Abstract
Background:
Oral mucositis is the most frequently occurring painful and dose-limiting side-effect of radiation of the head and neck region. Few studies demonstrated that oral glutamine suspension may significantly reduce the duration and severity of objective oral mucositis during radiotherapy.
Materials and Methods:
A randomized, prospective single institutional case control study was performed between April 2012 and November 2012 comparing the influence of oral glutamine on radiation induced mucositis in head and neck malignancy patients. Seventy biopsy proven patients with head and neck cancer receiving primary or adjuvant radiation therapy were randomized to receive either oral glutamine suspension daily 2h before radiation in the study arm (10 g in 1000 ml of water) (n = 35) or nothing before radiation; control arm (n = 35).
Results and Analysis:
Total 32 patients (91.43%) in the glutamine arm and total 34 patients (97.15%) developed mucositis. Grade 3 mucositis (14.29%) and grade 4 mucositis (2.86%) in the study arm (who received oral glutamine) were significantly less (P = 0.02 and P = 0.04, respectively) in the glutamine arm. The mean duration of grade 3 or worse mucositis (grade 3 and grade 4) was significantly less (6.6 days vs. 9.2 days) in study arm with P < 0.001. Mean time of onset of mucositis was significantly delayed in patients who took glutamine in comparison to control arm with P < 0.001.
Conclusion:
Glutamine delays oral mucositis in the head neck cancer patients. Moreover, it reduces the frequency and duration of grade 3 and grade 4 mucositis.
Keywords: Duration, glutamine, mucositis, severity, radioprotectant, chemoradiotherapy, head and neck
Introduction
Radiotherapy is one of the cornerstones in the management of head and neck malignancies. However, it leads to unavoidable toxicities in the form of systemic alterations and local lesions such as mucositis, loss of taste, decreased salivation, microbial colonization, dysphagia, and osteoradionecrosis.[1,2] The local toxicities clearly outweigh the systemic complaints both in severity and difficulty in management.
Annually, there are approximately 400,000 cases of treatment-induced damage to the oral cavity.[1] Oral mucositis is the most frequently occurring painful and dose-limiting side-effect of therapeutic irradiation of the head and neck.[3] Conventional fractionation schedules cause grade 3 and grade 4 mucositis in approximately 25% of the Radiation Therapy Oncology Group (RTOG) studies[4,5] whereas, accelerated regimes like concomitant boost or hyperfractionation increase the same to 50%.[5] Addition of concomitant chemotherapy during the radiotherapy further aggravates these lesions leading to significant morbidity, odynophagia, dysguesia, and subsequent dehydration and malnutrition.[6] Furthermore, modifications and dose reductions in the treatment schedule, more so with concurrent chemotherapy, to allow for resolution of these lesions can directly compromise patient survival. Different interventions are currently practiced with varying benefits, but there is no consensus on the most effective way to prevent or treat this most distressing complication.[7]
Glutamine is the most abundant free amino acid in the body.[8] In several animal species, glutamine was shown to be the major respiratory fuel for the intestinal tract.[9,10] Moreover, reduction of plasma glutamine levels by administration of glutaminase caused edema and ulceration of the intestinal mucosa as well as patchy areas of necrosis.[11]
Glutamine may help decrease mucous membrane injury induced by radiation by altering the inflammatory response. Glutathione, a byproduct of glutamine metabolism protects against oxidant injury.[12,13] Glutathione is an antagonist to prostaglandin E2 (PGE2) production, which is a strong inflammatory mediator. Klimberg et al. used a rat breast cancer model to show that glutamine-supplemented rats with mammary tumors had greater glutamine and glutathione concentrations, and decreased PGE2 production than rats that received no glutamine.[14] In another study, PGE2 levels from the tissues obtained by serial mucosal biopsies from dogs experiencing acute radiation effects increased with increasing inflammation.[15]
In patients with cancer, marked glutamine depletion develops over time; cancer cachexia is marked by massive depletion of skeletal muscle glutamine. This can have a negative impact on the function of host tissues that are dependent upon adequate stores of glutamine for optimal functioning.[16,17] Furthermore, the extent of normal tissue damage from radiation or chemotherapy may be influenced by the presence of adequate tissue glutamine stores. Both of these facts suggest a possible therapeutic role for glutamine in the prevention of host normal tissue toxicity during cancer treatment.[18]
A recent pilot trial by Huang et al.[19] demonstrated that oral glutamine suspension may significantly reduce the duration and severity of objective oral mucositis during radiotherapy. It may also shorten the duration of ≥ grade 3 mucositis. Similar observations were made by Dr. Silvermann in his review article on oral mucositis.[20] However, an adequately powered randomized clinical study required to establish these findings has not been carried out so far, to the best of our knowledge.
