Abstract
A 47-year-old man presented with general fatigue and dark urine. The laboratory data showed increased levels of hepatic transaminases. The patient was positive for hepatitis B virus (HBV) markers and negative for anti-human immunodeficiency virus. The HBV-DNA titer was set to 7.7 log copies/mL. The patient was diagnosed with acute hepatitis B. The HBV infection route was obscure. The serum levels of hepatic transaminases decreased to normal ranges without any treatment, but the HBV-DNA status was maintained for at least 26 mo, indicating the presence of persistent infection. We isolated HBV from the acute-phase serum and determined the genome sequence. A phylogenetic analysis revealed that the isolated HBV was genotype H. In this patient, the elevated peak level of HBV-DNA and the risk alleles at human genome single nucleotide polymorphisms s3077 and rs9277535 in the human leukocyte antigen-DP locus were considered to be risk factors for chronic infection. This case suggests that there is a risk of persistent infection by HBV genotype H following acute hepatitis; further cases of HBV genotype H infection must be identified and characterized. Thus, the complete determination of the HBV genotype may be essential during routine clinical care of acute hepatitis B outpatients.
Keywords: Acute hepatitis, Chronic hepatitis, Genotyping, Hepatitis B virus, Single nucleotide polymorphisms
Core tip: Hepatitis B virus (HBV) genotype H infection is rare in Asia, particularly in Japan. Here, we report a case of acute hepatitis B caused by a genotype H strain with persistent infection, although most adult cases of acute hepatitis B are self-limiting in Japan. This case suggests that the HBV genotype H infection can be a risk factor for persistent infection. Therefore, it is necessary to investigate the characteristics of genotype H infection in an accumulation of cases. Thus, the complete determination of the HBV genotype may be essential in the routine clinical care of acute hepatitis B patients.
INTRODUCTION
Hepatitis B is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV); it represents a major global health problem. HBV can cause chronic liver diseases and increases the risk of death from cirrhosis and liver cancer. Worldwide, an estimated two billion people have been infected with HBV and more than 240 million have chronic infections[1]. The HBV genome consists of approximately 3200-nucleotides of DNA; the virus replicates using a reverse transcriptase enzyme that lacks proofreading ability. Therefore, HBV possesses diverse genetic variability, and the viral population is classified into at least eight genotypes that are designated A -H[2-6]. In Japan, genotypes B and C are prevalent among patients with chronic infections. However, in the last decades, the prevalent genotype in acute HBV infections has shifted from genotype C to A[7-9]. There are some differences in the clinical features and outcomes among the genotypes[10-13]. It has been reported that the persistent infection from acute hepatitis is prevalent in adults that are infected with genotype A HBV. Thus, determining the HBV genotype is of increasing importance even in routine clinical practice, although a reliable kit for determination of all HBV genotypes is still uncommon and is not yet covered by insurance. The host factors associated with persistent infection by HBV have also been reported, such as single nucleotide polymorphisms (SNPs) or genotypes in the human leukocyte antigen-DP locus. It may also be useful for identifying the patients who are prone to develop chronic hepatitis.
In this report, we describe a case of acute hepatitis B resulting from infection by a genotype H strain of HBV. Although the laboratory data and symptoms were not distinguishable from acute hepatitis B with other genotypes, this patient developed persistent infection.
