Abstract

Cycloisomerizations of enynes are probably the most representative carbon–carbon bond forming reactions catalyzed by electrophilic metal complexes. These transformations are synthetically useful because chemists can use them to build complex architectures under mild conditions from readily assembled starting materials. However, these transformations can have complex mechanisms. In general, gold(I) activates alkynes in the presence of any other unsaturated functional group by forming an (η2-alkyne)–gold complex. This species reacts readily with nucleophiles, including electron-rich alkenes. In this case, the reaction forms cyclopropyl gold(I) carbene-like intermediates. These can come from different pathways depending on the substitution pattern of the alkyne and the alkene. In the absence of external nucleophiles, 1,n-enynes can form products of skeletal rearrangement in fully intramolecular reactions, which are mechanistically very different from metathesis reactions initiated by the [2 + 2] cycloaddition of a Grubbs-type carbene or other related metal carbenes.
In this Account, we discuss how cycloisomerization and addition reactions of substituted enynes, as well as intermolecular reactions between alkynes and alkenes, are best interpreted as proceeding through discrete cationic intermediates in which gold(I) plays a significant role in the stabilization of the positive charge. The most important intermediates are highly delocalized cationic species that some chemists describe as cyclopropyl gold(I) carbenes or gold(I)-stabilized cyclopropylmethyl/cyclobutyl/homoallyl carbocations. However, we prefer the cyclopropyl gold(I) carbene formulation for its simplicity and mnemonic value, highlighting the tendency of these intermediates to undergo cyclopropanation reactions with alkenes.
We can add a variety of hetero- and carbonucleophiles to the enynes in the presence of gold(I) in intra- or intermolecular reactions, leading to the corresponding adducts with high stereoselectivity through stereospecific anti-additions. We have also developed stereospecific syn-additions, which probably occur through similar intermediates. The attack of carbonyl groups at the cyclopropyl carbons of the intermediate cyclopropyl gold(I) carbenes initiates a particularly interesting group of reactions. These trigger a cascade transformation that can lead to the formation of two C–C and one C–O bonds. In the fully intramolecular process, this stereospecific transformation has been applied for the synthesis of natural sesquiterpenoids such as (+)-orientalol F and (−)-englerin A.
Intra- and intermolecular trapping of cyclopropyl gold(I) carbenes with alkenes leads to the formation of cyclopropanes with significant increase in the molecular complexity, particularly in cases in which this process combines with the migration of propargylic alkoxy and related OR groups. We have recently shown this in the stereoselective total synthesis of the antiviral sesquiterpene (+)-schisanwilsonene by a cyclization/1,5-acetoxy migration/intermolecular cyclopropanation. In this synthesis, the cyclization/1,5-acetoxy migration is faster than the alternative 1,2-acyloxy migration that would result in racemization.
1. Introduction
Cycloisomerizations of enynes proceed by mechanistically complex, multistep transformations and can lead to complex architectures by fully intramolecular processes. The pioneering work on the electrophilic activation of enynes was carried out by the group of Trost in the 1980s using palladium catalysts.1 These early studies were followed by several groups that examined other electrophilic metals, mainly ruthenium2 and platinum.3−7 The potential of gold catalysis in organic synthesis was demonstrated with the development of efficient additions of alcohols and water to alkynes under mild conditions by Teles8 and Tanaka,9 as well as by the phenol synthesis discovered by Hashmi using gold(III).10 This synthesis of phenols by cyclization of furans with alkynes was shown to be mechanistically related to some metal-catalyzed cycloisomerization reactions.11,12 In 2004, our group13 and those of Fürstner14 and Toste15 reported that gold(I) complexes were the most active and selective catalysts for the cycloisomerization of enynes. A mechanistically related gold(I)-catalyzed Conia-ene reaction of β-ketoesters with alkynes was also reported by Toste in 2004.16 Henceforth, homogeneous gold(I) catalysis experienced an outburst leading to the discovery of a phenomenal amount of new synthetically useful transformations. In addition to the important synthetic achievements made in the past decade in this area, the nature of the gold–carbon bond in intermediates of type [LAuCHR]+, which are involved in many gold(I)-catalyzed transformations, has inspired certain debate on the role played by gold(I) in the stabilization of these carbocationic species.17−19
Several reviews have covered synthetic and mechanistic aspects of homogeneous gold catalysis.20−28 In this Account, we focus on the developments of gold(I) catalytic transformations derived from our early studies on the cycloisomerization of simple enynes that have led to the discovery of complex cascade reactions.
2. Gold(I)-Catalyzed Cyclization of Enynes
Broadly, gold(I) selectively activates alkynes in the presence of alkenes and other functional groups.20 The high alkynophilicity of gold(I) does not reflect any thermodynamic preference for its coordination to alkynes, but it correlates with the higher reactivity of the resulting (η2-alkyne)–gold(I) complexes toward nucleophilic attack.29 In analogy to that shown in related cyclizations catalyzed by platinum(II),3−6 activation of the alkyne functionality by gold(I) forms an (η2-alkyne)–metal complex 1 that reacts as an electrophile with the alkene to form cyclopropyl gold(I) carbene-like intermediates 2 or 3 by an anti-5-exo-dig or a 6-endo-dig cyclization, respectively (Scheme 1).13,30−33 Intermediates 2 can evolve to generate new rearranged carbenes 4 by the formal insertion of the terminal alkene carbon into the alkyne carbons. These new carbenes 4 undergo α-proton elimination to yield 1,3-dienes 5, the products of an overall double-cleavage rearrangement. In this process, both the alkyne and the alkene have been cleaved in an intramolecular transformation. Although products with both configurations have been observed in this rearrangement, often compounds Z-5 (R = H) are obtained.34,35 On the other hand, intermediates 3 of 6-endo-dig cyclization can lead to bicyclo[4.1.0]hept-2-ene derivatives 6 by α-proton elimination.36−39 Alternatively, isomerization of 3 by ring expansion of the cyclopropane gives (η2-cyclobutene)–gold(I) complexes 7. The opening of these gold(I) complexes can form complexes 8, precursor of 1,3-dienes 9, in a transformation in which only the alkene has been cleaved. Highly strained bicyclo[3.2.0]hept-5-enes, which are the free ligands of 7, have been isolated only in a few cases.30,40,41 Less strained cyclobutenes resulting from a formal [2 + 2] cycloaddition have been obtained in the cyclization of 1,7- and 1,8-enynes.6,30,31,37,42 Intermediates 7 can also undergo isomerization to give bicyclo[3.2.0]hept-2-ene derivatives 10.36,37 Similarly, 1,5-43,44 and 1,7-enynes undergo rearrangements with gold(I) catalysts by somewhat related pathways.30,45
Scheme 1. General Pathways for the Gold(I)-Catalyzed Cycloisomerization of 1,6-Enynes.

