Abstract
Reference intervals are ideally defined on apparently healthy individuals and should be distinguished from clinical decision limits that are derived from known diseased patients. Knowledge of physiological changes is a prerequisite for understanding and developing reference intervals. Reference intervals may differ for various subpopulations because of differences in their physiology, most obviously between men and women, but also in childhood, pregnancy and the elderly. Changes in laboratory measurements may be due to various physiological factors starting at birth including weaning, the active toddler, immunological learning, puberty, pregnancy, menopause and ageing. The need to partition reference intervals is required when there are significant physiological changes that need to be recognised. It is important that laboratorians are aware of these changes otherwise reference intervals that attempt to cover a widened inter-individual variability may lose their usefulness. It is virtually impossible for any laboratory to directly develop reference intervals for each of the physiological changes that are currently known, however indirect techniques can be used to develop or validate reference intervals in some difficult situations such as those for children. Physiology describes our life’s journey, and it is only when we are familiar with that journey that we can appreciate a pathological departure.
Introduction
Clinical investigation classically begins with gathering symptoms or examining for signs. The purpose is to identify the clinical features that aren’t typically found in healthy people as well as to detect those characteristics associated with a particular illness. Similarly, measurements made by clinical laboratories only have value when they can be compared to the values that are outside the usual spread of values found in health or within the spread of values typically found in disease. It is important these are two distinct questions i.e. ‘Is there evidence that the patient is not healthy?’ or ‘Is there evidence that a patient has a particular disease?’. Consequently we have separated two classes of thresholds that can be used by the clinical laboratory.1–5
The first class of thresholds are reference intervals which describe the typical range of results seen in a healthy reference population. These were historically known as the ‘normal ranges’ but this term has been formally identified as incorrect and superseded according to the international standard for quality in medical laboratories (ISO 15189).6 The second class of thresholds are called clinical decision limits, where values above or below this threshold are considered diagnostic for the presence of a specific disease or are associated with a significant higher risk of the adverse clinical outcome(s). The most obvious example is a fasting glucose ≥7.0 mmol/L or a HbA1c ≥6.5% (48 mmol/mol) being defined as decision limits for the diagnosis of diabetes mellitus based on associated clinical outcomes, including diabetic retinopathy,7–9 although this decision point has been debated.10–12
Reference intervals are defined with a high specificity for health (typically 95% or more) while clinical decision limits also consider sensitivity for disease. Receiver Operator Characteristic (ROC) curves are now widely used to balance the need for sensitivity and specificity. An ‘optimal’ cut-off derived using this technique may be neither a highly specific (95%) reference intervals nor a clinical decision limit based on high sensitivity. Whether ROC derived optimal limits, by balancing sensitivity and specificity, truly represent the best option can be debated according to clinical circumstances which may place higher importance on sensitivity or specificity. Therefore ‘optimal’ limits derived from ROC curves should be considered as an intermediate category of threshold which is neither a highly specific reference interval nor a sensitivity focussed clinical decision limit.
This article will focus on reference intervals, which are more widely used than clinical decision limits or ROC based cutoffs. It will also discuss the physiological aspects that are pre-requisite for defining reference intervals or agreeing on harmonised reference intervals. These physiological aspects are at least as important as the statistical aspects of reference intervals that are best obtained from the CLSI C28-A3 standard.13 This standard represents over 30 years of professional development in the theory and application of reference values largely developed by the International Federation of Clinical Chemistry (IFCC)14–20 which was integral in developing that CLSI standard.
The considerable resources and statistical effort that are required to define reference intervals may be wasted if they don’t consider the underlying clinical purpose of reference intervals. In the current age of personalised medicine, the aim is to understand each patient as an individual. For every investigation our question is ‘What do I expect as a result for this particular patient if he/she is healthy?’
In terms of biological variability theory, when group inter-individual variability (CVg) is much larger than the intra-individual variability (CVi), reference intervals are less useful for judging individual patients.21–25 Ideally the reference interval shouldn’t be much wider than each patient’s expected variations and if the ratio of CVi to CVg (the index of individuality) is below 0.6, reference intervals lose their utility.26 More recently Petersen et al. have showed that the influence of the index of individuality on usefulness of reference intervals is even more important when a second sample is taken to confirm an abnormality.27 Utility can be restored by stratifying (or partitioning) patients into similar groups. As exemplified by Fraser,28 urine creatinine has an index of individuality of 0.46 when viewed as a whole and a urine creatinine reference interval is not as sensitive, for example, when a man’s urine creatinine level falls to the lower values normally seen in women. Separating urine creatinine reference intervals into gender based limits reduces inter-individual variation and improves the index of individuality to above 1.4 (1.42 for women and 1.83 for men), a value that confirms the utility of reference intervals.26
As well as providing some background on the important physiological impacts on laboratory measurement from the literature, this paper will demonstrate that such physiological changes are also evident in the data generated by clinical laboratories and laboratory databases. While laboratory data is affected by results from diseased individuals, indirect techniques for investigating reference intervals can be used according to CLSI C28-A3,13 which states ‘the (indirect) techniques are perhaps more appropriately employed using data from individuals who are relatively healthy’. Furthermore, it is important to note that even known disease generally does not affect all analytes.29 Over the last several years, the Australian laboratories of the Sonic Healthcare pathology network have been involved in a project to harmonise the reference intervals across these Australian laboratories.30–34 The databases consist of a predominantly primary care population which is largely Caucasian, using common analytical techniques (Roche Modular biochemistry and Sysmex haematology). In our deliberations we have found that the changes in the population medians for these investigations reflect the major physiological changes already described in the literature as well as many subtle physiological changes that should also be considered when establishing reference intervals.
Physiology of Gender Based Reference Intervals
There are differences between men and women that cannot be disputed but, in terms of physiology, what are the factors behind those differences? The chromosomal differences between women and men are relatively small (46XX vs 46XY), yet they lead to profound sexual differences including the gonads, genitalia, breasts, hair and muscle. Each of these differences is largely understood at a biochemical level, from the impact of anti-mullerian hormone on the development of genitourinary tract in men,35,36 to the effect of sex steroids on pubertal development.37–41 There are other significant changes in biochemical tests that appear at puberty and are therefore attributable to these hormonal changes (Table 1). Haemoglobin and serum urate show a similar rise at puberty, but only in boys (Figure 1).
Table 1.
Changes in some common analytes at puberty and the sex hormones most likely to have caused the change.
| Analyte | Pubertal Change | Sex Steroid |
|---|---|---|
| Creatine kinase | Boys rise by 50 IU/L | Testosterone |
| Creatinine | Boys rise by 15 μmol/L | Testosterone |
| Albumin | Boys rise by 2 g/L | Testosterone |
| Haemoglobin | Boys rise by 20 g/L | Testosterone |
| Urate | Boys rise by 0.05 mmol/L | Testosterone |
| Cholesterol | Boys fall by 0.4 mmol/L | Testosterone |
| Globulin | Girls rise by 2 g/L | Oestradiol |
| Platelets | Girls rise by 25 × 106/L | Oestradiol |
| Bicarbonate | Girls fall by 1.5 mmol/L | Progesterone |
Figure 1.
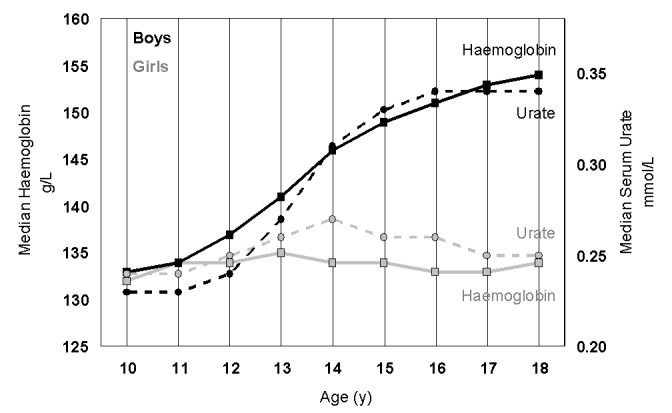
The increase in haemoglobin (squares, full line) and urate (circle, dotted lines) in girls (grey) and boys (black) between the ages of 10 and 18 years. For haemoglobin there were 45,939 girls and 33,361 boys and for urate there were 30,164 girls and 23,444 boys.
