Abstract
X-ray absorption spectroscopy has provided important insights into the structure and function of the Mn4Ca cluster in the oxygen-evolving complex of Photosystem II (PS II). The range of manganese extended x-ray absorption fine structure data collected from PSII until now has been, however, limited by the presence of iron in PS II. Using a crystal spectrometer with high energy resolution to detect solely the manganese Kα fluorescence, we are able to extend the extended x-ray absorption fine structure range beyond the onset of the iron absorption edge. This results in improvement in resolution of the manganese-backscatterer distances in PS II from 0.14 to 0.09 Å. The high resolution data obtained from oriented spinach PS II membranes in the S1 state show that there are three di-µ-oxo-bridged manganese-manganese distances of ~2.7 and ~2.8 Å in a 2:1 ratio and that these three manganese-manganese vectors are aligned at an average orientation of ~60° relative to the membrane normal. Furthermore, we are able to observe the separation of the Fourier peaks corresponding to the ~3.2 Å manganese-manganese and the ~3.4 Å manganese-calcium interactions in oriented PS II samples and determine their orientation relative to the membrane normal. The average of the manganese-calcium vectors at ~3.4 Å is aligned along the membrane normal, while the ~3.2 Å manganese-manganese vector is oriented near the membrane plane. A comparison of this structural information with the proposed Mn4Ca cluster models based on spectroscopic and diffraction data provides input for refining and selecting among these models.
Photosynthesis by green plants, algae, and cyanobacteria provides essentially all of the dioxygen in the biosphere as a byproduct of the electron transfer processes utilizing water as the ultimate electron source: 2H2O → O2 + 4H+ + 4e−.
Water oxidation is a light-driven reaction that is catalyzed by an oxygen-evolving complex (OEC)4 of Photosystem II (PS II) (1–4). The active site of the OEC is known to be a protein-bound complex containing four manganese and one calcium atom. This complex cycles through a series of five intermediate redox states that are referred to as S states (S0 to S4) (5). The S state transitions are driven by successive light-induced one-electron oxidations of the PS II reaction center. In each step the complex accumulates oxidizing equivalents until dioxygen is released during the spontaneous return from S4 to S0.
Many of the proposed mechanisms of water oxidation depend critically on knowledge of the Mn4Ca cluster structure. To date, structural models of the OEC complex have been suggested based on EPR techniques (6–9), x-ray absorption spectroscopy (XAS) (10–14), x-ray diffraction (XRD) (15–17), and infrared spectroscopy (Fourier transform infrared) (18). The XRD studies (3.0–3.8 Å resolution) have located the Mn4Ca cluster in the density map (16, 17) and confirmed the presence of calcium in the OEC cluster, as had been shown previously by EPR (19–21) and by extended x-ray absorption fine structure (EXAFS) spectroscopy (22, 23). A recent XAS study showed that the OEC complex is very susceptible to reduction and disruption during x-ray exposure, under the conditions used in collecting the published XRD data (24). Consequently, the precise location of the manganese and calcium atoms has not been reliably established within active OEC centers by XRD, as acknowledged in the most recent study (17).
Manganese XAS enables a detailed analysis of the Mn4Ca cluster in the OEC. X-ray absorption near-edge structure (XANES) contains information on the electronic structure and changes in oxidation states of the manganese that accompany S state transitions (25). EXAFS allows for a precise determination of manganese-backscatterer distances (26) and is, furthermore, very sensitive for establishing the permissible x-ray dose (24).
Recent EXAFS studies of the Mn4Ca cluster of the OEC have led to the conclusion that there are three manganese-manganese vectors in the range 2.7–2.9 Å reflecting di-µ-oxo-bridged manganese atom pairs (27), one manganese-manganese vector at 3.3 Å, and one or two manganese-calcium vectors at 3.4 Å (22, 23). The ability of the EXAFS technique to determine the presence of similar backscatterers at closely separated distances, ΔR, is dependent on Δk, the width of the k-space EXAFS data set (Å−1) (for details see supplemental data). The presence of iron, partly an integral component of the OEC and partly from adventitious sources, restricts the useful range in conventional manganese EXAFS to ~550 eV (Δk = 12.5 Å−1) above the manganese edge (iron K-edge at 7120 eV). Consequently, in PS II the manganese-manganese distance resolution is limited to Δr = 0.14 Å, meaning that two manganese-manganese vectors should differ greater than 0.14 Å to be resolved. Improvement in manganese-backscatterer distance resolution is critical for precise structural and mechanistic studies of the OEC. Conventional EXAFS spectra of PS II samples are based on the detection of the manganese Kα1,2 fluorescence (~5.9 keV) using a solid state detector of at best 150–200 eV from full width at half-maximum resolution (28–30), making it impossible to discriminate completely against the Fe Kα1,2 fluorescence at 6.4 keV (see Fig. 1). This limitation can be overcome by utilizing a crystal monochromator with high resolution (~1 eV) for the fluorescence detection (31, 32). Recently we showed that manganese EXAFS of the OEC can be collected up to ~1000 eV (k = 16.1 Å−1) above the manganese K-edge (27), improving the manganese-backscatterer distance resolution to 0.09 Å. This enabled us to study the heterogeneity in the manganese-manganese distances of solution samples in the S1 and S2 states, providing evidence for three manganese-manganese distances of ~2.7 and ~2.8 Å present in a 2:1 ratio (27). These improvements in determining the structural parameters are important for choosing among different proposed structural models, and they provide an opportunity for investigating the changes that occur as the Mn4Ca catalyst cycles through the S states.
FIGURE 1. Range-extended x-ray absorption spectroscopy.
Left, x-ray fluorescence of manganese and iron; Above, manganese Kα1 and Kα2 fluorescence peaks, with natural line width of ~5 eV, split by 11 eV. The multicrystal monochromator with 1-eV resolution is tuned to the manganese Kα1 peak. Below, fluorescence peaks of manganese and iron as detected using germanium detector. The fluorescence peaks are convoluted with the electronic window resolution of 150–200 eV of the germanium detector. This method of detection cannot resolve manganese Kα1 and Kα2 fluorescence peaks. Note different energy scales for the schemes shown above and below. Iron is an obligatory element in functional PS II complexes. Right, comparison of the PS II manganese K-edge EXAFS spectrum from an S1 state PS II sample obtained with a traditional 30-element energy-discriminating germanium detector with a spectrum collected using the high resolution crystal monochromator. Use of the high resolution detector eliminates the interference of iron and removes the limit of the energy range for manganese EXAFS data collection.
Additional geometric information about the spatial arrangement of manganese-backscatter vectors can be obtained if oriented PS II samples, such as oriented membranes or single crystals, are used for the measurement of EXAFS dichroism with linearly polarized synchrotron x-rays. Collection of the polarized EXAFS spectra on oriented PS II membranes at different angles between the membrane normal and the x-ray electric field vector results in dichroism that depends on how the particular absorber manganese-backscatter vector is aligned with respect to the electric field of the x-ray beam. Thus, the average orientation of a particular manganese-backscatter vector relative to the membrane normal and the average number of scatterers per absorbing atom can be determined (33–35).
Previous studies on oriented native and NH3-treated PS II membranes were based on conventional EXAFS. Average angles relative to the membrane normal of ~60° for the ~2.7 Å vectors (di-µ-oxo-bridged Mn2 units) and ~43° for the ~3.3 Å vectors (superposition of mono-µ-oxo-bridged manganese-manganese and manganese-calcium vectors) have been reported in two studies (34, 35), whereas another study reported an average angle of 80 ± 10° for the ~2.7 Å vectors without providing results for the ~3.3 Å vector (36). Because of the limited resolution, conventional EXAFS is not able to determine the orientations of the individual manganese-manganese and manganese-calcium vectors in the 3.2–3.4 Å region. In a complementary study, strontium K-edge polarized EXAFS of strontium-reactivated PS II membranes was used to predict the manganese-calcium orientation. It showed a lower and upper limit of 0 and 23°, respectively, for the average angle between the manganese-strontium vector(s) and the membrane normal and yielded an isotropic coordination number of manganese neighbors to strontium of either one or two (23). A recent polarized x-ray absorption spectroscopy study of PS II single crystals from cyanobacteria, using an x-ray dose below the threshold of damage, has derived feasible structures for the Mn4Ca cluster and the orientation of the cluster in the PS II crystal (14).
