Abstract
Assessment of liver fibrosis in chronic hepatitis C virus (HCV) infection is considered a relevant part of patient care and key for decision making. Although liver biopsy has been considered the gold standard for staging liver fibrosis, it is an invasive technique and subject to sampling errors and significant intra- and inter-observer variability. Over the last decade, several noninvasive markers were proposed for liver fibrosis diagnosis in chronic HCV infection, with variable performance. Besides the clear advantage of being noninvasive, a more objective interpretation of test results may overcome the mentioned intra- and inter-observer variability of liver biopsy. In addition, these tests can theoretically offer a more accurate view of fibrogenic events occurring in the entire liver with the advantage of providing frequent fibrosis evaluation without additional risk. However, in general, these tests show low accuracy in discriminating between intermediate stages of fibrosis and may be influenced by several hepatic and extra-hepatic conditions. These methods are either serum markers (usually combined in a mathematical model) or imaging modalities that can be used separately or combined in algorithms to improve accuracy. In this review we will discuss the different noninvasive methods that are currently available for the evaluation of liver fibrosis in chronic hepatitis C, their advantages, limitations and application in clinical practice.
Keywords: Liver fibrosis, Liver cirrhosis, Hepatitis C, Diagnosis, Elasticity imaging techniques
Core tip: There is increased interest in non-invasive markers of fibrosis, especially in chronic hepatitis C virus infection. Although several methodologies have been proposed over the last few years, the limited availability and concerns regarding the true accuracy of these techniques has restricted their clinical application. In this review we will discuss the different noninvasive methods that are currently available for the evaluation of liver fibrosis in chronic hepatitis C, their advantages, limitations and application in clinical practice.
INTRODUCTION
Assessment of liver fibrosis in chronic hepatitis C virus (HCV) infection is considered a relevant part of patient care and key for decision making. Liver fibrosis stage is probably the most robust prognostic factor in several liver diseases; including hepatitis C. Higher stages of fibrosis have been shown to be associated with progression to decompensated cirrhosis, the need for liver transplantation and liver-related death in HCV infection[1,2]. In addition, the severity of fibrosis may be used as a selection criterion for antiviral therapy and can indicate the need for further evaluations, such as surveillance for hepatocellular carcinoma (HCC) and esophageal varices screening[3,4]. For many years, liver biopsy has been considered the gold standard for staging liver fibrosis. Histological evaluation also provides information on necroinflammatory activity and other features such as steatosis and iron overload. Several scoring systems have been developed, the most common being the METAVIR, Scheuer’s, the Batts-Ludwig, the International Association for the Study of the Liver (IASL) and the Ishak scoring systems[5-9]. However, besides its advantages, liver biopsy is an invasive technique with associated morbidity. Minor complications are relatively common and about one fourth of patients have pain in the right upper quadrant or right shoulder after liver biopsy[10]. Severe complications are infrequent, with significant bleeding rates varying from 0.05% to 5.3% and mortality of less than 0.15% in the largest series[10]. The performance of liver biopsy for fibrosis staging has also been questioned and concerns regarding the possibility of sampling errors and significant intra- and inter-observer variability were raised over the last few years. Since biopsy represents 1/50000 of the liver, the heterogeneity of liver fibrosis in HCV infection and the inadequacy of sample size can cause considerable bias in the assessment of hepatic histology[11-13]. A study which included 124 patients with chronic HCV infection who underwent simultaneous laparoscopy-guided biopsies of the right and left hepatic lobes showed that 33.1% of the subjects had a difference of at least one stage between the two lobes[11]. Similarly, a study on virtual liver biopsy indicated that a non-fragmented specimen of at least 25 mm in length would be necessary to correctly evaluate fibrosis with a semiquantitative score, a goal not always achievable in daily practice[14].
Over the last few years, several noninvasive markers were proposed for liver fibrosis diagnosis in chronic HCV infection. Table 1 summarizes the major advantages and limitations of these methods in relation to liver biopsy. Besides the clear advantage of being noninvasive, a more objective interpretation of test results may overcome the mentioned intra- and inter-observer variability of liver biopsy. In addition, these tests can theoretically offer a more accurate view of fibrogenic events occurring in the entire liver with the advantage of providing frequent fibrosis evaluation without additional risk. However, in general, these tests show low accuracy in discriminating between intermediate stages of fibrosis and may be influenced by several hepatic and extra-hepatic conditions. In this review we will discuss the different noninvasive methods that are currently available for the evaluation of liver fibrosis in chronic hepatitis C, their advantages, limitations and application in clinical practice.
Table 1.
Major advantages and limitations of liver biopsy and noninvasive fibrosis markers
| Liver biopsy | Noninvasive markers | |
| Advantages | ||
| Validated scoring systems | Absence of significant discomfort and risks | |
| Differential diagnosis and associated conditions | Allows frequent re-evaluation | |
| Simultaneous evaluation of necro-inflammation | Objective interpretation | |
| Patient acceptance | ||
| Limitations | ||
| Invasive | Low accuracy to discriminate between intermediate stages of fibrosis | |
| High cost | Nonspecific for the liver (biomarkers) | |
| Sampling errors and intra- and inter-observer variability | Influence of several extra-hepatic factors |
NONINVASIVE MARKERS OF LIVER FIBROSIS
General considerations of noninvasive fibrosis markers
Because fibrosis denotes morphological changes, liver biopsy became the natural gold standard for staging the disease. However, the mentioned limitations of biopsy make it very difficult to interpret the “real” performance of surrogate markers of fibrosis in studies. In the vast majority of studies, the diagnostic performance of noninvasive markers of liver fibrosis was evaluated by calculating the area under the receiver operating characteristic (ROC) curve. The ROC curve is the plot that depicts the trade-off between the sensitivity and 1-specificity across a series of cut-off points when the diagnostic test is a continuously variable[15]. Mehta et al[16] demonstrated that, when a range of accuracies of biopsy and a range of prevalence of fibrosis are taken into account, even in the “best” scenario an area under receiver operating characteristic curve > 0.90 cannot be achieved even for a perfect marker. The perceived limitation in diagnostic accuracy of noninvasive markers of liver fibrosis is probably the major reason why these tests have not been widely adopted in clinical practice.
Noninvasive markers of liver fibrosis can be divided into two groups: serum biomarkers and imaging techniques. These methodologies will be presented separately and a combined approach will also be discussed in this review.
Serum biomarkers
Indirect (or class II) markers of liver fibrosis: This group comprises, in general, routine tests usually combined with other laboratory or clinical parameters in a specific model. The most commonly studied indirect markers in HCV infection include aspartate aminotransferase (AST), alanine aminotransferase (ALT), platelet count, gamma-glutamyltransferase (GGT), bilirubin, haptoglobin, apolipoprotein A1, and alpha-2-macroglobulin. Models that combined indirect markers are usually devised from retrospective studies and, as a rule for noninvasive fibrosis markers, are limited in discriminating between intermediate stages of fibrosis. Table 2 depicts the most common models including indirect markers proposed for fibrosis estimation in hepatitis C.
Table 2.
Selected models including indirect markers of liver fibrosis
| Score (original reference) | Variables | Performance in HCV patients1 |
|||||
| Significant fibrosis (≥ F2) |
Cirrhosis (≥ F4) |
||||||
| Median AUROC | Median sensitivity2 | Median specificity2 | Median AUROC | Median sensitivity2 | Median specificity2 | ||
| APRI[24] | AST and platelet count | 0.77 | 81% | 95% | 0.84 | 77% | 94% |
| FIB-43[41] | Age, AST, ALT and platelet count | 0.74 | 64% | 79% | 0.87 | 90% | 92% |
| Forns index[48] | Age, platelet count, cholesterol levels, and GGT | 0.76 | 88% | 94% | 0.87 | 98% | 91% |
| Fibro index[50] | Platelet count, AST, and gamma globulin | 0.76 | 94% | 97% | 0.86 | 70% | 91% |
Based on reference number 49;
Sensitivity values are presented for the lower cutoff and specificity for the upper cutoff (when multiple cutoffs are presented); 3Model originally proposed for HIV/HCV co-infected patients. HCV: Hepatitis C virus; AUROC: Area under the receiver operating characteristic curve; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; GGT: Gamma-glutamyltransferase; HIV: Human immunodeficiency virus.
The AST/ALT ratio has been used for several years as a noninvasive method for assessing the severity of chronic liver diseases, including chronic HCV infection[17-20]. Although some studies have found promising results, its performance as a noninvasive marker of fibrosis is generally low, especially in the diagnosis of less advanced stages of fibrosis[21,22]. In a recent study, we showed that the AST/ALT ratio had a low diagnostic accuracy in detecting significant fibrosis (AUROC of 0.661) as compared to other simple models, such as APRI (AUROC 0.793) and FIB-4 (AUROC 0.811)[23].
