Abstract
Objective:
The precise role of iron in immune regulation especially in children vulnerable to iron deficiency is not fully known. Hence, this study was conducted to evaluate the effects of iron deficiency anemia (IDA) and its treatment with oral iron supplementation on cell-mediated immunity (CMI) and humoral immunity (HMI) in children.
Materials and Methods:
A total of 40 children (<15 years) with IDA and 40 age-matched healthy children after satisfying the inclusion criteria were enrolled for this case-control study. Flow cytometric evaluation of absolute and relative numbers of cluster of differentiation 4 (CD4) and CD8 (cluster of differentiation 8) lymphocyte subgroups was carried out to assess the CMI and serum Immunoglobulin G (IgG), Immunoglobulin A (IgA), Immunoglobulin M (IgM) were measured to assess the HMI at baseline and 3 months post oral iron supplementation.
Results:
Significantly lower levels (P < 0.05) of CD4+ T-cells and decreased CD4:CD8 ratios were observed in the iron deficient children. Iron supplementation significantly improved the CD4+ cell counts and CD4:CD8 ratios. However, immunoglobulin levels weren’t different between the two groups.
Conclusions:
Although IDA did not influence HMI, it significantly impaired CMI, which was improved following iron supplementation for 3 months.
Keywords: Cell-mediated immunity, flow cytometry, humoral immunity, iron deficiency anemia
INTRODUCTION
Anemia is the most common nutritional disorder in the world mostly associated with iron deficiency (ID).[1] Children and women in the reproductive age group are especially vulnerable to ID. The significance of iron as an essential micronutrient is well-recognized and a variety of systemic, non-hematological effects of ID are known.[2] Iron is necessary for normal development of the immune system. Its deficiency affects the capacity to have an adequate immune response as it is necessary for immune cell proliferation and the generation of specific response to infection.[3] Despite proven reversible functional immunological defects, a clinically important relationship between states of ID and susceptibility to infections remain controversial.[4] Both ID and overload have negative effects on immune function; a balanced iron homeostasis is central to determining susceptibility toward and the fate of an infection.[5] Both experimental, and some clinical studies have emphasized the importance of iron in the integrity of the immune system especially the innate immunity (decreased bactericidal effect and respiratory burst of neutrophils) and the cellular component system of cell-mediated immunity (CMI) (decreased lymphocyte proliferation and delayed hypersensitivity responses).[3,4] Though, the circulating levels of total T-lymphocytes and their helper and cytotoxic subsets in ID have been investigated by many groups,[4,6,7,8,9] very few studies have been carried out in children.[10,11] The effects of ID on humoral immunity (HMI) remain controversial. Some reports indicate iron depletion may be responsible for decreased HMI while others did not report any change in serum immunoglobulin in iron deficiency anemia (IDA).[4,6,12,13] We therefore, designed this study to establish the altered status of these parameters in iron deficient children at baseline and at 3 months post iron supplementation therapy.
MATERIALS AND METHODS
Study design
This case-control study was conducted in children in the age group of less than 15 years,[14] i.e., including preschool-aged (0.00-4.99 years) and school-aged (5.00-14.99 years) children who came to our department for laboratory investigations after being referred from the departments of general medicine and pediatrics in 1½ year (01.01.2011-30.06.2012) of the study period. The study was carried out after obtaining the proper approval from the Ethical Committee of the Institution and after getting the informed consent from either parent of the child. Applying the inclusion and exclusion criteria, a total 40 children who were diagnosed as IDA during the study period after clinical and laboratory investigations and 40 healthy children of comparable age and sex fulfilling the inclusion criteria were considered as case and control group respectively. IDA cases fulfilling all of the following inclusion criteria: (i) Hemoglobin <10 g/dl, (ii) Mean corpuscular volume (MCV) <80 fl, (iii) Serum ferritin <20 ng/ml were included in the study as cases.
