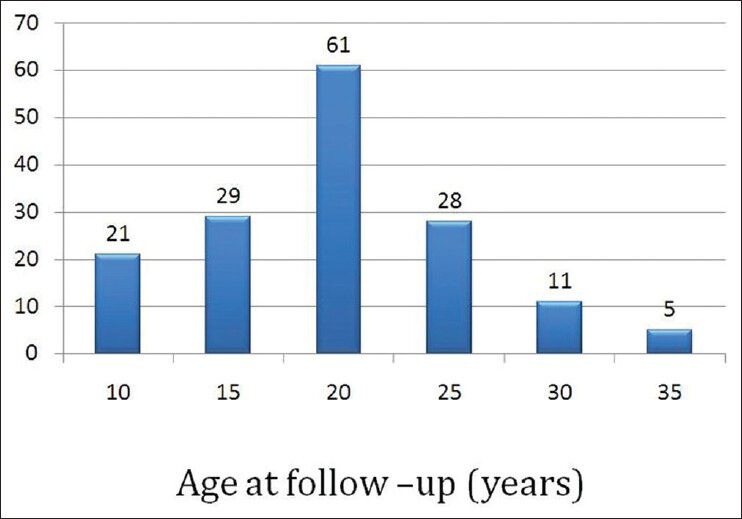
Age of the subjects during follow-up at the after-completion-of-treatment clinic
The duration of follow-up was 3-37 years; the median follow-up was 8 years, the majority (63%) being in the first decade of follow-up.
Three male participants consumed alcohol and one had a history of smoking (treated for Hodgkins lymphoma); none of the female subjects had a history of smoking or alcohol consumption. Most of the patients had a normal or low normal body mass index (26% and 68%, respectively).
The pattern of late effects detected is shown in Table 3. Male and female gonadal dysfunction was the commonest late toxic effect seen (25%). Of the men, 89% had oligospemia or azoospermia, while 78% of women reported gonadal dysfunction, amenorrhea or oligomenorrhea. All had received combination chemotherapy that included alkylating agents [Figure 2]. 7 persons (3 with Hodgkins lymphoma and 4 of ALL), four of whom with ALL had received prophylactic cranial irradiation of 18 Gy, did not attain “height for age”.
Table 3.
Late effects among survivors of childhood cancer at the time of follow-up at the after-completion-of-treatment clinic, Cancer Institute (WIA), Chennai, India
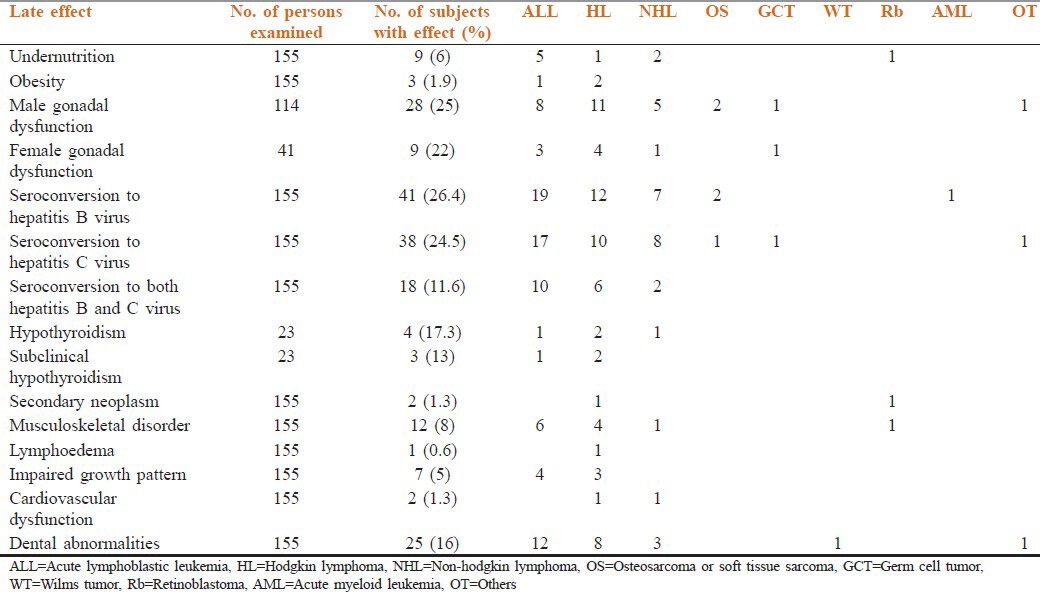
Figure 2.
