Abstract
Spinal glial gap junctions may play an important role in dorsal horn neuronal sensitization and neuropathic pain. In rats after an L5 spinal nerve ligation (SNL), we examined the effects of intrathecal injection of carbenoxolone (CBX), a gap junction decoupler, on neuropathic pain manifestations and on wide-dynamic range (WDR) neuronal activity in vivo. Intrathecal injection of CBX dose-dependently (0.1–50 μg, 10 μl) inhibited mechanical hypersensitivity in rats at 2-3 weeks post-SNL. However, the same doses of glycyrrhizic acid (an analogue of CBX but does not affect gap junctions) and mefloquine hydrochloride (a selective neuronal gap junction decoupler) were ineffective. Intrathecal CBX (5 μg) also attenuated heat hypersensitivity in SNL rats. Further, rats did not develop tachyphylaxis to CBX-induced inhibition of mechanical hypersensitivity after repetitive drug treatments (25 μg/day) during days 14-16 post-SNL. Electrophysiological study in SNL rats showed that spinal topical application of CBX (100 μg, 50 μl), which mimics intrathecal drug administration, attenuated WDR neuronal responses to mechanical stimuli and to repetitive intracutaneous electrical stimuli (0.5 Hz) that induce windup, a short-form of activity-dependent neuronal sensitization. Current findings suggest that the inhibition of neuropathic pain manifestations by intrathecal injection of CBX in SNL rats may involve an inhibition of glial gap junctions and an attenuation of WDR neuronal activity in the dorsal horn.
Keywords: Glia, gap junction, carbenoxolone, neuropathic pain
1. Introduction
Peripheral nerve injury may lead to hypersensitivity to mechanical and thermal stimuli, and increase dorsal horn neuron excitability. The neighboring glial cells may also play an important role in exaggerated spinal nociceptive transmission after nerve injury[1]. A principal mechanism of communication between glial cells is through gap junctions[2], which allow second messengers (e.g., cAMP, inositol trisphosphate), ions, and small hydrophilic molecules (e.g., ATP) to pass freely between the cells[3,4]. Mounting evidence suggests that spinal glial gap junctions may play an important role in pathologic pain conditions[5,6]. Blocking gap junctions with carbenoxolone (CBX), a disodium salt of 3’-0-hydrogen succinate of glycyrrhetic acid, induces analgesia in various animal models of persistent pain[5,7,8]. However, one caveat of previous studies is that the specificity of CBX toward glial gap junctions is uncertain, as CBX is a nonselective gap junction decoupler and may also directly affect neuron membrane properties[9]. Further, the mechanisms and the downstream events (e.g., changes in neuronal activity) that contribute to CBX-induced analgesia are not fully clear.
We sought to answer these questions in rats after an L5 spinal nerve ligation (SNL), which is a standardized and highly repeatable neuropathic pain model that has been widely studied[10,11]. We first examined whether intrathecal administration of CBX attenuates behavioral mechanical and heat hypersensitivity in SNL rats. We then attempted to use pharmacological tools to evaluate whether CBX-induced analgesia is largely through inhibiting glial gap junctions. Wide-dynamic range (WDR) neurons represent an important component in the network of spinal nociceptive transmission, and function as an essential cellular mediator of the central neuronal hyperexcitability underlying chronic pain[12,13]. WDR neurons display an action potential windup phenomenon to repetitive noxious stimuli, which reflects a short-term increase in neuronal excitability[12,14]. Finally, we conducted in vivo electrophysiological recording in SNL rats to examine whether spinal topical application of CBX, which mimics intrathecal drug administration, attenuates the responses of WDR neurons to mechanical stimuli and to repetitive intracutaneous electrical stimuli (0.5 Hz) that induce windup.
2. Materials and Methods
2.1. Animals
Experiments were performed on adult male Sprague–Dawley rats (250–350 g, Harlan Laboratories, Inc., Indianapolis, IN). All experimental procedures were conducted in strict accordance with the guidelines established by the Johns Hopkins University Animal Care and Use Committee as consistent with the National Institutes of Health Guide for the Use of Experimental Animals. All animals were euthanized with sodium pentobarbital (100–300 mg, i.p.) at the end of the experiment.
