Abstract
Reduced expression of the Indy (I'm Not Dead Yet) gene in D. melanogaster and C. elegans extends longevity. Indy and its mammalian homolog mINDY (Slc13a5, NaCT) are transporters of TCA cycle intermediates, mainly handling the uptake of citrate via the plasma membrane into the cytosol. Deletion of mINDY in mice leads to significant metabolic changes akin to caloric restriction, likely caused by reducing the effects of mINDY-imported citrate on fatty acid and cholesterol synthesis, glucose metabolism and ß-oxidation. This review will provide an overview on different mammalian SLC1 3 family members with a focus on mINDY (SLCl3A5) in glucose and energy metabolism and will highlight the role of mINDY as a putative therapeutic target for the treatment of obesity, non-alcoholic fatty liver disease and type 2 diabetes.
Keywords: INDY, citrate, insulin resistance, diabetes, obesity, longevity
Introduction
In D. melanogaster and C. elegans reduced expression of the non-electrogenic dicarboxylate and citrate transporter Indy (Acronym for I′m Not Dead Yet) promotes longevity in a manner akin to caloric restriction, one of the most reliable interventions to prolong life span over a wide range of species [1, 2]. In mammals, SLC13A5 encodes the Na+-coupled citrate transporter NaCT (we will use the alternative name mINDY throughout the review), which shares the highest sequence and functional similarity with D. melanogaster Indy.
mINDY-/- knockout mice are protected from diet induced obesity and insulin resistance that go along with excess caloric intake and aging [3]. The effect is mediated by a profound action of mINDY on mitochondrial metabolism in mice. Therefore, mINDY might serve as a therapeutic target for the treatment of obesity and type-2 diabetes. The purpose of this review is to summarize the role of mINDY in mammalian glucose and energy metabolism and describe the most recent advances on structure, expression, function and regulation of the protein.
The SLC13A family – an overview
The SLC13A family of Na+-coupled di- and tri-carboxylate/sulfate transporters comprises five genes, namely SLC13A1-5, encoding multi-spanning transporters with 8-13 transmembrane α-helices flanked by an intracellular N-terminus and an extracellular C-terminus, containing putative consensus glycosylation sites [4]. Orthologs of all five genes are found in prokaryotes and eukaryotes. All SLC13A family members contain numerous predicted consensus phosphorylation and N-myristyoylation sites for which the functional significance is unknown so far. Moreover, a highly conserved consensus sequence motif, the “sodium:sulfate symporter family signature’’, is found in each of the five family members [5].
The mammalian SLC13A Na+-coupled anion cotransporters are located in the plasma membrane of epithelial cells with ubiquitous expression, but mainly in liver, kidney, small intestine, placenta and cells of the central nervous system. They all play a variety of different physiological and pathophysiological roles in the different organs. SLC13A transporters mediate the Na+-coupled anion substrate movement across the plasma membrane of cells and are electrogenic with a general Na+:substrate ratio of 3:1.
SLC13A family members are divided in two functional groups: the Na+-sulfate cotransporters (NaS) mainly transporting sulfate, selenate and thiosulfate and the Na+- di- and tri-carboxylate cotransporters (NaDC) carrying Krebs-cycle intermediates such as succinate, citrate and α-ketoglutarate. While the SLC13A1 and SLC13A4 genes belong to the NaS, the SLC13A2, SLC13A3 and SLC13A5 genes represent the NaDC group.
SLC13A1 (also NaS1 or NaSi-1) is localized to the apical brush border membrane of the renal proximal tubules and intestinal epithelial cells [6–9]. SLC13A1 functions as an electrogenic pH-sensitive high affinity Na+-dependent SO-2 4 transporter with substrate preferences for the anions sulfate, thiosulfate, selenate and the cation Na+ [10, 11]. The human SLC13A1 transporter can be inhibited by molybdate, selenate, tungstate, selenate, succinate and citrate [6]. SLC13A1 deficient mice revealed numerous pathophysiological features, such as hyposulfatemia, hypersulfaturia, reduced body weight, postnatal growth and fertility, reduced circulating steroid levels, increased urinary glucocorticoid excretion and altered lipid and cholesterol metabolism within the liver [12–19]. The loss of SLC13A1 clearly shows its importance in maintaining sulfate homeostasis.
SLC13A2 is localized in epithelia with high metabolic needs specifically on the apical membrane of renal proximal tubular and small intestine cells where it reabsorbs intermediates of the tricarboxylic acid cycle like succinate, α-ketoglutarate and citrate. The activity and cell surface expression of SLC13A2 is dependent on its regulation by PKC-dependent, direct-phosphorylation independent pathways including the serum and glucocorticoid inducible kinases SGK1 and 3, PKB kinase and the NHE regulating factor 2 (NHERF2) [20, 21]. By taking up tricarboxylic acid (TCA) cycle intermediates into cells across the apical membrane, SLC13A2 plays an important role in oxidative metabolism. SLC13A2-/- mice are characterized by increased urinary excretion of diverse dicarboxylates [22].
