Abstract
Early life stress can permanently alter functioning of the hypothalamic-pituitary-adrenal (HPA) axis, which regulates the stress response and influences the perception of pain. Chronic pelvic pain patients commonly report having experienced childhood neglect or abuse, which increases the likelihood of presenting with comorbid chronic pain and/or mood disorders. Animal models of neonatal stress commonly display enhanced anxietylike behaviors, colorectal hypersensitivity, and disruption of proper neuro-immune interactions in adulthood. Here, we tested the hypothesis that early life stress impacts vaginal sensitivity by exposing mice to neonatal maternal separation (NMS) for 3hr/day during the first two (NMS14) or three (NMS21) postnatal weeks. As adults, female mice underwent vaginal balloon distension (VBD), which was also considered an acute stress. Before or after VBD, mice were assessed for anxiety-like behavior, hindpaw sensitivity, and changes in gene and protein expression related to HPA axis function and regulation. NMS21 mice displayed significantly increased vaginal sensitivity compared to naïve mice, as well as significantly reduced anxiety-like behavior at baseline, which was heightened following VBD. NMS21 mice exhibited significant thermal and mechanical hindpaw hypersensitivity at baseline and following VBD. NMS14 mice displayed no change in anxiety-like behavior and only exhibited significantly increased hindpaw mechanical and thermal sensitivity following VBD. Centrally, a significant decrease in negative regulation of the HPA axis was observed in the hypothalamus and hippocampus of NMS21 mice. Peripherally, NMS and VBD affected the expression of inflammatory mediators in the vagina and bladder. Corticotropin releasing factor (CRF) receptor and transient receptor potential (TRP) channel protein expression was also significantly, and differentially, affected in vagina, bladder, and colon by both NMS and VBD. Together these data indicate that NMS affects both central and peripheral aspects of the HPA axis, which may drive changes in vaginal sensitivity and the development of comorbid chronic pain and mood disorders.
Keywords: Vulvodynia, pain, stress, HPA axis, corticotropin releasing factor, TRP channels
Growth and development during the formative years, beginning prenatally and lasting until age 5, occurs at a pace exceeding that of any subsequent stage of life (Perry and Pollard, 1998). As a result, the nervous system is remarkably pliant to nurturing and adverse events during this period of development (Teicher et al., 2002). Psychological stressors, such as childhood neglect or abuse; low socioeconomic status; and witnessing domestic violence, parental discord, or crime in the home, have profound lifelong impacts on behavior and also serve as risk factors for functional pain disorders later in life (Grunau et al., 1994, Anand, 1998, Bennett et al., 1998, Moore and Kennedy, 2000). Accordingly, a significant subset of patients with functional pelvic pain disorders, including irritable bowel syndrome (IBS), interstitial cystitis (IC), and vulvodynia, report a history of adverse childhood events such as abuse or neglect (Chitkara et al., 2008, Jones et al., 2009, Peters et al., 2009). Subsets of these patients report stress-related symptom onset or increased severity, have difficulty coping with stressful situations, and suffer from depression and/or anxiety (Arnold et al., 2006, Larauche et al., 2012, Bullones Rodriguez et al., 2013).
The comorbidity between mood disorders and chronic pelvic pain is due, in part, to altered functioning of corticotropin releasing factor (CRF)-responsive regions of the brain, including the hypothalamic-pituitary-adrenal (HPA) axis, which regulates stress response and influences the perception of pain (Heim et al., 1998, Chang et al., 2009, Mayson and Teichman, 2009). Under stressful conditions, CRF is secreted from the paraventricular nucleus (PVN) of the hypothalamus and induces the systemic release of adrenocorticotropic hormone (ACTH) from the anterior pituitary corticotrophs. ACTH then stimulates glucocorticoid (cortisol in humans and corticosterone in rodents), synthesis and secretion from the adrenal cortex (Herman et al., 2005, Ulrich-Lai and Herman, 2009). Activity of the HPA axis is largely regulated by positive and negative feedback within the hypothalamus and from higher limbic structures, including the amygdala and hippocampus, which stimulate and inhibit CRF release, respectively (Herman et al., 2005, Ulrich-Lai and Herman, 2009). Glucocorticoid-driven negative feedback occurs through two receptors, glucocorticoid receptor (GR) and mineralocorticoid receptor (MR), which are relatively slow-acting and effect long-term changes in gene transcription (De Kloet et al., 1998). The two CRF receptors, CRF1 and CRF2, bind CRF and the related Urocortins (Ucn1-3) with varying affinity and work in opposition to one another to facilitate and depress activation of the HPA axis, respectively (Bale and Vale, 2004).
Neonatal maternal separation (NMS) in rodents has been used for several decades as a model of early life stress that significantly affects functioning of the HPA axis. NMS has generally been shown to increase the output of the HPA axis, evidenced as enhanced anxiety-like behaviors and prolonged release of ACTH and corticosterone following a stressful event (Romeo et al., 2003, Ladd et al., 2004, Plotsky et al., 2005, Aisa et al., 2008). CRF production in both the PVN and the central nucleus of the amygdala (CeA) (Plotsky et al., 2005, Aisa et al., 2008, Desbonnet et al., 2008), as well as altered hypothalamic and limbic CRF receptor and GR expression (Ladd et al., 2004, Plotsky et al., 2005, Aisa et al., 2008, O'Malley et al., 2011a), have been reported in NMS rats. Peripheral CRF release, in response to chronic stress, has been shown to promote neurogenic inflammation through mast cell degranulation and increased cytokine expression and neuropeptide release (Black, 2002). Mast cell infiltration and hypertrophy of sensory innervation have been reported in biopsies from patients with IBS (Barbara et al., 2004, Barbara et al., 2007, Akbar et al., 2008), IC (Larsen et al., 2008, Liu et al., 2012), and vulvodynia (Goetsch et al., 2010, Leclair et al., 2011). Involvement of transient receptor potential vanilloid 1 (TRPV1), the capsaicin receptor necessary for the development of inflammatory hyperalgesia (Caterina et al., 1999, Jones et al., 2005), has also been shown to contribute toward acute stress-induced colonic hypersensitivity in NMS rats (van den Wijngaard et al., 2009). Together these studies suggest that NMS increases the activity of the HPA axis resulting in central and peripheral changes underlying increased anxiety-like behaviors and visceral sensitivity and neurogenic inflammation.
Stress or direct organ irritation/inflammation during early development has been shown to permanently enhance sensitivity and nociceptive signaling within the gastrointestinal (Al–Chaer et al., 2000, Laird et al., 2001, Barreau et al., 2004, Winston et al., 2007, Christianson et al., 2010) and urinary (Randich et al., 2006b, DeBerry et al., 2007) systems of rodents, therefore, we hypothesized that early life stress would also increase sensitivity within the reproductive system concordant with impaired regulation of the HPA axis. Previous studies of early life stress in mice have largely performed NMS only during the stress hyporesponsive state (postnatal day [P]1–14) (Dent et al., 2007), and subsequently reported a mild anxiety-like phenotype (Millstein and Holmes, 2007), or a decrease in behavioral anxiety (Romeo et al., 2003, Savignac et al., 2011, Own and Patel, 2012). In the current study, we compared adult female mice that underwent NMS during the first two weeks of life to those that underwent NMS during the entire preweaning period (P1–21), to determine if stress following the stress hyporesponsive state, yet still during final synaptic maturation within the limbic system (Egorov and Draguhn, 2013), would result in a more pronounced phenotype. To assess vulnerability to adult stress exposure, mice were tested for anxiety-like behavior and hindpaw sensitivity, both prior to and following vaginal balloon distension (VBD), which quantified vaginal sensitivity and was considered an acute stress exposure. Protein and mRNA expression of mediators involved in regulating the HPA axis both centrally and peripherally, as well as protein expression of TRPV1, and the related TRPA1, within the vagina, bladder, and colon were measured to determine how early life and adult stress may influence comorbid pelvic pain disorders.
Experimental Procedures
Animals
Experiments were performed on female C57Bl/6 mice (Charles River, Wilmington, MA) born and housed in the Research Support Facility at the University of Kansas Medical Center. Mice were housed on a 12-hour light cycle from 600 to 1800 hours and received water and food ad libitum. All research performed conformed to the National Institute of Health Guide for the Care and Use of Laboratory Animals in accordance with the guidelines specified by the University of Kansas Medical Center Animal Care and Use Protocols.
Neonatal maternal separation
Beginning on postnatal day 1 (P1, date of birth was considered P0), pups were removed daily from their home cages for 180 minutes (1100 to 1400 hours) and placed as a litter, with a small amount of home bedding material, into a clean glass beaker and held at 34°C and 50% humidity. Each litter was weighed en masse prior to and at the end of the separation period. NMS14 mice underwent daily separation from P1 through P14 and then remained undisturbed, with the exception of routine animal husbandry, in their home cages until weaning at P22. NMS21 mice underwent daily separation from P1 through P21 and were weaned at P22. Naïve mice were born in-house and remained undisturbed, with the exception of daily weighing and routine animal husbandry, in their home cages until weaning at P22. Three separate cohorts of NMS21 mice and two separate cohorts of NMS14 mice were used in this study. Each cohort of NMS14 and NMS21 mice was compared to a corresponding naïve group of mice that were born, housed, and weaned during the same time frame to avoid potential complications arising from variations in prenatal shipping conditions, housing environment, and investigator handling.