2.2. L5 SNL
The SNL model was produced as described previously[13]. In brief, the rats were anesthetized with isoflurane (2%, Abbott Laboratories, North Chicago, IL), and the left L5 spinal nerve was ligated with a 6-0 silk suture and cut distally under aseptic conditions.
2.3 Behavioral tests
Hypersensitivity to punctuate mechanical stimulation with von Frey filaments (0.38, 0.57, 1.23, 1.83, 3.66, 5.93, 9.13, 13.1 g) was determined with the up-down method[15]. Abrupt paw withdrawal, licking, and shaking were considered positive responses. The paw withdrawal threshold (PWT) was determined according to the method and formula provided by Dixon[16]. Paw withdrawal latency (PWL) to radiant heat stimuli (cut-off time: 20 seconds) was measured with a plantar stimulator analgesia meter (IITC model 390, Woodland Hills, CA)[17]. To minimize experimenter bias, the investigator who performed the behavioral tests was blinded to the drug treatment conditions.
2.4. Intrathecal injection
Drugs were administrated through an implanted intrathecal catheter. For catheter implantation, a small slit was cut on the atlanto-occipital membrane, into which a saline-filled PE-10 tubing (6–7 cm) was inserted. After completing the experiment, intrathecal drug delivery was confirmed by injecting lidocaine (400 μg/20 μl, Hospira, Lack Forest, IL), which resulted in a temporary motor paralysis of the lower limbs.
2.5. Dorsal horn recordings
Extracellular recordings of dorsal horn neuron activity were obtained with microelectrodes as described previously[13]. Briefly, WDR neurons located in deep laminae (400–1200 μm to the dorsal surface) of the lumbar spinal segment were recorded. WDR neurons were characterized by using mechanical stimuli with intensities that ranged from mild to noxious texture[13]. We examined WDR neurons with defined receptive fields (RF) in the plantar region of the hindpaw. For mechanical test, we briefly mapped the RF with a von Frey monofilament (10 g), and a "sensitive site" in the RF was identified for application of von-Frey stimulation (0.6 g–15 g, 3 s). In separate experiment, windup of C-fiber–mediated responses (i.e., C-component) was examined by delivering a train of 16 electrical pulses (supra-C-fiber threshold, 2.0 ms, 0.5 Hz) through a pair of fine stimulating electrodes inserted subcutaneously in the RF[13]. Test module was applied before and 30–60 min after the drug treatment.
2.6. Drugs
Carbenoxolone disodium salt (CBX), glycyrrhizic acid (GCA), and mefloquine hydrochloride (MFQ) were purchased from Sigma-Aldrich. CBX was dissolved in saline (0.9%); GCA and MFQ were dissolved in DMSO initially and then further diluted to the final concentration with saline.
2.7. Data analysis
To establish the dose-response functions for CBX, we calculated percent maximum possible effect (%MPE) values with the equation: %MPE = [(post-drug PWT) − (pre-drug PWT)]/[(cut-off) − (pre-drug PWT)] × 100, where cutoff PWT=21.5 g. The methods for statistical comparisons in each study are given in the figure legends. STATISTICA 6.0 software (StatSoft, Inc., Tulsa, OK) was used to conduct all statistical analyses. The Tukey honestly significant difference (HSD) post-hoc test was used to compare specific data points in ANOVA. Data are expressed as mean ± SEM; P<0.05 was considered significant in all tests.