According to DFT calculations, the syn-5-exo-dig cyclization via intermediates 11 does not compete with the other two pathways.30,31 Exocyclic carbene intermediate 2, formed in the anti-5-exo-dig pathway, can also give rise to products of single-cleavage rearrangement 9 through transition state TS2–12 and intermediates 12.30
The pathway followed by a particular enyne is highly dependent on its substitution pattern. Thus, 1,6-enyne 13a with a terminal alkyne and a disubstituted alkene reacts with a cationic catalyst formed in situ from [Au(PPh3)Cl] and AgBF4 to form exclusively single-cleavage rearrangement diene 14a (Scheme 2).13,30 An identical product 14a was obtained from 1,6-enyne 13b, with the methyl substituent at the alkyne, in an equally highly selective double-cleavage rearrangement.37 Although the gold(I)-catalyzed single-cleavage rearrangement is usually a stereospecific process in which the configuration of the alkene is retained,13,30,46 reaction of (E)-1,6-enynes such as 13c–f, bearing strongly electron-donating substituents at the terminal alkene carbon, react anomalously with cationic gold(I) catalyst A(36,47,48) to give selectively Z-configured dienes 14b–e.49 The same Z-preference was observed with other highly electrophilic gold(I) or platinum(II) catalysts. The Z-isomers of enynes 13c and 13d also give rise to Z-dienes with gold(I) or platinum(II) catalysts.49 The stereochemically anomalous rearrangement remains mechanistically puzzling.
Scheme 2. Single- and Double-Cleavage Rearrangement of 1,6-Enynes.

It is important to emphasize that, in contrast to Pd(II)1 and Pt(II),5,50,51 gold(I) does not promote Alder–ene cycloisomerizations of 1,n-enynes.30 The Alder–ene cycloisomerization would require the simultaneous coordination of gold(I) to the alkyne and the alkene, which is not favorable for a metal that prefers a linear bicoordination. Furthermore, the oxidation of gold(I) to form a gold(III) metallacycle, a mandatory step in an Alder–ene cycloisomerization, is also a very unlikely process.37,52
Conventionally, we prefer to depict complexes of type 2 as cyclopropyl gold(I) carbenes, to highlight their propensity to undergo cyclopropanation reactions, although DFT calculations showed that these are highly delocalized species that can also be described as gold(I)-stabilized cyclopropylmethyl/cyclobutyl/homoallyl carbocations.20,30 The bond between Au and C in gold(I) carbenes [LAu=CHR]+ has been described as a half-double bond.19
Cyclizations of 1,5-enynes also proceed through species that are intermediate between a bicyclic gold(I) carbene and an open carbocation.53 The highly electrophilic carbene of the intermediate species formed in these cycloisomerizations can even undergo formal C–H insertion reactions at β-C–H bonds leading to new cyclopropanes.54
3. Gold(I)-Catalyzed Nucleophilic Additions to Enynes
In the presence of alcohols or water, gold(I) catalyzes the addition of these nucleophiles to the enynes leading to products of alkoxy- or hydroxycyclization (Scheme 3).13,30 The overall process is an anti addition of an electrophile (the (η2-alkyne)–gold(I) complex) and a heteronucleophile to an alkene. Therefore, this reaction is stereospecific, as illustrated in the methoxycyclizations of diastereomers 13g and 13h, which afford diastereomeric adducts 15a and 15b, respectively, by attack of MeOH to intermediate 16a (Scheme 3). In these transformations, the catalyst was generated by protonolysis of the gold(I)–carbon bond of precatalyst [Au(PPh3)Me] with a strong Brønsted acid. These processes follow the Markovnikov regiochemistry, which is further illustrated by the reaction of substrate 13i in MeOH to form six-membered ring 15c through intermediates of type 16b. Related additions to 1,5-enynes are also stereospecific.55−57
Scheme 3. anti-Addition of Heteronucleophiles to 1,6-Enynes.
A few exceptions have been observed with the most polarized substrates. Thus, whereas reaction of enyne 13j in MeOH as solvent gives the product of methoxycyclization 15d as a single anti isomer, in agreement with the general behavior observed by other 1,6-enynes in similar reactions catalyzed by gold13,30 or platinum,6 when the reaction of 13j was performed with only 5 equiv of MeOH, adduct 15d was obtained as a 3:2 anti/syn mixture of stereoisomers (Scheme 3).49
Additions of carbon nucleophiles to enynes can also be carried out in the presence of gold(I).58−61 Thus, for example, reaction of 1,6-enyne 13k with indole, an electron-rich heteroarene, leads to adducts 17a and 17b by nucleophilic attack at the cyclopropyl or carbene carbons, respectively, of intermediate 18 (Scheme 4).58,59 Adduct 17a was favored using phosphine–gold(I) complex A, whereas complex B with an NHC ligand directed the nucleophilic attack at the carbene carbon, leading to adduct 17b. This result can be explained by the enhancement of the carbene-like character of the intermediate 18 by the highly donating NHC ligand. The 6-endo-dig cyclization pathway predominates in the case of the addition of indole to phenyl-substituted enyne 13l, which leads stereospecifically to adduct 19, while in the case of substrate 13m, the electron-rich arene attacks at the most substituted alkene carbon leading to 20.59 The addition of 1,3-dicarbonyl compounds and allyl silanes to 1,6-enynes, as well as similar additions of diverse carbon nucleophiles to 1,5-enynes are also catalyzed by cationic gold(I) catalysts.59
Scheme 4. Addition of Arenes and Heteroarenes to 1,6-Enynes.

Related intramolecular arylations of 1,6-enynes,62,63 as well as additions of carboxylic acids to enynes,17 have been proposed to take place in a concerted manner following the Stork–Eschenmoser model for cyclizations of squalene and oxidosqualene. However, the results of Schemes 3 and 4 and other related studies64 are best accommodated if distorted cationic cyclopropyl gold(I) carbenes are involved as discrete intermediates. A similar type of intermediate is probably also involved in processes in which two carbon bonds are formed by electrophilic syn-addition to the alkene. An illustrative case is the intramolecular [4 + 2] cyclization of aryl alkynes with alkenes to form tricyclic derivatives (Scheme 5).36,38 This reaction of 1,6-enynes such as 13n is stereospecific and, according to DFT calculations, proceeds stepwise through intermediate 22, which evolves by a Friedel–Crafts-type reaction to form the final tricyclic derivative 21a.38 Similarly, 1-naphthyl substituted 1,6-enyne 13o gives tetracyclic derivative 21b, and a related 1,7-enyne 23 gives rise to 21c. Recently, we have obtained enantiomeric excesses up to 88% in the same [4 + 2] cycloadditions of aryl-substituted 1,6-enynes using chiral gold(I) phosphite complexes derived from 3,3′-bis(triphenylsilyl)-1,1′-bi-2-naphthol.65 Chiral biphosphine gold(I) catalysts had also been used for this type of [4 + 2] cycloadditions of aryl-substituted 1,6-enynes.66 The reaction of benzyl-substituted 1,5-enyne 24 also occurs in a similar manner to afford 25,53 with the skeleton of the natural product (+)-pycnanthuquinone C (26).
