Abstract
Introduction:
Hypothyroidism is associated with significant neurocognitive deficits because hypothyroidism prevents the brain from adequately sustaining the energy consuming processes needed for neurotransmission, memory, and other higher brain functions. Hence, the study was done to assess the cognitive functions of newly diagnosed subclinical and clinical hypothyroid patients by evoked response potential P300.
Materials and Methods:
75 patients each of newly diagnosed subclinical and clinical hypothyroid patients attending endocrinology clinic and 75 healthy age and sex matched euthyroid controls were considered for the study. P300 was recorded with Record Medicare System Polyrite, Chandigarh using auditory “oddball paradigm”. The data was analyzed using ANOVA followed by post Tukey's test.
Results:
Newly diagnosed clinical hypothyroid patients showed a significant increase in P300 latency compared to control (P < 0.05) and subclinical cases (P < 0.01) while there was no significant difference between the P300 latency of subclinical cases and control group. Also, there was no significant difference in P300 amplitude among the three groups.
Conclusion:
P300 latency in case of newly diagnosed hypothyroid clinical cases is significantly increased compared to newly diagnosed subclinical cases and control.
Keywords: Cognition, event related potentials, hypothyroidism, P300
INTRODUCTION
Low thyroid function irrespective of age has been found to have detrimental effect on cognitive functions because hypothyroidism prevents the brain from adequately sustaining the energy (glucose)-consuming processes needed for neurotransmission, memory and other higher brain functions. Low brain uptake of glucose is commonly associated with deterioration of cognition.[1] Hypothyroidism is associated with significant neurocognitive deficits involving attention and concentration, memory, perceptual function, defective language, executive functions, development of a range of intellectual, disorientation in space and time, loss of autonomy, emotional depersonalization and other mental defects.[2]
Hypothyroidism in non-demented older adults is associated with impairments in learning, word fluency, visual-spatial abilities, some aspect of attention, visual scanning and motor speed.[3] Cognitive functions can be assessed by evoked potential e.g., P300 wave of event related potential (ERP).[4] Some researchers have found that subclinical hypothyroidism is associated with changes in mood and cognitive functioning while other were of the opinion that no correlation exists between these two.[5,6]
MATERIALS AND METHODS
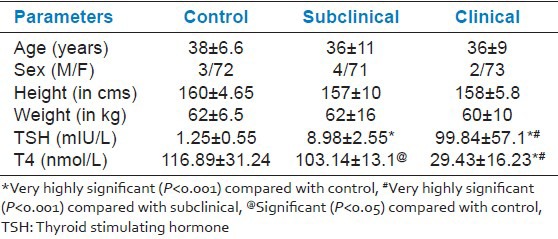
The present study was conducted in the department of Physiology in collaboration with the department of Medicine, PGIMS, Rohtak, India. It included 75 patients of newly diagnosed clinical and subclinical hypothyroidism patients attending endocrinology clinic and 75 healthy age and sex matched euthyroid controls [Table 1]. The subjects were divided into three groups:
Table 1.
Height, weight matching along with hormone level
Group I: Comprised of age and sex matched 75 healthy euthyroid subjects
Group II: Comprised of 75 newly diagnosed patients of subclinical hypothyroidism of either sex below the age of 50 years
Group III: Comprised of 75 newly diagnosed patients of clinical hypothyroidism of either sex below the age of 50 years.
Inclusion criteria
Hypothyroidism was diagnosed by increased thyroid stimulating hormone (TSH) and decreased thyroid hormone level. To avoid the effect of aging over cognition, we have included subjects below 50 years of age.
For subclinical hypothyroidism TSH level > 5.1mIU/L and T4 ≥57.9 nmol/L.
For clinical hypothyroidism TSH level > 5.1mIU/L and T4 <57.9 nmol/L.[7]
Exclusion criteria
Patients with any other major medical disorder which can affect cognitive function, i.e. diabetes mellitus, anaemia, hypertension, chronic obstructive pulmonary disease, acute or chronic liver and kidney disease
History of drug abuse including alcoholism
Neurological or psychiatric illness
Altered sensorium
Patients who don’t co-operate during the study period.