Physiology of Childhood
Children are not little adults and reference intervals for any paediatric biomarker should be developed specifically for children and include well-recognised developmental changes.42 While sexual characteristic changes across puberty are profound, the earlier changes during the growth of a child, from birth to puberty are also significant. One of the most important tools in confirming a healthy child is the growth chart.43 The growth represented in a child’s height or weight is not linear and typically has two growth spurts; one in the toddler age group (1 to 3 years) and one at adolescence (age 9 to 13 for girls and 11 to 16 for boys). Serum alkaline phosphatase (ALP) also demonstrates these two peaks, with the age of onset for the adolescent growth peak being earlier in pubertal girls and than pubertal boys (Figure 2).44,45 Other bone markers also showing these differences.46 This understanding is very important in setting paediatric reference intervals for alkaline phosphatase, as elevations of this enzyme can be considered the most common biochemical abnormality in Ricket’s47 and important48 or essential for its diagnosis.49
Figure 2.
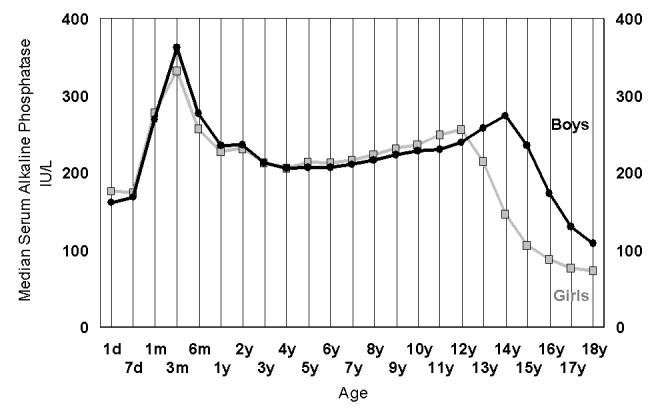
The changes in alkaline phosphatase plotted as the median value for each age group in 42,725 girls (grey) and 38,402 boys (black) derived from a laboratory population of predominantly outpatient children having a multiple biochemical analysis (screening) protocol.
These periods of bone growth also correspond to changes in calcium and phosphate metabolism (Figure 3). The higher calcium and phosphate levels during the ‘toddler’ growth surge has no gender differences, whereas the fall in phosphate levels during adolescence is earlier in girls than boys, mimicking the gender related delay in skeletal growth.50
Figure 3.
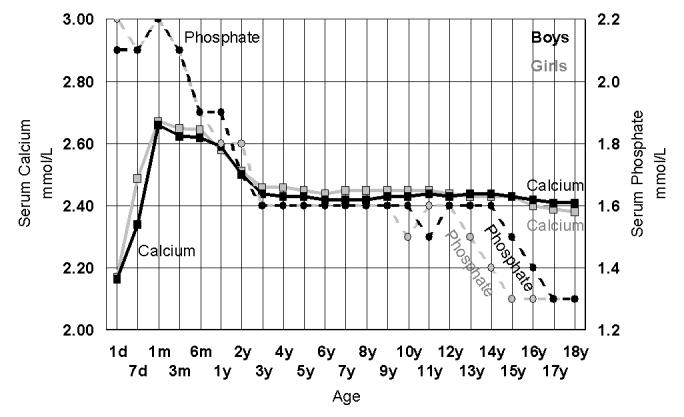
The changes in serum calcium (cresolpthalien complexone) (squares, full line) and serum phosphate (circles, dotted lines) plotted as the median value for each age group in 42,725 girls (grey) and 38,402 boys (black) derived from a laboratory multiple biochemical analysis population of predominantly outpatient children.
Skeletal height is the most obvious measure of childhood growth, but this is also accompanied by changes in muscle mass. Serum creatinine is a marker of muscle mass in children (especially as their renal function is usually intact), and it is interesting to note the changes in serum creatinine in children (Figure 4); the trends show a gender related increase in creatinine that parallels the adolescent rise in haemoglobin. However, we can see that both creatinine and haemoglobin show an almost linear increase from 6 months to 12 years, without any gender differences in that period.51–56 It is important to note that before puberty there aren’t any childhood surges in the rise of creatinine or haemoglobin that may be related to skeletal growth peaks, and therefore the most likely drivers of these changes are increasing physical activity and increased oxygen delivery requirements.
Figure 4.
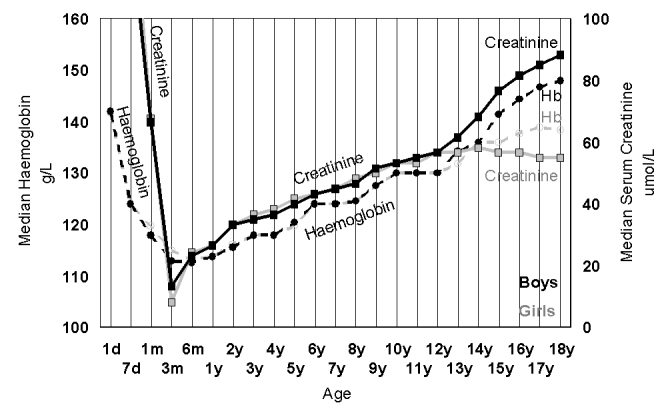
The increase in median creatinine (Roche rate blanked modified Jaffe) (squares, full line) and median haemoglobin (circle, dotted lines) in girls (grey) and boys (black) between the ages of 10 and 18 years. For haemoglobin there were 62,971 girls and 48,289 boys and for urate there were 42,725 girls and 38,391 boys.
It is well known that respiratory rate and heart rate fall during childhood,57,58 therefore how can we reconcile this with increasing physical activity and oxygen demand? One of the answers comes from looking at the bicarbonate changes in childhood (Figure 5). The rising serum bicarbonate in childhood has been described in many studies59–61 but perhaps not fully appreciated as the consequence of a gradual fall in respiratory rate across childhood57,58 and a continuing increase in absolute oxygen requirement. This leads to rising pCO2 across childhood62,63 which will result in rising bicarbonate.64–66 Furthermore, due to the issues of electrolyte charge, the increase in bicarbonate (by 5 to 7 mmol/L) has influence on the more subtle changes in other electrolytes across in childhood such as sodium which rises by 2 to 3 mmol/L (Figure 5) and chloride (not shown in Figure 5) which falls by 2 to 3 mmol/L.67 As these measured ion differences are balanced, they are not the cause of changes in the rising anion gap in childhood (rises in infancy and falls from age 2). This rising anion gap is caused by the changes in ‘unmeasured’ ions, especially rising albumin in infancy and falling phosphate.
Figure 5.
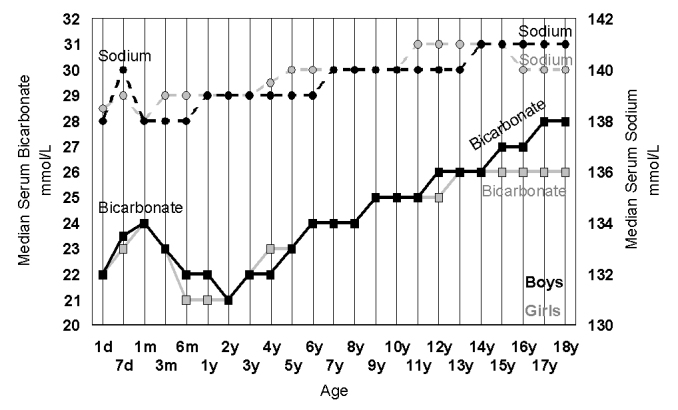
The increase in serum bicarbonate (squares, solid lines) and serum sodium (circles, dotted lines) in 42,725 girls (grey) and 38,391 boys (black) during childhood. The rise in childhood is related to the fall in respiratory rate across childhood and consequent increase in pCO2, the source of serum bicarbonate.
