Abstract
Cancer patients have unique problems associated with hepatitis C virus (HCV) infection and treatment not seen in the general population. HCV infection poses additional challenges and considerations for the management of cancer, and vice versa. HCV infection also can lead to the development of cancer, particularly hepatocellular carcinoma and non-Hodgkin lymphoma. In severely immunocompromised cancer patients, diagnosis of HCV infection requires increased reliance on RNA detection techniques. HCV infection can affect chemotherapy, and delay of HCV infection treatment until completion of chemotherapy and achievement of cancer remission may be required to decrease the potential for drug-drug interactions between antineoplastic agents and HCV therapeutics and potentiation of side effects of these agents. In addition, hematopoietic stem cell transplant (HSCT) recipients have an increased risk of early development of cirrhosis and fibrosis. Whether this increased risk applies to all patients regardless of cancer treatment is unknown. Furthermore, patients with cancer may have poorer sustained virological responses to HCV infection treatment than do those without cancer. Unfortunately, not all cancer patients are candidates for HCV infection therapy. In this article, we review the challenges in managing HCV infection in cancer patients and HSCT recipients.
Keywords: Hepatitis C virus, Cancer, Hematopoietic stem cell transplant, Chemotherapy, Treatment, Antiviral, Pegylated interferon, Ribavirin
Core tip: Hepatitis C virus (HCV) infection adds complexity to treatment considerations of cancer patients. This is amplified by the absence of standard of care guidelines for the management of cancer patients with HCV infection. Not only can HCV infection result in the development hepatocellular cancer and non-Hodgkin lymphoma, but the presence of HCV infection in cancer patients can affect the treatment of malignancies with antineoplastic chemotherapies. Side effects of HCV therapy can be exacerbated in cancer patients due to underlying cytopenias and comorbidities. In those patients treated for HCV infection, cancer patients have poorer response to treatment than the general non-cancer population.
INTRODUCTION
Hepatitis C virus (HCV) is the most common blood-borne pathogen in the United States, infecting an estimated 2.7-3.9 million people or roughly 1.0%-1.5% of the population[1]. Chronic HCV infection is the principal cause of liver disease-related deaths and the leading indication for liver transplantation in the United States[2]. HCV infection can also result in the development of hepatocellular carcinoma (HCC) and lymphoma, especially non-Hodgkin lymphoma[3,4]. In 2010, the incidence of new HCV infections in the United States was estimated to be about 17000 per year[1]. Although effective treatments that clear HCV infection are available, most affected individuals do not know they are infected[1]. Cancer patients pose unique challenges to the management and treatment of chronic HCV. In this patient population, there is an increased risk of liver disease progression, occurrence of occult HCV infection, development of viral reactivation, and no standard of care to guide how and when to treat such patients. In this article, we review the challenges in managing HCV infection in cancer patients and hematopoietic stem cell transplant (HSCT) recipients.
HCV INFECTION PREVALENCE IN CANCER PATIENTS
Although chronic HCV infection is common in cancer patients, studies of HCV infection in this population are lacking. Hence, the natural history of HCV infection and infection treatment outcomes in cancer patients are poorly understood. The known facts about HCV infection in cancer patients are limited. Estimates of the prevalence of HCV infection in cancer patients range from 1.5% to 32%[5-8].
DOES HCV INFECTION MANAGEMENT DIFFER IN PATIENTS WITH AND WITHOUT CANCER?
Management of HCV infection is more challenging in patients with cancer than in those without it given several diagnostic and therapeutic differences in these two patient groups (Table 1).
Table 1.
Differences on hepatitis C virus infection in cancer patients compared to those without cancer
| Occurrence of occult infection |
| Higher risk of developing early cirrhosis |
| Higher rate of fibrosis progression |
| Development of viral reactivation |
| No standard of care treatment |
| Worse virological outcome |
Diagnostic challenges
Cancer patients, especially those with hematologic malignancies, can have false-negative results of serological tests using antibodies against HCV. In some of these severely immunocompromised patients, conventional detection of anti-HCV antibodies is not sufficient for diagnosis, necessitating polymerase chain reaction analysis for HCV RNA quantitation in blood to identify patients with occult infections[9-12]. Additionally, following chemotherapy, HCV reactivation can occur[13] as described below.