The AST-to-Platelet Ratio Index (APRI) is calculated as (AST/upper limit of normal range)/platelet count (109/L) × 100. This index was originally proposed by Wai et al[24] in 2003 and became one of the most studied noninvasive fibrosis markers in chronic HCV infection. The APRI is based on the rationale that worsening of fibrosis and increasing portal pressure are associated with reduced production of thrombopoietin by hepatocytes, increased platelet sequestration within the spleen and reduced clearance of AST[25-27]. A meta-analysis exploring the performance of this model in HCV infection was published in 2011 and included 40 studies and a total of 8739 subjects[28]. This study showed only a moderate degree of accuracy for APRI in the detection of HCV-related fibrosis. The summary AUROC of the APRI for the diagnosis of significant fibrosis (≥ F2 according to METAVIR), severe fibrosis (≥ F3), and cirrhosis was 0.77, 0.80, and 0.83, respectively[28]. In this meta-analysis, the best cutoff for diagnosing significant fibrosis was 0.7, with a summary sensitivity and specificity of 77% and 72%, respectively. For the detection of cirrhosis, the optimal cutoff was 1.0, with a summary sensitivity and specificity of 76% and 72%, respectively. A threshold of 2.0 exhibited a specificity of 91% for diagnosing cirrhosis, but a low sensitivity of 46%[28]. A major advantage of APRI is that it was validated in special populations, such as HIV/HCV co-infection[28-37], in whom the overall performance seems to be lower than in HCV mono-infected individuals[28], and adjusted cutoffs may increase sensitivity and specificity, as we previously demonstrated[30]. APRI also proved to be a valuable tool in hemodialysis patients with chronic HCV infection. In a study which included 203 subjects, we demonstrated good performance of this model, especially in excluding significant fibrosis, with a negative predictive value (NPV) of 93% for a prevalence of significant fibrosis of 24%[38]. These results were further validated in a cohort of 279 hemodialysis patients[39] and Canbakan et al[40] showed that APRI was superior to FibroTest® in this population.
The FIB-4 is also a noninvasive method for the evaluation of liver fibrosis, based on simple variables such as age, AST, ALT and platelet count. It was initially proposed by researchers in the APRICOT study (AIDS Pegasys Ribavirin International Coinfection Trial) to evaluate the presence of liver fibrosis in HIV/HCV coinfected patients[41]. It was subsequently validated in a large cohort of HCV mono-infected patients in whom values < 1.45 had a NPV of 94.7% to exclude severe fibrosis (F3-F4) with a sensitivity of 74.3%[42]. A FIB-4 value higher than 3.25 had a positive predictive value (PPV) of 82.1% with a specificity of 98.2%[42]. The authors also showed a similar performance between FIB-4 and FibroTest®[42]. Several other studies reported that the FIB-4 index had a variable degree of accuracy in HCV-infected subjects[43-47]. We performed a comparison between FIB-4 and APRI and found similar AUROCs for both models (0.811 vs 0.793)[23]. However, the proportion of biopsies that could have been correctly avoided was substantially higher with FIB-4 than with APRI (63% vs 47%) suggesting that FIB-4 is probably a more useful tool for incorporation into daily practice[23].
The Forns index is based on age and three additional simple tests: platelet count, cholesterol levels, and GGT[48]. In the original study, the AUROC was 0.86 for the estimation group and 0.81 for the validation group in diagnosing significant fibrosis[48]. However, in a recent systematic review, the median AUROC from 22 studies was 0.76 for significant fibrosis and 0.87 for cirrhosis[49]. When evaluating those studies that performed direct comparisons, the Forns index and APRI showed a very similar performance for both significant fibrosis and cirrhosis[49].
The Fibroindex was originally proposed for diagnosis of HCV-related fibrosis and includes the following variables: platelet count, AST, and gamma globulin[50]. In the original study, this model exhibited a higher AUROC (0.83) for diagnosing significant fibrosis as compared to APRI and the Forns index[50]. Fibro index was also correlated significantly with variation in fibrosis stage when a subset of 30 patients who had undergone a liver biopsy twice was evaluated[50]. Nevertheless, in the mentioned systematic review, the median AUROC for significant fibrosis detection was 0.76 and for cirrhosis was 0.86[49]. Direct comparisons showed no superiority of Fibroindex over APRI for both significant fibrosis and cirrhosis detection[49].
Direct (or class I) markers of liver fibrosis: Multiple etiologies of liver disease, including chronic HCV infection, can lead to liver fibrosis through integrated signaling networks that regulate the deposition of extracellular matrix[51]. This sequence of events drives the activation of hepatic stellate cells into a myofibroblast-like phenotype that is contractile, proliferative and fibrogenic[51]. Collagen and other extracellular matrix (ECM) components are deposited as the liver generates a wound-healing response to encapsulate injury[51]. The direct (or class I) markers of liver fibrosis are usually fragments of the liver matrix components produced by hepatic stellate cells during the process of ECM remodeling, usually reflecting the deposition or removal of ECM[52]. The most studied direct markers are hyaluronic acid (HA), YKL-40, laminin, fibronectin, alpha-2-macroglobulin, procollagen type I carboxy terminal peptide (PICP), procollagen type III amino-terminal peptide (PIIINP), N-terminal propeptide of type II collagen, metalloproteinases (MMPs), tissue inhibitors of matrix metalloproteinases (TIMPs) and transforming growth factor-b1 (TGF-b1).
As stated for indirect tests, direct biomarkers are usually combined in composite scores to increase the diagnostic performance (Table 3). It is important to point out that these models are not liver-specific and have limitations, such as low accuracy for intermediate stages of fibrosis and limited availability (patented formulas or tests not routinely performed). Below, we discuss the most studied models combining direct and indirect biomarkers for the diagnosis of liver fibrosis in chronic hepatitis C.
Table 3.
Most studied models combining direct and indirect biomarkers for the diagnosis of liver fibrosis in chronic hepatitis C
| Score (original reference) | Variables | Performance in HCV patients1 |
|||||
| Significant fibrosis (≥ F2) |
Cirrhosis (≥ F4) |
||||||
| Median AUROC | Median sensitivity2 | Median specificity2 | Median AUROC | Median sensitivity2 | Median specificity2 | ||
| FibroTest[53] | Age, sex, serum haptoglobin, α2-macroglobulin, apolipoprotein A1, GGT, and total bilirubin | 0.79 | 92% | 96% | 0.86 | 85% | 81% |
| ELF[56] | Age, TIMP-1, PIIINP and hyaluronic acid | 0.81 | 85% | 70% | 0.88 | - | - |
| Fibrometer[61] | Platelet count, prothrombin index, AST, α2-macroglobulin, hyaluronic acid, blood urea nitrogen and age | 0.82 | 69% | 81% | 0.91 | - | - |
| FIBROspect II[63] | TIMP-1, α2-macroglobulin and hyaluronic acid | 0.86 | 80% | 70% | - | - | - |
| Hepascore[66] | Age, sex, α2-macroglobulin, hyaluronic acid, GGT and total bilirubin | 0.79 | 66% | 79% | 0.89 | 72% | 86% |
Based on reference number 49;
Sensitivity values are presented for the lower cutoff and specificity for the upper cutoff (when multiple cutoffs are presented). ELF: Enhanced liver fibrosis; HCV: Hepatitis C virus; AUROC: Area under the receiver operating characteristic curve; GGT: Gamma-glutamyltransferase; TIMP: Tissue inhibitor of matrix metalloproteinases; PIIINP: Procollagen type III amino-terminal peptide; AST: Aspartate aminotransferase.
The FibroTest® score was originally proposed in 2001 and is one of the most validated models for predicting liver fibrosis in HCV-infected patients[53]. It is computed by accessing a proprietary website and entering the patient’s age, sex, and serum haptoglobin, α2-macroglobulin, apolipoprotein A1, GGT, and total bilirubin. A meta-analysis published in 2007 showed a pooled adjusted AUROC of 0.83 for the FibroTest® in HCV patients[54]. However, the systematic review published by Chou and Wasson[49] revealed that when only studies that performed direct comparisons were considered, the APRI was associated with only a slightly lower AUROC than the FibroTest® for significant fibrosis (median difference between AUROCs, -0.03; range, -0.10 to 0.07), but there was no difference for cirrhosis (median difference between AUROCs, 0.0; range, -0.04 to 0.06). In addition, the FibroTest® was not superior to FIB-4 and other models such as the Fibrometer® and Hepascore® for the diagnosis HCV-related fibrosis, as shown in the same review[49]. Beyond the absence of a clear superiority of the FibroTest® over other free and readily available models, and given the variability of components of assays and analyzers, FibroTest® assays can only be performed in validated laboratories. In addition, the existence of hemolysis or Gilbert syndrome can lead to false-positive results and must be taken into account[55].
The enhanced liver fibrosis (ELF) score provides a single value by an algorithm combining age as well as quantitative serum measurements of TIMP-1, PIIINP and HA[56]. Age was removed from the simplified ELF score[56]. It was originally proposed in a cohort study including 1021 subjects with various chronic liver diseases (496 with chronic hepatitis C). In contrast to the good performance observed for alcoholic liver disease (AUROC 0.944) and non-alcoholic fatty liver disease (AUROC 0.870), a relatively low accuracy was observed in chronic HCV infection (AUROC 0.773)[56]. ELF was further validated in HCV patients[57-59], but it was not superior to APRI for the detection of both significant fibrosis and cirrhosis in studies that performed a direct comparison between the two models[49,58]. A recent study has shown that the ELF score is significantly influenced by gender (higher values in men) and age (higher scores in older persons), and this should be taken into account when interpreting the results of this scoring system[60].
