Abstract
Traditional metabolic engineering analyzes biosynthetic and physiological pathways, identifies bottlenecks, and makes targeted genetic modifications with the ultimate goal of increasing the production of high-value products in living cells. Such efforts have led to the development of a variety of organisms with industrially relevant properties. However, there are a number of cellular phenotypes important for research and the industry for which the rational selection of cellular targets for modification is not easy or possible. In these cases, strain engineering can be alternatively carried out using “inverse metabolic engineering”, an approach that first generates genetic diversity by subjecting a population of cells to a particular mutagenic process, and then utilizes genetic screens or selections to identify the clones exhibiting the desired phenotype. Given the availability of an appropriate screen for a particular property, the success of inverse metabolic engineering efforts usually depends on the level and quality of genetic diversity which can be generated. Here, we review classic and recently developed combinatorial approaches for creating such genetic diversity and discuss the use of these methodologies in inverse metabolic engineering applications.
Keywords: inverse metabolic engineering, genetic engineering, microbes, genetic screening, mutagenesis
1. Introduction
Metabolic engineering has been a well established scientific discipline for over two decades now [1]. During this period, advances in the understanding of the functional role of thousands of genes in various organisms, and the development of theoretical and experimental tools for determining the flow of metabolites through different biochemical pathways [2, 3], have provided hints for numerous potential cellular targets whose modification can lead to optimal metabolism and improved properties.
More recently, advances in sequencing technologies have resulted in an explosion in the number of sequenced microbial genomes, revealing a plethora of novel enzymes, biochemical reactions and pathways, while the development of efficient methodologies for performing directed protein evolution has enabled the engineering of enzymes with tailored activities [4, 5]. Furthermore, the emergence of more global approaches for analysing cell function, such as systems biology, have contributed to our understanding of how biochemical pathways operate, not in the form of sequential “isolated” reactions, but instead as complex, interdependent and dynamic networks of such reactions [6]. Finally, synthetic biology has extended the capabilities of classical metabolic engineering as it has provided the rationale and tools to combine biological components and generate designed gene networks, artificial metabolic pathways, and organisms with man-made genomes [7, 8].
These technologies, in combination with the significantly advanced analytical tools available today for analyzing levels of DNA, RNA, proteins and metabolites in the cell, have enabled the development of engineered prokaryotic and eukaryotic organisms with the ability to produce a wide array of chemicals useful for research and the industry: amino acids [9], antibiotics [10], alkaloids [11], isoprenoids [12], vitamines, fragrances, peptides and polyketides [13], organic solvents and biofuels [14, 15], nutraceuticals, polymers [16] and others.
A prerequisite for the success of classical (forward) metabolic engineering is a detailed knowledge of the biochemical pathways involved in the biosynthesis of a particular metabolite or in the appearance of a desired phenotype. This knowledge is necessary in order to be able to make targeted and rationally selected genetic modifications. However, a variety of cellular properties important for the industry and research, such as resistance to organic solvents and optimal production of certain metabolites, are still poorly characterized and frequently arise from modifications in pathways that involve genes of unknown function or ones that would be impossible to predict by rational engineering, possibly because they act by indirect or compensatory mechanisms. In these cases, where educated guesses about possible interventions are hard or impossible to make, a different strategy can be applied, which is termed “inverse metabolic engineering” [17]. In this type of metabolic engineering, the desired property is first linked to a readily detectable phenotype, e.g. changes in cell growth, color, fluorescence etc. Then, random genetic modifications, such as chromosomal point mutations, gene deletions, gene over-expressions etc., are introduced into the host so that a library of cells with genetic diversity is generated. This library is then screened and the clones that exhibit the desired phenotype are identified. Genetic analysis of the isolated clones subsequently can reveal the factors responsible for the improved properties and lead to enhanced understanding of the (previously unidentified) biochemical processes involved.
Given the availability of an effective genetic screening or selection system for the desired property, the potential success of an inverse metabolic effort is highly dependent on the level and quality of genetic diversity which can be generated. Here, we review combinatorial approaches for generating genetic diversity within a host cell population, and discuss the applications of these methodologies in inverse metabolic engineering. Emphasis is placed on approaches which have been developed in recent years.
2. Classical approaches in inverse metabolic engineering
2.1. Spontaneous mutagenesis
Spontaneously acquired mutations leading to increased fitness under specific growth conditions is the classical adaptive evolution paradigm. This has been used by mankind for millennia, and by the industry and academia for decades in order to generate useful organisms for a variety of applications. Numerous successes in inverse metabolic engineering mediated through spontaneous mutagenesis have been reported and include increased tolerance to isobutanol and ethanol [18, 19], growth on citrate [20]; production of D-lactate [21] and hard-to-express recombinant proteins in Escherichia coli [22]; production of 1,3-propanediol in Klebsiella pneumoniae [23]; xylose and galactose utilization in Saccharomyces cerevisiae [24, 25]. Analysis of the genetic lesions that lead to improved phenotypes in spontaneously evolved strains used to be a complicated or impossible process for variants carrying multiple and distantly located mutations, but the recent development of -omics technologies coupled with the increased throughput and decreased cost of sequencing technologies has made this a very tractable task [19–21, 26].
2.2. Random mutagenesis with chemical mutagens
Libraries of cells containing lesions randomly distributed over the entire chromosome can be readily generated by exposing a population to sub-lethal doses of mutagenic chemicals, such as N-methyl-N′-nitro-N-nitrosoguanidine and ethyl methanesulfonate or other mutagenic agents, such as UV irradiation. The use of such agents has resulted in the development of microbial strains with the ability to produce enhanced amounts of isobutanol [27], full-length IgG antibodies [28] and membrane proteins [29].
2.3. Transposon mutagenesis
Genes whose products have a negative impact on a desired property can be readily identified by transposon mutagenesis. This type of mutagenesis results in random insertions of transposable elements throughout the genome with concomitant functional disruption of the gene sequence that received the insertion. Transposon mutagenesis has led to the development of improved strains and the identification of inhibitory roles for genes involved in the production of biomass [30], lycopene [31], and membrane proteins [32] in E. coli; riboflavin production in Bacillus subtilis [33]; and poly-3-hydroxybutyrate in Synechocystis sp. PCC6803 [34], to name a few examples. Very useful tools for studying the effect of gene knockouts is the Keio collection, a publicly available library of all single knockouts of all the non-essential E. coli K-12 genes [35] and the yeast deletion collection [36]. The utility of these libraries in inverse metabolic engineering has already been demonstrated [37] and it is expected that such libraries will be increasingly used in the coming years.
2.4. Gene overexpression libraries
Genes, gene fragments or fragments of entire operons that favorably affect a desired property can be isolated from vector libraries co-expressing genomic fragments. Genomic libraries have been screened in order to identify genes that enhance alcohol tolerance/production and galactose fermentation in S. cerevisiae [38–40]; acetate and butanol tolerance [41, 42], lycopene [43] and membrane protein production [44] in E. coli; butyrate tolerance in Clostridium acetobutylicum [45], and in other cases. In addition, individual enhancer genes can be identified using the ASKA library, a library of all the E. coli open reading frames (ORFs) transcribed from the strong and inducible T5lac promoter [46] or the FLEXgene collection, an analogous library encoding yeast ORFs from S. cerevisiae [47], both of which are publicly available. Again, such collections have already been used successfully for inverse metabolic engineering applications [48, 49]. To explore the functional genomic content of unculturable organisms, similar screens can also be carried out by constructing and screening metagenomic libraries [50].
Finally, additive positive effects from genes and/or operons distantly located within a genome can be identified by a recently developed tool termed coexisting/coexpressing genomic libraries (CoGeLs) [51]. CoGeLs allow the simultaneous screening of two genomic libraries encoded in different vectors with compatible origins of replication which can coexist in the same host. These vectors can be regular bacterial plasmids that contain small- or medium-size inserts (up to 10 kbases) or fosmids, which are low-copy number vectors where large DNA fragments (∼40 kbases) can be inserted. Using a combination of two plasmid-encoded E. coli genomic libraries, Nicolaou et al. demonstrated that CoGeLs can be used to identify combinations of distantly located factors that impart increased acid resistance in E. coli [51].
