Abstract
Hepatitis C virus (HCV) is a global health concern which is responsible for most of the liver diseases. Currently, there is no vaccine available for prevention of HCV infection due to the high degree of strain variation. The current standard of care is a combination of pegylated interferon α with ribavirin and boceprevir/telaprevir. This treatment was partially effective and had significant side effects. Hence, there is a need to develop new antiviral agents that interfere with different stages of the HCV life cycle. Recent advances in the understanding of both the cellular and molecular mechanisms of HCV replication have provided the basis for novel therapeutic strategies. Several hundred plant species and their phyto-constituents have been isolated for screening against HCV, and some have been shown to have great medicinal value in preventing and/or ameliorating viral diseases in pre-clinical and clinical trials. This review summarizes medicinal plants and their phytochemicals which inhibit different stages of HCV life cycle and discuss their potential use in HCV therapy.
Keywords: Hepatitis C virus, Medicinal plants, Anti-hepatitis C virus drugs
Core tip: Medicinal plants have always caught the attention of researchers to develop antiviral drugs against dreadful viral diseases. Many plant species are being tested against hepatitis C virus (HCV) to find a possible cure for it and, hopefully, in the future these medicinal plants can serve as an important source for developing anti-HCV drugs.
INTRODUCTION
Hepatitis C virus (HCV) is a dreadful viral disease and has always been a global health issue. According to estimation, about 170 million people are affected, with the highest infection rates in Africa and Asia[1]. Each year approximately 3-4 million people are affected and > 350000 individuals die due to liver disease. HCV causes an acute infection which can eventually progress to chronic infection and can cause permanent liver damage, hepatocellular carcinoma (HCC), cirrhosis and death[2-5]. Acute to chronic progression is rapid in people who are older, human immunodeficiency virus (HIV) co-infected, consume more than 50 g of alcohol daily, or immunosuppressed and patients undergoing organ transplantation[6]. HCV belongs to the Flaviviridae family and has a positive single stranded RNA genome of 9.6 Kb. The genome of HCV has 5’ untranslated region (UTR) which works as an internal ribosomal entry site (IRES). The 5’ UTR is 324-341 in length and the IRES is considered important for Cap-independent translation of viral RNA[7,8]. This entry site (IRES) leads to the translation of an open reading frame (ORF) that encodes a 3010 amino acid poly protein precursor which is ultimately cleaved by host and viral proteases into 10 viral proteins in the order of NH (2) -Core-E1-E2-p7-NS2-NS3-NS4A-NS4B-NS5A-NS5B-COOH (Figure 1)[9]. According to past research, the structural proteins (Core, E and E2) and the nonstructural proteins (NS3 protease and NS5B RNA dependent RNA polymerase) have been considered the best targets to develop novel molecular inhibitors. Among these proteins, NS3 in association with NS4A has been hugely investigated due to its protease and helicase domains that are important in viral replication[1,10-12]. The life cycle of HCV is illustrated in Figure 2. HCV has six major genotypes with a series of subtypes[13]. In 2012, a new sequence has been found that is being named as subtype 7a[14]. The prevalence of genotype 3a is related to steatosis that leads to liver fibrosis[15,16].
Figure 1.
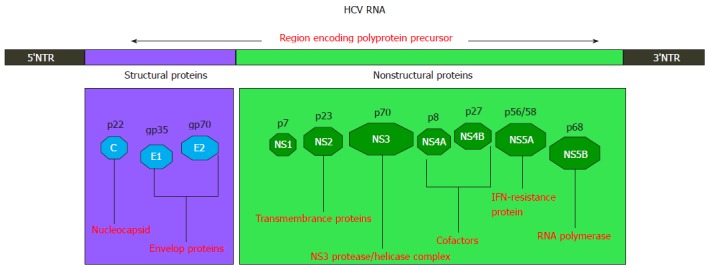
Hepatitis C virus genome organization. HCV: Hepatitis C virus; IFN: Interferon.
Figure 2.