In this work, we applied range-extended EXAFS to study the dichroism of the Mn4Ca cluster in oriented PS II membranes from spinach chloroplasts. The study shows: (i) the separation of the manganese-manganese (~3.2 Å) and manganese-calcium (~3.4 Å) vectors, which allows independent analysis of their orientation relative to the membrane normal; (ii) the determination of the dichroism characteristics of the three short manganese-manganese vectors (two at 2.7 Å and one at 2.8 Å) and their orientation in the PS II membrane. These results are used to discuss the structure and orientation of the Mn4Ca cluster in the PS II membrane.
MATERIALS AND METHODS
Sample Preparation and Characterization
PS II samples were prepared from spinach as previously described (37). They typically contain 4 manganese per 200–250 chlorophylls. The oxygen evolution rates for the PS II samples used in this study are between 400 and 500 µmol O2/(mg chlorophylls · h). The membranes were resuspended in 50 mm MES buffer, pH = 6.0, containing 0.4 m sucrose and 5 mm CaCl2 and pelleted by centrifugation at 4 °C (39,000 × g, 1 h). One or two drops of 50 mm MES buffer were added to the pellet, and the resulting paste was painted onto Mylar tape. The PS II membranes were dried under a stream of cold nitrogen gas at 4 °C in the dark for ~1 h, as described previously (38). This process was repeated five to seven times to generate samples with a sufficiently thick sample layer for the x-ray absorption experiment.
The paint-and-dry cycles produce one-dimensionally ordered samples with a preferred orientation of the PS II membrane normal perpendicular to the substrate surface. The extent of orientation (mosaic spread, which is the half-width of the Gaussian distribution of the angle of the membrane normal to the substrate normal in the PS II samples) was assessed from the angle dependence of the Tyr Dox and cytochrome b559 EPR signals (see supplemental data). X-band EPR spectroscopy was performed with a Varian E-109 spectrometer, a standard TE102 cavity, and an Air Products liquid helium cryostat. The samples used in this study displayed a mosaic spread of 15–20°. After drying the samples, their integrity was assayed by monitoring the amount of S2 multiline signal formed upon sample illumination at 195 K. The amplitude of the manganese signal was the same as that obtained from randomly oriented membranes at a similar concentration. Manganese K-edge XANES spectra of oriented samples can be used to reconstruct the solution XANES spectrum, which is very sensitive to the manganese oxidation state and damaged PS II centers containing Mn2+. The two spectra are indistinguishable, indicating the intact state of the oriented samples.
Data Collection
The x-ray spectra were recorded on the BioCAT undulator beamline 18-ID at the Advanced Photon Source (Argonne, IL). The energy of the incident x-rays was selected by means of a nitrogen-cooled silicon double-crystal monochromator at (111) orientation, yielding ~1-eV resolution. The monochromator energy was calibrated using the pre-edge peak energy of KMnO4 at 6543.3 eV. Higher harmonics from the monochromator were rejected by the focusing mirror. The incident beam intensity was set to ~4 × 1012 photons/s (~60% of the flux available at 18-ID) at a beam size of 1 × 2 mm2. This allowed us to perform fast EXAFS scans in continuous mode before the onset of radiation damage; 15 s per sweep, energy range 6500 to 7500 eV in 1 eV increments, one sweep per spot on sample, 15–20 different spots per sample depending on orientation, ~100 samples per orientation. The EXAFS scan parameters were chosen subsequent to and on the basis of a radiation damage study of the samples. XANES spectra were collected under identical conditions (number of photons, time and temperature that were used for subsequent EXAFS measurements), and the inflection point energy of the XANES spectra was monitored for any shifts to establish the safe x-ray dose (24). Radiation damage measurements were determined for both 15 and 75° orientations of the samples used in the study and were repeated each time we had x-ray beamtime at the synchrotron sources to account for any changes in the beam characteristics. A second 15-s sweep of the EXAFS for some samples was collected and the spectra were unchanged, providing additional confirmation of the absence of radiation damage. To avoid unnecessary sample exposure, a beam shutter was automatically inserted when data were not being collected. The manganese Kα fluorescence was detected by four spherically bent germanium analyzers (8.9 cm diameter, 85 cm radius of curvature) using the (333) Bragg reflection in a Rowland geometry. The analyzer energy was tuned to the manganese Kα1 peak at 5899 eV at a Bragg angle of 74.84°. A nitrogen-cooled solid state (germanium) detector was placed at the common focus of the four crystals on the intersecting Rowland circles. The analyzer bandwidth of 0.8 eV was determined by measuring the elastically scattered peak.
Experimental procedures and limitations for measuring range-extended EXAFS past multiple K- or L-edges, and the design and operation of the spectrometer have been described previously (32, 39). All samples were measured below 10 K in a liquid He cooled cryostat (Oxford CF1208).
Data Analysis
For each EXAFS scan, the energy was calibrated using the KMnO4 pre-edge reference peak (6543.3 eV), and the intensity was normalized by I0 before averaging. Approximately 1000 scans were averaged for each orientation of PS II membranes relative to the x-ray e-vector with a custom Matlab program. Data reduction of the EXAFS spectra was done as described previously (10, 40). Curve fitting was performed using ab initio calculated phases and amplitudes from the FEFF8 program from the University of Washington (41). These phases and amplitudes were used in the EXAFS Equation 1, which is described below and contains a sinusoidal function that gives the distance and an amplitude function that contains information about the scattering atom and the number of such neighboring atoms.
| (Eq. 1) |
The neighboring atoms to the central atom(s) are divided into j shells, with all atoms with the same atomic number and distance from the central atom grouped into a single shell. Within each shell, the coordination number Nj denotes the number of neighboring atoms in shell j at a distance of Rj from the central atom, i. feffj is the ab initio amplitude function for shell j, and the Debye-Waller term e−2σj2k2 accounts for damping due to both static and thermal disorder in absorber-back-scatterer distances. The mean free path term e−2Rj/λj(k) reflects losses due to inelastic scattering, where λj(k) is the electron mean free path. The oscillations in the EXAFS spectrum are reflected in the sinusoidal term sin(2kRj + αij(k)), where αij(k) is the ab initio phase function for shell j. This sinusoidal term shows the direct relation between the frequency of the EXAFS oscillations in k-space and the absorber-backscatterer distance. The EXAFS equation (Equation 1) was used to fit the experimental Fourier isolates using N, R, and σ2 as variable parameters. Fit details and evaluation of fit qualities are given in the supplemental data.
The spatial resolution in EXAFS is inversely related to the spectral range. Several formulas can be found in the EXAFS literature describing the resolution limits of the method, such as ΔRΔk ≈ 1, ΔRkmax = π/2 and ΔRΔk = π/2 (40, 42); for more details see the supplemental data (40).
For a detailed explanation of the theory of polarized EXAFS see the supplemental data. Angle θ is the angle between the x-ray e-vector and the membrane normal, and ϕ denotes the relative orientation of the manganese-backscatterer (manganese-manganese or manganese-calcium) vector of interest to the membrane normal.
RESULTS
Manganese X-ray Absorption Spectra
X-ray absorption manganese K-edge spectra of oriented membranes in the S1 state are shown in Fig. 2. Data were collected for two orientations in which the sample normal is placed at either 15 or 75° to the direction of the e-vector of the polarized x-rays. The edge positions and post-edge shape exhibit a marked angle dependence, as reported previously for oriented PS II membranes (34). Dichroism of the manganese K-edge spectra is even more obvious in the second-derivative spectra (Fig. 2, bottom). The powder manganese XANES spectrum created from the spectra collected from oriented membranes at two different orientations is identical to that obtained from a frozen solution sample. This result provides independent confirmation that the oriented samples are intact and not damaged by x-rays.
