Abstract
Purpose
Pluronic block copolymers are non-ionic surfactants with demonstrated sensitizing activity in chemotherapy and hyperthermia in various tumor cell lines. In the current study we investigated the potential activity of Pluronic as a radiosensitizing agent.
Materials and methods
As a possible mechanism, the effect of Pluronic on Hsp70 and Hsp90 was examined. Gli36 human glioma cells were treated with radiation alone as well as with a combination treatment of Pluronic and radiation.
Results
Clonogenic cell survival assays show that Pluronic has an elevated effect on radiosensitization (50% high, p < 0.01), even with radiation doses as low as 2 Gy. The Hsp90 level was reduced 24 h after the combined treatment in both in vitro and in vivo. Similarly, Hsp70 levels were also decreased 24 h post treatment. When Gli36 cells were exposed to Pluronic before and during irradiation, DNA DSB: double-strand breaks repair was reduced, and elevated apoptosis was also seen in tumor xenografts.
Conclusion
Data suggest the potential use of L10 as a radiosensitizer. While the mechanism of sensitization requires additional investigation, the presented results indicate that the effect may be due, in part, to a decrease in Hsp90 and 70 levels and increased DNA damage.
Keywords: Radiation, Pluronic, heat shock protein, DNA damage, apoptosis
Introduction
Radiation therapy is a widespread and effective treatment modality for many cancer patients. However, there are several limiting factors of radiotherapy, including dose tolerance limitations of surrounding normal tissue and radiation resistance of many tumors (Shintani et al. 2003). The effectiveness of radiation therapy is often determined by the radiosensitivity of the tumor cells (Gerweck et al. 2006). Therefore, there is significant research interest in finding novel treatment approaches to selectively increase the radiosensitivity of tumor cells.
Pluronics, relatively non-toxic and biocompatible agents, have been recognized as sensitizers for low-grade hyperthermia and chemotherapy (Alakhov et al. 1996, Exner et al. 2005, Krupka et al. 2006, Krupka et al. 2007, Batrakova et al. 2010). Pluronic is a triblock co-polymer composed of hydrophilic poly(ethylene oxide) (PEO) and hydrophobic poly(propylene oxide) (PPO) in the form of PEOx-PPOy-PEOx (where the x and y values differ in each type of Pluronic, leading to structure-dependent differences in properties (Figure 1A) (Kabanov et al. 2002). The ability of Pluronic to sensitize cells to low-grade hyperthermia and chemotherapy is, at least in part, due to increased cell death by modulating fluidity of the cell membrane, depleting intracellular ATP (Kabanov et al. 2003) and changing the P-glycoprotein drug efflux pump in multidrug resistant (MDR) cells (Batrakova et al. 1999, 2001, Batrakova and Kabanov 2008). Recently, we have shown that thermosensitization by Pluronic also partially depends on its ability to decrease the expression of (heat shock protein) Hsp70 both in vivo and in vitro (Perera et al. 2011). In the current study we hypothesized that, in addition to depressing expression of Hsp70, Pluronic can also decrease the expression of Hsp90 in glioblastomamultiforme (GBM).
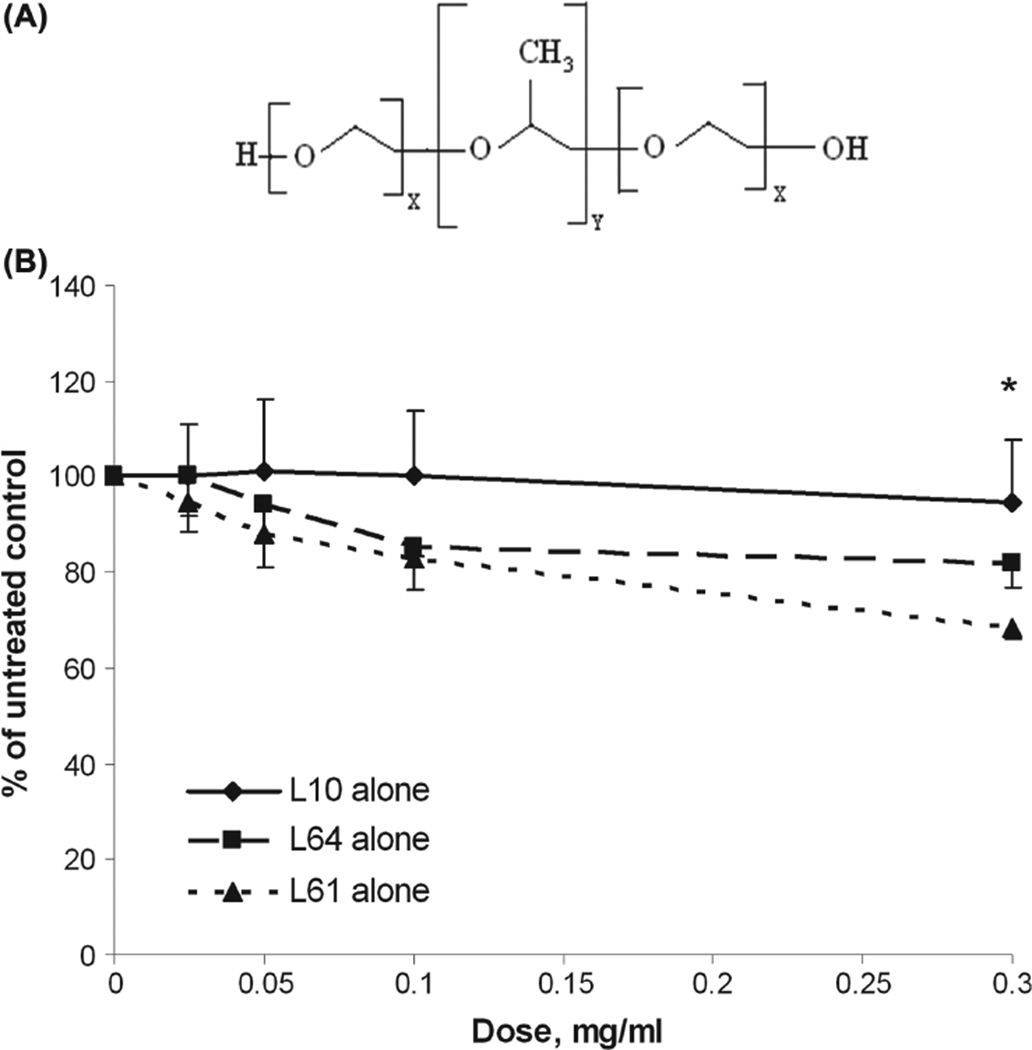
Figure 1.
Mitochondrial enzyme activity for Gli36 cells after treatment with Pluronic. (A) Schematic diagram of Pluronic block copolymers. (B) Mitochondrial enzyme activity of Gli36 cells after exposure to increasing concentrations of Pluronic L10 (critical micellar concentration (cmc) data has not been published), L64 (cmc = 1.4 × 10−4 mg/ml), or L61(cmc = 2.2 × 10−1 mg/ml) for 1 h. Cell viability was assayed 24 h after treatment using the WST-1 assay. The data were averaged and normalized to the untreated control to generate dose-response curves (n = 3, mean ± SD). * At a dose of 0.3 mg/ml, the mitochondrial enzyme activities of each Pluronic treatment group as measured by WST-1 assay were significantly different from each other (p < 0.05).
GBM is a devastating adult brain tumor with typical survival less than 1 year following the time of diagnosis (Louis 2006). Current treatment regimens for GBM are limited due to the resistance of malignant glioma cells to conventional therapies, which include surgical resection followed by radiation and chemotherapy. Often, phenotypic alteration of the tumor following treatment causes resistance to apoptosis and renewed tumor cell proliferation, followed by intense local invasion of tumor into the surrounding parenchyma, and ultimately GBM progression (Furnari et al. 2007). Therefore, much attention has been focused on studying the molecular machinery behind disease progression, particularly its radioresistance. A commonly studied target is Hsp90, a chaperone protein that protects its client protein from conformational changes and degradation (Morimoto et al. 1997). The expression of Hsp90 is known to be up-regulated in malignant as compared to normal tissues (Ferrarini et al. 1992).
Furthermore, many Hsp90 client proteins are involved in metastasis, invasion, and angiogenesis (Neckers 2002). Hsp90 stabilizes proteins such as Raf-1, Akt and ErbB2 (Schulte et al. 1995, Sato et al. 2000, Bull et al. 2004) that protect cells from radiation-induced death (Pirollo et al. 1997, Gupta et al. 2001, Tanno et al. 2004). Several studies have demonstrated that inhibition of Hsp90 causes inactivation of the client proteins, increasing tumor cell death in culture and in tumor models (Bisht et al. 2003, Enmon et al. 2003, Machida et al. 2003, Russell et al. 2003, Bull et al. 2004, Harashima et al. 2005, Yin et al. 2005, Peng et al. 2010, Zhao et al. 2011). However, inhibition of Hsp90 also causes up-regulation of Hsp70, ultimately limiting the effect of Hsp90 inhibition on cancer cell death (Drysdale et al. 2006). Therefore, there has been considerable interest in inhibiting both Hsp90 and Hsp70 in order to increase the radiosensitivity of tumor cells.