HSCT recipients with HCV infection have a greater risk of early cirrhosis and higher rate of fibrosis progression than do HCV-infected patients who do not undergo this transplantation. For example, researchers observed an expected median time to the development of cirrhosis of 18 years in HSCT recipients with chronic HCV infection, but 40 years in control patients with chronic HCV infection who did not receive HSCTs[14]. Cirrhosis may be a contraindication to high-dose conditioning regimens for HSCTs. Therefore, physicians should consider performing a liver biopsy before the start of conditioning therapy if they have a clinical suspicion of cirrhosis or extensive fibrosis resulting from chronic viral infection, including HCV infection, irrespective of the virus’s genotype[10,14].
Therapeutic challenges
No standard of treatment exists for patients with cancer and HCV infection. Treatment of this infection can mitigate specific risks in certain cancer patients. Unfortunately, evidence-based knowledge regarding the effects of treatment of HCV infection in cancer patients is lacking. This is likely reflective of exclusion of cancer patients from trials of HCV therapeutics owing in part to baseline hematological abnormalities frequently observed in cancer patients.
In a retrospective study, we have observed poor efficacy of antiviral treatment in HCV-infected cancer patients as a concern, as well. A sustained virological responses (SVRs) was observed in 11 (41%) of 27 cancer patients with chronic HCV infection[15]. Of the 11 patients, 3 had genotype 1 infection, and none of these patients achieved SVR.
HCV GENOTYPE DISTRIBUTION IN CANCER PATIENTS
HCV is genetically heterogeneous, with seven unique identifiable genotypes[16,17]. These genotypes exhibit variable geographical distribution worldwide, with genotype 1 occurring most frequently in the United States, followed by genotypes 2 and 3[18]. In a retrospective multicenter study, we examined the distribution of HCV genotypes in 636 patients with proven HCV infection (detectable HCV RNA). We found that genotype 1 was the predominant genotype in cancer patients, with a prevalence of about 66%; in comparison, the prevalence of this genotype was 84% in immunocompetent patients and 99% in patients co-infected with HCV and human immunodeficiency virus[19]. Also, genotypes 2 and 3 were more common in cancer patients than in the general population[19]. HCV genotype 2 was most prevalent in lymphoma patients and resulted in a threefold greater risk of lymphoma than did the other genotypes[19]. These findings provide evidence that the distribution of HCV genotypes 1, 2, and 3 differs by underlying disease status among patients in the same geographic region and suggest a carcinogenic association for some genotypes.
TREATMENT OF HCV INFECTION IN CANCER PATIENTS
Cancer patients benefit from treatment of their HCV infections in several ways[20]. As in the general population[21], the potential benefits of SVRs to this treatment include longevity of response and impact on clinical events such as prevention of liver disease progression, and improved survival.
The durability of SVR seems to be unaffected by chemotherapy in cancer patients. In one study at our center of 30 HCV-infected cancer patients who had SVRs before cancer diagnosis and then received chemotherapy or immunosuppressive therapy, none of them had post-SVR relapse of their infections following cancer treatment[22].
Compared with those who do not have responses to pegylated interferon (pegIFN) alpha-2 and ribavirin therapy, non cancer patients who undergo successful treatment of HCV infections have fewer hepatic complications, lower liver disease-related mortality rates, and lower HCC incidence rates[21]. Similarly, among 140 cancer patients with chronic HCV infection seen in our institution, we observed progression of liver disease to cirrhosis and portal hypertension in 7% and 9% of the untreated patients, respectively[15]. None of the patients who received treatment of HCV infection had progression of liver disease. Furthermore, HCV infection treatment is associated with reduced risk of cancer recurrence and improved survival rates in patients with HCV-related HCC[23,24]. HCV infection increases mortality risk in the posttransplantation period in HSCT recipients[25]. Therefore, HCV infection treatment should be considered for all eligible HSCT recipients[10], including cancer patients.
In some instances, HCV infection can be eradicated prior to the start of immunosuppressive therapy for cancer. This is of particular interest to oncologists, because HCV reactivation can occur following chemotherapy[13] and lead to discontinuation of or dose reduction for potentially life-saving chemotherapy[26].