The Fibrometer® is a patented and commercially available proprietary panel of tests that combines platelet count, prothrombin index, AST, α2-macroglobulin, HA, blood urea nitrogen and age[61]. In addition to the typical results of fibrosis stage corresponding to the METAVIR system, the Fibrometer® may also indicate the amount of liver fibrosis as a percentage of fibrous tissue within the liver (area of fibrosis)[62]. In the systematic review published by Chou and Wasson, the Fibrometer® exhibited a pooled AUROC of 0.82 for significant fibrosis and 0.91 for cirrhosis[49]. Direct comparisons showed that the Fibrometer® performed better than APRI and the FibroTest® in detecting both significant fibrosis and cirrhosis[43,49].
FIBROspect II® is also a commercially available panel that includes TIMP-1, α2-macroglobulin and HA levels[63]. It was generated from a cohort of 696 HCV-infected patients and exhibited an AUROC of 0.831 for diagnosing significant fibrosis in the original study[63]. FIBROspect II® was subsequently validated[64,65] and the pooled AUROC in the mentioned systematic review was 0.86 for significant fibrosis[49].
The Hepascore® is a patented model that combines age, sex and four biomarkers (α2-macroglobulin, HA, GGT and total bilirubin)[66]. It was devised in a cohort of chronic HCV patients[66] and further validated in several studies[67-72]. An interesting advantage of Hepascore® is that it can be totally automated using a single analyzer and only one serum sample[69]. However, data from comparative studies showed that this test was not superior to APRI and FibroTest® in diagnosing significant fibrosis and only slightly better for the detection of cirrhosis[49].
Imaging techniques
Transient hepatic elastography (TE) by FibroScan®: TE, as assessed by FibroScan® (Echosens, Paris, France), is a simple non-invasive method to measure liver stiffness, based on unidimensional transient elastography, a technique that uses elastic waves and low frequency ultrasounds (50 Hz). The equipment is composed of a probe, an in-built ultrasound system and an electronic unit for data processing. Through a transductor, low amplitude vibrations produced by the probe are transmitted to liver tissue. Simultaneously, the ultrasound system generates pulses that track and determine the rapidity of propagation of elastic waves within the parenchyma. The velocity of propagation is directly related to elasticity: the harder the tissue, the faster the propagation of elastic waves. Hence, tests with high results generally indicate the presence of significant fibrosis in liver parenchyma. The final result is the median of all valid acquisitions, which is considered to be representative of the hepatic elasticity, expressed in kilopascals (kPa), within a range of 2.5-75.0 kPa. The test is simple, fast (usually performed in less than 5 min), and can be easily carried out in both inpatient and outpatient settings. Neither special preparation nor laboratory tests are necessary.
TE has been evaluated in several non-viral chronic liver diseases[73-75] in both adults and children[76], it was, initially projected and then validated in patients with chronic hepatitis C[77-81].
Several studies have shown a significant correlation between TE and fibrosis stage, as assessed by the METAVIR scoring system[78-80], as well as by computer-assisted morphometric image analysis[81]. TE by the FibroScan® shows a similar performance for predicting significant fibrosis and higher accuracy to identify liver cirrhosis, as compared to other noninvasive tests. This has been shown by Castera et al[78] who evaluated and compared the performance of FibroScan®, FibroTest® (FT, Biopredictive, Paris, France) and the AST-to-platelet ratio index (APRI) in 183 HCV patients. AUROCs of FibroScan®, FibroTest® and APRI for the diagnosis of significant fibrosis (F ≥ 2) were 0.83, 0.85 and 0.78, respectively. To predict the presence of cirrhosis (F4), the AUROCs for each method were 0.95, 0.87 and 0.83, respectively. The diagnostic superiority of TE was further confirmed in a study of 298 HCV patients by comparing the performance of FibroScan®, FT, APRI, Lok index, platelet count (PC), prothrombin index (PI) and AST/ALT ratio (AAR) for the early detection of cirrhosis. In this study, TE was the most accurate method for predicting cirrhosis (AUROCs: TE 0.96 vs FT 0.82, Lok and APRI 0.80, PC 0.79, PI 0.73, AAR 0.61, P < 0.0001)[82].
The most validated TE cutoff points are 7.1 kPa to identify patients with significant fibrosis and 12.5 kPa to recognize those with cirrhosis[78,82,83]. TE values ≥ 14.6 kPa exhibit a positive predictive value of 90% to predict liver cirrhosis, with a positive likelihood ratio of 35.5[82].
TE was also evaluated in special populations of HCV patients, such as those with HCV-HIV co-infection[84,85] and post-transplant hepatitis C[86-88], with similar accuracy to that observed in the general population of HCV patients.
Besides estimating fibrosis stage, TE has been used to identify cirrhotic patients at risk of developing portal hypertension and liver-related complications[82,85,89,90]. However, several cutoff values have been used and many of these studies have found weak correlation coefficients, particularly among patients with high HVPG (> 10 mmHg). Altogether, these data indicate that the relationship between TE and HVPG is insufficiently linear to be clinically useful.
In contrast to HCV, few studies with appropriate methodology have evaluated the accuracy of TE in patients with chronic hepatitis B virus (HBV) infection[83,91-94]. An algorithm has been proposed to adjust the interpretation of TE values according to ALT levels. Values < 6.0 kPa and < 7.5 kPa accurately predict the absence of advanced fibrosis or cirrhosis in patients with serum ALT levels inferior to the upper limit of normality (ULN) and in subjects with ALT activity between 1 and 5 times the ULN, respectively[93]. Similarly, TE values > 9.0 kPa and > 12.0 kPa predict advanced fibrosis or cirrhosis in subjects with normal ALT and in those with ALT 1-5 times the ULN, respectively. A recent study suggested that TE exhibits a similar diagnostic performance in HBV infection as compared to HCV patients[83].
Acoustic radiation force impulse imaging: Although the acoustic radiation force impulse imaging technique has been developed by two companies, Siemens and Philips, most clinical studies have used the Siemens S2000 conventional ultrasound equipment which uses short-duration acoustic pulses (push-pulses) emitted with a frequency around 2.6 MHz. The compression induced by the pulse in the evaluated tissue generates shear waves which propagate perpendicularly into the tissue. These shear waves are tracked by the pulse-echo ultrasound acquisitions and their velocity of propagation is measured inside a small region-of-interest (ROI) of 5 mm × 10 mm located up to 8 cm in depth. The stiffer the tissue, the faster the shear wave velocity, which means that the speed of the shear wave increases with the severity of liver fibrosis[95-97]. The results are expressed in meters per second (m/s), ranging from 0.5 to 4.4 m/s (± 20% accuracy over the range). Higher values are generally found in the left hepatic lobe, but higher accuracy is obtained with ARFI measurements in the right hepatic lobe[98-100]. Although not formally recommended by the manufacturer, good quality technical parameters (especially an interquartile ratio < 30%) yielded better correlations between elastometry measurements and liver fibrosis, as well as higher accuracies for predicting fibrosis stages than those with inadequate parameters[101,102]. Evaluating 106 subjects with HCV infection, Bota et al[101] observed discordance of at least two stages of fibrosis between ARFI results and histological assessment in 31.7% of patients; in multivariate analysis, female gender and IQR ≥ 30% were associated with discordance. Finally, as observed with TE, high aminotransferase levels (> 5 times the upper limit of normal) are associated with higher liver stiffness as assessed by ARFI, which should be taken into account in order to interpret results adequately[103]. It is intuitive to consider that the impact of higher aminotransferase levels reflects the influence of higher levels of necroinflammatory activity, which has indeed been demonstrated by Chen et al[104].
ARFI elastography is an easy, fast (usually performed within five minutes), and reproducible noninvasive method for liver fibrosis assessment, especially in cirrhotic patients. However, ARFI reproducibility was lower in women, in patients with high BMI (≥ 25 kg/m2), in the presence of ascites and in the absence of liver cirrhosis[105]. Being included into a conventional ultrasound machine and the possibility of being performed in patients with ascites are relevant advantages of ARFI elastography over TE by FibroScan®. A standard procedure should include measuring in a supine position with the convex transducer (4C1) without specific breathing maneuvers (the patient is simply asked to stop breathing for a moment). Elastometry values increase after food intake, and measurements should be performed in the fasting state (or at least 3 h after the last meal)[106,107].
Although in a limited number of studies, the method has been evaluated in a variety of liver conditions, such as metabolic liver diseases[108-112], autoimmune liver diseases[113], hepatic tumors[114,115], and chronic hepatitis B[116,117]. However, like TE, ARFI has been most studied in patients with chronic HCV infection[118-128]. In a pooled meta-analysis, Friedrich-Rust et al[129] evaluated the original data of 518 patients from eight studies (73% with HCV). The optimal cut-offs for diagnosing significant fibrosis (F ≥ 2), advanced fibrosis (F ≥ 3) and cirrhosis were 1.34, 1.55, and 1.80 m/s, respectively, with diagnostic accuracies (represented by AUROCs) of 0.87, 0.91, and 0.93, respectively. In a large, international multicenter study, Sporea et al[119] retrospectively evaluated 914 HCV subjects. They observed a significant positive correlation between liver stiffness by ARFI and fibrosis stage (Spearman r = 0.654; P < 0.0001), and a good diagnostic performance for predicting fibrosis stage according to the METAVIR score. Although with significant overlapping of ARFI measurements for fibrosis F0-F2, advanced fibrosis (F ≥ 3) and cirrhosis could be accurately excluded. Generally, the correlation with histological fibrosis was similar between TE and ARFI elastography. Nevertheless, TE was better than ARFI for predicting the presence of liver cirrhosis and fibrosis (F ≥ 1)[119]. A recent meta-analysis which included 13 studies and 1163 patients with different hepatopathies demonstrated that for the detection of both significant fibrosis (F ≥ 2) and cirrhosis, the diagnostic performance of ARFI and TE were comparable, but ARFI showed a higher rate of reliable measurements as compared to TE[130].