Oral administration of glutamine is a convenient way of providing nutrients to patients with the preserved oral intake. Therefore, we proceeded to perform this study where the utility of glutamine was tested in head neck cancer patients undergoing radiotherapy at our clinic.
Materials and Methods
A randomized, prospective single institutional case control study was performed between April 2012 and November 2012 comparing the influence of oral glutamine on radiation induced mucositis in head and neck malignancy patients. Adult subjects with biopsy-proven malignant neoplasms of the head and neck region, who were assigned to receive radical radiotherapy and performance status not worse than 50% according to the Karnofsky performance status scale, were included. Patients who had received prior radiotherapy, or in whom the intent of radiotherapy was palliative were excluded. Permission from the Institutional Ethical Committee was obtained. After obtaining informed consent, 70 patients with head and neck cancer receiving definitive or adjuvant radiation therapy were randomized (1:1) to receive either oral glutamine suspension daily 2 hours before radiation; study arm (10 g in 1000 ml of water) (n = 35) or nothing before radiation; control arm (n = 35). Glutamine crystalline powder in sachets, each containing 10 g, 1 sachet dissolved in 1 liter of water was consumed daily within 2 hours before radiation. Patients were instructed to swish their mouths first with the glutamine solution and then swallow within 2 hours before radiation treatment 5 days/week on treatment days only. Patients who took upto 800 ml of glutamine solution on an average were also accepted. Radiation was administered at 2 Gy/fraction daily, 5 days a week. We evaluated the grading of oral mucositis every week during external beam radiotherapy. We stopped radiation when the patients developed grade 3 or grade 4 mucositis.
Prior to allocation, the patients were stratified by Radiotherapy (RT) treatment field (facial, cervical, and cervico-facial) and by age (<60 and >60 years). Age stratification was used because at age 60 years, around 40% of the population reported xerostomia,[21] which was an important factor for mucositis.
Mucositis was scored weekly using the World Health Organization method as follows: Grade 1, soreness and erythema; grade 2, erythema or ulcers but can eat solid-foods; grade 3, ulcers, can take liquid only; and grade 4, cannot swallow even liquids.[22]
Weekly grading of mucositis, onset and duration of mucositis were recorded and compared. Gender, race, tumor histology, tumor stage, tumor treatment modality, and RT area were analyzed as categorical variables; significance was tested by Fisher exact test for categorical variables, and by t-test for continuous variables. Frequencies and Fisher exact tests were used to analyze contingency outcomes by an independent variable and by patient characteristics. A two-sided P < 0.05 was considered statistically significant in all tests.
Results and Analysis
A total of 70 patients (35 in the glutamine arm and 35 in the control arm) were enrolled on the study. There was no drop out in either arm. For our study purpose, we divided both the study and the control arm according to their demographic characteristics (age and sex), subsites of head and neck malignancies (oral cavity cancers, laryngeal, and hypopharyngeal cancers, ca nasopharynx, stage of the disease, treatment provided, and radiation portals, which are shown in Table 1. Baseline characteristics showed no statistically significant difference between the study arm and control arm.
Table 1.
Baseline characteristics of study and control arm
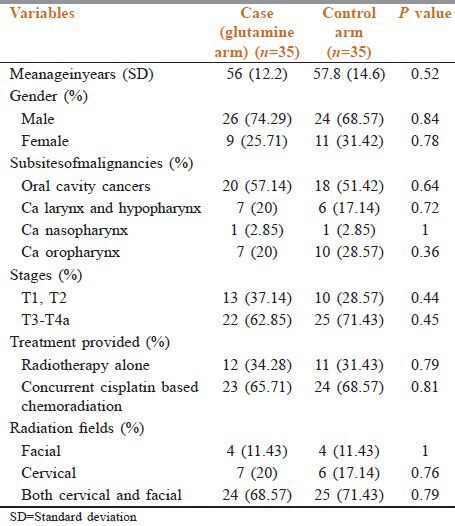
Total 32 patients (91.43%) in the glutamine arm and total 34 patients (97.15%) developed mucositis. Regarding the severity of mucositis, though there is no statistically significant difference in number of patients who developed grade 1 and grade 2 mucositis, but the number of patients who developed grade 3 mucositis (14.29%) and grade 4 mucositis (2.86%) in the study arm (who received oral glutamine) was significantly less (P = 0.02 and P = 0.04, respectively) as compared to the control arm (37.14% patients developed grade 3 mucositis and 17.14% patients developed grade 4 mucositis) [Table 2 and Figure 1].