CHALLENGES IN MANAGING HCV INFECTION THERAPY IN CANCER PATIENTS
Side effects of treatment of HCV infections are common in the general population[27-29]. Adverse events of HCV therapy that, in our experience, can be worse in patients than in those without it are listed in Table 2. For example, HCV infection treatment-related anemia and other blood abnormalities can be more profound in cancer patients. Not uncommonly, pretreatment baseline hemoglobin levels are decreased, especially in patients with hematological malignancies. As reported in patients without cancer, initiation of HCV infection treatment can be associated with a rapid drop in hemoglobin level in recipients of first-generation protease inhibitors (e.g., telaprevir, boceprevir) along with pegIFN alpha-2 plus ribavirin. This adverse effect seems to be more common in these patients than in recipients of dual therapy with pegIFN and ribavirin alone[30]. Similarly, other types of cytopenia can be encountered in cancer patients at baseline before treatment of HCV infection, such as leukopenia, thrombocytopenia, and neutropenia. These effects also can be profound in these patients upon initiation of HCV infection therapy, necessitating dose reduction for anti-HCV medications.
Table 2.
Side effects of drugs used to treat hepatitis C virusinfection that can be exacerbated in hepatitis C virus-infected cancer patients
| Drugs | Side effects |
| Interferon and ribavirin | Fatigue |
| Flu-like syndrome | |
| Nausea and vomiting | |
| Low-grade fever | |
| Weight loss | |
| Irritability | |
| Insomnia | |
| Depression | |
| Anemia | |
| Thrombocytopenia | |
| Neutropenia | |
| Pruritus | |
| Rashes | |
| Dyspnea | |
| Poor appetite | |
| Difficulty concentrating | |
| Severe infections (bacterial, fungal, or viral) | |
| First generation protease inhibitors | |
| Boceprevir | Anemia |
| Renal impairment | |
| Telaprevir | Anemia |
| Rash | |
| Gastrointestinal side effects (diarrhea and rectal burning) | |
| Renal impairment | |
Use of hematopoietic growth factors may be required to restore baseline hemoglobin levels, white blood cell counts, and platelet counts and help maintain and complete full-dose antiviral therapy for chronic HCV infections in cancer patients. In a series of cancer patients receiving treatment of HCV infection at our institution from 2009 to 2012, 24 (86%) of 28 patients experienced antiviral-induced hepatotoxic effects[31]. We administered growth factors (e.g., erythropoietin, granulocyte-macrophage colony-stimulating factor, thrombopoietin) to 15 patients (54%); with 9 (60%) of these patients received more than one growth factor. Patients who completed antiviral therapy were numerically more likely to have received growth factors than were those who did not (83% vs 63%, P = 0.6). More patients undergoing dual therapy than those undergoing triple therapy with first-generation protease inhibitors were able to complete their antiviral regimens when growth factors were included (0% vs 50%, P = 0.1). Overall, maintenance of treatment with full-dose antivirals was more common in those who received growth factors than in those who did not (100% vs 33%, P = 0.01); this was especially true for pegIFN alpha-2 (100% vs 17%, P = 0.04) and ribavirin (75% vs 36%, P = 0.3). We observed no major side effects with the use of other growth factors. The effect of dose reduction for anti-HCV medications and/or growth factors on the efficacy of treatment of this infection in cancer patients remains to be determined.
Psychiatric disorders, such as severe depression, adjustment disorder, anxiety, and bipolar disorder, are not uncommon in cancer patients[32]. Furthermore, depression is a major comorbidity in HCV-infected cancer patients, even in untreated individuals[33]. Uncontrolled major depression is an exclusionary criterion for initiation of HCV infection treatment with interferon[2]. Cancer patients may experience depression at any time throughout the course of their disease, ranging from prior to and during cancer treatment to even the post treatment survivorship period[34,35].
HCV reactivation is a rare yet important problem in cancer patients, particularly those with hematological malignancies[13]. If a patient with chronic HCV infection and cancer experiences viral reactivation while receiving chemotherapy, the management options are to reduce doses in the current chemotherapy regimen, select an alternative chemotherapy regimen with no overlapping hepatotoxic effects, and discontinue chemotherapy.
HOW AND WHEN CAN CANCER PATIENTS RECEIVE TREATMENT OF HCV INFECTION?