Combined approach
Algorithms combining different fibrosis tests have been proposed to improve the accuracy of noninvasive methods for the correct diagnosis of liver fibrosis in HCV infection. They use two serum-based models either simultaneously or in a sequential procedure[72,131,132]. They may also be based on agreement between a blood test and an imaging technique[133,134]. The Leroy algorithm was proposed in a study that evaluated six non-invasive scores in 180 HCV patients[72]. In this approach, the APRI and FibroTest® were calculated simultaneously and concordant results below the lower cutoffs (FibroTest® < 0.22 and APRI < 0.5) could rule out significant fibrosis with a NPV of 94.1%. Results above the upper cutoffs (FibroTest® > 0.59 and APRI > 2) exhibited a PPV for significant fibrosis of 96.7% and for extensive fibrosis (F3-F4) of 92.2%[72]. However, only 32% of patients presented concordant results. Therefore, a significant proportion of subjects are expected to require a liver biopsy when using this system[72]. The sequential algorithm (SAFE for fibrosis evaluation) biopsy algorithm was proposed in a study that included 2035 HCV patients[131]. The SAFE biopsy for simultaneous detection of significant fibrosis and cirrhosis produced only 52 (2.6%) misclassified cases, with an overall accuracy of 97.4%. However, as stated for the Leroy algorithm, liver biopsy would be required in the majority of cases (64%)[131].
A different approach was proposed by Castéra et al[133] in an algorithm combining FibroTest® and TE simultaneously. In this method (called the Castera algorithm or Bordeaux algorithm), the diagnosis of significant fibrosis is based in the finding of concordant results for both methods, and liver biopsy is recommended in discordant results or for those individuals in whom liver stiffness measurement was not possible[133]. For the diagnosis of significant fibrosis, the number of saved liver biopsies was significantly higher using Castera than the SAFE biopsy algorithm (71.9% vs 48.3%, respectively). However, accuracy of the SAFE biopsy algorithm was significantly higher than the Castera algorithm (97.0% vs 87.7%, respectively).
More recently, a combination of Fibrometer® and FibroScan® was proposed in a study including 1785 patients with chronic hepatitis C[134]. The so-called FM+FS classification includes 6 fibrosis classes (F0/1; F1/2; F2±1; F2/3; F3±1 and F4) and requires no liver biopsy[134]. Using this approach, the proportion of discordant results decreased as compared to the SAFE biopsy and Castera algorithm. Although this new classification of liver fibrosis appears to be more a formalization of uncertainty, it may also be an astute tactic for the practical application of noninvasive fibrosis markers, allowing a more precise interpretation of its results. However, the employment of two relatively expensive methods will increase costs and may limit application of the FM+FS classification.
Although algorithms including every previously studied noninvasive marker are obviously not available, based on the above data, a simple and plausible approach would be to simultaneously perform two tests (serum and imaging technique), and reserve liver biopsy for discordant results (Figure 1). We suggest using models based on simple blood tests, such as APRI or FIB-4, as there is no clear evidence for the use of more complex and expensive tests in this setting. Similar methodology was proposed in other review articles[135,136]. Using this system, it is probable that liver biopsy will still be necessary in a significant proportion of patients. However, the number of misclassifications is likely to be low.
Figure 1.
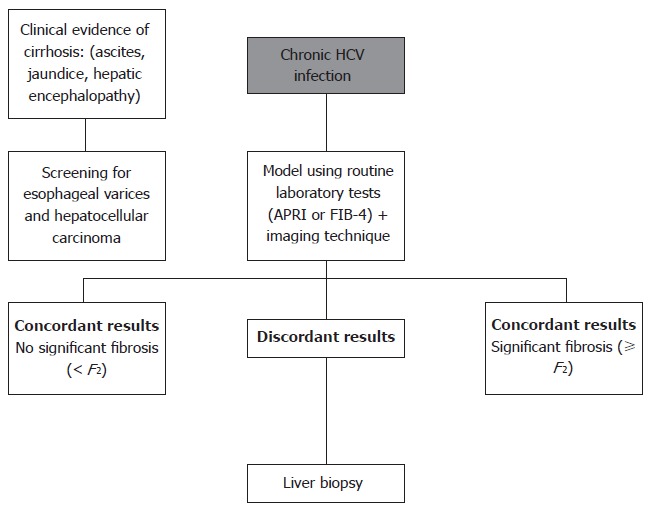
Suggested algorithm for diagnosing liver fibrosis in chronic hepatitis C. Patients with concordant results in two techniques (serum and imaging) may be followed without liver biopsy and histological analysis can be reserved for those with discordant results. HCV: Hepatitis C virus.
COST-EFFECTIVENESS OF NONINVASIVE MARKERS OF FIBROSIS
The total cost of each strategy for diagnosing liver fibrosis depends on several factors such as the need for hospitalization or sedation in the case of liver biopsy, potential risks, the modality of noninvasive tests and finally, the diagnostic accuracy. Although the procedure protocol varies greatly, liver biopsy usually requires short-term hospitalization, the administration of sedatives and specialized nursing staff for post-biopsy care. In addition, ultrasonography is often used to mark or guide the biopsy. These particularities and the need for interpretation are responsible for the relatively high cost of liver biopsy, ranging from $1200 in the United Kingdom[137] to $2200 in the United States[138]. There are limited data regarding the cost-effectiveness of noninvasive strategies for liver fibrosis assessment, especially for indirect markers. However, recent studies evaluating the FibroScan® and FibroTest® showed a favorable cost-effectiveness profile for both noninvasive tests as compared to the traditional approach based on liver biopsy[139-142]. There is a need for studies evaluating the cost-effectiveness of different strategies of noninvasive liver fibrosis assessment, such as the combined approach and the use of indirect markers. This information would be of special interest in low-income countries where the costs of liver biopsy are expected to be lower, but with limited availability.
CONCLUSION
The accurate diagnosis of liver fibrosis is essential for decision-making in chronic hepatitis C. Even though, over the last decade, remarkable achievements have been made in the noninvasive diagnosis of fibrosis, this is an evolving field and there is still room for improvement. It is possible that, in the near future, the incorporation of other methodologies such as genetic, proteomic, and metabolomics profiles will allow the diagnosis of fibrosis at earlier stages, even permitting the identification of stellate cell activation in pre-fibrotic stages. In addition, extensive validation of currently available tools, including the investigation of their prognostic value may extend the applicability of noninvasive fibrosis markers in clinical practice.
Although noninvasive tests are now routinely used in several countries, they are still very limited in differentiating between early stages of fibrosis, and this fact, at least in part, may be related to liver biopsy limitations. Along with the improvement in current noninvasive markers, there is also a need for changes in the way we look at fibrosis in the near future.