The main function of SLC13A2 is renal handling of citrate and therefore important in the formation of kidney stones and nephrolithiasis [20, 23]. Since the SGK1 signaling pathway, contributing to the regulation of renal function and arterial blood pressure, activates SLC13A2, the protein may also be important in the regulation of blood pressure, in addition to its role in water re-absorption [24].
SLC13A3 is conserved over a wide range of species and has been detected in zebrafish, xenophus frog, mouse and human [25]. SLC13A3 is expressed in liver, brain, kidney, placenta, pancreas, eye and optic nerve and is located on the apical membrane of placenta and synaptosomes and on the basolateral membrane of hepatocytes and renal proximal tubular cells. Slc13a3 is found mainly in astrocytes and at lower degree in neurons within the central nervous system [26–31]. SLC13A3 shows a substrate preference for succinate, α-ketoglutarate and citrate and is inhibited by TCA cycle intermediates, such as fumarate, oxaloacetate or malate [32]. So far, no SLC13A3 deficient mouse model has been described, but it was reported that renal mRNA and protein expression levels increase with age in humans and rats [33]. Moreover, SLC13A3 activity and plasma membrane expression are regulated via PKC-dependent and -independent mechanisms [34, 35]. Moreover, SLC13A3 seems to be involved in the regulation of cellular senescence by a mechanism including the NAD-(+)-dependent histone deacetylase sirtuin-1 (SIRT1) [36]. SLC13A3 expression has been shown to be regulated by the transcription factor paired-like homeodomain transcription factor 2 (PITX2) in ocular cells and was suggested to be important in the inborn metabolic disease Axenfeld-Rieger syndrome type 1 (ARS1), an autosomal dominant disorder of morphogenesis. ARS1 is caused by mutations in PITX2 leading to abnormal eye anterior segment development which could result in glaucomatous blindness [37]. Moreover SLC13A3 was identified by genomic analysis to be associated with hypertension and type-2 diabetes [38–40].
SLC13A4 is mainly expressed in placenta and testis and at a lower extent in brain, heart, thymus, liver and tonsils [41, 42]. The Na+-dependent transport of sulfate can be inhibited by substrates with similar structures like molybdate and selenate most likely by substrate competition [42]. So far no SLC13A4 null mouse model has been described, and remains to be determined whether or not SLC13A4 is involved in any human disorder (for an overview see Table 1).
Table 1.
Relevant data on SLC13A family members.
| Gene | Main expression | Substrates | Physiological Relevance |
|---|---|---|---|
| Slc13a1 | Renal proximal tubulus | Sulfate, Thiosulfate | sulfate homeostasis |
| Intestinal epithelial cells | Selenite | ||
| Na+ | |||
| Slc13a2 | Metabolically relevant epithelia | Succinate | Tri/Dicarboxylate homeostasis |
| Renal proximal tubulus | α-Ketoglutarate | ||
| Intestinal epithelial cells | Citrate | ||
| Slc13a3 | Brain | Succinate | Tri/Dicarboxylate homeostasis cellular senescence (?), |
| Kidney | α-Ketoglutarate | Axenfeld-Rieger Syndrome type I | |
| Placenta | Citrate | ||
| Liver Pancreas | |||
| Eye optic nerve | |||
| Slc13a4 | Placenta | Sulfate | Unknown |
| Testis | |||
| Brain Heart | |||
| Thymus | |||
| Liver | |||
| Tonsils | |||
| Slc13a5 | Liver | Citrate | Tri/Dicarboxylate homeostasis metabolic processes such as fatty acid synthesis, gluconeogenesis |
| Testis | Succinate | ||
| Brain | Malate Fumarate |
SLC13A5 – structure, expression and function
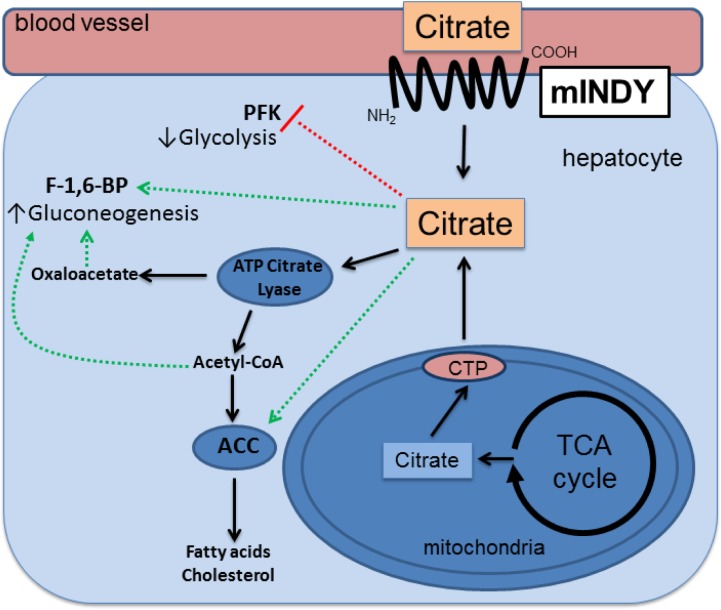
Cytosolic citrate is known as the prime carbon source for the synthesis of fatty acids, triacylglycerols, cholersterols and low-density lipoproteins. Moreover citrate leads to the activation of fatty acid synthesis and affects glycolysis and ß-oxidation [43–45]. An overview is provided in Figure 1. Main organs for fatty acid synthesis are the liver and white adipose tissue. Fatty acid synthesis has been shown to directly correlate with cytosolic citrate concentrations, partially depending on the direct import across the plasma membrane by mINDY [46, 47].
