Abstract
Nocardia otitidiscaviarum is a rare clinical isolate of primary cutaneous infections. This report describes a case of lymphocutaneous nocardiosis caused by N. otitidiscaviarum. Analysis of 16S ribosomal RNA gene of the isolate and the type strain of N. otitidiscaviarum DSM 43242 showed 100% similarity. The partial gene sequence of 1439 bp was submitted to GenBank. (EU031786). The isolate was susceptible only to amikacin, minocycline, linezolid and resistant to remaining other routine anti-nocardial drugs. The patient was free of nocardiosis after 12 weeks of treatment with amikacin and linezolid. We reviewed four other cases of lymphocutaneous nocardiosis caused by N. otitidiscaviarum.
Keywords: 16S ribosomal RNA genes, linezolid, lymphocutaneous nocardiosis, nocardia otitidiscaviarum
INTRODUCTION
Nocardiosis is a rare and potentially life threatening infection caused by members of the genus nocardia (Family Actinomycetaceae). These saprophytic microorganisms are opportunistic pathogens that pose a threat to immunocompromised individuals. Nocardia species are, aerobic, Gram-positive coccobacillary, high G+C content organisms that stain partially acid-fast, and often show branching morphology upon microscopic observation. Cutaneous nocardiosis presents either as a part of disseminated infection or as a primary infection resulting from traumatic inoculation.[1]
Nocardia brasiliensis followed by N. asteroides are common nocardia species associated with primary cutaneous infection.[2] The most common causative agent of lymphocutaneous nocardiosis is nocardia brasiliensis, isolated from approximately 80% of cases. Nocardia otitidiscaviarum has been infrequently reported as a cause of either localized or disseminated infection in humans.[3] Only four other cases of lymphocutaneous nocardiosis caused by nocardia otitidiscaviarum are reported. Hence, we reviewed the literature of lymphocutaneous nocardiosis caused by nocardia otitidiscaviarum in English language.
CASE REPORT
A 60-year-old male with a history of chronic obstructive pulmonary disease (COPD) was admitted to medical ward of our hospital. He presented with a month long history of breathlessness, cough with expectoration (Grade 4), high continuous fever, and ulcers on the right thigh which had been present for 15 days. One month prior, he sustained thorn prick injury while working in the field. An ulcer on right upper third of thigh began as a papule and gradually ulcerated over a period of two weeks. Many erythematous, painful nodules developed on the whole thigh and enlarged inguinal lymph nodes with inflammatory lymphangitic streaks.
General physical examination revealed mild pallor with clubbing (Grade 3). Vital parameters like blood pressure, pulse rate were within normal limits and respiratory rate was high. Local cutaneous examination revealed two ulcers on the right thigh and inguinal lymph nodes were enlarged and tender. The largest ulcer on the upper third of the right thigh measured 3 × 1.5 cm, excavated 0.5 cm deep with undermined edges with irregular margins. Another ulcer at mid thigh level was covered by scab and an ulcer in evolution was present just above the right knee.
The patient presented microcytic hypochromic blood picture and relative neutrophilia with 10.2 gm% hemoglobin. Erythrocyte sedimentation rate was 95 mm at the end of the first hour. Biochemical tests like, blood sugar, urea, creatinine were within normal limits. ELISA for HIV1 and two antibodies were negative. Chest X-ray revealed increased bronchovascular markings in para-hilar region with emphysematous changes. Gram stain of sputum revealed plenty of gram-positive capsulated diplococci and culture on blood agar yielded growth of Streptococcus pneumoniae.
Gram-stained smear from the discharge of ulcerative lesion revealed numerous pus cells and few irregularly stained gram-positive thin branching filaments. Modified Kinyoun's staining demonstrated acid-fast branching filamentous structures. Culture on Sabouraud dextrose agar, blood agar, and Lőwenstein-Jensen media yielded growth after 72 h, showing dry chalky, white-folded colonies with irregular margin. Biochemically, the isolate showed production of urease and reduced nitrates to nitrites. The isolate was identified as nocardia otitidiscaviarum by its ability to digest xanthine, hypoxanthine, and urea but not casein, gelatin, tyrosine.