Exclusion criteria for cases were: (i) Children with a history of receiving iron, other hematinic or multivitamins in preceding 3 months (ii) children with chronic blood loss or recent acute blood loss (iii) children with chronic illness, acute or chronic infection at the time of the study (iv) children with clinical features of malnutrition (v) children with serum albumin <3.5 g/dl (vi) children with C-reactive protein, CRP ≥0.6 mg/dl (vii) cases with a history of taking immunosuppressant drugs, radiotherapy or chemotherapy (viii) children with any disease that can alter serum antibodies such as immunodeficiency disease, autoimmune disease, malignancy, chronic infection etc., (ix) cases with no improvement in clinical features and laboratory parameters after 3 months of only oral iron supplementation.
Children in the ID group were given oral iron supplementation,[15] (6 mg/kg/day of elemental iron in two to three divided doses) for 3 months after treatment of parasitic infestations (tablet albendazole in a single dose of 200 mg for children <2 years and 400 mg for children >2 years). All the parameters were measured again after completion of oral iron therapy.
Control group was selected by using the following inclusion criteria (i) children not fulfilling any of the above mentioned criteria of IDA (ii) children with no history of chronic blood loss or recent acute blood loss (iii) children with no history of chronic illness, acute or chronic infection at the time of the study (iv) children with no clinical features of malnutrition (v) children with serum albumin ≥3.5 g/dl (vi) children with CRP <0.6 mg/dl (vii) children with no history of taking immunosuppressant drugs, radiotherapy or chemotherapy.
Sampling: 8 ml venous blood was collected under aseptic precautions from each child. In addition of making two good peripheral smears, 2 ml blood was placed in EDTA (Ethylene Diamine Tetra-acetic Acid) containing vial for complete blood count in an automated hematology analyzer (KX-21, Sysmex Corporation, Japan). A reticulocyte count was also performed on the sample using new methylene blue. 4 ml blood was taken in plain glass tube and serum was used for estimation of serum iron and total iron binding capacity (TIBC) colorimetrically (Dimension RxL, Siemens), serum ferritin by two-site sandwich immunoassay using the direct chemiluminometric technology (ADVIA Centaur, Siemens), serum immunoglobulins (IgG, IgA, IgM) by radial immunodiffusion (Diffu-Plate, Biocientifica S.A., Argentina), CRP and serum albumin. Rest of the blood was placed in EDTA containing vacutainer tube for flow cytometric determination of CD4+ and CD8+ cells.
Flow cytometry procedure: Peripheral blood (100 μL) was surface stained with fluorochrome-conjugated (Fluorescein Isothiocyanate, and phycoerythrin,) antibodies to CD3, CD4, and CD8 along with appropriate isotype controls (mouse IgG1) to distinguish non-specific “background” staining from specific antibody staining [Figure 1a]. After incubation for 15 min at room temperature, fluorescence-activated cell scanner (FACS) Lysing Solution (2 ml; BD Biosciences) was added and incubated for an additional 10 min at room temperature. Cells were centrifuged (400 g for 5 min), and the resultant pellet was washed with phosphate buffered saline and analyzed on a FACS Calibur flow cytometer, BD Biosciences. Lymphocytes were gated in the R1 region [Figure 1b] on the basis of characteristic linear forward and side scatter features and fluorescence was measured on a logarithmic scale, using CellQuest Pro software (BD Biosciences).
Figure 1a.
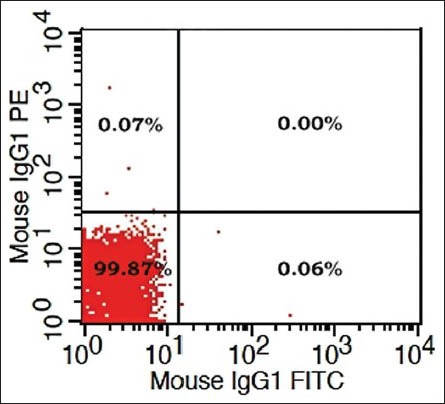
Isotype control (IgG1) to exclude non-specific background staining
Figure 1b.