Figure 1.
Schematic representation of metabolic pathways affected by intracellular citrate molecules.
Initially, the significance of Indy in lifespan and metabolic regulation has been described in D. melanogaster, where it encodes an electroneutral tricarboxylate carrier, mainly expressed in organs involved in energy homeostasis and preferentially transporting citrate across the plasma membrane in flies [2, 46, 48–54]. Long lived flies with reduced expression of Indy showed a reduction in whole body fat stores and less expression of insulin like proteins, comparable to levels in calorically restricted wildtype flies [55].
The mammalian Indy homolog, mINDY, or SLC13A5, was identified as the latest member of the SLC13 gene family and consists of 11 transmembrane domains [46]. The bacterial INDY homologue protein binds one molecule of citrate and one molecule of sodium. Here, the binding sides exist of conserved amino acid proteins, serving as the structural basis of the transporter specificity, which is conserved over a wide range of species [48]. The Na+:citrate stoichiometry for mINDY is 4:1, which is in contrast to the other SLC13 family members. The SLC13A5 gene encodes the Na+-coupled citrate transporter mINDY (NaCT) and shares the highest sequence and functional similarity with D. melanogaster Indy. In humans, the INDY gene is located on chromosome 17p13 with a size of approximately 30kb consisting of 12 exons [46]. A splice variant lacking 43 N-terminal residues has been reported by Pajor and colleagues, the function and tissue distribution are still unknown [56]. Mammalian tissue distribution studies have localized predominant INDY expression within the liver, but also in testis and brain[3, 46, 51]. Within neuronal tissues, mINDY shows an exclusive expression pattern in neurons but not in astrocytes [27, 29].
mINDY has a vast substrate specificity for the tricarboxylate citrate and exhibits the inward electrogenic Na+-coupled substrate cotransport. It has been reported, that mINDY mediated citrate transport is pH-sensitive and maximal activity is exhibited at pH 7.0-7.5, whereas the transporter is inhibited in acidic and alkaline pH [52].
The transporter shows lower affinities to other Krebs-cycle intermediates such as succinate, malate or fumarate [46, 51, 52]. mINDY is less selective to other organic anions since it is not mediating the uptake of glutarate derivates into neurons [57]. Moreover, mINDY is Li+-sensitive, varying largely between species. While human mINDY is stimulated by Li+, it is inhibited or unaffected in other species [47, 58].
Several lines of evidence suggest that transcriptional regulation of mINDY is mediated by the nutritional state, suggesting metabolic factors being involved in the regulation. For example, Indy expression is reduced by caloric restriction in flies [55], whereas caloric restriction increases INDY expression in bicyclus anynana butterflies [59]. In mice, starvation over 36 hours reduces mINDY expression in the liver [3]. In contrast, gavaging large amounts of olive oil induced INDY expression strongly in the liver in rats, as identified by microarray assays [60]. Etcheverry and colleagues could show that the regulation of mINDY is mediated by epigenetic mechanisms. Profiling of whole genome integrative analysis of methylation and gene expression in glioblastoma patients possessed several genes, showing an inverse correlation between promoter methylation and expression level in glioblastomas. Moreover, INDY was down-regulated with hypermethylation of its promoter [61].
Recently, we have demonstrated that mINDY expression and citrate uptake was induced by physiological concentrations of the hormone glucagon via a cAMP and cAMP-responsive-element-binding-protein (CREB)- dependent mechanism. The promoter sequence of mINDY was identified including a CREB binding site within this fragment, identifying mINDY as a CREB-dependent glucagon target gene, which is induced in the short term fasting status and type-2 diabetes [62].
Since mINDY is predominately expressed in the liver, a metabolically highly active organ, it may play a role in various metabolic processes in which citrate has an important function such as mitochondrial energy production (TCA cycle), fatty acid synthesis, cholesterol synthesis, glycolysis and gluconeogenesis [58].
Citrate is imported from the circulation across the plasma membrane into the liver by an electrogenic citrate-sodium co-transport via mINDY (NaCT) and other family members of the SLC13 transporter family. Citrate generated within the mitochondria by each turn of the TCA cycle also enters the cytoplasm when intracellular energy stores are abundant. Cytoplasmic citrate is converted to acetyl-CoA by ATP-citrate lyase (ACLY). The conversion of acetyl-CoA to malonyl-CoA by acetyl-CoA carboxylase (ACC) is the first step in fatty acid synthesis. ACC gets allosterically activated by citrate. The product from this reaction, malonyl-CoA, serves as the donor of C2-acetly groups in each turn of the fatty acid synthesis reaction cycle. The ACLY reaction also yields oxaloacetate, which can be decarboxylated and phosphorylated to form phosphoenolpyruvate (PEP), through phosphoenolpyruvate carboxykinase. A (PEPCK), opening into gluconeogenesis. Moreover, acetyl-CoA can be routed into cholesterol synthesis via several enzymes including 3-hydroxy-3-methylglutaryl-coenzym-A-reductase (HMG-CoA-reductase).Green lines indicate activating and red lines inhibitory connections. ATP – Adenosine triphosphate, CTP – citrate transport protein, F-1,6-BP – Froctose-1,6-bisphosphatase, PFK – Phosphofructokinase, TCA – tricarboxylic acid.