3. Recently developed approaches in inverse metabolic engineering
In recent years, a number of new approaches have been developed that attempt to create more “global” changes on cellular pathways and physiology as means of generating complex phenotypes more effectively. The majority of those strategies aim at modifying the transcriptional landscape of an organism, e.g. by generating libraries of randomized transcription factors or by mutating components of the RNA polymerase. Since certain components of the transcriptional machinery in prokaryotic as well as eukaryotic organisms regulate the expression of a wide repertoire of genes, subtle changes in these components can have a pronounced effect on the transcriptome of the cell, thus offering the potential for the emergence of diverse and complex phenotypes [52]. Some examples of these methods are described below and are summarized in Table 1.
Table 1.
Combinatorial genome engineering approaches which have been applied to inverse metabolic engineering applications.
| Method | Targeted cellular component | Target organism | Engineered phenotype | References |
|---|---|---|---|---|
| Spontaneous chromosomal mutagenesis | Chromosome | Escherichia coli | Ethanol and isobutanol tolerance; D-lactate, and hard-to-express protein production | [18, 19, 22] |
| Saccharomyces cerevisiae | Xylose consumption | [25] | ||
| Klebsiella pneumoniae | 1,3-propanediol production | [23] | ||
|
| ||||
| Chromosomal mutagenesis using chemical mutagens or mutator genes | Chromosome | E. coli | isobutanol, membrane protein, and full-length IgG production | [27–29] |
|
| ||||
| Transposon mutagenesis | All individual chromosomal genes | E. coli | Biomass, lycopene, and recombinant membrane protein production | [30–32] |
| Bacillus subtilis | Riboflavin production | [33] | ||
| Synechocystis PCC6803 | Poly-3-hydroxybutyrate production | [34] | ||
| S. cerevisiae | Isoprenoid production | [37] | ||
|
| ||||
| Genomic libraries and related approaches (individual gene overexpression libraries, CoGeLs) | Chromosomal fragments | E. coli | Acetate, glutamate, butanol, antibiotic and toxin tolerance; lycopene and membrane protein production | [41–44, 48, 49, 51] |
| S. cerevisiae | Alcohol tolerance and production; galactose fermentation | [38–40] | ||
| Clostridium acetobotulicum | Butyrate tolerance | [45] | ||
|
| ||||
| Global transcription machinery engineering (gTME) | General sigma factor σ70, stationary phase sigma factor σS, RNA polymerase α subunit, cAMP receptor protein (CRP), histone-like nucleoid structuring protein H-NS, H-NS-interacting haemolysin expression modulating protein Hha, Deinococcus radiodurans global regulator IrrE | E. coli | Ethanol, butanol, isobutanol, pentanol, 3-pentanol, acetate, butyrate, high osmolarity, and SDS tolerance; lycopene, L-tyrosine, and hyaluronic acid production | [61, 66, 67, 69, 71–73, 75, 76, 109] |
| Transcription factor Spt15p and TATA-binding protein Taf25p | S. cerevisiae | Ethanol tolerance and production; xylose fermentation; corn cob acid hydrolysate tolerance | [67] [70, 74] | |
| General sigma factor RpoD | Lactobacillus plantarum | lactic acid and hydrochloric acid tolerance | [68] | |
|
| ||||
| Libraries of artificial zinc fingers | Zinc finger domains fused to transcriptional activators, repressors or without fusion partner | S. cerevisiae | Tolerance to heat and osmotic stress; ketoconazole resistance | [57] |
| Mouse neuroblastoma cells | Neurogenesis, differentiation of neuroblasts to osteoblasts, proliferation rate | [57] | ||
| E. coli | Tolerance to butanol, heat, cold, and osmotic stress | [58–60] | ||
|
| ||||
| Multiplex automated genome engineering (MAGE) | Multiple rationally selected genomic loci | E. coli | Lycopene and indigo production; incorporation of artificial amino acids | [79, 80, 84] |
|
| ||||
| Trackable multiplex recombineering (TRMR) | >95% of all individual E. coli genes | E. coli | Tolerance to salicin, D-fucose, methylglyoxal, valine, acetate and lignocellulosic hydrolysate | [85, 86] |
|
| ||||
| Ribosome engineering | Ribosomal components or RNA polymerase subunits | Streptomyces strains | Actinorhodin, fredericamycin, formycin, actinomycin, piperidamycin production | [88–92, 96] |
| B. subtilis | Amylase and protease production | [93] | ||
| Pseudomonas putida | Resistance to toluene, m-xylene, and 4-hydroxybenzoate | [97] | ||
|
| ||||
| Genome shuffling | Chromosome | Streptomyces fradiae | Tylosin production | [100] |
| A strain of Lactobacillus | Tolerance to lactic acid | [102] | ||
| Sphingobium chlorophenolicum | Degradation of pentachlorophenol | [110] | ||
| E. coli | Butanol and antibiotic tolerance | [104, 107] | ||
| S. cerevisiae, Pichia stipitis | Ethanol production | [105] | ||
3.1 Zinc finger-based artificial transcription factors
Zinc fingers are highly specific DNA-binding protein domains which recognize three-base pair sequences and are found in many proteins that regulate transcription in a variety of organisms. One transcription factor can include several of these motifs. The ones that contain more fingers recognize larger stretches of DNA and are, generally, more specific about the genomic loci that they bind. Currently, at least one natural or engineered zinc finger exists for every possible triplet of DNA bases (4x4x4 = 64 possible triplets) [53–55]. Park et al. took advantage of the wide repertoire of DNA-binding specificities and the highly modular way with which these proteins can be assembled [56] to generate libraries of artificial transcriptional activators and repressors that can activate or silence practically any gene within a eukaryotic genome [57], with the ultimate goal of evolving complex phenotypes. First, they selected 40 and 25 zinc fingers with diverse DNA-binding specificities and used them to create random combinatorial combinations of three- and four-finger proteins, respectively. They then fused these randomly assembled finger triads and quadrads to a transcriptional activation domain, a transcriptional repressor domain, or no domain, to generate a library of artificial factors which can potentially regulate the transcription of genes close to their DNA-binding site. Expression of the generated three- and four-finger libraries fused to the Gal4 activation domain, the Ume6 repression domain, or no domain in S. cerevisiae resulted in the emergence of novel phenotypes such as growth arrest, and resistance to heat, osmotic stress, and the mycocidal antibiotic ketoconazole [57]. Replacement of the activation and repression domains with p65 and the Krüppel-associated box (KRAB), expression of the transcription factor library in mammalian cells, and screening for the emergence of complex phenotypes resulted in the identification of (i) one p65-containing zinc finger activator, which could induce neurogenesis of the mouse neuroblastoma cell line Neuro2A; (ii) one zinc finger activator that induced the differentiation of murine myoblasts to osteoblasts; and (iii) one inhibitor and one enhancer of cell proliferation [57]. Artificial zinc finger transcription factors can also be used to impart novel phenotypes in lower organisms such as bacteria, either in the absence of a transcriptional regulation domain [58], or in the presence of an activator, such as the cAMP receptor protein (CRP) [59, 60].
3.2 Global transcription machinery engineering (gTME)
gTME is another powerful new tool that enables the reprogramming of the cellular transcriptome through random mutagenesis of specific components of the global transcriptional machinery of a microbial organism. Initially, the components which were selected for mutagenesis were the E. coli general sigma factor σ70 [61], and the S. cerevisiae TATA-binding transcription factor Spt15p and the TATA-binding protein associated factor Taf25p [62]. The rationale behind the choice of σ70 as a target was that it regulates the expression of about 1,000 genes, which are important for normal exponential growth in bacteria [63], while missense mutations in rpoD, the gene encoding σ70, have been shown to result in changes in the binding preferences of the RNA polymerase-σ factor complex [64, 65]. Similarly, Spt15p and Taf25p were chosen because they are regarded as two of the main DNA-binding proteins that regulate the promoter specificities of the three RNA polymerases in yeast. These factors were mutated by error-prone PCR to form combinatorial libraries of random mutant alleles, cloned into appropriate vectors, and expressed in E. coli and yeast strains that contained the native genomic copy of the corresponding factor. After performing relevant genetic screens and selections, variants of the targeted factors were identified, whose expression resulted in the emergence of industrially important properties. In E. coli, several multiple point and truncation rpoD mutants were isolated that conferred increased tolerance to ethanol (up to 60 g/L), to sodium dodecyl sulfate (SDS), and to both ethanol and SDS, as well as enhanced production of lycopene (10-50% increase depending on the parental strain) [61]. In yeast, a triple point variant of Spt15p was isolated that exhibited a 13-fold increase in growth at specific glucose concentrations and a volumetric production of ethanol enhanced by ∼70% compared to the parental S. cerevisiae strain [62].