Table 2.
Comparison of the number of patients who developed mucositis in both arms
Figure 1.
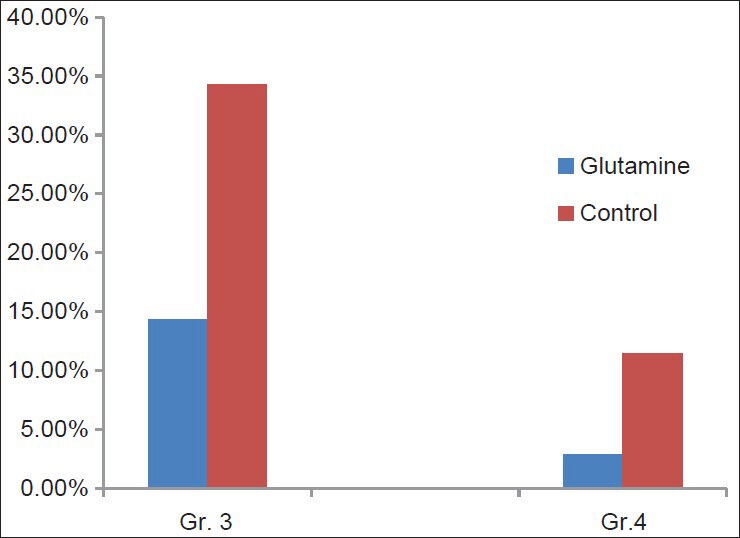
Bardiagram comparing the incidence of grade 3 and grade 4 mucositis in both arms
The mean duration of grade 3 mucositis or worse (grade 3 and grade 4) was significantly less (6.6 days vs. 9.2 days) in the study arm who received oral glutamine before radiation with P < 0.001 as shown in Table 3.
Table 3.
Comparison of duration of mucositis between two arms

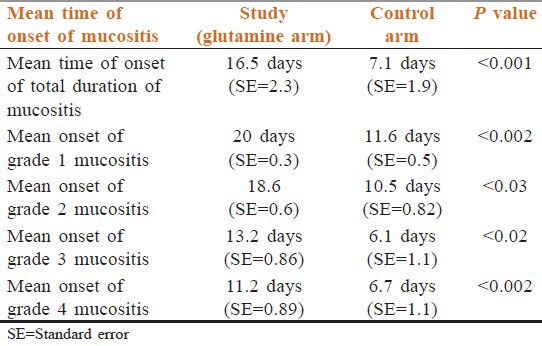
As evidenced from Table 4, the mean time to onset of mucositis was significantly earlier in the control arm who did not receive glutamine as compared to study arm (who received glutamine) with P < 0.001;thus, glutamine delayed the onset of mucositis. Separate analysis of different grades of mucosal toxicities similarly showed delayed onset of all grades of mucositis in patients who received glutamine as compared to control arm [Table 4].
Table 4.
Comparison of time of onset of mucositis between the two groups
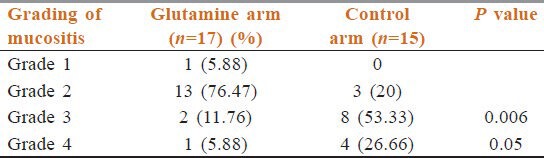
Patients above 60 years of age suffered from mucositis both in glutamine and control arm. However, grade 3 and grade 4 mucositis were less in the glutamine arm (11.76% and 5.88%) compared to the control arm (53.33%and 26.66%) [Table 5].
Table 5.
Mucositis above the age of 60 years
Patients who underwent both face and neck radiation suffered more severe mucositis than patients who underwent only face or neck radiation. Grade 3 or worse mucositis was seen in 52% (13 out of 25 face and neck irradiation) in the control arm. However, the same was seen much less in the glutamine arm (20.83%) (5 out of 24 face and neck irradiation) (P = 0.01).
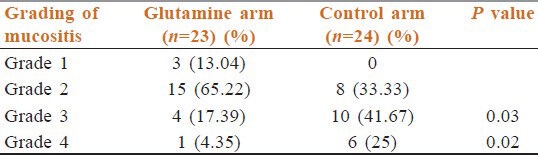
Patients who received concurrent chemoradiation suffered more mucositis. However, patients on the glutamine arm suffered less grade 3 and grade 4 mucositis (17.39% and 4.35%) than patients in the control arm (41.67%and 25%) (both statistically significant) [Table 6].
Table 6.
