Abstract
Relapsed-Refractory Diffuse Large B Cell Lymphoma (RR DLBCL), which accounts for approximately one-third of patients with DLBCL, remains a major cause of morbidity and mortality. Managing RR DLBCL continues to be a challenge to the treating hemato-oncologist. Salvage high-dose chemotherapy followed by autologous stem cell transplantation is the standard of care for chemosensitive relapses in DLBCL. Various salvage regimens are available, but the quest for an optimal regimen continues. The addition of rituximab to the salvage regimen has improved the outcome of RR DLBCL. Several pertinent issues regarding the management of RR DLBCL are discussed in this short review.
Keywords: Activated B cell, autologous stem cell transplant, diffuse large B cell lymphoma, germinal center B, high dose chemotherapy, relapsed refractory
Introduction
Non-Hodgkin lymphoma (NHL) is the seventh most common malignancy.[1] Diffuse large B cell lymphomas (DLBCLs) are the commonest subtype of NHL. They constitute about 30 to 40% of adult NHLs.
Diffuse large B cell by definition is a large transformed B cell with nuclear diameter more than twice that of a normal lymphocyte. In the recent 2008 WHO classification, DLBCL is classified under the diagnostic heading of “mature B cell neoplasms.” DLBCL is a distinct group in itself with many subtypes and entities based on morphology, immunophenotypic characteristics, and clinical presentation. DLBCL Not Otherwise Specified (NOS) is the commonest variety.[2,3] Using gene-expression profiling, cell-of-origin studies suggested that there are at least 3 distinct subtypes of DLBCL: Activated B-cell (ABC), germinal center B-cell (GCB), and primary mediastinal DLBCL. These differ in the postulated stage of cell of origin, gene expression, and response to anthracycline-based chemotherapy. The GCB DLBCL has better response rate than ABC DLBCL.[4,5]
DLBCL could present de novo or as a histologic transformation of other low-grade B cell lymphomas like follicular or chronic lymphocytic leukemia/small lymphocytic lymphoma. The de novo DLBCLs have better prognosis than the latter group.[6] Rituximab with CHOP is the widely accepted first line regimen for the management of DLBCL.[7]
Relapsed refractory DLBCL
Approximately 50 to 60% of patients with DLBCL achieve and maintain complete remission after first-line therapy; 30 to 40% relapse and 10% have refractory disease.[8,9] Relapsed refractory DLBCL (RR-DLBCL) is defined as per criteria proposed by Cheson et al. [Table 1].[10] Patients with RR-DLBCL have a poor outlook. If left untreated, RR-DLBCL has a life expectancy of 3 to 4 months.[11]
Table 1.
Response criteria for NHL
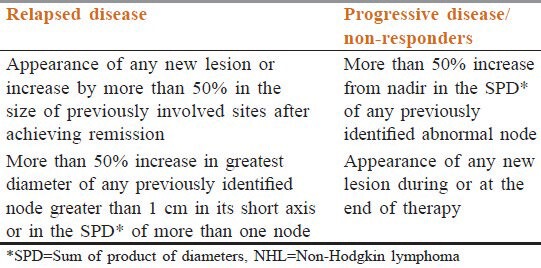
Diagnosis
Refractory disease is diagnosed during response assessment to primary treatment. Relapsed DLBCL can be clinically silent and is often diagnosed on routine follow-up. If clinical features and/or imaging findings suggest relapse, an excision biopsy should always be performed because RR-DLBCL has poor prognosis. Disease should be restaged at relapse with a CT scan of the chest/abdomen/pelvis and a bone marrow biopsy as it has prognostic value.[12] A PET-CT may further delineate extranodal and/or new site involvement.[13] Patients with CNS symptoms should be evaluated with CT-head and lumbar puncture for CSF cytology and flow cytometry. The IPI (international prognostic index) should be determined again at relapse.[12]
Standard treatment
High-dose therapy followed by autologous stem cell transplant (HD-ASCT) is the mainstay of therapy for RR-DLBCL. But, all patients are not fit or eligible for this therapeutic option. The treatment of RR-DLBCL is described under the headings of treatment for patients eligible for HD-ASCT and not eligible for HD-ASCT.