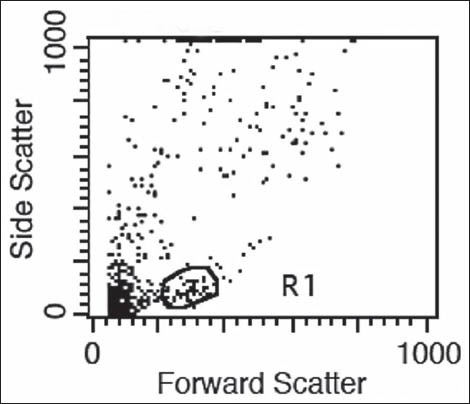
Gating of lymphocytes in the “R1” region
Statistical analysis
Student's (unpaired) and paired t-test, Pearson Chi-square test were utilized for statistical analysis using IBM SPSS statistics version 20. P < 0.05 was considered statistically significant.
RESULTS
We got 49 cases of IDA in our study period, but we had to exclude 9 cases as they were identified with one or more exclusion criteria. There was no significant difference in age, sex, weight and height between patients and the control group [Table 1]. At presentation, as regard to the hematological parameters, there was a significant decrease in hemoglobin concentration, RBC (Red blood cell) count, hematocrit, and MCV in IDA patients when compared to the control group. Furthermore, there was a significant increase in platelets count and RDW (Red cell distribution width). No significant difference in total leucocyte count, lymphocytic count and reticulocyte count was detected on comparing both groups [Table 2]. Serum iron and ferritin were significantly lower in IDA patients with a significant increase in TIBC [Table 3]. The percentage of CD4 + lymphocytes and CD4:CD8 ratio was significantly lower than the control group (P < 0.001) [Table 4]. However, there was no significant difference of CD8 + lymphocytes, serum IgG, IgA, and IgM levels in IDA and the control group. Following iron supplementation for 3 months, most of the hematological, immunological parameters and iron indices improved significantly [Tables 2–4]. Compared to the control group, hemoglobin concentration, RBC count, hematocrit, MCV, platelet counts, serum