After this initial success, a number of reports described the use of gTME to evolve bacterial and yeast strains exhibiting an array of industrially important phenotypes and properties, such as enhanced production of hyaluronic acid [66], L-tyrosine [67], and lactate [68]; increased to tolerance to ethanol, butanol, acetate, butyrate, lignocellulosic hydrolysates, and osmotic stress [69–73]; improved capacity to ferment xylose [74]; and regulated biofilm formation and dispersal [75, 76]. Furthermore, these studies showed that appropriate protein targets for gTME are not limited to σ70, Spt15p, and Taf25p, but can also be applied to additional components of the transcriptional machinery of an organism which can simultaneously affect the transcriptional profile of multiple genes, such as the α subunit of the E. coli RNA polymerase [67], CRP [69, 72], the histone-like nucleoid structuring protein H-NS [76], and the H-NS-interacting haemolysin expression modulating protein Hha [75]. In certain cases, it may be beneficial to target more than one of these factors simultaneously [77].
It is interesting to mention that improved cellular properties can be engineered not only by targeting endogenous global transcriptional regulators, but also by applying gTME to targets derived form heterologous hosts. For example, Chen et al. isolated variants of the global regulator IrrE from Deinococcus radiodurans (a bacterium with very high levels of resistance to radiation) that enhanced stress tolerances when expressed in E. coli [73]. However, not all global transcriptional regulators are appropriate gTME targets for a given property. This was demonstrated in the case of the E. coli stationary phase sigma factor σS, where effective variants for enhancing hyaluronic acid production in E. coli could not be identified, despite the fact that multiple enhancer variants could be identified when σ70 was subjected to mutagenesis [66]. Of course, one cannot rule out the possibility that σS variants useful for hyaluronic acid production in bacteria could exist but, due to the limitations in combinatorial protein library construction, they were not present in the screened rpoS library. Finally, but very importantly, transcriptional regulators which have been engineered by gTME are not only useful for conferring improved phenotypes to standard laboratory strains such as E. coli K12, but also for other microbes of industrial interest, such as Lactobacillus plantarum [68]. Once functional variants of gTME targets have been identified, the changes within the transcriptional landscape responsible for the appearance of the beneficial properties can be identified by DNA microarray analysis.
Based on the data described above, one can now claim that gTME is a relatively well established approach for inverse metabolic engineering applications. Through the already generated results, a number of important conclusions have been drawn, which will provide useful guidance for future gTME efforts. For example, Stephanopoulos and co-workers have tested gTME target libraries generated by low, medium, and high mutagenesis frequencies and have proposed that high-error rate mutagenic PCR pools exhibit increased probability to contain effective variants [68, 71]. This is consistent with the outcome from previous directed protein evolution efforts [78] and with the results of the analysis of the transcriptional regulator variants evolved by gTME, which has shown that the emergence of significantly improved phenotypes usually requires multiple mutations [61, 62, 66, 72, 73]. An interesting observation is that the effective variants are very often N- or C-terminal truncations of the original regulator [61, 66, 67, 71, 77]. Since improved phenotypes upon expression of the truncated regulators occur in the presence of the full-length chromosomal copy of the regulator gene, it is likely that the engineered properties arise from the combined action of the evolved truncation and the full-length, wild-type protein [67]. Alternatively, the evolved truncations could be acting as anti-sigma factors [77]. In cases where a truncated factor is responsible for the emergence of the desired phenotype, it may be more efficient to perform random mutagenesis using the truncated rather the full-length form of the protein [71].
As anticipated, the effects of the evolved transcriptional regulator proteins are phenotype-specific, i.e. a regulator evolved to impart a specific property will, in general, be ineffective in improving another desirable phenotype. However, in cases where different phenotypes are linked to similar cellular pathways, regulators evolved for one phenotype may have similar effects on another. For example, mutants of rpoA (the gene encoding the α subunit of the E. coli RNA polymerase) that resulted in enhanced resistance to 1-butanol were also found capable of enhancing bacterial resistance to isobutanol, 1-pentanol, and 3-pentanol [67]. For applications where two different unrelated cellular properties need to be simultaneously improved, an effective regulator for both properties can be evolved sequentially (first under conditions which are selective for property A and then the isolated variant can be mutated again and be subjected to selection for property B) or simultaneously (under conditions which are selective for both A and B) [61].
3.3. Multiplex automated genome engineering (MAGE)
One of the most promising recent genome engineering approaches is MAGE [79]. A key advantage of this technology is that it is capable of generating high levels of genetic variability within multiple genomic loci simultaneously. MAGE utilizes synthetic single-stranded DNA oligonucleotides and the Redα/Redβ recombinase system from bacteriophage lambda in order to introduce point mutations, insertions, and deletions into targeted genomic loci of a microbial organism, such as E. coli (Figure 1). Synthetic oligos carrying homology regions with the targeted genomic regions along with the desired modifications are electroporated into modified bacterial strains, which are deficient in the DNA mismatch repair gene mutS and which express the components of the λ Red recombinase system. By performing repeated cycles of this process, at least one targeted region can be modified in approximately 30% of the cells of a bacterial population in a period of time as short as two hours [79]. By utilizing prototype mutagenic oligos, Wang et al. estimated that they could generate a population that contained close to 1010 variations in sequence compared to the parental cells within a day. The efficiency with which a particular modification is made depends on the degree and length of the homology region between the genomic target and the mutagenic oligo. Continuous MAGE cycles can be carried out until the desired genetic diversity is achieved, a factor which depends on the number of the genomic loci that will be modified and the degree of sequence diversity that will be introduced at each locus. Church and co-workers have already built a prototype device that automates the entire process, thus allowing rapid and easy generation of cell populations with very high levels of genetic diversity [79]. As the prices for custom oligonucleotide synthesis decrease, the applicability of this technology has the potential to become very broad.
Figure 1.
Schematic representation of the MAGE approach. First, specific genes/genomic locations known/suspected to be involved in a particular cellular phenotype are targeted for modification. Then, synthetic oligonucleotides that introduce insertions, deletions, missense mutations, or other types of genetic lesions are synthesized and introduced into the target cell host by electroporation. Subsequently, the action of the λ Red recombinase system assists the introduction of the designed lesions into the target genomic loci. Finally, the beneficial mutations are selected and enriched by performing cycles of genetic screening or selection. The beneficial genomic alterations can be readily identified by DNA sequencing of the targeted genomic locations.