Footnotes
P- Reviewers: Mei S, Yoshiji H S- Editor: Qi Y L- Editor: Webster JR E- Editor: Liu XM
References
- 1.Everhart JE, Wright EC, Goodman ZD, Dienstag JL, Hoefs JC, Kleiner DE, Ghany MG, Mills AS, Nash SR, Govindarajan S, et al. Prognostic value of Ishak fibrosis stage: findings from the hepatitis C antiviral long-term treatment against cirrhosis trial. Hepatology. 2010;51:585–594. doi: 10.1002/hep.23315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawson A, Hagan S, Rye K, Taguri N, Ratib S, Zaitoun AM, Neal KR, Ryder SD, Irving WL. The natural history of hepatitis C with severe hepatic fibrosis. J Hepatol. 2007;47:37–45. doi: 10.1016/j.jhep.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 6.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 7.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 9.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 10.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 11.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 12.Siddique I, El-Naga HA, Madda JP, Memon A, Hasan F. Sampling variability on percutaneous liver biopsy in patients with chronic hepatitis C virus infection. Scand J Gastroenterol. 2003;38:427–432. doi: 10.1080/00365520310000825. [DOI] [PubMed] [Google Scholar]
- 13.Scheuer PJ. Liver biopsy size matters in chronic hepatitis: bigger is better. Hepatology. 2003;38:1356–1358. doi: 10.1016/j.hep.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 15.van Erkel AR, Pattynama PM. Receiver operating characteristic (ROC) analysis: basic principles and applications in radiology. Eur J Radiol. 1998;27:88–94. doi: 10.1016/s0720-048x(97)00157-5. [DOI] [PubMed] [Google Scholar]
- 16.Mehta SH, Lau B, Afdhal NH, Thomas DL. Exceeding the limits of liver histology markers. J Hepatol. 2009;50:36–41. doi: 10.1016/j.jhep.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giannini E, Botta F, Fasoli A, Ceppa P, Risso D, Lantieri PB, Celle G, Testa R. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci. 1999;44:1249–1253. doi: 10.1023/a:1026609231094. [DOI] [PubMed] [Google Scholar]
- 18.Giannini E, Risso D, Botta F, Chiarbonello B, Fasoli A, Malfatti F, Romagnoli P, Testa E, Ceppa P, Testa R. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003;163:218–224. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- 19.Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93:44–48. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- 20.Williams AL, Hoofnagle JH. Ratio of serum aspartate to alanine aminotransferase in chronic hepatitis. Relationship to cirrhosis. Gastroenterology. 1988;95:734–739. doi: 10.1016/s0016-5085(88)80022-2. [DOI] [PubMed] [Google Scholar]
- 21.Parise ER, Oliveira AC, Figueiredo-Mendes C, Lanzoni V, Martins J, Nader H, Ferraz ML. Noninvasive serum markers in the diagnosis of structural liver damage in chronic hepatitis C virus infection. Liver Int. 2006;26:1095–1099. doi: 10.1111/j.1478-3231.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- 22.Shaikh S, Memon MS, Ghani H, Baloch GH, Jaffery M, Shaikh K. Validation of three non-invasive markers in assessing the severity of liver fibrosis in chronic hepatitis C. J Coll Physicians Surg Pak. 2009;19:478–482. [PubMed] [Google Scholar]
- 23.Amorim TG, Staub GJ, Lazzarotto C, Silva AP, Manes J, Ferronato Mda G, Shiozawa MB, Narciso-Schiavon JL, Dantas-Correa EB, Schiavon Lde L. Validation and comparison of simple noninvasive models for the prediction of liver fibrosis in chronic hepatitis C. Ann Hepatol. 2012;11:855–861. [PubMed] [Google Scholar]
- 24.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 25.Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of “hypersplenic” thrombocytopenia. J Clin Invest. 1966;45:645–657. doi: 10.1172/JCI105380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamimoto Y, Horiuchi S, Tanase S, Morino Y. Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hepatology. 1985;5:367–375. doi: 10.1002/hep.1840050305. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki T, Takeshita A, Souda K, Kobayashi Y, Kikuyama M, Suzuki F, Kageyama F, Sasada Y, Shimizu E, Murohisa G, et al. Serum thrombopoietin levels in patients with chronic hepatitis and liver cirrhosis. Am J Gastroenterol. 1999;94:1918–1922. doi: 10.1111/j.1572-0241.1999.01231.x. [DOI] [PubMed] [Google Scholar]
- 28.Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, Zhan SH, Sun Y, Xuan SY. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53:726–736. doi: 10.1002/hep.24105. [DOI] [PubMed] [Google Scholar]
- 29.Singal AG, Thomassen LV, Gretch DR, Shuhart MC. Use of the AST to platelet ratio index in HCV/HIV co-infected patients. Aliment Pharmacol Ther. 2011;33:566–577. doi: 10.1111/j.1365-2036.2010.04560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carvalho-Filho RJ, Schiavon LL, Narciso-Schiavon JL, Sampaio JP, Lanzoni VP, Ferraz ML, Silva AE. Optimized cutoffs improve performance of the aspartate aminotransferase to platelet ratio index for predicting significant liver fibrosis in human immunodeficiency virus/hepatitis C virus co-infection. Liver Int. 2008;28:486–493. doi: 10.1111/j.1478-3231.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- 31.Al-Mohri H, Cooper C, Murphy T, Klein MB. Validation of a simple model for predicting liver fibrosis in HIV/hepatitis C virus-coinfected patients. HIV Med. 2005;6:375–378. doi: 10.1111/j.1468-1293.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 32.Loko MA, Castera L, Dabis F, Le Bail B, Winnock M, Coureau G, Bioulac-Sage P, de Ledinghen V, Neau D. Validation and comparison of simple noninvasive indexes for predicting liver fibrosis in HIV-HCV-coinfected patients: ANRS CO3 Aquitaine cohort. Am J Gastroenterol. 2008;103:1973–1980. doi: 10.1111/j.1572-0241.2008.01954.x. [DOI] [PubMed] [Google Scholar]
- 33.Kelleher TB, Mehta SH, Bhaskar R, Sulkowski M, Astemborski J, Thomas DL, Moore RE, Afdhal NH. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol. 2005;43:78–84. doi: 10.1016/j.jhep.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Macías J, Girón-González JA, González-Serrano M, Merino D, Cano P, Mira JA, Arizcorreta-Yarza A, Ruíz-Morales J, Lomas-Cabeza JM, García-García JA, et al. Prediction of liver fibrosis in human immunodeficiency virus/hepatitis C virus coinfected patients by simple non-invasive indexes. Gut. 2006;55:409–414. doi: 10.1136/gut.2005.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunes D, Fleming C, Offner G, O’Brien M, Tumilty S, Fix O, Heeren T, Koziel M, Graham C, Craven DE, et al. HIV infection does not affect the performance of noninvasive markers of fibrosis for the diagnosis of hepatitis C virus-related liver disease. J Acquir Immune Defic Syndr. 2005;40:538–544. doi: 10.1097/01.qai.0000184856.31695.bf. [DOI] [PubMed] [Google Scholar]
- 36.Shire NJ, Rao MB, Succop P, Buncher CR, Andersen JA, Butt AA, Chung RT, Sherman KE. Improving noninvasive methods of assessing liver fibrosis in patients with hepatitis C virus/human immunodeficiency virus co-infection. Clin Gastroenterol Hepatol. 2009;7:471–480, 480.e1-2. doi: 10.1016/j.cgh.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trang T, Petersen JR, Snyder N. Non-invasive markers of hepatic fibrosis in patients co-infected with HCV and HIV: comparison of the APRI and FIB-4 index. Clin Chim Acta. 2008;397:51–54. doi: 10.1016/j.cca.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 38.Schiavon LL, Schiavon JL, Filho RJ, Sampaio JP, Lanzoni VP, Silva AE, Ferraz ML. Simple blood tests as noninvasive markers of liver fibrosis in hemodialysis patients with chronic hepatitis C virus infection. Hepatology. 2007;46:307–314. doi: 10.1002/hep.21681. [DOI] [PubMed] [Google Scholar]
- 39.Liu CH, Liang CC, Liu CJ, Hsu SJ, Lin JW, Chen SI, Hung PH, Tsai HB, Lai MY, Chen PJ, et al. The ratio of aminotransferase to platelets is a useful index for predicting hepatic fibrosis in hemodialysis patients with chronic hepatitis C. Kidney Int. 2010;78:103–109. doi: 10.1038/ki.2010.74. [DOI] [PubMed] [Google Scholar]
- 40.Canbakan M, Senturk H, Canbakan B, Toptas T, Tabak O, Ozaras R, Tabak F, Balcı H, Sut N, Ozbay G. Validation of biochemical markers for the prediction of liver fibrosis and necroinflammatory activity in hemodialysis patients with chronic hepatitis C. Nephron Clin Pract. 2011;117:c289–c295. doi: 10.1159/000320751. [DOI] [PubMed] [Google Scholar]