iron, ferritin, and TIBC were normalized [Tables 2 and 3]. In addition, significant increase in the percentage of both CD4 + lymphocytes and CD4:CD8 ratio was observed with their values comparable to those in the control group [Table 4 and Figure 2].
Table 1.
Clinical variables in the iron deficient and control groups
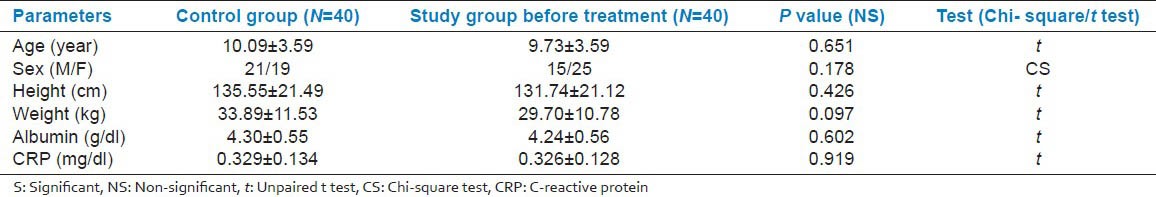
Table 2.
Comparison of hematological parameters among the control group (A), IDA group before treatment (B) and IDA group after treatment (C)
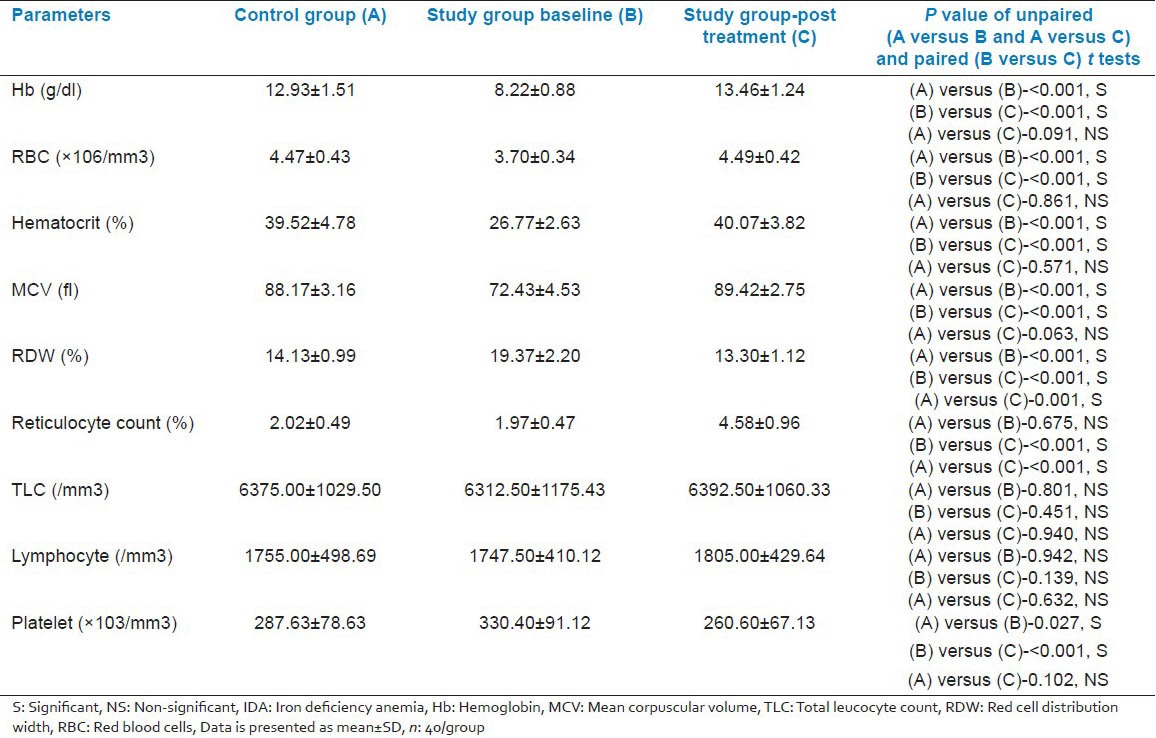
Table 3.
Comparison of iron parameters among the control group (A), IDA group before treatment (B) and IDA group after treatment (C)
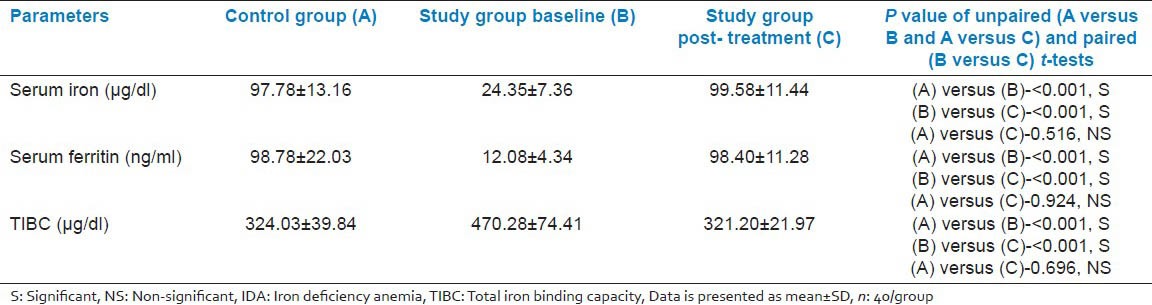
Table 4.
Comparison of immunological parameters among the control group (A), IDA group before treatment (B) and IDA group after treatment (C)
Figure 2 (a and b).
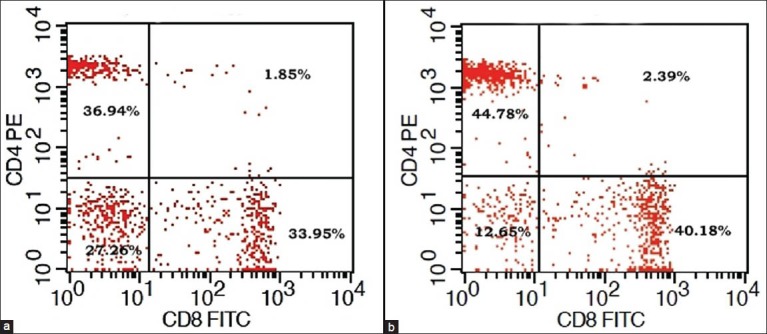
Dot plot diagram of flow cytometry showing CD4+ and CD8+ percentage of T-lymphocytes in a patient with iron deficiency anemia before and after iron supplementation
DISCUSSION
In the present study, in addition to common hematological parameters the changes in RDW, reticulocyte count and platelet count are comparable to the other well-known studies.[16,17] Number of mature T- and helper-inducer T-lymphocytes was found to be inversely related to iron status in a study by Berger et al.[10] Consistent with our findings, significantly lower levels of T-lymphocytes, CD4 + cells and decreased CD4:CD8 ratio were observed the iron deficient children.[18] These effects were improved following iron supplementation.