Regulation of citrate homeostasis by SLC13 members
Serum citrate concentration is relatively constant, ranging from 50-200 µM [62, 63]. Citrate absorption from nutritional sources in the small intestine seems to be mediated mainly via NaDC1 and NaDC3 [64, 65]. By this mechanism, more than 90% of an oral citrate load can be absorbed and hypocitraturia can be ameliorated [64, 66]. At physiological pH, most of serum citrate circulates in the form of triply charged citrate bound to divalent ions, such as calcium and magnesium, and is filtered freely at the glomerulus; reabsorption takes place predominantly in the proximal tubule via NaDC1 [65]. Moreover, citrate is also taken up into the kidney by removal from postglomerular blood. Interestingly, similar to our findings in the liver, citrate is also oxidized in the TCA cycle in the kidney and it has been reported to be metabolized to glucose in gluconeogenesis in the kidney [3, 62]. By these mechanisms, citrate contributes to meet the energy needs of the kidney [67–70]. In addition to kidney, citrate is also taken up by the liver via mINDY and NaDC3. There, it can be oxidized or it can be used for the synthesis of fatty acids and glucose (Figure 1) [62].
Circulating citrate is released from muscle, skin and bone. Citrate concentrations in bone exceed that of most other tissues and accounts for 70% of total body citrate content71. Citrate can also be taken up into the bone, being deposited in the mineral fraction and mINDY has recently identified in bone matrix, probably contributing to the function [71, 72]. Skeletal muscle, with its high capacity for oxidative substrate utilization is probably a major source of citrate release to the plasma pool. The isolated rat hindquarter releases considerable amounts of citrate [73]. Arterio-femoral plasma citrate difference in the working man is largely negative, and the working muscle can be considered as one of the major sources of plasma citrate during exercise [74]. Moreover, the human heart has also been shown to release citrate, and is has been postulated that similarly to liver and kidney, this function may contribute the regulation of myocardial lipid and glucose metabolism [75].
The physiological significance of the regulation of plasma citrate homeostasis is largely unknown. The plasma halflife of citrate in the dog is about 20 minutes, and citrate turnover rates have been estimated to equal 250µmol/h/L in man, with a tissue to plasma gradient of 3-4 to 1 [70]. Externally administered citrate is oxidized to a major extend and it might, hence, contribute to furnish cellular energy needs. Citrate concentrations undergo significant circadian rhythmicity peaking in the postprandial phase, a pattern that seems to be disturbed in type 1 diabetics [76, 77]. In these patients, insulin decreases circulating citrate levels. Prolonged fasting also reduces citrate levels in men. Moreover, we have found that glucagon increases the expression of SLC13A5 via the transcription factor CREB in rats, and by this mechanism, increases the uptake of citrate into the liver in early fasting [62].
Metabolic regulation through SLC13A5
INDY might be involved in metabolic regulation since it transports intermediates of the TCA cycle and reducing the expression of Indy promotes longevity in a manner akin to caloric restriction in flies [55, 78]. Caloric restriction was shown to prolong life span, or at least health span, in many species including primates [79–83].
Deletion of the Indy homolog ceNAC-2 in C.elegans leads to prolonged lifespan with a reduction in whole body fat stores [2, 53, 55, 84]. In D. melanogaster, Indy encodes a non-electrogenic, dicarboxylate and citrate transporter mainly expressed in organs of intermediary metabolism, i.e. the fat body, midgut and oenocyte [2]. The mammalian protein mINDY shares highest sequence and functional similarity with the fly Indy gene [46].
To examine the metabolic effects mINDY might have in mammals, we created a whole body knockout mouse model (mINDY-/- mouse). mINDY-/- mice displayed a reduction in the uptake of citrate from the circulation into the liver, but not kidney and adipose tissue, paralleled by elevated circulating citrate levels [3, 85]. mINDY-/- mice did not gain as much weight as control mice, a phenotype getting more pronounced with age. The observation was accompanied by a somewhat unexpected increase in oxygen consumption, carbon dioxide generation and resting energy expenditure. In this vein, hepatic mitochondrial density and gene expression of the peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC1-α) were increased in mINDY-/- mice. Hepatic microarray studies revealed important similarities between mINDY-/- mice and calorically restricted mice, sharing ∼ 80% of similar transcriptional changes, including increased expression of electron transport chain components.