CASE REPORT
A 47-year-old man living in Kawasaki, Japan, presented at our hospital with general fatigue and dark urine. Approximately 1 wk before visiting the hospital, the patient developed nausea, loss of appetite, and a feeling of fullness in the abdomen. Four days later, he noted darkening of his skin and urine. Upon admission, the patient’s laboratory data revealed elevated serum aspartate aminotransferase, alanine aminotransferase (ALT), lactate dehydrogenase, alkaline phosphatase, γ-glutamyltranspeptidase, and total bilirubin (T-Bil) levels (Table 1). The prothrombin activity was within the normal range (95%). Test for hepatitis B surface antigen (HBsAg; HISCL-2000i, Sysmex, Kobe, Hyogo, Japan), hepatitis B e-antigen (HBeAg; ARCHITECT® CLIA, Abbott Japan, Tokyo, Japan) and anti-hepatitis B core antigen (anti-HBc) IgM (ARCHITECT® CLIA) were positive. A test for HBV-DNA was also positive, exhibiting a titer of 7.7 log copies/mL (COBAS TaqMan HBV Test v2.0, Roche Diagnostics, Tokyo, Japan). HBsAg had not been detected 2 years previously when the patient had been admitted to another hospital for treatment of acute enterocolitis. Other hepatitis virus markers were negative. Therefore, the patient was diagnosed with acute hepatitis B. The genotype of the infecting HBV, as assessed by the Immunis HBV Genotype Immunis® HBV Genotype EIA Kit (Institute of Immunology, Tokyo, Japan), was determined as genotype C. The patient had not been abroad in the past 12 mo; he had no history of receiving blood or blood-related products, transfusions, or drug injections, and he reported no personal or family history of liver disease. The man was unmarried and declared that he was heterosexual, with no history of sexual contact with commercial sex workers or strangers. Anti-human immunodeficiency virus (HIV) was not detected. In the absence of medication, the patient’s condition and elevated ALT level improved within a month. Anti-HBe became detectable, and HBeAg disappeared 2 mo after onset of the symptoms. HBsAg became undetectable at 5 mo, but the patient still tested positive for HBV-DNA, a status that persisted for at least 26 mo following his presentation at our hospital (Figure 1). We are now preparing to administer anti-viral medication.
Table 1.
Laboratory findings at first visit to our hospital
| Hematology | Blood chemistry | Viral markers | Immunology | Coagulation | |||||
| WBC | 7400/μL | TP | 7.4 g/dL | Anti-HA IgM | (-) | IgA | 183 mg/dL | PT% | 95% |
| Neutrophil | 72.0% | Albumin | 4.5 g/dL | Anti-HCV | (-) | IgG | 1168 mg/dL | APTT | 36.4 s |
| Eosinophil | 1.0% | T-Bil | 11.1 mg/dL | HBsAg | (+) 197333 | IgM | 220 mg/dL | ||
| Basophil | 0.0% | D-Bil | 8.0 mg/dL | Anti-HBc IgM | (+) 25.5 C.O.I | ANA | × 40, homogeneous | ||
| Monocyte | 10.0% | AST | 1942 IU/L | HBeAg | (+) 253 C.O.I | ||||
| Lymphocyte | 17.0% | ALT | 2963 IU/L | Anti-HBe | (-) 0.0 % | ||||
| RBC | 457/μL | ALP | 612 IU/L | HBV-DNA | 7.7 log copies/mL | ||||
| Hemoglobin | 16.0 g/dL | γGTP | 756 IU/L | Anti-HIV | (-) | ||||
| Hematocrit | 46.4% | LDH | 739 IU/L | RPR | (-) | ||||
| Platelet | 36.6 × 104/μL | BUN | 8.2 mg/dL | TPHA | (+) | ||||
| Creatinine | 0.64 mg/dL | Anti-CMV IgG | (+) | ||||||
| T-Chol | 225 mg/dL | Anti-CMV IgM | (-) | ||||||
| Anti-EBV EBNA | (+) | ||||||||
| Anti-EBV EA IgG | (-) | ||||||||
| Anti-EBV VCA IgG | (+) | ||||||||
| Anti-EBV VCA IgM | (-) | ||||||||
WBC: White blood cells; RBC: Red blood cells; ANA: Antinuclear antibody; TP: Total protein; T-Bil: Total bilirubin; D-Bil: Direct bilirubin; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; γGTP: γ-glutamyltranspeptidase; LDH: Lactase dehydrogenase; BUN: Blood urea nitrogen; T-Chol: Total cholesterol; PT: Prothrombin activity; APTT: Activated partial thromboplastin time; C.O.I: Cutoff index; HA: Hepatitis A; HCV: Hepatitis C virus; HBsAg: Hepatitis B surface antigen; HBc: Hepatitis B core; HBeAg: Hepatitis B e-antigen; HBV: Hepatitis B virus; HIV: Human immunodeficiency virus; RPR: Rapid plasma regain; TPHA: Treponema pallidum hemagglutination assay; CMV: Cytomegalovirus; EBV: Epstein-Barr virus; EBNA: Epstein-Barr virus nuclear antigen; EA: Early antigen; VCA: Viral capsid antigen.
Figure 1.