Patients eligible for HD-ASCT
The landmark PARMA trial has established HDT-ASCT as the standard of care for RR-DLBCL. This approach salvages 30 to 40% of patients with DLBCL, who relapse after initial therapy.[14] Therefore, the initial approach to RR DLBCL management is to determine whether the patient is suitable for HD-ASCT.
For patients suitable for HD-ASCT, various salvage chemotherapeutic regimens are available. Before the Rituximab era, DHAP, ICE, MIME, and Mini-BEAM were some of the commonly used salvage therapies [Table 2].[14,15,16,17] Refractory DLBCL is also managed with these salvage regimens but has poor outcome.
Table 2.
Salvage chemotherapeutic regimens in the pre-rituximab era
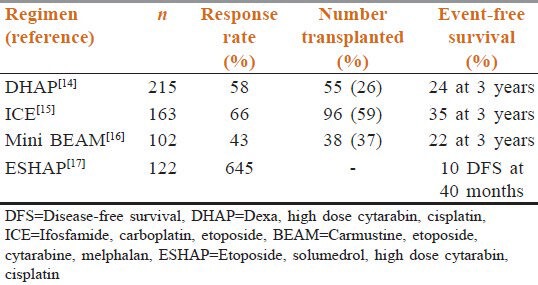
The overall response rates with MIME[18] and EPOCH[19] were 60% and 74%, respectively. The wide range of response rate in these trials is attributed not only to the differential efficacy of various chemotherapeutic drugs, but also to the patient population belonging to different age groups. At our center, we prefer R-DHAP as the HDT because it is cheaper and has fewer infectious complications than other HDTs. Our observation is supported by the good results observed in our subset of patients (unpublished personal observation).
Role of rituximab
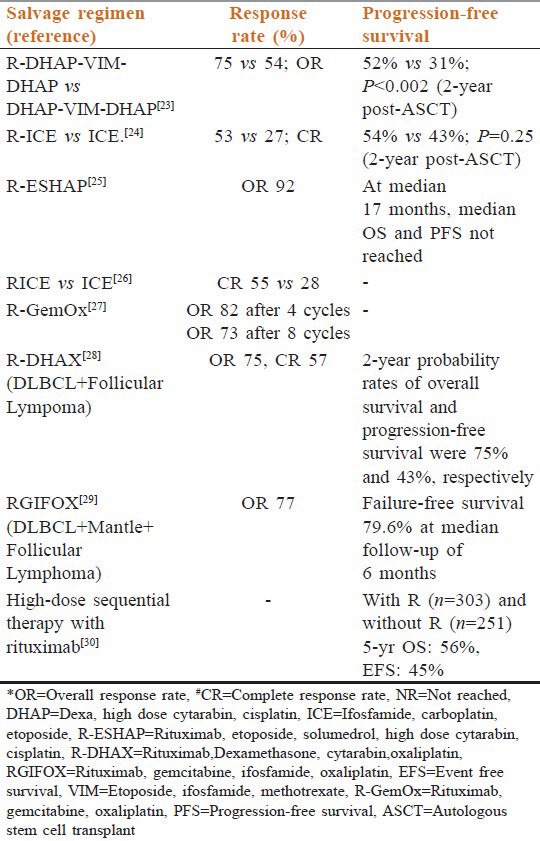
Rituximab monotherapy yielded good results in RR DLBCL.[20] Encouraged with these results, Rituximab was added to almost every salvage regimen available. Addition of Rituximab improved the response rates. This allowed more number of patients to undergo ASCT and improved the progression-free survival (PFS), disease-free survival, and overall survival (OS). The major drawback of these trials in the present scenario is that majority of patients had not been previously exposed to Rituximab. In the relapsed setting, the PFS and OS is better in R naïve patients (R-) than those who have been exposed to R previously (R+), especially for the early relapses (relapse within 12 months).[21,22] In the current scenario where majority patients have already been exposed to Rituximab, its role in the salvage chemotherapy needs to be reestablished, especially with respect to the emergence of Rituximab resistance, like in follicular lymphoma. Several other chemotherapeutic regimens have also shown increased response after addition of Rituximab to salvage chemotherapy. These responses are seen without any increase in toxicity and without affecting the stem cell collection [Table 3].[23,24,25,26,27,28,29,30]
Table 3.