Experimental design
All naïve, NMS14, and NMS21 mice were subjected to vaginal balloon distension (VBD) as adults (between 9–36 weeks of age, Table 1). VBD was considered a stressor in these experiments, as colorectal distension (CRD) significantly elevated serum corticosterone levels in a separate cohort of naïve female mice (761.6 ± 83.7 ng/ml) compared to age-matched non-distended naïve female mice (236.8 ± 106 ng/ml; p < 0.05, Mann-Whitney test, n=5). Either prior to (Baseline group) or following VBD (post- VBD group), mice underwent open field, thermal analgesiometer, and von Frey monofilament testing, as described in Table 1. With the exception of naïve mice in the NMS14 cohort, separate groups of mice were used for baseline and post-VBD behavioral measurements. All mRNA and protein analysis was performed on tissue from the same cohort of naïve and NMS21 mice that was euthanized a week after VBD testing or from age-matched non-VBD exposed mice.
Table 1.
Age of mice at experimental time points.
| Open Field |
Thermal Hindpaw |
Mechanical Hindpaw |
Vaginal Balloon Distension |
Open Field |
Thermal Hindpaw |
Mechanical Hindpaw |
|
|---|---|---|---|---|---|---|---|
| NMS14 | |||||||
| Baseline* | 6 | 9 | 10 | 12–13 | |||
| Post-VBD* | 12–13 | 15 | 15.5 | 17 | |||
| VBD only | 9–10 | ||||||
| NMS21 | |||||||
| Baseline | 6 | 6.5 | 7 | 9 | |||
| Post-VBD | 9–11 | 12 | 14 | 14.5 | |||
| VBD only | 30–36 | ||||||
NMS14, NMS21, and corresponding naïve mice underwent behavioral or physiological testing at the above noted ages (in weeks). Mice were assessed for anxiety-like behaviors using an open field (OF) actimeter and/or thermal and mechanical hindpaw sensitivity either before (Baseline) or after (post-VBD) vaginal balloon distension (VBD). With the exception of the naïve mice in the NMS14 cohort, separate groups of mice were used for baseline and post-VBD measurements. All mRNA and protein expression analysis utilized tissue from naïve and NMS21 mice exposed to OF and/or hindpaw measurements prior to VBD and euthanized one week following VBD or from naïve and NMS21 mice born and euthanized at the same time, but without exposure to VBD.
Behavioral analysis
All mice underwent a 30-minute acclimatization period within the testing room for at least one day prior to each behavioral test. For both thermal and mechanical hindpaw sensitivity testing, the mice were allowed to acclimate to the apparatus for 30 minutes prior to testing and the experimenter was blinded to the group status of the mice.
Open field testing
Activity in NMS14 (baseline: n=5, post-VBD: n=8), NMS21 (baseline: n=8, post- VBD: n=8) and naïve (NMS14 cohort, baseline: n=6, post-VBD: n=8; NMS21 cohort, baseline: n=7, post-VBD: n=7) mice was measured using a Force Plate Actimeter (BASi, San Diego, CA), which consists of a rigid, low-mass horizontal plate (44cm×44cm) coupled to high sensitivity force transducers on each corner. A Plexiglas enclosure rests a few millimeters above the plate to create a transparent enclosure, all of which rests within a light and sound-attenuated box. Animals were individually placed into the middle of the testing arena and allowed to move freely for 10 minutes. During this time, the software recorded the distance traveled and position of the mouse. The total distance traveled and percent of time spent in the perimeter (outermost 8.25cm; increased time spent in the perimeter is indicative of anxiety (Bailey and Crawley, 2009)) was calculated and binned and the data from the second 5 min bin is reported here.
Thermal analgesiometer testing
NMS14 (baseline: n=5, post-VBD: n=8), NMS21 (baseline: n=8, post-VBD: n=8) and naïve (NMS14 cohort, baseline: n=6, post-VBD: n=8; NMS21 cohort, baseline: n=8, post-VBD: n=8) mice were placed in individual clear plastic chambers (11×5×3.5cm) on the 30°C heated glass surface of a thermal analgesiometer (UARDG; Department of Anesthesiology, University of California San Diego, La Jolla, CA). A high intensity light (4.25 Amperes) was directed at the plantar aspect of the hindpaw and the latency to withdrawal from the stimulus was automatically recorded within 0.01 second. Alternating hindpaws were tested for a total of three times per side with a minimum of 5 minutes between applications. The stimulus terminated automatically at 20 seconds to avoid tissue damage. Individual responses were averaged together/mouse and group means were determined as previously described (Christianson et al., 2003).
Von Frey monofilament testing
NMS14 (baseline: n=4, post-VBD: n=8), NMS21 (baseline: n=8, post-VBD: n=8) and naïve (NMS14 cohort, baseline: n=6, post-VBD: n=7; NMS21 cohort, baseline: n=8, post-VBD: n=8) mice were placed into individual clear plastic chambers (11×5×3.5cm) on a wire mesh screen elevated 55 cm above a table. The up-down method was performed to test mechanical sensitivity using a standard set of von Frey monofilaments (1.65, 2.36, 2.83, 3.22, 3.61, 4.08, 4.31, 4.74g; Stoelting, Wood Dale, IL)(Dixon, 1980). Beginning with the 3.22g monofilament, mice received a single application to the plantar surface of the right hindpaw. A negative response was followed by the next larger filament and a positive response (considered a brisk withdrawal of the paw) was followed by the next smaller gram filament. The experimenter continued to move up or down the series, depending on the previously elicited response, for an additional four applications after the first positive response was observed for a minimum of five or a maximum of nine total monofilament applications. The value in log units of the final von Frey monofilament applied in the trial series was used to calculate a 50% g threshold for each mouse and group means were determined as previously described (Chaplan et al., 1994).
Electromyographic electrode implantation and vaginal balloon distension
The visceromotor response (VMR) to vaginal balloon distension (VBD) was evaluated in adult (≥9 weeks) NMS14 (n=19) and NMS21 (n=19) mice, along with corresponding naïve cohorts (n=13 and 20, respectively). Electrode implantation was performed as previously described (Christianson and Gebhart, 2007). Under inhaled isoflurane (4% induction, 2.5% maintenance) and aseptic conditions, the bare ends of two Teflon-coated stainless steel wires (3mm; Grass Technologies, West Warwick, RI) were inserted into the right lateral abdominal musculature, secured via 5-0 prolene sutures, tunneled subcutaneously to a small incision made in the nape of the neck and externalized for access during testing. Skin incisions were closed using 5-0 silk suture. Following recovery from anesthesia, mice were housed singly and allowed to recover for a minimum of 4 days before undergoing testing.
To facilitate balloon insertion and obtain proper restraint during VBD, mice were briefly sedated with inhaled isoflurane and a custom-made latex balloon (1cm in length) was inserted into the vagina and secured to the base of the tail with tape. The mouse was then placed into a Broome-style rodent restraint (Kent Scientific, Torrington, CT), the free ends of the electrode wires were attached to a differential amplifier (Model 1700, A–M Systems, Sequim, WA), and the mice were allowed to recover from anesthesia for 30 minutes. VBD was produced by inflating the balloon with air from a compressed nitrogen tank equipped with a dual-stage low delivery pressure regulator (Matheson- Linweld, Kansas City, MO) and a separate pressure monitor (World Precision Instruments, Sarasota, FL) was used to regulate the pressure inside of the balloon. Each pressure (40, 60, 80, 100 and 120mmHg) was applied three times for 20 seconds with a 4-minute rest period in between. A custom-made distension control device (The University of Iowa Medical Instruments, Iowa City, IA) was used to control the gas flow through the system. Electromyographic (EMG) activity was amplified, filtered and recorded on a personal computer with Spike 2 software (Cambridge Electronic Design, Cambridge, UK) for off-line analysis. VMR was quantified by measuring the area under the curve for the entire distension period divided by the duration of the distension and expressed as a percent of baseline activity (10s prior to VBD).
mRNA extraction and qRT-PCR
Mice were overdosed with inhaled isoflurane (>5%) and transcardially perfused with ice cold 0.9% saline. Brains were removed and frozen on dry ice. Hypothalamus, hippocampus, and amygdala were dissected, immediately snap frozen in liquid nitrogen, and stored at −80°C. The entire length of the vagina (not including the cervix), urinary bladder, and 1.5cm distal segment of the colon were removed, bisected longitudinally (to facilitate both mRNA and protein [see below] analysis), snap frozen in liquid nitrogen, and stored at −80°C. Total RNA was isolated from dissected tissues using Trizol reagent (Ambion, Austin, TX) and RNeasy Mini Kit (Qiagen, Valencia, CA). The concentration and purity were determined using a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) and cDNA was synthesized from total RNA (0.63 µg) using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative RT-PCR was performed using SsoAdvanced SYBR Green Supermix (Bio-Rad) and a Bio-Rad iCycler IQ real time PCR system with indicated 20µM primers (Integrated DNA Technologies, Coralville, IA) listed in Table 2. GAPDH was used as a control gene for brain tissues and β-actin was used as a control for vagina, bladder, and colon.
Table 1.