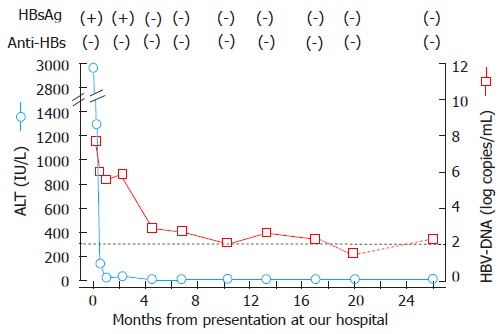
Clinical course of the patient infected with the genotype H strain. The dotted line indicates the detection limit of HBV-DNA (2.1 log copy/mL); the titer of the HBV-DNA was below the lower limit at 18 mo. HBsAg: Hepatitis B surface antigen; Anti-HBs: Antibody to hepatitis B surface antigen; ALT: Alanine aminotransferase; HBV: Hepatitis B virus.
For further analysis of the HBV infecting this patient, HBV-DNA was extracted from the acute-phase serum using a QIAamp DNA Blood Mini kit (QIAGEN, Valencia, CA). The entire HBV genome sequence was determined after polymerase chain reaction (PCR) amplification using the following primers [the number of nucleotides (nt) added to the primers were deduced from the prototype HBV/C clone, with accession no. AB246344]. For the amplification of half of the HBV genome, the outer primers were 5’-ATTCCACCAAGCTCTGCTAGATCCCAGAGT-3’ (nt 10-39) and 5’-GGTGCTGGTGAACAGACCAATTTATGCCTA-3’ (nt 1813-1784), and the inner primers were 5’-CCTATATTTTCCTGCTGGTGGCTCCAGTTC-3’ (nt 46-75) and 5’-TAGCCTAATCTCCTCCC CCAACTCCTCCCA-3’ (nt 1760-1731). For the other half of the HBV genome, the outer primers were 5’- ACGTCGCATGGAGACCACCGTGAACGCCCA-3’ (nt 1601-1630) and 5’-AAGTCCACCACGAGTCTAGACTCTGTGGTA-3’ (nt 266-237), and the inner primers were 5’-CCAGGTCTTGCCCAAGGTCTTACATAAGAG-3’ (nt 1631-1660) and 5’-CCCGCCTGTAACACGAGCAGGGGTCCTAGG-3’ (nt 207-178). The PCR was performed in a thermal cycler for 30 cycles (94 °C, 30 s; 60 °C, 30 s; 72 °C, 30 s) with TAKARA LA Taq® DNA polymerase (TAKARA, Shiga, Japan). The amplified fragments were sequenced directly with an automated DNA sequencer (3500 Genetic Analyzer, Applied Biosystems, Foster City, CA, United States).
The genome of the infecting HBV (designated as B-MHJ9014) was 3215 bases in size. A phylogenetic analysis was performed with this strain and several database reference strains. B-MHJ9014 sorted with the genotype-H branch of the tree and clustered with the genotype-H strains previously isolated from Japanese patients (Figure 2). The substitutions at nt 1762 and nt 1764 (the basal core promoter region) and at nt 1896 (the precore region) were not observed. The length of the deduced amino acid sequences of the S, X, Core, and P proteins were identical to those encoded by other genotype H strains in the databases. The α determinant region of the S protein of B-MHJ9014 harbored an amino acid polymorphism (phenylalanine to leucine) at residue 134. The predicted B-MHJ9014 reverse transcriptase did not include any of the amino acid substitutions known to be associated with nucleotide analog resistance. To assess the complexity of the infecting virus, S region sequences from 51 clones in acute phase serum were determined. The detected sequences were genotype H and were closely related to the consensus sequence determine by direct sequencing with 1-3 amino acids polymorphisms (data not shown).
Figure 2.
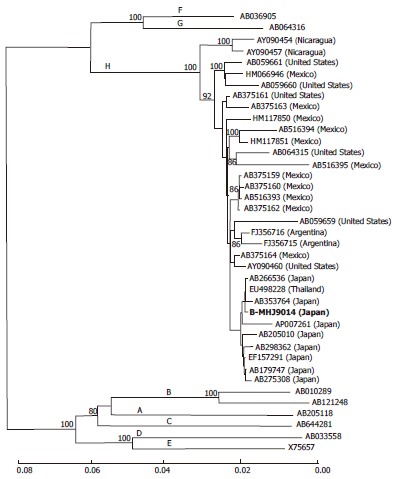
A phylogenetic trees constructed using the neighbor-joining method with the full hepatitis B virus genome sequence of the isolated and reference strains. The strain isolated in this case (B-MHJ9014) is shown in bold. The horizontal bar indicates the number of nucleotide substitutions per site. The reference sequences are shown with the DDBJ/EMBL/GenBank accession numbers. The HBV genotypes are indicated on each branch. The bootstrap values (> 80%) are indicated at the nodes as a percentage of the data obtained from 1000 resamplings. HBV: Hepatitis B virus.