Salvage chemotherapeutic regimens in the rituximab era
Gold standard salvage chemotherapy
An ideal salvage chemotherapeutic regimen should have higher response rates with minimal toxicity and should not have adverse effect on the stem cell harvest. Many options are available as salvage chemotherapy. RDHAP and RICE are the two widely used regimens worldwide. Which one is the best regimen? This issue has been addressed in the recently completed multicenter phase 2 CORAL STUDY, with an initial randomization between R-ICE x 3 vs. R-DHAP x 3 followed by BEAM-ASCT [Table 4]. A second randomization then allocated patients to maintenance treatment with Rituximab vs observation. In the first phase results of the trial, there was no significant difference in the response rate, 3-year EFS, or OS between the 2 salvage regimens.[22] However, an updated analysis of the CORAL study revealed that patients with GCB DLBCL (but not non-GCB DLBCL) appeared to benefit from salvage treatment with R-DHAP rather than R-ICE.[31]
Table 4.
CORAL study

Patients ineligible for HD-ASCT
According to standard bone marrow transplant guidelines, patients with severe concomitant medical or psychiatric illness, active central nervous system involvement, or HIV seropositivity are considered ineligible for ASCT. Other criteria for ineligibility includes a bilirubin level >2 mg/dL, creatinine level >1.5 mg/dL, low cardiac ejection fraction (<50%), and a forced expiratory volume in 1 second <50% and/or carbon monoxide diffusion test <50% of predicted level.[32] These patients have little chance at prolonged control of disease with a dismal outcome. The treatment option for these patients includes participation in phase 1/2 clinical trials with novel and experimental agents (vide infra in the future trends section). Patients are often offered palliation with radiotherapy[33] radioimmunoconjugates[34] or rituximab monotherapy.[35]
Prognostic factors in relapsed/refractory DLBCL:
-
Relapsed vs refractory disease
- Relapsed patients have higher OR than refractory patients.[24]
-
Age Adjusted International Prognostic Factors Index
- Patients with more risk factors (stage III/IV disease, serum LDH level greater than normal, and Karnofsky performance status less than 80%) perform worse than patients with less risk factors.[12]
-
Rituximab naïve status
- Rituximab naïve patients have better response rate.[21]
-
Time to relapse
Interestingly, the molecular subtype GCB or ABC have no prognostic value for relapsed/refractory DLBCL.[37,38]
Relapse after HD-ASCT
The prognosis of patients who relapse after HD-ASCT is extremely poor and the median survival is approximately 3 months.[39] The optimal management of patients with DLBCL relapsing after HD-ASCT is difficult and no standard treatment has been defined. Palliation,[40] second ASCT or non-myeloablative allogeneic stem cell transplant (AlloSCT)[41] are some of the options available for these patients. Second ASCT is rarely feasible.
Can we predict which patients will relapse or prove refractory?
Search for the predictors of relapse or refractory disease in DLBCL is on. The probable candidates in line are IPI at presentation,[42] CNS status,[43] immunoblastic histology,[44] molecular markers such as c-myc,[45] stromal signatures,[46] and interval PET scan.[47] Simple markers like absolute monocyte to absolute lymphocyte ratio at presentation[48] can also be useful in resource-constrained settings. In future, the treatment of DLBCL will be tailored according to the risk of relapse. Further studies need to validate this approach.
Future trends:
Various efforts are been made to improve the outcome of RR DLBCL.
Addition of rituximab post ASCT as maintenance/consolidation[49]
Addition of other monoclonal antibodies to rituximab[50]
Use of radioimmunoconjugates as palliation, as augmentation of HDT, or as part of conditioning regimen[51]
Use of novel agents like bortezomib,[52] enzastaurin,[53] everolimus,[54] lenalidomide,[55] fostamatinib,[56] etc.
Conclusions
High-dose chemotherapy followed by ASCT is the ideal treatment for eligible chemosensitive patients with RR DLBCL
The gold standard regimen for salvage chemotherapy does not exist. Superiority of one regimen (RICE or RDHAP) over other is not established, though some trends may emerge on further maturation of data from the CORAL study
Salvage regimen should contain Rituximab with platinum-based chemotherapy, as this combination is capable of producing high OR and CR rates
Various options for palliation are available for patients who cannot be offered HDT followed by ASCT
Advances are needed to improve the outcomes of RR DLBCL by developing newer drugs for salvage and for optimizing ASCT.
