Abstract
Bioprocesses conducted under conditions with restricted O2 supply are increasingly exploited for the synthesis of reduced biochemicals using different biocatalysts. The model facultative aerobe Escherichia coli, the microbial cell factory par excellence, has elaborate sensing and signal transduction mechanisms that respond to the availability of electron acceptors and alternative carbon sources in the surrounding environment. In particular, the ArcBA and CreBC two-component signal transduction systems are largely responsible for the metabolic regulation of redox control in response to O2 availability and carbon source utilization, respectively. Significant advances in the understanding of the biochemical, genetic, and physiological duties of these regulatory systems have been achieved in recent years. This situation allowed to rationally-design novel engineering approaches that ensure optimal carbon and energy flows within central metabolism, as well as to manipulate redox homeostasis, in order to optimize the production of industrially-relevant metabolites. In particular, metabolic flux analysis provided new clues to understand the metabolic regulation mediated by the ArcBA and CreBC systems. Genetic manipulation of these regulators proved useful for designing microbial cells factories tailored for the synthesis of reduced biochemicals with added value, such as poly(3-hydroxybutyrate), under conditions with restricted O2 supply. This network-wide strategy is in contrast with traditional metabolic engineering approaches, that entail direct modification of the pathway(s) at stake, and opens new avenues for the targeted modulation of central catabolic pathways at the transcriptional level.
Keywords: Escherichia coli, ArcBA, CreBC, reduced biochemicals, redox homeostasis, polyhydroxyalkanoates, metabolic flux analysis
INTRODUCTION
Anoxic fermentation of different carbon sources is rapidly gaining momentum in biotechnological setups aimed at obtaining reduced biochemicals. Relevant examples in this sense include (but are certainly not limited to) the production of polyhydroxyalkanoates (PHAs) [1, 2], ethanol [3, 4], 1,3-propanediol (1,3-PDO) [5, 6], succinate [7], and D-lactate [8]. These reduced biochemicals possess great commercial interest, and are usually synthesized by different microorganisms through the activity of both native and heterologous pathways. In spite of the fact that several bacterial species are currently being used in biotechnological setups, E. coli remains as the microbial cell factory (MCF) par excellence, mainly because this well- characterized enterobacterium can easily and rapidly grow on cheap substrates and can be modified as desired through a broad variety of molecular tools. As fermentation technologies designed for the production of bulk bioproducts need further innovation to became economically and environmentally sound [9], there is an increasing interest in methodologies that optimize the production of biochemicals, such as those listed above, according to both market demands and bioprocess energy requirements. It has been proposed that the most probable avenue for future improvements of these strategies will rely on a combination of efficient fermentation processes and in the manipulation of the MCF metabolism employing metabolic engineering strategies.
Metabolic manipulations to enhance the synthesis of metabolic products include several approaches to increase the availability of substrates needed for its formation and/or to eliminate competing pathways that lead to the formation of by-products, which sometimes conduces to undesired phenotypes. An alternative strategy that has been scarcely exploited for the design and optimization of MCFs is the network-wide manipulation of metabolic fluxes by means of mutations in global regulators. In fact, and depending on environmental circumstances, this approach can outperform the more traditional metabolic engineering strategy based on the direct manipulation of the gene(s) involved in the pathway(s) of interest. In the present mini-review, we summarize the recent advances and current state on the use of redox and/or regulatory E. coli mutants as MCFs for the production of reduced biochemicals and recombinant proteins. In the first part, we present some general aspects of the microbial metabolism that are subjected to control by environmental conditions through the regulation exerted by two-component signal transduction systems, namely ArcBA and CreBC. The application of targeted mutants in these regulatory systems in processes aimed at the synthesis of PHAs, under conditions with restricted O2 supply, is then presented along with other examples of products with commercial value. Future directions for improvement of these redox MCFs are finally discussed under the light of synthetic biology, computer-aided modeling, and other in silico strategies.
REGULATION OF CENTRAL METABOLIC PATHWAYS IN E. coli BY ENVIRONMENTAL CONDITIONS: NEW LESSONS FOR AN OLD HISTORY
As metabolic engineering approaches become more and more complex in the pursuit of the ideal MCF for the production of reduced biochemicals, the need of a complete understanding of cell physiology and metabolic network operativity under micro-oxic and anoxic growth conditions also becomes apparent. Recent genome-wide models revealed new aspects of the regulation of these pathways, which have a deep impact in the energy and redox homeostasis of the cells and, consequently, on the strategies implemented in order to manipulate these traits. A brief summary of the core E. coli metabolism is presented below, and the different pathways for D-glucose utilization are depicted in Fig. 1 along with some key regulatory checkpoints within the native metabolic network.
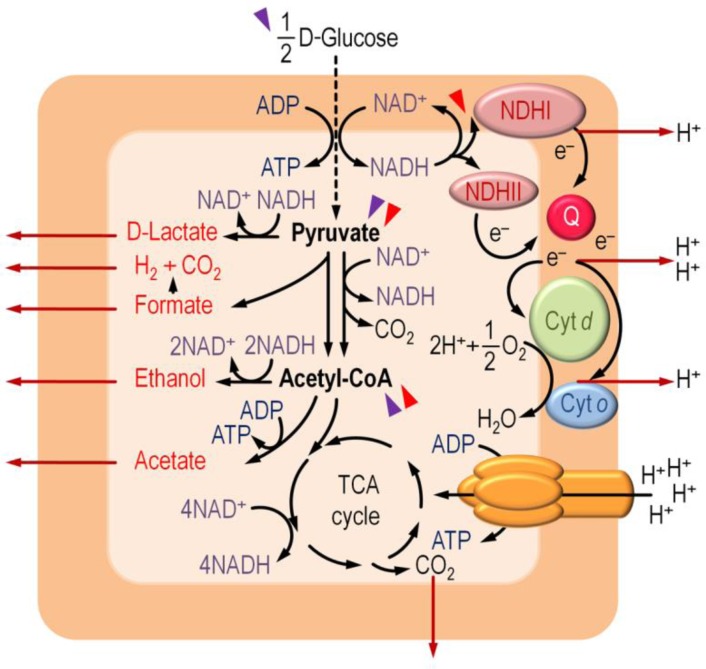
Figure 1.
Simplified representation of the oxic and anoxic pathways for D-glucose catabolism in E. coli. Oxic pathways are sketched to the right of the outline, and anoxic pathways are represented to the left along with the main fermentation metabolites formed (in red). The initial catabolic steps of D-glucose through the Embden-Meyerhof-Parnas pathway, which are independent of the presence of O2, are indicated by a dashed vertical arrow. Central metabolites relevant for the production of the reduced biochemicals discussed in the text are shown in boldface. The double arrow representing the conversion of pyruvate into acetyl-CoA illustrate the activity of either the pyruvate dehydrogenase complex (mostly under oxic conditions, right), or pyruvate-formate lyase (mostly under anoxic conditions, left). Secretion of fermentation metabolites and active H+ pumping are indicated by red arrows. Slanted arrowheads identify metabolic steps that are subjected to regulation by the ArcBA system (red) and/or the CreBC system (purple). Abbreviations are as follows: NDHI and NDHII, NADH:ubiquinone oxido-reductases I and II, respectively; Q, (ubi)quinone/(ubi)quinol; Cyt d and Cyt o; cytochromes d and o oxidases, respectively; CoA, coenzyme A; TCA cycle, tricarboxylic acid cycle. Nota bene, although the TCA cycle is depicted as an entirely oxic sequence of reactions in this scheme, the reductive branch, active under conditions with restricted O2 supply, produces succinate as a fermentation metabolite.
The initial steps in the catabolism of D-glucose, the preferred carbon source of E. coli and the substrate most often used in biotechnological setups, are independent of O2 availability. D-Glucose is split into the Embden-Meyerhof-Parnas (EMP) pathway and the pentose phosphate (PP) pathway at the D-glucose-6-P branching point [10]. The oxidation of D-glucose-6-P to D-ribose-5-P via the PP pathway generates reducing equivalents (i.e., NADPH) that are mostly used for biosynthesis and to quell stressful conditions. Degradation of D-glucose-6-P by the EMP pathway, in contrast, generates pyruvate, which under oxic conditions is decarboxylated by the pyruvate dehydrogenase complex to produce acetyl-coenzyme A (CoA), NADH, and CO2 [11, 12]. Oxidation of acetyl-CoA in the tricarboxylic acid (TCA) cycle generates electron carriers (i.e., NADH and FADH2), channeled into the electron-transport chain to finally reduce O2 to H2O, creating a H+ gradient used to generate ATP by means of ATP synthase. D-Glucose is completely oxidized to CO2 and H2O to conserve energy during oxic respiration, thus giving the maximum ATP yield.