In general, we recommend treatment of HCV infection in cancer survivors without evidence of metastatic disease, which compromises life expectancy. In our practice, we wait at least 6 mo after cancer remission before initiating myelosuppressive HCV infection treatment with pegIFN and ribavirin to allow patients’ bone marrow stem cell counts to recover. However, the patient’s cancer can relapse during this period. A discussion with the treating oncologist is recommended to determine the time after which the patient’s cancer is unlikely to relapse. Given our limited clinical understanding of potential drug-drug interactions between HCV therapeutics and chemotherapeutics and chemotherapy tolerability in HCV-infected cancer patients, simultaneous treatment of cancer (e.g., chemotherapy, radiation therapy) and HCV infection should be avoided. Also, HCV infection treatment can be administered to HSCT recipients who meet the following criteria: complete remission of the original disease, a period of at least 2 years since the HSCT, no evidence of protracted acute or chronic graft-vs-host disease, no immunosuppressive therapy for at least 6 mo, and normal blood counts and serum creatinine levels[10]. An overview for managing cancer patients with chronic HCV infection is outlined in Figure 1. Clinical experience with use of direct-acting antivirals in cancer survivors is very limited and and thus requires further research.
Figure 1.
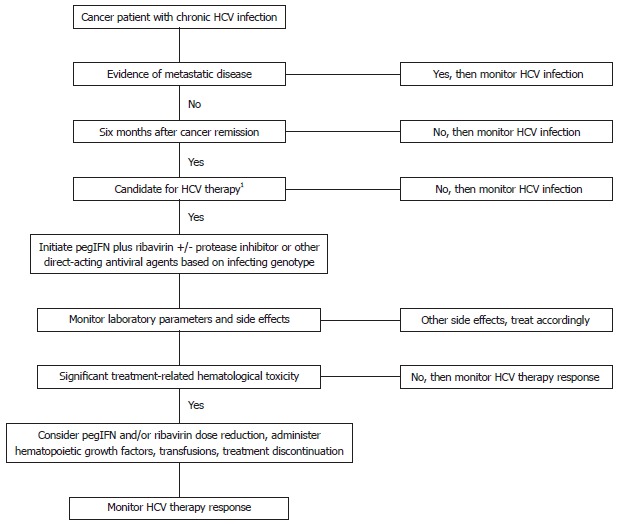
Algorithm for managing chronic hepatitis C virus infection in cancer patients. 1At least 6 mo after cancer remission before initiating myelosuppressive hepatitis C virus (HCV) treatment with interferon-based therapy. Wait 2 years after hematopoietic stem cell transplant before initiation of interferon-based HCV therapy for patients with normal serum creatinine, normal blood counts, off immunosuppressive therapy > 6 mo, and without evidence of original disease recurrence or graft versus host disease[10]. PegIFN: Pegylated interferon.
CONCLUSION
HCV infection is a unique challenge in the management of cancer patients. These challenges include an increased risk for liver disease progression, occurrence of occult HCV infection and development of viral reactivation. The potential manifestation of underlying psychiatric disorders before or during HCV therapy requires close monitoring and intervention as warranted. All these challenges are confounded by the fact that there is no standard of care for HCV management and treatment of patients with malignancies. HCV-infected cancer patients suitable for antiviral therapy may benefit from it, because of the HCV-related complications or poor survival rates seen in cancer patients and HSCT recipients with HCV infection who do not receive this therapy. However, this treatment requires close monitoring for side effects. Use of hematopoietic growth factors enables continuation of antiviral treatment in some cases. However, antiviral dose reductions may be necessary because of underlying baseline hematological deficiencies or severe adverse treatment responses. Experience with direct-acting antivirals in this patient population is very limited. The cost-effectiveness and benefit-adverse reactions ratio have yet to be clearly delineated. More research is required to establish these parameters. More effective and better tolerated antivirals are urgently needed to treat HCV-infected cancer patients.
ACKNOWLEDGMENTS
We thank Don Norwood for editorial assistance.