Acknowledgment
All IHTM Staff
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Cancer Facts and Figures 2012. American Cancer Society. [Last accessed in 2013 Jan]. Available from: http://www.cancer.org .
- 2.de Leval L, Hasserjian RP. Diffuse Large B-Cell Lymphomas and Burkitt Lymphoma. Hematol Oncol Clin North Am. 2009;23:791–827. doi: 10.1016/j.hoc.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood. 2011;117:5019–32. doi: 10.1182/blood-2011-01-293050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large B cell lymphomas. N Engl J Med. 2008;359:2313–23. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenz G, Staudt LM. Aggressive lymphomas. N Engl J Med. 2010;362:1417–29. doi: 10.1056/NEJMra0807082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garvin AJ, Simon RM, Osborne CK, Merrill J, Young RC, Berard CW. An autopsy study of histologic progression in non Hodgkin's lymphomas: 192 cases from the National Cancer Institute. Cancer. 1983;52:393–8. doi: 10.1002/1097-0142(19830801)52:3<393::aid-cncr2820520302>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-b-cell lymphoma. N Engl J Med. 2002;346:235–42. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 8.Perry AR, Goldstone AH. High-dose therapy for diffuse large-cell lymphoma in first remission. Ann Oncol. 1998;9(Suppl 1):S9–14. doi: 10.1093/annonc/9.suppl_1.s9. [DOI] [PubMed] [Google Scholar]
- 9.Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a Standard Regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's Lymphoma. N Engl J Med. 1993;328:1002–6. doi: 10.1056/NEJM199304083281404. [DOI] [PubMed] [Google Scholar]
- 10.Cheson BD, Horning SJ, Coiffier B, Shipp MA, Fisher RI, Connors JM, et al. Report of an international workshop to standardize response criteria for non-Hodgkin lymphomas. J Clin Oncol. 1999;17:1244–53. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 11.Pfreundschuh M, Trümper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–91. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 12.Hamlin PA, Zelenetz AD, Kewalramani T, Qin J, Satagopan JM, Verbel D, et al. Age-adjusted International Prognostic Index predicts autologous stem cell transplantation outcome for patients with relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2003;102:1989–96. doi: 10.1182/blood-2002-12-3837. [DOI] [PubMed] [Google Scholar]
- 13.Weeks JC, Yeap BY, Canellos GP, Shipp MA. Value of follow-up procedures in patients with large-cell lymphoma who achieve a complete remission. J Clin Oncol. 1991;9:1196–203. doi: 10.1200/JCO.1991.9.7.1196. [DOI] [PubMed] [Google Scholar]
- 14.Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy sensitive non-Hodgkin lymphoma. N Engl J Med. 1995;333:1540–5. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 15.Moskowitz CH, Bertino JR, Glassman JR, Hedrick EE, Hunte S, Coady-Lions N, et al. Ifosfamide, carboplatin and etoposide: A highly effective cyto-reduction and peripheral blood progenitor cell mobilization regimen for transplant-eligible patients with non-Hodgkins lymphoma. J Clin Oncol. 1999;17:3776–85. doi: 10.1200/JCO.1999.17.12.3776. [DOI] [PubMed] [Google Scholar]
- 16.Girouard C, Dufresne J, Imrie K, Stewart AK, Brandwein J, Prince HM, et al. Salvage chemotherapy with mini-BEAM for relapsed or refractory non-Hodgkins lymphoma prior to autologous bone marrow transplantation. Ann Oncol. 1997;7:675–80. doi: 10.1023/a:1008294725992. [DOI] [PubMed] [Google Scholar]
- 17.Velasquez WS, McLaughlin P, Tucker S, Hagemeister FB, Swan F, Rodriguez MA, et al. ESHAP - an effective chemotherapy regimen in refractory and relapsing lymphoma: A 4-year follow-up study. J Clin Oncol. 1994;12:1169–76. doi: 10.1200/JCO.1994.12.6.1169. [DOI] [PubMed] [Google Scholar]