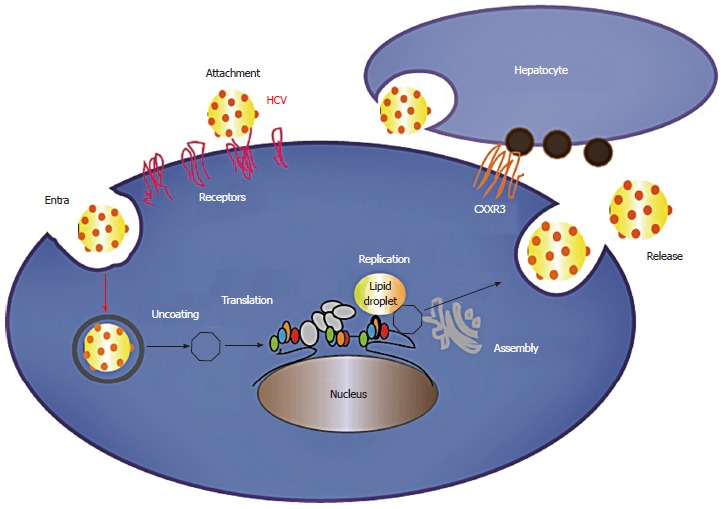
Hepatitis C virus life cycle.
To date, many medicinal plants have been tested against HCV and have proved beneficial as antiviral mediators. The reasons to prefer medicinal plants over traditional medicines are their fewer side effects, low cost and multiple target activities[17]. The phytochemicals of the medicinal plants, such as limonoids, alkaloids, lignana, organosulfur, furyl, thiophenes, polylines, terpenoids, flavonoids, polyphenolics, sulphides, saponins, coumarins, chlorophyllins, are considered important due to their efficiency at hampering viral entry, blocking/limiting the RNA/DNA genome replication and their anti-oxidant activity[18]. Currently, there are few antiviral drugs that can efficiently work against HCV as most of the antiviral drugs show side effects and many of the viruses acquire resistance against them; thus, there is a strong need to develop antiviral compounds that can suppress HCV without side effects. Therefore, medicinal plants due to their magical powers are being investigated to discover antiviral agents that can efficiently target the entry or replication of HCV virus and are believed to be our future inhibitors for this dreadful disease.
CURRENT TREATMENT
HCV is a major concern worldwide and clearing it in its early phase to avoid liver cirrhosis and HCC has always been the target for researchers. To date, there is no authenticated vaccine available in the market and current approved treatment (standard of care) is a combination therapy having pegylated interferon alpha (PegIFN-α) injections and antiviral nucleoside analogue ribavirin (RBV) used for 24-48 wk depending upon the type of genotype. All the genotypes of HCV show different sustained virological response (SVR) and genotype 1 which is regarded as most problematic genotype shows the clearance of HCV in 50% of the cases. Similarly, genotype 2 infection shows clearance in only 80% of the cases[19-22].
This combination therapy has several considerable side effects such as fever, anemia, flu and depression[23]. Several combinations of IFN are in clinical trials, such as taribavirin which is a prodrug of ribavirin and albinterferon which is the combination of IFN-α and human albumin[24,25]. The side effects caused by current treatment raised the need to develop antiviral compounds that can suppress or eliminate the infection without toxicity and side effects. This directed the investigation to test antiviral agents against HCV viral proteins. HCV NS3 protease has been investigated as a drug target[26,27]. The inhibition of NS3/4A protease interferes with the replication of HCV genome and also restores the pathways of the innate immunity[28]. To date, several NS3/4A protease inhibitors have been investigated and recently, two NS3 protease inhibitors, boceprevir and telaprevir, have been approved by the Food and Drug Administration (FDA) as combination therapy along with PegIFN-α and RBV for HCV genotype 1 patients[29]. Despite the availability of current treatment, there is a dire need to screen antiviral agents that can target all four genotypes with the same efficiency and without any side effects. This increases the significance of the medicinal plants as antiviral agents as they are less toxic, less costly and are easily accessed.