Primers used for real-time PCR analysis
| Gene | Forward (5’ – 3’) | Reverse (3’ – 5’) |
|---|---|---|
| CRF | CCTCAGCCGGTTCTGATCC | GCGGAGGAAGTATTCTTCACCC |
| Ucn2 | ACCCGTGTCATACTCTCCCTG | CAGCCTTGTAACGAGCCTG |
| CRF1 | CCCTGCCTTTTTCTACGGTGT | TTCCCGGTAGCCATTGTTTGT |
| CRF2 | CCTGTGGACACTTTTGGAGCA | TGTTGCAGTAGGTGTAGGGAC |
| GR | GACTCCAAAGAATCCTTAGCTCC | CTCCACCCCTCAGGGTTTTAT |
| MR | GAAAGGCGCTGGAGTCAAGT | CCATGTAGCTGTTCTCATTGGT |
| IL10 | GCTGGACAACATACTGCTAACC | ATTTCCGATAAGGCTTGGCAA |
| IL6 | CTGCCAGAGACTTCCATCCAGTT | GAAGTAGGGAAGGCCGTGG |
| TNFα | ATGGGCTTTCCGAATTCAC | GAGGCAACCTGACCACTCTC |
| Art | GGCCAACCCTAGCTGTTCT | TGGGTCCAGGGAAGCTT |
| NGF | ACACTCTGATCACTGCGTTTTTG | CCTTCTGGGACATTGCTATCTGT |
| GAPDH | ATGTGTCCGTCGTGGATCTGA | ATGCCTGCTTCACCACCTTCTT |
| β-actin | AGTGTGACGTTGACATCCGTA | GCCAGAGCAGTAATCTCCTTCT |
Western blot
Total protein was isolated from approximately 50 mg of snap-frozen vagina, bladder, and colon using Cell Extraction Buffer (Invitrogen, Grand Island, NY) containing Halt protease and phosphatase inhibitors (ThermoFisher Scientific, Waltham, MA) and Na3VO4 (Sigma, St. Louis, MO). Protein concentrations were determined using a DC protein assay (ThermoFisher). Samples were reduced by heating to 95°C for 5 minutes in the presence of 2-mercaptoethanol, subjected to SDS-PAGE (Criterion 4% to 12% Bis-Tris gels; Bio-Rad, Hercules, CA), and transferred to Nitrocellulose transfer membrane (Whatman GmbH, Dassel, Germany) by Criterion Blotter wet transfer (Bio- Rad). The membranes were blocked for 1 hour at room temperature in 5% milk in Tris-buffered saline with Tween-20 (TBST) and incubated overnight at 4°C with antisera to CRF1 (1:500; Millipore, Billerica, MA), CRF2 (1:800; Millipore), TRPV1 (1:1000; Alomone Labs, Jerusalem, Israel), or TRPA1 (1:1000; Aviva Systems Biology, San Diego, CA) and GAPDH (1:2000; Cell Signaling Technology, Danvers, MA) diluted in 5% milk in TBST. Membranes were then washed with TBST and incubated for 1 hour with antirabbit secondary antibody (1:10,000; Cell Signaling, Danvers, MA). Densitometry was performed using Quantity One 4.6.9 software (Bio-Rad).
Statistics
Calculations were made using Microsoft Excel and statistical analysis was performed using Student’s t-test, and 1-way or 2-way (with or without repeated measures) analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) or Bonferroni’s posttest (IBM SPSS Statistics, IBM Corporation, Armonk, NY; GraphPad Prism, GraphPad Software, La Jolla, CA), as denoted in the manuscript. All data are expressed as mean ± SEM. A p value of less than 0.05 was considered significant.
Results
NMS for 21 days impacts vaginal sensitivity
The effect of NMS and length of separation on vaginal sensitivity was determined by measuring the VMR during VBD in adult female NMS14, NMS21, and corresponding naïve mice. Similar to VMR during colorectal distension (Christianson et al., 2010), the EMG activity of the abdominal musculature significantly increased in response to greater intraballoon pressure applied within the vagina (Figure 1A–B). NMS14 mice did not display significantly increased vaginal sensitivity compared to naïve counterparts (Figure 1C). In contrast, NMS21 mice had significantly greater VMR over the entire distension series than their naïve counterparts and at every individual pressure beyond the lowest in posthoc comparison (Figure 1D).
Figure 1.
Vaginal sensitivity was significantly affected by neonatal maternal separation (NMS). The visceromotor response (VMR) of NMS14 (A), NMS21 (B), and corresponding naïve mice was measured by recording the electromyographic (EMG) activity of the abdominal musculature during graded balloon distension of the vagina (VBD). C) No significant difference in VMR was determined between NMS14 and naïve mice (p > 0.05). D) NMS21 mice had a significantly higher VMR than naïve mice over the entire distension series (p < 0.01), as well as at every pressure above the lowest applied. *,**,***p < 0.05, 0.01, 0.001, two-way RM ANOVA and Fisher’s LSD.
NMS and VBD affect anxiety-like behaviors
To determine the effect of NMS and length of separation on anxiety-like behavior, female NMS14, NMS21 and corresponding naïve mice were subjected to open field testing on a force plate actimeter to measure exploratory behavior either prior to or following VBD. NMS14 mice did not differ significantly from naïve mice with regards to time spent in the perimeter of the open field, either prior to or following VBD (Figure 2A). In contrast, NMS21 mice displayed significantly reduced anxiety-like behaviors at baseline, measured as less time spent in the perimeter of the open field (Figure 2B). Following VBD, NMS21 mice spent significantly more time in the perimeter of the open field than naïve mice or baseline NMS21 measurements, indicating an increase in anxiety-like behaviors (Figure 2B).
Figure 2.
Anxiety-like behavior and hindpaw sensitivity were dose-dependently affected by neonatal maternal separation (NMS) and vaginal balloon distension (VBD). The percent of time spent in the perimeter of an open field actimeter was recorded as a measure of behavioral anxiety in NMS14 (A), NMS21 (B), and corresponding naïve mice, both prior to (Baseline) and following VBD (Post-VBD). A) No significant effect of NMS or VBD on exploratory behavior was observed within the NMS14 group. B) NMS21 mice displayed a significant decrease in the percent of time spent in the perimeter of the open field at baseline compared to naïve mice. Following VBD, NMS21 mice spent a significantly larger percentage of their time in the perimeter compared to both post-VBD naïve mice and baseline NMS21 mice. C) The withdrawal latency of the hindpaw to a thermal stimulus was significantly shorter in NMS14 mice only following VBD, compared to both post-VBD naïve and NMS14 baseline measurements. D) NMS21 mice displayed a significantly shorter withdrawal latency to thermal stimulation of the hindpaw than naïve mice, both at baseline and post-VBD. E) The withdrawal threshold of the hindpaw to a mechanical stimulus was significantly lower in NMS14 mice only following VBD, compared to post-VBD naïve mice. F) NMS21 mice also displayed a significantly lower withdrawal threshold to mechanical stimulation of the hindpaw than naïve mice, both at baseline and post-VBD. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. naïve, ##p < 0.01, ####p < 0.0001 vs. baseline; two-way ANOVA and Bonferroni posttest.
Length of NMS affects hindpaw sensitivity
The impact of NMS and length of separation on hindpaw thermal and mechanical sensitivity was determined either prior to or following VBD. At baseline, NMS14 mice showed no significant difference in thermal or mechanical hindpaw sensitivity compared to naïve counterparts (Figure 2C, E). Following VBD, NMS14 mice displayed significantly reduced thermal withdrawal latencies and mechanical withdrawal thresholds, compared to naïve counterparts (Figure 2C, E), indicative of hypersensitivity. In comparison, NMS21 mice had significantly lower thermal withdrawal latencies and mechanical withdrawal thresholds both at baseline and following VBD, compared to naïve counterparts (Figure 2D, F). VBD had no significant effect on naïve thermal (Figure 2C–D) or mechanical (Figure 2E–F) hindpaw sensitivity in either cohort.
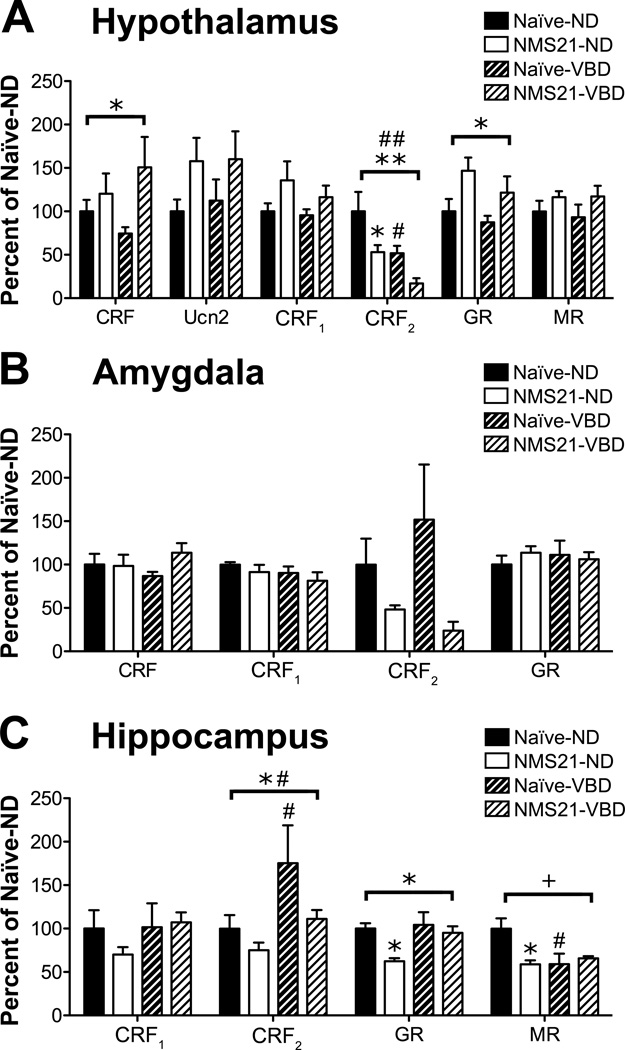
NMS and VBD disrupted regulatory gene expression of the HPA axis
To determine the impact of NMS and VBD on gene expression within central structures involved in the regulation and output of the HPA axis, we used RT-PCR to measure CRF, Ucn2, CRF1, CRF2, GR, and MR mRNA levels in the hypothalamus, amygdala, and hippocampus of naïve and NMS21 mice with and without exposure to VBD. In the hypothalamus, NMS had a significant effect on increasing CRF and GR mRNA levels, as well as inducing a trend towards increased Ucn2 and CRF1 mRNA levels (Figure 3A). NMS, as well as VBD, significantly decreased CRF2 mRNA levels in the hypothalamus, with non-distended NMS21 mice and post-VBD naïve mice both expressing significantly less CRF2 mRNA than non-distended naïve mice (Figure 3A). In the amygdala, only CRF2 was affected by NMS with a trend towards decreased expression (Figure 3B). In the hippocampus, NMS significantly decreased the mRNA levels of CRF2 and GR (Figure 3C). In opposition to the effect of VBD on CRF2 in the hypothalamus, VBD had a significant impact on increasing CRF2 mRNA levels in the hippocampus, particularly in naïve mice (Figure 3C). NMS and VBD had a significant interaction effect on MR mRNA levels in the hippocampus, with non-distended NMS21 mice and post-VBD naïve mice both expressing significantly less MR mRNA than nondistended naïve mice (Figure 3C).