- 18.Cabanillas F, Hagemeister FB, McLaughlin P, Velasquez WS, Riggs S, Fuller L, et al. Results of MIME salvage regimen for recurrent or refractory lymphoma. J Clin Oncol. 1987;5:407–12. doi: 10.1200/JCO.1987.5.3.407. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez M, Chabner BA, Pearson D, Steinberg SM, Jaffe ES, Cheson BD, et al. Role of a Doxorubicin containing regimen in relapsed and resistant lymphomas: An 8-year follow-up study of EPOCH. J Clin Oncol. 2000;18:3633–42. doi: 10.1200/JCO.2000.18.21.3633. [DOI] [PubMed] [Google Scholar]
- 20.Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab for the treatment of patients with relapsing or refractory aggressive lymphoma: A multicenter phase II study. Blood. 1998;92:1927–32. [PubMed] [Google Scholar]
- 21.Martín A, Conde E, Arnan M, Canales MA, Deben G, Sancho JM, et al. R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: The influence of prior exposure to rituximab on outcome. A GEL/TAMO study. Haematologica. 2008;93:1829–36. doi: 10.3324/haematol.13440. [DOI] [PubMed] [Google Scholar]
- 22.Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-Cell lymphoma in the Rituximab Era. J Clin Oncol. 2010;28:4184–90. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vellenga E, van Putten WL, van’t Veer MB, Zijlstra JM, Fibbe WE, van Oers MH, et al. Rituximab improves the treatment results of DHAP-VIM-DHAP and ASCT in relapsed/progressive aggressive CD20+NHL: A prospective randomized HOVON trial. Blood. 2008;111:537–43. doi: 10.1182/blood-2007-08-108415. [DOI] [PubMed] [Google Scholar]
- 24.Kewalramani T, Zelenetz AD, Nimer SD, Portlock C, Straus D, Noy A, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103:3684–8. doi: 10.1182/blood-2003-11-3911. [DOI] [PubMed] [Google Scholar]
- 25.Hicks L, Buckstein R, Mangel J, Piliotis E, Imrie K, Cheung MC, et al. Rituximab increases response to ESHAP in relapsed, refractory, and transformed aggressive B-cell lymphoma. Blood. 2006;108 Abstract 3067. [Google Scholar]
- 26.Zelenetz AD, Hamlin P, Kewalramani T, Yahalom J, Nimer S, Moskowitz CH. Ifosfamide, carboplatin, etoposide (ICE) based second line chemotherapy for the management of relapsed and refractory aggressive non Hodgkin's lymphoma. Ann Oncol. 2003;14(Suppl 1):i5–10. doi: 10.1093/annonc/mdg702. [DOI] [PubMed] [Google Scholar]
- 27.El Gnaoui T, Dupuis J, Belhadj K, Jais JP, Rahmouni A, Copie-Bergman C, et al. Rituximab, gemcitabine and oxaliplatin: An effective salvage regimen for patients with relapsed or refractory B cell Lymphoma not candidates for high dose therapy. Ann Oncol. 2007;18:1363–8. doi: 10.1093/annonc/mdm133. [DOI] [PubMed] [Google Scholar]
- 28.Lignon J, Sibon D, Madelaine I, Brice P, Franchi P, Briere J, et al. Rituximab, dexamethasone, cytarabine, and oxaliplatin (R-DHAX) is an effective and safe salvage regimen in relapsed/refractory B-cell non-Hodgkin lymphoma. Clin Lymph Myeloma Leuk. 2010;10:262–9. doi: 10.3816/CLML.2010.n.055. [DOI] [PubMed] [Google Scholar]
- 29.Corazzelli G, Russo F, Capobianco G, Marcacci G, Della Cioppa P, Pinto A. Gemcitabine, ifosfamide, oxaliplatin and Rituximab (R-GIFOX), a new effective cytoreductive/mobilizing salvage regimen for relapsed and refractory aggressive non-Hodgkin's lymphoma: Results of a pilot study. Ann Oncol. 2006;17(Suppl 4):iv18–24. doi: 10.1093/annonc/mdj994. [DOI] [PubMed] [Google Scholar]
- 30.Tarella C, Zanni M, Magni M, Benedetti F, Barbui T, Boccadoro M, et al. Benefit of rituximab addition to high-dose programs with autograft for B-cell lymphoma: A multicenter GITIL survey on 957 patients. Blood. 2006;108 Abstract 207. [Google Scholar]