To assess the presence of human genome SNPs in the HLA-DP locus that are associated with persistent infection by HBV[14,15], a blood specimen was obtained from the patient (who had previously provided informed consent). Genomic DNA was extracted from buffy coat samples with the QIAamp DNA Mini kit (QIAGEN); DNA for SNPs rs3077 and rs9277535 were amplified with the appropriate primers and TAKARA LA Taq® DNA polymerase and were sequenced directly. The patient was homozygous (G/G) at both of these SNPs; these alleles are considered to be risk alleles for persistent infection.
DISCUSSION
HBV genotype H was first reported in 2002[5]. Infections by this genotype have been found mainly in Nicaragua, Mexico, and California; this genotype is considered to be rare in Asia, particularly in Japan[5,16-18]. However, since the first recognition of genotype H in Japan in 2005, eight strains have been isolated from Japanese patients (Table 2)[18-25]. All reported genotype H strains were isolated from male patients aged 35 to 65 years old, and the major route of infection was sexual transmission (5/8, 62.5%). Four cases (50%) represent transmissions that occurred in Japan. Co-infection with HIV was not common (2/8, 25%). These characteristics were similar to the case described here. All isolated strains from Japanese patients clustered together as a branch on the phylogenetic tree; therefore, it is possible that a specific strain of genotype H has emerged and spread in Japan. Presumably, the infrequent use of a reliable and convenient detection kit for genotype H infection has hampered the correct diagnosis of genotype H infection; some cases may be misdiagnosed and considered to be infections by other genotypes. In fact, in the current case, our HBV isolate was originally identified as genotype C by the commercial kit that is covered by insurance in Japan. This kit was developed before the discovery of genotype H; thus, such a misidentification is a potential risk, as noted in the kit’s instruction manual. The clinical features of genotype H infection remain obscure. There is a growing need for an accumulation of genotype H infection cases. To this end, the use of a reliable HBV genotyping kit that can correctly distinguish all genotypes is essential for routine clinical practice.
Table 2.
Genotype H strains reported in Japan
| No. | Patient |
Hypothesized source of infection |
HIV infection1 | Clinical feature | Accession number (Ref.) | ||
| Age | Gender | Route | Place | ||||
| 1 | 52 | Male | Unknown | Japan | NA | Unknown blood donor | AB179747, [18] |
| 2 | 61 | Male | Sexual contact (heterosexual) | Thailand | NA | Chronic | AB205010, [19] |
| 3 | 46 | Male | Sexual contact (bisexual) | South America | (+) | Chronic | AP007261, [20] |
| 4 | 38 | Male | Sexual contact (homosexual) | Unknown | NA | Chronic | AB298362, [21] |
| 5 | 65 | Male | Unknown | Japan | NA | Acute | EF157291, [22] |
| 6 | 35 | Male | Unknown | Japan | NA | Acute | AB266536, [23] |
| 7 | 60 | Male | Sexual contact (homosexual) | Japan | (-) | Acute | AB275308, [24] |
| 8 | 60 | Male | Sexual contact (heterosexual) | Unknown | (+) | Chronic | AB353764, [25] |
| 9 | 47 | Male | Unknown | Japan | (-) | Acute to chronic | AB846650, this paper |
NA: Not available; HIV: Human immunodeficiency virus.