FIGURE 2. Manganese K-edge spectra of oriented PS II membranes.
Top, S1 state manganese K-edge x-ray spectra of oriented PS II membranes. The membrane normal of the PS II samples was oriented at 15° (solid line) or 75° (dashed line) with respect to the x-ray e-vector during data acquisition. Bottom, the corresponding second derivatives of the manganese K-edge spectra shown above.
The k3-weighted EXAFS spectra of the S1 state of PS II oriented with the membrane normal at 15 or 75° to the x-ray e-vector are shown in Fig. 3. The spectra are distinctly dichroic. The region of the photoelectron wave vector from 3.5 to 11.5 Å−1 (denoted by the dashed line in Fig. 3), which is accessible by conventional EXAFS, agrees with results published earlier for oriented PS II membranes in the S1 state (34). Clear differences can be seen in the spectra between the two orientations.
FIGURE 3. Manganese K-edge EXAFS spectra of oriented PS II membranes.
Manganese K-edge EXAFS spectra (k3-weighted) from oriented PS II membrane samples in the S1 state obtained with a high resolution spectrometer (range-extended EXAFS) at orientations of 15° (solid line) and 75° (dashed line) of the sample normal with respect to the x-ray e-vector. The dashed line at k = 11.5 Å−1 denotes the spectral limit of a conventional EXAFS experiment due to the iron edge. The range-extended EXAFS method allows data collection above the iron edge.
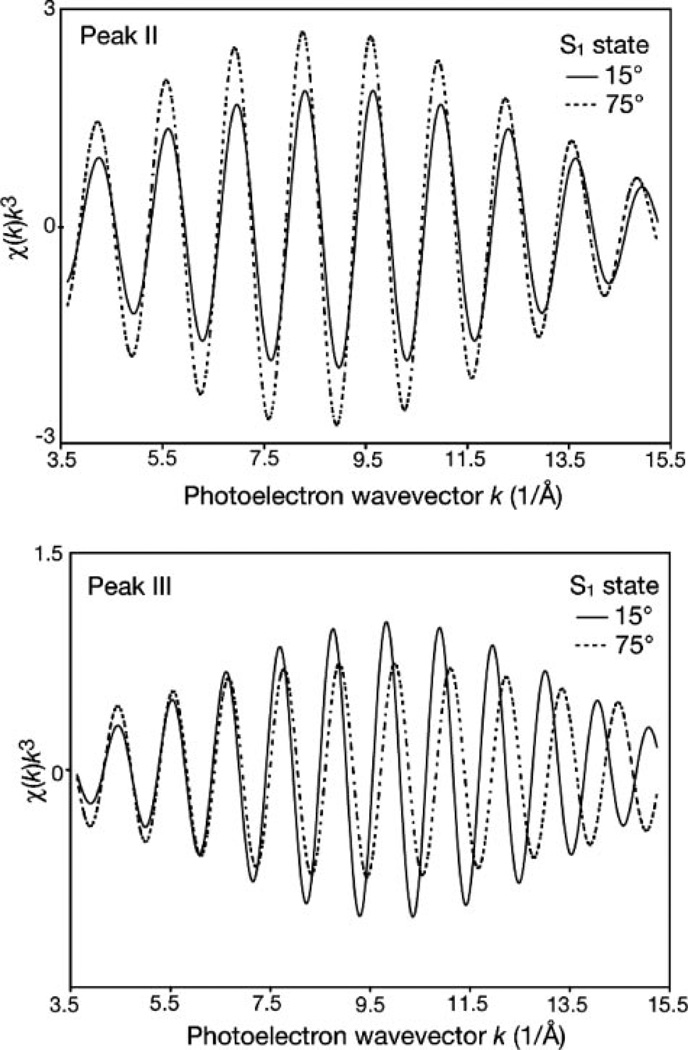
Fig. 4A shows the Fourier transforms of the range-extended EXAFS (k3-weighted) at 15 or 75°. The Fourier transforms exhibit well defined peaks, labeled I, II, IIIA, and IIIB, corresponding to the shells of backscatterers at different “apparent” distances, R′, from the manganese absorber. The apparent distance is shorter than the actual distance due to a phase shift induced by the interaction of the given absorber-scatterer pair with the photoelectron. For comparison, the Fourier transforms of the range-extended EXAFS spectra of both orientations, but truncated at 11.5 Å−1, are shown in Fig. 4B. Significant improvement in spectral resolution is observed for the range-extended EXAFS data (Fig. 4A). Increased spectral resolution reveals the orientation dependence of peaks II and IIIA and IIIB. The intensity of peak II, which consists of three manganese-manganese distances at 2.7 and 2.8 Å (see below) changes significantly between 15 and 75°, with higher intensity at 75°. Peak III shows a complex nature containing at least two peaks, IIIA and IIIB, with distances of 3.2 and 3.4 Å, respectively. Peak IIIA is more intense at 75° but has a decreased intensity at 15°. Peak IIIB is more intense at 15° but is close to the noise level at 75°.
FIGURE 4. Fourier transforms of the EXAFS spectra from oriented PS II membranes.
A, FT of manganese K-edge EXAFS spectra (Fig. 3) from oriented PS II membrane samples in the S1 state obtained with a high resolution spectrometer (range-extended EXAFS) at orientations of 15° (solid line) and 75° (dashed line) of the membrane normal with respect to the x-ray e-vector. The k range was 3.5–15.2 Å−1. Fourier peaks in A and B appear at an apparent distance R′ that is shorter than the actual distance R by ~0.5 Å due to a phase shift. B, Fourier transforms of the same data as in A, but the k range was truncated at 11.5 Å−1 for comparison with earlier published polarized EXAFS data obtained with conventional EXAFS. C, same as in A, but a phase correction was done using the EXAFSPAK suite of programs by Drs. Graham George and Ingrid Pickering (Stanford Synchrotron Radiation Laboratory), which results in the conversion of the apparent distance R′ into an approximate real distance R.
Previous calcium EXAFS studies of native PS II (22), strontium EXAFS of strontium-substituted PS II (23), and manganese EXAFS of calcium-depleted PS II (43) have demonstrated the contribution of both manganese-manganese (~3.2 Å) and manganese-calcium (~3.4 Å) vectors to Fourier transform (FT) peak III. The current observation (Fig. 4A) shows the distinct manganese-manganese and manganese-calcium vectors contributing to peak III at different distances and orientations. An assignment of peak IIIB to the manganese-calcium vectors can be made, taking into account the longer distance and different dichroism (oriented predominantly along the membrane normal). The dichroic behavior of peak IIIB (see below) is similar to that reported previously for the manganese-strontium vectors (23). In a like manner peak IIIA, which has a maximum at a shorter distance, can be assigned to the manganese-manganese vector. The intensity of this peak remains sizable also at the 15° orientation, and further analysis (see below) is needed to evaluate the orientation of this vector relative to the membrane normal.
The significant decrease of the half-width of the EXAFS FT peaks obtained in the range-extended EXAFS experiments (compare Fig. 4, A and B) results in good separation of peaks I and II. When phase correction is applied in the Fourier transform of the range-extended EXAFS (k3-weighted) at 15 or 75°, an additional peak, termed I′, is seen in Fig. 4C. Manganese-oxygen or manganese-chlorine interactions may be expected at a distance of ~2.2 Å (44), although any assignment of this feature must await further studies. Range-extended EXAFS on PS II preparations with bromine substituted for chlorine has the potential for clarifying the involvement of the halide cofactor in the OEC.