Footnotes
P- Reviewers: Quer J, Shi Z S- Editor: Zhai HH L- Editor: A E- Editor: Ma S
References
- 1.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Teo CG, Jewett A, Baack B, Rein DB, Patel N, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61:1–32. [PubMed] [Google Scholar]
- 2.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004;127:1372–1380. doi: 10.1053/j.gastro.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 4.Gisbert JP, García-Buey L, Pajares JM, Moreno-Otero R. Prevalence of hepatitis C virus infection in B-cell non-Hodgkin’s lymphoma: systematic review and meta-analysis. Gastroenterology. 2003;125:1723–1732. doi: 10.1053/j.gastro.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Fujii Y, Kaku K, Tanaka M, Yosizaki M, Kaneko T, Matumoto N. Hepatitis C virus infection in patients with leukemia. Am J Hematol. 1994;46:278–282. doi: 10.1002/ajh.2830460405. [DOI] [PubMed] [Google Scholar]
- 6.Markovic S, Drozina G, Vovk M, Fidler-Jenko M. Reactivation of hepatitis B but not hepatitis C in patients with malignant lymphoma and immunosuppressive therapy. A prospective study in 305 patients. Hepatogastroenterology. 1999;46:2925–2930. [PubMed] [Google Scholar]
- 7.Faggioli P, De Paschale M, Tocci A, Luoni M, Fava S, De Paoli A, Tosi A, Cassi E. Acute hepatic toxicity during cyclic chemotherapy in non Hodgkin’s lymphoma. Haematologica. 1997;82:38–42. [PubMed] [Google Scholar]
- 8.Vento S, Cainelli F, Longhi MS. Reactivation of replication of hepatitis B and C viruses after immunosuppressive therapy: an unresolved issue. Lancet Oncol. 2002;3:333–340. doi: 10.1016/s1470-2045(02)00773-8. [DOI] [PubMed] [Google Scholar]
- 9.Torres HA, Boeckh M, Chemaly RF. Viral infections. In: Yeung SJ, Escalante C, Gagel RF, editors. Internal medicine care of cancer patient. 1st ed. Shelton, CT: BC Decker Inc; 2009. pp. 151–161. [Google Scholar]
- 10.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, Wingard JR, Young JA, Boeckh MJ. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rai RR, Mathur A, Mathur D, Udawat HP, Nepalia S, Nijhawan S, Mathur A. Prevalence of occult hepatitis B & C in HIV patients infected through sexual transmission. Trop Gastroenterol. 2007;28:19–23. [PubMed] [Google Scholar]
- 12.Locasciulli A, Cavalletto D, Pontisso P, Cavalletto L, Scovena E, Uderzo C, Masera G, Alberti A. Hepatitis C virus serum markers and liver disease in children with leukemia during and after chemotherapy. Blood. 1993;82:2564–2567. [PubMed] [Google Scholar]
- 13.Torres HA, Davila M. Reactivation of hepatitis B virus and hepatitis C virus in patients with cancer. Nat Rev Clin Oncol. 2012;9:156–166. doi: 10.1038/nrclinonc.2012.1. [DOI] [PubMed] [Google Scholar]
- 14.Peffault de Latour R, Lévy V, Asselah T, Marcellin P, Scieux C, Adès L, Traineau R, Devergie A, Ribaud P, Espérou H, et al. Long-term outcome of hepatitis C infection after bone marrow transplantation. Blood. 2004;103:1618–1624. doi: 10.1182/blood-2003-06-2145. [DOI] [PubMed] [Google Scholar]
- 15.Torres HA, Mahale P, Davila M, Mulanovich V, Granwehr B, Kontoyiannis DP, Raad II. Hepatitis C virus infection in cancer patients: the story of a forgotten population. Oct 21-24; Canada. In: Abstracts of the 48th Annual Meeting of the Infectious Diseases Society of America: 2010. p. Vancouver, 2010. [Google Scholar]
- 16.Simmonds P, Bukh J, Combet C, Deléage G, Enomoto N, Feinstone S, Halfon P, Inchauspé G, Kuiken C, Maertens G, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology. 2005;42:962–973. doi: 10.1002/hep.20819. [DOI] [PubMed] [Google Scholar]