The isolate was sent to Aerobic Actinomycetes Laboratory, Centers for Disease Control and Prevention, Atlanta, Georgia, USA for confirmation and molecular analysis. 16S rRNA gene sequencing was done. Purified genomic DNA was amplified using the Expand High Fidelity PCR System (Roche Diagnostics Corporation, Indianapolis, IN, USA) with primers fL1 5’- CCGAATTCGTCG ACAACA GA GTTTGATCCTGGCTCAG - 3’ and rL1 5’- CCCGGG ATCCAAGC TTACGGCTACCTTG TTA CGACTT -3’.[4] Cycle sequencing was performed utilizing 16 universal primers[5]; excess dyes were removed with magnetic carboxylate beads (Agencourt Bioscience, Beverly MA) and reactions were sequenced on an ABI 3100 (Applied Biosystems).
The 16S rRNA gene sequences were assembled in Accelrys SeqMerge and trimmed to a minimum of two confirming reads. The sequence (>1400 bp) generated was compared to the GenBank database gene sequences derived from related nocardia and submitted by other investigators.
Analysis of 16S rRNA gene of the isolate and type strain of N. otitidiscaviarum DSM 43242 showed 100% similarity. The partial gene sequence (1439 bp) was submitted to GenBank, accession number EU031786.
The patient was relieved of breathlessness and cough with expectoration, following treatment with amoxicillin and clavulanic acid 625 mg twice daily for eight days. For nocardiosis the patient was initially treated with oral trimethoprim-sulfamethoxazole (TMP-SMX, 80-400 mg), two tablets twice daily for one week with no clinical improvement. Antibiotic susceptibility testing of the isolate by MIC method carried out at CDC Atlanta revealed that it was susceptible only to amikacin (MIC = 4), minocycline (MIC = 1), and linezolid (MIC = 2) and resistant to remaining other routine anti-nocardial drugs. The patient was treated with amikacin and linezolid for four weeks; once a clinical cure was evident the patient was asked to continue linezolid orally for another eight weeks. Four months later, there was no evidence of nocardiosis in the patient.
DISCUSSION
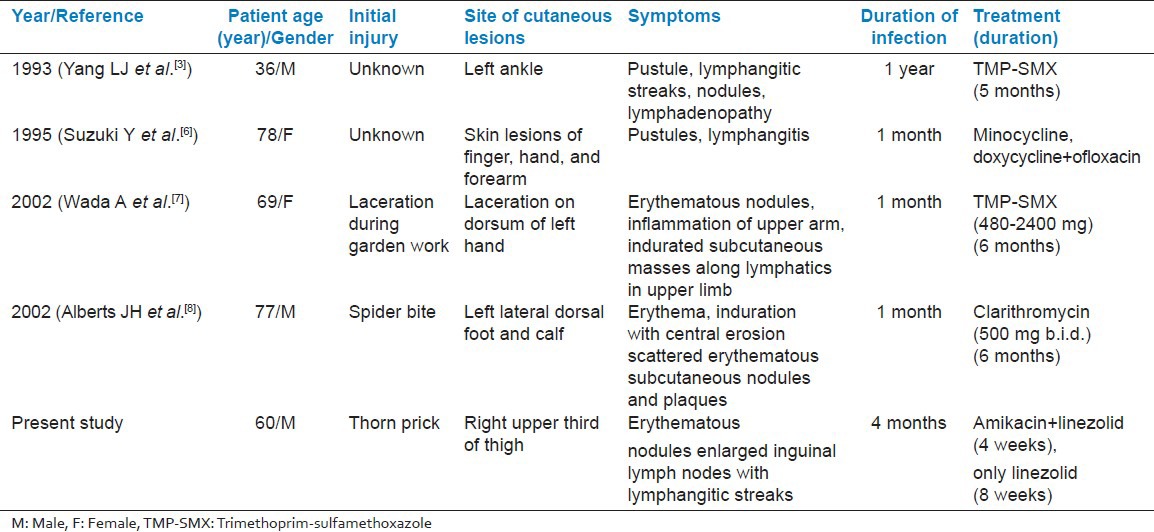
We found four additional cases in English-language literature. Yang L J et al.[3] in 1993, Suzuki Y et al.[6] in 1995, Wada A et al.[7] in 2002 and Alberts JH et al.[8] again in 2002 reported cases of lymphocutaneous nocardiosis caused by nocardia otitidiscaviarum. Out of which first case was from Taiwan and two cases were reported from Japan and one case from USA. Here we are presenting the first case report of lymphocutaneous nocardiosis, caused by nocardia otitidiscaviarum from India. The age range of patients in all reported cases was 60-78 years, including our case, three were male and two female. All the cases presented with lymphocutaneous nocardiosis and predominant risk factor was trauma in two cases, in another two cases cause was not known and remaining one case with spider bite. Three cases were mildly immunocompromised, as a result of corticosteroid use and other two were healthy individuals. The disease duration varied from few days to few months. The condition of two patients improved with TMP-SMX but another two cases did not respond with TMP-SMX and were then switched over to minocycline, doxycycline, clarithromycin and ofloxacin but our case was treated with amikacin and linezolid for one month and continued for eight weeks with only linezolid as the isolate was susceptible only to these drugs. Our patient was a known case of COPD, admitted with chief complaint of breathlessness and prolonged fever. We isolated S. pneumoniae from sputum and N. otitidiscaviarum from thigh lesions. This ruled out the possibility of pulmonary nocardiosis, though COPD is major associated disease with pulmonary nocardiosis.