The Fourier transforms shown in Fig. 4, A and C, provide the basis for drawing qualitative conclusions about possible distances in the manganese-backscatterer pairs and their preferential orientations relative to the membrane normal. Reliable quantitative results can be obtained by fitting the experimental data using the EXAFS Equation 1, as described under “Materials and Methods” and in the supplemental data. The assignment of each peak and a detailed analysis of the actual distance and orientation of each vector are described below. We will concentrate on FT peaks II and III, because these are from manganese-manganese and manganese-calcium backscattering and provide the most reliable information about the Mn4Ca structure and orientation.
Curve Fitting of EXAFS FT Peak II
Fits of Fourier peak II were carried out both separately and in conjunction with peak III (peaks II + III). The large differences in amplitude and envelope shapes of the oscillation of the peak II (and III) isolates at the two different orientations reflect the dichroism observed in the Fourier transform amplitudes (Fig. 5). The quality of the fits is judged using the fit error parameters Φ and ε2; to know how they are determined see the supplemental data (note that ε2 is normalized to the number of fit parameters). Fit error parameters reflect the deviation between the simulations and isolates. Fits 1–4 in Table 1 show the results from fitting one and two manganese-manganese shells to peak II at the 15 and 75° orientations of the S1 state. Addition of the second manganese-manganese shell (fits 3 and 4 in Table 1) results in considerable improvement of the fit quality. With two different manganese-manganese distances at 2.7 and 2.8 Å, the fit error Φ and ε2 decreased by ~40% for the two-shell fit relative to the one-shell fit. In our previous range-extended EXAFS study with isotropic solution samples we concluded that peak II is best interpreted as consisting of 2.7 Å and 2.8 Å manganese-manganese vectors (di-µ-oxo-bridged manganese-manganese moieties) with a 2:1 ratio in both S1 and S2 states (27). Our present results on oriented samples support this conclusion.
FIGURE 5. Fourier isolates of peak II and III from oriented PS II membranes.
Fourier isolates of peak II (top) and peak III (bottom) of one-dimensionally oriented PS II samples in the S1 state with the membrane normal at 15° and 75° with respect to the x-ray e-vector. The data are k3-weighted from 3.5 to 15.2 Å−1 (see Fig. 4A). For the back transform the individual Fourier peaks II and III were isolated by applying a Hamming window to the first and last 15% of the chosen range, leaving the middle 70% untouched. For typical isolates the range was ~0.7 Å for peak II and ~0.8–0.9 Å for peak III.
TABLE 1.
One- and two-shell fits of Fourier Peak II of oriented PS II membranes (S1 state) at angles of 15 and 75° between the membrane normal and the e-vector of the linearly polarized x-ray beam
| Fit # | Angle | Shell | R(Å) | Nappa | σ2 (Å2) × 103 | Φ (×103) | ε2 (×105) |
|---|---|---|---|---|---|---|---|
| One shell | |||||||
| 1 | 15° | Manganese-Manganese | 2.73 | 0.94 | 3.0 | 0.20 | 0.068 |
| 2 | 75° | Manganese-Manganese | 2.74 | 1.48 | 3.6 | 0.18 | 0.063 |
| Two shells | |||||||
| 3 | 15° | Manganese-Manganese | 2.72 | 0.70 | 1b | 0.12 | 0.040 |
| Manganese-Manganese | 2.82 | 0.33 | 1b | ||||
| 4 | 75° | Manganese-Manganese | 2.72 | 0.97 | 1b | 0.11 | 0.036 |
| Manganese-Manganese | 2.83 | 0.54 | 1b |
Errors are estimated to be 25% for Napp numbers.
Parameter was fixed.
The Napp values are higher for the 75° orientation compared with those for the 15° orientation. The orientation dependence of data extracted with a one-shell fit of Peak II (Tables 1 and supplemental Table S1, fits 1 and 2) results in Niso = 1.3 ± 0.3, which corresponds to two or three manganese-manganese interactions at an average angle of 〈ϕ〉 = 63 ± 5°, for a 2.74 ± 0.02 Å manganese-manganese vector. Note that two manganese-manganese vectors correspond to Niso = 1.0, which is at the lower border of the error bar; taking into account that the EXAFS technique tends to underestimate N values, the Niso = 1.3 ± 0.3 obtained favors three manganese-manganese interactions (expected Niso = 1.5). The angle is in agreement with that reported earlier based on conventional polarized EXAFS data (34, 35). The new range-extended data show that peak II contains interactions at 2.7 and 2.8 Å, which can be analyzed separately (Table 1, fits 3 and 4). Fig. 6 shows linear plots of Napp derived from fits 3 and 4 in Table 1 (solid squares) against 3cos2θ−1 (see supplemental Equation S6). Third points (open squares) were obtained from extended EXAFS measurements of isotropic PS II S1 in solution (see supplemental Table S1). Linear fits using only two data points from oriented samples (solid lines) or using three data points including the isotropic values (dashed lines) are nearly identical and result in the same Niso and 〈ϕ〉 values. Supplemental Fig. S7 shows more traditional polar plots of the Napp derived from fits 1 to 4 in Table 1 and supplemental Table S1 and plotted with respect to the detection angle (θ). Analysis of the orientation dependence of the 2.72 ± 0.02 Å manganese-manganese vector results in Niso = 0.88 ± 0.2 (two manganese-manganese interactions) at an average angle 〈ϕ〉 = 61 ± 5°, with respect to the membrane normal. The 2.83 ± 0.02 Å manganese-manganese vector exhibits Niso = 0.46 ± 0.12 (one manganese-manganese interaction) at an angle 〈ϕ〉 64 ± 10° with respect to the membrane normal.
FIGURE 6. Linear plot of polarized manganese EXAFS data from Fourier peak II.
Linear plots of the x-ray absorption dichroism of Peak II for oriented PS II samples in the S1 state. The Napp values (solid squares) are derived from two-shell curve fits of FT peak II (Fig. 4A and Table 1) and are plotted against 3cos2θ−1 (see supplemental Equation S6). Best fits are shown for the different manganese-manganese vectors as solid lines. Additional third points (open squares) were obtained from extended EXAFS measurements of isotropic PS II S1 in solution (see supplemental Table S1). Fits with three data points including the isotropic values (dashed lines) are very close to those obtained using only oriented sample data.
Curve Fitting of EXAFS FT Peak III
Curve fitting results for peak III are shown in Table 2. Analysis of peak III has been problematic in EXAFS studies of PS II because of the relatively weak intensity and correspondingly low signal-to-noise ratio. As mentioned above, peak III was found to exhibit dichroism and to consist of at least two different manganese-backscatterer vectors (34, 35). Both of these conclusions are strengthened by the new range-extended EXAFS measurements. Combinations of manganese, calcium, carbon, and oxygen were tested to fit peak III (10, 34, 40). Distances of 3.1–3.4 Å are reported between manganese atoms bridged by a single µ2- or µ3-oxo unit. In addition, for carboxylate- or histidine-derived ligands, a highly disordered shell of carbon atoms at 2.9–3.3 Å may be expected from the next-nearest neighbor atoms to the metal (46–51). However, attempts to include manganese-light atom (oxygen or carbon) shells into peak III fits result in increased fit errors (data not shown). On the basis of differences in apparent distances (R′), dichroism for peak IIIA and IIIB, and previous studies of oriented strontium-reactivated PS II membranes (23), we conclude that the manganese-calcium vector contributes mainly at 15° and the manganese-manganese vector at 75°. One-shell fits presented in Table 2 (rows 1 and 2) agree well with the above assignment and support a longer distance for the manganese-calcium vector compared with that for the manganese-manganese vector. Estimation of the minor contributions of the manganese-calcium vector at 75° orientation and the manganese-manganese vector at 15° orientation is required to determine the orientations of those vectors relative to the membrane normal, but isolating peaks of weak intensities results in unreliable two-shell fits. As described in an earlier study (23), we can either assume an Napp ≈ 0 for contributions close to the noise level or estimate the upper limit of the Napp based on proportionality of the reduced amplitude of FT peak IIIA or IIIB to the peak maximum at the same R′ in the complementary orientation. When the measured amplitude was close to the calculated noise level (between 4 and 10 Å in the FTs), as for the manganese-calcium interaction, the noise level in the FT spectrum was used to estimate the upper limit ofNapp. The upper limits of the Napp for manganese-manganese and manganese-calcium interactions are listed in Table 2.