- 41.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 42.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 43.Zarski JP, Sturm N, Guechot J, Paris A, Zafrani ES, Asselah T, Boisson RC, Bosson JL, Guyader D, Renversez JC, et al. Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: the ANRS HCEP-23 study. J Hepatol. 2012;56:55–62. doi: 10.1016/j.jhep.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 44.Hsieh YY, Tung SY, Lee K, Wu CS, Wei KL, Shen CH, Chang TS, Lin YH. Routine blood tests to predict liver fibrosis in chronic hepatitis C. World J Gastroenterol. 2012;18:746–753. doi: 10.3748/wjg.v18.i8.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahmad W, Ijaz B, Javed FT, Gull S, Kausar H, Sarwar MT, Asad S, Shahid I, Sumrin A, Khaliq S, et al. A comparison of four fibrosis indexes in chronic HCV: development of new fibrosis-cirrhosis index (FCI) BMC Gastroenterol. 2011;11:44. doi: 10.1186/1471-230X-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Güzelbulut F, Çetınkaya ZA, Sezıklı M, Yaşar B, Ozkara S, Övünç AO. AST-platelet ratio index, Forns index and FIB-4 in the prediction of significant fibrosis and cirrhosis in patients with chronic hepatitis C. Turk J Gastroenterol. 2011;22:279–285. doi: 10.4318/tjg.2011.0213. [DOI] [PubMed] [Google Scholar]
- 47.Schiavon LL, Filho RJ, Narciso JL, Sampaio JP, Lanzoni VP, Ferraz ML, Silva AE. Expanding the applicability of noninvasive fibrosis markers in HIV/HCV co-infected patients. Hepatology. 2007;45:257–258. doi: 10.1002/hep.21507. [DOI] [PubMed] [Google Scholar]
- 48.Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 49.Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807–820. doi: 10.7326/0003-4819-158-11-201306040-00005. [DOI] [PubMed] [Google Scholar]
- 50.Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology. 2007;45:297–306. doi: 10.1002/hep.21520. [DOI] [PubMed] [Google Scholar]
- 51.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baranova A, Lal P, Birerdinc A, Younossi ZM. Non-invasive markers for hepatic fibrosis. BMC Gastroenterol. 2011;11:91. doi: 10.1186/1471-230X-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 54.Poynard T, Morra R, Halfon P, Castera L, Ratziu V, Imbert-Bismut F, Naveau S, Thabut D, Lebrec D, Zoulim F, et al. Meta-analyses of FibroTest diagnostic value in chronic liver disease. BMC Gastroenterol. 2007;7:40. doi: 10.1186/1471-230X-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Poynard T, Munteanu M, Imbert-Bismut F, Charlotte F, Thabut D, Le Calvez S, Messous D, Thibault V, Benhamou Y, Moussalli J, et al. Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem. 2004;50:1344–1355. doi: 10.1373/clinchem.2004.032227. [DOI] [PubMed] [Google Scholar]
- 56.Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, Hubscher S, Roskams T, Pinzani M, Arthur MJ. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 57.Catanzaro R, Milazzo M, Arona S, Sapienza C, Vasta D, Arcoria D, Marotta F. Diagnostic accuracy of enhanced liver fibrosis test to assess liver fibrosis in patients with chronic hepatitis C. Hepatobiliary Pancreat Dis Int. 2013;12:500–507. doi: 10.1016/s1499-3872(13)60079-x. [DOI] [PubMed] [Google Scholar]
- 58.Petersen JR, Stevenson HL, Kasturi KS, Naniwadekar A, Parkes J, Cross R, Rosenberg WM, Xiao SY, Snyder N. Evaluation of the Aspartate Aminotransferase/Platelet Ratio Index and Enhanced Liver Fibrosis Tests to Detect Significant Fibrosis Due to Chronic Hepatitis C. J Clin Gastroenterol. 2013:Epub ahead of print. doi: 10.1097/MCG.0b013e3182a87e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guéchot J, Trocmé C, Renversez JC, Sturm N, Zarski JP. Independent validation of the Enhanced Liver Fibrosis (ELF) score in the ANRS HC EP 23 Fibrostar cohort of patients with chronic hepatitis C. Clin Chem Lab Med. 2012;50:693–699. doi: 10.1515/cclm-2011-0858. [DOI] [PubMed] [Google Scholar]
- 60.Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013;59:236–242. doi: 10.1016/j.jhep.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Calès P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konaté A, Gallois Y, Ternisien C, Chevailler A, Lunel F. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373–1381. doi: 10.1002/hep.20935. [DOI] [PubMed] [Google Scholar]
- 62.Calès P, Boursier J, Oberti F, Hubert I, Gallois Y, Rousselet MC, Dib N, Moal V, Macchi L, Chevailler A, et al. FibroMeters: a family of blood tests for liver fibrosis. Gastroenterol Clin Biol. 2008;32:40–51. doi: 10.1016/S0399-8320(08)73992-7. [DOI] [PubMed] [Google Scholar]
- 63.Patel K, Gordon SC, Jacobson I, Hézode C, Oh E, Smith KM, Pawlotsky JM, McHutchison JG. Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J Hepatol. 2004;41:935–942. doi: 10.1016/j.jhep.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Patel K, Nelson DR, Rockey DC, Afdhal NH, Smith KM, Oh E, Hettinger K, Vallée M, Dev A, Smith-Riggs M, et al. Correlation of FIBROSpect II with histologic and morphometric evaluation of liver fibrosis in chronic hepatitis C. Clin Gastroenterol Hepatol. 2008;6:242–247. doi: 10.1016/j.cgh.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Snyder N, Nguyen A, Gajula L, Soloway R, Xiao SY, Lau DT, Petersen J. The APRI may be enhanced by the use of the FIBROSpect II in the estimation of fibrosis in chronic hepatitis C. Clin Chim Acta. 2007;381:119–123. doi: 10.1016/j.cca.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 66.Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, Kench J, Farrell G, McCaughan GW, Jeffrey GP. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867–1873. doi: 10.1373/clinchem.2005.048389. [DOI] [PubMed] [Google Scholar]
- 67.Kalantari H, Hoseini H, Babak A, Yaran M. Validation of hepascore as a predictor of liver fibrosis in patients with chronic hepatitis C infection. Hepat Res Treat. 2011;2011:972759. doi: 10.1155/2011/972759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boursier J, de Ledinghen V, Zarski JP, Rousselet MC, Sturm N, Foucher J, Leroy V, Fouchard-Hubert I, Bertrais S, Gallois Y, et al. A new combination of blood test and fibroscan for accurate non-invasive diagnosis of liver fibrosis stages in chronic hepatitis C. Am J Gastroenterol. 2011;106:1255–1263. doi: 10.1038/ajg.2011.100. [DOI] [PubMed] [Google Scholar]
- 69.Guéchot J, Lasnier E, Sturm N, Paris A, Zarski JP. Automation of the Hepascore and validation as a biochemical index of liver fibrosis in patients with chronic hepatitis C from the ANRS HC EP 23 Fibrostar cohort. Clin Chim Acta. 2010;411:86–91. doi: 10.1016/j.cca.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 70.Becker L, Salameh W, Sferruzza A, Zhang K, ng Chen R, Malik R, Reitz R, Nasser I, Afdhal NH. Validation of hepascore, compared with simple indices of fibrosis, in patients with chronic hepatitis C virus infection in United States. Clin Gastroenterol Hepatol. 2009;7:696–701. doi: 10.1016/j.cgh.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 71.Bourliere M, Penaranda G, Ouzan D, Renou C, Botta-Fridlund D, Tran A, Rosenthal E, Wartelle-Bladou C, Delasalle P, Oules V, et al. Optimized stepwise combination algorithms of non-invasive liver fibrosis scores including Hepascore in hepatitis C virus patients. Aliment Pharmacol Ther. 2008;28:458–467. doi: 10.1111/j.1365-2036.2008.03742.x. [DOI] [PubMed] [Google Scholar]
- 72.Leroy V, Hilleret MN, Sturm N, Trocme C, Renversez JC, Faure P, Morel F, Zarski JP. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol. 2007;46:775–782. doi: 10.1016/j.jhep.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 73.Adhoute X, Foucher J, Laharie D, Terrebonne E, Vergniol J, Castéra L, Lovato B, Chanteloup E, Merrouche W, Couzigou P, et al. Diagnosis of liver fibrosis using FibroScan and other noninvasive methods in patients with hemochromatosis: a prospective study. Gastroenterol Clin Biol. 2008;32:180–187. doi: 10.1016/j.gcb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 74.Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, Nozaki Y, Yonemitsu K, Higurashi T, Takahashi H, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD) Dig Liver Dis. 2008;40:371–378. doi: 10.1016/j.dld.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 75.Corpechot C, El Naggar A, Poujol-Robert A, Ziol M, Wendum D, Chazouillères O, de Lédinghen V, Dhumeaux D, Marcellin P, Beaugrand M, et al. Assessment of biliary fibrosis by transient elastography in patients with PBC and PSC. Hepatology. 2006;43:1118–1124. doi: 10.1002/hep.21151. [DOI] [PubMed] [Google Scholar]
- 76.de Lédinghen V, Le Bail B, Rebouissoux L, Fournier C, Foucher J, Miette V, Castéra L, Sandrin L, Merrouche W, Lavrand F, et al. Liver stiffness measurement in children using FibroScan: feasibility study and comparison with Fibrotest, aspartate transaminase to platelets ratio index, and liver biopsy. J Pediatr Gastroenterol Nutr. 2007;45:443–450. doi: 10.1097/MPG.0b013e31812e56ff. [DOI] [PubMed] [Google Scholar]
- 77.Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 78.Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 79.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 80.Arena U, Vizzutti F, Abraldes JG, Corti G, Stasi C, Moscarella S, Milani S, Lorefice E, Petrarca A, Romanelli RG, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut. 2008;57:1288–1293. doi: 10.1136/gut.2008.149708. [DOI] [PubMed] [Google Scholar]
- 81.Nitta Y, Kawabe N, Hashimoto S, Harata M, Komura N, Kobayashi K, Arima Y, Shimazaki H, Nakano T, Murao M, et al. Liver stiffness measured by transient elastography correlates with fibrosis area in liver biopsy in patients with chronic hepatitis C. Hepatol Res. 2009;39:675–684. doi: 10.1111/j.1872-034X.2009.00500.x. [DOI] [PubMed] [Google Scholar]