MEDICINAL PLANTS AGAINST HCV
Viral infections with high mortality and morbidity rate are the leading cause of human deaths worldwide. All viruses start their life cycle through attachment and entry into the host cell and then increase their progeny by transcription and replication of the genome. The RNA viruses such as influenza, HIV and HCV have become a matter of concern as these are highly variable and lack an RNA dependent RNA polymerase proofreading mechanism[30]. Development of vaccines against viral diseases such as polio, mumps and smallpox have controlled these diseases but infections like HIV and HCV have been hard to target because of variation in genotypes. Infectious diseases have widely been treated using the medicinal plants and about 25% of current medicines have compounds from medicinal plants. There are plenty of plants that are known for their magical medicinal properties and these plants can serve as an important reservoir for drug discovery against infectious diseases. Current separation techniques have enabled researchers to find active compounds of plants as antiviral agents and to overcome the challenge of emerging infectious disease in human population. There is a wide range of medicinal plants which are being used to extract natural compounds that are being used for their antiviral activity. Liver diseases have been treated around the world using numerous medicinal plants and their formulations and this has given confidence to researchers to investigate the effect of these medicinal plants against HCV in more depth[31].
HCV infection is a leading cause of deaths among patients. To date, many drugs have been tested against HCV and many of them have successfully completed clinical trials but the problem of viral resistance against these drugs and side effects caused by these drugs have marked a question of developing better therapeutics against HCV[32]. At the present, drug discovery is being focused on medicinal herbs for HCV due to the lack of appropriate standard therapy. Acetonic and methanolic extracts of Acacia nilotica (AN) have shown novel inhibition of HCV titer in-vitro confirmed by real-time PCR[33]. Preclinical evaluation of the lyophilized juice of ginger and aqueous extracts of Milk thistle (MSE) has demonstrated anti HCV effects in the HepG2 cell line. Both of these plants have shown effective antiviral activity at concentrations of 300 μg/mL and 100 μg/mL respectively[34]. Recently, medicinal plants from Indonesia have been tested for their antiviral activity against HCV. Ethanolic extracts of Indonesian plants were analyzed in the Huh 7.5 cell line and HCV strains of 9 different genotypes namely 1a-71, 1b and 2b. Among the tested plants, Toona sureni leaves (TSL) showed IC50 value of 13.9, Melicope latifolia leaves (MLL) showed IC50 of 3.5, Melanolepis multiglandulosa stem (MMS) exhibited IC50 of 17.1 and Ficus fistulosa leaves (FFL) showed IC50 of 15.0. Among all of these, MLL, TSL, FFL and MMS exhibited antiviral activity against all genotypes of HCV and thus, it is suggested that these plants may prove good candidates to develop novel inhibitory drugs against HCV though, there is a need to further investigate these plants to develop drugs for the effective inhibition of HCV[35].
MEDICINAL PLANTS AGAINST HCV CORE
To date, many plants have been tested against HCV core protein and some of these plants have been found as a source of novel inhibitory drugs to suppress HCV infection. Some of these plants have been discussed in this review. In recent years, Pakistan has tested various medicinal plants in vitro and found significant inhibitory effects on HCV titer. Among these plants are glycyrrhizin (GL) which inhibited HCV titer in dose dependent mode and also exhibited synergistic effects when administered with interferon. GL inhibited the core protein of genotype 3a at the mRNA and protein level and thus has been suggested as a future drug that can help to decrease the viral titer of HCV[36]. Another plant Silybum marianum (SM) was tested by the same research group and two pure fractions of SM exhibited inhibition of HCV core protein of genotype 3a which was confirmed through western blotting. The research group suggested that the combination of SM with interferon shows promising results in treating HCV[37].
MEDICINAL PLANTS AGAINST NS3 PROTEASE
During the recent decades, advancements in drug discovery have revealed NS3/4A protease as an important drug target in overcoming HCV infection. Many inhibitors against NS3 have successfully entered clinical trials but there is still need to improve their functionality and efficiency. Various medicinal plants are also being investigated to develop anti HCV drugs that are not only efficient but are also easily available to developing countries due to their low cost[38]. Methanolic and water extracts of medicinal plants used in Sudanese traditional medicine such as Boswellia carterii, Acacia nilotica, Quercus infectoria, Embelia schimperi, Trachyspermum ammi, Q. infectoria, Piper cubeba and Syzygium aromaticum have been tested against HCV protease and showed more than 90% at 100 μg/mL[39]. An in-silico approach has been used to test Accacia nilotica phytochemicals against NS3/4A protease and found that they may serve as a potential drug candidate with relatively simple structural changes against HCV NS3/4A protease[40]. Solanum nigrum (SN) has also been tested against HCV and its methanolic and chloroform extracts exhibited significant inhibition against HCV protease in liver infected cells[41]. Recently, Viola yedoensis has been investigated to find an anti HCV compound targeting protease. Using the various chromatographic procedures, 3 coumarins have been isolated and characterized from Viola yedoensis. Among the isolated compounds, a dimeric coumarin 5, 5’-bi (6,7-dihydroxycoumarin) has significantly inhibited NS3/4A protease with IC50 value of 0.5 μg/ml. Thus, this dicoumarin can serve as an important molecular template to design novel anti HCV drugs[42].