3. Results
3.1. Intrathecal injection of CBX inhibits mechanical and heat hypersensitivity in SNL rats
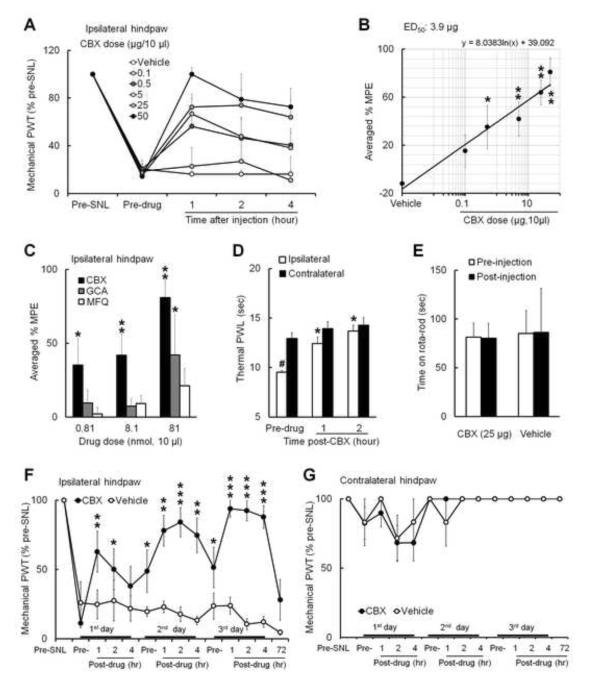
Mechanical hypersensitivity is an important manifestation of neuropathic pain. Intrathecal injection of CBX (0.1 μg: n=5; 0.5 μg: n=6; 5 μg: n=7; 25 μg: n=8; 50 μg: n=5, 10 μl) dose-dependently increased PWT in the ipsilateral (left) hind paw of rats at 2–3 weeks post-SNL (Fig. 1A). The %MPEs of CBX at 1, 2, and 4 h after injection were calculated, and the averaged %MPEs were used to plot the dose-response function (Fig. 1B, ED50=3.9 μg/10 μl). The %MPEs of CBX at doses of 0.5, 5, and 50 μg were significantly greater than that of vehicle (n=6). Because CBX may exert actions other than inhibiting gap junctions, we next tested whether intrathecal GCA (an analogue of CBX that shares similar structure and multiple properties of CBX but does not block gap junctions)[5,18-20], and MFQ (a selective neuronal gap junction decoupler) also alleviate mechanical hypersensitivity. At 0.81 and 8.1 nmol doses (10μl), CBX significantly increased %MPE from the vehicle-treated group. However, at the same doses, neither GCA nor MFQ significantly increased %MPE (Fig. 1C). Although %MPE of GCA at 81 nmol dose was higher than that of vehicle, rats exhibited severe signs of discomfort (e.g., frequent vocalizing, escaping) after injection. CBX only induced very mild and short-lasting irritation (1-2 min) at the highest dose (50 μg). Intrathecal CBX (5 μg, 10μl) also inhibited heat hypersensitivity, as indicated by the significant increase of ipsilateral PWL from pre-drug level after injection (Fig. 1D). Rota-rod tests showed that CBX (25 μg, 10 μl) did not induce motor dysfunction in SNL rats at 1 h post-injection (Fig. 1E).
Fig. 1. Intrathecal injection of carbenoxolone inhibits mechanical and heat hypersensitivity in nerve-injured rats.
(A) At 2–3 weeks after an L5 spinal nerve ligation (SNL), intrathecal injection of carbenoxolone (CBX, 0.1 μg: n=5; 0.5 μg: n=6; 5 μg: n=7; 25 μg: n=8; 50 μg: n=5; vehicle: n=6, 10 μl) dose-dependently increased ipsilateral paw withdrawal threshold (PWT) of rats from pre-drug value, reflecting attenuated mechanical hypersensitivity. (B) The dose-response function of CBX-induced inhibition of mechanical hypersensitivity was established based on the averaged % MPE at 1, 2, and 4 h after drug injection. *p<0.05, **p<0.01 versus vehicle, one-way ANOVA. (C) The averaged % MPEs of the same molar doses of intrathecal CBX (0.5 μg/0.81 nmol, 5 μg/8.1 nmol, 50 μg/81 nmol, 10 μl), glycyrrhizic acid (GCA, 0.81 nmol: n=6; 8.1 nmol: n=5, 81 nmol: n=5) and mefloquine hydrochloride (MFQ, 0.81 nmol: n=5; 8.1 nmol: n=5, 81 nmol: n=6) on inhibiting mechanical hypersensitivity. *p<0.05, **p<0.01 versus vehicle, two-way ANOVA. (D) The ipsilateral paw withdrawal latency (PWL) to heat stimuli was shorter than that on the contralateral side (n=6), reflecting heat hypersensitivity in ipsilateral hindpaw. Intrathecal injection of CBX (5 μg) increased ipsilateral PWL from pre-drug level at 1 and 2 h after injection. #p<0.05 versus contralateral side, Student t-test. *p<0.05 versus pre-drug, one-way repeated-measures ANOVA. (E) SNL rats exhibited no motor dysfunction at 1 h post-CBX (25 μg, n=6) or vehicle (saline, n=4) injection. (F) Increase in ipsilateral PWT after repeated intrathecal infusions of CBX (25 μg, 10 μl, n=8, once/day) and not vehicle (n=5) on three consecutive days in rats from day 14 post-SNL. *p<0.05, **p<0.01, ***p<0.001 versus pre-drug value on first day, two-way mixed model ANOVA. (G) The contralateral PWT was not changed after CBX and vehicle treatment. Data are expressed as mean ± SEM.