In the current study, we investigated the effect of Pluronic triblock copolymer as a sensitizing agent for radiation therapy of Gli36 cells in vitro and evaluated the in vivo therapeutic potential of combining radiation treatment with Pluronic in nude mice bearing Gli36 xenografts. Cells were treated with Pluronic, radiation or Pluronic + radiation combined treatment. Treated cells were analyzed for cell viability, colony-forming ability, and the level of Hsp90 and Hsp70 proteins. Radiation-induced DNA damage was also analyzed as histone γ-H2AX foci formation by flow cytometry and as tail moment by the comet assay. In vivo studies were carried out by irradiating tumors with or without Pluronic, and analyzing tissue samples for expression of Hsp90 and Hsp70 proteins and radiation-induced apoptosis.
Materials and methods
Formulation of Pluronic solutions
Pluronics L10, L61, and L64 (molecular weight (Mw) of 3200, 2000, and 2900 Da; average PPO/PEO units of 49.7/7.3, 31/4.55, and 30/26.4, respectively (Chiappetta and Sosnik 2007) were used in the initial in vitro screening of radiosensitization. Pluronic L61 was generously donated by BASF (Shreveport, LA, USA). Pluronic L10 and L64 were purchased from Sigma Aldrich (Milwaukee, WI, USA). Pluronic stock solutions were prepared by dissolving 25 mg/ml of each polymer in incomplete (without serum) Dulbecco’s minimal essential medium (DMEM) overnight at 4°C. Solutions were sterilized by passage through a sterile 0.22 µm syringe filter (Millipore, Billerica, MA, USA), further diluted in DMEM to prepare test solutions (0.1–0.3 mg/ml) and stored at 4°C until use.
Cell culture
Human glioblastoma Gli36 cells (kindly provided by Dr James Basilion, Departments of Radiology and Biomedical Engineering and the Case Center for Molecular Imaging, CWRU, OH, USA) were cultured in complete DMEM medium (containing 10% fetal bovine serum and 1% penicillin/streptomycin; Life Technologies, Grand Island, NY, USA). Cells were cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Cells were passaged at 90% confluence. Twenty four hours before treatment, cells were detached with 0.25% trypsin-EDTA and plated onto 96-well or six-well plates (1 × 105 cells/ml) as required for each assay (200 µl and 3 ml per well in 96-well plates and six-well plates, respectively).
Drug sensitivity assay
Cell viability was measured using mitochondrial succinate dehydrogenase assay (WST-1 assay; Biosciences, San Francisco, CA, USA). After a cell incubation period of 24 h in 96-well plates, the culture medium was aspirated, and cells were treated with 100 µl of Pluronic (L61, L64, and L10) test solutions (0.1–0.3 mg/ml) for 1 h at 37°C. After the treatment, cells were replenished with fresh medium and recovered at 37°C in a humidified incubator for 24 h. The assay was performed following the manufacturer’s directions. Briefly, the medium was removed and 100 µl of diluted reagent (1:9 with incomplete medium) was added and incubated at 37°C for 1 h. The optical density at 450 nm was determined using a plate reader (TECAN; Durham, NC, USA). All studies were repeated in triplicate. Using data obtained from this set of experiments, the best lead candidate, Pluronic L10, was selected and utilized exclusively in all following experiments.
Protein biomarker analysis
For immunoblot analysis, whole cell lysates were prepared as follows. First, cells were cultured in Petri dishes and exposed to 3 Gy γ-radiation (3.2 Gy/min) with or without 0.3 mg/ml L10 treatment (1 h), and returned to the incubator. Five or 24 h after treatment, cells were lysed on ice for 15–30 min in a lysis buffer (Cell Signaling Technology, Beverly, MA, USA) and centrifuged at 10,000 g for 10 min at 4°C. A Bio-Rad (Hercules, CA, USA) protein assay kit was used to determine protein concentration in the supernates. Protein was electrophoresed on sodium dodecyl sulfate/polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat dry milk in TBST buffer (0.1% Tween-20, 20 mM Tris-HCl, pH 7.5, and 140 mM NaCl). Membranes were then incubated with primary antibodies against Hsp90, Hsp70, and β-actin (Enzo Life Science, Farmingdale, NY, USA), followed by secondary antibody/horseradish peroxidase conjugates (Millipore). The SNAP id system (Millipore) was used for the antibody incubation. Horseradish peroxidase (HRP) substrate-luminal reagent (Millipore) was used to detect chemiluminescence signal, which was photographed by Alpha Imager HP (Proteinsimple, Santa Clara, CA, USA). For in vivo Hsp analysis, tumor xenografts were excised from each group of nude mice and tissue samples were prepared by homogenizing the piece of dissected tumor in lysis buffer, followed by centrifugation at 4°C and collecting the supernate. The Western blot analysis was carried out as described above.
Clonogenic survival
Cell survival was measured by the colony formation ability of treated cells using a clonogenic assay. Cells were plated in six-well plates and after 24 h, irradiated with γ-rays (0–8 Gy) using a cesium-137 chamber with or without 1 h pretreatment with L10 (0.1 and 0.3 mg/ml). Untreated and treated cells were trypsinized, counted, diluted and seeded in six-well plates in duplicates and cultured for 10 days. Colonies were fixed with methanol (Fisher, Pittsburgh, PA, USA), stained with May-Grunwald stain (EMD Chemicals, Gibbstown, NJ, USA) and Giemsa solution (Sigma Aldrich, St Louis, MO, USA). Colonies containing more than 50 cells were counted using ImageJ (National Institutes of Health, Bethesda, MD, USA; http://imagej.nih.gov/ij/). The assay was repeated in triplicate and results used to calculate the surviving fraction. The mean surviving fraction (S) for cell samples after irradiation with radiation doses (D) were fitted to the linear quadratic (LQ) model (Equation 1).
| (1) |
where α and β are constants derived from the linear quadratic fit of the curve.
The dose enhancement factor (DEF), which is the dose reducing survival to 10% for radiation alone divided by that for radiation in the presence of Pluronic, was calculated from the dose-response curves. The mean inactivation dose (MID) was calculated as described earlier (Latz et al. 1998). All curve fitting and clonogenic survival curve analysis was calculated using a custom algorithm in MATLAB®.
Neutral comet assay
Single cell electrophoresis assay or comet assay was performed for evaluating the formation of DNA double-strand breaks (DSB) following exposure to 0.3 mg/ml L10 alone, radiation alone (3 Gy), and combined treatment. At different post treatment time points (t = 0, 3, 6, 24 h), treated cells were collected, embedded in agarose gel on a slide (Trevigen, Gaithersburg, MD, USA), and subjected to lysis followed by electrophoresis under neutral conditions. During electrophoresis, damaged DNA travels away from the nucleus while intact DNA remains in place without migrating. The slides were stained with SYBER green and examined under a Zeiss Axio Observer Z1 motorized FL inverted microscope (Jena, Germany). The amount of DNA damage was measured by analyzing the tail moment of migrated DNA using Komet 6.0 software (Kinetic Imaging, Liverpool, UK). Comet tail moment is calculated as the product of tail length and the fraction of DNA in the tail for each treatment and each time point (Sun et al. 2006). All comets were quantified by three independent trials (scored 75 cells in each).
Measurement of γ-H2AX
More quantitative results of DNA damage were obtained using flow cytometric analysis of γ-H2AX. Cells were irradiated (3 Gy) with and without 1 h pretreatment with 0.3 mg/ml L10. At different time points (t = 0, 1, 2, 4, 24 h) post treatment, cells were trypsinized, collected (~ 106 cells), and fixed with methanol. Cell samples were rinsed with PBS, centrifuged and resuspended in 200 µl of mouse monoclonal anti-phospho-histone H2AX antibody (1:500 dilution; Millipore) at 4°C for 1 h. Cells were washed with PBS/BSA and then resuspended in 4,6-diamidino-2-phenylindole (DAPI; Sigma Aldrich). DNA DSB were determined by the presence of γ-H2AX staining as detected by flow cytometric analysis. The measurements were conducted using a BD LSR II flow cytometer (BD Biosciences, San Jose, CA, USA), and cells were excited with the 488 nm laser. The data analysis was performing using WinList 3D 6.0 (Verity Software House, Topsham, ME, USA).