Figure 3.
Neonatal maternal separation (NMS) and vaginal balloon distension (VBD) significantly impacted negative regulatory feedback onto the hypothalamic-pituitary-adrenal (HPA) axis. Gene expression was measured by RT-PCR in the hypothalamus (A), amygdala (B), and hippocampus (C) of naïve and NMS21 mice that either did not (Naïve-ND and NMS21-ND) or did undergo VBD (Naïve-VBD and NMS21-VBD). A) In the hypothalamus, NMS significantly increased CRF and GR mRNA expression and induced a trend towards increased Ucn2 and CRF1 mRNA expression. NMS and VBD both significantly decreased CRF2 mRNA expression with NMS21-ND and naïve-VBD expressing significantly less CRF2 mRNA than naïve-ND. B) NMS caused a trend towards decreased CRF2 mRNA expression in the amygdala (p = 0.051). C) In the hippocampus, NMS significantly decreased the mRNA expression of CRF2 and GR with NMS21-ND expressing significantly less GR mRNA than naïve-ND, whereas VBD significantly increased CRF2 mRNA expression with naïve-VBD expressing significantly more CRF2 mRNA naïve-ND. NMS and VBD had a significant interaction effect on decreasing MR mRNA expression in the hippocampus with NMS21-ND and naïve-VBD both expressing significantly less MR than naïve-ND. Housekeeping gene: β-actin. Brackets indicate significant impact of NMS (*,**p <0.05, 0.01), VBD (#,##p < 0.05, 0.01) or an NMS/VBD interaction (+p < 0.05), two-way ANOVA; *p < 0.05 vs. naïve, # p < 0.05 vs. baseline, Bonferroni posttest.
NMS and VBD impact adult vaginal inflammatory mediator, CRF receptor, and TRP channel expression patterns
We performed RT-PCR to determine mRNA levels of inflammatory mediators shown to be affected by NMS (O'Malley et al., 2011b, Dimatelis et al., 2012, Lennon et al., 2013) and Western blotting to determine the potential contribution of peripheral CRF receptor and TRP channel protein expression on vaginal sensitivity following NMS and/or VBD. NMS alone did not significantly impact inflammatory mediator expression within the vagina. However, NMS and VBD had a significant interaction effect on both IL10 and TNFα mRNA levels, with post-VBD NMS21 mice expressing significantly higher levels of both genes than post-VBD naïve mice (Figure 4A). VBD was shown to significantly impact Artemin (Art) mRNA levels, with post-VBD NMS21 mice expressing significantly higher levels of Art mRNA than non-distended NMS21 mice. NMS and VBD also had a significant interaction effect on CRF1 protein expression in the vagina, with post-VBD NMS21 mice expressing significantly less CRF1 protein than post-VBD naïve mice (Figure 4B). Despite being significantly altered in central structures, the protein expression of CRF2 was not altered by either NMS or VBD in the vagina (Figure 4B). NMS also did not affect protein expression of TRPV1 or TRPA1 in the vagina; however VBD significantly decreased the expression of both proteins compared to non-distended counterparts (Figure 4C).
Figure 4.
Neonatal maternal separation (NMS) and vaginal balloon distension (VBD) significantly disrupted gene and protein expression within the vagina. RT-PCR (A) and Western blot (B–C) was performed on vagina from naïve and NMS21 mice that either did not (Naïve-ND and NMS21-ND) or did undergo VBD (Naïve-VBD and NMS21-VBD). A) NMS and VBD had a significant interaction effect on IL10 mRNA expression with NMS21-VBD expressing significantly more IL10 mRNA than NMS21-ND or naïve-VBD. NMS and VBD also had a significant interaction effect on TNFα mRNA expression with NMS21-VBD expressing significantly more TNFα mRNA than naïve-VBD. VBD significantly increased Artemin (Art) mRNA expression with NMS21-VBD expression significantly more Art than NMS21-ND. IL6 and nerve growth factor (NGF) transcripts were not affected by either NMS or VBD. B) Representative Western blots are shown for CRF1, CRF2, and corresponding GAPDH protein expression with bands at 55, 47, and 35kD, respectively. NMS and VBD had a significant interaction effect on CRF1 protein expression in the vagina with NMS21-VBD expressing significantly less CRF1 protein than naïve-VBD. CRF2 protein expression in the vagina was unaffected by NMS or VBD. C) Representative Western blots are shown for TRPV1, TRPA1, and corresponding GAPDH protein expression with bands at 85, 127, and 35kD, respectively. VBD significantly decreased the protein expression of both TRPV1 and TRPA1 in the vagina. Naïve-VBD and NMS21-VBD both expressed significantly less TRPV1 than their non-distended counterparts. NMS21-VBD also expressed significantly less TRPA1 protein than NMS21-ND. Housekeeping gene: GAPDH (A). Brackets indicate significant impact of VBD (#,####p < 0.05, 0.0001) or an NMS/VBD interaction (+,++p < 0.05, 0.01), two-way ANOVA; *,**p < 0.05, 0.01 vs. naïve, #,### p < 0.05, 0.001 vs. baseline, Bonferroni posttest.
Influence of NMS and VBD on inflammatory mediator, CRF receptor, and TRP channel expression patterns in the bladder and colon
Up to 50% of women with chronic pelvic pain experience symptoms from more than one disorder (Zondervan et al., 1999, Arnold et al., 2006, Latthe et al., 2006, Carrico et al., 2009, Rodriguez et al., 2009, Green et al., 2010, Gardella et al., 2011, Warren et al., 2011). To determine how NMS and VBD impact neighboring viscera, which have been shown to be affected by early adverse events (Al–Chaer et al., 2000, Barreau et al., 2004, Randich et al., 2006b, Christianson et al., 2010), we performed RTPCR to measure mRNA levels of inflammatory mediators and Western blotting to assess CRF receptor and TRP channel protein expression in the bladder and colon. In the bladder, NMS significantly increased IL10 and NGF mRNA levels and VBD significantly decreased IL6 mRNA (Figure 5A). In the colon, only a trend towards NMS-induced increased IL10 mRNA levels was observed. Peripheral CRF receptor expression was differentially affected in the bladder and colon by NMS and VBD (Figure 6A). In the bladder, CRF1 protein expression was significantly increased by VBD with post-VBD NMS21 bladder expressing significantly more CRF1 than non-distended NMS bladder (Figure 5B). CRF1 protein expression was not impacted by NMS or VBD in the colon; however NMS and VBD had a significant interaction effect on CRF2 in the colon with non-distended NMS21 expressing significantly more CRF2 protein than non-distended naïve or post-VBD NMS21 (Figure 6B). As in vagina, TRPV1 protein expression was significantly decreased post-VBD in both bladder and colon (Figure 5C and 6C). An additional significant interaction effect of NMS and VBD was observed on TRPV1 expression in the bladder with NMS21 post-VBD bladder expressing significantly less TRPV1 than naïve post-VBD bladder (Figure 5C). TRPA1 protein expression was differentially affected by NMS and VBD in bladder and colon. In the bladder, a significant effect of VBD and a significant interaction effect of NMS and VBD on TRPA1 protein expression was observed with naïve post-VBD bladder expressing significantly more TRPA1 protein than non-distended naïve bladder (Figure 5C). In the colon, NMS significantly increased TRPA1 protein expression, while VBD significantly decreased TRPA1 protein expression (Figure 6C).
Figure 5.
Neonatal maternal separation (NMS) and vaginal balloon distension (VBD) significantly disrupted gene and protein expression within the bladder. RT-PCR (A) and Western blot (B–C) was performed on bladder from naïve and NMS21 mice that either did not (Naïve-ND and NMS21-ND) or did undergo VBD (Naïve-VBD and NMS21-VBD). A) NMS significantly increased both IL10 and nerve growth factor (NGF) mRNA expression. VBD significantly decreased IL6 mRNA expression. Neither NMS nor VBD had a significant effect on TNFα or Artemin (Art) mRNA expression in the bladder. B) Representative Western blots are shown for CRF1, CRF2, and corresponding GAPDH protein expression with bands at 55, 47, and 35kD, respectively. VBD significantly increased CRF1 protein expression in the bladder with NMS21-VBD expressing significantly more CRF1 protein than NMS21-ND. CRF2 protein expression in the bladder was not significantly affected by either NMS or VBD. C) Representative Western blots are shown for TRPV1, TRPA1, and corresponding GAPDH protein expression with bands at 85, 127, and 35kD, respectively. TRPV1 protein expression in the bladder was significantly affected by VBD alone and by an interaction effect of NMS and VBD. NMS21-VBD bladder expressed significantly less TRPV1 than either NMS21-ND or naïve-VBD bladder and naïve-VBD expressed significantly less TRPV1 than naïve-ND bladder. In contrast, TRPA1 protein expression was significantly increased by VBD and there was also a significant interaction effect of NMS and VBD. Naïve-VBD bladder expressed significantly more TRPA1 than naïve21-ND bladder. Housekeeping gene: GAPDH (A). Brackets indicate significant impact of NMS (*p < 0.05), VBD (#,##,####p < 0.05, 0.01, 0.0001) or an NMS/VBD interaction (+,++p < 0.05, 0.01), two-way ANOVA; **p < 0.01 vs. naïve, #,##,#### p < 0.05, 0.01, 0.0001 vs. baseline, Bonferroni posttest.