MEDICINAL PLANT PHYTOCHEMICALS AS HCV INHIBITORS
Medicinal plants have shown potential against viral infections and investigation of their active compounds has taken antiviral research to a new horizon. Currently, there are many plant derivatives being tested against HCV and some of them have shown significant inhibition in entry, replication and assembly steps of the viral life cycle, described in Table 1.
Table 1.
Medicinal plant phytochemicals as anti- hepatitis C virus agents
| Phytochemicals | Viral step | Effect |
| Diosgenin[43] | Replication | Inhibition of transcription factor 3 and signal transducer |
| Silymarin/silibinin[37,44,45] | Viral entry (viral attachment) Replication | Inhibition of core protein and NS5 RNA-dependent RNA polymerase |
| Iridoids[46,47] | Viral entry | Blockage of E2 and CD81 contact |
| Naringenin[48,49] | Viral assembly | Suppression of core protein activity |
| EGCG[50,51] | Viral entry | Disturbance in glycoprotein activity and Inhibition of cell-cell transmission |
| Quercetin[52,53] | Replication | Inhibition of IRES activity and NS3 polymerase |
| Ladanein[54] | Post attachment entry step | Inhibition of receptor interactions, virus endocytosis, or membrane fusion |
| Luteolin and apigenin[55] | Replication | Inhibition of NS5B polymerase activity |
IRES: Internal ribosomal entry site. EGCG: Epigallocatechin gallate.
Diosgenin (3β-hydroxy-5-spirostene), which is a plant-derived sapogenin, has effectively blocked the replication of the HCV subgenomic replicon system at both the mRNA and the protein level. A decrease in activator of transcription factor 3 and signal transducer has been observed. The EC50 value of diosgenin was 3.8 μmol with no cellular toxicity. In another antiviral system, it showed inhibition of viral replication at 20 μmol concentration[43].
Silymarin, which is isolated from Silybum marianum has been tested against HCV and is found to be effective in inhibiting the viral activity of HCV. Silymarin has been tested against the HCV core protein of genotype 3a and was found to be effective in inhibiting the core expression[37]. Silibinin, which is a combination of two diastereoisomers, is the major component of silymarin responsible for anti HCV activity[44]. The antiviral effect of silibinin has been tested against NS5 RNA-dependent RNA polymerase with IC50 values of 75-100 μmol[45].
HCV entry has been blocked using different iridoids from Lamium album using HCVpp. The aqueous extract of Lamium album containing lamiridosins A/B (1/2) and iridoids aglycone epimers have reduced HCVpp entry by disturbing the contact of HCV envelope 2 proteins (E2) with the CD81 receptor[46]. The 4-[(1,2-dihydro-2-oxo-3H-indol-3-ylidene) amino]-N (4,6-dimethyl-2-pyrimidiny) benzene sulphonamide and its derivatives have also been evaluated for anti-HCV activity and have shown inhibitory effects. The replication of HCV RNA has been blocked by 5-fluoro derivative of Isatin in Huh 5-2 cells[47].
Naringenin is a predominant flavanone found in the grapefruit and has been tested on the HCV particles[48]. Naringenin has suppressed the activity of core protein in Huh 7 cells and has also effectively blocked the assembly of HCV particles. It showed maximum inhibition at 200 μmol concentration with IC50 value of 109 μmol[49].