TABLE 2.
Fits of Fourier Peak III of oriented PS II membranes (S1 state) at angles of 15 and 75° between the membrane normal and the e-vector of the linearly polarized x-ray beam
| Fit # | Angle | Shell | R(Å) | Napp | σ2 (Å2) × 103 | Φ (×103) | ε2 (×105) |
|---|---|---|---|---|---|---|---|
| Peak III isolate | |||||||
| 1 | 15° | Manganese-calcium | 3.37 | 0.77a | 2b | 0.42 | 0.14 |
| 2 | 75° | Manganese-manganese | 3.24 | 0.46a | 2b | 0.49 | 0.17 |
| 15° | Manganese-manganese | 0.22c | |||||
| 75° | Manganese-calcium | 0.11d |
Errors are estimated to be 25% for Napp numbers.
Parameter was fixed.
Napp was estimated from relative peak heights in the Fourier transform.
The Napp was estimated from noise level in the Fourier transform.
Fig. 7 shows Napp for the manganese-manganese component of Peak III derived from Table 2 plotted against 3cos2θ−1, as was done for Peak II in Fig. 6. A third point (open squares) was obtained from extended EXAFS measurements of isotropic PS II S1 in solution (supplemental Table S2). A linear fit using only two data points from oriented samples (solid lines) and a fit using three data points including the isotropic values (dashed lines) are similar and, within experimental error, result in similar Niso and 〈ϕ〉 values. The orientation dependence of Napp for the manganese-manganese 3.2 ± 0.02 Å vector (see Table 2) results in Niso = 0.39 ± 0.1 and 〈ϕ〉 = 70°. These values are consistent with a single manganese-manganese 3.2 Å vector, for which the expected value of Niso is 0.5.
FIGURE 7. Linear plot of polarized manganese EXAFS data from Fourier peak III.
Linear plots of the x-ray absorption dichroism of the manganese-manganese (3.20 ± 0.02 Å) (black) and manganese-calcium (3.40 ± 0.02 Å) (gray) vectors for oriented PS II samples in the S1 state. The Napp values were derived from one-shell fits (solid squares) of FT peak III (Fig. 4A and Table 2) and are plotted with respect to 3cos2θ−1 (estimation of Napp for manganese-manganese vector at θ = 15° and for manganese-calcium vector at θ = 75° is described in the text). The best fits of Napp versus θ to supplemental Equation S6 are shown for the manganese-manganese and manganese-calcium vectors (solid line) taking into account the experimentally determined mosaic spread of Ω = 20°. Additional third points (open squares) were obtained from range-extended EXAFS measurements of PS II S1 solution (see supplemental Table S2). Fits with three data points including the isotropic values (dashed lines) are close to that obtained using only oriented sample data. The results from Figs. 6 and 7 and the polar plots in the supporting information are summarized in Table 3.
For the manganese-calcium 3.4 Å distance, the expected value for Niso is 0.25 for one manganese-calcium vector or 0.50 for two vectors. If we assume that Napp = 0 at 75°, the angle dependence ofNapp for this component results inNiso = 0.29 ± 0.09 for 〈ϕ〉 = 0°. This value favors one manganese-calcium interaction at 3.40 ± 0.02 Å; however, taking into account the error range and previous data of Cinco et al. (22) the possibility of two interactions at this distance cannot be excluded (22). The upper limit of this angle was estimated to be 18° in this work and 23° by Cinco et al. (23) for the strontium-manganese interaction. Despite relatively high uncertainty, we can conclude that this vector is aligned near to the membrane normal.
DISCUSSION
FT Peak II; Three Short Manganese-Manganese Interactions in the OEC
From a number of EXAFS (12, 13, 33, 52, 53) and EPR studies (6, 7, 54, 55), it is known that a major structural motif of the OEC is the di-µ-oxo-bridged Mn2 unit. Conventional EXAFS studies could not settle the question of whether there are two or three di-µ-oxo-bridged manganese-manganese moieties in the native S1 and S2 states (40, 53, 56). The uncertainty in the determination of the numbers of absorber-backscatterer vectors by EXAFS has prevented a clear solution to this problem. However, conventional EXAFS studies more clearly show that there is heterogeneity in the manganese-manganese distances in the range of 2.7–2.85 Å for the S0 state (40), for samples poised in the S2 g = 4.1 state (57), as well as for the chemically modified F−- (58) and the NH3-treated (35) S2 state samples. For those states with two manganese-manganese distances, the number of di-µ-oxo-bridged manganese-manganese interactions can be determined with a higher accuracy, considering that only an integral number of each interaction is allowed. Re-evaluation of earlier results by Robblee et al. (40) demonstrated that they are more consistent with a 2:1 ratio, with the shorter distance predominating, supporting OEC models containing three 2.7–2.85 Å manganese-manganese interactions. For the native S1 and S2 states, with less distance heterogeneity, the results of the conventional EXAFS were still not conclusive (40).
The recent range-extended EXAFS study of PS II in solution provides evidence supporting the presence of three di-µ-oxo-bridged manganese-manganese vectors in the native S1 and S2 states (27). The conclusion is based on the fits of the Fourier peak II and II + III isolates, which demonstrated that: (i) two distinct manganese-manganese vectors contribute to peak II (2.7 and 2.8 Å); (ii) there is an unequal distribution of the coordination numbers of N1 (2.7 Å) and N2 (2.8 Å), which would be consistent with the presence of three di-µ-oxo-bridged manganese-manganese moieties; (iii) the fit clearly improved when the N1/N2 ratio is close to 2:1 with Ntot ≈ 1.5. The difference between the two di-µ-oxo-bridged manganese-manganese distances is approximately 0.1 Å for both the S1 and the S2 state, which explains why the traditional EXAFS study with a distance resolution of 0.14 Å was unable to reveal such distance heterogeneity.
The current polarized EXAFS data from oriented PS II membranes in the S1 state support the conclusions summarized above for the S1 state in solution. The fit qualities for peak II shown in Table 1 demonstrate significant improvement if two manganese-manganese vectors are introduced. The x-ray absorption linear dichroism from oriented PS II membranes demonstrates that the average manganese-manganese (~2.7 Å) vector and manganese-manganese (~2.8 Å) vector both have similar orientation of ~60° to the membrane normal, as summarized in Table 3. The averaged (~2.7–2.8 Å) vector is oriented at ~63°, which is the same as the value reported earlier from conventional EXAFS studies with oriented PS II membranes (34).
TABLE 3.
| Vector R(Å) | Niso | Angle to membrane normal, (ϕ) | |
|---|---|---|---|
| Peak II, one shell fit | Manganese-manganese | ||
| 2.74 ± 0.02 | 1.30 ± 0.30 | 63 ± 5° | |
| Peak II, two shell fit | Manganese-manganese | ||
| 2.72 ± 0.02 | 0.88 ± 0.20 | 61 ± 5° | |
| 2.82 ± 0.02 | 0.46 ± 0.12 | 64 ± 10° | |
| Peak III, estimation of the Napp limits are included |
Manganese-manganese | ||
| 3.20 ± 0.02 | 0.39 ± 0.10 | >70° | |
| Manganese-calcium | |||
| 3.40 ± 0.02 | 0.29 ± 0.09 | <18° (<23°)a |
Value from Cinco et al. (23).
Several possible reasons for manganese-manganese distance heterogeneity can be suggested: 1) differences in the redox states of manganese atoms resulting in the 2.7 Å and 2.8 Å manganese-manganese vectors; 2) differences in types of µ-oxo-bridges (µ2-oxo versus µ3-oxo) connecting manganese atoms; 3) protonation of the µ-oxo bridge. Protonation of the µ-oxo bridge lowers the manganese-oxygen bond order, which causes an increase in the manganese-manganese distance. Studies of model compounds support these possible reasons for distance heterogeneity (59–61).