- 31.Thieblemont C, Briere J, Mounier N, Voelker HU, Cuccuini W, Hirchaud E, et al. The Germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: A bio-CORAL study. J Clin Oncol. 2011;29:4079–87. doi: 10.1200/JCO.2011.35.4423. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez J, Caballero MD, Gutierrez A, Solano C, Arranz R, Lahuerta JJ, et al. Autologous stem-cell transplantation in diffuse large B-cell non-Hodgkin's lymphoma not achieving complete response after induction chemotherapy: The GEL/TAMO experience. Ann Oncol. 2004;15:1504–9. doi: 10.1093/annonc/mdh391. [DOI] [PubMed] [Google Scholar]
- 33.Murthy V, Thomas K, Foo K, Cunningham D, Johnson B, Norman A, et al. Efficacy of palliative low-dose involved-field radiation therapy in advanced lymphoma: A phase II study. Clin Lymphoma Myeloma. 2008;8:241–5. doi: 10.3816/CLM.2008.n.032. [DOI] [PubMed] [Google Scholar]
- 34.Morschhauser F, Illidge T, Huglo D, Martinelli G, Paganelli G, Zinzani PL, et al. Efficacy and safety of Yttrium-90 ibritumomab tiuxetin in patients with relapsed and refractory diffuse large B-cell lymphoma not appropriate for autologous stem-cell transplantation. Blood. 2007;110:54–8. doi: 10.1182/blood-2007-01-068056. [DOI] [PubMed] [Google Scholar]
- 35.Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab (Anti-CD20 Monoclonal Antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: A multicenter phase II study. Blood. 1998;92:1927–32. [PubMed] [Google Scholar]
- 36.Guglielmi C, Gomez F, Philip T, Hagenbeek A, Martelli M, Sebban C, et al. Time to relapse has prognostic value in patients with aggressive lymphoma enrolled onto the Parma trial. J Clin Oncol. 1998;16:3264–9. doi: 10.1200/JCO.1998.16.10.3264. [DOI] [PubMed] [Google Scholar]
- 37.Moskowitz CH, Zelenetz AD, Kewalramani T, Hamlin P, Lessac-Chenen S, Houldsworth J, et al. Cell of origin, germinal center versus non germinal center, determined by immunohistochemistry on tissue microarray, does not correlate with outcome in patients with relapsed and refractory DLBCL. Blood. 2005;106:3383–5. doi: 10.1182/blood-2005-04-1603. [DOI] [PubMed] [Google Scholar]
- 38.Moskowitz CH. Pretreatment prognostic factors and outcome in patients with relapsed or primary-refractory diffuse large B-cell lymphoma treated with second-line chemotherapy and autologous stem cell transplantation. Ann Oncol. 2006;17(Suppl 4):iv37–9. doi: 10.1093/annonc/mdj998. [DOI] [PubMed] [Google Scholar]
- 39.Vose JM, Bierman PJ, Anderson JR, Kessinger A, Pierson J, Nelson J, et al. Progressive disease after high-dose therapy and autologous transplantation for lymphoid malignancy: Clinical course and patient follow-up. Blood. 1992;80:2142–8. [PubMed] [Google Scholar]
- 40.Pircher A, Gassner EM, Steurer M, Wolf D. Durable complete remission in a patient with recurrent DLBCL receiving rituximab monotherapy after high-dose chemotherapy and autologous stem cell transplantation. BMJ Case Rep 2012. 2012 doi: 10.1136/bcr.02.2012.5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rezvani AR, Norasetthada L, Gooley T, Sorror M, Bouvier ME, Sahebi F, et al. Non-myeloablative allogeneic haematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: A multicentre experience. Br J Haematol. 2008;143:395–403. doi: 10.1111/j.1365-2141.2008.07365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anon The international non-Hodgkin's lymphoma prognostic factors project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–94. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 43.Boehme V, Schmitz N, Zeynalova S, Loeffler M, Pfreundschuh M. CNS events in elderly patients with aggressive lymphoma treated with modern chemotherapy (CHOP-14) with or without rituximab: An analysis of patients treated in the RICOVER-60 trial of the German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL) Blood. 2009;113:3896–902. doi: 10.1182/blood-2008-10-182253. [DOI] [PubMed] [Google Scholar]