Consequently, on a high fat diet, mINDY-/- mice showed less body weight gain compared to control mice. Moreover, fat mass was significantly reduced by almost 50% and relative lean mass was increased as measured by nuclear magnet resonance. Hepatic triglyceride content was reduced upon mINDY deficiency concomitant with reduced liver lipid deposits. Moreover, the hepatic lipid oxidation marker, beta-hydroxybutyrate, was increased in high caloric fed mice by 62%. Measurements of lipid oxidation in primary hepatocytes isolated from high caloric diet fed mINDY-/- mice revealed a reduced incorporation of citrate into fatty acids and sterols. Together, these data indicate that high fat diet fed mINDY knockout mice have elevated lipid oxidation and reduced lipid synthesis rates compared to controls [3].
AMP-activated protein kinase (AMPK) is activated by increased intracellular adenosine monophosphate (AMP) stores and inhibited when adenosine triphosphate (ATP) reservoirs are high. Hence, AMPK is able to serve as a cellular energy sensor, coordinating insulin sensitivity, lipid-oxidation and -synthesis and mitochondrial biogenesis via the transcriptional co-activator PGC1-α [86, 87] In the mINDY-/- mice, ATP stores were reduced and AMPK phosphorylation was increased, suggesting an increased activation of AMPK through reduced intracellular biochemical energy stores [3].
Feeding mice a high fat diet serves as a well-known model to study obesity related impairment in glucose metabolism [88–92]. For this purpose, mINDY-/- mice were fed a high fat diet, and the impact of mINDY deletion on glucose metabolism was studied in vivo, by intraperitoneal glucose tolerance tests (ipGTT) first. Basal plasma glucose and insulin concentrations were decreased in mINDY-/- mice compared to control mice. Then, the gold standard to study insulin sensitivity, the hyperinsulinemic euglycemic clamp, demonstrated improved insulin sensitivity with reduced basal and clamp endogenous hepatic glucose production upon mINDY deletion. Additionally, peripheral glucose uptake by the gastrocnemius muscle was increased in INDY deletion mice. This protection from fat-induced muscle insulin resistance was accompanied by reduced content of skeletal muscle DAGs as major mediators of insulin resistance [93–97].
Hepatic insulin resistance is strongly associated with hepatic lipid accumulation [98]. A simple unifying hypothesis that has been proposed to explain these observations is that insulin resistance develops when there is an imbalance between supply and utilization of intracellular lipid leading to net accumulation of intracellular diacylglycerol (DAG hypothesis) [93, 98, 99]. Increased DAG content in turn results in activation of novel protein kinase C's (PKC's) and subsequent impairment in insulin signaling. With high-fat feeding, this condition is achieved due to the continued, high supply of dietary fat, which exceeds the capacity of hepatocytes to oxidize the fatty acids, store the fatty acids as neutral lipid or export fatty acids in VLDL (very low-density lipoprotein) particles. In contrast, with aging, declines in mitochondrial function may contribute to net accumulation of intracellular DAGs [99]. Consistent with this hypothesis, mINDY-/- mice showed reduced hepatic DAG concentrations, decreased membrane PKCɛ content, and protection from hepatic insulin resistance associated with high-fat feeding and aging. Confirmation of this key interaction between DAG, activation of PKCɛ, and insulin resistance has been demonstrated in numerous other rodent models of fatty liver associated hepatic insulin resistance [97, 100–106].
SLC13A5 – therapeutic aspects
Reducing INDY expression has been proven beneficial in terms of life span and/or metabolic regulation in all species tested so far. Therefore, it seems plausible to speculate that mINDY might be an interesting target for the treatment of metabolic disease, such as obesity, non-alcoholic fatty liver disease and type 2 diabetes [48, 107, 108]. To date, no specific therapeutic agents to modulate mINDY function or expression have been reported. Interestingly, human mINDY activity was described to be stimulated by Lithium, in concentrations that are observed during the treatment of bipolar disorders. Sun and colleagues reported on compounds, which inhibit the citrate transport protein (CTP) on the inner mitochondrial membrane [109, 110]. Moreover they could identify a compound with selectivity for mINDY over CTP [111]. Whether or not such a compound will be amenable to therapeutic intervention remains to be determined. The high concentration needed to inhibit mINDY with this molecule makes it unlikely to become clinically relevant. The discovery of a more potent compound modulating mINDY function could provide a useful tool to delineate the structure and function of mINDY. Ultimately, a putative inhibitor of mINDY holds the potential to induce the beneficial effects of caloric restriction, without requiring severe caloric restriction in mammals.
Acknowledgements
This work was supported by grants from the German Research Foundation (DFG, Bl1292/4-1) and the Fritz-Thyssen-Foundation (Az 10.12.2.140).
Competing Interests
The authors have declared that no competing interests exist.