Some metabolic changes in childhood seem to occur at the time of weaning. While digestive changes such as the reduction in lactase and increase in sucrase may be programmed for the expected change in diet,68 metabolic programming may also be affected by nutritional experiences such as formula feeding.69 The gut microbiota has also been implicated in the relationship between diet and metabolism.70 The serum calcium pattern in infants and toddlers60 shown in Figure 3 reflects the understood nutritional importance of milk. It is also interesting to look at the changes in cholesterol and triglycerides (Figure 6). While cholesterol rises at weaning (typically 6 months) and stays high, triglycerides rise at the same time but then fall back by age 5 years.60
Figure 6.
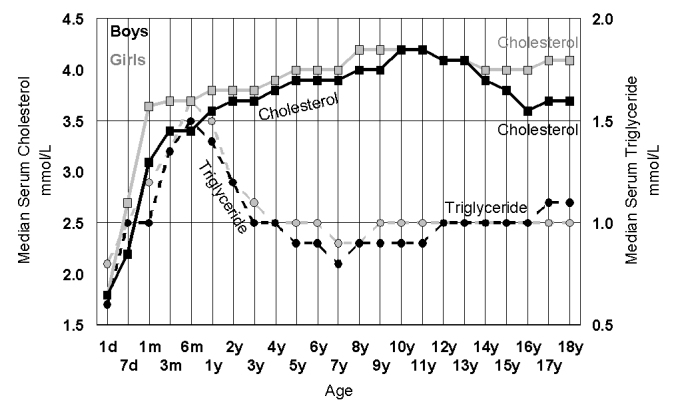
The increase in median values for random serum cholesterol (squares, solid lines) triglycerides (circles, dotted lines) in 42,725 girls (grey) and 38,391 boys (black) during childhood. While both cholesterol and triglyceride rise in infancy, cholesterol stays high while triglycerides fall back by age 3.
Finally, the exposure to different antigens in food as well as different microbes represents one of the most important ‘behind the scenes’ changes in childhood; the training of the immune system. During weaning and in the toddler age group, neutrophils and globulins rise to their plateau at age 5,71 whereas lymphocytes have an enormous peak in infancy and then gradually fall to adult levels (Figure 7).72 The rise in globulins can be shown to be due to the rise in immunoglobulins.73
Figure 7.

The changes in serum globulin (biuret protein – BCG albumin) (squares, solid line), neutrophil count (circles, dotted lines) and lymphocyte counts (stars, dashed line) in girls (grey) and boys (black) during childhood. For serum globulin there were 42,275 girls and 38,391 boys and for neutrophil and lymphocyte count there were 62,971 girls and 48,289 boys.
Physiology of Pregnancy
There are many important hormonal changes in pregnancy that ultimately impact on numerous aspects of physiology, such as the expansion in extracellular fluid volume74 and corresponding increase in renal filtration.75 These fluid changes are largely responsible for the typical falls in the concentration of most serum constituents. There are very few clinical laboratory measurements that rise in normal pregnancy, the most obvious being those related to the rise in oestrogens and progesterone. While we know that the synthetic oestrogens in the oral contraceptive pill increase transferrin levels and that the rise in oestrogens in pregnancy may do the same, most of the transferrin rise in pregnancy is in the third trimester and it is therefore misleading to attribute this solely to oestrogen. Furthermore, it is also know that iron stores are often depleted at this stage of rapid growth.76
The rise in alkaline phosphatase in pregnancy is largely due production of placental alkaline phosphatase.77 While most laboratorians are aware that alkaline phosphatase is higher in pregnant women, during the first and second trimester alkaline phosphatase levels are actually lower than in non-pregnant women and it is mainly in the third trimester that placental growth results in higher alkaline phosphatase levels.78,79 This increase is mimicked by another placental product, Cystatin C,80 which similarly undermines its usual clinical diagnostic role for renal function. An important biochemical measurement in the third trimester of pregnancy is serum urate, as increases are an important risk marker of pre-eclampsia.81 Obstetricians are aware that urate normally rises in the third trimester and risk thresholds change depending on the stage of the third trimester (Figure 8).82
Figure 8.
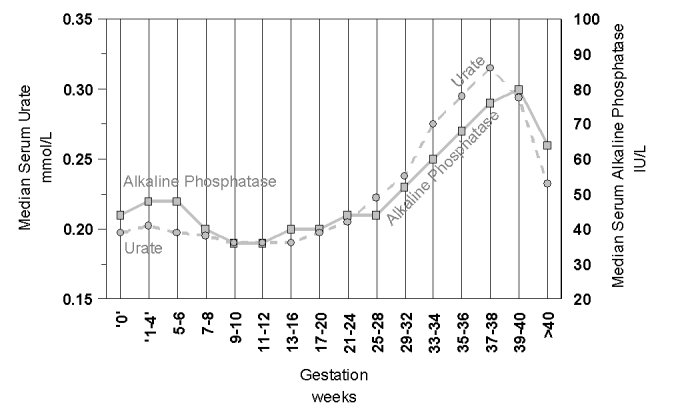
The changes in serum urate (squares, solid line) and alkaline phosphatase (circles, dotted line) in 30,321 pregnant women of varying gestational age.
Figure 5 clearly shows that the median serum bicarbonate level in young women is 26 mmol/L and approximately 2 mmol/L lower than young men (28 mmol/L). This difference is because young women generally have lower pCO2 than men.83 In pregnancy, the pCO2 and bicarbonate levels are even lower,84 with bicarbonate a further 2 mmol/L lower than in non-pregnant women. All these changes are known to be due to the effect of progesterone which increases respiratory activity in pregnancy.85
Physiological Changes in Adults
The most profound physiological transition in adulthood is the menopause. Hormonal changes in the menstrual cycle affect breathing86 and, not surprisingly, the loss of the respiratory stimulation by oestrogen and progesterone ‘allows’ postmenopausal women to respire at a similar rate to men. Postmenopausal women adapt to the higher levels of pCO287,88 unless hormone (especially progesterone) replacement therapy is given.89 Serum bicarbonate levels correspondingly rise by 1 or 2 mmol/L in postmenopausal women. Whilst such subtle changes in bicarbonate are seldom of any clinical concern, the physiological importance of this menopausal change could be clinically important, since a rise in bicarbonate results in an increase in complexed calcium90 and will increase the filtered renal load of calcium91 which, combined with a decrease in renal calcium reabsorption at menopause,92 can result in increased renal calcium loss at the menopause. Inevitably this will have to be replaced by diet or resorbed from bone.93,94 The changes in median bicarbonate, calcium and alkaline phosphatase in serum shown in Figure 9 seem to be directly related to menopause and these physiological changes deserve much closer attention when we are trying to understand the physiology of the menopause and reference intervals across the climacteric.
Figure 9.
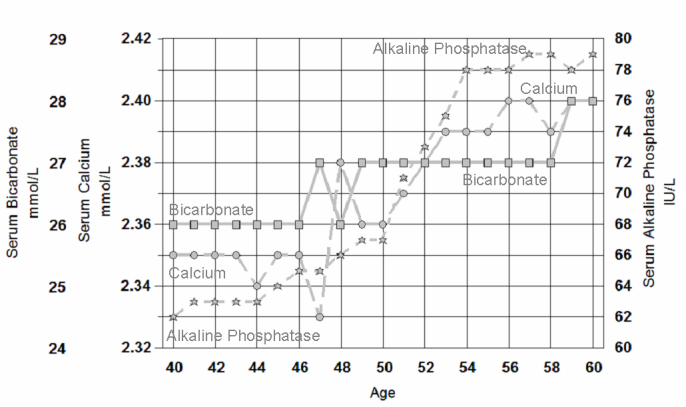
The changes in median serum bicarbonate (squares, solid line), median serum calcium (cresolpthalein complexone) (circles, dashed line) and median serum alkaline phosphatase (stars, dotted lines) in 74,032 women from 40 to 60 years of age.