In separate experiments, rats that received repetitive intrathecal CBX (25 μg/day, 10 μl, n=8) and not saline (n=5) on days 14–16 post-SNL exhibited increased ipsilateral PWT on each treatment day (Fig. 1F). Intriguingly, the pre-drug PWTs on the second and third CBX treatment day were also significantly higher than that on the first day, indicating a carryover effect. The contralateral PWT was not significantly changed after treatment (Fig. 1G).
3.2. CBX inhibits WDR neurons in SNL rats
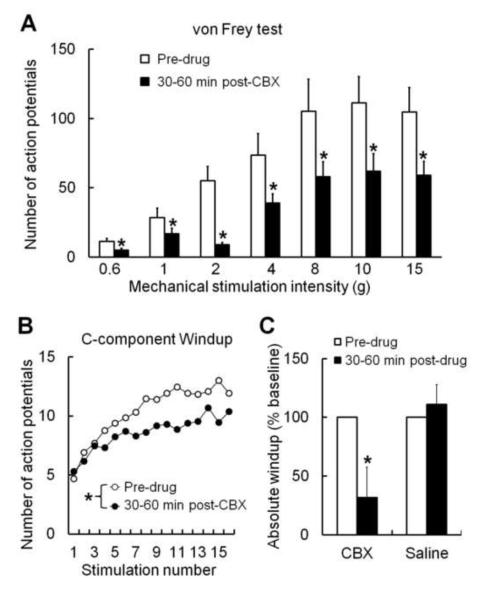
Since only CBX, but not GCA and MFQ, inhibited neuropathic pain, we next explored potential cellular mechanisms for CBX-induced analgesia by recording WDR neurons in SNL rats. Spinal topical application of CBX (100 μg/50 μl, n=10), but not saline (data not shown), significantly reduced WDR neuronal response to graded mechanical stimulation (von Frey filament, 0.6–15 g, 3 s) at 30–60 min post-drug (Fig. 2A). WDR neuron also receives C-fiber inputs, and its response to a suprathreshold electrical stimulus can be separated into an early A-component (0–75 ms) and a later C-component (75–500 ms)[13]. The C-component evoked by each stimulus in the train of 0.5 Hz windup-inducing electrical stimulation was used to plot windup curves (Fig. 2B). The windup curve and the absolute windup value were significantly decreased at 30-60 min post-CBX (100 μg/50 μl, n=13), but not saline (n=8, Fig. 2B,C). Absolute windup = (total number of action potentials evoked by the train) − [16 × (number of action potentials evoked by the first stimulus of the train)].
Fig. 2. Spinal topical application of carbenoxolone attenuates WDR neuronal activity in nerve-injured rats.