Animal studies
Animals were handled according to a protocol approved by the Institutional Animal Care and Use Committee at Case Western Reserve University and were in accordance with all applicable protocols and guidelines in regard to animal use. In all procedures, the animals were anesthetized with IP injection of ketamine/xylazine cocktail. Gli36 (3 × 105) cells were suspended in growth medium and Matrigel (BD Biosciences) in 1:2 ratio and injected subcutaneously into the flank of athymic nude mice (28 total mice). After 10 days, mice were equally divided into seven groups. Three groups (control group and radiation only groups [t = 5 and t = 24 h]) received 100 µl of intravenous saline and the other four groups (L10 only [t = 5 and t = 24 h] and L10 + radiation groups [t = 5 and t = 24 h]) received 100 µl of 0.3 mg/ml L10 by intravenous injection (IV). One hour after L10 or saline administration, flank tumors in radiation only and L10 + radiation groups were irradiated in a Cs-137 chamber (3 Gy) covering the rest of the body with a customized lead jig for shielding. Five or 24 h post treatment, animals were euthanized by carbon dioxide inhalation, and tumors were excised and stored at − 80°C for protein analysis and histology studies.
Apoptosis analysis by TUNEL assay
The apoptosis of irradiated tumor xenografts was analyzed using the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (Promega, Madison, WI, USA). Mice bearing tumor xenografts were irradiated as explained above and tumors were excised at 5 and 24 h after treatment. Tumor tissue sections were fixed in 4% paraformaldehyde for 15 min, washed, and labeled with solution containing terminal deoxynucleotidyl transferase (TdT) reaction buffer, TdT enzyme, and biotinylated nucleotide for 1 h at 37°C in humidified atmosphere. The tissue sections were washed and DAPI was added for positive staining of cells. The TUNEL staining was observed at 20 × under a Zeiss Axio Observer Z1 motorized FL inverted microscope (Jena, Germany). Images of entire tissue sections were obtained by montage of each section made using automated tiling function of the microscope and analyzed for the positive staining using AxioVision V 4.8 software (Thornwood, NY, USA). The percentage of apoptotic regions relative to the total number of cancer sites was used as a measure of the efficacy of its treatment
Statistical analysis
All data are presented as mean ± SD (standard deviation) unless otherwise noted. Cell viability data are normalized with respect to untreated control. Statistical significance of differences between experimental groups in the clonogenic survival, comet, γ-H2AX, and TUNEL assays were derived using one-way ANOVA model with a Tukey’s range test for multicomparison. All statistical calculations were performed using the statistics toolbox in MATLAB®.
Results
Pluronics are relatively non-toxic to Gli36 cells
Figure 1B shows the relative cell viabilities 24 h after 1 h exposure to Pluronic L10, L64, and L61concentrations ranging from 0–0.3 mg/ml. Results demonstrated decrease of mitochondrial succinate dehydrogenase activity is not significant at the lowest dose (~ 0.025 mg/ml) of each Pluronic. However, cells were more sensitive to higher doses (0.1–0.3 mg/ml) of L64 and L61. Mitochondrial enzyme activity of cells treated with 0.3 mg/ml of L64 was 81 ± 5% of untreated control. The most reduction of enzyme activity was observed in cells treated with 0.3 mg/ml of Pluronic L61, which reduced cell viability to 70 ± 3% of untreated control. As seen in Figure 1B, L10 treatment maintained enzyme activity higher than 94 ± 13% for all tested concentrations. At a dose of 0.3 mg/ ml, the enzyme activity of each Pluronic treatment group was significantly different from the others (p < 0.05). The results demonstrate relatively high enzyme activity (thus low toxicity) of L10 to Gli36 cells. On the basis of mitochondrial enzyme activity data, L10 at 0.3 mg/ml was selected for subsequent radiation experiments.
Pluronic L10 sensitizes Gli36 cells to radiation
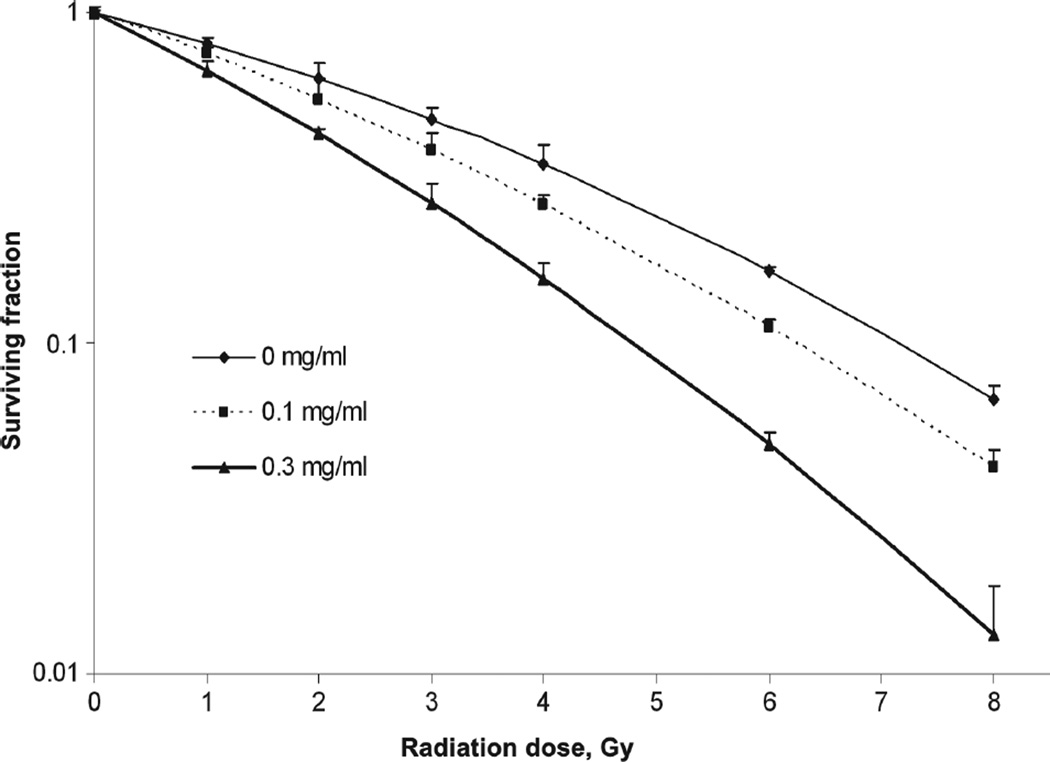
To test the effects of Pluronic L10 on radiosensitivity of Gli36 cells, clonogenic assays were performed after exposure to 0–8 Gy of radiation with and without 1 h pretreatment with 0.1 or 0.3 mg/ml L10. The cell survival curves for each treatment are presented in Figure 2. A significant radiosensitizing effect (p < 0 .01) of 0.3 mg/ml L10 was achieved compared to radiation only at each radiation dose. The data were fit to the linear-quadratic model (Equation 1), and the calculated parameters of radiosensitivity are presented in Table I. The dominant change was found in the alpha (α) coefficient, which was increased by both concentrations of L10, indicating a steeper slope of the initial component of the radiation survival curves (increased by 41 and 99% by 0.1 and 0.3 mg/ml, respectively). The Table also includes the dose enhancement factor (DEF), which is the ratio of the dose reducing the surviving fraction to 0.1 in the absence vs. presence of L10. DEF 0.1 was 1.14 for 0.1 mg/ml L10 and 1.52 for 0.3 mg/ml L10. A third indication of radiosensitization by Pluronic L10 was the mean inactivation dose (MID), which was decreased as the L10 concentration increased. These data indicate that Pluronic L10 is a radiosensitizer for Gli36 cells.
Figure 2.
Clonogenic survival of Gli36 cells. Cells were exposed to radiation doses of 0–8 Gy, with and without 1 h pretreatment with Pluronic L10 (0.1 and 0.3 mg/ml). After 10 days incubation, colonies were fixed and stained, and colonies containing > 50 cells were counted. Surviving fraction was determined by dividing the plating efficiency of irradiated cultures by the plating efficiency of un-irradiated cultures, which was 70.8% without L10 and 65.9% and 61.9% for 0.1 and 0.3 mg/ml L10, respectively. Survival curve data from three independent experiments are expressed as mean with ± SD. * The difference between radiation and L10 + radiation values were significant at each dose (p < 0.05).
Table I.