When O2 is not available, E. coli undergoes anoxic respiration as long as an alternative electron acceptor is present in the surrounding environment [13]. Electron-transport chains of E. coli comprise dehydrogenases and terminal oxidases linked to a complex (ubi)quinone pool [14]. The presence of a particular electron acceptor under anoxic conditions results in the transcriptional activation of specific oxido-reductases and dehydrogenases and, concomitantly, in the repression of alternative oxido-reductases [14, 15]. This transcriptional control is exerted by global regulatory proteins (e.g., Fnr, ArcA, NarQ, NarX), which respond to changing levels of electron acceptors (O2 in the case of Fnr and ArcA, and nitrate in the case of NarQ and NarX) to maximize metabolic efficiency [10, 14–17], see also next section).
In the absence of any terminal acceptor, substrate-level phosphorylation is used for energy production and redox homeostasis is achieved by transferring electrons from reducing equivalents to an internal electron acceptor, such as pyruvate or acetyl-CoA [18]. Mixed acid fermentation [19, 20] thereby generates the overflow metabolites acetate, ethanol, formate, D-lactate, and succinate. Under anoxic conditions, pyruvate generated through the EMP pathway is processed by the radical enzyme pyruvate formate-lyase (PFL), leading to the production of acetyl-CoA and formate (with a lesser contribution of the mostly aerobic pyruvate-dehydrogenase complex [21, 22]). Expression of the pfl genes is controlled by several transcription factors [23–28], and PFL is mainly active in the absence of O2 [29], although it can contribute to acetyl-CoA formation under micro-oxic conditions [30, 31]. Formate produced by PFL can be converted to H2 and CO2 by formate-hydrogen lyase [32–34]. On the other hand, acetyl-CoA produced by PFL can be converted into ethanol or acetate. Reduction of acetyl-CoA to ethanol is catalyzed by an acetaldehyde/alcohol dehydrogenase (AdhE), and production of acetate from acetyl-CoA generates ATP by substrate-level phosphorylation [35]. This two-step reaction is catalyzed by the sequential action of the enzymes phosphotransacetylase (Pta) and acetate kinase (AckA), encoded by the ackA-pta operon. The metabolic fate of acetyl-CoA largely depends on the amount and type of the carbon source used and the availability of reducing equivalents. As mentioned above, minor amounts of D-lactate and succinate are also produced during mixed acid fermentation. D-Lactate is formed by the reduction of pyruvate by the NADH-dependent D-lactate dehydrogenase (LdhA) [36]. Succinate is produced by the reductive decarboxylation of P-enol-pyruvate to fumarate by fumarate reductase, as a result of the repression of the oxidative branch in the TCA cycle [37, 38]. The presence of alternative carbon sources in the culture medium also alters the pattern of metabolic pathways active within the cell. Although not extensively considered in the present mini-review, some interesting approaches designed for metabolic engineering applications involve the use of glycerol (see next sections), pentoses, and short-chain fatty acids [39–43].
All the above mentioned metabolic pathways are ultimately regulated by the availability of O2, and the delicate redox and energy balances are tightly controlled at several levels. On top of them all, transcriptional regulation constitutes the predominant mechanism whereby the activity of the relevant pathway(s) is temporally and spatially coordinated in order to meet homeostasis. The next section describes the main patterns of global regulation that ultimately ensure the network-wide adjustment of metabolic pathway activity.
REGULATORY TASKS OF THE ArcBA AND CreBC TWO-COMPONENT SYSTEMS ON THE CENTRAL METABOLIC PATHWAYS OF E. coli
Signal transduction pathways are involved in intercellular interactions and communication of environmental conditions to the interior of the cell. The final outcome of such a signaling pathway is the activation of specific transcription factor(s) that, in turn, control(s) gene expression. Regulation of gene expression is a very complex process, and transcriptional regulators can be subdivided in global and local regulators depending on the number of operons (i.e., regulons) they control. Global regulators control the expression of a vast number of genes, which might be physically scattered along the genome and normally belong to different functional clusters. According to the data available in the EcoCyc database [44], E. coli K12 strain MG1655 contains forty master (i.e., global) regulators and σ factors. Perhaps surprisingly, only seven global regulators control the expression of ca. half of all genes; i.e., ArcA, Crp, Fis, Fnr, Ihf, Lrp, and NarL [45]. In contrast to these global regulators, local regulators control the expression of only a few genes, and ca. one-fifth of all the transcriptional regulators so far described control the expression of just one or two genes.
O2 functions as an electron acceptor and substrate for catabolism in a wide variety of bacteria, and most facultative anaerobe microorganisms are able to thrive across a wide range of O2 availability conditions. As hyperoxic conditions may produce adverse effects in bacteria by inducing oxidative stress, micro-aerobic and facultative anaerobic bacteria have evolved elaborate sensing and signal transduction mechanisms in order to adapt their metabolism in response to O2 availability [46]. As explained before, the choice of energy generation pathways is determined by the accessibility to electron acceptors. Sophisticated and often interrelated regulatory networks switch the expression of these pathways on and off as needed [13, 15]. In the hierarchical regulation system for energy transduction, adaptive responses to O2 are mainly coordinated by the Fnr and ArcA global regulators [47]. The transcriptional regulation exerted by Fnr is mostly related to strict anoxic conditions, and its effects have been reviewed elsewhere [48, 49].
The ArcBA (anoxic redox control) two-component signal transduction system modulates at the transcriptional level the expression of many operons according to the redox state of the environment [50–53]. ArcB is a transmembrane sensor kinase which under anoxic or micro-oxic conditions undergoes stable phosphorylation and then transphosphorylates the response regulator ArcA. The main targets for repression by ArcA∼P are the genes that encode enzymes involved in oxic respiration. On the other hand, the cytochrome d oxidase, with high O2 affinity, and genes encoding fermentation enzymes such as PFL, are activated under anoxic conditions. The effects of ArcA∼P on the transcription patterns has been analyzed at the whole-genome level, and it was shown that many other genes, far beyond those directly involved in redox metabolism, are putative targets for ArcA regulation. In fact, it was found that ca. 150 operons involved in energy metabolism, nutrients transport, bacterial survival, catabolism of diverse carbon sources, and transcriptional regulation, were included in the Arc modulon. Later on, statistical analysis of genome-wide DNA microarrays led to the striking conclusion that the transcription of at least 1,139 genes of E. coli could be either directly or indirectly regulated by ArcA∼P [54].
CreBC (carbon source responsive) is also a global sensing and regulation system responsive to both the carbon source and the O2 availability [55]. The cre locus comprises creABCD and was formerly known as phoM locus. While creA is a hypothetical open reading frame and creD, also known as cet, encodes an inner-membrane protein of unknown function; creB and creC encode a two-component system, i.e., a cytoplasmic regulator and a sensor kinase, respectively. Following the discovery of CreBC and its recognition as a sensing/regulatory pair, genes modulated bona fide by this system proved very elusive. Based on genome-wide sequence analysis and using bioinformatic tools, Avison et al. [55] were able to define a so-called cre tag sequence, to which CreB is known to bind in vitro, in order to describe the Cre regulon. So far, the Cre regulon comprises seven genes activated by CreBC (among them, ackA-pta, talA, and creD) and one repressed (malE), and the expression pattern of these genes responds to the nature of the carbon source used to grow the cells and the O2 availability. Indeed, expression of genes modulated by CreBC is activated under fermentative growth conditions using glycolytic carbon sources, as well as under oxic conditions with low-molecular-weight fermentation products as the substrate, such as formate or pyruvate. Yet, very little is known about this system and the extent of its regulation on the central metabolism of E. coli.