Mucositis in patients receiving concurrent chemoradiation
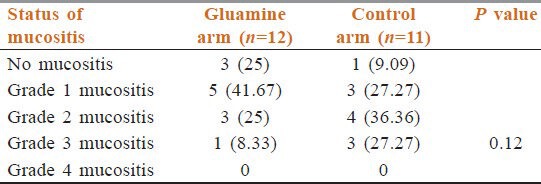
In patients undergoing only radiation, there was no grade 4 mucositis in either arm. Grade 3 mucositis was less in the glutamine arm (8.33%) than patients in the control arm (27.27%) but, this was not statistically significant [Table 7].
Table 7.
Mucositis in patients receiving only radiation
Discussion
The challenge of oral mucositis still continues to plague therapeutic irradiation, especially in head and neck cancers. Different interventions are currently practiced with varying benefits, but there is no consensus on the most effective way to prevent or treat this distressing complication.[7]
Glutamine has already proved to be efficacious against radiation and chemotherapy induced intestinal injury[12,23] and stomatitis.[24] A recent trial of oral glutamine was conducted in patients of metastatic breast cancer treated with paclitaxel and melphalan as a conditioning regime for autologous stem cell transplantation. The decrease in duration and incidence of oral mucositis (grade>=2) was statistically significant (P = 0.048 and P = 0.026 respectively) with glutamine (Saforis) compared to the placebo arm.[24] A recent pilot trial by Huang et al.[19] and a study carried out by Silvermann[20] demonstrated that oral glutamine suspension may significantly reduce the duration and severity of oral mucositis during radiotherapy.
Our study showed that oral glutamine delays the development of mucositis. The mean time of onset of mucositis is significantly delayed in patients who received glutamine with P < 0.001. The mean duration of grade 3 mucositis or worse (grade 3 and grade 4) was significantly less (6.6 days vs. 9.2 days) in the glutamine arm.
The mean total duration of mucositis as well as all the different grades were significantly lesser in the glutamine arm. Although the incidence of mucositis as a whole was not decreased significantly, but incidence and duration of grade 3 and grade 4 mucositis were significantly less with glutamine with P values 0.02 and 0.04, respectively.
Chance of mucositis is much higher in elderly patients. In the present study, glutamine showed beneficial effects by reducing grade 3 and worse mucositis in patients above 60 years of age.
Patients who undergo concurrent chemoradiotherapy may develop severe mucositis for which treatment may have to be stopped temporarily in many cases. In our study, we noted that patients in glutamine arm suffered less grade 3 and grade 4 mucositis (statistically significant).
Though, less grade 3 and worse mucositis was seen in patients undergoing only radiation in glutamine arm, but it was not statistically significant.
However, our results may encourage further studies on this issue recruiting number of patients.
Conclusion
Our study shows the efficacy of glutamine in all aspects of oral mucositis in head and neck cancer irradiation-appearance, incidence, duration, and severity. It is really encouraging to note that patients of head and neck cancer suffering from severe mucositis due to standard treatment (radiation with or without concurrent chemotherapy) can be alleviated to a great extent simply by such a feasible and affordable option. However, the results need to be warranted by future studies with the larger samples in order to be recommended as a standard protocol.
Footnotes
Source of Support: Glutamine packets were supplied free of cost to patients by GLS Pharmaceuticals for the trial after obtaining institutional ethical committee approval.
Conflict of Interest: None declared.