Scheme 5. Formal [4 + 2] Cycloaddition of Arylalkynes with Alkenes.
4. Gold(I)-Catalyzed Intermolecular Reactions of Alkynes with Alkenes
The parent intermolecular reaction between alkynes and alkenes catalyzed by gold(I) was a challenge since all the conceivable products are themselves substituted alkenes, which can compete with the initial alkene leading to oligomerization products. In addition, electron-rich alkenes, which would be the best partners for this reaction, would coordinate preferentially with gold(I), thus reducing the concentration of the active (η2-alkyne)–gold(I) complex. After much experimentation with different gold(I) complexes, cyclobutenes 27 were obtained as the products of this intermolecular reaction by using cationic gold(I) complex D with a very bulky phosphine (Scheme 6).67 The observed regiochemistry of this [2 + 2] cycloaddition is consistent with a reaction proceeding by electrophilic addition to the alkene via TS28–29 to form a highly distorted cyclopropyl gold(I) carbene 29, which undergoes ring expansion through TS29–30 to give (η2-cyclobutene)–gold(I) complex 30. Intermediate 29 was also trapped intramolecularly with an alkene to form the corresponding cyclopropane.67 This process has been extended for the synthesis of large macrocycles such as 32 from enyne 31 by intramolecular [2 + 2] cycloaddition (Scheme 7).68
Scheme 6. [2 + 2] Cycloaddition of Arylalkynes with Alkenes.

Scheme 7. Macrocyclization via [2 + 2] Cycloaddition of Alkynes with Alkenes.
Interestingly, the intermolecular reaction of propiolic acid with alkenes proceeds through regioisomeric cyclopropyl gold(I) carbene intermediates 35, in which gold bonds to the internal carbon of the alkyne (Scheme 8).69 Asymmetrically substituted alkenes, such as styrene, give lactones 33 by attack of the carboxylic acid to the most substituted carbon of the alkene. On the other hand, alkenes with two identical, or very similar, substituents evolve by 1,3-migration to form stereospecifically 1,3-dienes 34.
Scheme 8. Divergent Pathways in the Reaction of Propiolic Acid with Alkenes.

It is interesting that a very similar transition state to TS34–35 for the formation of 1,3-dienes from propiolic acid69 had been also proposed in a seemingly different context. Electrophilic gold(I) catalysts promote the retro-Buchner reaction of 7-substituted 1,3,5-cycloheptatrienes, generating substituted gold(I) carbenes and a molecule of benzene.70 This reaction proceeds by retrocyclopropanation of the norcaradienes, which are in tautomeric equilibrium with the cycloheptatrienes. Other related retrocyclopropanations have been observed in the presence of gold(I).71,72 In the case of 7-cyclopropylcycloheptatriene 36, the reaction leads selectively to Z,Z-1,4-diphenyl-1,3-butadiene (Z,Z-37), whose formation can be rationalized by the evolution of cyclopropyl gold(I) carbene 38 through TS38–37 by 1,3-shift of a CHPh fragment (Scheme 9). This transition state is also very similar to that involved in the single cleavage rearrangement (TS2–12, Scheme 1). The ring expansion of 38 to form cyclobutene 39, which would have afforded E,E-37 by conrotatory opening, was not observed in this system.70
Scheme 9. Generation and Evolution of a Cyclopropyl Gold(I) Carbene by Retro-Buchner Reaction of 7-Cyclopropyl-1,3,5-cycloheptatriene 36.

5. Gold(I)-Catalyzed Cyclopropanation of Enynes
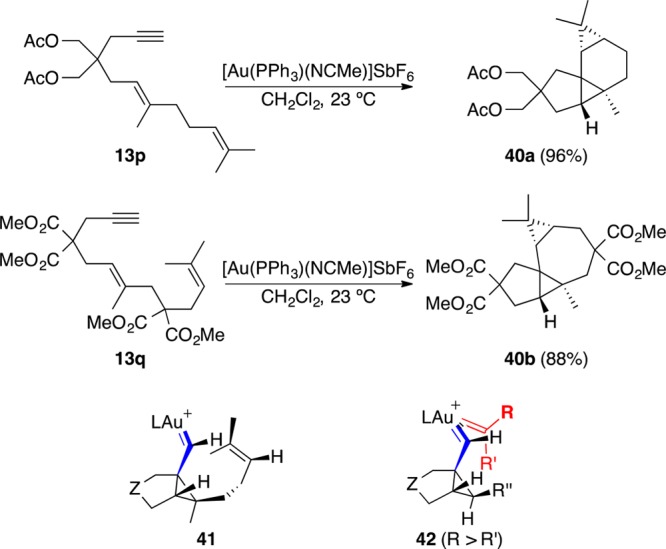
The carbene-like character of the intermediates formed in metal-catalyzed cycloisomerizations is more clearly manifested in intra- and intermolecular cyclopropanation of alkenes.2,7,73 Thus, reaction of dienynes 13p and 13q with gold(I) leads stereoselectively to tetracyclic compounds 40a and 40b (Scheme 10). These cyclopropanations occur through intermediates such as 41 or 42 for intermolecular processes,74,75 in a concerted although highly asynchronous manner. Intramolecular cyclopropanations of 1,5-enynes proceed similarly through an endo-carbene.53 However, cyclopropanation of 1,6-enynes occurs stepwise for more polarized alkenes such as styrenes, although the overall process is still stereospecific since formation of the second carbon–carbon bond occurred with a very small activation energy.75 Other theoretical calculations also suggest that the cyclopropanation of electron-rich alkenes by gold(I) carbenes proceeds by a stepwise mechanism.76
Scheme 10. Cyclopropanation of Alkenes via Cyclopropyl Gold(I) Carbene Intermediates.
Dienynes such as 13r substituted with OR groups at the propargylic position react with gold(I) catalysts by intramolecular 1,5-migration of OR groups to form tricyclic compounds 43a,b, which are structurally related to the sesquiterpenes globulol and epiglobulol (Scheme 11).77 This result is consistent with a reaction occurring via intermediate 44, in which the OR group attacks the cationic center to form bridged system 45. Opening of 45 then leads to an α,β-unsaturated gold carbene/allyl gold cation 46a, which undergoes intramolecular cyclopropanation with the alkene at the side chain to give 43a. In the presence of CD3OD, intermolecular addition of this external nucleophile to 44 leads to 47, which then gives rise to 43b-d3 via 46b.
Scheme 11. Gold(I)-Catalyzed 1,5-Migration of OR Groups in Dienyne 13r.