The intracellular distribution of metabolites (and, consequently, the phenotypic traits of the cell) is regulated at several levels. Transcriptional regulation is thought to be the principal type of regulation in bacteria, yet the way in which it controls metabolic fluxes is not well understood. Several studies have recently tried to clarify the correlation between metabolic networks and transcriptional control by means of metabolic flux analysis in global regulatory mutants. A breakthrough in fluxome quantification strategies was the use of 13C-substrates, which proceed through the entire metabolic network and propagate their labeling pattern into the pools of downstream metabolites, thus allowing to calculate the flux(es) through the pathway(s) at stake [56–59]. Since ArcA has been identified as a major global regulator, several works were dedicated to unravel its role in controlling the E. coli central metabolism. One of them dealt with different global regulatory mutants of E. coli grown on D-glucose batch cultures [60]. This study provided novel insights into the regulation brought about by the ArcBA system, demonstrating that, unexpectedly, the control of fully oxic and anoxic fluxes through the TCA cycle was exerted by ArcA in an ArcB-independent fashion. 13C-labeling experiments were also performed in ∆arcA and ∆fnr mutants in D-glucose − limited chemostat cultures [61]. These mutations were shown to affect the flux distribution in the core metabolic pathways, mainly through the availability of reducing equivalents.
As both ArcBA and CreBC regulate the expression of genes encoding enzymes of the central catabolic pathways (see Supplementary Table S1 for details), the next relevant question was whether there is a shared control of metabolism by the two systems. 13C-Based metabolic flux analysis conducted for a wild-type E. coli strain and its isogenic ∆creB, ∆arcA, and ∆creB ∆arcA derivatives in D-glucose- and O2-limited chemostat experiments demonstrated that both ArcBA and CreBC exert an important influence on central carbon catabolic fluxes [62]. In particular, it was observed that both the P-enol-pyruvate and the acetyl-CoA metabolic nodes are subjected to regulation by CreB and ArcA, strongly influencing the fate of the carbon atoms at these branching points. Interestingly, the first steps on D-glucose catabolism did not seem to be affected, and most of the carbon source was channeled through the EMP pathway without evident differences between the wild-type strain and its mutant derivatives.
At this point, it is relevant to consider that the genotype-phenotype relationships in regulatory mutants are usually very complex, and the deletion of global regulators may have an impact beyond the redox and catabolic traits of the cells. The combined approaches discussed in this section for the analysis of regulatory mutants are relevant since deletion of global regulatory genes is expected to affect the entire cellular and metabolic landscape in a rather difficult-to-predict fashion. Moreover, possible cross-talk mechanisms between these two-component systems [63, 64] could also contribute to the complex biochemical signalization in the resulting MCFs, and modulation of the Arc and/or Cre signalization by different levels of fermentation metabolites [65] cannot be ruled out. One way or the other, the results of the studies discussed above underscored the potential of arc and cre mutants as MCFs, and they were utilized for the production of different reduced biochemicals, as detailed in the next sections.
BACTERIAL POLYHYDROXYALKANOATES
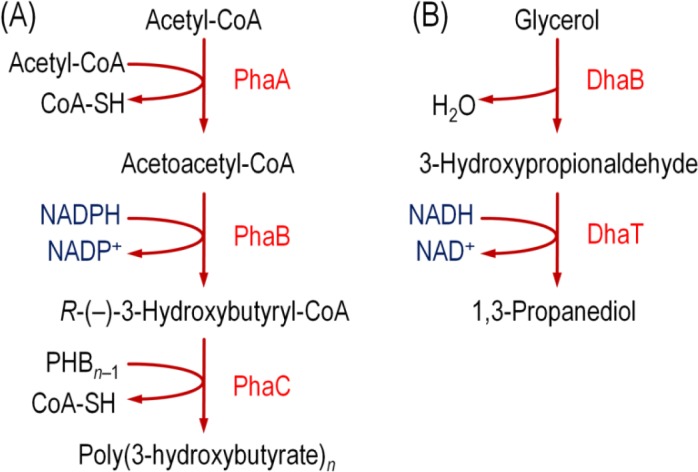
PHAs are synthesized naturally by a wide variety of bacterial species as a reserve material for carbon and energy [2, 66]. These ubiquitous polymers attract increasing industrial interest as renewable, biodegradable, biocompatible, and extremely versatile thermoplastics [2]. In fact, PHAs are the only H2O-proof thermoplastic materials which are also fully biodegradable in both oxic and anoxic environments. Two classes of PHAs are distinguished according to their monomer composition: short-chain length (SCL) PHAs, and medium-chain length (MCL) PHAs. SCL-PHAs are composed of 3-hydroxyacid monomers with a chain length of three to five carbon atoms, such as poly(3-hydroxybutyrate) (PHB, the most common and widely distributed PHA in nature); whereas MCL-PHAs contain 3-hydroxyacid monomers with six to sixteen carbon atoms. All of these polymers are optically active R-(—)-compounds, and give rise to isotactic carbon chains. The structural versatility of PHAs is partly due to the wide substrate range of the polymer-synthesizing enzymes, and it endows PHAs with an extended spectrum of mechanical and physical properties, a clear advantage vis-à-vis to other bioplastics. More than 200 different monomer constituents have been found so far in these polymers [67]. PHB is synthesized from acetyl-CoA in a three-step pathway (Fig. 2A), that uses NADPH as cofactor.
Figure 2.
Biosynthetic pathways of two model reduced biochemicals, poly(3-hydroxybutyrate) and 1,3-propanediol. Biochemical steps that consume reducing equivalents are highlighted in blue. Abbreviations are as follows: CoA, coenzyme A; PhaA, 3-ketoacyl-CoA thiolase; PhaB, acetoacetyl-CoA reductase; PhaC, poly(3-hydroxyalkanoate) synthase; DhaB, glycerol dehydratase; DhaT, 1,3-propanediol oxidoreductase. In most of the E. coli recombinants described in the text, the phaBAC genes were obtained from Azotobacter sp. strain FA8 [70].
The design and development of efficient MCFs for PHA production received much attention in the last few decades. Although different microorganisms have been exploited for polymer production, E. coli is most often used as the biocatalyst [68] as it does not accumulate PHAs naturally, thus offering the flexibility to manipulate both native and heterologous pathways for PHA synthesis. Current high-yield bioprocesses for the synthesis of PHAs require fully oxic conditions, meaning that they are high energy-consuming processes. The environmental impact of replacing oil-derived plastics with biopolymers has been the subject of several studies, among them, those regarding technical-scale PHB production in bioreactors [69]. The main conclusion of these studies is that when the total amount of energy invested is taken into consideration [i.e., the energy needed for sterilization, aeration, and agitation (both in the bioreactor and downstream processing), as well as the energy for the production of agricultural feed-stocks used as carbon sources], the environmental performance of PHAs equals that of petrochemical polymers. This situation clearly calls for alternative bioprocesses and biocatalysts to meet the technical and environmental requirements of a sound production process.
Metabolic networks are the most obvious targets for rational design of sustainable PHA production processes. As stated above, E. coli arc mutants are unregulated for respiration under micro-oxic conditions. Some enzymes of the TCA cycle are not repressed and the pool of reducing equivalents is elevated, and thus available to be funneled into reduced biochemicals. The PHB biosynthetic genes from Azotobacter sp. strain FA8 [70] were introduced in a ΔarcA strain, and the polymer accumulation was evaluated under micro-oxic conditions in a semi-synthetic medium containing D-glucose as the carbon source. A PHB content of ca. 35% (w/w) was attained in such a redox mutant, while the wild-type strain failed to produce PHB under the same culture conditions [71]. Within the same conceptual framework, PHA synthesis was evaluated in E. coli CT1061, an arcA and creC constitutive mutant. The latter mutation confers enhanced carbon source consumption, while retaining the markedly reducing intracellular environment typical of arc mutants (with a NADH/NAD+ ratio of ca. 1 mol/mol) [72]. E. coli CT1061 actually outperformed cre and arc individual mutants as a redox MCF. Increased PHB yields on substrate were observed in D-glucose- or glycerol-supplemented semi-synthetic media [73], especially in micro-oxic fed-batch cultures with glycerol supplementation. Being the main by-product of the biodiesel industry, glycerol became a cheap substrate for bioprocesses in the last few years. Since C atoms are in a more reduced state than those in hexoses, the use of glycerol as a biotechnological substrate is especially attractive in bioprocesses designed to produce reduced biochemicals [74, 75]. The micro-oxic fed-batch cultures afore mentioned allowed a 2.6-fold increase in the PHB volumetric productivity when compared to batch cultures. After 60 h, biomass and PHB concentrations reached 21.17 and 10.81 g/L, respectively, resulting in a PHB content of 51% (w/w) [76]. Ethanol was co-produced as a valuable by-product of this process [77], making it even more interesting in terms of sustainability, as two industrially-relevant reduced biochemicals were obtained from glycerol, both intracellularly (PHB) and extracellularly (ethanol).