Figure 6.
Neonatal maternal separation (NMS) and vaginal balloon distension (VBD) significantly disrupted protein expression within the colon. RT-PCR (A) and Western blot (B–C) was performed on colon from naïve and NMS21 mice that either did not (Naïve-ND and NMS21-ND) or did undergo VBD (Naïve-VBD and NMS21-VBD). A) NMS only had a moderate effect on mRNA expression in the colon, with a trend towards increased IL10 mRNA expression in NMS21 colon. Neither NMS nor VBD had a significant effect on IL6, TNFα, Artemin (Art), or nerve growth factor (NGF) mRNA expression in the colon. B) Representative Western blots are shown for CRF1, CRF2, and corresponding GAPDH protein expression with bands at 55, 47, and 35kD, respectively. NMS and VBD had a significant interaction effect on CRF2 protein expression in the colon with NMS21-ND expressing higher CRF2 protein than both naïve-ND and NMS21-VBD colon. CRF1 protein expression in the colon was not significantly affected by either NMS or VBD. C) Representative Western blots are shown for TRPV1, TRPA1, and corresponding GAPDH protein expression with bands at 85, 127, and 35kD, respectively. Similar to its effects on vagina and bladder, VBD significantly decreased TRPV1 protein expression in the colon with naïve-VBD expressing significantly less TRPV1 than naïve-ND colon. NMS and VBD both separately induced a significant increase in TRPA1 protein expression in the colon. NMS21-ND colon expressed significantly more TRPA1 protein than naïve-ND colon. Housekeeping gene: GAPDH (A). Brackets indicate significant impact of NMS (*p < 0.05), VBD (#p < 0.05) or an NMS/VBD interaction (++p < 0.01), two-way ANOVA; *p < 0.05 vs. naïve, # p < 0.05 vs. baseline, Bonferroni posttest.
Discussion
Patients suffering from chronic pelvic pain syndromes, particularly IBS, IC, and vulvodynia, commonly report that stress initiates or exacerbates existing symptoms (Arnold et al., 2006, Larauche et al., 2012, Bullones Rodriguez et al., 2013). A history of early adverse events increases the likelihood of diagnosis of one or more of these pain syndromes, as well as comorbidity with depression and/or anxiety (Grunau et al., 1994, Anand, 1998, Bennett et al., 1998, Moore and Kennedy, 2000). The current study demonstrates that early life stress has a dose response-like effect on vaginal and hindpaw sensitivity and anxiety-like behaviors in adult female mice. Changes in mRNA and protein expression related to the regulation and output of the HPA axis may underlie these behavioral outcomes.
Clinical evidence suggests a strong link between child abuse or neglect and the development of vulvodynia in adulthood (Moore and Kennedy, 2000, Harlow and Stewart, 2005, Latthe et al., 2006, Sack et al., 2007, Nguyen et al., 2009). A recent study reported that patients with provoked vestibulodynia have a lower vaginal distension threshold than control patients, suggesting that vulvodynia may be associated with vaginal allodynia (Farmer et al., 2013). In the current study, mice that experienced the greatest amount of neonatal stress – in the form of NMS for 21 days, displayed robust vaginal allodynia by generating a significantly enhanced VMR at every intraballoon pressure greater than the lowest applied. This response differs markedly from what we (Christianson et al., 2010) and others (Randich et al., 2006a, DeBerry et al., 2007, Winston et al., 2007) have observed following neonatal irritation or inflammation in the pelvic viscera where VMR was generally increased only at the highest applied pressures. An earlier study of colorectal sensitivity in NMS rats reported an increase in VMR at both low and high intraballoon pressures following acute water avoidance stress (Coutinho et al., 2002), which has been shown to impact visceral sensitivity (Schwetz et al., 2005, Robbins et al., 2007). The difference in outcomes between NMS and neonatal visceral irritation/inflammation likely involves recruitment of higher structures involved in regulating the HPA axis. The lack of effect of NMS for 14 days on vaginal sensitivity was striking considering a recent publication by Maloney et al., (Moloney et al., 2012), that demonstrated increased VMR during colorectal distension in male mice that underwent maternal separation combined with unpredictable maternal stress from P1–14. It is likely that variances in the neonatal stress paradigm used, organs of interest, and the sex of the mice that were studied underlie the observed differences between the two studies.
To our knowledge, this is the first report of measuring VMR to quantify physiological responses to graded distension of the vagina in conscious mice. Previous studies by Berkley, et al., have measured escape behaviors in awake rats (Berkley et al., 2001), as well as VMR in anesthetized rats (Nagabukuro and Berkley, 2007), in response to VBD, and demonstrated significant effects of estrous cycle (Cason et al., 2003) and cyst burden and innervation (McAllister et al., 2009, McAllister et al., 2012) in an experimental model of endometriosis. Miranda et al. (Miranda et al., 2011), also reported an estrous cycle effect on VMR during colorectal distension in awake rats that received intravesicular zymosan as neonates to induce cystitis. Importantly, both studies reported that the effect of the experimental treatment was large enough to negate the estrous cycle effect. Based on these observations and the unknown effect of collecting vaginal smears on vaginal sensitivity in NMS mice, the estrous cycle of the mice in this study was unknown.
The observations of the impact of NMS on hindpaw sensitivity are novel, as previous studies have shown either no impact (Lariviere et al., 2006) or a decrease (Weaver et al., 2007) in thermal hindpaw sensitivity in adult female NMS rats. No literature could be found assessing thermal hindpaw sensitivity in mice or mechanical hindpaw thresholds in either rodent species following NMS. Viscero-somatic convergence between the hindpaw and bladder or colon has been previously demonstrated following inflammation of either organ (Traub and Wang, 2004, Bielefeldt et al., 2006) or the hindpaw (Bielefeldt et al., 2006) of adult rodents. Neonatal irritation of the colon (Christianson et al., 2010), bladder (Randich et al., 2006b) or vagina (unpublished observation, manuscript in preparation), has been shown to have differential effects on hindpaw sensitivity, suggesting that the nature, location, and timing of an early adverse event can dramatically affect the outcome of considered “secondary” symptomology. Vaginal and/or cervical stimulation has previously been shown to be antinociceptive and decrease hindpaw sensitivity and/or completely block hindpaw withdrawal from noxious stimuli (Komisaruk and Wallman, 1977). However, these measurements were taken during vaginal/cervical stimulation and not as a consequence of the stress generated during the visceral stimulation, therefore cervical stimulation likely did not influence the observations in the current study.
Stressful events experienced early in life can greatly increase the likelihood of developing anxiety- or depression-like symptoms during adulthood (Heim et al., 2001, Veenema et al., 2008). NMS in rats has generally been shown to increase anxiety-like behaviors and the duration of ACTH and corticosterone release following a stressful event (Ladd et al., 2004, Plotsky et al., 2005, Aisa et al., 2008), whereas NMS in mice has been shown to generate a mild anxiety-like phenotype (Millstein and Holmes, 2007), or decrease behavioral anxiety (Romeo et al., 2003, Savignac et al., 2011, Own and Patel, 2012). In the current study, we observed no change in anxiety-like behavior in NMS14 mice and a decrease in anxiety-like behavior in NMS21 mice at baseline, which was reversed following VBD. There was an observable difference in anxiety-like behavior between the two naïve groups that corresponded to the NMS14 and NMS21 mice. Considering that corresponding naïve mice were weighed daily at the time of separation, the additional week of brief daily separations experienced by the NMS21 naïve group may have contributed to this difference, as brief daily separations have been shown to reduce anxiety-like behaviors {McIntosh, 1999 #72}. This observation illustrates the importance of testing corresponding naïve and NMS groups to control for differences in environmental stressors that may influence behavioral outcomes. Changes in gene expression within the hypothalamus of NMS mice, both at baseline and following VBD, suggest that increased HPA output, resulting from diminished negative feedback, may underlie the increase in anxiety-like behaviors. NMS simultaneously increased CRF and decreased CRF2 mRNA levels. The trend towards an increase in Ucn2 mRNA in NMS21 mice may be a compensatory action, as VBD also decreased CRF2 mRNA in naïve mice, but did not show a corresponding increase in Ucn2. GR was also increased in NMS21 hypothalamus, which could be a compensatory response to decreased hippocampal GR expression (Victoria et al., 2013) or GR resistance (Silverman and Sternberg, 2012). Up-regulation of both CRF1 protein expression (O'Malley et al., 2011a) and binding (Plotsky et al., 2005) in the hypothalamus of NMS rats has been previously reported and supports our findings here.