Comparison of α and β parameters, the dose enhancement factor (DEF), and the mean inactivation dose (MID) without and with Pluronic L10.
| Group | α(Gy−1) | β(Gy−2) | α/β | DEF0.1 | MID |
|---|---|---|---|---|---|
| IR alone | 0.192 | 0.018 | 10.6 | 1.00 | 3.50 |
| + L10 (0.1 mg/ml) | 0.272 | 0.015 | 17.6 | 1.14 | 3.02 |
| + L10 (0.3 mg/ml) | 0.383 | 0.019 | 19.4 | 1.52 | 2.39 |
Effect of Pluronic L10 on Hsp90 level in vitro
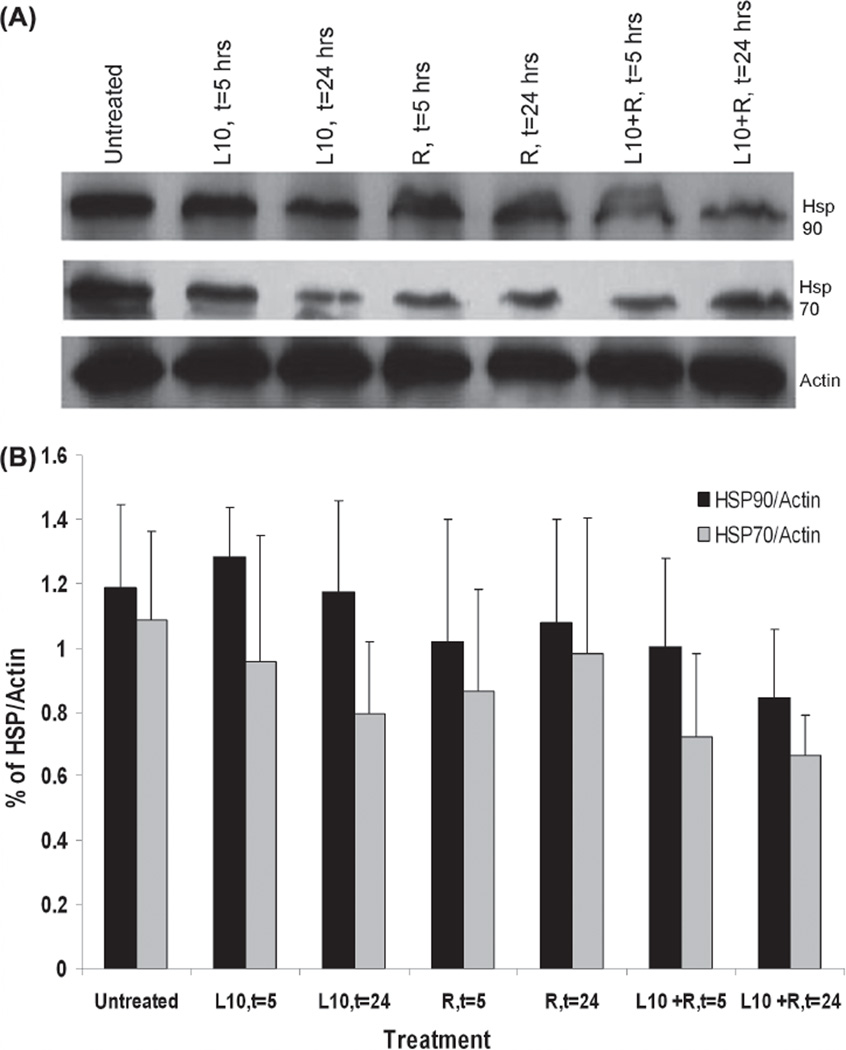
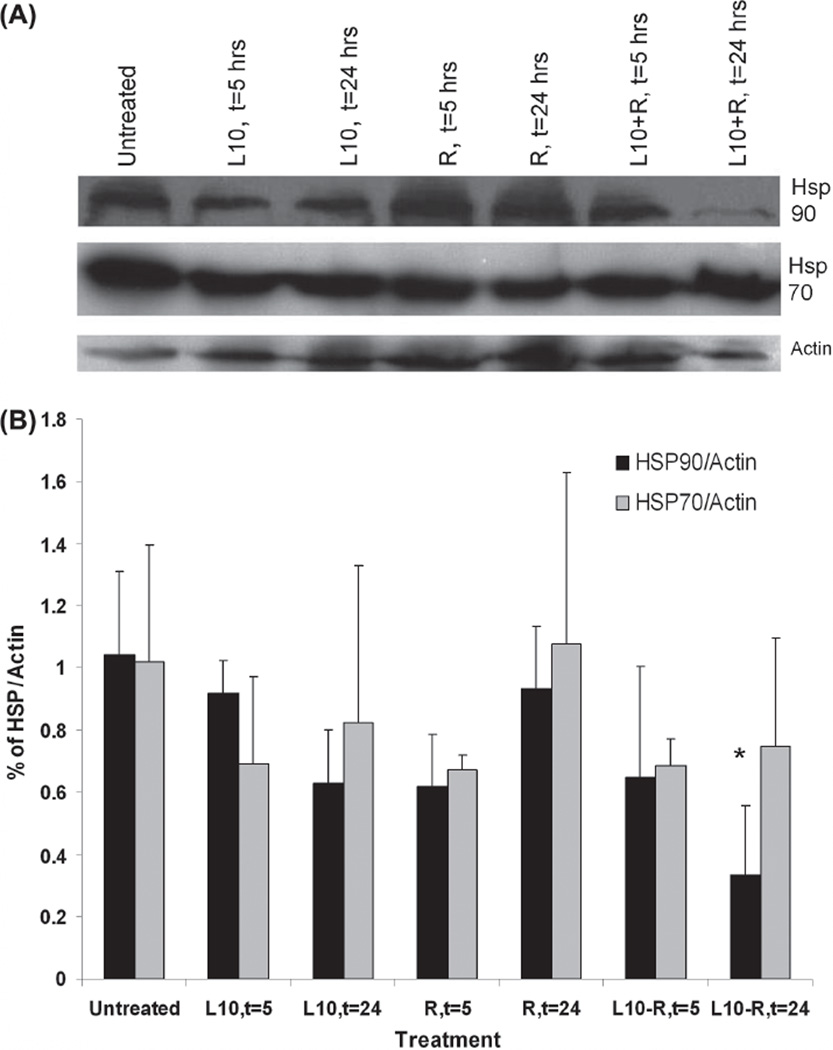
Hsp90 and Hsp70 expression in Gli36 cells was determined by immunoblot analysis for cells that were irradiated (3 Gy) with or without 1 h pretreatment with Pluronic L10. Figure 3 shows Hsp90 and Hsp70 protein levels in lysates recovered after 5 or 24 h of post-treatment incubation. There was no significant decrease in either protein at 5 h after treatment. However, by 24 h after the combined treatment, it appeared that the Hsp90 and Hsp70 levels were reduced by 30% and 39%, respectively, compared to the untreated control, although the differences were not statistically significant.
Figure 3.
Immunoblot analysis of Hsp90 and Hsp70 in Gli36 cells in vitro. (A) Representative Western blot of in vitro Hsp90 and Hsp70 levels after 1 h pretreatment with 0.3 mg/ml Pluronic L10 (L10), 3 Gy radiation only (R), and combined treatment (L10 + R) at different time points post-treatment (t = 5, t = 24 h). (B) Hsp expression at different time points was normalized versus actin. Each peak is the mean of three independent measurements ± SD.