Other 1,6-enynes bearing different OR groups at the propargylic position react similarly to form α,β-unsaturated gold carbenes/allyl-gold cations related to 46. Thus, enyne 13s with an allyloxy group gave stereoselectively tricyclic compound 48a by cyclization, 1,5-migration, and, finally, an intramolecular cyclopropanation (Scheme 12).73 By appending the alkene to the 1,6-enyne through a silicon tether in substrate 13t, we also obtained cyclic siloxane 48b with high stereoselectivity.78 In an intermolecular variant of this process, reaction of 1,6-enyne 13u with alkene 49 gave 50, which was converted into the antiviral sesquiterpene (+)-schisanwilsonene (51) by a stereoselective route that includes a divinyl cyclopropane rearrangement. In this last example, it is interesting to remark that the cyclization/1,5-acetoxy migration is faster that the alternative 1,2-acyloxy migration that would lead to racemization.78
Scheme 12. Reactions of 1,6-Enynes via 1,5-Migration of OR.

6. Gold(I)-Catalyzed Cascade Reactions of Oxoenynes
1,6-Enyne 13v with a carbonyl group at the alkenyl side chain reacts in the presence of gold(I) to give oxatricyclic derivative 52a by a cascade [2 + 2 + 2] alkyne/alkene/carbonyl cycloaddition in which two C–C and one C–O bonds are formed (Scheme 13).79 Diene 53 was also obtained as a minor product. This reaction probably takes place by nucleophilic opening of the cyclopropane ring of intermediate 54 by the carbonyl group to form an oxonium cation 55, which gives 56 by a Prins-type intramolecular reaction closing a seven-membered ring. Intermediate 56 then gives oxatricyclic derivative 52a by metal elimination or diene 53 by a fragmentation process. The [2 + 2 + 2] cycloaddition of substrates 13w and 13x led to more functionalized tricyclic products 52b and 52c, which were transformed into the natural products (+)-orientalol F (57)80 and (−)-englerin A (58).81 Another total synthesis of 58 used a very similar gold(I) catalyzed reaction as the key step.82 The remarkable stereochemical control exerted by the propargylic stereocenter in the cyclizations of substrates 13w and 13x is identical to that observed in the cyclization proceeding via 1,5-OR migration through intermediate 44 (Scheme 11).77 Interestingly, attack of a carbonyl group to the cyclopropyl gold carbene is faster than the 1,5-migration of the propargylic OR groups.
Scheme 13. Intramolecular [2 + 2 + 2] Alkyne/Alkene/Carbonyl Cycloaddition of Oxo-1,6-enynes.

Intermolecular reactions of 1,6-enynes with carbonyl compounds in the presence of gold(I) catalysts lead to a variety of products depending on the substitution pattern of the alkene.83−85 Thus, for example, 1,6-enyne 13y reacts with 2,4,6-trimethylbenzaldehyde to give the product of formal [2 + 2 + 2] cycloaddition 59a, along with diene 60a, resulting from a metathesis-type reaction (Scheme 14).83 When the reaction was performed with 13z and 1-pyrenecarboxaldehyde, 1,3-diene 60b was obtained as the major compound. Formation of the [2 + 2 + 2] cycloaddition products of type 59 can be explained by attack of the aldehyde to cyclopropyl gold(I) intermediate 61 to give oxonium cation 62, followed by Prins cyclization to form tetrahydropyranyl cation 63 and metal elimination. Metathesis-type products 60 could be formed by a fragmentation of 63, analogous to that observed in the intramolecular gold(I)-catalyzed reaction of oxo-1,6-enynes (Scheme 13).79
Scheme 14. Intermolecular [2 + 2 + 2] Alkyne/Alkene/Carbonyl Cycloaddition of 1,6-Enynes with Aldehydes.

Oxo-1,5-enynes such as E- and Z-64 also undergo gold(I)-catalyzed cyclization to form tricyclic derivatives 65a and 65b, respectively (Scheme 15).86 When gold(I) complexes with donating ligands are used as catalysts, the major cycloisomerization pathway proceeding through intermediates 66 and 67 is stereospecific. However, the stereoselectivity is only moderate in the case of E-64, which is consistent with the existence of two competitive pathways, supporting again the proposal for stepwise processes via discrete intermediates in gold(I) catalyzed cascade reactions.
Scheme 15. Intramolecular Alkyne/Alkene/Carbonyl Cycloaddition of Oxo-1,5-enynes.

The intermolecular gold(I)-catalyzed reaction of terminal alkynes with oxoalkenes of type 68 leads to 8-oxabicyclo[3.2.1]oct-3-enes 69 by a similar [2 + 2 + 2] cycloaddition process through intermediates 70 in which two C–C and one C–O bonds are formed (Scheme 16).87
Scheme 16. Intermolecular [2 + 2 + 2] Alkyne/Alkene/Carbonyl Cycloaddition of Alkynes with Oxoalkenes.

7. Concluding Remarks
Many reactions of 1,n-enynes and related substrates catalyzed by gold(I) bear certain resemblance with carbocationic processes promoted by Brønsted or Lewis acids. However, gold(I) catalysts orchestrate complex reactions with exquisite regio- and stereocontrol, by stabilizing the key reactive cationic intermediates. Although in a few cases the reactions proceed through open carbocations, most transformations are stereospecific. The basic mechanistic pathways involved in the cycloisomerization of 1,n-enynes are reasonably well understood, although still the factors that control the many competitive pathways are still rather obscure, particularly in intermolecular reactions. Nevertheless, complex cascade transformations can now be designed based on relatively simple principles. This journey to gain mechanistic insight into this family of complex transformations has also led to the discovery of robust, yet highly reactive cationic catalysts such as A and D bearing bulky phosphines, which are among the most useful gold(I) catalysts.
Acknowledgments
We thank the MICINN (Grant CTQ2010-16088/BQU), the AGAUR (Grant 2009 SGR 47), the European Research Council (Advanced Grant No. 321066), and the ICIQ Foundation for financial support. C.O. acknowledges the receipt of a FPU fellowship from the Ministry of Education and Science. We also thank the past and present graduate students of our group at the Universidad Autónoma de Madrid (UAM) and the ICIQ, notably Carolina Fernández-Rivas, María Méndez, Belén Martín-Matute, M. Paz Muñoz-Herranz, Cristina Nevado, Cristina Nieto-Oberhuber, Catalina Ferrer, Eloísa Jiménez-Núñez, Elena Herrero-Gómez, Patricia Pérez-Galán, Verónica López-Carrillo, Mihai Raducan, Nicolas Delpont, César Rogelio Solorio-Alvarado, Núria Huguet, Morgane Gaydou, Yahui Wang, and Anna Homs, as well as our theoretical collaborators Diego J. Cárdenas (UAM), Elena Buñuel (UAM), Feliu Maseras (ICIQ), and Maria Besora (ICIQ) for their contributions to the understanding of the complex mechanisms triggered by gold catalysts.