[9] This proved that the N. otitidiscaviarum infection is restricted locally as lymphocutaneous infection. Meanwhile the patient was relieved of respiratory symptoms with Amoxiclav 625 mg for eight days but thigh lesions did not respond. The other four reviewed cases presented only with lymphocutaneous infection. Details of the reviewed cases are given in Table 1.
Table 1.
Characteristics of reported cases of lymphocutaneous infections
N. otitidiscaviarum was first isolated by Snijders[10] in 1924. Gordon and Mihm were first to define the biochemical criterion for its identification thus distinguishing it from other species of nocardia. They proposed the name nocardia caviae. Currently this organism is identified as N. otitidiscaviarum as per Bergey's manual of systematic bacteriology.[11]
N. otitidiscaviarum is less pathogenic than other species of nocardia. The pathogenicity of N. otitidiscaviarum is influenced by number of factors such as route of infection, inoculum size, strain variability and other contributory factors.[12] Following cutaneous inoculation, nocardia may spread through the lymphatics to lymph nodes resulting in lymphocutaneous nocardiosis, which usually begins as an ulcerated plaque at the site of injury, followed by lymphangitis and subcutaneous erythematous nodules along the lymphatic drainage often with deep regional lymphadenopathy. This is also known as sporotrichoid nocardiosis.[1] Nocardia otitidiscaviarum has been infrequently reported as a cause of either localized or disseminated infection in humans. In one large series of nocardial infections, only 10 (2.9%) of 347 cases were due to N. otitidiscaviarum.[10]
Sulphonamides have comprised the cornerstone for treatment of nocardiosis since 1940s. The drug of choice most typically is TMP-SMX.[13] Newer antimicrobials are desirable because of increasing number of cases of resistance to sulphonamides, especially N. farcinica and N. otitidiscaviarum have demonstrated inconsistent susceptibility to sulphonamides.[14] Because of development of intolerance to TMP-SMX and adverse effect of long term use of TMP-SMX leads one to think of other alternative highly active oral agents. Reports suggest that linezolid is the first antimicrobial to be active against all clinically significant species of nocardia. Because of its activity and availability as an oral agent, linezolid has the potential to be the primary drug for treatment of nocardiosis. The clinical experience with successful use of linezolid for treatment of nocardiosis has been reported.[15]
The optimal duration of therapy for N. otitidiscaviarum infection is unknown but most reports mention treatment for several months and in general duration of treatment for nocardiosis ranges six weeks for localized infection to >one year for disseminated infections.[16]
ACKNOWLEDGMENTS
We thank June Brown, Aerobic Actinomycetes Laboratory, Centers for Disease Control and Prevention, Atlanta, Georgia, USA for confirmation and molecular analysis of our isolate nocardia otitidiscaviarum.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Moeller CA, Burton CS. Primary lymphocutaneous Nocardia brasiliensis infection. Arch Dermatol. 1986;122:1180–2. [PubMed] [Google Scholar]