FT Peak III; Orientation of the Long Manganese-Manganese and Manganese-Calcium Vectors Relative to the Membrane Normal
Fits to EXAFS data allow consideration of some relevant questions about the chemical nature of backscatterers contributing to peak III and, now, about the orientation of those manganese-backscatterer vectors relative to the membrane normal. Calcium was included in the fit combination because it has been implicated as a structural element of the OEC through O2 evolution activity (19, 45, 62– 66), EPR (20), and calcium- and strontium-EXAFS experiments (22, 23). In conventional EXAFS experiments it was noticed previously that the addition of the manganese-calcium vector to the manganese-manganese long interaction improves fit qualities. The evidence that peak III cannot be a result of only manganese-calcium interactions came from a study in which the manganese EXAFS spectrum of calcium-depleted PS II showed a peak III with decreased intensity (43). High resolution range-extended EXAFS data on oriented PS II membranes provide new experimental support for the conclusion that peak III contains both manganese-manganese and manganese-calcium interactions; the combination of oriented preparations and range-extended EXAFS allows the two types of interactions to be resolved. Information about the relative orientation of the manganese-strontium vectors was reported in the earlier study of strontium-reconstituted, oriented PS II membranes (23).
Results of analysis of the x-ray absorption linear dichroism from peak III of the oriented PS II samples are summarized in Table 3. The orientation of the averaged manganese-calcium vector is similar to that reported for strontium-reconstituted, oriented PS II membranes (23). The manganese-manganese (~3.2 Å) vector is oriented more toward the membrane plane. The average of both vectors is in agreement with the results from conventional EXAFS: ~43° for the ~3.3 Å vectors (combination of mono-µ-oxo-bridged manganese-manganese and manganese-calcium vectors) (34).
Structure and Orientation of the Mn4Ca Cluster in PS II
The structural information about the Mn4Ca cluster in the S1 state, based on polarized EXAFS data, is summarized in Table 3. Topological models for the Mn4 cluster compatible with the EXAFS data (27) and containing three short 2.7–2.8 Å manganese-manganese vectors and one manganese-manganese interaction at 3.2Åare shown in Fig. 8A. Previously, dichroism characteristics of only the averages of the short manganese-manganese vectors at ~2.7 Å and the long manganese-manganese and manganese-calcium interactions at ~3.3 Å were known (34). Those data were not sufficient to restrict the possible orientations of the proposed models with respect to the PS II membrane or the PS II protein frame. With the new range-extended EXAFS data, we can for the first time determine independently the angle dependence for three different (2.8 Å, averaged 2.7 Å, and 3.2 Å) manganese-manganese vectors relative to the membrane normal. This allows us to impose additional restrictions on proposed structural models. The importance of the results in Table 3 is that they restrict the angles of the manganese-manganese or manganese-calcium vectors relative to the membrane normal for all three manganese-manganese vectors simultaneously.
FIGURE 8. Cluster models compatible with polarized range-extended EXAFS.
A, models for the Mn4Ca cluster compatible with the range-extended manganese EXAFS data with three short 2.7–2.8 Å manganese-manganese distances and one longer manganese-manganese distance at 3.2 Å. B, Mn4 models developed from Fig. 8A topological core structures and their proposed orientation relative to the membrane normal consistent with polarized range-extended EXAFS (Table 3). Note that in the membrane plane there is a rotational ambiguity which is always present for one-dimensionally oriented samples such as layered membranes. We emphasize that there may be other models that can be tested in this manner (this would include structural and optical isomers of listed models). C, the orientation of the average manganese-calcium vector in relation to the 3.2 Å manganese-manganese vector. The cones represent a range for the average manganese-calcium vector(s) along the membrane normal (~18°), and the 3.2 Å manganese-manganese vector toward the membrane plane (~20°), respectively.
Knowledge of the angles between the membrane normal and each of three different vectors involving manganese-manganese interactions allows us to determine whether one or more orientations for any particular model are consistent with the dichroism data. For this purpose we used the averaged 2.7, 2.8, and 3.2 Å manganese-manganese vectors, and we illustrate this approach for each of the models in Fig. 8A; the orientations shown in Fig. 8B are in agreement with the dichroism measurements. We emphasize that there may be other models that can be tested in this manner (this would include structural and optical isomers of listed models). There are two possibilities for the placement of the 2.8 Å manganese-manganese vector for Model I and three possibilities for Model II. As structures Ia and Ib and IIa, IIb, and IIc in Fig. 8B demonstrate, different placement of the 2.8 Å manganese-manganese vectors results in rather small changes in the orientation of models, as follows from the close values of the angle of 2.8 Å manganese-manganese vector and averaged angle of two 2.7 Å manganese-manganese vectors to the membrane normal (~60°, Table 3). Uncertainty in the angle between the 3.2 Å manganese-manganese vector and membrane normal (>70, Table 3) results in a subset of model orientations as this angle changes within the determined range; however, this does not produce dramatic changes in the model orientations. Model III has a high degree of rotational freedom for the 3.2 Å manganese-oxygen-manganese mono-µ-oxo unit that results in multiple solutions; in Fig. 8B we show only one such example. There are many possibilities for the placement of calcium in the models shown in Fig. 8B, and the average orientation of the manganese-calcium vector can best be described to be within a cone about the membrane normal, as shown in Fig. 8C. Using the results of polarized EXAFS (Table 3), the range of possible orientations of the models to the membrane normal can be dramatically reduced as illustrated in Fig. 8B. However, the following uncertainties remain: (i) rotational ambiguity in the membrane plane, which is always present for one-dimensionally oriented samples such as oriented membranes and (ii) multiple possibilities for calcium coordination; present data do not allow us to distinguish clearly whether there is one or two manganese-calcium interactions as well as precise angle between the manganese-calcium vector and the membrane normal.
The geometric information obtained from polarized EXAFS measurements on spinach membranes provides an important tool for testing proposed Mn4Ca models from other studies. For example, results from this study can be compared with the models from x-ray crystal structures from cyanobacteria. The PS II x-ray structures at 3.5 and 3.0 Å resolution (16, 17) indicate three manganese-calcium interactions at 3.2–3.4 Å distances, with average manganese-calcium angles of ~40° and ~34° to the membrane normal for the two different structures, respectively (16, 17). Those angles are larger than the upper limit from this study of ~18° and from the strontium-reconstituted PS II membrane study (23) of ~23°. In Ferreira et al. (16) two manganese-manganese interactions at ~3.2 Å were modeled in the OEC structure with an average angle of ~48° to the membrane normal, which is different from the lower limit of ~70° from this study. In Loll et al. (17), the ~3.2 Å manganese-manganese interactions form an average angle of ~61°, which is closer to the results in this study. However, the OEC in Loll et al. (17) contain two ~2.7 Å and two ~3.2 Å manganese-manganese vectors, compared with the three ~2.7 Å and one ~3.2 Å manganese-manganese distances required by EXAFS data.
Recently the polarized EXAFS measurements on PS II single crystals of the thermophilic cyanobacterium Thermosynechococcus elongatus combined with XRD resulted in a set of high resolution structures for the Mn4Ca cluster (14). Model I from Yano et al. (14) has all three (averaged 2.7, 2.8, and 3.2 Å) manganese-manganese vectors oriented differently relative to the membrane normal compared with those determined in this study (Table 3) and is less favored (14). Models II and III from Yano et al. (14) are similar to IIa in this study (Fig. 8B), with the ~60° orientation of the 2.8 Å manganese-manganese vector and ~80° orientation of the 3.2 Å manganese-manganese vector relative to the membrane normal; however, the averaged orientation of the 2.7 Å manganese-manganese vectors to the membrane normal is ~40° compared with ~60° (Table 3). This difference in orientation of the averaged 2.7 Å vectors with respect to the membrane normal could be due to the inherent errors in the determination of the angles in this method, or we speculate that this variation in orientation could reflect the differences in PS II from thermophilic cyanobacteria versus spinach. Also note that the resolution of the two experiments is different; analysis of the single crystal EXAFS data involve considerations of the protein unit cell symmetry, while oriented membranes have a unique axis along the membrane normal with rotational uncertainty in the plane of the membrane.