In Japan, most cases of acute hepatitis B are self-limiting, but some cases have been reported to have progressed to persistent infections[9,26-29]. Among the reported cases of genotype H infection, 4 strains were isolated from chronic hepatitis patients; in all cases, the infection was ascribed to sexual contact (Table 2)[19-21,25]. In our case, the HBV-DNA persisted for at least 26 mo. To our knowledge, this report represents the only case of genotype H infection in which chronic hepatitis was observed following acute infection. HBsAg was no longer detected at 4 mo from onset by HISCL-2000i. This disappearance was also confirmed by ARCHITECT® HBsAg (CMIA, Abbott Japan, Tokyo, Japan). In the S protein analysis, we found an amino acid polymorphism in the α determinant region. This polymorphism may affect the sensitivity for detecting HBsAg. HIV infection, a well-known risk factor for prolonged HBV infection[30], was not detected in our patient. Recently, the risk factors for HBV persistent infection have been reported in an analysis of a cohort that excluded patients co-infected with HIV[29]. In that report, infection with genotype A, elevated peak levels of HBV-DNA, and attenuated peak levels of ALT were suggested as risk factors for chronic infection. In the case described here, the peak level of HBV-DNA was 7.7 log copy/mL, which was consistent with increased risk for chronic infection. However, our patient exhibited a peak level of ALT of 2963 IU/L, which is a value that would classify this individual in the self-limiting group. Therefore, the clinical features of this case did not completely fit the risk factors associated with the establishment of chronic infection in the previous analysis[29]. Another reported risk factor for chronic HBV infection is the presence of certain SNP alleles. Specifically, selected SNPs around the HLA-DP locus have been reported to be associated with chronic hepatitis B in Asians[14,15]. With the informed consent of our patient, we determined the sequences for these SNPs (rs3077 and rs9277535) and found that this patient harbored risk alleles at both polymorphisms. This factor may have contributed to the establishment of chronic infection in this case.
In conclusion, we report a case of acute hepatitis B caused by a genotype H strain of HBV. This patient exhibited persistent infection. Our finding suggests that the infection of HBV genotype H can be a risk factor for persistent infection. We believe that it is necessary to use kits that are capable of accurate genotyping to permit an accumulation of cases and to investigate the clinical features of genotype H infection in routine clinical practice.
COMMENTS
Case characteristics
The main symptoms were nausea, loss of appetite, and a feeling of fullness in the abdomen.
Clinical diagnosis
The patient was a case of acute hepatitis B caused by a genotype H strain with persistent infection.
Differential diagnosis
The hepatitis B virus (HBV) genotype was considered to be important to predict the outcome and clinical features.
Laboratory diagnosis
To diagnose this patient, the detection of HBV markers and the complete determination of the HBV genotype were essential.
Treatment
The anti-viral treatment was not administered because we expected this case was self-limiting. Authors are now preparing medication.
Experiences and lessons
The infection of HBV genotype H can be a risk factor for persistent infection and the complete determination of HBV genotype is important.
Peer review
To conclude the association between HBV genotype H and chronic infection, the accumulation of cases of genotype H infection is essential.
Footnotes
Supported by Japan Society for the Promotion of Science and the Ministry of Health, Labour and Welfare and the Ministry of Education, Culture, Sports, Science and Technology of Japan
P- Reviewers: Chan KM, Chun YH, Rodriguez-Frias F S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
References
- 1.World Health Organization. Hepatitis B Fact Sheet. Accessed August. 2013. Available from: http://www.whoint/ mediacentre/factsheets/fs204/en/indexhtml. [Google Scholar]