The main goal of a successful heterologous expression system based on plasmids is to achieve the highest tolerable protein or metabolite yield and thus to walk the thin line between high levels of heterologous gene expression and the metabolic capacity of the host. An unexpected (and most welcome) trait of MCFs bearing arc and cre mutations is an enhanced ability to maintain plasmids that encode the biosynthetic genes for reduced biochemicals (e.g., ethanol and PHB), even in the absence of the selective pressure imposed by addition of antibiotics [78]. This side effect was shown to be mostly related to the capacity enabled by the plasmid-encoded bioreactions to regenerate NAD(P)+, needed to continue cell growth.
Another target of global regulation exploited for the heterologous accumulation of PHB in E. coli is the AtoSC two-component system, which regulates the catabolism of short-chain fatty acids. Theodorou et al. [79] recently demonstrated that PHB synthesis from D-glucose is impaired in a ΔatoSC and ΔatoDAEB mutant, and that this phenotype can be restored by ectopic expression of Ato components in the recombinants. The authors hinted an important role of the AtoSC system in polymer accumulation and also proposed that this regulatory system could be an important target for metabolic engineering manipulations. In a former study, Pettinari et al. [80] proposed the use of a transcriptional activator of the ato genes from Bacillus megaterium as an elegant strategy to obtain different types of PHAs in recombinant E. coli MCFs.
A different approach was the use of anoxic promoters to drive the transcription of the pha genes from Cupriavidus necator in E. coli recombinants under conditions with restricted O2 supply [81]. Among the nine native promoters tested, PadhE was the most effective in promoting micro-oxic PHB synthesis. The same research group recently reported the construction of an E. coli recombinant that carries the pha genes from C. necator, as well as the genes encoding hydrogenase 3 and/or acetyl-CoA synthetase [82]. The resulting strain had the advantage of co-producing PHB and H2 under micro-oxic conditions in a synthetic medium containing D-glucose and acetate as the carbon sources.
1,3-PROPANEDIOL
1,3-PDO is used in many synthetic reactions, particularly as a monomer for condensations to produce polyesters, polyethers, and polyurethanes [83]. Polymers made with this compound are biodegradable and have enhanced chemical properties, such as higher light stability and solubility [6]. 1,3-PDO is primarily produced through chemical synthesis from petroleum derivatives (acrolein and ethylene) in processes that involve high pressure and temperature, but it can also be obtained by microbial fermentation, a more environmentally-favorable process that has lower costs, especially considering its production from glycerol, the abundant by-product of the biodiesel synthesis [84]. 1,3-PDO was originally identified by August Freund in a glycerol fermentation process with a mixed culture containing Clostridium pasteurianum [85], and was later found to be produced by the fermentation of glycerol by many Gram-positive and Gram-negative bacteria, including Citrobacter, Clostridium, Enterobacter, Klebsiella, and Lactobacillus species [86]. As the anoxic growth on glycerol generates an excess of reducing equivalents, it causes a redox imbalance. In order to meet redox homeostasis, cells synthesize products that serve as an electron sink, such as 1,3-PDO. This molecule is synthesized in two steps: [i] dehydrogenation of glycerol to 3-hydroxypropionaldehyde, and [ii] an NADH-dependent reduction of this intermediate to 1,3-PDO, that is secreted into the medium [86, 87] (Fig. 2B). In microorganisms that produce 1,3-PDO naturally, the enzymes involved in glycerol metabolism are a glycerol dehydratase, a 1,3-PDO oxidoreductase, a glycerol dehydrogenase, and a dihydroxyacetone phosphate kinase. These enzymes are encoded in the dha operon, which has been characterized in K. pneumoniae, C. freundii, and C. butyricum [6].
Many natural 1,3-PDO producers are not suitable for industrial production because of their particular culture conditions and, in the case of pathogens, because special safety precautions are required [5]. For these reasons, there has been recent interest in converting glycerol to 1,3-PDO by recombinant E. coli strains. Such MCFs also permit the study of the effects of different mutations in genes involved in metabolism and global regulators in order to improve the synthesis of 1,3-PDO. Several research groups have reconstructed the 1,3-PDO pathway in E. coli, normally containing genes from K. pneumoniae or C. butyricum [88, 89]. The highest 1,3-PDO production yield with glycerol as the sole carbon source reported so far was in a recombinant E. coli strain expressing dhaB1 and dhaB2 from C. butyricum, which encode the vitamin B12-independent glycerol dehydratase DhaB1 and its activating factor, DhaB2, respectively, tandemly arrayed with yqhD from E. coli, which encodes the 1,3-PDO oxidoreductase isoenzyme YqhD, an NADPH-dependent dehydrogenase [90].
Generally, it can be considered that availability of NADH is one of the main factors limiting the production of 1,3-PDO [5]. Several metabolic manipulations have been performed in natural producers to optimize the synthesis of 1,3-PDO. For example, in K. pneumoniae the reductive glycerol pathway has been enhanced, and the oxidative glycerol pathway or competing metabolite pathways have been eliminated, increasing 1,3-PDO yields [84]. Another strategy involves the manipulation of cofactor availability. In K. oxytoca, this has been achieved by introducing a formate dehydrogenase from Candida boidinii to regenerate NADH [89]. On the other hand, manipulation of redox metabolism in E. coli can be achieved using mutations redox regulators, such as ArcA, to enhance the synthesis of reduced metabolites [91]. This strategy has also been applied to increase the availability of reducing equivalents for the synthesis of 1,3-PDO in E. coli recombinants containing multiple genetic modifications [92].
RECOMBINANT PROTEIN PRODUCTION
Several E. coli strains have been examined as potential MCFs to produce recombinant proteins at high titres [93], most often in high-cell-density cultures. A comprehensive overview of the main E. coli strains used in recombinant protein production processes and their characteristics has been recently published by Waegeman and Soetaert [94]. Although E. coli B and E. coli K12 are equally used as hosts for recombinant protein production (ca. 47% and 53%, respectively), E. coli BL21 is by far the most commonly used MCF (ca. 35%) in academia [95, 96]. This figure is probably even higher in industrial setups.
Acetate formation in E. coli cultures grown under either oxic or micro-oxic/anoxic conditions still represents a major problem in the industrial application of this microorganism [35, 97, 98]. As hinted before, acetate is mostly produced when respiratory pathways are saturated and NADH can no longer be re-oxidized [99]. This, in turn, causes a shunt in the main catabolic pathways by re-directing carbon precursors into acetate (the synthesis of which does not generate NADH, in contrast with the four molecules of NADH generated by each turn of the TCA cycle, Fig. 1), and thus allowing to achieve redox homeostasis. A plethora of different strategies have been devised to increase recombinant protein formation and to decrease acetate formation, including optimization of the bioprocess conditions as well as metabolic engineering of production hosts [100]. These attempts can be categorized in three main classes: [i] deleting genes involved in acetate formation, [ii] avoiding overflow metabolism by limiting D-glucose uptake via alteration of the degree of oxidation of the carbon source, applying alternative feeding strategies, or by engineering the D-glucose uptake system, and [iii] circumventing overflow metabolism by re-directing central metabolic fluxes and preserving sufficient precursors for amino acids synthesis, the actual building blocks of proteins.