- 44.Engelhard M, Brittinger G, Huhn D, Gerhartz HH, Meusers P, Siegert W, et al. Subclassification of diffuse large B-cell lymphomas according to the Kiel classification: Distinction of centroblastic and immunoblastic lymphomas is a significant prognostic risk factor. Blood. 1997;89:2291–7. [PubMed] [Google Scholar]
- 45.Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009;114:3533–7. doi: 10.1182/blood-2009-05-220095. [DOI] [PubMed] [Google Scholar]
- 46.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, et al. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–23. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang DH, Ahn JS, Byun BH, Min JJ, Kweon SS, Chae YS, et al. Interim PET/CT-based prognostic model for the treatment of diffuse large B cell lymphoma in the post-rituximab era. Ann Hematol. 2012 doi: 10.1007/s00277-012-1640-x. in press. [DOI] [PubMed] [Google Scholar]
- 48.Porrata LF, Ristow K, Habermann TM, Ozsan N, Dogan A, Macon W, et al. Absolute monocyte/lymphocyte count prognostic score is independent of immunohistochemically determined cell of origin in predicting survival in diffuse large B-cell lymphoma. Leuk Lymphoma. 2012;53:2159–65. doi: 10.3109/10428194.2012.690605. [DOI] [PubMed] [Google Scholar]
- 49.Haioun C, Mounier N, Emile JF, Bologna S, Coiffier B, Gisselbrecht C, et al. Rituximab compared to observation after high-dose consolidative first-line chemotherapy (HDC) with autologous stem cell transplantation in poor-risk diffuse large B-cell lymphoma: Updated results of the LNH98-B3 GELA study. J Clin Oncol. 2007;25 Abstract 8012. [Google Scholar]
- 50.Strauss SJ, Morschhauser F, Rech J, Repp R, Solal-Celigny P, Zinzani PL, et al. Multicenter phase II trial of immunotherapy with the humanized anti-CD22 antibody, epratuzumab, in combination with rituximab, in refractory or recurrent non-Hodgkin's lymphoma. J Clin Oncol. 2006;24:3880–6. doi: 10.1200/JCO.2006.05.6291. [DOI] [PubMed] [Google Scholar]
- 51.Gisselbrecht C, Vose J, Nademanee A, Gianni AM, Nagler A. Radioimmunotherapy for stem cell transplantation in Non-Hodgkin's lymphoma: In pursuit of a complete response. Oncologist. 2009;14(Suppl 2):41–51. doi: 10.1634/theoncologist.2009-S2-41. [DOI] [PubMed] [Google Scholar]
- 52.Dunleavy K, Pittaluga S, Czuczman MS, Dave SS, Wright G, Grant N, et al. Differential efficacy of bortezomib plus chemotherapy within molecular subtypes of diffuse large B-cell lymphoma. Blood. 2009;113:6069–76. doi: 10.1182/blood-2009-01-199679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Portlock CS. Enzastaurin, a Targeted PKCβ Inhibitor, in Relapsed or Refractory DLBCL: A promising new strategy based on gene expression signature. Curr Oncol Rep. 2007;9:371. [PubMed] [Google Scholar]
- 54.Witzig TE, Reeder CB, LaPlant BR, Gupta M, Johnston PB, Micallef IN, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed aggressive lymphoma. Leukemia. 2011;25:341–7. doi: 10.1038/leu.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hernandez-Ilizaliturri FJ, Deeb G, Zinzani PL, Pileri SA, Malik F, Macon WR, et al. Higher response to lenalidomide in relapsed/refractory diffuse large B-cell lymphoma in nongerminal center B-cell-like than in germinal center B-cell-like phenotype. Cancer. 2011;117:5058–66. doi: 10.1002/cncr.26135. [DOI] [PubMed] [Google Scholar]
- 56.Friedberg JW, Sharman J, Sweetenham J, Johnston PB, Vose JM, Lacasce A, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115:2578–85. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]