References
- 1.Anderson RM, Weindruch R (2012) The caloric restriction paradigm: implications for healthy human aging. Am J Hum Biol 24: 101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogina B, Reenan RA, Nilsen SP, Helfand SL (2000) Extended life-span conferred by cotransporter gene mutations in Drosophila. Science 290: 2137–2140 [DOI] [PubMed] [Google Scholar]
- 3.Birkenfeld AL, Lee HY, Guebre-Egziabher F, Alves TC, Jurczak MJ, et al. (2011) Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metab 14: 184–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markovich D (2008) Expression cloning and radiotracer uptakes in Xenopus laevis oocytes. Nat Protoc 3: 1975–1980 [DOI] [PubMed] [Google Scholar]
- 5.Markovich D (2001) Physiological roles and regulation of mammalian sulfate transporters. Physiol Rev 81: 1499–1533 [DOI] [PubMed] [Google Scholar]
- 6.Lee A, Beck L, Markovich D (2000) The human renal sodium sulfate cotransporter (SLC13A1; hNaSi-1) cDNA and gene: organization, chromosomal localization, and functional characterization. Genomics 70: 354–363 [DOI] [PubMed] [Google Scholar]
- 7.Beck L, Markovich D (2000) The mouse Na(+)-sulfate cotransporter gene Nas1. Cloning, tissue distribution, gene structure, chromosomal assignment, and transcriptional regulation by vitamin D. J Biol Chem 275: 11880–11890 [DOI] [PubMed] [Google Scholar]
- 8.Markovich D, Forgo J, Stange G, Biber J, Murer H (1993) Expression cloning of rat renal Na + /SO4(2-) cotransport. Proc Natl Acad Sci U S A 90: 8073–8077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotscher M, Custer M, Quabius ES, Kaissling B, Murer H, et al. (1996) Immunolocalization of Na/SO4-cotransport (NaSi-1) in rat kidney. Pflugers Arch 432: 373–378 [DOI] [PubMed] [Google Scholar]
- 10.Markovich D, Aronson PS (2007) Specificity and regulation of renal sulfate transporters. Annu Rev Physiol 69: 361–375 [DOI] [PubMed] [Google Scholar]
- 11.Busch AE, Waldegger S, Herzer T, Biber J, Markovich D, et al. (1994) Electrogenic cotransport of Na+ and sulfate in Xenopus oocytes expressing the cloned Na + SO4(2-) transport protein NaSi-1. J Biol Chem 269: 12407–12409 [PubMed] [Google Scholar]
- 12.Dawson PA, Beck L, Markovich D (2003) Hyposulfatemia, growth retardation, reduced fertility, and seizures in mice lacking a functional NaSi-1 gene. Proc Natl Acad Sci U S A 100: 13704–13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson PA, Steane SE, Markovich D (2004) Behavioural abnormalities of the hyposulphataemic Nas1 knock-out mouse. Behav Brain Res 154: 457–463 [DOI] [PubMed] [Google Scholar]
- 14.Dawson PA, Steane SE, Markovich D (2005) Impaired memory and olfactory performance in NaSi-1 sulphate transporter deficient mice. Behav Brain Res 159: 15–20 [DOI] [PubMed] [Google Scholar]
- 15.Dawson PA, Gardiner B, Grimmond S, Markovich D (2006) Transcriptional profile reveals altered hepatic lipid and cholesterol metabolism in hyposulfatemic NaS1 null mice. Physiol Genomics 26: 116–124 [DOI] [PubMed] [Google Scholar]
- 16.Dawson PA, Gardiner B, Lee S, Grimmond S, Markovich D (2008) Kidney transcriptome reveals altered steroid homeostasis in NaS1 sulfate transporter null mice. J Steroid Biochem Mol Biol 112: 55–62 [DOI] [PubMed] [Google Scholar]
- 17.Dawson PA, Huxley S, Gardiner B, Tran T, McAuley JL, et al. (2009) Reduced mucin sulfonation and impaired intestinal barrier function in the hyposulfataemic NaS1 null mouse. Gut 58: 910–919 [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Kesby JP, Muslim MD, Steane SE, Eyles DW, et al. (2007) Hyperserotonaemia and reduced brain serotonin levels in NaS1 sulphate transporter null mice. Neuroreport 18: 1981–1985 [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Dawson PA, Hewavitharana AK, Shaw PN, Markovich D (2006) Disruption of NaS1 sulfate transport function in mice leads to enhanced acetaminophen-induced hepatotoxicity. Hepatology 43: 1241–1247 [DOI] [PubMed] [Google Scholar]
- 20.Pajor AM, Sun N (1999) Protein kinase C-mediated regulation of the renal Na(+)/dicarboxylate cotransporter, NaDC-1. Biochim Biophys Acta 1420: 223–230 [DOI] [PubMed] [Google Scholar]
- 21.Boehmer C, Embark HM, Bauer A, Palmada M, Yun CH, et al. (2004) Stimulation of renal Na+ dicarboxylate cotransporter 1 by Na + /H+ exchanger regulating factor 2, serum and glucocorticoid inducible kinase isoforms, and protein kinase B. Biochem Biophys Res Commun 313: 998–1003 [DOI] [PubMed] [Google Scholar]
- 22.Ho HT, Ko BC, Cheung AK, Lam AK, Tam S, et al. (2007) Generation and characterization of sodium-dicarboxylate cotransporter-deficient mice. Kidney Int 72: 63–71 [DOI] [PubMed] [Google Scholar]