Range-extended EXAFS provides an important technical development that allows differentiation of the 2.7 and 2.8 Å manganese-manganese, the 3.2 Å manganese-manganese, and the 3.4 Å manganese-calcium interactions. Detailed information about the orientation of manganese-manganese and manganese-calcium vectors in the OEC in the S1 state provide a critical starting point for the analysis of the structural changes in the OEC throughout the catalytic S state cycle.
Supplementary Material
Acknowledgments
We thank Prof. Stephen P. Cramer at the Lawrence Berkeley National Laboratory for suggesting the range-extended EXAFS experiment and are grateful to him for providing access to the crystal analyzer (National Institutes of Health Grant GM-65440, National Science Foundation Grant CHE-0213592, and Office of Biological and Environmental Research, Department of Energy (to S. P. Cramer)). Dr. Olga Krupina is acknowledged for helpful discussions. Synchrotron facilities were provided by Advanced Photon Source operated by the Department of Energy, Office of Basic Energy Sciences under Contract W-31-109-ENG-38. BioCAT is a National Institutes of Health-supported Research Center (Grant RR-08630). Stanford Synchrotron Radiation Laboratory is operated by Stanford University for the United States Department of Energy, Office of Basic Energy Sciences.
Footnotes
This work was supported in part by National Institutes of Health Grant GM-55302 and by the Director, Office of Science, Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences and of the Department of Energy under Contract DE-AC02-05CH11231. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental data (including Equations S1–S7), Figs. S1–S8, Tables S1–S3, and Refs. 1–9.
This article was selected as a Paper of the Week.
The abbreviations used are: OEC, oxygen-evolving complex; EXAFS, extended x-ray absorption fine structure; FT, Fourier transform; PS II, photosystem II; XANES, x-ray absorption near edge spectroscopy; XAS, x-ray absorption spectroscopy; XRD, x-ray diffraction; MES, 4-morpholineethanesulfonic acid.
REFERENCES
- 1.Debus RJ. Biochim. Biophys. Acta. 1992;1102:269–352. doi: 10.1016/0005-2728(92)90133-m. [DOI] [PubMed] [Google Scholar]
- 2.Rutherford AW, Zimmermann J-L, Boussac A. In: The Photosystems: Structure, Function, and Molecular Biology. Barber J, editor. BV Amsterdam: Elsevier Science Publishers; 1992. pp. 179–229. [Google Scholar]
- 3.Ort DR, Yocum CF, editors. Oxygenic Photosynthesis: The Light Reactions. The Netherlands: Kluwer Academic Publishers, Dordrecht; 1996. [Google Scholar]
- 4.Wydrzynski T, Satoh S. Photosystem II: The Light-Driven Water:Plastoquinone Oxidoreductase. The Netherlands: Springer, Dordrecht; 2005. [Google Scholar]
- 5.Kok B, Forbush B, McGloin M. Photochem. Photobiol. 1970;11:457–475. doi: 10.1111/j.1751-1097.1970.tb06017.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller AF, Brudvig GW. Biochim. Biophys. Acta. 1991;1056:1–18. doi: 10.1016/s0005-2728(05)80067-2. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa K, Ono T-a, Inoue Y, Kusunoki M. Chem. Phys. Lett. 1999;300:9–19. [Google Scholar]
- 8.Carrell TG, Tyryshkin AM, Dismukes GC. J. Biol. Inorg. Chem. 2002;7:2–22. doi: 10.1007/s00775-001-0305-3. [DOI] [PubMed] [Google Scholar]
- 9.Britt RD, Campbell KA, Peloquin JM, Gilchrist ML, Aznar CP, Dicus MM, Robblee J, Messinger J. Biochim. Biophys. Acta. 2004;1655:158–171. doi: 10.1016/j.bbabio.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 10.DeRose VJ, Mukerji I, Latimer MJ, Yachandra VK, Sauer K, Klein MP. J. Am. Chem. Soc. 1994;116:5239–5249. [Google Scholar]
- 11.Yachandra VK, Sauer K, Klein MP. Chem. Rev. 1996;96:2927–2950. doi: 10.1021/cr950052k. [DOI] [PubMed] [Google Scholar]
- 12.Penner-Hahn JE. Struct. Bond. 1998;90:1–36. [Google Scholar]
- 13.Sauer K, Yano J, Yachandra VK. Photosynth. Res. 2005;85:73–86. doi: 10.1007/s11120-005-0638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yano J, Kern J, Sauer K, Latimer M, Pushkar Y, Biesiadka J, Loll B, Saenger W, Messinger J, Zouni A, Yachandra VK. Science. 2006;314:821–825. doi: 10.1126/science.1128186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamiya N, Shen JR. Proc. Natl. Acad. Sci. U.S.A. 2003;100:98–103. doi: 10.1073/pnas.0135651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S. Science. 2004;303:1831–1838. doi: 10.1126/science.1093087. [DOI] [PubMed] [Google Scholar]
- 17.Loll B, Kern J, Saenger W, Zouni A, Biesiadka J. Nature. 2005;438:1040–1044. doi: 10.1038/nature04224. [DOI] [PubMed] [Google Scholar]
- 18.Chu H-A, Hillier W, Law NA, Babcock GT. Biochim. Biophys. Acta. 2001;1503:69–82. doi: 10.1016/s0005-2728(00)00216-4. [DOI] [PubMed] [Google Scholar]
- 19.Boussac A, Rutherford AW. Chem. Scr. 1988;28A:123–126. [Google Scholar]
- 20.Boussac A, Rutherford AW. Biochemistry. 1988;27:3476–3483. [Google Scholar]
- 21.Boussac A, Zimmermann J-L, Rutherford AW. Biochemistry. 1989;28:8984–8989. doi: 10.1021/bi00449a005. [DOI] [PubMed] [Google Scholar]
- 22.Cinco RM, Holman KLM, Robblee JH, Yano J, Pizarro SA, Bellacchio E, Sauer K, Yachandra VK. Biochemistry. 2002;41:12928–12933. doi: 10.1021/bi026569p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cinco RM, Robblee JH, Messinger J, Fernandez C, Holman KLM, Sauer K, Yachandra VK. Biochemistry. 2004;43:13271–13282. doi: 10.1021/bi036308v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yano J, Kern J, Irrgang K-D, Latimer MJ, Bergmann U, Glatzel P, Pushkar Y, Biesiadka J, Loll B, Sauer K, Messinger J, Zouni A, Yachandra VK. Proc. Natl. Acad. Sci. U.S.A. 2005;102:12047–12052. doi: 10.1073/pnas.0505207102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Messinger J, Robblee JH, Bergmann U, Fernandez C, Glatzel P, Visser H, Cinco RM, McFarlane KL, Bellacchio E, Pizarro SA, Cramer SP, Sauer K, Klein MP, Yachandra VK. J. Am. Chem. Soc. 2001;123:7804–7820. doi: 10.1021/ja004307+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yachandra VK. In: Photosystem II: The Light-Driven Water:Plastoquinone Oxidoreductase. Wydrzynski T, Satoh S, editors. The Netherlands: Springer, Dordrecht; 2005. pp. 235–260. [Google Scholar]