The greater impact of the three week-long NMS period on all behavioral and physiological measurements in the current study, compared to the standard two weeklong NMS period, may be due to disruption of proper limbic structure maturation. The peak period of hippocampal neurogenesis overlaps with the stress hyporesponsive period (P1–14) (Sapolsky and Meaney, 1986); however, mature firing patterns do not emerge until the third postnatal week (Egorov and Draguhn, 2013). Limbic structures have been shown to influence pain and anxiety through HPA- and non-HPA-mediated mechanisms. Systemic and local corticosterone production increases CRF expression in the CeA of the amygdala through a GR-dependent mechanism (Cook, 2002). The level of CRF expression within the amygdala has been shown to mediate pain effects, which can be blocked by CRF1 antagonist treatment, but not CRF2 antagonist (Ji et al., 2013). This observation was supported by earlier work demonstrating that basal CRF1 signaling contributes to pain-related synaptic facilitation and CRF2 exerts a latent inhibitory influence (Fu and Neugebauer, 2008). Observations in the current study support this earlier work, as a trend toward decreased CRF2 mRNA expression was detected in amygdala of NMS mice, suggesting that a loss of inhibition within the amygdala could be contributing towards the increase in vaginal sensitivity and anxiety-like behaviors. Hippocampal inhibition of the HPA axis can be compromised by chronic stress or longterm high dose corticosteroid treatment, resulting in decreased GR expression (Herman et al., 2005). Observations in the current study support this mechanism, as NMS significantly decreased both GR and MR protein expression in the hippocampus, potentially compromising hippocampal inhibition of the HPA axis. Acute adult stress, in the form of VBD, also significantly impacted mRNA levels in the hippocampus, specifically increasing CRF2 and decreasing MR levels. The increase in CRF2 mRNA levels was likely compensatory in response to either heightened HPA output or decreased hippocampal MR levels following VBD. The lack of a similar increase in CRF2 in NMS21 hippocampus suggests potential disruption in proper limbic response following acute stress. A prolonged decrease in hippocampal MR expression has been previously reported to occur following a single sustained acute stress in rats and has been theorized to contribute to post-traumatic stress disorder (Liberzon et al., 1999).
Up to 50% of women with chronic pelvic pain experience symptoms from more than one disorder, creating a greater negative impact on quality of life and complicating already less-than-optimal treatment strategies (Zondervan et al., 1999, Arnold et al., 2006, Latthe et al., 2006, Carrico et al., 2009, Rodriguez et al., 2009, Green et al., 2010, Gardella et al., 2011, Warren et al., 2011). A potential role for CRF in comorbidity has been established for psychological and chronic pain disorders. A significant correlation has been observed between IBS and depression/anxiety in patients with high cortisone levels (Tache et al., 2005). Epidemiological studies have also found a link between anxiety and voiding disorders (Klausner and Steers, 2004); and IC patients with concomitant comorbid diagnoses of fibromyalgia, chronic fatigue syndrome, or rheumatoid arthritis had a higher mean afternoon cortisol level and increased pain during bladder filling than IC patients with no additional diagnoses (Lutgendorf et al., 2002). Downstream propagation of neurogenic inflammation resulting from dysregulation of the HPA axis has been proposed as a possible underlying mechanism for numerous functional pain syndromes (Black, 2002). In the current study, we observed significant effects of NMS, VBD, and interaction effects of both on all three visceral structures. Examination of mRNA levels of genes previously shown to be affected by NMS or involved in mediating pelvic hypersensitivity (Clarke et al., 2009, O'Malley et al., 2011b, Dimatelis et al., 2012, Lennon et al., 2013, Lu et al., 2013) revealed possible disruption of downstream HPA axis effects. For example, NMS and VBD had a significant interaction effect on IL10 and TNFα mRNA levels in the vagina, which resulted in a significant increase in NMS21 vagina post-VBD, but not in naïve vagina. The lack of baseline changes in NMS21 vagina suggests that the response to acute stress in these animals is altered, compared to that in naïve mice, producing an altered downstream local cytokine response to VBD. This observation is supported by the significant interaction effect of NMS and VBD on CRF1 protein expression in the vagina, where naïve vagina showed a trend toward increased CRF1 protein expression, which was significantly lower in NMS21 vagina, suggesting dysregulation of peripheral CRF receptor expression. Cytokine and growth factor mRNA levels were also significantly altered by NMS and VBD, separately, in the bladder. With the exception of NMS producing a significant increase in NGF mRNA levels, the impact of NMS on IL10 levels and VBD on IL6 levels was largely anti-inflammatory, suggesting that these interventions may result in lowered bladder sensitivity. Spinally-administered CRF2 antagonist was shown to reverse stress-induced bladder sensitivity in the rat, whereas CRF1 antagonist had no effect (Robbins and Ness, 2008), however, the role of peripheral CRF receptors in bladder sensitivity has not yet been established. Future studies will determine whether the increase in CRF1 protein expression in the bladder inhibits or drives bladder sensitivity following acute stress. The expression pattern of CRF2 in the colon was similar to that reported by O’Malley et al., (O'Malley et al., 2010), which showed a baseline increase in CRF2 protein expression in NMS rats that was reversed by open field stress. However, they also reported a similar effect on CRF1 protein expression, which was not evident in our study; however, the species studied, length of NMS, and nature of the adult stressor likely contributes to this discrepancy. It is unclear whether the VBD effect on expression changes resulted from stress or from a physiological response to organ distension. Due to the close proximity of the investigated pelvic organs, it is possible that pressure exerted onto the bladder and colon during VBD could have impacted gene expression within these organs, as well. Regardless, these data support that NMS and VBD have effects on the vagina, as well as on the immediately adjacent viscera.
The TRP family of receptors has been shown to contribute towards acute inflammatory colorectal hypersensitivity (Jones et al., 2007, D'Aldebert et al., 2011, Engel et al., 2011), as well as to colonic hypersensitivity resulting from neonatal colon insult (Winston et al., 2007, Christianson et al., 2010). Local upregulation of TRPA1 and/or TRPV1 protein expression has also been reported following acute inflammation of the colon (Yang et al., 2008, Xia et al., 2012) and bladder (Merrill et al., 2012). In nondistended mice, we only observed a significant increase in TRPA1 protein expression in NMS colon. However, VBD had a significant effect on TRPV1 and TRPA1 protein expression in every tissue type examined. VBD significantly decreased TRPV1 protein expression in naïve and NMS vagina, bladder and colon; and NMS and VBD had a significant interaction effect on decreasing TRPV1 expression in bladder. VBD had a similar effect on TRPA1 protein expression in the vagina and colon; however, VBD significantly increased TRPA1 protein expression in the bladder, most prominently in naïve bladder. Although, bladder and colon sensitivity were not assayed in the current study, it is likely that TRP channel expression did not contribute towards the observed vaginal hypersensitivity in the NMS21 mice. It is striking that distension of an adjacent organ would significantly decrease TRPV1 protein expression in both bladder and colon. Future studies assaying less invasive forms of stress will address whether this and the aforementioned expression changes were due to potential physical deformation of the tissue or if they were truly stress-mediated.
Conclusion
This study provides evidence that early life stress in female mice has significant effects on vaginal sensitivity, anxiety-like behaviors, and central and peripheral expression of mediators involved in the regulation and output of the HPA axis. Exposure to neonatal stress had a dose response-like effect on most behavioral outcomes, which were further enhanced by exposure to adult stress in the form of VBD. Together with previous studies on the effect of NMS on psychological disturbances and pelvic organ sensitivity, the findings here demonstrate that neonatal stress in females can impact adult pain processing associated not only with the bladder and colon, but also with the reproductive tract and may provide a potential mechanism for the development of comorbid chronic pelvic pain syndromes.
Highlights.
Vaginal and hindpaw sensitivity was increased in mice that underwent 3 weeks of NMS
NMS and VBD reduced expression of negative feedback regulators of the HPA axis
NMS differentially affected inflammatory expression within the pelvic viscera
VBD influenced neuro-immune expression in the vagina, colon and bladder
Acknowledgments
This work was supported by NIH grants R03 DK080182 (JAC), P20 GM104936 (JAC), core support from RR016475 and HD002528, and The Madison and Lila Self Fellowship Program (ANP). We would like to thank Bilal Hassan for assistance with neonatal maternal separation and Drs. Sarah Tague and Doug Wright for their insight during manuscript preparation.