Recording electrodes for ERP P300
The volume conducted evoked responses were picked up from scalp by using disc type of Ag/AgCl2 electrodes. Two reference electrodes was attached to left and right mastoid, designated as A1 and A2 respectively, one active electrode on vertex labeled as Cz and one as ground electrode to forehead termed as Fpz. All the electrodes were plugged to a junction box. Impedance between skin and electrode was monitored and kept below 5 K ohms. Recommended montages for ERP p300 were: Channel 1: Cz -A1, Channel 2: Cz -A2 and Ground: Fpz.
Procedure for P300
P300 was recorded in context of a standard auditory oddball paradigm. Target or rare tone and non target or frequent tone of 70 dB was applied on both ears simultaneously through headphones applied to ears of subject. Rare tone and frequent tone was of 2 KHz and 1 KHz respectively. Rare tone stimuli were given in 20% and frequent tone stimuli in 80% frequency in random. Stimulus frequency was 1 stimulus per two sec. Total numbers of stimuli given were 160. The signals were picked by electrodes and were filtered, amplified, averaged, displayed on the screen of RMS EMG EP MK2 and recorded. Latency and amplitude of P300 wave were measured.
Statistical analysis
All the data gathered were tabulated and were analyzed using SPSS 14th version. For analysis ANOVA was used followed by Tukey's test.
RESULTS
The TSH was significantly higher (P < 0.001) in clinical hypothyroid patients compared with subclinical and controls. While T4 was significantly lower (P < 0.001) in clinical hypothyroid patients when compared with subclinical and controls as shown in Table 1. On comparison between subclinical and control, TSH was significantly higher (P < 0.001) and T4 was significantly lower (P < 0.05) in subclinical group. Height and weight were comparable amongst the three groups.
Table 2 shows that newly diagnosed clinical hypothyroid patients showed a significant increase in P300 latency compared to control (P < 0.01) and subclinical cases (P < 0.05) while there was no significant difference between the P300 latency of subclinical cases and control group. Also, there was no significant difference in P300 amplitude among the three groups [Figures 1–3].
Table 2.
Comparison of ERP P300 values of subclinical clinical and control cases
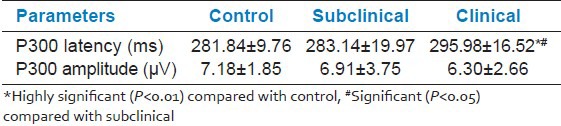
Figure 1.
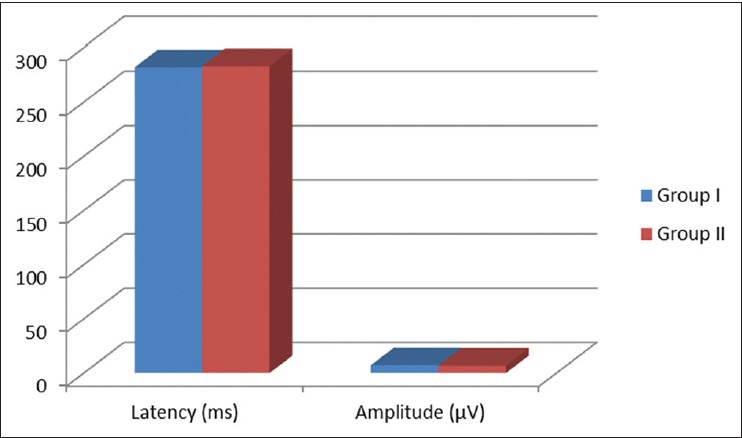
Comparison of P300 latency and amplitude between control (Group I) and newly diagnosed subclinical hypothyroid patients (Group II)
Figure 3.