- 27.Yano J, Pushkar Y, Glatzel P, Lewis A, Sauer K, Messinger J, Bergmann U, Yachandra VK. J. Am. Chem. Soc. 2005;127:14974–14975. doi: 10.1021/ja054873a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott RA. In: Structural and Resonance Techniques in Biological Research. Rousseau DL, editor. Orlando, FL: Academic Press; 1984. pp. 295–362. [Google Scholar]
- 29.Cramer SP. In: X-ray Absorption: Principles, Applications, Techniques of EXAFS, SEXAFS and XANES. Koningsberger DC, Prins R, editors. New York: Wiley; 1988. pp. 257–326. [Google Scholar]
- 30.Yachandra VK. Methods Enzymol. 1995;246:638–675. doi: 10.1016/0076-6879(95)46028-4. [DOI] [PubMed] [Google Scholar]
- 31.Bergmann U, Cramer SP. SPIE Conference on Crystal and Multilayer Optics. San Diego, CA: SPIE; 1998. pp. 198–209. [Google Scholar]
- 32.Glatzel P, de Groot FMF, Manoilova O, Grandjean D, Weckhuysen BM, Bergmann U, Barrea R. Phys. Rev. B. 2005;72:014–117. [Google Scholar]
- 33.George GN, Prince RC, Cramer SP. Science. 1989;243:789–791. doi: 10.1126/science.2916124. [DOI] [PubMed] [Google Scholar]
- 34.Mukerji I, Andrews JC, DeRose VJ, Latimer MJ, Yachandra VK, Sauer K, Klein MP. Biochemistry. 1994;33:9712–9721. doi: 10.1021/bi00198a042. [DOI] [PubMed] [Google Scholar]
- 35.Dau H, Andrews JC, Roelofs TA, Latimer MJ, Liang W, Yachandra VK, Sauer K, Klein MP. Biochemistry. 1995;34:5274–5287. doi: 10.1021/bi00015a043. [DOI] [PubMed] [Google Scholar]
- 36.Schiller H, Dittmer J, Iuzzolino L, Dörner W, Meyer-Klaucke W, Solé VA, Nolting H-F, Dau H. Biochemistry. 1998;37:7340–7350. doi: 10.1021/bi972329b. [DOI] [PubMed] [Google Scholar]
- 37.Berthold DA, Babcock GT, Yocum CF. FEBS Lett. 1981;134:231–234. [Google Scholar]
- 38.Rutherford AW. Biochim. Biophys. Acta. 1985;807:189–201. [Google Scholar]
- 39.Glatzel P, Bergmann U, Yano J, Visser H, Robblee JH, Gu WW, de Groot FMF, Christou G, Pecoraro VL, Cramer SP, Yachandra VK. J. Am. Chem. Soc. 2004;126:9946–9959. doi: 10.1021/ja038579z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robblee JH, Messinger J, Cinco RM, McFarlane KL, Fernandez C, Pizarro SA, Sauer K, Yachandra VK. J. Am. Chem. Soc. 2002;124:7459–7471. doi: 10.1021/ja011621a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rehr JJ, Albers RC. Rev. Mod. Phys. 2000;72:621–654. [Google Scholar]
- 42.Teo BK. EXAFS: Basic Principles and Data Analysis. Berlin: Springer-Verlag; 1986. [Google Scholar]
- 43.Latimer MJ, DeRose VJ, Yachandra VK, Sauer K, Klein MP. J. Phys. Chem. B. 1998;102:8257–8265. doi: 10.1021/jp981668r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pizarro SA, Visser H, Cinco RM, Robblee JH, Pal S, Mukhopadhyay S, Mok HJ, Sauer K, Wieghardt K, Armstrong WH, Yachandra VK. J. Bio. Inorg. Chem. 2004;9:247–255. doi: 10.1007/s00775-003-0520-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boussac A, Rappaport F, Carrier P, Verbavatz JM, Gobin R, Kirilovsky D, Rutherford AW, Sugiura M. J. Biol. Chem. 2004;279:22809–22819. doi: 10.1074/jbc.M401677200. [DOI] [PubMed] [Google Scholar]
- 46.Vincent JB, Christmas C, Huffman JC, Christou G, Chang HR, Hendrickson DN. J. Chem. Soc. Chem. Commun. 1987:236–238. [Google Scholar]
- 47.Christou G, Vincent JB. In: Metal Clusters in Proteins. Que L Jr, editor. Washington, DC: American Chemical Society; 1988. pp. 238–255. [Google Scholar]
- 48.Wieghardt K. Angew. Chem.-Int. Ed. Engl. 1989;28:1153–1172. [Google Scholar]
- 49.Cinco RM, Rompel A, Visser H, Aromí G, Christou G, Sauer K, Klein MP, Yachandra VK. Inorg. Chem. 1999;38:5988–5998. doi: 10.1021/ic991003j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Law NA, Caudle MT, Pecoraro VL. Adv. Inorg. Chem. 1999;46:305–440. [Google Scholar]
- 51.Mukhopadhyay S, Mandal SK, Bhaduri S, Armstrong WH. Chem. Rev. 2004;104:3981–4026. doi: 10.1021/cr0206014. [DOI] [PubMed] [Google Scholar]
- 52.Yachandra VK, DeRose VJ, Latimer MJ, Mukerji I, Sauer K, Klein MP. Science. 1993;260:675–679. doi: 10.1126/science.8480177. [DOI] [PubMed] [Google Scholar]
- 53.Dau H, Iuzzolino L, Dittmer J. Biochim. Biophys. Acta. 2001;1503:24–39. doi: 10.1016/s0005-2728(00)00230-9. [DOI] [PubMed] [Google Scholar]
- 54.Peloquin JM, Britt RD. Biochim. Biophys. Acta. 2001;1503:96–111. doi: 10.1016/s0005-2728(00)00219-x. [DOI] [PubMed] [Google Scholar]
- 55.Kulik LV, Epel B, Lubitz W, Messinger J. J. Am. Chem. Soc. 2005;127:2392–2393. doi: 10.1021/ja043012j. [DOI] [PubMed] [Google Scholar]
- 56.Kusunoki M, Takano T, Ono T, Noguchi T, Yamaguchi Y, Oyanagi H, Inoue Y. In: Photosynthesis: from Light to Biosphere. Mathis P, editor. The Netherlands: Kluwer Academic Publishers, Dordrecht; 1995. pp. 251–254. [Google Scholar]
- 57.Liang W, Latimer MJ, Dau H, Roelofs TA, Yachandra VK, Sauer K, Klein MP. Biochemistry. 1994;33:4923–4932. doi: 10.1021/bi00182a022. [DOI] [PubMed] [Google Scholar]
- 58.DeRose VJ, Latimer MJ, Zimmermann J-L, Mukerji I, Yachandra VK, Sauer K, Klein MP. Chem. Phys. 1995;194:443–459. [Google Scholar]
- 59.Baldwin MJ, Stemmler TL, Riggs-Gelasco PJ, Kirk ML, Penner-Hahn JE, Pecoraro VL. J. Am. Chem. Soc. 1994;116:11349–11356. [Google Scholar]
- 60.Pence LE, Caneschi A, Lippard SJ. Inorg. Chem. 1996;35:3069–3072. [Google Scholar]
- 61.Darovsky A, Kezerashvili V, Coppens P, Weyhermüller T, Hummel H, Wieghardt K. Inorg. Chem. 1996;35:6916–6917. doi: 10.1021/ic9610391. [DOI] [PubMed] [Google Scholar]
- 62.Boussac A, Maison-Peteri B, Etienne A-L, Vernotte C. Biochim. Biophys. Acta. 1985;808:231–234. [Google Scholar]
- 63.Grove GN, Brudvig GW. Biochemistry. 1998;37:1532–1539. doi: 10.1021/bi971356z. [DOI] [PubMed] [Google Scholar]
- 64.Ono T-A, Inoue Y. FEBS Lett. 1988;227:147–152. [Google Scholar]
- 65.Chen C, Kazimir J, Cheniae GM. Biochemistry. 1995;34:13511–13526. doi: 10.1021/bi00041a031. [DOI] [PubMed] [Google Scholar]
- 66.Ananyev GM, Dismukes GC. Biochemistry. 1997;36:11342–11350. doi: 10.1021/bi970626a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.