Abbreviations
- HPA
Hypothalamic-pituitary-adrenal
- NMS
neonatal maternal separation
- VBD
vaginal balloon distension
- CRF
corticotropin releasing factor
- TRP
transient receptor potential
- IBS
irritable bowel syndrome
- IC
interstitial cystitis
- PVN
paraventricular nucleus
- ACTH
adrenocorticotropic hormone
- GR
glucocorticoid receptor
- MR
mineralocorticoid receptor
- Ucn
Urocortin
- CeA
central nucleus of the amygdala
- P
postnatal
- CRD
colorectal distension
- VMR
visceromotor response
- EMG
electromyographic
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aisa B, Tordera R, Lasheras B, Del Rio J, Ramirez MJ. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–1226. doi: 10.1016/j.neuroscience.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al–Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- Anand KJ. Clinical importance of pain and stress in preterm neonates. Biol Neonate. 1998;73:1–9. doi: 10.1159/000013953. [DOI] [PubMed] [Google Scholar]
- Arnold LD, Bachmann GA, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstetrics and gynecology. 2006;107:617–624. doi: 10.1097/01.AOG.0000199951.26822.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KR, Crawley JN. Anxiety-Related Behaviors in Mice. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. Boca Raton (FL): 2009. [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annual review of pharmacology and toxicology. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, Pasquinelli G, Morselli-Labate AM, Grady EF, Bunnett NW, Collins SM, Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Barreau F, Cartier C, Ferrier L, Fioramonti J, Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. 2004;127:524–534. doi: 10.1053/j.gastro.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bennett EJ, Tennant CC, Piesse C, Badcock CA, Kellow JE. Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut. 1998;43:256–261. doi: 10.1136/gut.43.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley KJ, Cason A, Jacobs H, Bradshaw H, Wood E. Vaginal hyperalgesia in a rat model of endometriosis. Neuroscience letters. 2001;306:185–188. doi: 10.1016/s0304-3940(01)01906-1. [DOI] [PubMed] [Google Scholar]
- Bielefeldt K, Lamb K, Gebhart GF. Convergence of sensory pathways in the development of somatic and visceral hypersensitivity. Am J Physiol Gastrointest Liver Physiol. 2006;291:G658–G665. doi: 10.1152/ajpgi.00585.2005. [DOI] [PubMed] [Google Scholar]
- Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain, behavior, and immunity. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Bullones Rodriguez MA, Afari N, Buchwald DS. Evidence for overlap between urological and nonurological unexplained clinical conditions. The Journal of urology. 2013;189:S66–S74. doi: 10.1016/j.juro.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico DJ, Sherer KL, Peters KM. The relationship of interstitial cystitis/painful bladder syndrome to vulvodynia. Urologic nursing. 2009;29:233–238. [PubMed] [Google Scholar]
- Cason AM, Samuelsen CL, Berkley KJ. Estrous changes in vaginal nociception in a rat model of endometriosis. Horm Behav. 2003;44:123–131. doi: 10.1016/s0018-506x(03)00121-1. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Chang L, Sundaresh S, Elliott J, Anton PA, Baldi P, Licudine A, Mayer M, Vuong T, Hirano M, Naliboff BD, Ameen VZ, Mayer EA. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of neuroscience methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chitkara DK, van Tilburg MA, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. The American journal of gastroenterology. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Bielefeldt K, Malin SA, Davis BM. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain. 2010;151:540–549. doi: 10.1016/j.pain.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson JA, Gebhart GF. Assessment of colon sensitivity by luminal distension in mice. Nature protocols. 2007;2:2624–2631. doi: 10.1038/nprot.2007.392. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Ryals JM, McCarson KE, Wright DE. Beneficial actions of neurotrophin treatment on diabetes-induced hypoalgesia in mice. J Pain. 2003;4:493–504. doi: 10.1016/j.jpain.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Clarke G, Quigley EM, Cryan JF, Dinan TG. Irritable bowel syndrome: towards biomarker identification. Trends in molecular medicine. 2009;15:478–489. doi: 10.1016/j.molmed.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Cook CJ. Glucocorticoid feedback increases the sensitivity of the limbic system to stress. Physiology & behavior. 2002;75:455–464. doi: 10.1016/s0031-9384(02)00650-9. [DOI] [PubMed] [Google Scholar]
- Coutinho SV, Plotsky PM, Sablad M, Miller JC, Zhou H, Bayati AI, McRoberts JA, Mayer EA. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am J Physiol Gastrointest Liver Physiol. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- D'Aldebert E, Cenac N, Rousset P, Martin L, Rolland C, Chapman K, Selves J, Alric L, Vinel JP, Vergnolle N. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology. 2011;140:275–285. doi: 10.1053/j.gastro.2010.09.045. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocrine reviews. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- DeBerry J, Ness TJ, Robbins MT, Randich A. Inflammation-induced enhancement of the visceromotor reflex to urinary bladder distention: modulation by endogenous opioids and the effects of early-in-life experience with bladder inflammation. J Pain. 2007;8:914–923. doi: 10.1016/j.jpain.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent G, Choi DC, Herman JP, Levine S. GABAergic circuits and the stress hyporesponsive period in the rat: ontogeny of glutamic acid decarboxylase (GAD) 67 mRNA expression in limbic-hypothalamic stress pathways. Brain research. 2007;1138:1–9. doi: 10.1016/j.brainres.2006.04.082. [DOI] [PubMed] [Google Scholar]
- Desbonnet L, Garrett L, Daly E, McDermott KW, Dinan TG. Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2008;26:259–268. doi: 10.1016/j.ijdevneu.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Dimatelis JJ, Pillay NS, Mutyaba AK, Russell VA, Daniels WM, Stein DJ. Early maternal separation leads to down-regulation of cytokine gene expression. Metabolic brain disease. 2012;27:393–397. doi: 10.1007/s11011-012-9304-z. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annual review of pharmacology and toxicology. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Draguhn A. Development of coherent neuronal activity patterns in mammalian cortical networks: common principles and local hetereogeneity. Mechanisms of development. 2013;130:412–423. doi: 10.1016/j.mod.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Engel MA, Leffler A, Niedermirtl F, Babes A, Zimmermann K, Filipovic MR, Izydorczyk I, Eberhardt M, Kichko TI, Mueller-Tribbensee SM, Khalil M, Siklosi N, Nau C, Ivanovic-Burmazovic I, Neuhuber WL, Becker C, Neurath MF, Reeh PW. TRPA1 and substance P mediate colitis in mice. Gastroenterology. 2011;141:1346–1358. doi: 10.1053/j.gastro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Farmer MA, Maykut CA, Huberman JS, Huang L, Khalife S, Binik YM, Apkarian AV, Schweinhardt P. Psychophysical properties of female genital sensation. Pain. 2013 doi: 10.1016/j.pain.2013.05.028. [DOI] [PubMed] [Google Scholar]
- Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:3861–3876. doi: 10.1523/JNEUROSCI.0227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardella B, Porru D, Nappi RE, Dacco MD, Chiesa A, Spinillo A. Interstitial cystitis is associated with vulvodynia and sexual dysfunction--a case-control study. The journal of sexual medicine. 2011;8:1726–1734. doi: 10.1111/j.1743-6109.2011.02251.x. [DOI] [PubMed] [Google Scholar]
- Goetsch MF, Morgan TK, Korcheva VB, Li H, Peters D, Leclair CM. Histologic and receptor analysis of primary and secondary vestibulodynia and controls: a prospective study. American journal of obstetrics and gynecology. 2010;202:614 e611–614 e618. doi: 10.1016/j.ajog.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Archives of general psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RV, Whitfield MF, Petrie JH, Fryer EL. Early pain experience, child and family factors, as precursors of somatization: a prospective study of extremely premature and fullterm children. Pain. 1994;56:353–359. doi: 10.1016/0304-3959(94)90174-0. [DOI] [PubMed] [Google Scholar]
- Harlow BL, Stewart EG. Adult-onset vulvodynia in relation to childhood violence victimization. American journal of epidemiology. 2005;161:871–880. doi: 10.1093/aje/kwi108. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hanker JP, Hellhammer DH. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosomatic medicine. 1998;60:309–318. doi: 10.1097/00006842-199805000-00017. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. The American journal of psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Progress in neuro-psychopharmacology & biological psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Ji G, Fu Y, Adwanikar H, Neugebauer V. Non-pain-related CRF1 activation in the amygdala facilitates synaptic transmission and pain responses. Molecular pain. 2013;9:2. doi: 10.1186/1744-8069-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GT, Power C, Macfarlane GJ. Adverse events in childhood and chronic widespread pain in adult life: Results from the 1958 British Birth Cohort Study. Pain. 2009;143:92–96. doi: 10.1016/j.pain.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Jones RC, 3rd, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133:184–194. doi: 10.1053/j.gastro.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Jones RC, 3rd, Xu L, Gebhart GF. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:10981–10989. doi: 10.1523/JNEUROSCI.0703-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner AP, Steers WD. Corticotropin releasing factor: a mediator of emotional influences on bladder function. The Journal of urology. 2004;172:2570–2573. doi: 10.1097/01.ju.0000144142.26242.f3. [DOI] [PubMed] [Google Scholar]
- Komisaruk BR, Wallman J. Antinociceptive effects of vaginal stimulation in rats: neurophysiological and behavioral studies. Brain research. 1977;137:85–107. doi: 10.1016/0006-8993(77)91014-9. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biological psychiatry. 2004;55:367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Laird JM, Martinez-Caro L, Garcia-Nicas E, Cervero F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain. 2001;92:335–342. doi: 10.1016/S0304-3959(01)00275-5. [DOI] [PubMed] [Google Scholar]
- Larauche M, Mulak A, Tache Y. Stress and visceral pain: from animal models to clinical therapies. Experimental neurology. 2012;233:49–67. doi: 10.1016/j.expneurol.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere WR, Sattar MA, Melzack R. Inflammation-susceptible Lewis rats show less sensitivity than resistant Fischer rats in the formalin inflammatory pain test and with repeated thermal testing. Journal of neurophysiology. 2006;95:2889–2897. doi: 10.1152/jn.00608.2005. [DOI] [PubMed] [Google Scholar]