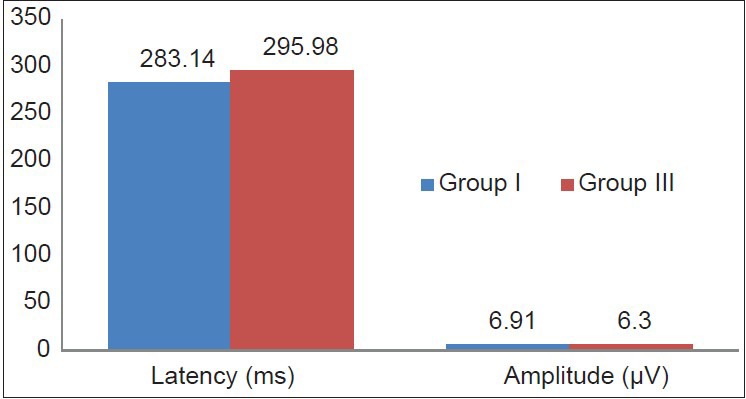
Comparison of P300 latency and amplitude between newly diagnosed subclinical (Group II) and clinical (Group III) hypothyroid patients
Figure 2.
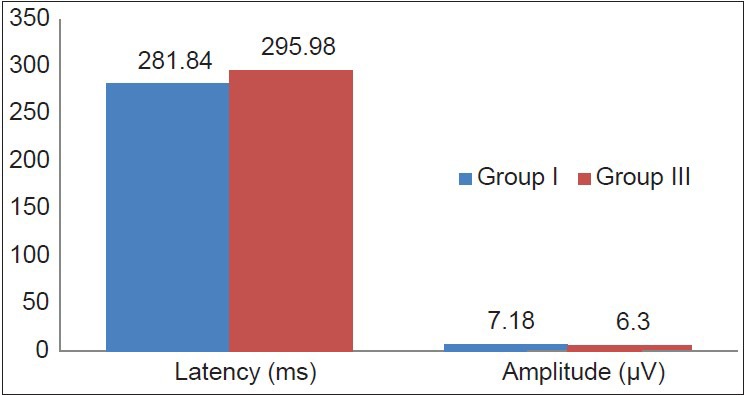
Comparison of P300 latency and amplitude between control (Group I) and newly diagnosed clinical hypothyroid patients (Group III)
Table 3 shows that the correlation coefficient between P300 latency and T4 was negative (−0.546) and statistically significant, while with TSH, it was positive (0.267), though statistically non-significant. The correlation coefficient between P300 amplitude and T4 was positive (0.042) and with TSH, it was also positive (0.159), but none was statistically significant.
Table 3.
Correlation of hormone level with P300 latency and amplitude in group II

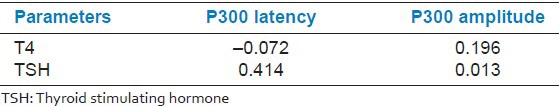
Table 4 shows that the correlation coefficient between P300 latency and TSH was positively correlated (0.414) and statistically significant, while with T4, it was negatively correlated (−0.072) and non-significant. The correlation coefficient between P300 amplitude and T4 was positive (0.196), while with TSH, it was negative (−0.013), though both were statistically non-significant.
Table 4.
Correlation of hormone level with P300 latency and amplitude in group III
DISCUSSION
Normal brain function requires thyroid hormones. Thyroid dysfunction is related to nervous system disorder involving GABA as reported by clinical studies.[8] The role of thyroid with regard to cognition is not yet clear.[9] Studies done recently shows that the cases previously considered asymptomatic may be having many non specific subjective features. In these patients there may be fine but subtle difference from euthyroid. The transition from normal euthyroid to hypothyroid is continuous and hence while assessing a case both the degree and duration of the case has to be considered. Subclinical hypothyroidism is marked by increase in serum TSH level while thyroid hormone level in serum is normal. One result of this which is postulated is there is an alteration on cognitive function and mood. Our result in Table 2 shows that there is significant difference in P300 latency between clinical and subclinical cases while there is highly significant difference between clinical and controls.
Our finding is supported by studies made by Tutuncu et al. who reported that there is delay of P300 latency in both mild and severe case of hypothyroidism.[10] Hala et al. and Jensovsky et al. also derived the same finding when studying the effect of hyper and hypo thyroidism on P300 latency.[11] On the contrary studies by Osterweil et al. showed no difference in P300 latency.[12] Studies by Mahmoud et al. also showed no difference.[13]
We further went ahead to find out a correlation between T4, TSH and P300 latency and amplitude which showed significant negative correlation in clinical cases when T4 was compared with P 300 latency while the rest have been non significant as shown in Tables 3 and 4.