Epigallocatechin-3-gallate (EGCG) is found in green tea extract and recently this compound has been found significant in inhibiting HCV entry. EGCG had no effect on HCV assembly, replication and release but it efficiently inhibited cell-culture-derived HCV (HCVcc) entry into hepatocellular cell lines and this effect was independent of the HCV genotypes[50]. The effect of EGCG on HCV entry blockage has also been confirmed by another research group using a new anti HCV molecule screening assay[51]. Thus, EGCG may serve as an important anti HCV drug by blocking the entry of virus into host cells.
Quercetin, present in vegetables, fruits, grains and leaves has been investigated as an anti HCV agent. Quercetin not only reduced IRES activity but also its augmentation by NS5A. Thus quercetin reduced viral production without any toxic effects[52]. Similarly, in another study quercetin suppressed RNA replication in a subgenomic RNA replicon and also inhibited replication in a model containing NS3 substrates suggesting that it may be related to NS3 protein of HCV and blocks replication by targeting the NS3 protease. The effects of quercetin were nontoxic and were significant[53].
Ladanein (BJ486K) is a flavone isolated from the Marrubium peregrinum L. (Lamiaceae). Ladanein has shown effective inhibition of the post attachment entry step of HCV with an IC50 value of 2.5 μmol. In combination with cyclosporine, it showed interesting synergetic results against all the genotypes of HCV. This provides clues to further investigate ladanein for its anti HCV potential in patients[54]. Luteolin and apigenin have also been identified as anti HCV agents with IC50 values of 4.3 μmol and 7.9 μmol respectively. Among both compounds, luteolin showed persuasive inhibition of NS5B polymerase activity with an IC50 of 1.12 μmol[55].
Footnotes
P- Reviewers: Cong WM, Kato T, Liu ZH, Ma Q, Zhu X S- Editor: Qi Y L- Editor: O’Neill M E- Editor: Ma S
References
- 1.Romański B, Bokowski W. [Evaluation of the therapeutic effect of sodium cromoglycate in patients with gastric and duodenal ulcers, sensitive to exogenous allergens] Pol Tyg Lek. 1986;41:181–184. [PubMed] [Google Scholar]
- 2.World Health Organization. Hepatitis C. 2013. p. Fact sheet No.164. [Google Scholar]
- 3.O'Sullivan A, Brody M. Discharge planning for the mentally disabled. QRB Qual Rev Bull. 1986;12:55–67. doi: 10.1016/s0097-5990(16)30010-0. [DOI] [PubMed] [Google Scholar]
- 4.Jahan S, Ashfaq UA, Khaliq S, Samreen B, Afzal N. Dual behavior of HCV Core gene in regulation of apoptosis is important in progression of HCC. Infect Genet Evol. 2012;12:236–239. doi: 10.1016/j.meegid.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Aaronson NK, Calais da Silva F, de Voogt HJ. Subjective response criteria and quality of life. Prog Clin Biol Res. 1988;269:261–273. [PubMed] [Google Scholar]
- 6.Naggie S. Management of hepatitis C virus infection: the basics. Top Antivir Med. 2012;20:154–161. [PMC free article] [PubMed] [Google Scholar]
- 7.Tsukiyama-Kohara K, Iizuka N, Kohara M, Nomoto A. Internal ribosome entry site within hepatitis C virus RNA. J Virol. 1992;66:1476–1483. doi: 10.1128/jvi.66.3.1476-1483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka T, Lau JY, Mizokami M, Orito E, Tanaka E, Kiyosawa K, Yasui K, Ohta Y, Hasegawa A, Tanaka S. Simple fluorescent enzyme immunoassay for detection and quantification of hepatitis C viremia. J Hepatol. 1995;23:742–745. doi: 10.1016/0168-8278(95)80043-3. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Cerezo H, Dalziel K. L-alpha-glycerophosphate dehydrogenase from beef liver. Methods Enzymol. 1975;41:259–264. doi: 10.1016/s0076-6879(75)41059-x. [DOI] [PubMed] [Google Scholar]