- 2.Okamoto H, Tsuda F, Sakugawa H, Sastrosoewignjo RI, Imai M, Miyakawa Y, Mayumi M. Typing hepatitis B virus by homology in nucleotide sequence: comparison of surface antigen subtypes. J Gen Virol. 1988;69(Pt 10):2575–2583. doi: 10.1099/0022-1317-69-10-2575. [DOI] [PubMed] [Google Scholar]
- 3.Norder H, Couroucé AM, Magnius LO. Complete genomes, phylogenetic relatedness, and structural proteins of six strains of the hepatitis B virus, four of which represent two new genotypes. Virology. 1994;198:489–503. doi: 10.1006/viro.1994.1060. [DOI] [PubMed] [Google Scholar]
- 4.Stuyver L, De Gendt S, Van Geyt C, Zoulim F, Fried M, Schinazi RF, Rossau R. A new genotype of hepatitis B virus: complete genome and phylogenetic relatedness. J Gen Virol. 2000;81:67–74. doi: 10.1099/0022-1317-81-1-67. [DOI] [PubMed] [Google Scholar]
- 5.Arauz-Ruiz P, Norder H, Robertson BH, Magnius LO. Genotype H: a new Amerindian genotype of hepatitis B virus revealed in Central America. J Gen Virol. 2002;2002:2059–2073. doi: 10.1099/0022-1317-83-8-2059. [DOI] [PubMed] [Google Scholar]
- 6.Kurbanov F, Tanaka Y, Mizokami M. Geographical and genetic diversity of the human hepatitis B virus. Hepatol Res. 2010;40:14–30. doi: 10.1111/j.1872-034X.2009.00601.x. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi M, Arase Y, Ikeda K, Tsubota A, Suzuki Y, Saitoh S, Kobayashi M, Suzuki F, Akuta N, Someya T, et al. Clinical characteristics of patients infected with hepatitis B virus genotypes A, B, and C. J Gastroenterol. 2002;37:35–39. doi: 10.1007/s535-002-8130-z. [DOI] [PubMed] [Google Scholar]
- 8.Tamada Y, Yatsuhashi H, Masaki N, Nakamuta M, Mita E, Komatsu T, Watanabe Y, Muro T, Shimada M, Hijioka T, et al. Hepatitis B virus strains of subgenotype A2 with an identical sequence spreading rapidly from the capital region to all over Japan in patients with acute hepatitis B. Gut. 2012;61:765–773. doi: 10.1136/gutjnl-2011-300832. [DOI] [PubMed] [Google Scholar]
- 9.Yotsuyanagi H, Ito K, Yamada N, Takahashi H, Okuse C, Yasuda K, Suzuki M, Moriya K, Mizokami M, Miyakawa Y, et al. High levels of hepatitis B virus after the onset of disease lead to chronic infection in patients with acute hepatitis B. Clin Infect Dis. 2013;57:935–942. doi: 10.1093/cid/cit348. [DOI] [PubMed] [Google Scholar]
- 10.Kao JH, Chen PJ, Lai MY, Chen DS. Hepatitis B genotypes correlate with clinical outcomes in patients with chronic hepatitis B. Gastroenterology. 2000;118:554–559. doi: 10.1016/s0016-5085(00)70261-7. [DOI] [PubMed] [Google Scholar]
- 11.Orito E, Mizokami M, Sakugawa H, Michitaka K, Ishikawa K, Ichida T, Okanoue T, Yotsuyanagi H, Iino S. A case-control study for clinical and molecular biological differences between hepatitis B viruses of genotypes B and C. Japan HBV Genotype Research Group. Hepatology. 2001;33:218–223. doi: 10.1053/jhep.2001.20532. [DOI] [PubMed] [Google Scholar]
- 12.Chu CJ, Lok AS. Clinical significance of hepatitis B virus genotypes. Hepatology. 2002;35:1274–1276. doi: 10.1053/jhep.2002.33161. [DOI] [PubMed] [Google Scholar]
- 13.Miyakawa Y, Mizokami M. Classifying hepatitis B virus genotypes. Intervirology. 2003;46:329–338. doi: 10.1159/000074988. [DOI] [PubMed] [Google Scholar]
- 14.Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, Hosono N, Kubo M, Tsunoda T, Kamatani N, Kumada H, et al. A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet. 2009;41:591–595. doi: 10.1038/ng.348. [DOI] [PubMed] [Google Scholar]
- 15.Nishida N, Sawai H, Matsuura K, Sugiyama M, Ahn SH, Park JY, Hige S, Kang JH, Suzuki K, Kurosaki M, et al. Genome-wide association study confirming association of HLA-DP with protection against chronic hepatitis B and viral clearance in Japanese and Korean. PLoS One. 2012;7:e39175. doi: 10.1371/journal.pone.0039175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flichman D, Galdame O, Livellara B, Viaut M, Gadano A, Campos R. Full-length genome characterization of hepatitis B virus genotype H strain isolated from serum samples collected from two chronically infected patients in Argentina. J Clin Microbiol. 2009;47:4191–4193. doi: 10.1128/JCM.01337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roman S, Tanaka Y, Khan A, Kurbanov F, Kato H, Mizokami M, Panduro A. Occult hepatitis B in the genotype H-infected Nahuas and Huichol native Mexican population. J Med Virol. 2010;82:1527–1536. doi: 10.1002/jmv.21846. [DOI] [PubMed] [Google Scholar]