Pluronic L10 slows the repair of DNA double-strand breaks
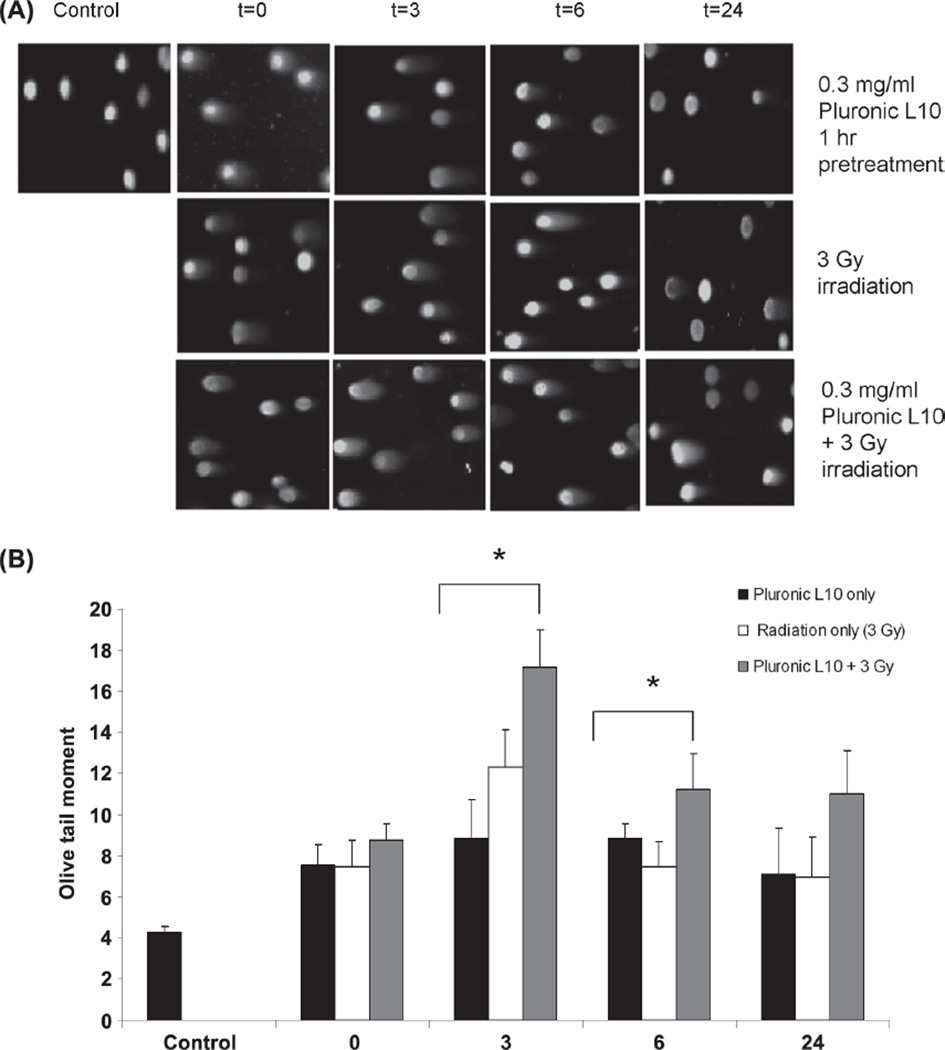
As shown in Figure 4, cells containing damaged DNA were observed as a comet with a bright head and elongated tail. In contrast, healthy DNA emerged as an intact nucleus with no tail. Seventy-five randomly selected, individual, and non-overlapping cells were scored for DNA damage analysis. As expected, DNA damage was observed after treatment with radiation alone and L10 + radiation. Three hours after treatment, all samples showed maximum DNA damage. The Olive tail moment of the irradiated cells was 12.4 ± 1.7, but when radiation was combined with L10, the tail moment significantly increased to 17.2 ± 1.7 (p < 0.01). Six hours after treatments, Olive tail moment decreased for radiation only and combined treatment groups. However 24 h after the combined treatment, the Olive tail moment was 58% higher than for the individual treatments at the same time point.
Figure 4.
Comet images of Gli36 cells obtained from neutral single-cell gel electrophoresis (SCGE). (A) Comet images of Gli36 cells after treatment with 0.3 mg/ml L10 (1 h), 3 Gy radiation, and combination of L10 and radiation. (B) Comet tail moment was calculated as the product of tail length and the fraction of DNA in the tail for each treatment and each time point. The data are the mean of two individual trials (scored 75 cells in each) ± SD. * p < 0.01 as compared to the other treatment conditions at the same time points. “ This Figure is reproduced in color in the online version of International Journal of Radiation Biology.”
Pluronic L10 increases γ-H2AX focus formation in Gli36 cells
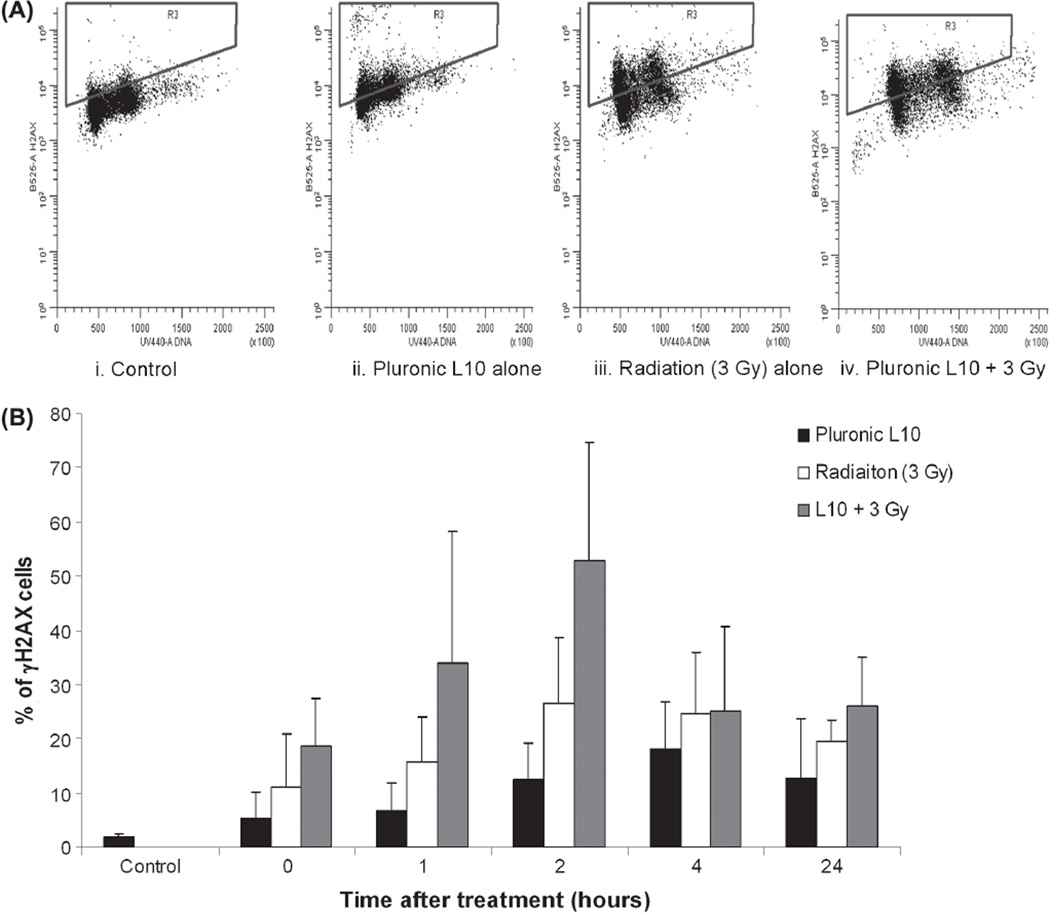
To further elucidate the effect of L10 on DNA damage repair, the level of phosphorylated histone H2AX (γ-H2AX) was analyzed by flow cytometry. Cells were exposed to L10 for 1 h, irradiated with 3 Gy and at different time points, cell samples were fixed, stained and analyzed for γ-H2AX staining. As demonstrated in Figure 5, immediately after combined treatment, γ-H2AX staining was increased compared to the untreated and control groups. The level of γ-H2AX foci reached a maximum 2 h after the combined treatment. After the 2 h time point, phosphorylation of γ-H2AX decreased with time. Similar to the comet assay results, the level of γ-H2AX remained higher than the initial level 24 h after the combined treatment and higher than that of the individual treatments.
Figure 5.
Flow cytometric detection of γ-H2AX formation in Gli36 cells after irradiating with Pluronic L10. (A) The bivariate distribution of cells 2 h after treatment with 0.3 mg/ml Pluronic L10, 3 Gy, or Pluronic L10 + 3 Gy. Y axis is γ-H2AX staining and X axis is the DNA content. Notice the presence of increased γ-H2AX staining (as gated) after combined treatment. (B) Percentage of cells with γ-H2AX staining after irradiation (3 Gy) with and without pretreatment with 0.3 mg/ml Pluronic L10. Analysis was carried out by flow cytometry after γ-H2AX staining of treated cells. Two hours after treatment, cells treated with both Pluronic L10 and radiation show the highest percentage of γ-H2AX staining. Values are an average of three independent experiments ± SD.
Pluronic L10 decreases the level of Hsp90 in tumor xenografts
To investigate the in vivo potential of combining L10 and irradiation, Gli36 tumors were inoculated in nude mice. The tumor xenografts were irradiated with and without L10, and tissue lysates were collected as described in Methods. Figure 6 shows a representative immunoblot of in vivo Hsp analysis. Hsp90 levels within tumor tissues decreased significantly by 67.9% at 24 h after the combined treatment compared to the untreated control tumors (p < 0.05). In addition, 24 h after the combined treatment, there was a trend toward a decrease in Hsp70 levels by 26.6% compared to the untreated control; the decrease was not statistically significant.
Figure 6.
Representative Western blot of Hsp90 and Hsp70 protein analysis from Gli36 tumor xenografts (A) Hsp90 and Hsp70 expresion after 1 h pretreatment of 0.3 mg/ml Pluronic L10 (L10), 3 Gy radiation only (R), and combined treatment (L10 + R) at different times of post-treatment (t = 5, t = 24 h). (B) Hsp expression at different time points normalized versus actin. Each peak is the mean of four individual experiments ± SD. * p < 0.01 combined treatment vs. untreated control.