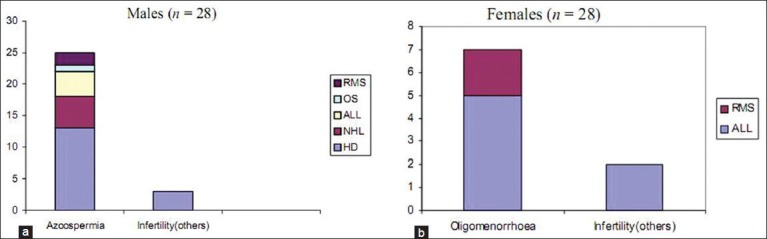
(a and b) Patterns of gonadal dysfunction among survivors of childhood cancer at the time of follow-up at the after-completion-of-treatment clinic, Cancer Institute, Chennai, India
Hypothyroidism was seen in 4 of 23 subjects tested. Two had had Hodgkin lymphoma, one had had non-Hodgkin lymphoma and one had been treated for ALL. All had received radiotherapy to the head and neck. Three had a thyroid profile suggesting subclinical hypothyroidism.
Two second cancers were observed. A 1-year-old female child, found to have bilateral retinoblastoma in 1968 and treated with external beam radiotherapy (50 Gy), presented in February 2005 with a pre-auricular swelling and lymphadenopathy and ectomesenchymoma of the parotid gland was diagnosed. An 8-year-old boy in whom stage II Hodgkin lymphoma had been diagnosed in 1973 underwent staging laparotomy and was treated with combination chemotherapy and “mantle” radiotherapy. In May 2005, he presented with dysphagia and was found to have carcinoma of the hypopharynx.
Two survivors out of 155 patients (1.3%) were found to have cardiovascular dysfunction (at 11 and 16 years of follow-up respectively). A 17-year-old male in whom lymphoblastic lymphoma was diagnosed in 1995 had received anthracycline-based chemotherapy (350 mg/m2 cumulative dose) and irradiation to the mediastinum (25 Gy). He was subsequently found to have dilated cardiomyopathy with congestive cardiac failure in 2004. The other person was a 24-year-old male survivor of Hodgkin lymphoma (cumulative dose of anthracyclines 200 mg/m2) and had received subtotal nodal irradiation (30 Gy) and was in the 16th year of follow-up. He was asymptomatic but was incidentally found to have mitral valve regurgitation. Echocardiography done at diagnosis was within normal limits.
Post-mantle field radiotherapy lymphedema was seen in one person. One survivor treated for ALL developed avascular necrosis of the head of the femur (who was 5 years at the time of diagnosis). Two were found to have gynecomastia. Ultrasonography of the abdomen and pelvis revealed no abnormal findings. The QOL assessment showed a high QOL for 31 (55.3%) persons and an average QOL for 21 (37.5%). Neurocognitive assessment was performed on 20 survivors of ALL, all of whom had received prophylactic cranial irradiation (range: 18-24 Gy median dose, 20 Gy) and 12 doses of intrathecal methotrexate as per the MCP 841 protocol. The mean age of the children in this group was 17.3 years and 93% were male. The median duration of follow-up was 9.3 years. 3 (15%) of the subjects had a score suggesting mental deficiency (score ≤69), while 12 (60%) had average scores (90-109).
Discussion
Our results show that the most common long-term sequelae of therapy for childhood cancer were impaired reproductive capacity and abnormal growth. Obesity was uncommon and most of the survivors had a good QOL. The limitations of the study include the fact that the study population comprised only the survivors who agreed to attend the ACT clinic and that hormonal evaluations were not performed in all subjects, owing to financial constraints.