Biographies
Carla Obradors was born in Manresa (Catalonia, Spain) in 1987. She completed her B.Sc. in Chemistry (2010) at the Universitat Autònoma de Barcelona (UAB) and joined the group of Prof. Antonio M. Echavarren at the Institute of Chemical Research of Catalonia (ICIQ) with a FPU fellowship. She received a Special Master Thesis Award from the Universitat Rovira i Virgili (Tarragona) in 2012.
Antonio M. Echavarren was born in Bilbao in 1955 (Basque Country, Spain) and obtained his Ph.D. at the Universidad Autónoma de Madrid (UAM, 1982) with Prof. Francisco Fariña. After a postdoctoral stay in Boston College with Prof. T. Ross Kelly, he joined the UAM as an Assistant Professor (1984–86). Following a two year period as a NATO-fellow in the group of Prof. John K. Stille in Fort Collins (Colorado State University), he joined the Institute of Organic Chemistry of the CSIC in Madrid, where he stayed until 1992. That year he returned to the UAM as a Professor of Organic Chemistry. He is also Professor of Research of the CSIC since 2004. He moved in 2004 to Tarragona as a Group Leader at the Institute of Chemical Research of Catalonia (ICIQ). He received the 2004 Janssen-Cylag Award in Organic Chemistry and the 2010 Gold Medal of the Royal Spanish Chemical Society.
The authors declare no competing financial interest.
References
- Trost B. M. Palladium-Catalyzed Cycloisomerizations of Enynes and Related Reactions. Acc. Chem. Res. 1990, 23, 34–42. [Google Scholar]
- Chatani N.; Kataoka K.; Murai S.; Furukawa N.; Seki Y. Construction of Novel Polycyclic Ring Systems by Transition-Metal-Catalyzed Cycloisomerization of Ene-Ene-Ynes. Interception of a Carbenoid Intermediate in Skeletal Reorganization of Enynes. J. Am. Chem. Soc. 1998, 120, 9104–9105. [Google Scholar]
- Chatani N.; Furukawa N.; Sakurai H.; Murai S. PtCl2-Catalyzed Conversion of 1,6- and 1,7-Enynes to 1-Vinylcycloalkenes. Anomalous Bond Connection in Skeletal Reorganization of Enynes. Organometallics 1996, 15, 901–903. [Google Scholar]
- Oi S.; Tsukamoto I.; Miyano S.; Inoue Y. Cationic Platinum-Complex-Catalyzed Skeletal Reorganization of Enynes. Organometallics 2001, 20, 3704–3709. [Google Scholar]
- Fürstner A.; Szillat H.; Stelzer F. Novel Rearrangements of Enynes Catalyzed by PtCl2. J. Am. Chem. Soc. 2000, 122, 6785–6786. [Google Scholar]
- Méndez M.; Muñoz M. P.; Echavarren A. M. Platinum-Catalyzed Hydroxy- and Alkoxycyclization of Enynes. J. Am. Chem. Soc. 2000, 122, 11549–11550. [Google Scholar]
- Mainetti E.; Mouriès V.; Fensterbank L.; Malacria M.; Marco-Contelles J. The Effect of a Hydroxy Protecting Group on the PtCl2-Catalyzed Cyclization of Dienynes–A Novel, Efficient, and Selective Synthesis of Carbocycles. Angew. Chem., Int. Ed. 2002, 41, 2132–2135. [PubMed] [Google Scholar]
- Teles J. H.; Brode S.; Chabanas M. Cationic Gold(I) Complexes: Highly Efficient Catalysts for the Addition of Alcohols to Alkynes. Angew. Chem., Int. Ed. 1998, 37, 1415–1418. [DOI] [PubMed] [Google Scholar]
- Mizushima E.; Sato K.; Hayashi T.; Tanaka M. Highly Efficient AuI-Catalyzed Hydration of Alkynes. Angew. Chem., Int. Ed. 2002, 41, 4563–4565. [DOI] [PubMed] [Google Scholar]
- Hashmi A. S. K.; Frost T. M.; Bats J. W. Highly Selective Gold-Catalyzed Arene Synthesis. J. Am. Chem. Soc. 2000, 122, 11553–11554. [Google Scholar]
- Martín-Matute B.; Cárdenas D. J.; Echavarren A. M. Pt(II)-catalyzed Intramolecular Reaction of Furans with Alkynes. Angew. Chem., Int. Ed. 2001, 40, 4754–4757. [DOI] [PubMed] [Google Scholar]
- Martín-Matute B.; Nevado C.; Cárdenas D. J.; Echavarren A. M. Intramolecular Reactions of Alkynes with Furans and Electron Rich Arenes Catalyzed by PtCl2: The Role of Platinum Carbenes as Intermediates. J. Am. Chem. Soc. 2003, 125, 5757–5766. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; Muñoz M. P.; Buñuel E.; Nevado C.; Cárdenas D. J.; Echavarren A. M. Cationic Gold(I) Complexes: Highly Alkynophilic Catalysts for the exo- and endo-Cyclization of Enynes. Angew. Chem., Int. Ed. 2004, 43, 2402–2406. [DOI] [PubMed] [Google Scholar]
- Mamane V.; Gress T.; Krause H.; Fürstner A. Platinum- and Gold-Catalyzed Cycloisomerization Reactions of Hydroxylated Enynes. J. Am. Chem. Soc. 2004, 126, 8654–8655. [DOI] [PubMed] [Google Scholar]
- Luzung M. R.; Markham J. P.; Toste F. D. Catalaytic Isomerization of 1,5-Enynes to Bicyclo[3.1.0]hexenes. J. Am. Chem. Soc. 2004, 126, 10858–10859. [DOI] [PubMed] [Google Scholar]
- Kennedy-Smith J. J.; Staben S. T.; Toste F. D. Gold(I)-Catalyzed Conia-Ene Reaction of β-Ketoesters with Alkynes. J. Am. Chem. Soc. 2004, 126, 4526–4527. [DOI] [PubMed] [Google Scholar]
- Fürstner A.; Morency L. On the Nature of the Reactive Intermediates in Gold-Catalyzed Cycloisomerization Reactions. Angew. Chem., Int. Ed. 2008, 47, 5030–5033. [DOI] [PubMed] [Google Scholar]
- Hashmi A. S. K. “High Noon” in Gold Catalysis: Carbene versus Carbocation Intermediates. Angew. Chem., Int. Ed. 2008, 47, 6754–6756. [DOI] [PubMed] [Google Scholar]