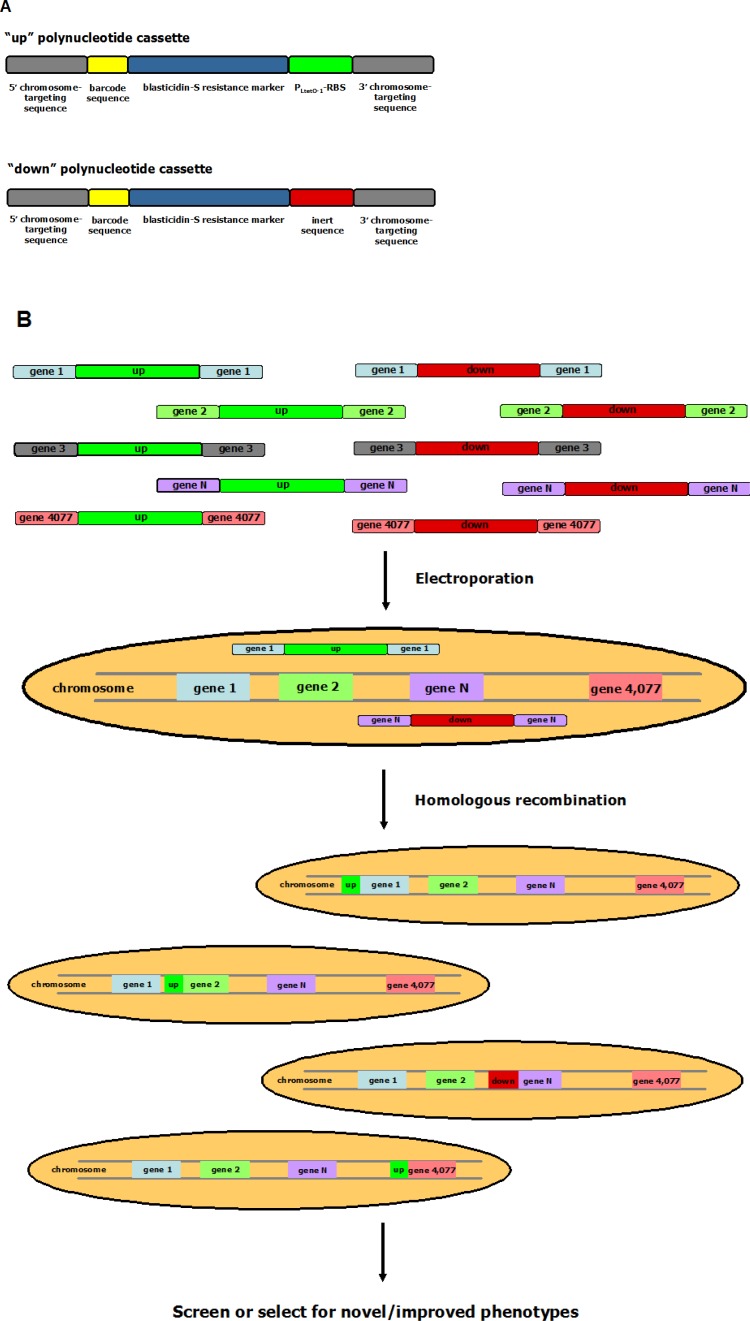
Figure 2.
Schematic representation of the TRMR approach. (A). Synthetic DNA oligos in TRMR used for targeted gene up- and down-regulation in a microbial organism. These include gene-specific 5′- and 3′-terminal homology regions (grey), a gene-specific molecular barcode sequence (yellow), a blasticidin-S resistance marker for selecting for cells where successful recombination has occurred (blue), and a sequence which results, in general, in up- (green) or down-regulation (red) of the gene located immediately downstream from this sequence. The sequence responsible for the up-regulation of gene expression contains the strong and inducible PLtetO-1 promoter [108] and a ribosome-binding site (RBS), while the sequence responsible for gene down-regulation contains an inert sequence. (B). Gene-specific synthetic oligos the lead to up- and down-regulation were generated for each one of the 4,077 genes of E. coli MG1655, yielding a total of 8,154 gene-targeting oligos. These oligos can then be introduced into living microbial cells by electroporation. Successful homologous recombination (selected for by the emergence of a resistance phenotype to the antibiotic blasticidin) can then lead to the creation of a microbial cell library, where each member expresses a different gene at higher or lower levels compared to the parental cells. Genes that have an enhancing or inhibitory role for a particular cellular property can then be identified by subjecting the TRMR-generated library to an appropriate genetic screen or selection, followed by sequencing or microarray analysis.
In order to demonstrate potential applications of MAGE in cellular programming, Wang et al. utilized the approach to enhance lycopene production in E. coli. The ribosome-binding sequences (RBSs) of twenty genes previously reported to affect lycopene production were randomized simultaneously, while four other such genes were targeted for inactivation by introduction of internal stop codon mutations. After performing 35 cycles of MAGE and screening for colonies with intense red pigmentation, the authors identified variants carrying RBS sequence modifications in four genes (dxs, dxr, idi, ispA) acting at the beginning and the end of the lycopene biosynthesis pathway, while three out of the four targets for inactivation were found to contain stop codons. Some of the isolated mutants were found to produce up to five-fold more lycopene than the parental bacterial strains. This corresponded to a yield of 9 mg/g of dry cell weight, higher than all other previously reported efforts [79]. In addition to enhanced metabolite production, MAGE has additionally been applied to bacterial genomic reprogramming in order to increase the incorporation of artificial amino acids into engineered proteins [80].
More recent improvements of the technology [81–83] have resulted in increased efficiency of the MAGE process, which has allowed for efficient multi-loci insertion of longer sequences (>10 bases) of DNA within a microbial genome (co-selection MAGE or CoS-MAGE) [84]. By using CoS-MAGE, Wang et al. were able to rapidly generate engineered E. coli, where the promoters of up to seventeen endogenous genes could be replaced with the strong and orthogonal-to-E. coli T7 promoter. Screening for enhanced production of the blue dye indigo in the generated T7 knock-in strains led to isolation of variants which can produce more than two-fold more dye (∼9 mg/g of dry cell weight) than the parental strain [84]. Despite the tremendous potential of MAGE and CoS-MAGE for application in inverse metabolic engineering efforts, one should consider that these approaches can only be applied in cases where genes relevant to a particular cellular property have already been identified previously by other techniques.
3.4 Trackable multiplex recombineering (TRMR)
Very recently, Gill and co-workers have developed an elegant methodology for constructing libraries of genetically modified microorganisms based on homologous recombination of pools of synthetic oligos [85]. To demonstrate the technology, the authors constructed two sets of polynucleotide cassettes each of which comprised 5′ and 3′ recognition sequences for homologous recombination with the genomic region immediately upstream of the start codon of each one of the 4,077 protein-coding genes of E. coli MG1655, interrupted by a gene-specific tracking sequence (barcode oligoncleotide), a blasticidin-S resistance marker for selection of successful recombination, and either an “up” cassette or a “down” cassette. The “up” cassette consisted of the sequences of the strong inducible promoter PLtetO-1 and a ribosome-binding site, whereas the “down” cassette comprised a transcriptionally and translationally inert sequence. The function of the up cassette was to generally up-regulate the expression of its target gene, while the down cassette was intended to down-regulate gene expression. After introduction of this library of polynucleotides into E. coli cells by electroporation and selection in antibiotic-containing media for clones where successful homologous recombination took place, the investigators were able to generate libraries of modified bacteria where each member contained a different up-regulated or down-regulated gene. This pool of 2 x 4,077 = 8,154 mutant strains was subsequently subjected to selection for growth under conditions that normally do not permit growth of wild-type E. coli. These included media that contain salicin as the sole carbon source, media where the presence of D-fucose inhibits the ability of E. coli to utilize arabinose as a carbon source, and lethal concentrations of methylglyoxal (due to extensive oxidative damage), valine (due to feedback inhibition of the biosynthesis of leucine and isoleucine), and lignocellylosic hydrolysate (due to the presence of a cocktail of different growth inhibitors). Warner et al. reported that clones resistant to these conditions could emerge from the described TRMR libraries with frequencies 100-fold higher compared to the same cells lacking the introduced polynucleotide libraries [85]. Subsequently, the isolated clones could be easily characterized by DNA sequencing or microarray analysis using their corresponding molecular barcode sequences to identify the genes responsible for these complex phenotypes. In this manner, already known as well as novel genes involved in the emergence of these cellular properties could be identified.