Impaired reproductive capacity was the most common long-term toxic effect. All the subjects had received alkylating agents, which are widely used in treating childhood cancer but are often responsible for gonadal toxicity. Spermatogenesis is highly sensitive to cyclophosphamide, with a dose-effect relationship that is exacerbated by co-administration of other alkylating agents, like procarbazine.[11] Furthermore, permanent azoospermia results from radiation doses greater than 3-4 Gy.[12] In females, the risks of menstrual irregularity, ovarian failure and infertility increase with age at treatment. A Canadian study of female childhood cancer survivors also showed increased incidence of infertility and early menopause.[13]
Obesity has been reported in survivors of pediatric ALL, the highest risk (in a study conducted in the USA) being found in girls treated at 4 years of age or younger with cranial irradiation at doses of greater than 20 Gy.[8] Although growth hormone level was not measured in our study, 5% of our survivors had not attained the expected height for age and four of these had received cranial irradiation, the other three may be explained due to nutritional causes. Growth hormone deficiency, delayed or precocious puberty and hypopituitarism can all occur in childhood cancer survivors, hypothalamic dysfunction being the most common abnormality seen after cranial irradiation.[9]
A study in North America of 118 ALL survivors treated with 24 Gy cranial irradiation showed that 74% had an impaired growth pattern due to growth hormone deficiency.[10]
The seroconversion rates to hepatitis B and hepatitis C infection were 26% and 25%, respectively, whereas in a prospective study in the USA, the conversion rates were 16% and 3.2% respectively.[14] This high seroconversion rate may be due to the long duration of follow-up of survivors and the fact that the patients had received treatment and blood transfusion prior to the standard 3rd generation antibody testing. Currently, seroconversion rates at our center are 6% for hepatitis B and 1.2% for hepatitis C. This subset of survivors in our study will require close monitoring, as they are at an increased risk of developing cirrhosis and hepatocellular carcinoma. None of these persons showed features of cirrhosis or portal hypertension on sonography.
The 16% of survivors who had dental caries were given a dental consultation and appropriate treatment. Salivary gland irradiation incidental to treatment of head-and-neck malignancies or Hodgkin lymphoma can cause qualitative and quantitative changes in salivary flow that can result in an increased incidence of dental caries.[15]
Hypothyroidism was seen in 4 of 23 subjects in whom it was studied (17%). All had received radiation therapy to the head and neck. Subclinical hypothyroidism was seen in an additional three. The incidence of thyroid dysfunction depends on the dose of radiation, the length of follow-up and the biochemical criteria used to make the diagnosis.[16] Only persons who had received radiotherapy to the brain and head and neck region and who are susceptible to hormonal dysfunction were screened for thyroid function.
Both the patients who developed a second cancer had received irradiation and the second neoplasm developed within or adjacent to the field of irradiation. In the St. Jude study[17] also, irradiated survivors had a higher cumulative incidence of second neoplasms than non-irradiated survivors. In a recent publication from the childhood cancer survivor study, it was noted that the risk of developing a second malignancy was significantly elevated following all childhood cancers except central nervous system neoplasms and was highest following neuroblastoma and soft-tissue sarcoma.[18]
Three of the survivors had had a splenectomy, which increases the risk of life-threatening invasive bacterial infection.[19] In our series, however, no severe or life-threatening infections were seen as all were appropriately vaccinated.
Two of the survivors who had had a retinoblastoma had undergone enucleation. In survivors of retinoblastoma, a small orbital volume can result from either enucleation or radiation therapy. Being <1 year old at the time of treatment can increase the risk, but this finding is not consistent across studies. Better management of prosthetic implants and newer methods of delivering radiation therapy are likely to reduce the risk.[20] None of our survivors developed cataract, although they have received external beam radiotherapy.
Of the 55 survivors assessed, our study showed average and good QOL in 93%. In a recent series in India, severe psychological problems were encountered in 12% of survivors.[21] As a group, childhood cancer survivors appear to be within the normal expected range in terms of psychosocial adjustment,[22] though subtle concerns related to physical impairments, vocational discrimination, economic burden and early and open communication within the family were identified.[23]
Although a baseline neurocognitive assessment was not performed, our study showed significant neurocognitive dysfunction with poor academic performance in 3 of 20 (6%) survivors of ALL. This is consistent with the results of previous cross-sectional studies.[24] Risk factors for neurocognitive sequelae in childhood cancer include radiation dose, young age at the time of treatment, combined chemo and radiotherapy and female sex.[25] Deficits in fine motor skills, visual-spatial ability, verbal and nonverbal memory, psychomotor speed and shifting of attention, auditory perception, word fluency, contingency naming and the ability to follow directions have all been reported.[26] Deficit in fine motor skills, verbal and nonverbal and word fluency were the main deficits noted in our study.