- Benitez D.; Shapiro N. D.; Tkatchouk E.; Wang Y.; Goddard W. A. III; Toste D. F. A Bonding Model for Gold(I) Carbene Complexes. Nat. Chem. 2009, 1, 482–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Sun J.; Kozmin S. A. Gold and Platinum Catalysis of Enyne Cycloisomerization. Adv. Synth. Catal. 2006, 348, 2271–2296. [Google Scholar]
- Jiménez-Núñez E.; Echavarren A. M. Gold-Catalyzed Cycloisomerizations of Enynes: A Mechanistic Perspective. Chem. Rev. 2008, 108, 3326–3350. [DOI] [PubMed] [Google Scholar]
- Gorin D. J.; Sherry B. D.; Toste F. D. Ligand Effects in Homogeneous Au Catalysis. Chem. Rev. 2008, 108, 3351–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelet V.; Toullec P. Y.; Genêt J. P. Cycloisomerization of 1,n-Enynes: Challenging Metal-Catalyzed Rearrangements and Mechanistic Insights. Angew. Chem., Int. Ed. 2008, 47, 4268–4315. [DOI] [PubMed] [Google Scholar]
- Fürstner A. Gold and Platinum Catalysis–a Convenient Tool for Generating Molecular Complexity. Chem. Soc. Rev. 2009, 38, 3208–3221. [DOI] [PubMed] [Google Scholar]
- Shapiro N. D.; Toste F. D. A Reactivity-Driven Approach to the Discovery and Development of Gold-Catalyzed Organic Reactions. Synlett 2010, 675–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L.-P.; Hammond G. B. Recent Advances in the Isolation and Reactivity of Organogold Complexes. Chem. Soc. Rev. 2012, 41, 3129–3139. [DOI] [PubMed] [Google Scholar]
- Rudolph M.; Hashmi A. S. K. Gold Catalysis in Total Synthesis–an Update. Chem. Soc. Rev. 2012, 41, 2448–2462. [DOI] [PubMed] [Google Scholar]
- Obradors C.; Echavarren A. M. Intriguing Mechanistic Labyrinths in Gold(I) Catalysis. Chem. Commun. 2013, 10.1039/C3CC45518A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mota M.; Cabello N.; Maseras F.; Echavarren A. M.; Pérez-Ramírez J.; Lopez N. Selective Homogeneous and Heterogeneous Gold Catalysis with Alkynes and Alkenes: Similar Behavior, Different Origin. ChemPhysChem 2008, 9, 1624–1629. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; López S.; Muñoz M. P.; Cárdenas D. J.; Buñuel E.; Nevado C.; Echavarren A. M. Divergent Mechanisms for the Skeletal Rearrangement and [2 + 2] Cycloaddition of Enynes Catalyzed by Gold. Angew. Chem., Int. Ed. 2005, 44, 6146–6148. [DOI] [PubMed] [Google Scholar]
- Escribano-Cuesta A.; Pérez-Galán P.; Herrero-Gómez E.; Sekine M.; Braga A. A. C.; Maseras F.; Echavarren A. M. The Role of Cyclobutenes in Gold(I)-Catalysed Skeletal Rearrangement of 1,6-Enynes. Org. Biomol. Chem. 2012, 10, 6105–6111. [DOI] [PubMed] [Google Scholar]
- Ferrer C.; Raducan M.; Nevado C.; Claverie C. K.; Echavarren A. M. Missing Cyclization Pathways and New Rearrangements Unveiled in the Gold(I) and Platinum(II)-Catalyzed Cyclization of 1,6-Enynes. Tetrahedron 2007, 63, 6306–6316. [Google Scholar]
- Soriano E.; Marco-Contelles J. Mechanistic Insights on the Cycloisomerization of Polyunsaturated Precursors Catalyzed by Platinum and Gold Complexes. Acc. Chem. Res. 2009, 42, 1026–1036. [DOI] [PubMed] [Google Scholar]
- Ota K.; Chatani N. Rh(II)-catalyzed skeletal reorganization of enynes involving selective cleavage of C–C triple bonds. Chem. Commun. 2008, 2906–2907. [DOI] [PubMed] [Google Scholar]
- Ota K.; Lee S. I.; Tang J.-M.; Takachi M.; Nakai H.; Morimoto T.; Sakurai H.; Kataoka K.; Chatani N. Rh(II)-Catalyzed Skeletal Reorganization of 1,6- and 1,7-Enynes through Electrophilic Activation of Alkynes. J. Am. Chem. Soc. 2009, 131, 15203–15211. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; López S.; Echavarren A. M. Intramolecular [4 + 2] Cycloadditions of 1,3-Enynes or Arylalkynes with Alkenes with Highly Reactive Cationic Phosphine Au(I) Complexes. J. Am. Chem. Soc. 2005, 127, 6178–6179. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; Muñoz M. P.; López S.; Jiménez-Núñez E.; Nevado C.; Herrero-Gómez E.; Raducan M.; Echavarren A. M. Gold(I)-Catalyzed Cyclizations of 1,6-Enynes: Alkoxycyclizations and exo/endo Skeletal Rearrangements. Chem.—Eur. J. 2006, 12, 1677–1693. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; Pérez-Galán P.; Herrero-Gómez E.; Lauterbach T.; Rodríguez C.; López S.; Bour C.; Rosellón A.; Cárdenas D. J.; Echavarren A. M. Gold(I)-Catalyzed Intramolecular [4 + 2] Cycloadditions of Arylalkynes or 1,3-Enynes with Alkenes: Scope and Mechanism. J. Am. Chem. Soc. 2008, 130, 269–279. [DOI] [PubMed] [Google Scholar]
- Lee Y. T.; Kang Y. K.; Chung Y. K. Au(I)-Catalyzed Cycloisomerization Reaction of Amide- or Ester-Tethered 1,6-Enynes to Bicyclo[3.2.0]hept-6-en-2-ones. J. Org. Chem. 2009, 74, 7922–7934. [DOI] [PubMed] [Google Scholar]
- Lee S. I.; Kim S. M.; Choi M. R.; Kim S. Y.; Chung Y. K. Au(I)-Catalyzed Cyclization of Enynes Bearing an Olefinic Cycle. J. Org. Chem. 2006, 71, 9366–9372. [DOI] [PubMed] [Google Scholar]
- Brooner R. E. M.; Brown T. J.; Widenhoefer R. A. Direct Observation of a Cationic Gold(I)–Bicyclo[3.2.0]hept-1(7)-ene Complex Generated in the Cycloisomerization of a 7-Phenyl-1,6-enyne. Angew. Chem. Int. Ed. 2013, 52, 6259–6261. [DOI] [PubMed] [Google Scholar]