- 23.Bergeron MJ, Burzle M, Kovacs G, Simonin A, Hediger MA (2011) Synthesis, maturation, and trafficking of human Na + -dicarboxylate cotransporter NaDC1 requires the chaperone activity of cyclophilin B. J Biol Chem 286: 11242–11253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meinild AK, Loo DD, Pajor AM, Zeuthen T, Wright EM (2000) Water transport by the renal Na(+)-dicarboxylate cotransporter. Am J Physiol Renal Physiol 278: F777–783 [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Fei YJ, Kekuda R, Yang-Feng TL, Devoe LD, et al. (2000) Structure, function, and genomic organization of human Na(+)-dependent high-affinity dicarboxylate transporter. Am J Physiol Cell Physiol 278: C1019–1030 [DOI] [PubMed] [Google Scholar]
- 26.Markovich D, Murer H (2004) The SLC13 gene family of sodium sulphate/carboxylate cotransporters. Pflugers Arch 447: 594–602 [DOI] [PubMed] [Google Scholar]
- 27.Fujita T, Katsukawa H, Yodoya E, Wada M, Shimada A, et al. (2005) Transport characteristics of N-acetyl-L-aspartate in rat astrocytes: involvement of sodium-coupled high-affinity carboxylate transporter NaC3/NaDC3-mediated transport system. J Neurochem 93: 706–714 [DOI] [PubMed] [Google Scholar]
- 28.Bai X, Chen X, Feng Z, Hou K, Zhang P, et al. (2006) Identification of basolateral membrane targeting signal of human sodium-dependent dicarboxylate transporter 3. J Cell Physiol 206: 821–830 [DOI] [PubMed] [Google Scholar]
- 29.Yodoya E, Wada M, Shimada A, Katsukawa H, Okada N, et al. (2006) Functional and molecular identification of sodium-coupled dicarboxylate transporters in rat primary cultured cerebrocortical astrocytes and neurons. J Neurochem 97: 162–173 [DOI] [PubMed] [Google Scholar]
- 30.Wada M, Shimada A, Fujita T (2006) Functional characterization of Na+ -coupled citrate transporter NaC2/NaCT expressed in primary cultures of neurons from mouse cerebral cortex. Brain Res 1081: 92–100 [DOI] [PubMed] [Google Scholar]
- 31.Lamp J, Keyser B, Koeller DM, Ullrich K, Braulke T, et al. (2011) Glutaric aciduria type 1 metabolites impair the succinate transport from astrocytic to neuronal cells. J Biol Chem 286: 17777–17784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Tsukaguchi H, Chen XZ, Berger UV, Hediger MA (1999) Molecular and functional analysis of SDCT2, a novel rat sodium-dependent dicarboxylate transporter. J Clin Invest 103: 1159–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Chen X, Zhu H, Peng L, Hong Q (2003) Relationship between aging and renal high-affinity sodium-dependent dicarboxylate cotransporter-3 expression characterized with antifusion protein antibody. J Gerontol A Biol Sci Med Sci 58: B879–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hagos Y, Burckhardt BC, Larsen A, Mathys C, Gronow T, et al. (2004) Regulation of sodium-dicarboxylate cotransporter-3 from winter flounder kidney by protein kinase C. Am J Physiol Renal Physiol 286: F86–93 [DOI] [PubMed] [Google Scholar]
- 35.Srisawang P, Chatsudthipong A, Chatsudthipong V (2007) Modulation of succinate transport in Hep G2 cell line by PKC. Biochim Biophys Acta 1768: 1378–1388 [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Zacchia M, Tian X, Wan L, Sakamoto A, et al. (2010) Acid regulation of NaDC-1 requires a functional endothelin B receptor. Kidney Int 78: 895–904 [DOI] [PubMed] [Google Scholar]
- 37.Strungaru MH, Footz T, Liu Y, Berry FB, Belleau P, et al. (2011) PITX2 is involved in stress response in cultured human trabecular meshwork cells through regulation of SLC13A3. Invest Ophthalmol Vis Sci 52: 7625–7633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarvari M, Kallo I, Hrabovszky E, Solymosi N, Toth K, et al. (2010) Estradiol replacement alters expression of genes related to neurotransmission and immune surveillance in the frontal cortex of middle-aged, ovariectomized rats. Endocrinology 151: 3847–3862 [DOI] [PubMed] [Google Scholar]
- 39.Simino J, Shi G, Arnett D, Broeckel U, Hunt SC, et al. (2011) Variants on chromosome 6p22.3 associated with blood pressure in the HyperGEN study: follow-up of FBPP quantitative trait loci. Am J Hypertens 24: 1227–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bento JL, Palmer ND, Zhong M, Roh B, Lewis JP, et al. (2008) Heterogeneity in gene loci associated with type 2 diabetes on human chromosome 20q13.1. Genomics 92: 226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Girard JP, Baekkevold ES, Feliu J, Brandtzaeg P, Amalric F (1999) Molecular cloning and functional analysis of SUT-1, a sulfate transporter from human high endothelial venules. Proc Natl Acad Sci U S A 96: 12772–12777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markovich D, Regeer RR, Kunzelmann K, Dawson PA (2005) Functional characterization and genomic organization of the human Na(+)-sulfate cotransporter hNaS2 gene (SLC13A4). Biochem Biophys Res Commun 326: 729–734 [DOI] [PubMed] [Google Scholar]