(A) The number of action potential responses of WDR neurons to graded mechanical stimulation (von Frey filament, 0.6–15 g) was decreased at 30–60 min after spinal topical application of carbenoxolone (CBX, 100 μg/50 μl, n=10) in rats with spinal nerve ligation (SNL). *p < 0.05 versus pre-drug, two-way repeated-measures ANOVA. (B) The number of action potentials in the C-component of WDR neurons progressively increased (i.e., windup) in response to repetitive intracutaneous electrical stimulation (16 pulses, supra-C threshold intensity, 2.0 ms, 0.5 Hz). The windup curve was depressed at 30-60 minutes post-CBX (n=13), but not saline (n=8, data not shown). *p < 0.05 versus pre-drug, two-way repeated-measures ANOVA. (C) The absolute windup value was also significantly decreased after CBX, but not saline, treatment. *p < 0.05 versus pre-drug, paired t-test. Data are expressed as mean ± SEM.
4. Discussion
The current study suggests that intrathecal CBX, but not GCA and MFQ, dose-dependently attenuates mechanical hypersensitivity during the maintenance phase of neuropathic pain in rats (2-3 weeks post-SNL). Further, spinal topical application of CBX inhibited WDR neurons in SNL rats.
Mechanical hypersensitivity is an important manifestation of neuropathic pain in patients and is also observed in animal models of neuropathic pain[21]. CBX has been used to block gap junctions and hemichannels and induces analgesia in pathological pain conditions[6-8,22]. In current study, the inhibition of mechanical and heat hypersensitivity from a single bolus injection of CBX in SNL rats was long-lasting (e.g., for hours at the higher dose), and was not associated with motor dysfunction. Yet, the current finding differs from a recent study which showed that CBX failed to attenuate the fully developed mechanical hypersensitivity induced by oxaliplatin, a chemotherapy drug, in rats[6]. Although the reasons for this discrepancy remain to be determined, it may be partially due to differences in glial activation and etiology of neuropathic pain resulted from mononeuropathy and chemotherapy-induced neuropathy. We further demonstrated that repetitive intrathecal CBX treatment through days 14–16 post-SNL did not cause acute desensitization or tachyphylaxis to its pain-inhibition. Yet, whether analgesic tolerance may develop after prolonged treatment with CBX (e.g., weeks) remains to be determined. Glial cell activation and enhanced gap junction function were suggested to be important to neuropathic pain, and a previous study showed that CBX did not affect normal heat and mechanical sensitivity in non-injured animals[5]. In line with this notion, the contralateral (uninjured side) PWL in SNL rats did not significantly change after CBX. Yet, we cannot exclude the possibility that CBX may affect normal nociception at higher doses.
Glycyrrhetinic acid derivative, such as CBX, may bind to gap junction channels and induce conformational modification to close the channel[23]. Accordingly, CBX is often used to disrupt intercellular communication via gap junctions[24]. Yet, CBX also has other actions and is not specific to glia gap junctions. For example, CBX has a mineralocorticoid agonist effect[25], and can block other channels including P2X7 receptor-associated Pan-1 channels[26]. Therefore, we further evaluated if CBX-induced pain inhibition may involve a blocking of glial gap junctions. Since there is no selective glial gap junction blocker available and downregulation of spinal glial gap junction protein attenuated neuropathic pain by itself [27], we attempted to address this question by comparing the effects of CBX on neuropathic pain with that of GCA and MFQ. GCA is an analogue of CBX that has similar structure and shares most known properties of CBX except for affecting gap junctions[5,18-20]. Intrathecal GCA did not inhibit neuropathic mechanical hypersensitivity at the doses that are effective for CBX. Although the highest dose of GCA (81 nmol) increased PWT, it was associated with severe side effects. Thus, the apparent pain inhibition from the high dose GCA may have also been due to stress and distraction caused by its side effects. Unlike GCA, CBX did not induce significant side effects even at the highest dose tested (50 μg). It is possible that GCA may exert actions other than that of CBX and hence causes side effects. Finally, intrathecal administration of a selective neuronal gap junction decoupler, MFQ, did not alleviate mechanical hypersensitivity. In view of current findings and that CBX was widely used as a potent blocker of the major astroglia-to-astroglia and astroglia-to-neuron gap junction in previous studies [24,28], we infer that pain inhibition from intrathecal CBX in SNL rats may be attributable (but not exclusively) to its inhibition of glial gap junctions. Yet, it needs to be noted that current findings from conventional pharmacological studies may not represent as direct evidence for CBX-induced pain inhibition that is exclusively through inhibiting glial gap junctions. Future study is needed to determine which type of glial cell (microglia or astrocyte) is mostly affected by CBX. Astrocyte activation was prominent in the spinal cord after nerve injury[29], and astrocyte gap junction was suggested to play an important role in neuropathic pain[30].