Pluronic L10 sensitizes tumors to radiotherapy in nude mice by increasing apoptosis
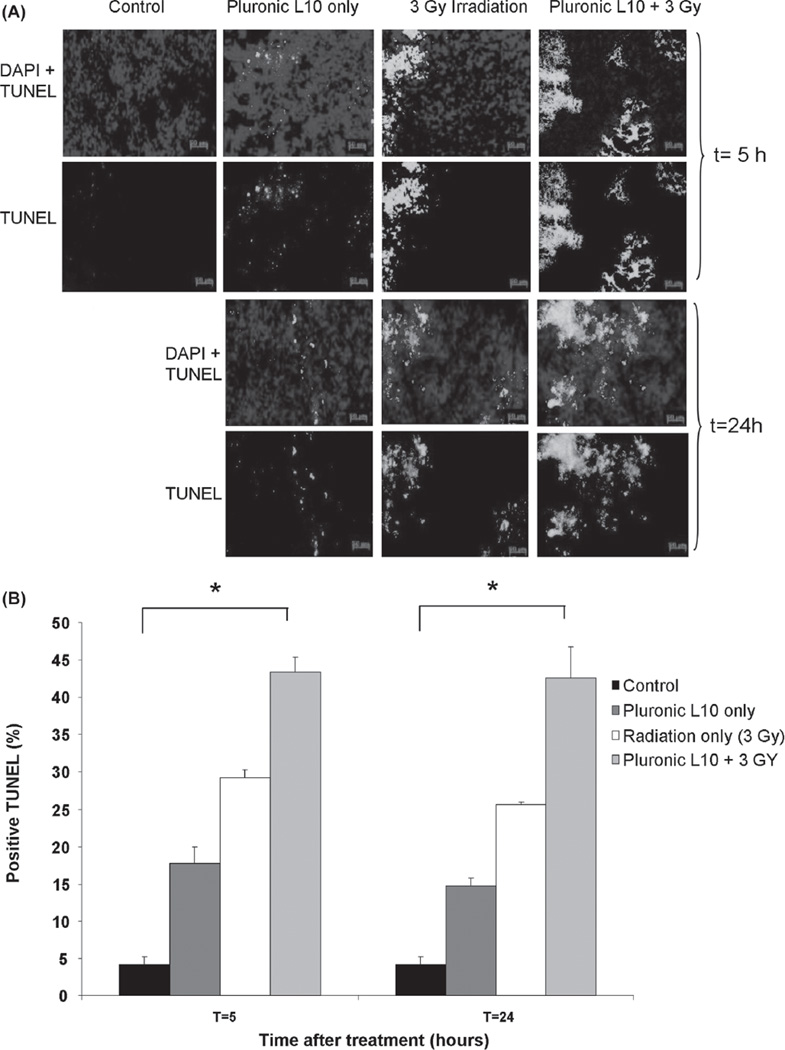
Histological examination of Gli36 tumor xenografts using the TUNEL assay (Figure 7) demonstrated differences in apoptosis between each treatment condition. Apoptosis at both time points (5 and 24 h after treatment) was significantly elevated in combined-treatment samples (43.3 ± 2.0% at t = 5 h and 42.5 ± 4.0% at t = 24 h) compared to those receiving individual treatments (Pluronic L10 only: 17.8 ± 2.0% at t = 5 h and 14.7 ± 1.0% at t = 24 h, Radiation only: 29.2 ± 1.0% at t = 5 h and 25.6 ± 1.0% at t = 2 4 h) with p < 0.01. One day after the combined treatment the level of apoptosis remained the same as observed at 5 h. These data suggest an additive effect of L10 in elevation of apoptosis in the Gli36 tumor xenograft model.
Figure 7.
TUNEL staining of Gli36 tumor sections (A) TUNEL staining after irradiation with 3 Gy with and without 1 h pretreatment with Pluronic L10 (0.3 mg/ml). Apoptotic nuclei are shown in green and normal nuclei are shown in blue, stained by DAPI. (B) TUNEL positive cells are expressed as the percentage of total cell number. The data obtained from three individual tumor xenografts are presented as mean value ± SD. * p < 0.01. “ Th is Figure is reproduced in color in the online version of International Journal of Radiation Biology.”
Discussion
Our analysis indicates that Gli36 cells acquired enhanced sensitivity to ionizing radiation after being treated with Pluronic L10. Pluronic block copolymers have a large spectrum of properties by which they are distinguished from one another, including molecular weight, EO and PO units, hydrophilic-lipophilic balance (HLB), and the critical micelle concentration (CMC), that could influence their functionality and sensitizing effects for hyperthermia, chemotherapy, and radiotherapy. Pluronics have demonstrated high circulation time in the body having a half life (t 1/2) of 60–90 h following intravenous administration (Batrakovaet al. 2004). Taking advantage of these sensitizing properties, Pluronic L61 has been incorporated into a novel formulation of doxorubicin (SP1049C) currently undergoing Phase II clinical trials (Batrakova and Kabanov 2008).
Based on our preliminary cell viability data, Pluronic L10 was less toxic to Gli36 cells than the other Pluronics tested (L61 and L64) and was utilized for studying the effect of Pluronic in radiosensitization in GBM. Pluronic L10 has a molecular weight of 3200 Da, is composed of 49.7 PPO units and 7.3 EEO units, and has a calculated HLB of 2 (Krupka and Exner 2011). Due to the high ratio of PPO: EEO segment, Pluronic L10 has a highly lipophilic nature and likely has an increased capability to change cellular membrane fluidity. Higher values of DEF 0.1 and enhanced α / β ratio for the combined treatment obtained from the clonogenic assays indicate that L10 functions as a sensitizer for radiation therapy. Additionally, our Western blot data demonstrate somewhat decreased Hsp90 levels 24 h after combined treatment (L10 treatment followed by γ-irradiation) compared to untreated controls. Hsp90 supports a diverse range of client proteins involved with radioresistance (e.g., Raf-1, Akt and ErbB2), and the reduction of Hsp90 levels suppresses those signaling molecules involved in tumor cell survival following radiation-induced injury (Schulte et al. 1995, Pirollo et al. 1997, Sato et al. 2000, Gupta et al. 2001, Bull et al. 2004, Tanno et al. 2004). Previous studies with known inhibitors of Hsp90 (such as 17-AAG and 17-DMAG) have shown that inhibition of Hsp90 in many cancers results in a concomitant increased expression of Hsp70 (Drysdale et al. 2006). The accompanying increase in Hsp70 protects cells from various factors, including oxidative stress, and increases cancer cell survival after the therapy (Drysdale et al. 2006, Harrison et al. 2008). Interestingly, our results demonstrate that L10 has the ability to modulate the expression of not only Hsp90 but Hsp70 as well. Additionally, activation of both Hsp transcription (Price and Calderwood 1991) and its chaperoning function (Shpund and Gershon 1997) have been shown to be ATP-dependent processes. As stated previously, Pluronic has been shown to deplete intracellular ATP levels, which may provide a mechanism for the suppression of Hsp70 and 90 expression following radiation injury.
Phosphorylation of H2AX is a reporter of DNA damage, and quantification of the γ-H2AX in cell culture is a method to follow the DNA DSB level that is induced by radiation therapy. Measuring γ-H2AX staining by flow cytometry offers quantitative results. The neutral comet assay provides another selective measurement of DNA DSB. The DNA damage analysis from both the comet assay and γ-H2AX flow cytometry demonstrates the reduced capacity of cells exposed to the combined treatment to remove DSB, resulting in a higher level of residual DSB after treatment with both Pluronic and radiation as compared to radiation alone.
Most GBM express a DNA repair phenotype that relates to its inherent radioresistance and poor clinical outcomes. Homologous recombination (HR) is one of the pathways for DSB repair, which involves Rad51 recombinase in conjunction with BRCA1 and BRCA2 (Wang et al. 2011). BRCA2 is a recognized client protein of Hsp90, and therefore Hsp90 inhibitors (such as 17-AAG) result in degradation of BRCA2 and promote destruction of Rad51 (Noguchi et al. 2006, Dungey et al. 2009). In a similar manner, the decreased expression of Hsp90 with treatment of Pluronic L10 in GBM may lead to suppression of the level of BRCA2 and other proteins in the DSB repair pathway. In-depth studies of the gene and protein expression of BRCA1 and BRCA2, and Rad51 are necessary to elucidate the underlying pathway by which Pluronic L10 increases the cells ability to recover from DNA damage after exposure to radiation. Additionally, it has been reported that Hsp70 decreases the lag time between the G2/M phase transition after irradiation, which decreases p53 expression and consequently improves resistance to treatment (Lee et al. 2001). Therefore the reduction in Hsp70 expression may lead to a G2/M phase arrest and increased apoptosis.