More recently, the same laboratory demonstrated that the ability of TRMR to assess the effect of up- and down-regulating individual genes on desired cellular properties at a genome-wide scale can be combined with the more in-depth search for the optimal transcription levels of genes relevant to a specific phenotype, which is offered by MAGE [86]. In that work, Sandoval et al. first used the TRMR libraries described above to identify genes which are involved in enhanced bacterial resistance to corn stover hydrolysate, acetate, and low pH, and to rank their relevance in these improved phenotypes. Then, they generated MAGE-like bacterial libraries, where the RBSs preceding the start codons of the genes identified as relevant in the previous step for the property in question were randomized. Based on the calculated efficiency of the process, the authors estimated that they could generate a population that contained a significant percentage of single, double, triple and quadruple mutants (i.e. mutants where the RBSs of one, two, three, and four genes were modified, respectively) after thirteen cycles of recombination. These libraries were then re-subjected to genetic selection for improved phenotypes. In the case of the resistance phenotype to low pH, one clone was identified with improved growth characteristics compared both to the wild-type as well as the single mutant isolated by TRMR [86], thus demonstrating the potential power of combining the two methodologies. In the case of the other two selection conditions which were tested, however, no multi-gene mutant could be isolated that performed better than the single-gene variants identified by TRMR only. This highlighted the importance of epistatic interactions between the different genes associated with a particular phenotype. It remains to be seen whether such combinations of TRMR and MAGE will yield strains with improved characteristics for a variety of cellular properties.
3.5 Ribosome engineering
Another very interesting approach for generating complex phenotypes in microbes is “ribosome engineering”. Here, microbial cells are exposed to lethal concentrations of antibiotics that inhibit ribosomal function, such as chloramphenicol, rifampicin, streptomycin, kanamycin, spectinomycin, and others, and clones resistant to different levels of these compounds are selected [87]. Resistance to these antibiotics frequently arises from mutations in components comprising the ribosome, such as ribosomal proteins and RNA, or in other factors that affect mRNA translation, such as elements of the RNA polymerase. These changes can result in aberrant protein synthesis and in enhanced production of specific proteins. In certain cases, some of these overproduced proteins are encoded by genes which are normally in a dormant state. This altered proteome can in turn lead to the emergence of improved or even novel cellular properties. In one of the first demonstrations of the approach, Ochi and co-workers showed that mutations in rpsL, the gene encoding the ribosomal protein S12, which confer resistance to streptomycin in Streptomyces lividans and Streptomyces coelicolor result in increased production of the antibiotic actinorhodin [88, 89]. Similar effects could also be observed with rpsL mutations that confer resistance to paromomycin [90]. Spontaneous mutations in rpsL can impart resistance to high levels of streptomycin and are rare as they occur with a frequency of 10−9-10−10 [87]. Low-level resistance to streptomycin can be acquired about 1,000 times more frequently by other types of mutations, such as in the gene rsmG, which encodes a 16S RNA methyltransferase [91]. Such mutations can also lead to enhanced antibiotic production and, very interestingly, combination of the identified rpsL and rsmG mutations results in an additive increase in antibiotic yields [91].
Streptomycin tolerance mutations can have a beneficial effect in the production of other useful products apart from actinorhodin in different bacteria. For example, mutants of Streptomyces chattanoogensis could produce up to 26-fold more fredericamycin, while mutants of Streptomyces antibioticus and Streptomyces lavendulae were able to overproduce formycin and actinomycin [92]. Furthermore, streptomycin-tolerant mutants of B. subtilis can accumulate enhanced amounts of enzymes, such as amylases and proteases [93]. Mutations in other translational machinery components, e.g. the gene rpoB that encodes a subunit of the RNA polymerase, which confer resistance to antibiotics other than streptomycin, such as rifampicin and gentamicin, can also have enhancing effects in antibiotic production [94]. Very importantly, the presence of mutations of this type in a specific bacterium can lead to the production of antibiotics which are not normally produced by the particular organism [95] or even in the production of completely novel compounds with antibiotic activities [96]. Finally, mutations conferring resistance to streptomycin, rifampicin, and gentamicin can also impart other types of industrially relevant phenotypes, such as enhanced tolerance to organic solvents and chemicals, as exemplified by strains of Pseudomonas putida whose growth could be sustained in significantly enhanced concentrations of toluene, m-xylene, 4-hydroxybenzoate and others [97]. It should be mentioned that the mutations conferring resistance to the different antibiotics can be combined to provide synergistic effects in the desired phenotypes [98] and introduction of these mutations in industrial microbial strains can be as effective in improving desired phenotypes as in the laboratory organism in which they were originally identified [99].
3.6 Genome shuffling
In cases where strains with improved cellular phenotypes have been created by one of the techniques discussed above, whole-genome recombination or “shuffling” can be applied to generate hybrid organisms that combine the beneficial mutations in a synergistic manner to further improve the desired properties [100]. Genome shuffling is typically carried out by a technique termed protoplast fusion [101]. Protoplast fusion involves enzymic removal of the cell wall (e.g. with lysozyme in bacteria), exposure to osmotic stabilizers so as to maintain the protoplast structure intact, and the addition of fusogenic chemical agents, such as polyethylene glycol (PEG), that induce the formation of membrane fusions between the generated protoplasts. Alternatively to the addition of PEG, protoplast fusion can take place more efficiently by electrofusion, i.e. by exposure to low-strength electric fields. It has been shown that repeated rounds of genome shuffling can result in the rapid emergence of complex phenotypes, such as improved production of the antibiotic tylosin from Streptomyces fradiae [100], tolerance to low pH of an industrial strain of Lactobacillus [102], degradation of pentachlorophenol in Sphingobium chlorophenolicum [103], butanol tolerance in E. coli [104], production of ethanol in yeast [105] etc. However, genome shuffling in Gram-negative bacteria, such as E. coli, is rather inefficient [104, 106] and, thus, the technology needs to be improved before it can become one of widespread use.
Kao and co-workers have recently attempted to address this issue by employing the fertility factor plasmid F to generate a system of continuous “sexual” exchange of genetic material between cells of a normally asexual organism, such as E. coli [107]. In this system, E. coli cells were engineered to become chromosomal DNA donors by incorporating the F factor into their genome along with a pair of sequences that act as origins of transfer, while deletion of the genes traS and traT, which encode for the proteins TraS and TraT that mediate surface exclusion, increased significantly the frequency of mating and DNA transfer. Winkler and Kao demonstrated that the emergence of a complex phenotype which can arise from mutations in multiple genomic loci that have a small beneficial impact, such as resistance to chloramphenicol, can be accelerated significantly in this system [107]. An important advantage of this approach is that evolution can be carried out continuously in liquid culture, a feature that makes the process considerably easier than classical genome shuffling.
4. Outlook
Numerous studies have demonstrated that inverse metabolic engineering can be a very powerful approach for generating engineered strains with complex cellular properties. The availability of a variety of methods that allow the generation of large libraries of mutant organisms carrying different types of genetic profiles holds the potential to greatly expand the repertoire of phenotypes which can be accessed. Analysis of the generated variants will subsequently provide a better understanding of the cellular processes which are critical for the emergence of the desired properties. The acquired knowledge can then be used as a starting point for guiding targeted pathway modifications through classical metabolic engineering or for additional rounds of inverse metabolic engineering, until improvements reach a high enough level to become attractive for academic and industrial applications.
Acknowledgements
This work was supported by the research grant “Synergasia” 09ΣYN-21-1078 of the General Secretariat of Research and Technology – Greece.
Competing Interests
The authors have declared that no competing interests exist.