None of these menopausal changes has a corresponding change in men. However men do have a gradual loss of the gender related differences that appeared at puberty. The gradual age-related decline in haemoglobin is much more obvious in men and can be related to the decline in testosterone levels with age.95,96
Some may argue that any decline in function with human ageing is due to the accumulation of pathology rather than a physiological phenomenon.97 As much as investigators have tried to understand ageing with concepts such as ‘inflammaging’,98 telomere shortening,99 oxidative damage,100 or hormonal deficiency and metabolic decline,101 the explanation of ageing remains elusive. It is possible that human design has a built in ‘expiry date’102 (or at least a ‘best before date’). Studies in the healthiest elderly show significant changes including an age related decline in respiratory function (e.g. falling pO2),103–105 renal function (falling eGFR)106–108 and cardiac status (rising high sensitivity troponin levels).109–113 Many of the non-hormonal changes that occur with ageing are subtle compared to the hormonal changes such as for dehydroepiandrosterone sulphate (DHEAS).114 Whether these deteriorations represent normal ageing or the accumulation of pathology may be ‘academic’, because either way they may represent the known increasing health risk in the elderly. Age related reference intervals have the effect of ‘normalising’ physiological decline through maintaining age related specificity (i.e. 95%), but this necessarily also results in a decrease in sensitivity for disease and the associated mortality risks of ageing.
The Challenge of Partitioning
The partitioning of reference intervals into separate subclasses according to age, gender, ethnicity or ‘other’ is advisable when a clinical foundation or a logical physiological basis exists.115 Partitioning is a valuable tool for enhancing the diagnostic power of reference intervals.116 The partitioning of reference intervals is important but may also be the most complicated part of defining reference intervals. The partitioning into male and female seems the easiest step, but when should this be done? If the answer is where there is a significant difference between genders, what do we mean by significant difference? Differences have been generally considered as statistical differences. If there was a statistically significant difference for serum sodium of 1 mmol/L, how confident can we be of the usefulness of that difference in any particular individual when our routine assays cannot distinguish that difference? The balance between analytical quality and the quality of clinical interpretation is described in the Stockholm hierarchy,117 where the ultimate measure of quality is established by its relationship with clinical outcome. This ideal goal of defining analytical quality goals based on clinical outcome has not yet been applied to most measurements in laboratory medicine, let alone to the related issue of the impact of the quality of reference intervals on clinical interpretation and outcome.118 Therefore, as we are also unable to determine whether differences in the quality of partitioning of reference intervals will impact on clinical outcomes, we need to apply lesser approaches to the suitability of partitioning such as clinical opinion, statistics or laboratory consensus. Statistical partitioning methods by Stinton et al,119 Harris and Boyd120 and Ichihara and Kawai,121 are essentially based on an arbitrary distance between two distributions, although improvements can be made by applying a prevalence adjusted distance.116 These methods don’t apply where there are more than two partitions to compare (as with almost all measurands). Gender can be assessed as two partitions, but what of age or pregnancy or where there is a continuous change? In order to compare the appropriate partitions, knowledge of the physiological changes affecting that measure is required.
As previously mentioned, when intra-individual variability is much tighter than inter-individual variability (index of individuality is below 0.6), reference intervals lose their usefulness. However, by partitioning reference intervals (to reduce inter-individual variation), the ratio of intra- to inter-individual variation can be increased above 1.4.
The dominant form of partitioning applied in clinical laboratory medicine is by social consensus: adulthood begins at age 18 (or 21), gestational age in pregnancy is divided into three trimesters, adult age can be divided into decades and old age is the age of retirement which is about 65 to 70 years. In this review, I hope to have demonstrated in the preceding discussions that while physiological changes often coincide with social and commonly used partitions such as weaning, adolescence, pregnancy, menopause and retirement, many of these physiological changes do not follow such partitions. For example, does physiology of pregnancy have any relevance to our arbitrary division of the nine months of pregnancy into three trimesters? Partitioning in adolescence should ideally be linked to the pubertal Tanner stages, but this requires considerable effort for laboratorians to develop this understanding as well as an effort by clinicians to apply these partitions. Childhood definitions are particularly problematic as, for example, thyroid stimulating hormone (TSH) falls to adult levels by age 12, while ALP falls to adult levels by age 21 in boys. It is difficult to create a set of partitions that reflects all the changes in childhood and this is probably why many reference interval studies in children settle on dividing childhood in 5 year blocks. The social divisions of childhood: newborn, infant, toddler, preschool, primary school and secondary school, are also more understandable for anxious parents who may otherwise be confused when trying to understand nuances of their child’s pathology reports. While there may be pragmatic reasons to apply these social divisions, as well as tools to decide whether these partitions lead to statistically significant differences, we should consider in these discussions, the scientific understanding of how physiology affects reference distributions.
Conclusion
Reference intervals should represent our understanding of physiology and the way it normally affects laboratory tests. This understanding is vital for maintaining the high specificity of reference intervals. The lack of a full appreciation of the importance of this understanding of physiology became obvious to our pathology network over the many years we have spent reviewing our reference intervals using direct and indirect reference interval data. We were fortunate to have access to hundreds of thousands of patient results that are largely an outpatient population where screening using multiple biochemical analysis is common and satisfies the CLSI C28-A3 requirement that indirect techniques are most appropriate using data from individuals who are relatively healthy.13 Indeed it would be nearly impossible to create corresponding data using expensive direct reference interval projects. If we need 120 healthy people to develop one direct reference interval, that requirement becomes 240 if there are gender differences, 360 more for the three trimesters of pregnancy and hundreds more if we are to consider age related partitioning for the elderly. We would also require hundreds more for the various stages of childhood122 which is why CLSI C28-A3 specifically states that indirect techniques ‘are used when it is deemed too difficult to collect samples from healthy subjects (e.g. paediatrics)’13 and investigators have used the indirect approach successfully in both paediatrics123 and the elderly.124 It is, therefore, virtually impossible to perform direct reference interval studies with enough individuals to represent all the physiological differences that are known to exist.125 Indirect reference intervals usually compare very well with those derived directly,126 but may reveal previously unsuspected differences.127
As stated by Fraser: ‘appreciation of the biological changes that occur over the span of life is a necessary prerequisite to deciding whether stratification of reference values according to age is likely to be necessary’.128 Human physiology describes our expected journey through life. We cannot define pathological departures from that journey without first understanding the journey itself.
Acknowledgments
I wish to acknowledge the contributions of the pathologists and scientists of the Sonic Healthcare network who have been on the journey of rediscovering the physiological changes in each analyte during our fortnightly teleconferences over the last 8 years. In particular I would like to thank David Kanowski, Grahame Caldwell, Michael Metz, Tina Yen, Sydney Sacks, Nick Taylor, Robert Flatman, Lee Price, Zhong Lu, John Bothman, Richard Hanlon, Alan McNeil, Bev Robotham, Ellen Maxwell, Sarah Hall and Peter Joseph as well as the CEO of Sonic Healthcare, Dr Colin Goldschmidt, who ensures that medical leadership across the organisation drives quality and innovation. This article is based on my previously unpublished ‘Current Concepts’ lecture given across Australia and New Zealand in 2012 for the Australasian Association of Clinical Biochemists and I also acknowledge the encouragement from the Harmonisation Committee of that Association. I also declare my recent membership of the IFCC Committee on Reference Intervals and Decision Limits.
Footnotes
Competing Interests: None declared.