- Larsen MS, Mortensen S, Nordling J, Horn T. Quantifying mast cells in bladder pain syndrome by immunohistochemical analysis. BJU international. 2008;102:204–207. doi: 10.1111/j.1464-410X.2008.07576.x. discussion 207. [DOI] [PubMed] [Google Scholar]
- Latthe P, Mignini L, Gray R, Hills R, Khan K. Factors predisposing women to chronic pelvic pain: systematic review. In: Clinical research, editor. BMJ. Vol. 332. 2006. pp. 749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclair CM, Goetsch MF, Korcheva VB, Anderson R, Peters D, Morgan TK. Differences in primary compared with secondary vestibulodynia by immunohistochemistry. Obstetrics and gynecology. 2011;117:1307–1313. doi: 10.1097/AOG.0b013e31821c33dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon EM, Maharshak N, Elloumi H, Borst L, Plevy SE, Moeser AJ. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10−/− mice. Inflammatory bowel diseases. 2013;19:712–719. doi: 10.1097/MIB.0b013e3182802a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Lopez JF, Flagel SB, Vazquez DM, Young EA. Differential regulation of hippocampal glucocorticoid receptors mRNA and fast feedback: relevance to post-traumatic stress disorder. Journal of neuroendocrinology. 1999;11:11–17. doi: 10.1046/j.1365-2826.1999.00288.x. [DOI] [PubMed] [Google Scholar]
- Liu HT, Shie JH, Chen SH, Wang YS, Kuo HC. Differences in mast cell infiltration, E-cadherin, and zonula occludens-1 expression between patients with overactive bladder and interstitial cystitis/bladder pain syndrome. Urology. 2012;80:225 e213–225 e228. doi: 10.1016/j.urology.2012.01.047. [DOI] [PubMed] [Google Scholar]
- Lu S, Peng H, Wang L, Vasish S, Zhang Y, Gao W, Wu W, Liao M, Wang M, Tang H, Li W, Li W, Li Z, Zhou J, Zhang Z, Li L. Elevated specific peripheral cytokines found in major depressive disorder patients with childhood trauma exposure: a cytokine antibody array analysis. Comprehensive psychiatry. 2013;54:953–961. doi: 10.1016/j.comppsych.2013.03.026. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Kreder KJ, Rothrock NE, Hoffman A, Kirschbaum C, Sternberg EM, Zimmerman MB, Ratliff TL. Diurnal cortisol variations and symptoms in patients with interstitial cystitis. The Journal of urology. 2002;167:1338–1343. [PubMed] [Google Scholar]
- Mayson BE, Teichman JM. The relationship between sexual abuse and interstitial cystitis/painful bladder syndrome. Current urology reports. 2009;10:441–447. doi: 10.1007/s11934-009-0070-3. [DOI] [PubMed] [Google Scholar]
- McAllister SL, Dmitrieva N, Berkley KJ. Sprouted innervation into uterine transplants contributes to the development of hyperalgesia in a rat model of endometriosis. PloS one. 2012;7:e31758. doi: 10.1371/journal.pone.0031758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister SL, McGinty KA, Resuehr D, Berkley KJ. Endometriosis-induced vaginal hyperalgesia in the rat: role of the ectopic growths and their innervation. Pain. 2009;147:255–264. doi: 10.1016/j.pain.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L, Girard BM, May V, Vizzard MA. Transcriptional and translational plasticity in rodent urinary bladder TRP channels with urinary bladder inflammation, bladder dysfunction, or postnatal maturation. J Mol Neurosci. 2012;48:744–756. doi: 10.1007/s12031-012-9867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neuroscience and biobehavioral reviews. 2007;31:3–17. doi: 10.1016/j.neubiorev.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Miranda A, Mickle A, Schmidt J, Zhang Z, Shaker R, Banerjee B, Sengupta JN. Neonatal cystitis-induced colonic hypersensitivity in adult rats: a model of viscero-visceral convergence. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2011;23 doi: 10.1111/j.1365-2982.2011.01724.x. 683-e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney RD, O'Leary OF, Felice D, Bettler B, Dinan TG, Cryan JF. Early-life stress induces visceral hypersensitivity in mice. Neuroscience letters. 2012;512:99–102. doi: 10.1016/j.neulet.2012.01.066. [DOI] [PubMed] [Google Scholar]
- Moore J, Kennedy S. Causes of chronic pelvic pain. Bailliere's best practice & research. 2000;14:389–402. doi: 10.1053/beog.1999.0082. [DOI] [PubMed] [Google Scholar]
- Nagabukuro H, Berkley KJ. Influence of endometriosis on visceromotor and cardiovascular responses induced by vaginal distention in the rat. Pain. 2007;132(Suppl 1):S96–S103. doi: 10.1016/j.pain.2007.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen RH, Swanson D, Harlow BL. Urogenital infections in relation to the occurrence of vulvodynia. The Journal of reproductive medicine. 2009;54:385–392. [PubMed] [Google Scholar]
- O'Malley D, Dinan TG, Cryan JF. Alterations in colonic corticotropin-releasing factor receptors in the maternally separated rat model of irritable bowel syndrome: differential effects of acute psychological and physical stressors. Peptides. 2010;31:662–670. doi: 10.1016/j.peptides.2010.01.004. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Dinan TG, Cryan JF. Neonatal maternal separation in the rat impacts on the stress responsivity of central corticotropin-releasing factor receptors in adulthood. Psychopharmacology. 2011a;214:221–229. doi: 10.1007/s00213-010-1885-9. [DOI] [PubMed] [Google Scholar]
- O'Malley D, Liston M, Hyland NP, Dinan TG, Cryan JF. Colonic soluble mediators from the maternal separation model of irritable bowel syndrome activate submucosal neurons via an interleukin-6-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2011b;300:G241–G252. doi: 10.1152/ajpgi.00385.2010. [DOI] [PubMed] [Google Scholar]
- Own LS, Patel PD. Maternal behavior and offspring resiliency to maternal separation in c57bl/6 mice. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Perry BD, Pollard R. Homeostasis, stress, trauma, and adaptation. A neurodevelopmental view of childhood trauma. 1998;7:33–51. viii. [PubMed] [Google Scholar]
- Peters KM, Killinger KA, Ibrahim IA. Childhood symptoms and events in women with interstitial cystitis/painful bladder syndrome. Urology. 2009;73:258–262. doi: 10.1016/j.urology.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Randich A, Uzzell T, Cannon R, Ness TJ. Inflammation and enhanced nociceptive responses to bladder distension produced by intravesical zymosan in the rat. BMC urology. 2006a;6:2. doi: 10.1186/1471-2490-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randich A, Uzzell T, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006b;7:469–479. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- Robbins MT, DeBerry J, Ness TJ. Chronic psychological stress enhances nociceptive processing in the urinary bladder in high-anxiety rats. Physiology & behavior. 2007;91:544–550. doi: 10.1016/j.physbeh.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins MT, Ness TJ. Footshock-induced urinary bladder hypersensitivity: role of spinal corticotropin-releasing factor receptors. J Pain. 2008;9:991–998. doi: 10.1016/j.jpain.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez MA, Afari N, Buchwald DS. Evidence for overlap between urological and nonurological unexplained clinical conditions. The Journal of urology. 2009;182:2123–2131. doi: 10.1016/j.juro.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Hormones and Behavior. 2003;43:561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Sack M, Lahmann C, Jaeger B, Henningsen P. Trauma prevalence and somatoform symptoms: are there specific somatoform symptoms related to traumatic experiences? The Journal of nervous and mental disease. 2007;195:928–933. doi: 10.1097/NMD.0b013e3181594846. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain research. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- Savignac HM, Dinan TG, Cryan JF. Resistance to early-life stress in mice: effects of genetic background and stress duration. Frontiers in behavioral neuroscience. 2011;5:13. doi: 10.3389/fnbeh.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwetz I, McRoberts JA, Coutinho SV, Bradesi S, Gale G, Fanselow M, Million M, Ohning G, Tache Y, Plotsky PM, Mayer EA. Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. Am J Physiol Gastrointest Liver Physiol. 2005;289:G704–G712. doi: 10.1152/ajpgi.00498.2004. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Annals of the New York Academy of Sciences. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tache Y, Million M, Nelson AG, Lamy C, Wang L. Role of corticotropin-releasing factor pathways in stress-related alterations of colonic motor function and viscerosensibility in female rodents. Gender medicine. 2005;2:146–154. doi: 10.1016/s1550-8579(05)80043-9. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. The Psychiatric clinics of North America. 2002;25:397–426. vii–viii. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Wang G. Colonic inflammation decreases thermal sensitivity of the forepaw and hindpaw in the rat. Neuroscience letters. 2004;359:81–84. doi: 10.1016/j.neulet.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wijngaard RM, Klooker TK, Welting O, Stanisor OI, Wouters MM, van der Coelen D, Bulmer DC, Peeters PJ, Aerssens J, de Hoogt R, Lee K, de Jonge WJ, Boeckxstaens GE. Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterology and motility : the official journal of the European Gastrointestinal Motility Society. 2009;21:e1107–e1194. doi: 10.1111/j.1365-2982.2009.01339.x. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Reber SO, Selch S, Obermeier F, Neumann ID. Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology. 2008;149:2727–2736. doi: 10.1210/en.2007-1469. [DOI] [PubMed] [Google Scholar]
- Victoria NC, Inoue K, Young LJ, Murphy AZ. Long-term dysregulation of brain corticotrophin and glucocorticoid receptors and stress reactivity by single early-life pain experience in male and female rats. Psychoneuroendocrinology. 2013;38:3015–3028. doi: 10.1016/j.psyneuen.2013.08.013. [DOI] [PubMed] [Google Scholar]
- Warren JW, van de Merwe JP, Nickel JC. Interstitial cystitis/bladder pain syndrome and nonbladder syndromes: facts and hypotheses. Urology. 2011;78:727–732. doi: 10.1016/j.urology.2011.06.014. [DOI] [PubMed] [Google Scholar]
- Weaver SA, Diorio J, Meaney MJ. Maternal separation leads to persistent reductions in pain sensitivity in female rats. J Pain. 2007;8:962–969. doi: 10.1016/j.jpain.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Xia CM, Gulick MA, Yu SJ, Grider JR, Murthy KS, Kuemmerle JF, Akbarali HI, Qiao LY. Up-regulation of brain-derived neurotrophic factor in primary afferent pathway regulates colon-to-bladder cross-sensitization in rat. Journal of neuroinflammation. 2012;9:30. doi: 10.1186/1742-2094-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Li Y, Zuo X, Zhen Y, Yu Y, Gao L. Transient receptor potential ankyrin-1 participates in visceral hyperalgesia following experimental colitis. Neuroscience letters. 2008;440:237–241. doi: 10.1016/j.neulet.2008.05.093. [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Yudkin PL, Vessey MP, Dawes MG, Barlow DH, Kennedy SH. Patterns of diagnosis and referral in women consulting for chronic pelvic pain in UK primary care. British journal of obstetrics and gynaecology. 1999;106:1156–1161. doi: 10.1111/j.1471-0528.1999.tb08141.x. [DOI] [PubMed] [Google Scholar]