References
- 1.Dose AM. The symptom experience of mucositis, stomatitis, and xerostomia. Semin Oncol Nurs. 1995;11:248–55. doi: 10.1016/s0749-2081(05)80005-1. [DOI] [PubMed] [Google Scholar]
- 2.Zlotolow IM. General consideration in prevention and treatment of oral manifestation of cancer therapies. In: Berget AP, Weissman DE, editors. Principles and Practice of Supportive Oncology. Philadelphia, PA: Lippincott Raven; 1998. p. 237. [Google Scholar]
- 3.Vissink A, Jansma J, Spijkervet FK, Burlage FR, Coppes RP. Oral sequele of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- 4.Lee DJ, Cosmatos D, Marcial VA, Fu KK, Rotman M, Cooper JS, et al. Results of an RTOG phaseIII trial (RTOG 85-27) comparing radiotherapy plus etanidazole with radiotherapy alone for locally advanced head and neck carcinomas. Int J Radiat Oncol Biol Phys. 1995;32:567–76. doi: 10.1016/0360-3016(95)00150-W. [DOI] [PubMed] [Google Scholar]
- 5.Fu KK, Pajak TF, Trotti A, Jones CU, Spencer SA, Phillips TL, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 6.Bitran JD, Samuels B, Klein L, Hanauer S, Johnson L, Martinec J, et al. Tandem high-dose chemotherapy supported by hematopoietic progenitor cells yields prolonged survival in stage IV breast cancer. Bone Marrow Transplant. 1996;17:157–62. [PubMed] [Google Scholar]
- 7.Mead GM. Management of oral mucositis associated with cancer chemotherapy. Lancet. 2002;359:815–6. doi: 10.1016/s0140-6736(02)07960-6. [DOI] [PubMed] [Google Scholar]
- 8.Krebs H. Glutamine metabolism in the anima lbody. In: Mora J, Palacios R, editors. Glutamine: Metabolism, enzymology and regulation. NewYork: Academic Press; 1980. pp. 319–25. [Google Scholar]
- 9.Windmueller HG, Spaeth AE. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem. 1974;249:5070–9. [PubMed] [Google Scholar]
- 10.Windmueller HG, Spaeth AE. Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for post absorptive rat small intestine. J Biol Chem. 1978;253:69–76. [PubMed] [Google Scholar]
- 11.Baskerville A, Hambleton P, Benbough JE. Pathological features of glutaminase toxicity. Br J Exp Pathol. 1980;61:132–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Rouse K, Nwokedi E, Woodliff JE, Epstein J, Klimberg VS. Glutamine enhances selectivity of chemotherapy through changes in glutathione metabolism. Ann Surg. 1995;221:420–6. doi: 10.1097/00000658-199504000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savarese DM, Savy G, Vahdat L, Wischmeyer PE, Corey B. Prevention of chemotherapy and radiation toxicity with glutamine. Cancer Treat Rev. 2003;29:501–13. doi: 10.1016/s0305-7372(03)00133-6. [DOI] [PubMed] [Google Scholar]
- 14.Klimberg VS, Kornbluth J, Cao Y, Dang A, Blossom S, Schaeffer RF. Glutamine suppresses PGE 2 synthesis and breast cancer growth. J Surg Res. 1996;63:293–7. doi: 10.1006/jsre.1996.0263. [DOI] [PubMed] [Google Scholar]
- 15.Shewchuk LD, Baracos VE, Field CJ. Dietary L-glutamine supplementation reduces the growth of the Morris Hepatoma7777 in exercise-trained and sedentary rats. J Nutr. 1997;127:158–66. doi: 10.1093/jn/127.1.158. [DOI] [PubMed] [Google Scholar]
- 16.Ardawi MS, Newsholme EA. Glutamine metabolism in lymphocytes of the rat. Biochem J. 1983;212:835–42. doi: 10.1042/bj2120835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klimberg VS, Souba WW, Dolson DJ, Salloum RM, Hautamaki RD, Plumley DA, et al. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990;66:62–8. doi: 10.1002/1097-0142(19900701)66:1<62::aid-cncr2820660113>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 18.Huang EY, Leung SW, Wang CJ, Chen HC, Sun LM, Fang FM, et al. Oral glutamine to alleviate radiation-induced oral mucositis: A pilot randomized trial. Int J Radiat Oncol Biol Phys. 2000;46:535–9. doi: 10.1016/s0360-3016(99)00402-2. [DOI] [PubMed] [Google Scholar]
- 19.Silverman S., Jr Diagnosis and management of oral mucositis. J Support Oncol. 2007;5(Suppl 1):13–21. [PubMed] [Google Scholar]
- 20.Billings RJ, Proskin HM, Moss ME. Xerostomia and associated factors in a community-dwelling adult population. Community Dent Oral Epidemiol. 1996;24:312–6. doi: 10.1111/j.1600-0528.1996.tb00868.x. [DOI] [PubMed] [Google Scholar]
- 21.Pico JL, Avila-Garavito A, Naccache P. Mucositis: Its Occurrence, Consequences, and Treatment in the Oncology Setting. Oncologist. 1998;3:446–51. [PubMed] [Google Scholar]
- 22.Souba WW, Klimberg VS, Copeland EM., 3rd Glutamine nutrition in the management of radiation enteritis. JPENJ Parenter Enteral Nutr. 1990;14:106S–8. doi: 10.1177/014860719001400414. [DOI] [PubMed] [Google Scholar]
- 23.Anderson PM, Schroeder G, Skubitz KM. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer. 1998;83:1433–9. doi: 10.1002/(sici)1097-0142(19981001)83:7<1433::aid-cncr22>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Cockerham MB, Weinberger BB, Lerchie SB. Oral glutamine for the prevention of oral mucositis associated with high-dose paclitaxel and melphalan for autologous bone marrow transplantation. Ann Pharmacother. 2000;34:300–3. doi: 10.1345/aph.19168. [DOI] [PubMed] [Google Scholar]