References
- 1.Lindstedt G, Tryding N. [There is difference between decision limits and reference intervals. Reference intervals are based on measurements in healthy individuals, decision limits on measurements in patients] Lakartidningen. 2007;104:2076–9. [PubMed] [Google Scholar]
- 2.Sikaris K. Application of the stockholm hierarchy to defining the quality of reference intervals and clinical decision limits. Clin Biochem Rev. 2012;33:141–8. [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen PH, Jensen EA, Brandslund I. Analytical performance, reference values and decision limits. A need to differentiate between reference intervals and decision limits and to define analytical quality specifications. Clin Chem Lab Med. 2012;50:819–31. doi: 10.1515/cclm-2011-0844. [DOI] [PubMed] [Google Scholar]
- 4.Boyd JC. Defining laboratory reference values and decision limits: populations, intervals, and interpretations. Asian J Androl. 2010;12:83–90. doi: 10.1038/aja.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceriotti F, Henny J. Are my laboratory results normal? Considerations to be made concerning reference intervals and decision limits. eJIFCC. 2008;19:1–9. [PMC free article] [PubMed] [Google Scholar]
- 6.International Organization for Standardization (ISO) Medical Laboratories – Particular Requirements for Quality and Competence. Geneva: ISO; 2012. ISO 15189. [Google Scholar]
- 7.Xin Z, Yuan MX, Li HX, Hua L, Feng JP, Shi J, et al. Evaluation for fasting and 2-hour glucose and HbA1c for diagnosing diabetes based on prevalence of retinopathy in a Chinese population. PLoS One. 2012;7:e40610. doi: 10.1371/journal.pone.0040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabir MM, Hanson RL, Dabelea D, Imperatore G, Roumain J, Bennett PH, et al. Plasma glucose and prediction of microvascular disease and mortality: evaluation of 1997 American Diabetes Association and 1999 World Health Organization criteria for diagnosis of diabetes. Diabetes Care. 2000;23:1113–8. doi: 10.2337/diacare.23.8.1113. [DOI] [PubMed] [Google Scholar]
- 9.Cho NH, Kim TH, Woo SJ, Park KH, Lim S, Cho YM, et al. Optimal HbA1c cutoff for detecting diabetic retinopathy. Acta Diabetol. 2013;50:837–42. doi: 10.1007/s00592-013-0452-3. [DOI] [PubMed] [Google Scholar]
- 10.Cheng YJ, Gregg EW, Geiss LS, Imperatore G, Williams DE, Zhang X, et al. Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the U.S. population: Implications for diabetes diagnostic thresholds. Diabetes Care. 2009;32:2027–32. doi: 10.2337/dc09-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong TY, Liew G, Tapp RJ, Schmidt MI, Wang JJ, Mitchell P, et al. Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet. 2008;371:736–43. doi: 10.1016/S0140-6736(08)60343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonas JB, Xu L, Xie XW, Wang YX. Relationship between fasting glucose and retinopathy for diagnosis of diabetes: results from a population-based study in urban and rural China. Retina. 2010;30:1223–7. doi: 10.1097/IAE.0b013e3181ce74ae. [DOI] [PubMed] [Google Scholar]
- 13.Clinical and Laboratory Standards Institute (CLSI) Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved guideline – Third Edition. Wayne, PA, USA: CLSI; 2008. CLSI Document C28-A3. [Google Scholar]
- 14.Saris NE. The International Federation of Clinical Chemistry, IFCC Section (1978) no. 2. International Federation of Clinical Chemistry provisional recommendation on the theory of reference values (1978). Part 1. The concept of reference values. J Clin Chem Clin Biochem. 1979;17:337–9. [PubMed] [Google Scholar]
- 15.PetitClerc C, Wilding P. International Federation of Clinical Chemistry (IFCC), Scientific Committee, Clinical Section. The theory of reference values. Part 2. Selection of individuals for the production of reference values. J Clin Chem Clin Biochem. 1984;22:203–8. [PubMed] [Google Scholar]
- 16.Solberg HE, PetitClerc C. International Federation of Clinical Chemistry (IFCC), Scientific Committee, Clinical Section, Expert Panel on Theory of Reference Values. Approved recommendation (1988) on the theory of reference values. Part 3. Preparation of individuals and collection of specimens for the production of reference values. J Clin Chem Clin Biochem. 1988;26:593–8. [PubMed] [Google Scholar]
- 17.Solberg HE, Stamm D, International Federation of Clinical Chemistry IFCC IFCC recommendation—theory of reference values. Part 4. Control of analytical variation in the production, transfer and application of reverence values. Clin Chim Acta. 1991;202:S5–11. doi: 10.1016/0009-8981(91)90266-f. [DOI] [PubMed] [Google Scholar]
- 18.International Federation of Clinical Chemistry, Scientific Committee, Clinical Section Expert Panel on Theory of Reference Values (EPTRV). The theory of reference values. Part 5. Statistical treatment of collected reference values. Determination of reference limits. Clin Chim Acta. 1984;137:97F–114F. [PubMed] [Google Scholar]
- 19.Dybkaer R. International federation of clinical chemistry (IFCC)1),2) the theory of reference values. Part 6. Presentation of observed values related to reference values. J Clin Chem Clin Biochem. 1982;20:841–5. [PubMed] [Google Scholar]
- 20.Solberg HE. A guide to IFCC recommendations on reference values. J Int Fed Clin Chem. 1993;5:162–5. [PubMed] [Google Scholar]
- 21.Harris EK. Statistical aspects of reference values in clinical pathology. Prog Clin Pathol. 1981;8:45–66. [PubMed] [Google Scholar]
- 22.Van Steirteghem AC, Robertson EA, Young DS. Variance components of serum constituents in healthy individuals. Clin Chem. 1978;24:212–22. [PubMed] [Google Scholar]
- 23.Jiménez CV. Usefulness of reference limits and evaluation of significant differences. An example of the biological variation of serum rheumatoid factors. Ann Biol Clin (Paris) 1994;52:529–33. [PubMed] [Google Scholar]
- 24.Iglesias N, Petersen PH, Ricós C. Power function of the reference change value in relation to cut-off points, reference intervals and index of individuality. Clin Chem Lab Med. 2005;43:441–8. doi: 10.1515/CCLM.2005.078. [DOI] [PubMed] [Google Scholar]
- 25.Andersen TB, Erlandsen EJ, Frøkiaer J, Eskild-Jensen A, Brøchner-Mortensen J. Comparison of within- and between-subject variation of serum cystatin C and serum creatinine in children aged 2–13 years. Scand J Clin Lab Invest. 2010;70:54–9. doi: 10.3109/00365510903556308. [DOI] [PubMed] [Google Scholar]
- 26.Harris EK. Effects of intra- and interindividual variation on the appropriate use of normal ranges. Clin Chem. 1974;20:1535–42. [PubMed] [Google Scholar]
- 27.Petersen PH, Sandberg S, Fraser CG, Goldschmidt H. Influence of index of individuality on false positives in repeated sampling from healthy individuals. Clin Chem Lab Med. 2001;39:160–5. doi: 10.1515/CCLM.2001.027. [DOI] [PubMed] [Google Scholar]
- 28.Fraser CG. Inherent biological variation and reference values. Clin Chem Lab Med. 2004;42:758–64. doi: 10.1515/CCLM.2004.128. [DOI] [PubMed] [Google Scholar]
- 29.Carlsson L, Lind L, Larsson A. Reference values for 27 clinical chemistry tests in 70-year-old males and females. Gerontology. 2010;56:259–65. doi: 10.1159/000251722. [DOI] [PubMed] [Google Scholar]
- 30.Lubke S, Sikaris KA, Kanowski D, Flatman R, Norman P, Badrick T. Validation of electrolyte reference intervals. Clin Biochem Rev. 2005;26(Suppl):S28. [Google Scholar]