Acetate has been postulated to be a potential signaling molecule in the activation of the Arc system [65], adding a further level of complexity to the ArcBA-mediated metabolic responses in E. coli. Vemuri et al. [101] reasoned that if cells are provided with an efficient mechanism to reduce high NADH/NAD+ ratios (that result in production of acetate) while preventing the Arc-dependent repression of the TCA cycle, additional carbon skeletons would be available for both biomass generation and recombinant protein production. The authors constructed an E. coli arcA mutant that overexpresses a NADH oxidase from Streptococcus pneumoniae [101]. Using steady-state, D-glucose − limited chemostat cultures, they were able to expose a strong correlation between acetate formation and the intracellular redox ratio [102]. Moreover, delay of acetate overflow in the engineered strain allowed to attain a titer of the model recombinant protein β-galactosidase ca. 2-fold higher than that of the wild-type strain.
Recent studies also allowed to explain a particular phenotype of the industrially-relevant E. coli BL21. By comparing the biomass yields and 13C-metabolic flux analysis of a ΔarcA ΔiclR double knock-out constructed in two genetic backgrounds (E. coli MG1655 and BL21) grown either under D-glucose-excess or D-glucose-limited conditions, it turned out that the metabolic similarity between both strains arise from mutations that affect both arcA expression and the promoter region of the gene encoding IclR, a local regulator which controls the transcription of the glyoxylate pathway genes within the aceBAK operon [103, 104]. These redox MCFs proved adequate for industrial purposes, as isocitrate was directly converted into succinate and malate, preventing carbon loss as CO2 and considerably diminishing the synthesis of acetate.
OTHER METABOLIC ENGINEERING STRATEGIES FOR THE MANIPULATION OF THE REDOX HOMEOSTASIS
Although several strategies and approaches complementary to those discussed in this mini-review have been explored for the manipulation of the redox state in MCFs designed for reduced biochemicals production, some of them deserve special attention because of their relevance. A recent study by Bidart et al. [105] exploited the possibility of using partial deletions in the ArcB regulator in order to obtain graded phenotypes. This constituted a first-case study on the effects of confined deletions in a global regulator (as opposed to the elimination of the entire gene) for metabolic engineering endeavors. A gradual impact of these deletions on the distribution of metabolic fluxes was observed under anoxic growth conditions, and most of the changes could be traced to the redox state of the cells. The incremental differences observed both in the redox homeostasis and central carbon fluxes among the mutants make them potentially attractive MCFs for biotechnological purposes.
Other studies dealt with the direct manipulation of the redox state of the cell by stimulating high NAD(P)H/NAD(P)+ ratios. For instance, Zhou et al. [106] manipulated the transcriptional control of the genes encoding the components of the pyruvate dehydrogenase complex by using promoters of the pfl genes. Doubling of the reducing power output was achieved when D-glucose was converted into acetyl-CoA through the EMP pathway followed by oxidation of pyruvate by the anoxically-active pyruvate dehydrogenase complex (i.e., D-glucose → 2acetyl-CoA + 4NADH). Martínez et al. [107] constructed a recombinant E. coli strain by replacing gapA, encoding the native NAD+-dependent glyceraldehyde-3-phosphate dehydrogenase, with gapC from Clostridium acetobutylicum, which encodes a NADP+-dependent glyceraldehyde-3-phosphate dehydrogenase. The recombinant produced 2 moles of NADPH, instead of NADH, per mole of D-glucose consumed. The effectiveness of the NADPH enhancing system was analyzed in the recombinant MCFs by evaluating the production of lycopene and ɛ-caprolactone, the synthesis of which consumes NADPH, as model systems. The authors elegantly demonstrated that the synthesis of both heterologous molecules was favored in the recombinant MCFs when compared to the parental strain. Another interesting approach reported by Zhu et al. [108] showed that controlled respiration levels in E. coli can be exploited to allow wide operating windows in terms of O2 availability, thus allowing for the formation of reduced bioproducts even under fully oxic conditions. The authors added different amounts of coenzyme Q1 (an ubiquinone analogue) to oxic cultures of ΔubiCA mutant strains (i.e., lacking the critical enzymes involved in ubiquinone synthesis). In this case, the target metabolite was ethanol, using glycerol as the carbon source. The recombinant MCF, carrying the pdc and adhB genes from Zymomonas mobilis, produced high ethanol titers under conditions in which the original strain was not able to synthesize any reduced by-product derived from the oxic glycerol catabolism.
SUMMARY AND OUTLOOK
From the data presented in the preceding sections, it is clear that the relative lack of knowledge on the cellular wiring of regulatory networks under conditions relevant to both laboratory and industrial applications represents one of the most significant hurdles to be overcome for the efficient design of MCFs. However, a suite of different strategies that could offer interesting alternatives to tackle this issue has emerged in recent years.
In the first place, genome-scale and specific metabolic models are starting to play an important role in deciphering the cellular and metabolic characteristics of microbial cells in silico [109, 110]. The number of genome-scale metabolic models is rapidly increasing and their quality is also improving. Strategies devised to incorporate experimental data, such as high-throughput -omics data, have also been developed to enhance the quality and the accuracy of metabolic models. In close connection with these in silico approaches, synthetic biology is rapidly emerging as a relevant field for the rational manipulation of MCFs. Synthetic biology can be defined as the engineering of biology, i.e., the synthesis of complex, biologically-based (or inspired) systems, which could display functions that do not exist in nature. The possibility of developing an entirely synthetic host for efficient target-molecule production presents great opportunities for further research. In this sense, synthetic biology can significantly advance metabolic engineering by both contributing tools (e.g., minimal genome hosts, vectors, genetic controllers, and characterized enzymes), and by aiding potential interventions to metabolism at one or many of the following levels: [i] enhancement in the rate of substrate uptake, [ii] reduction of flux(es) to undesirable by-products, and enhancement of precursors and cofactor availability, [iii] introduction of relevant heterologous pathway(s) and optimization of the activity of its constituent enzymes, and [iv] export of the product to the extracellular medium in order to shift the concentration equilibrium towards product formation.
This exciting and extremely dynamic scenario will certainly lead to the development of better strategies to manipulate central and peripheral pathways to enhance the production of reduced biochemicals and other molecules of industrial interest.
Supplementary Material
ACKNOWLEDGEMENTS
The work described in this mini-review was defrayed by generous grants from the Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET), the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT), and the University of Buenos Aires (UBACyT Programme). J.A.R., A.D.A., B.S.M., M.J.P., and P.I.N. are researchers from CONICET; and M.S.G. and M.P.M. are beneficiaries of graduate student fellowships from CONICET.
Abbreviations
MCF, microbial cell factory; PHAs, polyhydroxyalkanoates; PHB, poly(3-hydroxybutyrate); 1,3-PDO, 1,3-propanediol; EMP, Embden-Meyerhof-Parnas; PP, pentose phosphate; TCA cycle, tricarboxylic acid cycle; CoA, coenzyme A; PFL, pyruvate-formate lyase
Competing Interests
The authors have declared that no competing interests exist.