- 10.Foy E, Li K, Wang C, Sumpter R, Ikeda M, Lemon SM, Gale M. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science. 2003;300:1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- 11.Thomas ED. A review of the results of human marrow transplantation in Seattle. Nihon Ketsueki Gakkai Zasshi. 1977;40:863–872. [PubMed] [Google Scholar]
- 12.Kato N. Molecular virology of hepatitis C virus. Acta Med Okayama. 2001;55:133–159. doi: 10.18926/AMO/32025. [DOI] [PubMed] [Google Scholar]
- 13.Simmonds P, Holmes EC, Cha TA, Chan SW, McOmish F, Irvine B, Beall E, Yap PL, Kolberg J, Urdea MS. Classification of hepatitis C virus into six major genotypes and a series of subtypes by phylogenetic analysis of the NS-5 region. J Gen Virol. 1993;74(Pt 11):2391–2399. doi: 10.1099/0022-1317-74-11-2391. [DOI] [PubMed] [Google Scholar]
- 14.Nakano T, Lau GM, Lau GM, Sugiyama M, Mizokami M. An updated analysis of hepatitis C virus genotypes and subtypes based on the complete coding region. Liver Int. 2012;32:339–345. doi: 10.1111/j.1478-3231.2011.02684.x. [DOI] [PubMed] [Google Scholar]
- 15.Strocchi R, De Pasquale V, Guizzardi S, Govoni P, Facchini A, Raspanti M, Girolami M, Giannini S. Human Achilles tendon: morphological and morphometric variations as a function of age. Foot Ankle. 1991;12:100–104. doi: 10.1177/107110079101200207. [DOI] [PubMed] [Google Scholar]
- 16.Westin J, Nordlinder H, Lagging M, Norkrans G, Wejstål R. Steatosis accelerates fibrosis development over time in hepatitis C virus genotype 3 infected patients. J Hepatol. 2002;37:837–842. doi: 10.1016/s0168-8278(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 17.Jassim SA, Naji MA. Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol. 2003;95:412–427. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- 18.Naithani R, Huma LC, Holland LE, Shukla D, McCormick DL, Mehta RG, Moriarty RM. Antiviral activity of phytochemicals: a comprehensive review. Mini Rev Med Chem. 2008;8:1106–1133. doi: 10.2174/138955708785909943. [DOI] [PubMed] [Google Scholar]
- 19.Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, O’Grady J, Reichen J, Diago M, Lin A, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343:1666–1672. doi: 10.1056/NEJM200012073432301. [DOI] [PubMed] [Google Scholar]
- 20.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 21.Vasil'eva VI, Sumarokov AA, Kozhevnikova LK, Asratian AA, Gorbunov MA. [Frequency of finding hepatitis B viral markers in young children born of mothers carrying the HBs-antigen] Zh Mikrobiol Epidemiol Immunobiol. 1987;(2):32–35. [PubMed] [Google Scholar]
- 22.Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology. 2006;130:231–264; quiz 214-217. doi: 10.1053/j.gastro.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Shah PJ, Greenberg WM. Tolbutamide and fatal water intoxication. Br J Psychiatry. 1991;158:719–720. doi: 10.1192/bjp.158.5.719b. [DOI] [PubMed] [Google Scholar]
- 24.Rustgi VK. Albinterferon alfa-2b, a novel fusion protein of human albumin and human interferon alfa-2b, for chronic hepatitis C. Curr Med Res Opin. 2009;25:991–1002. doi: 10.1185/03007990902779186. [DOI] [PubMed] [Google Scholar]
- 25.Benhamou Y, Afdhal NH, Nelson DR, Shiffman ML, Halliman DG, Heise J, Chun E, Pockros PJ. A phase III study of the safety and efficacy of viramidine versus ribavirin in treatment-naïve patients with chronic hepatitis C: ViSER1 results. Hepatology. 2009;50:717–726. doi: 10.1002/hep.23073. [DOI] [PubMed] [Google Scholar]