- 31.Sikaris KA, Kanowski D, Caldwell G, Sack S, Flatman R. Consensus network reference intervals. Clin Biochem Rev. 2006;27(Suppl):S34. [Google Scholar]
- 32.Sikaris KA, Lu Z, Kanowski D, Price L, Flatman R, Caldwell G, et al. Defining Sonic Network reference intervals for pregnancy. Clin Biochem Rev. 2009;30(Suppl):S20. [Google Scholar]
- 33.Sikaris KA, Lu Z, Kanowski D, Price L, Flatman R, Caldwell G, et al. Defining Sonic Network reference intervals for children. Clin Biochem Rev. 2009;30(Suppl):S20. [Google Scholar]
- 34.Rowbotham B, Maxwell E, Sikaris KA. Indirect estimation of haematology reference intervals by data mining. Int J Lab Hematol. 2010;32(s1):138. [Google Scholar]
- 35.Grinspon RP, Rey RA. New perspectives in the diagnosis of pediatric male hypogonadism: the importance of AMH as a Sertoli cell marker. Arq Bras Endocrinol Metabol. 2011;55:512–9. doi: 10.1590/s0004-27302011000800003. [DOI] [PubMed] [Google Scholar]
- 36.Josso N, Belville C, di Clemente N, Picard JY. AMH and AMH receptor defects in persistent Müllerian duct syndrome. Hum Reprod Update. 2005;11:351–6. doi: 10.1093/humupd/dmi014. [DOI] [PubMed] [Google Scholar]
- 37.Shi L, Remer T, Buyken AE, Hartmann MF, Hoffmann P, Wudy SA. Prepubertal urinary estrogen excretion and its relationship with pubertal timing. Am J Physiol Endocrinol Metab. 2010;299:E990–7. doi: 10.1152/ajpendo.00374.2010. [DOI] [PubMed] [Google Scholar]
- 38.Reiter EO, Root AW. Hormonal changes of adolescence. Med Clin North Am. 1975;59:1289–304. doi: 10.1016/s0025-7125(16)31930-7. [DOI] [PubMed] [Google Scholar]
- 39.Porquet D. [Endocrine biochemistry of puberty] Ann Biol Clin (Paris) 1997;55:425–33. [PubMed] [Google Scholar]
- 40.Richmond EJ, Rogol AD. Male pubertal development and the role of androgen therapy. Nat Clin Pract Endocrinol Metab. 2007;3:338–44. doi: 10.1038/ncpendmet0450. [DOI] [PubMed] [Google Scholar]
- 41.Hiort O. Androgens and puberty. Best Pract Res Clin Endocrinol Metab. 2002;16:31–41. doi: 10.1053/beem.2002.0178. [DOI] [PubMed] [Google Scholar]
- 42.Goldman J, Becker ML, Jones B, Clements M, Leeder JS. Development of biomarkers to optimize pediatric patient management: what makes children different? Biomark Med. 2011;5:781–94. doi: 10.2217/bmm.11.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole TJ. Assessment of growth. Best Pract Res Clin Endocrinol Metab. 2002;16:383–98. doi: 10.1053/beem.2002.0209. [DOI] [PubMed] [Google Scholar]
- 44.Mora S, Cafarelli L, Erba P, Puzzovio M, Zamproni I, Giacomet V, et al. Differential effect of age, gender and puberty on bone formation rate assessed by measurement of bone-specific alkaline phosphatase in healthy Italian children and adolescents. J Bone Miner Metab. 2009;27:721–6. doi: 10.1007/s00774-009-0092-4. [DOI] [PubMed] [Google Scholar]
- 45.Shine B. Use of routine clinical laboratory data to define reference intervals. Ann Clin Biochem. 2008;45:467–75. doi: 10.1258/acb.2008.008028. [DOI] [PubMed] [Google Scholar]
- 46.Gajewska J, Ambroszkiewicz J, Laskowska-Klita T. [Some bone turnover markers in serum of healthy children and adolescents in relation to age and gender] Wiad Lek. 2005;58:476–80. [PubMed] [Google Scholar]
- 47.Soliman A, De Sanctis V, Adel A, El Awwa A, Bedair S. Clinical, biochemical and radiological manifestations of severe vitamin d deficiency in adolescents versus children: response to therapy. Georgian Med News. 2012;210:58–64. [PubMed] [Google Scholar]
- 48.Kovar I, Mayne P, Barltrop D. Plasma alkaline phosphatase activity: a screening test for rickets in preterm neonates. Lancet. 1982;1:308–10. doi: 10.1016/s0140-6736(82)91569-0. [DOI] [PubMed] [Google Scholar]
- 49.Ohata Y, Ozono K. [Updates on rickets and osteomalacia: guidelines for diagnosis of rickets and osteomalacia] Clin Calcium. 2013;23:1421–8. [PubMed] [Google Scholar]
- 50.Round JM. Plasma calcium, magnesium, phosphorus, and alkaline phosphatase levels in normal British schoolchildren. Br Med J. 1973;3:137–40. doi: 10.1136/bmj.3.5872.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boer DP, de Rijke YB, Hop WC, Cransberg K, Dorresteijn EM. Reference values for serum creatinine in children younger than 1 year of age. Pediatr Nephrol. 2010;25:2107–13. doi: 10.1007/s00467-010-1533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Finney H, Newman DJ, Thakkar H, Fell JM, Price CP. Reference ranges for plasma cystatin C and creatinine measurements in premature infants, neonates, and older children. Arch Dis Child. 2000;82:71–5. doi: 10.1136/adc.82.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jopling J, Henry E, Wiedmeier SE, Christensen RD. Reference ranges for hematocrit and blood hemoglobin concentration during the neonatal period: data from a multihospital health care system. Pediatrics. 2009;123:e333–7. doi: 10.1542/peds.2008-2654. [DOI] [PubMed] [Google Scholar]
- 54.Dapper DV, Nwauche CA, Siminialayi IM. Some haematological reference values for pre-primary and primary school aged children in Port Harcourt, Nigeria. Niger J Clin Pract. 2009;12:262–7. [PubMed] [Google Scholar]
- 55.Igarashi T, Itoh Y, Maeda M, Igarashi T, Fukunaga Y. Mean hemoglobin levels in venous blood samples and prevalence of anemia in Japanese elementary and junior high school students. J Nippon Med Sch. 2012;79:232–5. doi: 10.1272/jnms.79.232. [DOI] [PubMed] [Google Scholar]
- 56.El-Hazmi MA, Warsy AS. Normal reference values for hematological parameters, red cell indices, HB A2 and HB F from early childhood through adolescence in Saudis. Ann Saudi Med. 2001;21:165–9. doi: 10.5144/0256-4947.2001.165. [DOI] [PubMed] [Google Scholar]
- 57.Wallis LA, Healy M, Undy MB, Maconochie I. Age related reference ranges for respiration rate and heart rate from 4 to 16 years. Arch Dis Child. 2005;90:1117–21. doi: 10.1136/adc.2004.068718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fleming S, Thompson M, Stevens R, Heneghan C, Plüddemann A, Maconochie I, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377:1011–8. doi: 10.1016/S0140-6736(10)62226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greeley C, Snell J, Colaco, Beatey J, Bailey J, Bjorn S, et al. Paediatric reference ranges for electrolytes and creatinine. Clin Chem. 1993;39:1172. [Google Scholar]
- 60.Ghoshal AK, Soldin SJ. Evaluation of the Dade Behring Dimension RxL: integrated chemistry system-pediatric reference ranges. Clin Chim Acta. 2003;331:135–46. doi: 10.1016/s0009-8981(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 61.Chan MK, Seiden-Long I, Aytekin M, Quinn F, Ravalico T, Ambruster D, et al. Canadian Laboratory Initiative on Pediatric Reference Interval Database (CALIPER): pediatric reference intervals for an integrated clinical chemistry and immunoassay analyzer, Abbott ARCHITECT ci8200. Clin Biochem. 2009;42:885–91. doi: 10.1016/j.clinbiochem.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 62.Pianosi P, Wolstein R. Carbon dioxide chemosensitivity and exercise ventilation in healthy children and in children with cystic fibrosis. Pediatr Res. 1996;40:508–13. doi: 10.1203/00006450-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 63.Cassels DE, Morse M. Arterial blood gases and acid-base balance in normal children. J Clin Invest. 1953;32:824–36. doi: 10.1172/JCI102799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dong SH, Liu HM, Song GW, Rong ZP, Wu YP. Arterialized capillary blood gases and acid-base studies in normal individuals from 29 days to 24 years of age. Am J Dis Child. 1985;139:1019–22. doi: 10.1001/archpedi.1985.02140120065028. [DOI] [PubMed] [Google Scholar]