Glial cells and neurons have a close anatomical relationship in the spinal cord[31]. Neuron-glia interaction has been suggested to contribute to pain hypersensitivity after nerve injury[1,32]. For example, the reactive astrocytes release gliotransmitters (e.g., glutamate and ATP) as well as proinflammatory cytokines which may increase neuronal excitability and facilitate nociceptive transmission[33,34]. WDR neurons are candidates for T-cells in "gate control theory" of pain and play an important role in spinal pain transmission[35,36]. Nerve injury may increase WDR neuronal excitability[13,37]. We found that spinal topical application of CBX attenuated the evoked response of WDR neurons to mechanical stimuli in SNL rats. This finding is in line with a previous observation that CBX reduces medullary dorsal horn neuronal response to mechanical stimulation after mustard oil application to the tooth pulp[22]. Further, a recent study showed that CBX inhibited mechanical hypersensitivity in a rat model of orofacial neuropathic pain[38]. Pain hypersensitivity induced by trigeminal nerve injury and SNL may involve different mechanisms. Together, these findings suggest that an inhibition of central neuronal excitability may by a common cellular mechanism that, at least partially, underlies CBX-induced inhibition of neuropathic pain manifestations that arise from different etiologies.
In experimental animals, repetitive electrical stimulation of C-fibers progressively increases the response of WDR neurons, an electrophysiological phenomenon known as windup[12,14]. Windup may result from slow temporal summation of C-fiber–mediated responses of dorsal horn neurons and represents a form of short-term neuronal sensitization to repetitive noxious inputs[12,14]. Importantly, windup also occurs during natural stimulation of C-fibers[39,40]. The current study shows for the first time that spinal topical application of CBX can inhibit the development of activity-dependent neuronal sensitization in WDR neurons. Yet, since CBX may modulate synaptic transmission and neuronal membrane properties independent of its ability to block gap junctions[9,20], it remains to be determined whether the neuronal inhibition by CBX results primarily from an interruption of abnormal functional syncytium formed by glial cells via gap junctions. Examining effects of GCA and MFQ on WDR neurons may help to answer this question in the future.
In summary, our study suggests that intrathecal CBX attenuates neuropathic pain manifestations in SNL rats, and the pain inhibition may involve an inhibition of glial gap junctions and an attenuation of WDR neuronal activity in the dorsal horn. Thus, the spinal glial gap junctions can play an essential role in the maintenance of neuropathic pain, and may hence provide an important target of neuropathic pain treatment.
Intrathecal carbenoxolone (CBX) inhibited neuropathic pain manifestations in rats.
Neuropathic rats did not develop tachyphylaxis to repetitive CBX treatments.
CBX attenuated response of wide-dynamic range neurons (WDR) to mechanical stimuli.
CBX also inhibited windup, a short-form of neuronal sensitization, in WDR neurons.