Tumor xenografts generated from Gli36 cells exhibited enhanced radioresponse when L10 was also administered. Levels of Hsp90 as well as Hsp70 in tumor tissues were reduced 24 h after the combined treatment. Due to the heterogeneous cell populations present in the tumor xenografts tissue homogenate, slight differences occurred between the in vitro and in vivo Hsp expression (Inda et al. 2010, Durrett et al. 2011). Additionally, apoptosis was increased in the L10 + radiation treatment group compared to the untreated and radiation only controls at both 5 and 24 h after treatment. While it is unusual for apoptosis to become evident at 5 h, prior observations of apoptosis on the same time scale (~ 4–6 h after irradiation) have been reported in cells in which apoptosis is the primary method of cell death (Meyn 1997). To further evaluate the role of apoptosis in Gli36 cells and xenografts, other methods such as Annexin V staining and Caspase 3 analysis could be beneficial (Psarros et al. 2005).
Overall, these observations suggest that Pluronic L10 is an effective radiosensitizer, both in vitro and in vivo. The significant decrease in Hsp90 expression noted in vivo may in part explain the radiosensitization effects of L10 in Gli36 cells, but overall the mechanism behind the sensitization is currently uncertain. In addition, making Pluronic attractive for potential future clinical translation is the fact that Pluronic has been shown to enhance the transport of several materials, such as horseradish peroxidase (HRP) and leptin, across the blood-brain barrier (BBB) both in vitro and in vivo (Batrakova et al. 2005, Price et al. 2010). The penetrating ability of Pluronic in BBB may further enhance outcome of radiotherapy in GBM. Our preliminary results suggest a complex and multi-faceted mechanism behind the radiosensitization effects of L10 which may, at least in part, be due to reduction in Hsp70 and Hsp90 levels. Additional research is warranted to further elucidate the mechanisms and effects of Pluronic on radiation therapy.
Acknowledgements
We are grateful to the Case Comprehensive Cancer Center (P30 CA43703) for providing the following core facilities used in this research: Radiation Resources, Athymic Animal and Xenograft, Flow Cytometry and Translational Research. We also thank Michael Sramkoski (Flow Cytometry Core Facility) for assistance with the flow cytometry data analysis, and Dr John Pink (Translational Research Core Facility) for assistance with the comet assays. We thank Dr Pubudu Peiris, Dr Luis Solorio, and Joseph Meyers for their support during this study.
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award number R01CA136857 to AAE. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alakhov V, Moskaleva E, Batrakova EV, Kabanov AV. Hypersensitization of multidrug resistant human ovarian carcinoma cells by pluronic P85 block copolymer. Bioconjugate Chemistry. 1996;7:209–216. doi: 10.1021/bc950093n. [DOI] [PubMed] [Google Scholar]
- Batrakova E, Lee S, Li S, Venne A, Alakhov V, Kabanov A. Fundamental relationships between the composition of pluronic block copolymers and their hypersensitization effect in MDR cancer cells. Pharmaceutical Research. 1999;16:1373–1379. doi: 10.1023/a:1018942823676. [DOI] [PubMed] [Google Scholar]
- Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: Contributions of energy depletion and membrane fluidization. Journal of Pharmacology and Experimental Therapeutics. 2001;299:483–493. [PubMed] [Google Scholar]
- Batrakova EV, Li S, Li Y, Alakhov VY, Elmquist WF, Kabanov AV. Distribution kinetics of a micelle-forming block copolymer Pluronic P85. Journal of Controlled Release. 2004;100:389–397. doi: 10.1016/j.jconrel.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Batrakova EV, Vinogradov SV, Robinson SM, Niehoff ML, Banks WA, Kabanov AV. Polypeptide point modifications with fatty acid and amphiphilic block copolymers for enhanced brain delivery. Bioconjugate Chemistry. 2005;16:793–802. doi: 10.1021/bc049730c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakova EV, Kabanov AV. Pluronic block copolymers: Evolution of drug delivery concept from inert nanocarriers to biological response modifiers. Journal of Controlled Release. 2008;130:98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakova EV, Li S, Brynskikh AM, Sharma AK, Li Y, Boska M, Gong N, Mosley RL, Alakhov VY, Gendelman HE, Kabanov AV. Effects of pluronic and doxorubicin on drug uptake, cellular metabolism, apoptosis and tumor inhibition in animal models of MDR cancers. Journal of Controlled Release. 2010;143:290–301. doi: 10.1016/j.jconrel.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht KS, Bradbury CM, Mattson D, Kaushal A, Sowers A, Markovina S, Ortiz KL, Sieck LK, Isaacs JS, Brechbiel MW, Mitchell JB, Neckers LM, Gius D. Geldanamycin and 17-allylamino-17-demethoxygeldanamycin potentiate the in vitro and in vivo radiation response of cervical tumor cells via the heat shock protein 90-mediated intracellular signaling and cytotoxicity. Cancer Research. 2003;63:8984–8995. [PubMed] [Google Scholar]
- Bull EE, Dote H, Brady KJ, Burgan WE, Carter DJ, Cerra MA, Oswald KA, Hollingshead MG, Camphausen K, Tofilon PJ. Enhanced tumor cell radiosensitivity and abrogation of G2 and S phase arrest by the Hsp90 inhibitor 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin. Clinical Cancer Research. 2004;10:8077–8084. doi: 10.1158/1078-0432.CCR-04-1212. [DOI] [PubMed] [Google Scholar]
- Chiappetta DA, Sosnik A. Poly(ethylene oxide)-poly(propylene oxide) block copolymer micelles as drug delivery agents: improved hydrosolubility, stability and bioavailability of drugs. Eur J Pharm Biopharm. 2007;66:303–317. doi: 10.1016/j.ejpb.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Drysdale MJ, Brough PA, Massey A, Jensen MR, Schoepfer J. Targeting Hsp90 for the treatment of cancer. Current Opinion in Drug Discovery & Development. 2006;9:483–495. [PubMed] [Google Scholar]
- Dungey FA, Caldecott KW, Chalmers AJ. Enhanced radio-sensitization of human glioma cells by combining inhibition of poly(ADP-ribose) polymerase with inhibition of heat shock protein 90. Molecular Cancer Therapeutics. 2009;8:2243–2254. doi: 10.1158/1535-7163.MCT-09-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrett R, Foo J, Leder K, Mayberry J, Michor F. Intratumor heterogeneity in evolutionary models of tumor progression. Genetics. 2011;188:461–477. doi: 10.1534/genetics.110.125724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enmon R, Yang WH, Ballangrud AM, Solit DB, Heller G, Rosen N, Scher HI, Sgouros G. Combination treatment with 17-N-allylamino-17-demethoxy geldanamycin and acute irradiation produces supra-additive growth suppression in human prostate carcinoma spheroids. Cancer Research. 2003;63:8393–8399. [PubMed] [Google Scholar]
- Exner AA, Krupka TM, Scherrer K, Teets JM. Enhancement of carboplatin toxicity by Pluronic block copolymers. Journal of Controlled Release. 2005;106:188–197. doi: 10.1016/j.jconrel.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Ferrarini M, Heltai S, Zocchi MR, Rugarli C. Unusual expression and localization of heat-shock proteins in human tumor cells. International Journal of Cancer. 1992;51:613–619. doi: 10.1002/ijc.2910510418. [DOI] [PubMed] [Google Scholar]
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes & Development. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Gerweck LE, Vijayappa S, Kurimasa A, Ogawa K, Chen DJ. Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Cancer Research. 2006;66:8352–8355. doi: 10.1158/0008-5472.CAN-06-0533. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Bakanauskas VJ, Cerniglia GJ, Cheng Y, Bernhard EJ, Muschel RJ, McKenna WG. The Ras radiation resistance pathway. Cancer Research. 2001;61:4278–4282. [PubMed] [Google Scholar]
- Harashima K, Akimoto T, Nonaka T, Tsuzuki K, Mitsuhashi N, Nakano T. Heat shock protein 90 (Hsp90) chaperone complex inhibitor, radicicol, potentiated radiation-induced cell killing in a hormone-sensitive prostate cancer cell line through degradation of the androgen receptor. International Journal of Radiation Biology. 2005;81:63–76. doi: 10.1080/09553000400029460. [DOI] [PubMed] [Google Scholar]