CONCLUSION
P300 latency in case of newly diagnosed clinical hypothyroid patients was significantly increased in comparison to newly diagnosed subclinical cases and control groups.
ACKNOWLEDGMENT
Mr. Randhir (lab technician) for his cooperation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Text Begin ME, Langlois MF, Lorrain D, Cunnane SC. Thyroid function and cognition during aging. Curr Gerontol Geriatr Res. 2008 474868. [Google Scholar]
- 2.Samuels MH, Schuff KG, Carlson NE, Carello P, Janowsky JS. Health status, mood and cognition in experimentally induced subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:2545–51. doi: 10.1210/jc.2007-0011. [DOI] [PubMed] [Google Scholar]
- 3.Osterweil D, Syndulko K, Cohen SN, Pettler PD, Hershman JM, Cummings JL, et al. Cognitive function in non demented older adults with hypothyroidism. J Am Geriatr Soc. 1992;40:325–35. doi: 10.1111/j.1532-5415.1992.tb02130.x. [DOI] [PubMed] [Google Scholar]
- 4.Razvi S, Ingoe LE, McMillan CV, Weaver JU. Health status in patients with subclinical hypothyroidism. European J Endocrinol. 2005;152:713–7. doi: 10.1530/eje.1.01907. [DOI] [PubMed] [Google Scholar]
- 5.Roberts LM, Pattison H, Roalfe A, Franklyn J, Wilson S, Hobbs FD, et al. Is subclinical thyroid dysfunction in the elderly associated with depression or cognitive dysfunction? Ann Intern Med. 2006;145:573–81. doi: 10.7326/0003-4819-145-8-200610170-00006. [DOI] [PubMed] [Google Scholar]
- 6.Tandon OP, Verma A, Ram BK. Cognitive dysfunction in NIDDM: P 3 event related evoked potential study. Indian J Physiol Pharmacol. 1999;43:383–8. [PubMed] [Google Scholar]
- 7.Canaries GJ, Manowitz NR, Mayor G, Ridgway C. The colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–34. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 8.Weins SC, Trudeau VL. Thyroid hormone and gamma-aminobutyric acid (GABA) interactions in neuroendocrine systems. Comp Biochem Physiol. 2006;144:332–44. doi: 10.1016/j.cbpa.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 9.Constant El, Devolder AG, Ivanoiu A, Bol A, Labar D, Seghers A, et al. Cerebral blood flow and glucose metabolism in hypothyroidism: A positron emission tomography study. J Clin Endocirnol Metab. 2001;86:3864–70. doi: 10.1210/jcem.86.8.7749. [DOI] [PubMed] [Google Scholar]
- 10.Tutuncu NB, Karatas M, Sozay S. Prolonged P300 Latency in Thyroid Failure: A Paradox -P300 latency recovers later in mild hypothyroidism than in severe hypothyroidism. Thyroid. 2004;14:622–7. doi: 10.1089/1050725041692837. [DOI] [PubMed] [Google Scholar]
- 11.Jensovsky J, Ruzicka E, Spackova N, Hejdukova B. Changes in event related potential and cognitive processes in patients with subclinical hypothyroidism after thyroxine treatment. Endocr Regul. 2002;36:155–22. [PubMed] [Google Scholar]
- 12.Osterweil D, Syndulko K, Cohen SN, Pettler JP, Hershman JM, Cummings JL. Cognitive function in non-demented older adults with hypothyroidism. J Am Ger Soc. 1992;40:325–35. doi: 10.1111/j.1532-5415.1992.tb02130.x. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoud A, Sadek H, Shereen F, Akmal M, Ashraf MG, Nermeen AK, et al. Screening for Cognitive Dysfunction in Patients with Hypothyroidism. Egypt J Neurol Psychiat Neurosurg. 2008;45:175–84. [Google Scholar]