- 65.Burritt MF, Slockbower JM, Forsman RW, Offord KP, Bergstralh EJ, Smithson WA. Pediatric reference intervals for 19 biologic variables in healthy children. Mayo Clin Proc. 1990;65:329–36. doi: 10.1016/s0025-6196(12)62533-6. [DOI] [PubMed] [Google Scholar]
- 66.Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, et al. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem. 2012;58:854–68. doi: 10.1373/clinchem.2011.177741. [DOI] [PubMed] [Google Scholar]
- 67.Burritt MF, Slockbower JM, Forsman RW, Offord KP, Bergstralh EJ, Smithson WA. Pediatric reference intervals for 19 biologic variables in healthy children. Mayo Clin Proc. 1990;65:329–36. doi: 10.1016/s0025-6196(12)62533-6. [DOI] [PubMed] [Google Scholar]
- 68.Henning SJ. Biochemistry of intestinal development. Environ Health Perspect. 1979;33:9–16. doi: 10.1289/ehp.79339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel MS, Srinivasan M. Metabolic programming in the immediate postnatal life. Ann Nutr Metab. 2011;58(Suppl 2):18–28. doi: 10.1159/000328040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aggarwal J, Swami G, Kumar M. Probiotics and their Effects on Metabolic Diseases: An Update. J Clin Diagn Res. 2013;7:173–7. doi: 10.7860/JCDR/2012/5004.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hicks JM, Bjorn S, Beatey J, et al. Pediatric reference ranges for albumin, globulin and total protein on the Hitachi 747. Clin Chem. 1995;41:S93. [Google Scholar]
- 72.Falcão RP. Human blood lymphocyte subpopulations from birth to eight years. Clin Exp Immunol. 1980;39:203–7. [PMC free article] [PubMed] [Google Scholar]
- 73.Belldegrin A, Shoenfeld Y, Pick AI, Vana D. Age related distribution of serum immunoglobulin concentration in 1003 healthy children and adults. Biomedicine. 1980;33:8–12. [PubMed] [Google Scholar]
- 74.Cheek DB, Petrucco OM, Gillespie A, Ness D, Green RC. Muscle cell growth and the distribution of water and electrolyte in human pregnancy. Early Hum Dev. 1985;11:293–305. doi: 10.1016/0378-3782(85)90083-0. [DOI] [PubMed] [Google Scholar]
- 75.Winston J, Levitt MF. Renal function, renal disease and pregnancy. In: Cherry SH, Merkatz IR, editors. Complications of Pregnancy: Medical, Surgical, Gynecologic, Psychosocial and Perinatal. 4th ed. Baltimore: Williams & Wilkins; 1991. p. 502. [Google Scholar]
- 76.Horton DK, Adetona O, Aguilar-Villalobos M, Cassidy BE, Pfeiffer CM, Schleicher RL, et al. Changes in the concentrations of biochemical indicators of diet and nutritional status of pregnant women across pregnancy trimesters in Trujillo, Peru, 2004–2005. Nutr J. 2013;12:80. doi: 10.1186/1475-2891-12-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valenzuela GJ, Munson LA, Tarbaux NM, Farley JR. Time-dependent changes in bone, placental, intestinal, and hepatic alkaline phosphatase activities in serum during human pregnancy. Clin Chem. 1987;33:1801–6. [PubMed] [Google Scholar]
- 78.Bacq Y, Zarka O, Bréchot JF, Mariotte N, Vol S, Tichet J, et al. Liver function tests in normal pregnancy: a prospective study of 103 pregnant women and 103 matched controls. Hepatology. 1996;23:1030–4. doi: 10.1002/hep.510230514. [DOI] [PubMed] [Google Scholar]
- 79.Ekinci E, Lu ZX, Sikaris KA, Atanasovski D, Bittar I, Lam Q, et al. Alkaline phosphatase reference intervals in pregnancy. Clin Biochem Rev. 2012;33:S23. [Google Scholar]
- 80.Kristensen K, Larsson I, Hansson SR. Increased cystatin C expression in the pre-eclamptic placenta. Mol Hum Reprod. 2007;13:189–95. doi: 10.1093/molehr/gal111. [DOI] [PubMed] [Google Scholar]
- 81.Wu Y, Xiong X, Fraser WD, Luo ZC. Association of uric acid with progression to preeclampsia and development of adverse conditions in gestational hypertensive pregnancies. Am J Hypertens. 2012;25:711–7. doi: 10.1038/ajh.2012.18. [DOI] [PubMed] [Google Scholar]
- 82.Nwagha UI, Ejezie FE, Iyare EE. Evaluation of serum uric acid levels in normal pregnant Nigerian women. Niger J Clin Pract. 2009;12:83–6. [PubMed] [Google Scholar]
- 83.Klæstrup E, Trydal T, Pedersen JF, Larsen JM, Lundbye-Christensen S, Kristensen SR. Reference intervals and age and gender dependency for arterial blood gases and electrolytes in adults. Clin Chem Lab Med. 2011;49:1495–500. doi: 10.1515/CCLM.2011.603. [DOI] [PubMed] [Google Scholar]
- 84.Jensen D, Duffin J, Lam YM, Webb KA, Simpson JA, Davies GA, et al. Physiological mechanisms of hyperventilation during human pregnancy. Respir Physiol Neurobiol. 2008;161:76–86. doi: 10.1016/j.resp.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 85.Weissgerber TL, Wolfe LA, Hopkins WG, Davies GA. Serial respiratory adaptations and an alternate hypothesis of respiratory control in human pregnancy. Respir Physiol Neurobiol. 2006;153:39–53. doi: 10.1016/j.resp.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 86.Hadziomerović D, Moeller KT, Licht P, Hein A, Veitenhansel S, Kusmitsch M, et al. The biphasic pattern of end-expiratory carbon dioxide pressure: a method for identification of the fertile phase of the menstrual cycle. Fertil Steril. 2008;90:731–6. doi: 10.1016/j.fertnstert.2007.06.078. [DOI] [PubMed] [Google Scholar]
- 87.Kastrup A, Dichgans J, Niemeier M, Schabet M. Changes of cerebrovascular CO2 reactivity during normal aging. Stroke. 1998;29:1311–4. doi: 10.1161/01.str.29.7.1311. [DOI] [PubMed] [Google Scholar]
- 88.Preston ME, Jensen D, Janssen I, Fisher JT. Effect of menopause on the chemical control of breathing and its relationship with acid-base status. Am J Physiol Regul Integr Comp Physiol. 2009;296:R722–7. doi: 10.1152/ajpregu.90865.2008. [DOI] [PubMed] [Google Scholar]
- 89.Orr-Walker BJ, Horne AM, Evans MC, Grey AB, Murray MA, McNeil AR, et al. Hormone replacement therapy causes a respiratory alkalosis in normal postmenopausal women. J Clin Endocrinol Metab. 1999;84:1997–2001. doi: 10.1210/jcem.84.6.5797. [DOI] [PubMed] [Google Scholar]
- 90.Nordin BE, Need AG, Hartley TF, Philcox JC, Wilcox M, Thomas DW. Improved method for calculating calcium fractions in plasma: reference values and effect of menopause. Clin Chem. 1989;35:14–7. [PubMed] [Google Scholar]
- 91.Nordin BE, Need AG, Morris HA, Horowitz M. Biochemical variables in pre- and postmenopausal women: reconciling the calcium and estrogen hypotheses. Osteoporos Int. 1999;9:351–7. doi: 10.1007/s001980050158. [DOI] [PubMed] [Google Scholar]
- 92.Nordin BE, Need AG, Morris HA, O’Loughlin PD, Horowitz M. Effect of age on calcium absorption in postmenopausal women. Am J Clin Nutr. 2004;80:998–1002. doi: 10.1093/ajcn/80.4.998. [DOI] [PubMed] [Google Scholar]
- 93.Nordin BE, Horowitz M, Need A, Morris HA. Renal leak of calcium in post-menopausal osteoporosis. Clin Endocrinol (Oxf) 1994;41:41–5. doi: 10.1111/j.1365-2265.1994.tb03782.x. [DOI] [PubMed] [Google Scholar]
- 94.Giannini S, Nobile M, Dalle Carbonare L, Lodetti MG, Sella S, Vittadello G, et al. Hypercalciuria is a common and important finding in postmenopausal women with osteoporosis. Eur J Endocrinol. 2003;149:209–13. doi: 10.1530/eje.0.1490209. [DOI] [PubMed] [Google Scholar]