References
- 1.Verlinden RAJ, Hill DJ, Kenward MA, Williams CD, Radecka I (2007) Bacterial synthesis of biodegradable polyhydroxyalkanoates. J Appl Microbiol 102: 1437–1449 [DOI] [PubMed] [Google Scholar]
- 2.Gomez JGC, Méndez BS, Nikel PI, Pettinari MJ, Prieto MA, et al. (2012) Making green polymers even greener: towards sustainable production of polyhydroxyalkanoates from agroindustrial by-products In: Petre M, editor. Advances in applied biotechnology. Rijeka, Croatia: InTech; pp. 41–62 [Google Scholar]
- 3.Clomburg JM, Gonzalez R (2010) Biofuel production in Escherichia coli: the role of metabolic engineering and synthetic biology. Appl Microbiol Biotechnol 86: 419–434 [DOI] [PubMed] [Google Scholar]
- 4.Geddes CC, Nieves IU, Ingram LO (2011) Advances in ethanol production. Curr Opin Biotechnol 22: 312–319 [DOI] [PubMed] [Google Scholar]
- 5.Biebl H (1999) Microbial production of 1,3-propanediol. Appl Microbiol Biotechnol 52: 289–297 [DOI] [PubMed] [Google Scholar]
- 6.Kaur G, Srivastava AK, Chand S (2012) Advances in biotechnological production of 1,3-propanediol. Biochem Eng J 64: 106–118 [Google Scholar]
- 7.Thakker C, Martínez I, San KY, Bennett GN (2012) Succinate production in Escherichia coli . Biotechnol J 7: 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okano K, Tanaka T, Ogino C, Fukuda H, Kondo A (2010) Biotechnological production of enantiomeric pure lactic acid from renewable resources: recent achievements, perspectives, and limits. Appl Microbiol Biotechnol 85: 413–423 [DOI] [PubMed] [Google Scholar]
- 9.Vickers CE, Klein-Marcuschamer D, Krömer JO (2012) Examining the feasibility of bulk commodity production in Escherichia coli . Biotechnol Lett 34: 585–596 [DOI] [PubMed] [Google Scholar]
- 10.Gottschalk G (1986) Bacterial metabolism Springer Verlag, New York [Google Scholar]
- 11.Guest JR, Angier SJ, Russell GC (1989) Structure, expression, and protein engineering of the pyruvate dehydrogenase complex of Escherichia coli . Ann N Y Acad Sci 573: 76–99 [DOI] [PubMed] [Google Scholar]
- 12.Mattevi A, Obmolova G, Schulze E, Kalk KH, Westphal AHet al. (1992) Atomic structure of the cubic core of the pyruvate dehydrogenase multienzyme complex. Science 255: 1544–1550 [DOI] [PubMed] [Google Scholar]
- 13.Patschkowski T, Bates DN, Kiley PJ (2000) Mechanisms for sensing and responding to oxygen deprivation In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, D.C.: ASM Press; pp. 61–78 [Google Scholar]
- 14.Unden G, Bongaerts J (1997) Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim Biophys Acta 1320: 217–234 [DOI] [PubMed] [Google Scholar]
- 15.Gunsalus RP (1992) Control of electron flow in Escherichia coli: coordinated transcription of respiratory pathway genes. J Bacteriol 174: 7069–7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavicchioli R, Chiang RC, Kalman LV, Gunsalus RP (1996) Role of the periplasmic domain of the Escherichia coli NarX sensor-transmitter protein in nitrate-dependent signal transduction and gene regulation. Mol Microbiol 21: 901–911 [DOI] [PubMed] [Google Scholar]
- 17.Iuchi S, Weiner L (1996) Cellular and molecular physiology of Escherichia coli in the adaptation to aerobic environments. J Biochem 120: 1055–1063 [DOI] [PubMed] [Google Scholar]
- 18.Becker S, Vlad D, Schuster S, Pfeiffer P, Unden G (1997) Regulatory O2 tensions for the synthesis of fermentation products in Escherichia coli and relation to aerobic respiration. Arch Microbiol 168: 290–296 [DOI] [PubMed] [Google Scholar]
- 19.Clark DP (1989) The fermentation pathways of Escherichia coli . FEMS Microbiol Rev 5: 223–234 [DOI] [PubMed] [Google Scholar]
- 20.Böck A, Sawers G (1996) Fermentation In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology, vol. 1. Washington, D.C.: ASM Press; pp. 262–282 [Google Scholar]
- 21.de Graef MR, Alexeeva S, Snoep JL, Teixeira de Mattos MJ (1999) The steady-state internal redox state (NADH/NAD) reflects the external redox state and is correlated with catabolic adaptation in Escherichia coli . J Bacteriol 181: 2351–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murarka A, Clomburg JM, Moran S, Shanks JV, Gonzalez R(2010) Metabolic analysis of wild-type Escherichia coli and a pyruvate dehydrogenase complex (PDHC)-deficient derivative reveals the role of PDHC in the fermentative metabolism of glucose. J Biol Chem 285: 31548–31558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawers G, Böck A (1988) Anaerobic regulation of pyruvate formate-lyase from Escherichia coli K-12. J Bacteriol 170: 5330–5336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawers G, Wagner AFV, Böck A (1989) Transcription initiation at multiple promoters of the pfl gene by Eσ70-dependent transcription in vitro and heterologous expression in Pseudomonas putida in vivo . J Bacteriol 171: 4930–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawers G, Suppmann B (1992) Anaerobic induction of pyruvate formate-lyase gene expression is mediated by the ArcA and FNR proteins. J Bacteriol 174: 3474–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawers G (1993) Specific transcriptional requirements for positive regulation of the anaerobically inducible pfl operon by ArcA and FNR. Mol Microbiol 10: 737–747 [DOI] [PubMed] [Google Scholar]
- 27.Sirko A, Zehelein E, Freundlich M, Sawers G (1993) Integration host factor is required for anaerobic pyruvate induction of pfl operon expression in Escherichia coli . J Bacteriol 175: 5769–5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaiser M, Sawers G (1995) Nitrate repression of the Escherichia coli pfl operon is mediated by the dual sensors NarQ and NarX and the dual regulators NarL and NarP. J Bacteriol 177: 3647–3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner AFV, Frey M, Neugebauer FA, Schäfer W, Knappe J (1992) The free radical in pyruvate formate-lyase is located on glycine-734. Proc Natl Acad Sci USA 89: 996–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexeeva S, de Kort B, Sawers G, Hellingwerf KJ, Teixeira de Mattos MJ (2000) Effects of limited aeration and of the ArcAB system on intermediary pyruvate catabolism in Escherichia coli . J Bacteriol 182: 4934–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shalel-Levanon S, San KY, Bennett GN (2005) Effect of ArcA and FNR on the expression of genes related to the oxygen regulation and the glycolysis pathway in Escherichia coli under microaerobic growth conditions. Biotechnol Bioeng 92: 147–159 [DOI] [PubMed] [Google Scholar]
- 32.Birkmann A, Zinoni F, Sawers G, Böck A (1987) Factors affecting transcriptional regulation of the formate-hydrogen-lyase pathway of Escherichia coli . Arch Microbiol 148: 44–51 [DOI] [PubMed] [Google Scholar]
- 33.Rossmann R, Sawers G, Böck A (1991) Mechanism of regulation of the formate-hydrogen lyase pathway by oxygen, nitrate, and pH: definition of the formate regulon. Mol Microbiol 5: 2807–2814 [DOI] [PubMed] [Google Scholar]
- 34.Suppmann B, Sawers G (1994) Isolation and characterization of hypophosphite-resistant mutants of Escherichia coli: identification of the FocA protein, encoded by the pfl operon, as a putative formate transporter. Mol Microbiol 11: 965–982 [DOI] [PubMed] [Google Scholar]
- 35.Dittrich CR, Bennett GN, San KY (2005) Characterization of the acetate-producing pathways in Escherichia coli . Biotechnol Prog 21: 1062–1067 [DOI] [PubMed] [Google Scholar]
- 36.Garvie EI (1980) Bacterial lactate dehydrogenases. Microbiol Rev 44: 106–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashworth JM, Kornberg HL (1966) The anaplerotic fixation of carbon dioxide by Escherichia coli . Proc R Soc Lond B Biol Sci 165: 179–188 [DOI] [PubMed] [Google Scholar]
- 38.Cronan JE, LaPorte D (1996) Tricarboxylic acid cycle and glyoxylate bypass In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology, vol. 1. Washington, D.C.: ASM Press; pp. 206–216 [Google Scholar]