- Harrison EM, Sharpe E, Bellamy CO, McNally SJ, Devey L, Garden OJ, Ross JA, Wigmore SJ. Heat shock protein 90-binding agents protect renal cells from oxidative stress and reduce kidney ischemia-reperfusion injury. American Journal of Physiology. 2008;295:F397–F405. doi: 10.1152/ajprenal.00361.2007. [DOI] [PubMed] [Google Scholar]
- Inda MM, Bonavia R, Mukasa A, Narita Y, Sah DW, Vandenberg S, Brennan C, Johns TG, Bachoo R, Hadwiger P, Tan P, Depinho RA, Cavenee W, Furnari F. Tumor heterogeneity is an active process maintained by a mutant EGFR-induced cytokine circuit in glioblastoma. Genes and Development. 2010;24:1731–1745. doi: 10.1101/gad.1890510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanov AV, Batrakova EV, Alakhov VY. Pluronic block copolymers as novel polymer therapeutics for drug and gene delivery. Journal of Controlled Release. 2002;82:189–212. doi: 10.1016/s0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kabanov AV, Batrakova EV, Alakhov VY. An essential relationship between ATP depletion and chemosensitizing activity of Pluronic block copolymers. Journal of Controlled Release. 2003;91:75–83. doi: 10.1016/s0168-3659(03)00211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupka TM, Weinberg BD, Ziats NP, Haaga JR, Exner AA. Injectable polymer depot combined with radiofrequency ablation for treatment of experimental carcinoma in rat. Investigative Radiology. 2006;41:890–897. doi: 10.1097/01.rli.0000246102.56801.2f. [DOI] [PubMed] [Google Scholar]
- Krupka TM, Weinberg BD, Wu H, Ziats NP, Exner AA. Effect of intratumoral injection of carboplatin combined with pluronic P85 or L61 on experimental colorectal carcinoma in rats. Experimental Biology and Medicine (Maywood) 2007;232:950–957. [PubMed] [Google Scholar]
- Krupka TM, Exner AA. Structural parameters governing activity of Pluronic triblock copolymers in hyperthermia cancer therapy. International Journal of Hyperthermia. 2011;27:663–671. doi: 10.3109/02656736.2011.599828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz D, Fleckenstein K, Eble M, Blatter J, Wannenmacher M, Weber KJ. Radiosensitizing potential of gemcitabine (2′,2′-difluoro-2′-deoxycytidine) within the cell cycle in vitro. International Journal of Radiation Oncology, Biology, Physics. 1998;41:875–882. doi: 10.1016/s0360-3016(98)00105-9. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Choi SA, Lee KH, Chung HY, Kim TH, Cho CK, Lee YS. Role of inducible heat shock protein 70 in radiation-induced cell death. Cell Stress Chaperones. 2001;6:273–281. doi: 10.1379/1466-1268(2001)006<0273:roihsp>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN. Molecular pathology of malignant gliomas. Annual Review of Pathology. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]
- Machida H, Matsumoto Y, Shirai M, Kubota N. Geldanamycin, an inhibitor of Hsp90, sensitizes human tumour cells to radiation. International Journal of Radiation Biology. 2003;79:973–980. doi: 10.1080/09553000310001626135. [DOI] [PubMed] [Google Scholar]
- Meyn RE. Apoptosis and response to radiation: Implications for radiation therapy. Oncology (Williston Park) 1997;11:349–356. discussion 356, 361, 365. [PubMed] [Google Scholar]
- Morimoto RI, Kline MP, Bimston DN, Cotto JJ. The heat-shock response: Regulation and function of heat-shock proteins and molecular chaperones. Essays in Biochemistry. 1997;32:17–29. [PubMed] [Google Scholar]
- Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends in Molecular Medicine. 2002;8:S55–S61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Yu D, Hirayama R, Ninomiya Y, Sekine E, Kubota N, Ando K, Okayasu R. Inhibition of homologous recombination repair in irradiated tumor cells pretreated with Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Biochemical and Biophysical Research Communications. 2006;351:658–663. doi: 10.1016/j.bbrc.2006.10.094. [DOI] [PubMed] [Google Scholar]
- Peng B, Xu L, Cao F, Wei T, Yang C, Uzan G, Zhang D. HSP90 inhibitor, celastrol, arrests human monocytic leukemia cell U937 at G0/G1 in thiol-containing agents reversible way. Molecular Cancer. 2010;9:79. doi: 10.1186/1476-4598-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RH, Krupka TM, Wu H, Traughber B, Dremann D, Broome AM, Exner AA. Role of Pluronic block copolymers in modulation of heat shock protein 70 expression. International Journal of Hyperthermia. 2011;27:672–681. doi: 10.3109/02656736.2011.608218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirollo KF, Hao Z, Rait A, Ho CW, Chang EH. Evidence supporting a signal transduction pathway leading to the radiation-resistant phenotype in human tumor cells. Biochemical and Biophysical Research Communications. 1997;230:196–201. doi: 10.1006/bbrc.1996.5922. [DOI] [PubMed] [Google Scholar]
- Price BD, Calderwood SK. Ca2+ is essential for multistep activation of the heat shock factor in permeabilized cells. Molecular and Cellular Biology. 1991;11:3365–3368. doi: 10.1128/mcb.11.6.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TO, Farr SA, Yi X, Vinogradov S, Batrakova E, Banks WA, Kabanov AV. Transport across the blood-brain barrier of pluronic leptin. The Journal of Pharmacology and Experimental Therapeutics. 2010;333:253–263. doi: 10.1124/jpet.109.158147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psarros TG, Mickey B, Gilio J, Drees J, Gall K, Carlson D, Giller C, Willis MS. Gliosarcoma cell death after radiosurgery in a rat model. Minimally Invasive Neurosurgery. 2005;48:142–148. doi: 10.1055/s-2004-830266. [DOI] [PubMed] [Google Scholar]
- Russell JS, Burgan W, Oswald KA, Camphausen K, Tofilon PJ. Enhanced cell killing induced by the combination of radiation and the heat shock protein 90 inhibitor 17-allylamino-17-demethoxygeldanamycin: A multitarget approach to radiosensitization. Clinical Cancer Research. 2003;9:3749–3755. [PubMed] [Google Scholar]
- Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proceedings of the National Academy of Sciences of the USA. 2000;97:10832–10837. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte TW, Blagosklonny MV, Ingui C, Neckers L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. The Journal of Biological Chemistry. 1995;270:24585–24588. doi: 10.1074/jbc.270.41.24585. [DOI] [PubMed] [Google Scholar]
- Shintani S, Mihara M, Li C, Nakahara Y, Hino S, Nakashiro K, Hamakawa H. Up-regulation of DNA-dependent protein kinase correlates with radiation resistance in oral squamous cell carcinoma. Cancer Science. 2003;94:894–900. doi: 10.1111/j.1349-7006.2003.tb01372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpund S, Gershon D. Alterations in the chaperone activity of HSP70 in aging organisms. Archives of Gerontology and Geriatrics. 1997;24:125–131. doi: 10.1016/s0167-4943(96)00745-5. [DOI] [PubMed] [Google Scholar]
- Sun Y, Huang YC, Xu QZ, Wang HP, Bai B, Sui JL, Zhou PK. HIV-1 Tat depresses DNA-PK(CS) expression and DNA repair, and sensitizes cells to ionizing radiation. International Journal of Radiation Oncology, Biology, Physics. 2006;65:842–850. doi: 10.1016/j.ijrobp.2006.02.040. [DOI] [PubMed] [Google Scholar]
- Tanno S, Yanagawa N, Habiro A, Koizumi K, Nakano Y, Osanai M, Mizukami Y, Okumura T, Testa JR, Kohgo Y. Serine/threonine kinase AKT is frequently activated in human bile duct cancer and is associated with increased radioresistance. Cancer Research. 2004;64:3486–3490. doi: 10.1158/0008-5472.CAN-03-1788. [DOI] [PubMed] [Google Scholar]
- Wang QE, Milum K, Han C, Huang YW, Wani G, Thomale J, Wani AA. Differential contributory roles of nucleotide excision and homologous recombination repair for enhancing cisplatin sensitivity in human ovarian cancer cells. Molecular Cancer. 2011;10:24. doi: 10.1186/1476-4598-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Zhang H, Burrows F, Zhang L, Shores CG. Potent activity of a novel dimeric heat shock protein 90 inhibitor against head and neck squamous cell carcinoma in vitro and in vivo. Clinical Cancer Research. 2005;11:3889–3896. doi: 10.1158/1078-0432.CCR-04-2272. [DOI] [PubMed] [Google Scholar]
- Zhao M, Ma J, Zhu HY, Zhang XH, Du ZY, Xu YJ, Yu XD. Apigenin inhibits proliferation and induces apoptosis in human multiple myeloma cells through targeting the trinity of CK2, Cdc37 and Hsp90. Molecular Cancer. 2011;10:104. doi: 10.1186/1476-4598-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]