- 26.Gluckman PD, Ballard PL, Kaplan SL, Liggins GC, Grumbach MM. Prolactin in umbilical cord blood and the respiratory distress syndrome. J Pediatr. 1978;93:1011–1014. doi: 10.1016/s0022-3476(78)81240-2. [DOI] [PubMed] [Google Scholar]
- 27.López-Labrador FX. Hepatitis C Virus NS3/4A Protease Inhibitors. Recent Pat Antiinfect Drug Discov. 2008;3:157–167. doi: 10.2174/157489108786242369. [DOI] [PubMed] [Google Scholar]
- 28.Bagliani A, Moia R. [Effects of a new anti-inflammatory agent in some phlebopathies of the lower extremities] Minerva Cardioangiol. 1979;27:481–488. [PubMed] [Google Scholar]
- 29.Coupland RM, Howell PR. An experience of war surgery and wounds presenting after 3 days on the border of Afghanistan. Injury. 1988;19:259–262. doi: 10.1016/0020-1383(88)90041-1. [DOI] [PubMed] [Google Scholar]
- 30.Coen DM. Antiviral drug resistance. Ann N Y Acad Sci. 1990;616:224–237. doi: 10.1111/j.1749-6632.1990.tb17843.x. [DOI] [PubMed] [Google Scholar]
- 31.Tilak JC, Adhikari S, Devasagayam TP. Antioxidant properties of Plumbago zeylanica, an Indian medicinal plant and its active ingredient, plumbagin. Redox Rep. 2004;9:219–227. doi: 10.1179/135100004225005976. [DOI] [PubMed] [Google Scholar]
- 32.Kitazato K, Wang Y, Kobayashi N. Viral infectious disease and natural products with antiviral activity. Drug Discov Ther. 2007;1:14–22. [PubMed] [Google Scholar]
- 33.Evers MP, Zelle B, Peeper DS, Mager WH, Planta RJ, Eriksson AW, Frants RR. Molecular cloning of a pair of human pepsinogen A genes which differ by a Glu----Lys mutation in the activation peptide. Hum Genet. 1987;77:182–187. doi: 10.1007/BF00272389. [DOI] [PubMed] [Google Scholar]
- 34.Jacob JR, Korba BE, You JE, Tennant BC, Kim YH. Korean medicinal plant extracts exhibit antiviral potency against viral hepatitis. J Altern Complement Med. 2004;10:1019–1026. doi: 10.1089/acm.2004.10.1019. [DOI] [PubMed] [Google Scholar]
- 35.Wahyuni TS, Tumewu L, Permanasari AA, Apriani E, Adianti M, Rahman A, Widyawaruyanti A, Lusida MI, Fuad A, Soetjipto D, et al. Antiviral activities of Indonesian medicinal plants in the East Java region against hepatitis C virus. Virol J. 2013;10:259. doi: 10.1186/1743-422X-10-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raisaro A, Caizzi V, Roda G, Minzioni G, Bargiggia G, Bertucci C, Recusani F, Tronconi L. [Doppler evaluation of the Sorin and Medtronic-Hall prostheses in the aortic position] G Ital Cardiol. 1988;18:206–212. [PubMed] [Google Scholar]
- 37.da Silva Filho CE, Muench A. [Hardness of a low gold (46%) content alloy] Rev Odontol Univ Sao Paulo. 1988;2:127–130. [PubMed] [Google Scholar]
- 38.Idrees S, Ashfaq UA. HCV infection and NS-3 serine protease inhibitors. Virol Mycol. 2013;2:112. [Google Scholar]
- 39.Hussein G, Miyashiro H, Nakamura N, Hattori M, Kakiuchi N, Shimotohno K. Inhibitory effects of sudanese medicinal plant extracts on hepatitis C virus (HCV) protease. Phytother Res. 2000;14:510–516. doi: 10.1002/1099-1573(200011)14:7<510::aid-ptr646>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 40.Murakami K, Kuwabara H, Miura S, Mochizuki H, Nagamine T, Tomioka S, Yamada S, Kobayashi S, Ogawa A. [A case of glomerulonephritis complicated by idiopathic retroperitoneal fibrosis-like disease] Nihon Naika Gakkai Zasshi. 1986;75:799–806. doi: 10.2169/naika.75.799. [DOI] [PubMed] [Google Scholar]