Long-term morbidity in childhood cancer survivors relates largely to treatment modality and the challenge remains to improve survival rates while reducing the incidence and severity of treatment-related late effects and to achieve optimal QOL. Our study showed impaired reproductive capacity to be the commonest long-term sequela, with a low incidence of obesity and good QOL. Future studies should include more active recruitment of subjects, to improve attendance at the ACT clinic, in the form of letters and telephone contacts.
Monitoring for late effects helps oncologists and physicians to make an early diagnosis and to intervene. It also helps in the development of safer treatment modalities, thereby improving the QOL of long-term survivors. The information provided by this ongoing study may allow clinicians to better monitor childhood cancer survivors in countries with limited resources.
Acknowledgments
The authors would like to thank Dr. Ama Rohatiner, Professor Elisabeth Heseltine and the International Network for Cancer Treatment and Research workshop for the help provided in preparation of this manuscript.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, et al., editors. Bethesda, MD: National Cancer Institute, SEER Program; 1999. Cancer Incidence and Survival among Children and Adolescents: United States Seer Program 1975-1995. NIH Pub. No. 99-4649. [Google Scholar]
- 2.Shanta MA, Gajalakshmi CK, Swaminatham R. India, Madras metropolitan tumour registry, 1982-1992. In: Parkin DM, Kramarova E, Draper GJ, Masuyer E, Michaelis J, Neglia J, et al., editors. Incidence of Childhood Cancer. Vol. 2. Lyon: International International Agency for Research on Cancer; 1998. pp. 169–71. IARC Scientific Publications No. 144. [Google Scholar]
- 3.Swaminathan R, Shanta V, Murugaiyan J, Sambandam T.S, Balasubramanian S, Sampath P. Chennai: National Cancer Registry Program, Cancer Institute (WIA); Madras Metropolitan Tumour Registry Technical Report 2009-2010. [Google Scholar]
- 4.Schwartz CL. Long-term survivors of childhood cancer: The late effects of therapy. Oncologist. 1999;4:45–54. [PubMed] [Google Scholar]
- 5.Vidhubala E, Kannan RR, Mani SC, Karthikesh K, Muthuvel R, Surendran V, et al. Validation of quality of life questionnaire for patients with cancer – Indian scenario. Indian J Cancer. 2005;42:138–44. doi: 10.4103/0019-509x.17058. [DOI] [PubMed] [Google Scholar]
- 6.Malin AJ. Indian Psychological Corporation Nagpur; 1966. Malin's intelligence scale for Indian children. [Google Scholar]
- 7.Prabha R. New Delhi: Manasayan; 1974. Wechler's adult performance intelligence scale-Indian adapatation (form PR) [Google Scholar]
- 8.Oeffinger KC, Mertens AC, Sklar CA, Yasui Y, Fears T, Stovall M, et al. Obesity in adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21:1359–65. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 9.Gurney JG, Kadan-Lottick NS, Packer RJ, Neglia JP, Sklar CA, Punyko JA, et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood cancer survivor study. Cancer. 2003;97:663–73. doi: 10.1002/cncr.11095. [DOI] [PubMed] [Google Scholar]
- 10.Leung W, Rose SR, Zhou Y, Hancock ML, Burstein S, Schriock EA, et al. Outcomes of growth hormone replacement therapy in survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 2002;20:2959–64. doi: 10.1200/JCO.2002.09.142. [DOI] [PubMed] [Google Scholar]
- 11.Ben Arush MW, Solt I, Lightman A, Linn S, Kuten A. Male gonadal function in survivors of childhood Hodgkin and non-Hodgkin lymphoma. Pediatr Hematol Oncol. 2000;17:239–45. doi: 10.1080/088800100276415. [DOI] [PubMed] [Google Scholar]