References
- 1.Bailey JE, Birnbaum S, Galazzo JL, Khosla C, Shanks JV (1990) Strategies and challenges in metabolic engineering. Ann N Y Acad Sci 589: 1–15 [DOI] [PubMed] [Google Scholar]
- 2.Papoutsakis ET (1984) Equations and calculations for fermentations of butyric acid bacteria. Biotechnol Bioeng 26: 174–187 [DOI] [PubMed] [Google Scholar]
- 3.Varma A, Palsson BO (1994) Metabolic Flux Balancing - Basic Concepts, Scientific and Practical Use. Bio-Technology 12: 994–998 [Google Scholar]
- 4.Jackel C, Kast P, Hilvert D (2008) Protein design by directed evolution. Annu Rev Biophys 37: 153–173 [DOI] [PubMed] [Google Scholar]
- 5.Dougherty MJ, Arnold FH (2009) Directed evolution: new parts and optimized function. Curr Opin Biotechnol 20: 486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ideker T, Galitski T, Hood L (2001) A new approach to decoding life: Systems biology. Annu Rev Genomics Hum Genet 2: 343–372 [DOI] [PubMed] [Google Scholar]
- 7.Andrianantoandro E, Basu S, Karig DK, Weiss R (2006) Synthetic biology: new engineering rules for an emerging discipline. Mol Syst Biol 2: 2006 0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, et al. (2010) Creation of a Bacterial Cell Controlled by a Chemically Synthesized Genome. Science 329: 52–56 [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Lee SY (2008) Towards systems metabolic engineering of microorganisms for amino acid production. Curr Opin Biotechnol 19: 454–460 [DOI] [PubMed] [Google Scholar]
- 10.Planson AG, Carbonell P, Grigoras I, Faulon JL (2011) Engineering antibiotic production and overcoming bacterial resistance. Biotechnol J 6: 812–825 [DOI] [PubMed] [Google Scholar]
- 11.Hawkins KM, Smolke CD (2008) Production of benzylisoquinoline alkaloids in Saccharomyces cerevisiae. Nat Chem Biol 4: 564–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, et al. (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440: 940–943 [DOI] [PubMed] [Google Scholar]
- 13.Khosla C (1997) Harnessing the biosynthetic potential of modular polyketide synthases. Chem Rev 97: 2577–2590 [DOI] [PubMed] [Google Scholar]
- 14.Atsumi S, Hanai T, Liao JC (2008) Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451: 86–U13 [DOI] [PubMed] [Google Scholar]
- 15.Peralta-Yahya PP, Zhang FZ, del Cardayre SB, Keasling JD (2012) Microbial engineering for the production of advanced biofuels. Nature 488: 320–328 [DOI] [PubMed] [Google Scholar]
- 16.Aldor IS, Keasling JD (2003) Process design for microbial plastic factories: metabolic engineering of polyhydroxyalkanoates. Curr Opin Biotechnol 14: 475–483 [DOI] [PubMed] [Google Scholar]
- 17.Bailey JE, Sburlati A, Hatzimanikatis V, Lee K, Renner WA, et al. (1996) Inverse metabolic engineering: A strategy for directed genetic engineering of useful phenotypes. Biotechnol Bioeng 52: 109–121 [DOI] [PubMed] [Google Scholar]
- 18.Yomano LP, York SW, Ingram LO (1998) Isolation and characterization of ethanol-tolerant mutants of Escherichia coli KO11 for fuel ethanol production. J Ind Microbiol Biotechnol 20: 132–138 [DOI] [PubMed] [Google Scholar]
- 19.Atsumi S, Wu TY, Machado IM, Huang WC, Chen PY, et al. (2010) Evolution, genomic analysis, and reconstruction of isobutanol tolerance in Escherichia coli. Mol Syst Biol 6: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blount ZD, Borland CZ, Lenski RE (2008) Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli. Proc Natl Acad Sci U S A 105: 7899–7906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Utrilla J, Licona-Cassani C, Marcellin E, Gosset G, Nielsen LK, et al. (2012) Engineering and adaptive evolution of Escherichia coli for d-lactate fermentation reveals GatC as a xylose transporter. Metab Eng 14: 469–476 [DOI] [PubMed] [Google Scholar]
- 22.Miroux B, Walker JE (1996) Over-production of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J Mol Biol 260: 289–298 [DOI] [PubMed] [Google Scholar]
- 23.Du C, Zhang Y, Li Y, Cao Z (2007) Novel redox potential-based screening strategy for rapid isolation of Klebsiella pneumoniae mutants with enhanced 1,3-propanediol-producing capability. Appl Environ Microbiol 73: 4515–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong KK, Vongsangnak W, Vemuri GN, Nielsen J (2011) Unravelling evolutionary strategies of yeast for improving galactose utilization through integrated systems level analysis. Proc Natl Acad Sci U S A 108: 12179–12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scalcinati G, Otero JM, Van Vleet JR, Jeffries TW, Olsson L, et al. (2012) Evolutionary engineering of Saccharomyces cerevisiae for efficient aerobic xylose consumption. FEMS Yeast Res 12: 582–597 [DOI] [PubMed] [Google Scholar]
- 26.Bro C, Nielsen J (2004) Impact of 'ome' analyses on inverse metabolic engineering. Metab Eng 6: 204–211 [DOI] [PubMed] [Google Scholar]
- 27.Smith KM, Liao JC (2011) An evolutionary strategy for isobutanol production strain development in Escherichia coli. Metab Eng 13: 674–681 [DOI] [PubMed] [Google Scholar]
- 28.Makino T, Skretas G, Kang TH, Georgiou G (2011) Comprehensive engineering of Escherichia coli for enhanced expression of IgG antibodies. Metab Eng 13: 241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massey-Gendel E, Zhao A, Boulting G, Kim HY, Balamotis MA, et al. (2009) Genetic selection system for improving recombinant membrane protein expression in E. coli. Protein Sci 18: 372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badarinarayana V, Estep PW 3rd, Shendure J, Edwards J, Tavazoie S, et al. (2001) Selection analyses of insertional mutants using subgenic-resolution arrays. Nat Biotechnol 19: 1060–1065 [DOI] [PubMed] [Google Scholar]
- 31.Alper H, Miyaoku K, Stephanopoulos G (2005) Construction of lycopene-overproducing E. coli strains by combining systematic and combinatorial gene knockout targets. Nat Biotechnol 23: 612–616 [DOI] [PubMed] [Google Scholar]
- 32.Skretas G, Georgiou G (2009) Genetic analysis of G protein-coupled receptor expression in Escherichia coli: inhibitory role of DnaJ on the membrane integration of the human central cannabinoid receptor. Biotechnol Bioeng 102: 357–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tannler S, Zamboni N, Kiraly C, Aymerich S, Sauer U (2008) Screening of Bacillus subtilis transposon mutants with altered riboflavin production. Metab Eng 10: 216–226 [DOI] [PubMed] [Google Scholar]
- 34.Tyo KE, Jin YS, Espinoza FA, Stephanopoulos G (2009) Identification of gene disruptions for increased poly-3-hydroxybutyrate accumulation in Synechocystis PCC 6803. Biotechnol Prog 25: 1236–1243 [DOI] [PubMed] [Google Scholar]
- 35.Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, et al. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2: 2006 0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, et al. (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285: 901–906 [DOI] [PubMed] [Google Scholar]
- 37.Ozaydin B, Burd H, Lee TS, Keasling JD (2012) Carotenoid-based phenotypic screen of the yeast deletion collection reveals new genes with roles in isoprenoid production. Metab Eng. [DOI] [PubMed] [Google Scholar]
- 38.Lee KS, Hong ME, Jung SC, Ha SJ, Yu BJ, et al. (2011) Improved galactose fermentation of Saccharomyces cerevisiae through inverse metabolic engineering. Biotechnol Bioeng 108: 621–631 [DOI] [PubMed] [Google Scholar]
- 39.Jin YS, Alper H, Yang YT, Stephanopoulos G (2005) Improvement of xylose uptake and ethanol production in recombinant Saccharomyces cerevisiae through an inverse metabolic engineering approach. Appl Environ Microbiol 71: 8249–8256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong ME, Lee KS, Yu BJ, Sung YJ, Park SM, et al. (2010) Identification of gene targets eliciting improved alcohol tolerance in Saccharomyces cerevisiae through inverse metabolic engineering. J Biotechnol 149: 52–59 [DOI] [PubMed] [Google Scholar]