Acknowledgements:
The authors thank Claire F. Levine, MS (Scientific Editor, Department of Anesthesiology/CCM, the Johns Hopkins University) for editing the manuscript. This study was supported by a research grant to Q.X. from China Scholarship Council (Beijing, China) and a grant to Y.G. from the National Institutes of Health (NS70814, Bethesda, Maryland, USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Ji RR, Berta T, Nedergaard M. Glia and pain: Is chronic pain a gliopathy? Pain. 2013 doi: 10.1016/j.pain.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chew SS, Johnson CS, Green CR, nesh-Meyer HV. Role of connexin43 in central nervous system injury. Exp. Neurol. 2010;225:250–261. doi: 10.1016/j.expneurol.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Burra S, Jiang JX. Regulation of cellular function by connexin hemichannels. Int. J. Biochem. Mol. Biol. 2011;2:119–128. [PMC free article] [PubMed] [Google Scholar]
- 4.Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia. 1998;24:50–64. [PubMed] [Google Scholar]
- 5.Spataro LE, Sloane EM, Milligan ED, Wieseler-Frank J, Schoeniger D, Jekich BM, Barrientos RM, Maier SF, Watkins LR. Spinal gap junctions: potential involvement in pain facilitation. J. Pain. 2004;5:392–405. doi: 10.1016/j.jpain.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Yoon SY, Robinson CR, Zhang H, Dougherty PM. Spinal astrocyte gap junctions contribute to oxaliplatin-induced mechanical hypersensitivity. J. Pain. 2013;14:205–214. doi: 10.1016/j.jpain.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roh DH, Yoon SY, Seo HS, Kang SY, Han HJ, Beitz AJ, Lee JH. Intrathecal injection of carbenoxolone, a gap junction decoupler, attenuates the induction of below-level neuropathic pain after spinal cord injury in rats. Exp. Neurol. 2010;224:123–132. doi: 10.1016/j.expneurol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Wei H, Hao B, Huang JL, Ma AN, Li XY, Wang YX, Pertovaara A. Intrathecal administration of a gap junction decoupler, an inhibitor of Na(+)-K(+)-2Cl(−) cotransporter 1, or a GABA(A) receptor agonist attenuates mechanical pain hypersensitivity induced by REM sleep deprivation in the rat. Pharmacol. Biochem. Behav. 2010;97:377–383. doi: 10.1016/j.pbb.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 9.Tovar KR, Maher BJ, Westbrook GL. Direct actions of carbenoxolone on synaptic transmission and neuronal membrane properties. J. Neurophysiol. 2009;102:974–978. doi: 10.1152/jn.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung JM, Kim HK, Chung K. Segmental spinal nerve ligation model of neuropathic pain. Methods Mol. Med. 2004;99:35–45. doi: 10.1385/1-59259-770-X:035. [DOI] [PubMed] [Google Scholar]
- 11.Shechter R, Yang F, Xu Q, Cheong YK, He SQ, Sdrulla A, Carteret AF, Wacnik PW, Dong X, Meyer RA, Raja SN, Guan Y. Conventional and Kilohertz-frequency Spinal Cord Stimulation Produces Intensity- and Frequency-dependent Inhibition of Mechanical Hypersensitivity in a Rat Model of Neuropathic Pain. Anesthesiology. 2013;119:422–432. doi: 10.1097/ALN.0b013e31829bd9e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 13.Guan Y, Wacnik PW, Yang F, Carteret AF, Chung CY, Meyer RA, Raja SN. Spinal cord stimulation-induced analgesia: electrical stimulation of dorsal column and dorsal roots attenuates dorsal horn neuronal excitability in neuropathic rats. Anesthesiology. 2010;113:1392–1405. doi: 10.1097/ALN.0b013e3181fcd95c. [DOI] [PubMed] [Google Scholar]
- 14.Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog. Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 15.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 16.Dixon WJ. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 17.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia 793. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 18.Duax WL, Ghosh D, Pletnev V. Steroid dehydrogenase structures. mechanism of action, and disease, Vitam. Horm. 2000;58:121–148. doi: 10.1016/s0083-6729(00)58023-6. [DOI] [PubMed] [Google Scholar]