- 41.Leitz T, Müller WA. Evidence for the involvement of PI-signaling and diacylglycerol second messengers in the initiation of metamorphosis in the hydroid Hydractinia echinata Fleming. Dev Biol. 1987;121:82–89. doi: 10.1016/0012-1606(87)90140-0. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Li MY, Wang LW, Gao J, Ma CM. Isolation, Identification, Quantification and Inhibitory Activity on HCV Protease of Coumarins from Viola yedoensis. Can. Chem. Trans. 2013;1:157–164. [Google Scholar]
- 43.Wang YJ, Pan KL, Hsieh TC, Chang TY, Lin WH, Hsu JT. Diosgenin, a plant-derived sapogenin, exhibits antiviral activity in vitro against hepatitis C virus. J Nat Prod. 2011;74:580–584. doi: 10.1021/np100578u. [DOI] [PubMed] [Google Scholar]
- 44.Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DY. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology. 2007;132:1925–1936. doi: 10.1053/j.gastro.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 45.Glass P, Miller M, Short B. Morbidity for survivors of extracorporeal membrane oxygenation: neurodevelopmental outcome at 1 year of age. Pediatrics. 1989;83:72–78. [PubMed] [Google Scholar]
- 46.Zhang H, Rothwangl K, Mesecar AD, Sabahi A, Rong L, Fong HH. Lamiridosins, hepatitis C virus entry inhibitors from Lamium album. J Nat Prod. 2009;72:2158–2162. doi: 10.1021/np900549e. [DOI] [PubMed] [Google Scholar]
- 47.McGarvey P, Helling RB, Lee JY, Engelke DR, el-Gewely MR. Initiation of rrn transcription in chloroplasts of Euglena gracilis bacillaris. Curr Genet. 1988;14:493–500. doi: 10.1007/BF00521275. [DOI] [PubMed] [Google Scholar]
- 48.Nahmias Y, Goldwasser J, Casali M, van Poll D, Wakita T, Chung RT, Yarmush ML. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology. 2008;47:1437–1445. doi: 10.1002/hep.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung R, Dickins J, Nicholson PW, Thomas AS, Smith HH, Larson HE, Deshmukh AA, Dobbs RJ, Dobbs SM. Compliance with anti-tuberculous therapy: a field trial of a pill-box with a concealed electronic recording device. Eur J Clin Pharmacol. 1988;35:401–407. doi: 10.1007/BF00561372. [DOI] [PubMed] [Google Scholar]
- 50.Ciesek S, von Hahn T, Colpitts CC, Schang LM, Friesland M, Steinmann J, Manns MP, Ott M, Wedemeyer H, Meuleman P, et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology. 2011;54:1947–1955. doi: 10.1002/hep.24610. [DOI] [PubMed] [Google Scholar]
- 51.Fukazawa H, Suzuki T, Wakita T, Murakami Y. A cell-based, microplate colorimetric screen identifies 7,8-benzoflavone and green tea gallate catechins as inhibitors of the hepatitis C virus. Biol Pharm Bull. 2012;35:1320–1327. doi: 10.1248/bpb.b12-00251. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez O, Fontanes V, Raychaudhuri S, Loo R, Loo J, Arumugaswami V, Sun R, Dasgupta A, French SW. The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatology. 2009;50:1756–1764. doi: 10.1002/hep.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bachmetov L, Gal-Tanamy M, Shapira A, Vorobeychik M, Giterman-Galam T, Sathiyamoorthy P, Golan-Goldhirsh A, Benhar I, Tur-Kaspa R, Zemel R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J Viral Hepat. 2012;19:e81–e88. doi: 10.1111/j.1365-2893.2011.01507.x. [DOI] [PubMed] [Google Scholar]
- 54.Haid S, Novodomská A, Gentzsch J, Grethe C, Geuenich S, Bankwitz D, Chhatwal P, Jannack B, Hennebelle T, Bailleul F, et al. A plant-derived flavonoid inhibits entry of all HCV genotypes into human hepatocytes. Gastroenterology. 2012;143:213–222.e5. doi: 10.1053/j.gastro.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 55.Antunes JL, Carmel PW, Zimmerman EA, Ferin M. Regeneration of the magnocellular system of the rhesus monkey following hypothalamic lesions. Ann Neurol. 1979;5:462–469. doi: 10.1002/ana.410050511. [DOI] [PubMed] [Google Scholar]