- 17.Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment Web resource. Hepatology. 2014;59:318–327. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nainan OV, Alter MJ, Kruszon-Moran D, Gao FX, Xia G, McQuillan G, Margolis HS. Hepatitis C virus genotypes and viral concentrations in participants of a general population survey in the United States. Gastroenterology. 2006;131:478–484. doi: 10.1053/j.gastro.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Torres HA, Nevah MI, Barnett BJ, Mahale P, Kontoyiannis DP, Hassan MM, Raad II. Hepatitis C virus genotype distribution varies by underlying disease status among patients in the same geographic region: a retrospective multicenter study. J Clin Virol. 2012;54:218–222. doi: 10.1016/j.jcv.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Torres HA, Adachi JA, Roach LR, Smith KM, Mahale PS, Davila M, Raad II. Hepatitis C clinic operated by infectious disease specialists at a comprehensive cancer center: help is on the way. Clin Infect Dis. 2012;54:740–742. doi: 10.1093/cid/cir930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pearlman BL, Traub N. Sustained virologic response to antiviral therapy for chronic hepatitis C virus infection: a cure and so much more. Clin Infect Dis. 2011;52:889–900. doi: 10.1093/cid/cir076. [DOI] [PubMed] [Google Scholar]
- 22.Mahale P, Okhuysen PC, Torres HA. Does Chemotherapy Cause Viral Relapse in Cancer Patients With Hepatitis C Infection Successfully Treated With Antivirals? Clin Gastroenterol Hepatol. 2013:Epub ahead of print. doi: 10.1016/j.cgh.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Arcaini L, Merli M, Passamonti F, Bruno R, Brusamolino E, Sacchi P, Rattotti S, Orlandi E, Rumi E, Ferretti V, et al. Impact of treatment-related liver toxicity on the outcome of HCV-positive non-Hodgkin’s lymphomas. Am J Hematol. 2010;85:46–50. doi: 10.1002/ajh.21564. [DOI] [PubMed] [Google Scholar]
- 24.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 25.Ramos CA, Saliba RM, de Pádua L, Khorshid O, Shpall EJ, Giralt S, Patah PA, Hosing CM, Popat UR, Rondon G, et al. Impact of hepatitis C virus seropositivity on survival after allogeneic hematopoietic stem cell transplantation for hematologic malignancies. Haematologica. 2009;94:249–257. doi: 10.3324/haematol.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahale P, Kontoyiannis DP, Chemaly RF, Jiang Y, Hwang JP, Davila M, Torres HA. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol. 2012;57:1177–1185. doi: 10.1016/j.jhep.2012.07.031. [DOI] [PubMed] [Google Scholar]
- 27.Jou JH, Muir AJ. In the clinic. Hepatitis C. Ann Intern Med. 2012;157:ITC6–1 - ITC6-16. doi: 10.7326/0003-4819-157-11-201212040-01006. [DOI] [PubMed] [Google Scholar]
- 28.Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355:2444–2451. doi: 10.1056/NEJMct061675. [DOI] [PubMed] [Google Scholar]
- 29.Mauss S, Hueppe D, Alshuth U. Renal impairment is frequent in chronic hepatitis C patients under triple therapy with telaprevir or boceprevir. Hepatology. 2014;59:46–48. doi: 10.1002/hep.26602. [DOI] [PubMed] [Google Scholar]
- 30.Perry CM. Telaprevir: a review of its use in the management of genotype 1 chronic hepatitis C. Drugs. 2012;72:619–641. doi: 10.2165/11208370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Torres HA, Mahale P, Saxena A, Afshar-Kharghan V, Kroll MH, Oo TH. Use of growth factors in treatment of hepatitis C viral infection in cancer patients. Blood. In: Abstracts of the 48th Annual Meeting of the Infectious Diseases Society of America: 2012. p. Annual Meeting of the American Society of Hematology in Atlanta; Georgia suppl; abstract 4725. [Google Scholar]
- 32.Reilly CM, Bruner DW, Mitchell SA, Minasian LM, Basch E, Dueck AC, Cella D, Reeve BB. A literature synthesis of symptom prevalence and severity in persons receiving active cancer treatment. Support Care Cancer. 2013;21:1525–1550. doi: 10.1007/s00520-012-1688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sockalingam S, Links PS, Abbey SE. Suicide risk in hepatitis C and during interferon-alpha therapy: a review and clinical update. J Viral Hepat. 2011;18:153–160. doi: 10.1111/j.1365-2893.2010.01393.x. [DOI] [PubMed] [Google Scholar]
- 34.Laoutidis ZG, Mathiak K. Antidepressants in the treatment of depression/depressive symptoms in cancer patients: a systematic review and meta-analysis. BMC Psychiatry. 2013;13:140. doi: 10.1186/1471-244X-13-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: a systematic review and meta-analysis. Lancet Oncol. 2013;14:721–732. doi: 10.1016/S1470-2045(13)70244-4. [DOI] [PubMed] [Google Scholar]