- 41.Reyes LH, Almario MP, Kao KC (2011) Genomic library screens for genes involved in n-butanol tolerance in Escherichia coli. PLoS One 6: e17678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandoval NR, Mills TY, Zhang M, Gill RT (2011) Elucidating acetate tolerance in E. coli using a genome-wide approach. Metab Eng 13: 214–224 [DOI] [PubMed] [Google Scholar]
- 43.Jin YS, Stephanopoulos G (2007) Multi-dimensional gene target search for improving lycopene biosynthesis in Escherichia coli. Metab Eng 9: 337–347 [DOI] [PubMed] [Google Scholar]
- 44.Skretas G, Makino T, Varadarajan N, Pogson M, Georgiou G (2012) Multi-copy genes that enhance the yield of mammalian G protein-coupled receptors in Escherichia coli. Metab Eng 14: 591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borden JR, Jones SW, Indurthi D, Chen Y, Papoutsakis ET (2010) A genomic-library based discovery of a novel, possibly synthetic, acid-tolerance mechanism in Clostridium acetobutylicum involving non-coding RNAs and ribosomal RNA processing. Metab Eng 12: 268–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kitagawa M, Ara T, Arifuzzaman M, Ioka-Nakamichi T, Inamoto E,et al. (2005) Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res 12: 291–299 [DOI] [PubMed] [Google Scholar]
- 47.Hu Y, Rolfs A, Bhullar B, Murthy TV, Zhu C,et al. (2007) Approaching a complete repository of sequence-verified protein-encoding clones for Saccharomyces cerevisiae. Genome Res 17: 536–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soo VW, Hanson-Manful P, Patrick WM (2011) Artificial gene amplification reveals an abundance of promiscuous resistance determinants in Escherichia coli. Proc Natl Acad Sci U S A 108: 1484–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skretas G, Georgiou G (2010) Simple genetic selection protocol for isolation of overexpressed genes that enhance accumulation of membrane-integrated human G protein-coupled receptors in Escherichia coli. Appl Environ Microbiol 76: 5852–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chauhan NS, Ranjan R, Purohit HJ, Kalia VC, Sharma R (2009) Identification of genes conferring arsenic resistance to Escherichia coli from an effluent treatment plant sludge metagenomic library. FEMS Microbiol Ecol 67: 130–139 [DOI] [PubMed] [Google Scholar]
- 51.Nicolaou SA, Gaida SM, Papoutsakis ET (2011) Coexisting/Coexpressing Genomic Libraries (CoGeL) identify interactions among distantly located genetic loci for developing complex microbial phenotypes. Nucleic Acids Res 39: e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santos CN, Stephanopoulos G (2008) Combinatorial engineering of microbes for optimizing cellular phenotype. Curr Opin Chem Biol 12: 168–176 [DOI] [PubMed] [Google Scholar]
- 53.Lee DK, Seol W, Kim JS (2003) Custom DNA-binding proteins and artificial transcription factors. Curr Top Med Chem 3: 645–657 [DOI] [PubMed] [Google Scholar]
- 54.Joung JK, Ramm EI, Pabo CO (2000) A bacterial two-hybrid selection system for studying protein-DNA and protein-protein interactions. Proc Natl Acad Sci U S A 97: 7382–7387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dreier B, Fuller RP, Segal DJ, Lund CV, Blancafort P, et al. (2005) Development of zinc finger domains for recognition of the 5'-CNN-3' family DNA sequences and their use in the construction of artificial transcription factors. J Biol Chem 280: 35588–35597 [DOI] [PubMed] [Google Scholar]
- 56.Segal DJ, Beerli RR, Blancafort P, Dreier B, Effertz K, et al. (2003) Evaluation of a modular strategy for the construction of novel polydactyl zinc finger DNA-binding proteins. Biochemistry 42: 2137–2148 [DOI] [PubMed] [Google Scholar]
- 57.Park KS, Lee DK, Lee H, Lee Y, Jang YS, et al. (2003) Phenotypic alteration of eukaryotic cells using randomized libraries of artificial transcription factors. Nat Biotechnol 21: 1208–1214 [DOI] [PubMed] [Google Scholar]
- 58.Park KS, Jang YS, Lee H, Kim JS (2005) Phenotypic alteration and target gene identification using combinatorial libraries of zinc finger proteins in prokaryotic cells. J Bacteriol 187: 5496–5499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee JY, Yang KS, Jang SA, Sung BH, Kim SC (2011) Engineering butanol-tolerance in escherichia coli with artificial transcription factor libraries. Biotechnol Bioeng 108: 742–749 [DOI] [PubMed] [Google Scholar]
- 60.Lee JY, Sung BH, Yu BJ, Lee JH, Lee SH, et al. (2008) Phenotypic engineering by reprogramming gene transcription using novel artificial transcription factors in Escherichia coli. Nucleic Acids Res 36: e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alper H, Stephanopoulos G (2007) Global transcription machinery engineering: a new approach for improving cellular phenotype. Metab Eng 9: 258–267 [DOI] [PubMed] [Google Scholar]
- 62.Alper H, Moxley J, Nevoigt E, Fink GR, Stephanopoulos G (2006) Engineering yeast transcription machinery for improved ethanol tolerance and production. Science 314: 1565–1568 [DOI] [PubMed] [Google Scholar]
- 63.Gregory BD, Nickels BE, Darst SA, Hochschild A (2005) An altered-specificity DNA-binding mutant of Escherichia coli sigma70 facilitates the analysis of sigma70 function in vivo. Mol Microbiol 56: 1208–1219 [DOI] [PubMed] [Google Scholar]
- 64.Gardella T, Moyle H, Susskind MM (1989) A mutant Escherichia coli sigma 70 subunit of RNA polymerase with altered promoter specificity. J Mol Biol 206: 579–590 [DOI] [PubMed] [Google Scholar]
- 65.Siegele DA, Hu JC, Walter WA, Gross CA (1989) Altered promoter recognition by mutant forms of the sigma 70 subunit of Escherichia coli RNA polymerase. J Mol Biol 206: 591–603 [DOI] [PubMed] [Google Scholar]
- 66.Yu H, Tyo K, Alper H, Klein-Marcuschamer D, Stephanopoulos G (2008) A high-throughput screen for hyaluronic acid accumulation in recombinant Escherichia coli transformed by libraries of engineered sigma factors. Biotechnol Bioeng 101: 788–796 [DOI] [PubMed] [Google Scholar]
- 67.Klein-Marcuschamer D, Santos CN, Yu H, Stephanopoulos G (2009) Mutagenesis of the bacterial RNA polymerase alpha subunit for improvement of complex phenotypes. Appl Environ Microbiol 75: 2705–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein-Marcuschamer D, Stephanopoulos G (2008) Assessing the potential of mutational strategies to elicit new phenotypes in industrial strains. Proc Natl Acad Sci U S A 105: 2319–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, Chong H, Ching CB, Jiang R (2012) Random mutagenesis of global transcription factor cAMP receptor protein for improved osmotolerance. Biotechnol Bioeng 109: 1165–1172 [DOI] [PubMed] [Google Scholar]
- 70.Liu H, Liu K, Yan M, Xu L, Ouyang P (2011) gTME for improved adaptation of Saccharomyces cerevisiae to corn cob acid hydrolysate. Appl Biochem Biotechnol 164: 1150–1159 [DOI] [PubMed] [Google Scholar]
- 71.Klein-Marcuschamer D, Stephanopoulos G (2010) Method for designing and optimizing random-search libraries for strain improvement. Appl Environ Microbiol 76: 5541–5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang H, Chong H, Ching CB, Song H, Jiang R (2012) Engineering global transcription factor cyclic AMP receptor protein of Escherichia coli for improved 1-butanol tolerance. Appl Microbiol Biotechnol 94: 1107–1117 [DOI] [PubMed] [Google Scholar]
- 73.Chen T, Wang J, Yang R, Li J, Lin M, et al. (2011) Laboratory-evolved mutants of an exogenous global regulator, IrrE from Deinococcus radiodurans, enhance stress tolerances of Escherichia coli. PLoS One 6: e16228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu H, Yan M, Lai C, Xu L, Ouyang P (2010) gTME for improved xylose fermentation of Saccharomyces cerevisiae. Appl Biochem Biotechnol 160: 574–582 [DOI] [PubMed] [Google Scholar]