- 19.Diao H, Xiao S, Howerth EW, Zhao F, Li R, Ard MB, Ye X. Broad gap junction blocker carbenoxolone disrupts uterine preparation for embryo implantation in mice. Biol. Reprod. 2013;89:31. doi: 10.1095/biolreprod.113.110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chepkova AN, Sergeeva OA, Haas HL. Carbenoxolone impairs LTP and blocks NMDA receptors in murine hippocampus. Neuropharmacology. 2008;55:139–147. doi: 10.1016/j.neuropharm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Tabo E, Jinks SL, Eisele JH, Jr., Carstens E. Behavioral manifestations of neuropathic pain and mechanical allodynia. and changes in spinal dorsal horn neurons, following L4-L6 dorsal root constriction in rats, Pain. 1999;80:503–520. doi: 10.1016/S0304-3959(98)00243-7. [DOI] [PubMed] [Google Scholar]
- 22.Chiang CY, Li Z, Dostrovsky JO, Sessle BJ. Central sensitization in medullary dorsal horn involves gap junctions and hemichannels. Neuroreport. 2010;21:233–237. doi: 10.1097/WNR.0b013e328336eecb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J. Pharmacol. Exp. Ther. 1988;246:1104–1107. [PubMed] [Google Scholar]
- 24.Iglesias R, Dahl G, Qiu F, Spray DC, Scemes E. Pannexin 1: the molecular substrate of astrocyte "hemichannels". J. Neurosci. 2009;29:7092–7097. doi: 10.1523/JNEUROSCI.6062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jellinck PH, Monder C, McEwen BS, Sakai RR. Differential inhibition of 11 beta-hydroxysteroid dehydrogenase by carbenoxolone in rat brain regions and peripheral tissues. J. Steroid Biochem. Mol. Biol. 1993;46:209–213. doi: 10.1016/0960-0760(93)90296-9. [DOI] [PubMed] [Google Scholar]
- 26.Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J. Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohara PT, Vit JP, Bhargava A, Jasmin L. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. J. Neurophysiol. 2008;100:3064–3073. doi: 10.1152/jn.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy JI, Dudek FE, Rash JE. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res. Brain Res. Rev. 2004;47:191–215. doi: 10.1016/j.brainresrev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 29.Okada-Ogawa A, Suzuki I, Sessle BJ, Chiang CY, Salter MW, Dostrovsky JO, Tsuboi Y, Kondo M, Kitagawa J, Kobayashi A, Noma N, Imamura Y, Iwata K. Astroglia in medullary dorsal horn (trigeminal spinal subnucleus caudalis) are involved in trigeminal neuropathic pain mechanisms. J. Neurosci. 2009;29:11161–11171. doi: 10.1523/JNEUROSCI.3365-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ji XT, Qian NS, Zhang T, Li JM, Li XK, Wang P, Zhao DS, Huang G, Zhang L, Fei Z, Jia D, Niu L. Spinal astrocytic activation contributes to mechanical allodynia in a rat chemotherapy-induced neuropathic pain model. PLoS. One. 2013;8:e60733. doi: 10.1371/journal.pone.0060733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren K. Emerging role of astroglia in pain hypersensitivity. Jpn. Dent. Sci. Rev. 2010;46:86. doi: 10.1016/j.jdsr.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 2013;716:106–119. doi: 10.1016/j.ejphar.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 33.Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- 34.Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia. 2006;54:716–725. doi: 10.1002/glia.20374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guan Y, Borzan J, Meyer RA, Raja SN. Windup in dorsal horn neurons is modulated by endogenous spinal mu-opioid mechanisms. J. Neurosci. 2006;26:4298–4307. doi: 10.1523/JNEUROSCI.0960-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 37.Chu KL, Faltynek CR, Jarvis MF, McGaraughty S. Increased WDR spontaneous activity and receptive field size in rats following a neuropathic or inflammatory injury: implications for mechanical sensitivity. Neurosci. Lett. 2004;372:123–126. doi: 10.1016/j.neulet.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 38.Wang H, Cao Y, Chiang CY, Dostrovsky JO, Sessle BJ. The gap junction blocker carbenoxolone attenuates nociceptive behavior and medullary dorsal horn central sensitization induced by partial infraorbital nerve transection in rats. Pain . 2013 doi: 10.1016/j.pain.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes AM, Rhodes J, Fisher G, Sellers M, Growcott JW. Assessment of the effect of dextromethorphan and ketamine on the acute nociceptive threshold and wind-up of the second pain response in healthy male volunteers. Br. J. Clin. Pharmacol. 2002;53:604–612. doi: 10.1046/j.1365-2125.2002.01602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J. Pain. 2007;8:893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]