- 82.Castéra L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, de Lédinghen V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol. 2009;50:59–68. doi: 10.1016/j.jhep.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 83.Cardoso AC, Carvalho-Filho RJ, Stern C, Dipumpo A, Giuily N, Ripault MP, Asselah T, Boyer N, Lada O, Castelnau C, et al. Direct comparison of diagnostic performance of transient elastography in patients with chronic hepatitis B and chronic hepatitis C. Liver Int. 2012;32:612–621. doi: 10.1111/j.1478-3231.2011.02660.x. [DOI] [PubMed] [Google Scholar]
- 84.de Lédinghen V, Douvin C, Kettaneh A, Ziol M, Roulot D, Marcellin P, Dhumeaux D, Beaugrand M. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175–179. doi: 10.1097/01.qai.0000194238.15831.c7. [DOI] [PubMed] [Google Scholar]
- 85.Vergara S, Macías J, Rivero A, Gutiérrez-Valencia A, González-Serrano M, Merino D, Ríos MJ, García-García JA, Camacho A, López-Cortés L, et al. The use of transient elastometry for assessing liver fibrosis in patients with HIV and hepatitis C virus coinfection. Clin Infect Dis. 2007;45:969–974. doi: 10.1086/521857. [DOI] [PubMed] [Google Scholar]
- 86.Carrión JA, Navasa M, Bosch J, Bruguera M, Gilabert R, Forns X. Transient elastography for diagnosis of advanced fibrosis and portal hypertension in patients with hepatitis C recurrence after liver transplantation. Liver Transpl. 2006;12:1791–1798. doi: 10.1002/lt.20857. [DOI] [PubMed] [Google Scholar]
- 87.Rigamonti C, Donato MF, Fraquelli M, Agnelli F, Ronchi G, Casazza G, Rossi G, Colombo M. Transient elastography predicts fibrosis progression in patients with recurrent hepatitis C after liver transplantation. Gut. 2008;57:821–827. doi: 10.1136/gut.2007.135046. [DOI] [PubMed] [Google Scholar]
- 88.Harada N, Soejima Y, Taketomi A, Yoshizumi T, Ikegami T, Yamashita Y, Itoh S, Kuroda Y, Maehara Y. Assessment of graft fibrosis by transient elastography in patients with recurrent hepatitis C after living donor liver transplantation. Transplantation. 2008;85:69–74. doi: 10.1097/01.tp.0000297248.18483.16. [DOI] [PubMed] [Google Scholar]
- 89.Foucher J, Chanteloup E, Vergniol J, Castéra L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Lédinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403–408. doi: 10.1136/gut.2005.069153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL, et al. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290–1297. doi: 10.1002/hep.21665. [DOI] [PubMed] [Google Scholar]
- 91.Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360–369. doi: 10.1111/j.1365-2893.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 92.Kim SU, Ahn SH, Park JY, Kang W, Kim do Y, Park YN, Chon CY, Han KH. Liver stiffness measurement in combination with noninvasive markers for the improved diagnosis of B-viral liver cirrhosis. J Clin Gastroenterol. 2009;43:267–271. doi: 10.1097/MCG.0b013e31816f212e. [DOI] [PubMed] [Google Scholar]
- 93.Chan HL, Wong GL, Choi PC, Chan AW, Chim AM, Yiu KK, Chan FK, Sung JJ, Wong VW. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16:36–44. doi: 10.1111/j.1365-2893.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 94.Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, de Lédinghen V, Beaugrand M. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int. 2009;29:242–247. doi: 10.1111/j.1478-3231.2008.01802.x. [DOI] [PubMed] [Google Scholar]
- 95.Mauldin FW, Zhu HT, Behler RH, Nichols TC, Gallippi CM. Robust principal component analysis and clustering methods for automated classification of tissue response to ARFI excitation. Ultrasound Med Biol. 2008;34:309–325. doi: 10.1016/j.ultrasmedbio.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nightingale K, Soo MS, Nightingale R, Trahey G. Acoustic radiation force impulse imaging: in vivo demonstration of clinical feasibility. Ultrasound Med Biol. 2002;28:227–235. doi: 10.1016/s0301-5629(01)00499-9. [DOI] [PubMed] [Google Scholar]
- 97.Piscaglia F, Marinelli S, Bota S, Serra C, Venerandi L, Leoni S, Salvatore V. The role of ultrasound elastographic techniques in chronic liver disease: Current status and future perspectives. Eur J Radiol. 2014;83:450–455. doi: 10.1016/j.ejrad.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 98.Piscaglia F, Salvatore V, Di Donato R, D’Onofrio M, Gualandi S, Gallotti A, Peri E, Borghi A, Conti F, Fattovich G, et al. Accuracy of VirtualTouch Acoustic Radiation Force Impulse (ARFI) imaging for the diagnosis of cirrhosis during liver ultrasonography. Ultraschall Med. 2011;32:167–175. doi: 10.1055/s-0029-1245948. [DOI] [PubMed] [Google Scholar]
- 99.Karlas T, Pfrepper C, Wiegand J, Wittekind C, Neuschulz M, Mössner J, Berg T, Tröltzsch M, Keim V. Acoustic radiation force impulse imaging (ARFI) for non-invasive detection of liver fibrosis: examination standards and evaluation of interlobe differences in healthy subjects and chronic liver disease. Scand J Gastroenterol. 2011;46:1458–1467. doi: 10.3109/00365521.2011.610004. [DOI] [PubMed] [Google Scholar]
- 100.Toshima T, Shirabe K, Takeishi K, Motomura T, Mano Y, Uchiyama H, Yoshizumi T, Soejima Y, Taketomi A, Maehara Y. New method for assessing liver fibrosis based on acoustic radiation force impulse: a special reference to the difference between right and left liver. J Gastroenterol. 2011;46:705–711. doi: 10.1007/s00535-010-0365-7. [DOI] [PubMed] [Google Scholar]
- 101.Bota S, Sporea I, Sirli R, Popescu A, Dănilă M, Sendroiu M. Factors that influence the correlation of acoustic radiation force impulse (ARFI), elastography with liver fibrosis. Med Ultrason. 2011;13:135–140. [PubMed] [Google Scholar]
- 102.Goertz RS, Sturm J, Pfeifer L, Wildner D, Wachter DL, Neurath MF, Strobel D. ARFI cut-off values and significance of standard deviation for liver fibrosis staging in patients with chronic liver disease. Ann Hepatol. 2013;12:935–941. [PubMed] [Google Scholar]
- 103.Bota S, Sporea I, Peck-Radosavljevic M, Sirli R, Tanaka H, Iijima H, Saito H, Ebinuma H, Lupsor M, Badea R, et al. The influence of aminotransferase levels on liver stiffness assessed by Acoustic Radiation Force Impulse Elastography: a retrospective multicentre study. Dig Liver Dis. 2013;45:762–768. doi: 10.1016/j.dld.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 104.Chen SH, Li YF, Lai HC, Kao JT, Peng CY, Chuang PH, Su WP, Chiang IP. Effects of patient factors on noninvasive liver stiffness measurement using acoustic radiation force impulse elastography in patients with chronic hepatitis C. BMC Gastroenterol. 2012;12:105. doi: 10.1186/1471-230X-12-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bota S, Sporea I, Sirli R, Popescu A, Danila M, Costachescu D. Intra- and interoperator reproducibility of acoustic radiation force impulse (ARFI) elastography--preliminary results. Ultrasound Med Biol. 2012;38:1103–1108. doi: 10.1016/j.ultrasmedbio.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 106.Goertz RS, Egger C, Neurath MF, Strobel D. Impact of food intake, ultrasound transducer, breathing maneuvers and body position on acoustic radiation force impulse (ARFI) elastometry of the liver. Ultraschall Med. 2012;33:380–385. doi: 10.1055/s-0032-1312816. [DOI] [PubMed] [Google Scholar]
- 107.Popescu A, Bota S, Sporea I, Sirli R, Danila M, Racean S, Suseanu D, Gradinaru O, Ivascu Siegfried C. The influence of food intake on liver stiffness values assessed by acoustic radiation force impulse elastography-preliminary results. Ultrasound Med Biol. 2013;39:579–584. doi: 10.1016/j.ultrasmedbio.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 108.Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640–647. doi: 10.1148/radiol.10091662. [DOI] [PubMed] [Google Scholar]
- 109.Friedrich-Rust M, Romen D, Vermehren J, Kriener S, Sadet D, Herrmann E, Zeuzem S, Bojunga J. Acoustic radiation force impulse-imaging and transient elastography for non-invasive assessment of liver fibrosis and steatosis in NAFLD. Eur J Radiol. 2012;81:e325–e331. doi: 10.1016/j.ejrad.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 110.Fierbinteanu Braticevici C, Sporea I, Panaitescu E, Tribus L. Value of acoustic radiation force impulse imaging elastography for non-invasive evaluation of patients with nonalcoholic fatty liver disease. Ultrasound Med Biol. 2013;39:1942–1950. doi: 10.1016/j.ultrasmedbio.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 111.Behrens CB, Langholz JH, Eiler J, Jenewein R, Naehrlich L, Fuchs K, Harth S, Krombach GA, Alzen GF. A pilot study of the characterization of hepatic tissue strain in children with cystic-fibrosis-associated liver disease (CFLD) by acoustic radiation force impulse imaging. Pediatr Radiol. 2013;43:552–557. doi: 10.1007/s00247-012-2560-6. [DOI] [PubMed] [Google Scholar]