- 39.Dellomonaco C, Rivera C, Campbell P, Gonzalez R (2010) Engineered respiro-fermentative metabolism for the production of biofuels and biochemicals from fatty acid-rich feedstocks. Appl Environ Microbiol 76: 5067–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JH, Block DE, Mills DA (2010) Simultaneous consumption of pentose and hexose sugars: an optimal microbial phenotype for efficient fermentation of lignocellulosic biomass. Appl Microbiol Biotechnol 88: 1077–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dellomonaco C, Clomburg JM, Miller EN, Gonzalez R (2011) Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals. Nature 476: 355–359 [DOI] [PubMed] [Google Scholar]
- 42.Jang YS, Kim B, Shin JH, Choi YJ, Choi Set al. (2012) Bio-based production of C2-C6 platform chemicals. Biotechnol Bioeng 109: 2437–2459 [DOI] [PubMed] [Google Scholar]
- 43.Lee JW, Na D, Park JM, Lee J, Choi Set al. (2012) Systems metabolic engineering of microorganisms for natural and non-natural chemicals. Nat Chem Biol 8: 536–546 [DOI] [PubMed] [Google Scholar]
- 44.Keseler IM, Collado-Vides J, Santos-Zavaleta A, Peralta-Gil M, Gama-Castro Set al. (2011) EcoCyc: a comprehensive database of Escherichia coli biology. Nucleic Acids Res 39: D583–D590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martínez-Antonio A, Collado-Vives J (2003) Identifying global regulators in transcriptional regulatory networks in bacteria. Curr Opin Microbiol 6: 482–489 [DOI] [PubMed] [Google Scholar]
- 46.Unden G, Becker S, Bongaerts J, Holighaus G, Schirawski Jet al. (1995) O2-sensing and O2-dependent gene regulation in facultatively anaerobic bacteria. Arch Microbiol 164: 81–90 [PubMed] [Google Scholar]
- 47.Green J, Paget MS (2004) Bacterial redox sensors. Nat Rev Microbiol 2: 954–966 [DOI] [PubMed] [Google Scholar]
- 48.Kiley PJ, Beinert H (1998) Oxygen sensing by the global regulator, FNR: the role of the iron-sulfur cluster. FEMS Microbiol Rev 22: 341–352 [DOI] [PubMed] [Google Scholar]
- 49.Unden G, Achebach S, Holighaus G, Tran HQ, Wackwitz Bet al. (2002) Control of FNR function of Escherichia coli by O2 and reducing conditions. J Mol Microbiol Biotechnol 4: 263–268 [PubMed] [Google Scholar]
- 50.Iuchi S, Lin ECC (1988) arcA (dye), a global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc Natl Acad Sci USA 85: 1888–1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lynch AS, Lin ECC (1996) Responses to molecular oxygen In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology, vol. 1. Washington, D.C.: ASM Press; pp. 1526–1538 [Google Scholar]
- 52.Alexeeva S, Hellingwerf KJ, Teixeira de Mattos MJ (2003) Requirement of ArcA for redox regulation in Escherichia coli under microaerobic but not anaerobic or aerobic conditions. J Bacteriol 185: 204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shalel-Levanon S, San KY, Bennett GN (2005) Effect of oxygen on the Escherichia coli ArcA and FNR regulation systems and metabolic responses. Biotechnol Bioeng 89: 556–564 [DOI] [PubMed] [Google Scholar]
- 54.Salmon KA, Hung SP, Steffen NR, Krupp R, Baldi Pet al. (2005) Global gene expression profiling in Escherichia coli K12: effects of oxygen availability and ArcA. J Biol Chem 280: 15084–15096 [DOI] [PubMed] [Google Scholar]
- 55.Avison MB, Horton RE, Walsh TR, Bennett PM (2001) Escherichia coli CreBC is a global regulator of gene expression that responds to growth in minimal media. J Biol Chem 276: 26955–26961 [DOI] [PubMed] [Google Scholar]
- 56.Wiechert W (2001) 13C metabolic flux analysis. Metab Eng 3: 195–206 [DOI] [PubMed] [Google Scholar]
- 57.Sauer U (2004) High-throughput phenomics: experimental methods for mapping fluxomes. Curr Opin Biotechnol 15: 58–63 [DOI] [PubMed] [Google Scholar]
- 58.Shimizu K (2004) Metabolic flux analysis based on 13C-labeling experiments and integration of the information with gene and protein expression patterns. Adv Biochem Eng Biotechnol 91: 1–49 [DOI] [PubMed] [Google Scholar]
- 59.Zamboni N, Sauer U (2009) Novel biological insights through metabolomics and 13C-flux analysis. Curr Opin Microbiol 12: 553–558 [DOI] [PubMed] [Google Scholar]
- 60.Perrenoud A, Sauer U (2005) Impact of global transcriptional regulation by ArcA, ArcB, Cra, Crp, Cya, Fnr, and Mlc on glucose catabolism in Escherichia coli . J Bacteriol 187: 3171–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu J, Shalel-Levanon S, Bennett GN, San KY (2006) Effect of the global redox sensing/regulation networks on Escherichia coli and metabolic flux distribution based on C-13 labeling experiments. Metab Eng 8: 619–627 [DOI] [PubMed] [Google Scholar]
- 62.Nikel PI, Zhu J, San KY, Méndez BS, Bennett GN (2009) Metabolic flux analysis of Escherichia coli creB and arcA mutants reveals shared control of carbon catabolism under microaerobic growth conditions. J Bacteriol 191: 5538–5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamamoto K, Hirao K, Oshima T, Aiba H, Utsumi Ret al. (2005) Functional characterization in vitro of all two-component signal transduction systems from Escherichia coli . J Biol Chem 280: 1448–1456 [DOI] [PubMed] [Google Scholar]
- 64.Groban ES, Clarke EJ, Salis HM, Miller SM, Voigt CA (2009) Kinetic buffering of cross talk between bacterial two-component sensors. J Mol Biol 390: 380–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rolfe MD, Ter Beek A, Graham AI, Trotter EW, Shahzad Asif HMet al. (2011) Transcript profiling and inference of Escherichia coli K-12 ArcA activity across the range of physiologically relevant oxygen concentrations. J Biol Chem 286: 10147–10154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role, and industrial uses of bacterial polyhydroxyalkanoates. Microbiol Rev 54: 450–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steinbüchel A, Valentin HE (1995) Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol Lett 128: 219–228 [Google Scholar]
- 68.Keshavarz T, Roy I (2010) Polyhydroxyalkanoates: bioplastics with a green agenda. Curr Opin Microbiol 13: 321–326 [DOI] [PubMed] [Google Scholar]
- 69.Harding KG, Dennis JS, von Blottnitz H, Harrison STL (2007) Environmental analysis of plastic production processes: comparing petroleum-based polypropylene and polyethylene with biologically based poly-β-hydroxybutyric acid using life cycle analysis. J Biotechnol 130: 57–66 [DOI] [PubMed] [Google Scholar]
- 70.Pettinari MJ, Vázquez GJ, Silberschmidt D, Rehm B, Steinbüchel Aet al. (2001) Poly(3-hydroxybutyrate) synthesis genes in Azotobacter sp. strain FA8. Appl Environ Microbiol 67: 5331–5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nikel PI, Pettinari MJ, Galvagno MA, Méndez BS (2006) Poly(3-hydroxybutyrate) synthesis by recombinant Escherichia coli arcA mutants in microaerobiosis. Appl Environ Microbiol 72: 2614–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nikel PI, Pettinari MJ, Ramirez MC, Galvagno MA, Méndez BS (2008) Escherichia coli arcA mutants: metabolic profile characterization of microaerobic cultures using glycerol as a carbon source. J Mol Microbiol Biotechnol 15: 48–54 [DOI] [PubMed] [Google Scholar]
- 73.Nikel PI, de Almeida A, Giordano AM, Pettinari MJ (2010) Redox driven metabolic tuning: carbon source and aeration affect synthesis of poly(3-hydroxybutyrate) in Escherichia coli . Bioeng Bugs 1: 291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dobson R, Gray V, Rumbold K (2012) Microbial utilization of crude glycerol for the production of value-added products. J Ind Microbiol Biotechnol 39: 217–226 [DOI] [PubMed] [Google Scholar]
- 75.Pettinari MJ, Mezzina MP, Méndez BS, Godoy MS, Nikel PI (2012) Glycerol as a substrate for bioprocesses in different O2 availability conditions In: De Santos Silva M, Costa Ferreira P editors. Glycerol: production, structure and applications. Hauppauge, N.Y.: Nova Science Publishers; pp. 139–156 [Google Scholar]