Interestingly the number of CD8+ lymphocytes are similar in the iron deficient and control groups;[10,11,18] however, relative percentage of CD8+ cells is enhanced in ID.[7]
Thibault et al.,[11] reported only qualitative rather than a quantitative defect in CMI in 3-36-month-old children. In this study, a decreased in vitro IL−2 production by lymphocytes without any change in lymphocyte counts in iron deficient children was observed. Such effects may be attributed to the difference in various states of lymphocyte maturity. Indeed the level of mature T-lymphocytes (CD4+ and CD8+) was significantly lower while that of the immature T-cells (CD1a+) was significantly higher in IDA children.[17] The mature T-cell count was significantly improved following iron supplementation. Concurring these results, Sejas et al.,[19] also stated that ID in children can significantly affect circulating immature lymphocyte subpopulations. A similar reduction in lymphocyte counts was also observed among pre-menopausal women with IDA.[20]
In our study, IDA was not associated with any major effects on HMI and is consistent with other studies by Ekiz et al.,[4] and Sadeghian et al.,[13] carried out on children and adult non-pregnant females respectively. Bagchi et al.,[21] also gave similar opinion regarding the effect of ID on HMI in children. Although our results are in contrast to Tang et al.[6] Feng et al.,[12] and Guzikowska et al.,[22] who reported a lower level of IgG or IgA in pregnant women or children with IDA, it was beyond the scope of the present study to explain this variation and differing results. Nevertheless, such variations may be attributed to differing selection criteria and sample size.[18] Hence, we envisage studies involving a larger number of children, belonging to well-defined age groups from different geographic areas for a conclusive interpretation in future.
CONCLUSIONS
Lower CD4+ lymphocyte levels and the CD4:CD8 ratio in children with ID may contribute to the decreased CMI, which can be restored by iron supplementation. The degree of anemia probably has a varying role on the extent of immunological compromise. Although IDA did not have major effects on HMI, our study clearly depicts a positive association of ID with impaired CMI, which warrants medical intervention.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Technical Consensus on Key Issues. Boston, MA: International Nutrition Foundation; 1999. UNICEF/UNU/WHO/MI. Preventing Iron Deficiency in Women and Children; pp. 1–60. [Google Scholar]
- 2.Hercberg S, Galan P. Biochemical effects of iron deprivation. Acta Paediatr Scand Suppl. 1989;361:63–70. doi: 10.1111/apa.1989.78.s361.63. [DOI] [PubMed] [Google Scholar]
- 3.Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131:568S–79S. doi: 10.1093/jn/131.2.568S. [DOI] [PubMed] [Google Scholar]
- 4.Ekiz C, Agaoglu L, Karakas Z, Gurel N, Yalcin I. The effect of iron deficiency anemia on the function of the immune system. Hematol J. 2005;5:579–83. doi: 10.1038/sj.thj.6200574. [DOI] [PubMed] [Google Scholar]
- 5.Weiss G. Iron. In: Hughes DA, Darlington LG, Bendich A, editors. Diet and Human Immune Function. Totowa, New Jersey (NJ), USA: Humana Press; 2004. pp. 203–15. Bendich A, editor Nutrition andhealth. [Google Scholar]
- 6.Tang YM, Chen XZ, Li GR, Zhou RH, Ning H, Yan H. Effects of iron deficiency anemia on immunity and infectious disease in pregnant women. Wei Sheng Yan Jiu. 2006;35:79–81. [PubMed] [Google Scholar]
- 7.Santos PC, Falcão RP. Decreased lymphocyte subsets and K-cell activity in iron deficiency anemia. Acta Haematol. 1990;84:118–21. doi: 10.1159/000205047. [DOI] [PubMed] [Google Scholar]