- 95.Ferrucci L, Maggio M, Bandinelli S, Basaria S, Lauretani F, Ble A, et al. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med. 2006;166:1380–8. doi: 10.1001/archinte.166.13.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Maggio M, Ceda GP, Lauretani F, Bandinelli S, Metter EJ, Guralnik JM, et al. Gonadal status and physical performance in older men. Aging Male. 2011;14:42–7. doi: 10.3109/13685538.2010.518179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harm K. [Reference ranges in geriatrics: a review of age dependence of selected blood components] Z Gerontol Geriatr. 1997;30:185–92. [PubMed] [Google Scholar]
- 98.Goto M. Inflammaging (inflammation + aging): A driving force for human aging based on an evolutionarily antagonistic pleiotropy theory? Biosci Trends. 2008;2:218–30. [PubMed] [Google Scholar]
- 99.Xi H, Li C, Ren F, Zhang H, Zhang L. Telomere, aging and age-related diseases. Aging Clin Exp Res. 2013;25:139–46. doi: 10.1007/s40520-013-0021-1. [DOI] [PubMed] [Google Scholar]
- 100.Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, Verdin E. The sirtuins, oxidative stress and aging: an emerging link. Aging (Albany NY) 2013;5:144–50. doi: 10.18632/aging.100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barzilai N, Gabriely I, Atzmon G, Suh Y, Rothenberg D, Bergman A. Genetic studies reveal the role of the endocrine and metabolic systems in aging. J Clin Endocrinol Metab. 2010;95:4493–500. doi: 10.1210/jc.2010-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonneux L, Barendregt JJ, Van der Maas PJ. The expiry date of man: a synthesis of evolutionary biology and public health. J Epidemiol Community Health. 1998;52:619–23. doi: 10.1136/jech.52.10.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruivo S, Viana P, Martins C, Baeta C. Effects of aging on lung function. A comparison of lung function in healthy adults and the elderly. Rev Port Pneumol. 2009;15:629–53. [PubMed] [Google Scholar]
- 104.Ren WY, Li L, Zhao RY, Zhu L. Age-associated changes in pulmonary function: a comparison of pulmonary function parameters in healthy young adults and the elderly living in Shanghai. Chin Med J (Engl) 2012;125:3064–8. [PubMed] [Google Scholar]
- 105.Vaz Fragoso CA, Lee PJ. The aging lung. J Gerontol A Biol Sci Med Sci. 2012;67:233–5. doi: 10.1093/gerona/glr249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rule AD, Cornell LD, Poggio ED. Senile nephrosclerosis--does it explain the decline in glomerular filtration rate with aging? Nephron Physiol. 2011;119(Suppl 1):6–11. doi: 10.1159/000328012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Christensson A, Elmståhl S. Estimation of the age-dependent decline of glomerular filtration rate from formulas based on creatinine and cystatin C in the general elderly population. Nephron Clin Pract. 2011;117:c40–50. doi: 10.1159/000319646. [DOI] [PubMed] [Google Scholar]
- 108.Glassock RJ, Winearls C. Ageing and the glomerular filtration rate: truths and consequences. Trans Am Clin Climatol Assoc. 2009;120:419–28. [PMC free article] [PubMed] [Google Scholar]
- 109.Giannoni A, Giovannini S, Clerico A. Measurement of circulating concentrations of troponin I and T in healthy subjects: a tool for monitoring myocardial tissue renewal? Clin Chem Lab Med. 2009;47:1167–77. doi: 10.1515/CCLM.2009.320. [DOI] [PubMed] [Google Scholar]
- 110.Bima A, Lu ZX, Botros M, Sikaris KA. Age and eGFR as predictors of high sensitivity Troponin T. Clin Biochem Rev. 2011;32(Suppl):S37. [Google Scholar]
- 111.Bima A, Sikaris K. Towards appreciating appropriate clinical responses to highly sensitive cardiac troponin assays. Intern Med J. 2012;42(Suppl 5):16–22. doi: 10.1111/j.1445-5994.2012.02893.x. [DOI] [PubMed] [Google Scholar]
- 112.Vasikaran SD, MacDonald SP, Sikaris KA. High sensitivity cardiac troponin assays for risk stratification and for the diagnosis of acute myocardial. Ann Clin Biochem. 2012;49:209–10. doi: 10.1258/acb.2012.012058. [DOI] [PubMed] [Google Scholar]
- 113.Vasikaran SD, Bima A, Botros M, Sikaris KA. Comment on: Cardiac troponin testing in the acute care setting: ordering, reporting, and high sensitivity assays--an update from the Canadian society of clinical chemists (CSCC); the case for age related acute myocardial infarction (AMI) cut-offs. Clin Biochem. 2012;45:513–4. doi: 10.1016/j.clinbiochem.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 114.Huber KR, Mostafaie N, Stangl G, Worofka B, Kittl E, Hofmann J, et al. Clinical chemistry reference values for 75-year old apparently healthy persons. Clin Chem Lab Med. 2006;44:1355–60. doi: 10.1515/CCLM.2006.247. [DOI] [PubMed] [Google Scholar]
- 115.Plebani M, Lippi G. Personalized (laboratory) medicine: a bridge to the future. Clin Chem Lab Med. 2013;51:703–6. doi: 10.1515/cclm-2013-0021. [DOI] [PubMed] [Google Scholar]
- 116.Lahti A. Partitioning biochemical reference data into subgroups: comparison of existing methods. Clin Chem Lab Med. 2004;42:725–33. doi: 10.1515/CCLM.2004.123. [DOI] [PubMed] [Google Scholar]
- 117.Kenny D, Fraser CG, Hyltoft Petersen P, Kallner A. Consensus Agreement: Conference on strategies to set global quality specifications in laboratory medicine. Scand J Clin Lab Invest. 1999;59:585. [PubMed] [Google Scholar]
- 118.Sikaris K. Application of the Stockholm hierarchy to defining the quality of reference intervals and clinical decision limits. Clin Biochem Rev. 2012;33:141–8. [PMC free article] [PubMed] [Google Scholar]
- 119.Stinton TJ, Cowley DM, Bryant SJ. Reference intervals for calcium, phosphate, and alkaline phosphatase as derived on the basis of multichannel-analyzer profiles. Clin Chem. 1986;32:76–9. [PubMed] [Google Scholar]
- 120.Harris EK, Boyd JC. On dividing reference data into subgroups to produce separate reference ranges. Clin Chem. 1990;36:265–70. [PubMed] [Google Scholar]
- 121.Ichihara K, Kawai T. Determination of reference intervals for 13 plasma proteins based on IFCC international reference preparation (CRM470) and NCCLS proposed guideline (C28-P, 1992): a strategy for partitioning reference individuals with validation based on multivariate analysis. J Clin Lab Anal. 1997;11:117–24. doi: 10.1002/(SICI)1098-2825(1997)11:2<117::AID-JCLA8>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shaw JL, Binesh Marvasti T, Colantonio D, Adeli K. Pediatric reference intervals: challenges and recent initiatives. Crit Rev Clin Lab Sci. 2013;50:37–50. doi: 10.3109/10408363.2013.786673. [DOI] [PubMed] [Google Scholar]
- 123.Shine B. Use of routine clinical laboratory data to define reference intervals. Ann Clin Biochem. 2008;45:467–75. doi: 10.1258/acb.2008.008028. [DOI] [PubMed] [Google Scholar]
- 124.Bock BJ, Dolan CT, Miller GC, Fitter WF, Hartsell BD, Crowson AN, et al. The data warehouse as a foundation for population-based reference intervals. Am J Clin Pathol. 2003;120:662–70. doi: 10.1309/W8J8-5AG4-WDG6-JGJ9. [DOI] [PubMed] [Google Scholar]
- 125.Henny J. The IFCC recommendations for determining reference intervals: strengths and limitations. J Lab Med. 2009;33:45–51. [Google Scholar]
- 126.Katayev A, Balciza C, Seccombe DW. Establishing reference intervals for clinical laboratory test results: is there a better way? Am J Clin Pathol. 2010;133:180–6. doi: 10.1309/AJCPN5BMTSF1CDYP. [DOI] [PubMed] [Google Scholar]
- 127.Kanno T, Ohgushi Y, Shibata T. Setting reference intervals without considering sex and age differences. Rinsho Byori. 2008;56:622–6. [PubMed] [Google Scholar]
- 128.Fraser CG. Inherent biological variation and reference values. Clin Chem Lab Med. 2004;42:758–64. doi: 10.1515/CCLM.2004.128. [DOI] [PubMed] [Google Scholar]