- 18.Ohnuma H, Yoshikawa A, Mizoguchi H, Okamoto H. Characterization of genotype H hepatitis B virus strain identified for the first time from a Japanese blood donor by nucleic acid amplification test. J Gen Virol. 2005;86:595–599. doi: 10.1099/vir.0.80732-0. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima A, Usui M, Huy TT, Hlaing NK, Masaki N, Sata T, Abe K. Full-length sequence of hepatitis B virus belonging to genotype H identified in a Japanese patient with chronic hepatitis. Jpn J Infect Dis. 2005;58:244–246. [PubMed] [Google Scholar]
- 20.Shibayama T, Masuda G, Ajisawa A, Hiruma K, Tsuda F, Nishizawa T, Takahashi M, Okamoto H. Characterization of seven genotypes (A to E, G and H) of hepatitis B virus recovered from Japanese patients infected with human immunodeficiency virus type 1. J Med Virol. 2005;76:24–32. doi: 10.1002/jmv.20319. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki F, Akuta N, Suzuki Y, Yatsuji H, Sezaki H, Arase Y, Kawamura Y, Hosaka T, Kobayashi M, Ikeda K, et al. Selection of a virus strain resistant to entecavir in a nucleoside-naive patient with hepatitis B of genotype H. J Clin Virol. 2007;39:149–152. doi: 10.1016/j.jcv.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Tamada Y, Yano K, Komatsu T, Yatsuhashi H, Ishibashi H, Takahashi K, Mishiro S. First Domestic Case of Acute Hepatitis Caused by an HBV genotype H Strain. Kanzo. 2007;48:109–111. [Google Scholar]
- 23.Kumagai I, Abe K, Oikawa T, Sato A, Sato S, Endo R, Takikawa Y, Suzuki K, Masuda T, Sainokami S, et al. A male patient with severe acute hepatitis who was domestically infected with a genotype H hepatitis B virus in Iwate, Japan. J Gastroenterol. 2007;42:168–175. doi: 10.1007/s00535-006-1963-2. [DOI] [PubMed] [Google Scholar]
- 24.Chihara N, Arase Y, Suzuki F, Suzuki Y, Kobayashi M, Akuta N, Hosaka T, Sezaki H, Yatsuji H, Kawamura Y, et al. Prolonged hepatitis after acute infection with genotype H hepatitis B virus. Intern Med. 2007;46:1847–1851. doi: 10.2169/internalmedicine.46.0163. [DOI] [PubMed] [Google Scholar]
- 25.Kanada A, Takehara T, Ohkawa K, Kato M, Tatsumi T, Miyagi T, Sakamori R, Yamaguchi S, Uemura A, Kohga K, et al. Early emergence of entecavir-resistant hepatitis B virus in a patient with hepatitis B virus/human immunodeficiency virus coinfection. Hepatol Res. 2008;38:622–628. doi: 10.1111/j.1872-034X.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y, Kobayashi M, Ikeda K, Suzuki F, Arfase Y, Akuta N, Hosaka T, Saitoh S, Kobayashi M, Someya T, et al. Persistence of acute infection with hepatitis B virus genotype A and treatment in Japan. J Med Virol. 2005;76:33–39. doi: 10.1002/jmv.20320. [DOI] [PubMed] [Google Scholar]
- 27.Ozasa A, Tanaka Y, Orito E, Sugiyama M, Kang JH, Hige S, Kuramitsu T, Suzuki K, Tanaka E, Okada S, et al. Influence of genotypes and precore mutations on fulminant or chronic outcome of acute hepatitis B virus infection. Hepatology. 2006;44:326–334. doi: 10.1002/hep.21249. [DOI] [PubMed] [Google Scholar]
- 28.Miyoshi T, Hiraoka A, Hidaka S, Shimizu Y, Ninomiya K, Utsunomiya H, Tazuya N, Tanihira T, Hasebe A, Miyamoto Y, et al. An adult patient with acute infection with hepatitis B virus genotype C that progressed to chronic infection. Intern Med. 2012;51:173–176. doi: 10.2169/internalmedicine.51.6142. [DOI] [PubMed] [Google Scholar]
- 29.Ito K, Yotsuyanagi H, Yatsuhashi H, Karino Y, Takikawa Y, Saito T, Arase Y, Imazeki F, Kurosaki M, Umemura T, et al. Risk factors for long-term persistence of serum hepatitis B surface antigen following acute hepatitis B virus infection in Japanese adults. Hepatology. 2014;59:89–97. doi: 10.1002/hep.26635. [DOI] [PubMed] [Google Scholar]
- 30.Gilson RJ, Hawkins AE, Beecham MR, Ross E, Waite J, Briggs M, McNally T, Kelly GE, Tedder RS, Weller IV. Interactions between HIV and hepatitis B virus in homosexual men: effects on the natural history of infection. AIDS. 1997;11:597–606. doi: 10.1097/00002030-199705000-00007. [DOI] [PubMed] [Google Scholar]