- 75.Hong SH, Lee J, Wood TK (2010) Engineering global regulator Hha of Escherichia coli to control biofilm dispersal. Microb Biotechnol 3: 717–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hong SH, Wang X, Wood TK (2010) Controlling biofilm formation, prophage excision and cell death by rewiring global regulator H-NS of Escherichia coli. Microb Biotechnol 3: 344–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Santos CN, Xiao W, Stephanopoulos G (2012) Rational, combinatorial, and genomic approaches for engineering L-tyrosine production in Escherichia coli. Proc Natl Acad Sci U S A 109: 13538–13543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drummond DA, Iverson BL, Georgiou G, Arnold FH (2005) Why high-error-rate random mutagenesis libraries are enriched in functional and improved proteins. J Mol Biol 350: 806–816 [DOI] [PubMed] [Google Scholar]
- 79.Wang HH, Isaacs FJ, Carr PA, Sun ZZ, Xu G, et al. (2009) Programming cells by multiplex genome engineering and accelerated evolution. Nature 460: 894–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Isaacs FJ, Carr PA, Wang HH, Lajoie MJ, Sterling B, et al. (2011) Precise manipulation of chromosomes in vivo enables genome-wide codon replacement. Science 333: 348–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Carr PA, Wang HH, Sterling B, Isaacs FJ, Lajoie MJ, et al. (2012) Enhanced multiplex genome engineering through co-operative oligonucleotide co-selection. Nucleic Acids Res 40: e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lajoie MJ, Gregg CJ, Mosberg JA, Washington GC, Church GM (2012) Manipulating replisome dynamics to enhance lambda Red-mediated multiplex genome engineering. Nucleic Acids Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mosberg JA, Gregg CJ, Lajoie MJ, Wang HH, Church GM (2012) Improving Lambda Red Genome Engineering in Escherichia coli via Rational Removal of Endogenous Nucleases. PLoS One 7: e44638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang HH, Kim H, Cong L, Jeong J, Bang D, et al. (2012) Genome-scale promoter engineering by coselection MAGE. Nat Methods 9: 591–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Warner JR, Reeder PJ, Karimpour-Fard A, Woodruff LB, Gill RT (2010) Rapid profiling of a microbial genome using mixtures of barcoded oligonucleotides. Nat Biotechnol. [DOI] [PubMed] [Google Scholar]
- 86.Sandoval NR, Kim JY, Glebes TY, Reeder PJ, Aucoin HR (2012) Strategy for directing combinatorial genome engineering in Escherichia coli. Proc Natl Acad Sci U S A 109: 10540–10545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ochi K (2007) From microbial differentiation to ribosome engineering. Biosci Biotechnol Biochem 71: 1373–1386 [DOI] [PubMed] [Google Scholar]
- 88.Shima J, Hesketh A, Okamoto S, Kawamoto S, Ochi K (1996) Induction of actinorhodin production by rpsL (encoding ribosomal protein S12) mutations that confer streptomycin resistance in Streptomyces lividans and Streptomyces coelicolor A3(2). J Bacteriol 178: 7276–7284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hesketh A, Ochi K (1997) A novel method for improving Streptomyces coelicolor A3(2) for production of actinorhodin by introduction of rpsL (encoding ribosomal protein S12) mutations conferring resistance to streptomycin. J Antibiot (Tokyo) 50: 532–535 [DOI] [PubMed] [Google Scholar]
- 90.Okamoto-Hosoya Y, Sato TA, Ochi K (2000) Resistance to paromomycin is conferred by rpsL mutations, accompanied by an enhanced antibiotic production in Streptomyces coelicolor A3(2). J Antibiot (Tokyo) 53: 1424–1427 [DOI] [PubMed] [Google Scholar]
- 91.Nishimura K, Hosaka T, Tokuyama S, Okamoto S, Ochi K (2007) Mutations in rsmG, encoding a 16S rRNA methyltransferase, result in low-level streptomycin resistance and antibiotic overproduction in Streptomyces coelicolor A3(2). J Bacteriol 189: 3876–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hosoya Y, Okamoto S, Muramatsu H, Ochi K (1998) Acquisition of certain streptomycin-resistant (str) mutations enhances antibiotic production in bacteria. Antimicrob Agents Chemother 42: 2041–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kurosawa K, Hosaka T, Tamehiro N, Inaoka T, Ochi K (2006) Improvement of alpha-amylase production by modulation of ribosomal component protein S12 in Bacillus subtilis 168. Appl Environ Microbiol 72: 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu H, Zhang Q, Ochi K (2002) Activation of antibiotic biosynthesis by specified mutations in the rpoB gene (encoding the RNA polymerase beta subunit) of Streptomyces lividans. J Bacteriol 184: 3984–3991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Inaoka T, Takahashi K, Yada H, Yoshida M, Ochi K (2004) RNA polymerase mutation activates the production of a dormant antibiotic 3,3'-neotrehalosadiamine via an autoinduction mechanism in Bacillus subtilis. J Biol Chem 279: 3885–3892 [DOI] [PubMed] [Google Scholar]
- 96.Hosaka T, Ohnishi-Kameyama M, Muramatsu H, Murakami K, Tsurumi Y, et al. (2009) Antibacterial discovery in actinomycetes strains with mutations in RNA polymerase or ribosomal protein S12. Nat Biotechnol 27: 462–464 [DOI] [PubMed] [Google Scholar]
- 97.Hosokawa K, Park NH, Inaoka T, Itoh Y, Ochi K (2002) Streptomycin-resistant (rpsL) or rifampicin-resistant (rpoB) mutation in Pseudomonas putida KH146-2 confers enhanced tolerance to organic chemicals. Environ Microbiol 4: 703–712 [DOI] [PubMed] [Google Scholar]
- 98.Hu H, Ochi K (2001) Novel approach for improving the productivity of antibiotic-producing strains by inducing combined resistant mutations. Appl Environ Microbiol 67: 1885–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tamehiro N, Hosaka T, Xu J, Hu H, Otake N, et al. (2003) Innovative approach for improvement of an antibiotic-overproducing industrial strain of Streptomyces albus. Appl Environ Microbiol 69: 6412–6417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang YX, Perry K, Vinci VA, Powell K, Stemmer WP, et al. (2002) Genome shuffling leads to rapid phenotypic improvement in bacteria. Nature 415: 644–646 [DOI] [PubMed] [Google Scholar]
- 101.Gokhale DV, Puntambekar US, Deobagkar DN (1993) Protoplast fusion: a tool for intergeneric gene transfer in bacteria. Biotechnol Adv 11: 199–217 [DOI] [PubMed] [Google Scholar]
- 102.Patnaik R, Louie S, Gavrilovic V, Perry K, Stemmer WP, et al. (2002) Genome shuffling of Lactobacillus for improved acid tolerance. Nat Biotechnol 20: 707–712 [DOI] [PubMed] [Google Scholar]
- 103.Dai MH, Copley SD (2004) Genome shuffling improves degradation of the anthropogenic pesticide pentachlorophenol by Sphingobium chlorophenolicum ATCC 39723. Applied and Environmental Microbiology 70: 2391–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Winkler J, Rehmann M, Kao KC (2010) Novel Escherichia coli hybrids with enhanced butanol tolerance. Biotechnol Lett 32: 915–920 [DOI] [PubMed] [Google Scholar]
- 105.Zhang W, Geng A (2012) Improved ethanol production by a xylose-fermenting recombinant yeast strain constructed through a modified genome shuffling method. Biotechnol Biofuels 5: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dai M, Ziesman S, Ratcliffe T, Gill RT, Copley SD (2005) Visualization of protoplast fusion and quantitation of recombination in fused protoplasts of auxotrophic strains of Escherichia coli. Metab Eng 7: 45–52 [DOI] [PubMed] [Google Scholar]
- 107.Winkler J, Kao KC (2012) Harnessing recombination to speed adaptive evolution in Escherichia coli. Metab Eng 14: 487–495 [DOI] [PubMed] [Google Scholar]
- 108.Lutz R, Bujard H (1997) Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res 25: 1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alper H, Stephanopoulos G (2007) Global transcription machinery engineering: a new approach for improving cellular phenotype. Metab Eng 9: 258–267 [DOI] [PubMed] [Google Scholar]
- 110.Dai M, Copley SD (2004) Genome shuffling improves degradation of the anthropogenic pesticide pentachlorophenol by Sphingobium chlorophenolicum ATCC 39723. Appl Environ Microbiol 70: 2391–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]