- 112.Karlas T, Hempel M, Tröltzsch M, Huster D, Günther P, Tenckhoff H, Mössner J, Berg T, Keim V, Wiegand J. Non-invasive evaluation of hepatic manifestation in Wilson disease with transient elastography, ARFI, and different fibrosis scores. Scand J Gastroenterol. 2012;47:1353–1361. doi: 10.3109/00365521.2012.719924. [DOI] [PubMed] [Google Scholar]
- 113.Righi S, Fiorini E, De Molo C, Cipriano V, Cassani F, Muratori L, Lenzi M, Morselli Labate AM, Serra C. ARFI elastography in patients with chronic autoimmune liver diseases: A preliminary study. J Ultrasound. 2012;15:226–231. doi: 10.1016/j.jus.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park H, Park JY, Kim do Y, Ahn SH, Chon CY, Han KH, Kim SU. Characterization of focal liver masses using acoustic radiation force impulse elastography. World J Gastroenterol. 2013;19:219–226. doi: 10.3748/wjg.v19.i2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frulio N, Laumonier H, Carteret T, Laurent C, Maire F, Balabaud C, Bioulac-Sage P, Trillaud H. Evaluation of liver tumors using acoustic radiation force impulse elastography and correlation with histologic data. J Ultrasound Med. 2013;32:121–130. doi: 10.7863/jum.2013.32.1.121. [DOI] [PubMed] [Google Scholar]
- 116.Sporea I, Sirli R, Bota S, Popescu A, Sendroiu M, Jurchis A. Comparative study concerning the value of acoustic radiation force impulse elastography (ARFI) in comparison with transient elastography (TE) for the assessment of liver fibrosis in patients with chronic hepatitis B and C. Ultrasound Med Biol. 2012;38:1310–1316. doi: 10.1016/j.ultrasmedbio.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 117.Friedrich-Rust M, Buggisch P, de Knegt RJ, Dries V, Shi Y, Matschenz K, Schneider MD, Herrmann E, Petersen J, Schulze F, et al. Acoustic radiation force impulse imaging for non-invasive assessment of liver fibrosis in chronic hepatitis B. J Viral Hepat. 2013;20:240–247. doi: 10.1111/j.1365-2893.2012.01646.x. [DOI] [PubMed] [Google Scholar]
- 118.Sporea I, Sirli R, Bota S, Fierbinţeanu-Braticevici C, Petrişor A, Badea R, Lupşor M, Popescu A, Dănilă M. Is ARFI elastography reliable for predicting fibrosis severity in chronic HCV hepatitis? World J Radiol. 2011;3:188–193. doi: 10.4329/wjr.v3.i7.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sporea I, Bota S, Peck-Radosavljevic M, Sirli R, Tanaka H, Iijima H, Badea R, Lupsor M, Fierbinteanu-Braticevici C, Petrisor A, et al. Acoustic Radiation Force Impulse elastography for fibrosis evaluation in patients with chronic hepatitis C: an international multicenter study. Eur J Radiol. 2012;81:4112–4118. doi: 10.1016/j.ejrad.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 120.Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595–604. doi: 10.1148/radiol.2523081928. [DOI] [PubMed] [Google Scholar]
- 121.Rizzo L, Calvaruso V, Cacopardo B, Alessi N, Attanasio M, Petta S, Fatuzzo F, Montineri A, Mazzola A, L’abbate L, et al. Comparison of transient elastography and acoustic radiation force impulse for non-invasive staging of liver fibrosis in patients with chronic hepatitis C. Am J Gastroenterol. 2011;106:2112–2120. doi: 10.1038/ajg.2011.341. [DOI] [PubMed] [Google Scholar]
- 122.Kircheis G, Sagir A, Vogt C, Vom Dahl S, Kubitz R, Häussinger D. Evaluation of acoustic radiation force impulse imaging for determination of liver stiffness using transient elastography as a reference. World J Gastroenterol. 2012;18:1077–1084. doi: 10.3748/wjg.v18.i10.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, Maniu A. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303–310. [PubMed] [Google Scholar]
- 124.Takahashi H, Ono N, Eguchi Y, Eguchi T, Kitajima Y, Kawaguchi Y, Nakashita S, Ozaki I, Mizuta T, Toda S, et al. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30:538–545. doi: 10.1111/j.1478-3231.2009.02130.x. [DOI] [PubMed] [Google Scholar]
- 125.Fierbinteanu-Braticevici C, Andronescu D, Usvat R, Cretoiu D, Baicus C, Marinoschi G. Acoustic radiation force imaging sonoelastography for noninvasive staging of liver fibrosis. World J Gastroenterol. 2009;15:5525–5532. doi: 10.3748/wjg.15.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Goertz RS, Zopf Y, Jugl V, Heide R, Janson C, Strobel D, Bernatik T, Haendl T. Measurement of liver elasticity with acoustic radiation force impulse (ARFI) technology: an alternative noninvasive method for staging liver fibrosis in viral hepatitis. Ultraschall Med. 2010;31:151–155. doi: 10.1055/s-0029-1245244. [DOI] [PubMed] [Google Scholar]
- 127.Haque M, Robinson C, Owen D, Yoshida EM, Harris A. Comparison of acoustic radiation force impulse imaging (ARFI) to liver biopsy histologic scores in the evaluation of chronic liver disease: A pilot study. Ann Hepatol. 2010;9:289–293. [PubMed] [Google Scholar]
- 128.Sporea I, Sirli RL, Deleanu A, Popescu A, Focsa M, Danila M, Tudora A. Acoustic radiation force impulse elastography as compared to transient elastography and liver biopsy in patients with chronic hepatopathies. Ultraschall Med. 2011;32 Suppl 1:S46–S52. doi: 10.1055/s-0029-1245360. [DOI] [PubMed] [Google Scholar]
- 129.Friedrich-Rust M, Nierhoff J, Lupsor M, Sporea I, Fierbinteanu-Braticevici C, Strobel D, Takahashi H, Yoneda M, Suda T, Zeuzem S, et al. Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212–e219. doi: 10.1111/j.1365-2893.2011.01537.x. [DOI] [PubMed] [Google Scholar]
- 130.Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, Peck-Radosavljevic M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138–1147. doi: 10.1111/liv.12240. [DOI] [PubMed] [Google Scholar]
- 131.Sebastiani G, Halfon P, Castera L, Pol S, Thomas DL, Mangia A, Di Marco V, Pirisi M, Voiculescu M, Guido M, et al. SAFE biopsy: a validated method for large-scale staging of liver fibrosis in chronic hepatitis C. Hepatology. 2009;49:1821–1827. doi: 10.1002/hep.22859. [DOI] [PubMed] [Google Scholar]
- 132.Bourliere M, Penaranda G, Renou C, Botta-Fridlund D, Tran A, Portal I, Lecomte L, Castellani P, Rosenthal-Allieri MA, Gerolami R, et al. Validation and comparison of indexes for fibrosis and cirrhosis prediction in chronic hepatitis C patients: proposal for a pragmatic approach classification without liver biopsies. J Viral Hepat. 2006;13:659–670. doi: 10.1111/j.1365-2893.2006.00736.x. [DOI] [PubMed] [Google Scholar]
- 133.Castéra L, Sebastiani G, Le Bail B, de Lédinghen V, Couzigou P, Alberti A. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J Hepatol. 2010;52:191–198. doi: 10.1016/j.jhep.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 134.Boursier J, de Ledinghen V, Zarski JP, Fouchard-Hubert I, Gallois Y, Oberti F, Calès P. Comparison of eight diagnostic algorithms for liver fibrosis in hepatitis C: new algorithms are more precise and entirely noninvasive. Hepatology. 2012;55:58–67. doi: 10.1002/hep.24654. [DOI] [PubMed] [Google Scholar]
- 135.Bhogal H, Sterling RK. Staging of liver disease: which option is right for my patient? Infect Dis Clin North Am. 2012;26:849–861. doi: 10.1016/j.idc.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 136.Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther. 2009;30:557–576. doi: 10.1111/j.1365-2036.2009.04062.x. [DOI] [PubMed] [Google Scholar]
- 137.Chalmers RJ, Kirby B, Smith A, Burrows P, Little R, Horan M, Hextall JM, Smith CH, Klaber M, Rogers S. Replacement of routine liver biopsy by procollagen III aminopeptide for monitoring patients with psoriasis receiving long-term methotrexate: a multicentre audit and health economic analysis. Br J Dermatol. 2005;152:444–450. doi: 10.1111/j.1365-2133.2005.06422.x. [DOI] [PubMed] [Google Scholar]
- 138.Crockett SD, Kaltenbach T, Keeffe EB. Do we still need a liver biopsy? Are the serum fibrosis tests ready for prime time? Clin Liver Dis. 2006;10:513–534, viii. doi: 10.1016/j.cld.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 139.Whitty JA, Tallis C, Nguyen KH, Scuffham PA, Crosland P, Hewson K, Pai Mangalore R, Black M, Holtmann G. Cost and time savings from a rapid access model of care using transient elastography to screen and triage patients with chronic Hepatitis C infection. J Med Econ. 2014;17:159–165. doi: 10.3111/13696998.2013.867271. [DOI] [PubMed] [Google Scholar]
- 140.Canavan C, Eisenburg J, Meng L, Corey K, Hur C. Ultrasound elastography for fibrosis surveillance is cost effective in patients with chronic hepatitis C virus in the UK. Dig Dis Sci. 2013;58:2691–2704. doi: 10.1007/s10620-013-2705-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.García-Jurado L, Oyagüez I, Casado MÁ, Tural C, González-García J, Ortega E, Pineda JA. [Evaluation of the costs of transient elastography (FibroScan(®)) in the diagnosis of liver fibrosis in HIV patients with hepatitis C virus] Enferm Infecc Microbiol Clin. 2012;30:294–299. doi: 10.1016/j.eimc.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 142.Liu S, Schwarzinger M, Carrat F, Goldhaber-Fiebert JD. Cost effectiveness of fibrosis assessment prior to treatment for chronic hepatitis C patients. PLoS One. 2011;6:e26783. doi: 10.1371/journal.pone.0026783. [DOI] [PMC free article] [PubMed] [Google Scholar]