- 2.Tsuboi R, Takamori K, Ogawa H, Mikami Y, Arai T. Lymphocutaneous Nocardiosis Caused by Nocardia asteroides-Case report and literature review. Arch Dermatol. 1986;122:1183–5. [PubMed] [Google Scholar]
- 3.Yang LJ, Chan HL, Chen WJ, Kuo TT. Lymphocutaneous nocardiosis caused by Nocardia caviae: The first case report from Asia. J Am Acad Dermatol. 1993;29:639–41. doi: 10.1016/s0190-9622(08)81870-1. [DOI] [PubMed] [Google Scholar]
- 4.Segonds C, Heulin T, Marty N, Chabanon G. Differentiation of Burkholderia species by PCR-restriction fragment length polymorphism analysis of the 16S rRNA gene and application to cystic fibrosis isolates. J Clin Microbiol. 1999;37:2201–8. doi: 10.1128/jcm.37.7.2201-2208.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morey RE, Galloway RL, Bragg SL, Steigerwalt AG, Mayer LW, Levett PN. Species-specific identification of Leptospiraceae by 16S rRNA gene sequencing. J Clin Microbiol. 2006;44:3510–6. doi: 10.1128/JCM.00670-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki Y, Toyama K, Utsugi K, Yazawa K, Mikami Y, Fujita M, et al. Primary lymphocutaneous nocardiosis due to Nocardia otitidiscaviarum: The first case report from Japan. J Dermatol. 1995;22:344–7. doi: 10.1111/j.1346-8138.1995.tb03400.x. [DOI] [PubMed] [Google Scholar]
- 7.Wada A, Matsuda S, Kubota H, Miura H, Iwamoto Y. Primary lymphocutaneous nocardiosis caused by Nocardia otitidiscaviarum. Hand Surg. 2002;7:285–7. doi: 10.1142/s021881040200114x. [DOI] [PubMed] [Google Scholar]
- 8.Alberts JH, Boyd AS. Nocardia otitidiscaviarum: An unusual nocardia species causing a primary lymphocutaneous infectious process in a mildly immunosuppressed patient. Skinmed. 2002;1:62–4. doi: 10.1111/j.1540-9740.2002.01740.x. [DOI] [PubMed] [Google Scholar]
- 9.Mari B, Montón C, Mariscal D, Luján M, Sala M, Domingo C. Pulmonary nocardiosis: Clinical experience in ten cases. Respiration. 2001;68:382–8. doi: 10.1159/000050531. [DOI] [PubMed] [Google Scholar]
- 10.Clark NM, Braun DK, Pasternak A, Chenoweth CE. Primary cutaneous Nocardia otitidiscaviarum infection: Case report and review. Clin Infect Dis. 1995;20:1266–70. doi: 10.1093/clinids/20.5.1266. [DOI] [PubMed] [Google Scholar]
- 11.Goodfellow M, Lechvalier MP. Genus Nocardia Trevisan 1889. In: Holt JG, Williams ST, Sharpe ME, editors. Bergey's Manual of Systematic bacteriology. Vol. 4. Baltimore: Williams and Wilkins; 1989. pp. 2350–61. [Google Scholar]
- 12.Beaman BL, Beaman L. Nocardia species: Host-parasite relationships. Clin Microbiol Rev. 1994;7:213–64. doi: 10.1128/cmr.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace RJ, Jr, Septimus EJ, Williams TW, Jr, Conklin RH, Satterwhite TK, Bushby MB, et al. Use of trimethoprim-sulfamethoxazole for treatment of infections due to Nocardia. Rev Infect Dis. 1982;4:315–25. doi: 10.1093/clinids/4.2.315. [DOI] [PubMed] [Google Scholar]
- 14.Stamm AM, McFall DW, Dismukes WE. Failure of sulfonamides and trimethoprim in the treatment of nocardiosis. Arch Intern Med. 1983;143:383–5. [PubMed] [Google Scholar]
- 15.Moylett EH, Pacheco SE, Brown-Elliott BA, Perry TR, Buescher ES, Birmingham MC, et al. Clinical experience with linezolid for the treatment of Nocardia infection. Clin Infect Dis. 2003;6:313–8. doi: 10.1086/345907. [DOI] [PubMed] [Google Scholar]
- 16.Lerner PI. Nocardia species. In: Mandell GL, Douglas RG Jr, Bennett JE, editors. Principles and practice of Infectious diseases. 3rd ed. New-York: Churchill Livingstone; 1990. pp. 1926–32. [Google Scholar]