- 8.Zimmer JP, Garza C, Heller ME, Butte N, Goldman AS. Postpartum maternal blood helper T (CD3+CD4+) and cytotoxic T (CD3+CD8+) cells: Correlations with iron status, parity, supplement use, and lactation status. Am J Clin Nutr. 1998;67:897–904. doi: 10.1093/ajcn/67.5.897. [DOI] [PubMed] [Google Scholar]
- 9.Luraschi A, Borgotti P, Gioria A, Fedeli P. Determination of lymphocyte subpopulations, defined with monoclonal antibodies, in patients with iron deficiency anemia. Minerva Med. 1991;82:557–63. [PubMed] [Google Scholar]
- 10.Berger J, Schneider D, Dyck JL, Joseph A, Aplogan A, Galan P, et al. Iron deficiency, cell mediated immunity and infection among 6-36 month old children living in rural Togo. Nutr Res. 1992;12:39–49. [Google Scholar]
- 11.Thibault H, Galan P, Selz F, Preziosi P, Olivier C, Badoual J, et al. The immune response in iron-deficient young children: Effect of iron supplementation on cell-mediated immunity. Eur J Pediatr. 1993;152:120–4. doi: 10.1007/BF02072487. [DOI] [PubMed] [Google Scholar]
- 12.Feng XB, Yang XQ, Shen J. Influence of iron deficiency on serum IgG subclass and pneumococcal polysaccharides specific IgG subclass antibodies. Chin Med J (Engl) 1994;107:813–6. [PubMed] [Google Scholar]
- 13.Sadeghian MH, Keramati MR, Ayatollahi H, Manavifar L, Enaiati H, Mahmoudi M. Serum immunoglobulins in patients with iron deficiency anemia. Indian J Hematol Blood Transfus. 2010;26:45–8. doi: 10.1007/s12288-010-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993-2005. Public Health Nutr. 2009;12:444–54. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 15.Lerner NB, Stills R. Iron-Deficiency anemia. In: Kliegman RM, Stanton BF, St. Geme JW III, Schor NF, Behrman RE, editors. Nelson Textbook of Pediatrics. 19th ed. Philadelphia (PA), USA: Elsevier Saunders; 2011. pp. 1655–7. [Google Scholar]
- 16.Andrews NC. Iron deficiency and related disorders. In: Greer JP, Foerster J, Rodgers JM, Paraskevas F, Glader B, Arber DA, et al., editors. Wintrobe's Clinical Hematology. 12th ed. Philadelphia (PA), USA: Wolters Kluwer Health/Lippincott Williams and Wilkins; 2009. pp. 810–34. [Google Scholar]
- 17.Attia MA, Essa SA, Nosair NA, Amin AM, El-Agamy OA. Effect of iron deficiency anemia and its treatment on cell mediated immunity. Indian J Hematol Blood Transfus. 2009;25:70–7. doi: 10.1007/s12288-009-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullick S, Rusia U, Sikka M, Faridi MA. Impact of iron deficiency anaemia on T lymphocytes and their subsets in children. Indian J Med Res. 2006;124:647–54. [PubMed] [Google Scholar]
- 19.Sejas E, Kolsteren P, Hoeree T, Roberfroid D. Iron supplementation in previously anemic Bolivian children normalized hematologic parameters, but not immunologic parameters. J Trop Pediatr. 2008;54:164–8. doi: 10.1093/tropej/fmm106. [DOI] [PubMed] [Google Scholar]
- 20.Reza Keramati M, Sadeghian MH, Ayatollahi H, Mahmoudi M, Khajedaluea M, Tavasolian H, et al. Peripheral blood lymphocyte subset counts in pre-menopausal women with iron-deficiency anaemia. Malays J Med Sci. 2011;18:38–44. [PMC free article] [PubMed] [Google Scholar]
- 21.Bagchi K, Mohanram M, Reddy V. Humoral immune response in children with iron-deficiency anaemia. Br Med J. 1980;280:1249–51. doi: 10.1136/bmj.280.6226.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guzikowska E, Cholewa Z, Bujniewicź E, Moskała H, Guzikowski K, Madeyski J. Various parameters of humoral and cellular immunity in children with iron deficiency. Pol Tyg Lek. 1989;44:718–9. [PubMed] [Google Scholar]