- 76.Nikel PI, Pettinari MJ, Galvagno MA, Méndez BS (2008) Poly(3-hydroxybutyrate) synthesis from glycerol by a recombinant Escherichia coli arcA mutant in fed-batch microaerobic cultures. Appl Microbiol Biotechnol 77: 1337–1343 [DOI] [PubMed] [Google Scholar]
- 77.Nikel PI, Ramirez MC, Pettinari MJ, Méndez BS, Galvagno MA (2010) Ethanol synthesis from glycerol by Escherichia coli redox mutants expressing adhE from Leuconostoc mesenteroides . J Appl Microbiol 109: 492–504 [DOI] [PubMed] [Google Scholar]
- 78.Nikel PI, Pettinari MJ, Galvagno MA, Méndez BS (2010) Metabolic selective pressure stabilizes plasmids carrying biosynthetic genes for reduced biochemicals in Escherichia coli redox mutants. Appl Microbiol Biotechnol 88: 563–573 [DOI] [PubMed] [Google Scholar]
- 79.Theodorou EC, Theodorou MC, Kyriakidis DA (2012) Involvement of the AtoSCDAEB regulon in the high molecular weight poly-(R)-3-hydroxybutyrate biosynthesis in phaCAB+ Escherichia coli . Metab Eng 14: 354–365 [DOI] [PubMed] [Google Scholar]
- 80.Pettinari MJ, Vázquez GJ, Krüger N, Vary PS, Steinbüchel Aet al. (1998) trans activation of the Escherichia coli ato structural genes by a regulatory protein from Bacillus megaterium: potential use in polyhydroxyalkanoate production. Appl Microbiol Biotechnol 49: 737–742 [DOI] [PubMed] [Google Scholar]
- 81.Wei XX, Shi ZY, Yuan MQ, Chen GQ (2009) Effect of anaerobic promoters on the microaerobic production of polyhydroxybutyrate (PHB) in recombinant Escherichia coli . Appl Microbiol Biotechnol 82: 703–712 [DOI] [PubMed] [Google Scholar]
- 82.Wang RY, Shi ZY, Chen JC, Wu Q, Chen GQ (2012) Enhanced co-production of hydrogen and poly-(R)-3-hydroxybutyrate by recombinant PHB producing E. coli over-expressing hydrogenase 3 and acetyl-CoA synthetase. Metab Eng 14: 496–503 [DOI] [PubMed] [Google Scholar]
- 83.Zeng AP, Biebl H (2002) Bulk chemicals from biotechnology: the case of 1,3-propanediol production and the new trends. Adv Biochem Eng Biotechnol 74: 239–259 [DOI] [PubMed] [Google Scholar]
- 84.Maervoet VET, De Mey M, Beauprez J, De Maeseneire S, Soetaert WK (2011) Enhancing the microbial conversion of glycerol to 1,3-propanediol using metabolic engineering. Org Process Res Dev 15: 189–202 [Google Scholar]
- 85.Freund A (1881) Über die Bildung und Darstellung von Trimethylenalkohol aus Glycerin. Monatsheft Für Chimie 2: 636–641 [Google Scholar]
- 86.Nakamura CE, Whited GM (2003) Metabolic engineering for the microbial production of 1,3-propanediol. Curr Opin Biotechnol 14: 454–459 [DOI] [PubMed] [Google Scholar]
- 87.Saxena RK, Anand P, Saran S, Isar J (2009) Microbial production of 1,3-propanediol: recent developments and emerging opportunities. Biotechnol Adv 27: 895–913 [DOI] [PubMed] [Google Scholar]
- 88.Altaras NE (1999) Metabolic engineering of a 1,2-propanediol pathway in Escherichia coli . Appl Environ Microbiol 65: 1180–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y, Huang Z, Du C, Li Y, Cao Z (2009) Introduction of an NADH regeneration system into Klebsiella oxytoca leads to an enhanced oxidative and reductive metabolism of glycerol. Metab Eng 11: 101–106 [DOI] [PubMed] [Google Scholar]
- 90.Tang X, Tan Y, Zhu H, Zhao K, Shen W (2009) Microbial conversion of glycerol to 1,3-propanediol by an engineered strain of Escherichia coli . Appl Environ Microbiol 75: 1628–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pettinari MJ, Nikel PI, Ruiz JA, Méndez BS (2008) ArcA redox mutants as a source of reduced bioproducts. J Mol Microbiol Biotechnol 15: 41–47 [DOI] [PubMed] [Google Scholar]
- 92.Cervin MA, Soucaille P, Valle F (2010) Process for the biological production of 1,3-propanediol with high yield. USA Patent US7745184. [Google Scholar]
- 93.Jana S, Deb JK (2005) Strategies for efficient production of heterologous proteins in Escherichia coli . Appl Microbiol Biotechnol 67: 289–298 [DOI] [PubMed] [Google Scholar]
- 94.Waegeman H, Soetaert W (2011) Increasing recombinant protein production in Escherichia coli through metabolic and genetic engineering. J Ind Microbiol Biotechnol 38: 1891–1910 [DOI] [PubMed] [Google Scholar]
- 95.Sevastsyanovich YR, Alfasi SN, Cole JA (2010) Sense and nonsense from a systems biology approach to microbial recombinant protein production. Biotechnol Appl Biochem 55: 9–28 [DOI] [PubMed] [Google Scholar]
- 96.Gonçalves GAL, Bower DM, Prazeres DMF, Monteiro GA, Prather KLJ (2012) Rational engineering of Escherichia coli strains for plasmid biopharmaceutical manufacturing. Biotechnol J 7: 251–261 [DOI] [PubMed] [Google Scholar]
- 97.Luli GW, Strohl WR (1990) Comparison of growth, acetate production, and acetate inhibition of Escherichia coli strains in batch and fed-batch fermentations. Appl Environ Microbiol 56: 1004–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.El-Mansi M (2004) Flux to acetate and lactate excretions in industrial fermentations: physiological and biochemical implications. J Ind Microbiol Biotechnol 31: 295–300 [DOI] [PubMed] [Google Scholar]
- 99.Andersen KB, von Meyenburg K (1980) Are growth rates of Escherichia coli in batch cultures limited by respiration?. J Bacteriol 144: 114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.De Mey M, De Maeseneire S, Soetaert W, Vandamme E (2007) Minimizing acetate formation in E. coli fermentations. J Ind Microbiol Biotechnol 34: 689–700 [DOI] [PubMed] [Google Scholar]
- 101.Vemuri GN, Eiteman MA, Altman E (2006) Increased recombinant protein production in Escherichia coli strains with overexpressed water-forming NADH oxidase and a deleted ArcA regulatory protein. Biotechnol Bioeng 94: 538–542 [DOI] [PubMed] [Google Scholar]
- 102.Vemuri GN, Altman E, Sangurdekar DP, Khodursky AB, Eiteman MA (2006) Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redox ratio. Appl Environ Microbiol 72: 3653–3661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Waegeman H, Beauprez J, Moens H, Maertens J, De Mey Met al. (2011) Effect of iclR and arcA knockouts on biomass formation and metabolic fluxes in Escherichia coli K12 and its implications on understanding the metabolism of Escherichia coli BL21 (DE3). BMC Microbiol 11: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Waegeman H, Maertens J, Beauprez J, De Mey M, Soetaert W (2011) Effect of iclR and arcA deletions on physiology and metabolic fluxes in Escherichia coli BL21 (DE3). Biotechnol Lett 34: 329–337 [DOI] [PubMed] [Google Scholar]
- 105.Bidart GN, Ruiz JA, de Almeida A, Méndez BS, Nikel PI (2012) Manipulation of the anoxic metabolism in Escherichia coli by ArcB deletion variants in the ArcBA two-component system. Appl Environ Microbiol 78: 8784–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou S, Iverson AG, Grayburn WS (2010) Doubling the catabolic reducing power (NADH) output of Escherichia coli fermentation for production of reduced products. Biotechnol Prog 26: 45–51 [DOI] [PubMed] [Google Scholar]
- 107.Martínez I, Zhu J, Lin H, Bennett GN, San KY (2008) Replacing Escherichia coli NAD-dependent glyceraldehyde 3-phosphate dehydrogenase (GAPDH) with a NADP-dependent enzyme from Clostridium acetobutylicum facilitates NADPH dependent pathways. Metab Eng 10: 352–359 [DOI] [PubMed] [Google Scholar]
- 108.Zhu J, Sánchez A, Bennett GN, San KY (2011) Manipulating respiratory levels in Escherichia coli for aerobic formation of reduced chemical products. Metab Eng 13: 704–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Herrgård M, Panagiotou G (2012) Analyzing the genomic variation of microbial cell factories in the era of “New Biotechnology”. Comput Struct Biotechnol J 3: e201210012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jouhten PT (2012) Metabolic modelling in the development of cell factories by synthetic biology. Comput Struct Biotechnol J 3: e201210009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.