- Odabachian Y.; Gagosz F. Cyclobutenes as Isolable Intermediates in the Gold(I)-Catalysed Cycloisomerisation of 1,8-Enynes. Adv. Synth. Catal. 2009, 351, 379–386. [Google Scholar]
- Zhang L.; Kozmin S. Gold-catalyzed cycloisomerization of siloxy enynes to cyclohexadienes. J. Am. Chem. Soc. 2004, 126, 11806–11807. [DOI] [PubMed] [Google Scholar]
- Sun J.; Conley M.; Zhang L.; Kozmin S. Au-and Pt-Catalyzed Cycloisomerizations of 1, 5-Enynes to Cyclohexadienes with a Broad Alkyne Scope. J. Am. Chem. Soc. 2006, 128, 9705–9710. [DOI] [PubMed] [Google Scholar]
- Cabello N.; Rodríguez C.; Echavarren A. M. Gold-Catalyzed Cyclizations of 1,7-Enynes. Synlett 2007, 1753–1758. [Google Scholar]
- Nieto-Oberhuber C.; López S.; Jiménez-Núñez E.; Echavarren A. M. The Mechanistic Puzzle of Transition Metal-Catalyzed Skeletal Rearrangements of Enynes. Chem.—Eur. J. 2006, 12, 5916–5923. [DOI] [PubMed] [Google Scholar]
- Herrero-Gómez E.; Nieto-Oberhuber C.; López S.; Benet-Buchholz J.; Echavarren A. M. Cationic η1/η2-Gold(I) Complexes of Simple Arenes. Angew. Chem., Int. Ed. 2006, 45, 5455–5459. [DOI] [PubMed] [Google Scholar]
- Pérez-Galán P.; Delpont N.; Herrero-Gómez E.; Maseras F.; Echavarren A. M. Metal-Arene Interactions in Dialkylbiarylphosphane Complexes of Copper, Silver, and Gold. Chem.—Eur. J. 2010, 16, 5324–5332. [DOI] [PubMed] [Google Scholar]
- Jiménez-Núñez E.; Claverie C. K.; Bour C.; Cárdenas D. J.; Echavarren A. M. cis-Selective Single-Cleavage Skeletal Rearrangement of 1,6-Enynes Reveals the Multifaceted Character of the Intermediates in Metal-Catalyzed Cycloisomerizations. Angew. Chem., Int. Ed. 2008, 47, 7892–7895. [DOI] [PubMed] [Google Scholar]
- Nevado C.; Cárdenas D. J.; Echavarren A. M. Reaction of Enol Ethers with Alkynes Catalyzed by Transition Metals: 5exo-dig versus 6endo-dig Cyclizations via Cyclopropyl Platinum or Gold Carbene Complexes. Chem.—Eur. J. 2003, 9, 2627–2635. [DOI] [PubMed] [Google Scholar]
- Nevado C.; Charruault L.; Michelet V.; Nieto-Oberhuber C.; Muñoz M. P.; Méndez M.; Rager M.-N.; Genêt J. P.; Echavarren A. M. On the Mechanism of Carbohydroxypalladation of Enynes. Additional Insights on the Cyclization of Enynes with Electrophilic Metal Complexes. Eur. J. Org. Chem. 2003, 706–713. [Google Scholar]
- Lauterbach T.; Livendahl M.; Rosellón A.; Espinet P.; Echavarren A. M. Unlikeliness of Pd-Free Gold(I)-Catalyzed Sonogashira Coupling Reactions. Org. Lett. 2010, 12, 3006–3009. [DOI] [PubMed] [Google Scholar]
- López-Carrillo V.; Huguet N.; Mosquera Á.; Echavarren A. M. Nature of the Intermediates in Gold(I)-Catalyzed Cyclizations of 1,5-Enynes. Chem.—Eur. J. 2011, 17, 10972–10978. [DOI] [PubMed] [Google Scholar]
- Horino Y.; Yamamoto T.; Ueda K.; Kuroda S.; Toste F. D. Au(I)-Catalyzed Cycloisomerizations Terminated by sp3 C–H Bond Insertion. J. Am. Chem. Soc. 2009, 131, 2809–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Kozmin S. Gold-Catalyzed Assembly of Heterobicyclic Systems. J. Am. Chem. Soc. 2005, 127, 6962–6963. [DOI] [PubMed] [Google Scholar]
- Böhringer S.; Gagosz F. Gold(I)-Catalyzed [4 + 2] Annelation/Nucleophilic Addition Sequence: Stereoselective Synthesis of Functionalized Bicyclo[4.3.0]nonenes. Adv. Synth. Catal. 2008, 350, 2617–2630. [Google Scholar]
- Buzas A.; Istrate F.; Le Goff X. F.; Odabachian Y.; Gagosz F. Gold(I)-Catalyzed [4 + 2] Cycloaddition of N-(Hex-5-enynyl) tert-Butyloxycarbamates. J. Organomet. Chem. 2009, 694, 515–519. [Google Scholar]
- Amijs C. H. M.; Ferrer C.; Echavarren A. M. Gold(I)-Catalysed Arylation of 1,6-Enynes: Different Site Reactivity of Cyclopropyl Gold Carbenes. Chem. Commun. 2007, 698–700. [DOI] [PubMed] [Google Scholar]
- Amijs C. H. M.; López-Carrillo V.; Raducan M.; Pérez-Galán P.; Ferrer C.; Echavarren A. M. Gold(I)-Catalyzed Intermolecular Addition of Carbon Nucleophiles to 1,5- and 1,6-Enynes. J. Org. Chem. 2008, 73, 7721–7730. [DOI] [PubMed] [Google Scholar]
- Leseurre L.; Chao C.-M.; Seki T.; Genin E.; Toullec P. Y.; Genêt J.-P.; Michelet V. Synthesis of Functionalized Carbo- And Heterocycles via Gold-Catalyzed Cycloisomerization Reactions of Enynes. Tetrahedron 2009, 65, 1911–1918. [Google Scholar]
- Böhringer S.; Gagosz F. Gold(I)-Catalyzed [4 + 2] Annelation/Nucleophilic Addition Sequence: Stereoselective Synthesis of Functionalized Bicyclo[4.3.0]nonenes. Adv. Synth. Catal. 2008, 350, 2617–2630. [Google Scholar]
- Chao C. M.; Vitale M. R.; Toullec P. Y.; Genêt J. P.; Michelet V. Asymmetric Gold-Catalyzed Hydroarylation/Cyclization Reactions. Chem.—Eur. J. 2009, 15, 1319–1323. [DOI] [PubMed] [Google Scholar]
- Sethofer S. G.; Mayer T.; Toste F. D. Gold(I)-Catalyzed Enantioselective Polycyclization Reactions. J. Am. Chem. Soc. 2010, 132, 8276–8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Galán P.; Martin N. J. A.; Campaña A. G.; Cárdenas D. J.; Echavarren A. M. Carbocations or Cyclopropyl Gold Carbenes in Cyclizations of Enynes. Chem.—Asian J. 2001, 6, 482–486. [DOI] [PubMed] [Google Scholar]