- 12.Sklar CA, Robison LL, Nesbit ME, Sather HN, Meadows AT, Ortega JA, et al. Effects of radiation on testicular function in long-term survivors of childhood acute lymphoblastic leukemia: A report from the children cancer study group. J Clin Oncol. 1990;8:1981–7. doi: 10.1200/JCO.1990.8.12.1981. [DOI] [PubMed] [Google Scholar]
- 13.Chiarelli AM, Marrett LD, Darlington G. Early menopause and infertility in females after treatment for childhood cancer diagnosed in 1964-1988 in Ontario, Canada. Am J Epidemiol. 1999;150:245–54. doi: 10.1093/oxfordjournals.aje.a009995. [DOI] [PubMed] [Google Scholar]
- 14.Segal BH. Infections in the cancer patient. In: Devita VT, Helmann S, editors. Principles and Practice of Oncology. 7th ed. Philadelphia: JB Lippincott; 2005. pp. 2477–84. [Google Scholar]
- 15.Makkonen TA, Nordman E. Estimation of long-term salivary gland damage induced by radiotherapy. Acta Oncol. 1987;26:307–12. doi: 10.3109/02841868709089980. [DOI] [PubMed] [Google Scholar]
- 16.Gleeson HK, Darzy K, Shalet SM. Late endocrine, metabolic and skeletal sequelae following treatment of childhood cancer. Best Pract Res Clin Endocrinol Metab. 2002;16:335–48. doi: 10.1053/beem.2002.0201. [DOI] [PubMed] [Google Scholar]
- 17.Pui CH, Cheng C, Leung W, Rai SN, Rivera GK, Sandlund JT, et al. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–9. doi: 10.1056/NEJMoa035091. [DOI] [PubMed] [Google Scholar]
- 18.Bassal M, Mertens AC, Taylor L, Neglia JP, Greffe BS, Hammond S, et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:476–83. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 19.Pickering LK, Peter G, Baker CJ, editors. 25th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2000. 2000 Red Book: Report of the Committee on Infectious Diseases. [Google Scholar]
- 20.Kaste SC, Chen G, Fontanesi J, Crom DB, Pratt CB. Orbital development in long-term survivors of retinoblastoma. J Clin Oncol. 1997;15:1183–9. doi: 10.1200/JCO.1997.15.3.1183. [DOI] [PubMed] [Google Scholar]
- 21.Goswami SS, Kurkre P, Arora B, Pradhan P, Deodher J, Dalvi N. Mumbai: Tata Memorial Center; 2006. Effectiveness of psychological assessment and interventions in coping and care of long term survivors of childhood cancer patients in India (UICC 2006 abstract) pp. 227–3. [Google Scholar]
- 22.Hymovich DP, Roehnert JE. Psychosocial consequences of childhood cancer. Semin Oncol Nurs. 1989;5:56–62. doi: 10.1016/0749-2081(89)90023-5. [DOI] [PubMed] [Google Scholar]
- 23.Gray RE, Doan BD, Shermer P, FitzGerald AV, Berry MP, Jenkin D, et al. Psychologic adaptation of survivors of childhood cancer. Cancer. 1992;70:2713–21. doi: 10.1002/1097-0142(19921201)70:11<2713::aid-cncr2820701124>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Butler RW, Hill JM, Steinherz PG, Meyers PA, Finlay JL. Neuropsychologic effects of cranial irradiation, intrathecal methotrexate, and systemic methotrexate in childhood cancer. J Clin Oncol. 1994;12:2621–9. doi: 10.1200/JCO.1994.12.12.2621. [DOI] [PubMed] [Google Scholar]
- 25.Brown RT, Sawyer MB, Antoniou G, Toogood I, Rice M, Thompson N, et al. A 3-year follow-up of the intellectual and academic functioning of children receiving central nervous system prophylactic chemotherapy for leukemia. J Dev Behav Pediatr. 1996;17:392–8. doi: 10.1097/00004703-199612000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Ciesielski KT, Yanofsky R, Ludwig RN, Hill DE, Hart BL, Astur RS, et al. Hypoplasia of the cerebellar vermis and cognitive deficits in survivors of childhood leukemia. Arch Neurol. 1994;51:985–93. doi: 10.1001/archneur.1994.00540220031012. [DOI] [PubMed] [Google Scholar]