- Delpont N.; Escofet I.; Pérez-Galán P.; Spiegl D.; Raducan M.; Bour C.; Sinisi R.; Echavarren A. M. Modular Chiral Gold(I) Phosphite Complexes. Catal. Sci. Technol. 2013, 3, 3007–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C.-M.; Vitale M. R.; Toullec P. Y.; Genêt J.-P.; Michelet V. Asymmetric Gold-Catalyzed Hydroarylation/Cyclization Reactions. Chem.—Eur. J. 2009, 15, 1319–1323. [DOI] [PubMed] [Google Scholar]
- López-Carrillo V.; Echavarren A. M. Gold(I)-Catalyzed Intermolecular [2 + 2] Cycloaddition of Alkynes with Alkenes. J. Am. Chem. Soc. 2010, 132, 9292–9294. [DOI] [PubMed] [Google Scholar]
- Obradors C.; Leboeuf D.; Aydin J.; Echavarren A. M. Gold(I)-Catalyzed Macrocyclization of 1,n-Enynes. Org. Lett. 2013, 15, 1576–1579. [DOI] [PubMed] [Google Scholar]
- Yeom H.-S.; Koo J.; Park H.-S.; Wang Y.; Liang Y.; Yu Z.-X.; Shin S. Gold-Catalyzed Intermolecular Reactions of Propiolic Acids with Alkenes: [4 + 2] Annulation and Enyne Cross Metathesis. J. Am. Chem. Soc. 2012, 134, 208–211. [DOI] [PubMed] [Google Scholar]
- Solorio-Alvarado C. R.; Wang Y.; Echavarren A. M. Cyclopropanation with Gold(I) Carbenes by Retro-Buchner Reaction from Cycloheptatrienes. J. Am. Chem. Soc. 2011, 133, 11952–11955. [DOI] [PubMed] [Google Scholar]
- Solorio-Alvarado C. R.; Echavarren A. M. Gold-Catalyzed Annulation/Fragmentation: Formation of Free Gold Carbenes by Retro-Cyclopropanation. J. Am. Chem. Soc. 2010, 132, 11881–11883. [DOI] [PubMed] [Google Scholar]
- Fedorov A.; Batiste L.; Bach A.; Birney D. M.; Chen P. Potential Energy Surface for (Retro-)Cyclopropanation: Metathesis with a Cationic Gold Complex. J. Am. Chem. Soc. 2011, 133, 12162–12171. [DOI] [PubMed] [Google Scholar]
- Nieto-Oberhuber C.; López S.; Muñoz M. P.; Jiménez-Núñez E.; Buñuel E.; Cárdenas D. J.; Echavarren A. M. Gold(I)-Catalyzed Intramolecular Cyclopropanation of Dienynes. Chem.—Eur. J. 2006, 12, 1694–1702. [DOI] [PubMed] [Google Scholar]
- López S.; Herrero-Gómez E.; Pérez-Galán P.; Nieto-Oberhuber C.; Echavarren A. M. Gold(I)-Catalyzed Intermolecular Cyclopropanation of Enynes with Alkenes: Trapping of Two Different Gold Carbenes. Angew. Chem., Int. Ed. 2006, 45, 6029–6032. [DOI] [PubMed] [Google Scholar]
- Pérez-Galán P.; Herrero-Gómez H.; Hog D. T.; Martin N. J. A.; Maseras F.; Echavarren A. M. Mechanism of the Gold-Catalyzed Cyclopropanation of Alkenes with 1,6-Enynes. Chem. Sci. 2011, 2, 141–149. [Google Scholar]
- Batiste L.; Fedorov A.; Chen P. Gold Carbenes via 1,2-Dialkoxycyclopropane Ring-Opening: A Mass Spectrometric and DFT Study of the Reaction Pathways. Chem. Commun. 2010, 46, 3899–3901. [DOI] [PubMed] [Google Scholar]
- Jiménez-Núñez E.; Raducan M.; Lauterbach T.; Molawi K.; Solorio C. R.; Echavarren A. M. Evolution of Propargyl Ethers into Allylgold Cations in the Cyclization of Enynes. Angew. Chem., Int. Ed. 2009, 48, 6152–6155. [DOI] [PubMed] [Google Scholar]
- Gaydou M.; Miller R. E.; Delpont N.; Ceccon J.; Echavarren A. M. Synthesis of (+)-Schisanwilsonene A by Tandem Gold-Catalyzed Cyclization-1,5-Migration-Cyclopropanation. Angew. Chem., Int. Ed. 2013, 52, 6396–6399. [DOI] [PubMed] [Google Scholar]
- Jiménez-Núñez E.; Claverie C. K.; Nieto-Oberhuber C.; Echavarren A. M. Prins Cyclizations in Au-Catalyzed Reactions of Enynes. Angew. Chem., Int. Ed. 2006, 45, 5452–5455. [DOI] [PubMed] [Google Scholar]
- Jiménez-Núñez E.; Molawi K.; Echavarren A. M. Stereoselective Gold-Catalyzed Cycloaddition of Functionalized Ketoenynes: Synthesis of (+)-Orientalol F. Chem. Commun. 2009, 7327–7329. [DOI] [PubMed] [Google Scholar]
- Molawi K.; Delpont N.; Echavarren A. M. Enantioselective Synthesis of (−)-Englerins A and B. Angew. Chem., Int. Ed. 2010, 49, 3517–3519. [DOI] [PubMed] [Google Scholar]
- Zhou Q.; Chen X.; Ma D. Asymmetric, Protecting-Group-Free Total Synthesis of (−)-Englerin A. Angew. Chem., Int. Ed. 2010, 49, 3513–3516. [DOI] [PubMed] [Google Scholar]
- Escribano-Cuesta A.; López-Carrillo V.; Janssen D.; Echavarren A. M. Gold-Catalyzed Reactions of 1,5- and 1,6-Enynes with Carbonyl Compounds: Cycloaddition vs. Metathesis. Chem.—Eur. J. 2009, 15, 5646–5650. [DOI] [PubMed] [Google Scholar]
- Schelwies M.; Dempwolff A. L.; Rominger F.; Helmchen G. Gold-Catalyzed Intermolecular Addition of Carbonyl Compounds to 1,6-Enynes. Angew. Chem., Int. Ed. 2007, 46, 5598–5601. [DOI] [PubMed] [Google Scholar]
- Schelwies M.; Moser R.; Dempwolff A. L.; Rominger F.; Helmchen G. Gold-Catalyzed Intermolecular Addition of Carbonyl Compounds to 1,6-Enynes: Reactivity, Scope, and Mechanistic Aspects. Chem.—Eur. J. 2009, 15, 10888–10900. [DOI] [PubMed] [Google Scholar]
- Huguet N.; Echavarren A. M. Gold-Catalyzed Cyclizations of Oxo-1,5-enynes. Synlett 2012, 23, 49–53. [Google Scholar]
- Obradors C.; Echavarren A. M. Intermolecular Gold-Catalyzed Cycloaddition of Alkynes with Oxoalkenes. Chem.—Eur. J. 2013, 19, 3547–3551. [DOI] [PubMed] [Google Scholar]