- 43.Spencer AF, Lowenstein JM (1962) The supply of precursors for the synthesis of fatty acids. J Biol Chem 237: 3640–3648 [PubMed] [Google Scholar]
- 44.Bloch K, Vance D (1977) Control mechanisms in the synthesis of saturated fatty acids. Annu Rev Biochem 46: 263–298 [DOI] [PubMed] [Google Scholar]
- 45.Ruderman NB, Saha AK, Vavvas D, Witters LA (1999) Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol 276: E1–E18 [DOI] [PubMed] [Google Scholar]
- 46.Inoue K, Zhuang L, Ganapathy V (2002) Human Na+ -coupled citrate transporter: primary structure, genomic organization, and transport function. Biochem Biophys Res Commun 299: 465–471 [DOI] [PubMed] [Google Scholar]
- 47.Gopal E, Miyauchi S, Martin PM, Ananth S, Srinivas SR, et al. (2007) Expression and functional features of NaCT, a sodium-coupled citrate transporter, in human and rat livers and cell lines. Am J Physiol Gastrointest Liver Physiol 292: G402–408 [DOI] [PubMed] [Google Scholar]
- 48.Mancusso R, Gregorio GG, Liu Q, Wang DN (2012) Structure and mechanism of a bacterial sodium-dependent dicarboxylate transporter. Nature 491: 622–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knauf F, Mohebbi N, Teichert C, Herold D, Rogina B, et al. (2006) The life-extending gene Indy encodes an exchanger for Krebs-cycle intermediates. Biochem J 397: 25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knauf F, Rogina B, Jiang Z, Aronson PS, Helfand SL (2002) Functional characterization and immunolocalization of the transporter encoded by the life-extending gene Indy. Proc Natl Acad Sci U S A 99: 14315–14319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoue K, Zhuang L, Maddox DM, Smith SB, Ganapathy V (2002) Structure, function, and expression pattern of a novel sodium-coupled citrate transporter (NaCT) cloned from mammalian brain. J Biol Chem 277: 39469–39476 [DOI] [PubMed] [Google Scholar]
- 52.Inoue K, Fei YJ, Zhuang L, Gopal E, Miyauchi S, et al. (2004) Functional features and genomic organization of mouse NaCT, a sodium-coupled transporter for tricarboxylic acid cycle intermediates. Biochem J 378: 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fei YJ, Liu JC, Inoue K, Zhuang L, Miyake K, et al. (2004) Relevance of NAC-2, an Na + -coupled citrate transporter, to life span, body size and fat content in Caenorhabditis elegans. Biochem J 379: 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogina B, Helfand SL (2013) Indy mutations and Drosophila longevity. Front Genet 4: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang PY, Neretti N, Whitaker R, Hosier S, Chang C, et al. (2009) Long-lived Indy and calorie restriction interact to extend life span. Proc Natl Acad Sci U S A 106: 9262–9267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pajor AM (2006) Molecular properties of the SLC13 family of dicarboxylate and sulfate transporters. Pflugers Arch 451: 597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brauburger K, Burckhardt G, Burckhardt BC (2011) The sodium-dependent di- and tricarboxylate transporter, NaCT, is not responsible for the uptake of D-, L-2-hydroxyglutarate and 3-hydroxyglutarate into neurons. J Inherit Metab Dis 34: 477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Inoue K, Zhuang L, Maddox DM, Smith SB, Ganapathy V (2003) Human sodium-coupled citrate transporter, the orthologue of Drosophila Indy, as a novel target for lithium action. Biochem J 374: 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pijpe J, Pul N, van Duijn S, Brakefield PM, Zwaan BJ (2011) Changed gene expression for candidate ageing genes in long-lived Bicyclus anynana butterflies. Exp Gerontol 46: 426–434 [DOI] [PubMed] [Google Scholar]
- 60.Martinez-Beamonte R, Navarro MA, Guillen N, Acin S, Arnal C, et al. (2011) Postprandial transcriptome associated with virgin olive oil intake in rat liver. Front Biosci (Elite Ed) 3: 11–21 [DOI] [PubMed] [Google Scholar]
- 61.Etcheverry A, Aubry M, de Tayrac M, Vauleon E, Boniface R, et al. (2010) DNA methylation in glioblastoma: impact on gene expression and clinical outcome. BMC Genomics 11: 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neuschafer-Rube F, Lieske S, Kuna M, Henkel J, Perry RJ, et al. (2013) The Mammalian INDY Homolog is Induced by CREB in a Rat Model of Type 2 Diabetes. Diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neuschafer-Rube F, Pathe-Neuschafer-Rube A, Hippenstiel S, Kracht M, Puschel GP (2013) NF-kappaB-dependent IL-8 induction by prostaglandin E(2) receptors EP(1) and EP(4). Br J Pharmacol 168: 704–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fegan J, Khan R, Poindexter J, Pak CY (1992) Gastrointestinal citrate absorption in nephrolithiasis. J Urol 147: 1212–1214 [DOI] [PubMed] [Google Scholar]
- 65.Pajor AM (2013) Sodium-coupled dicarboxylate and citrate transporters from the SLC13 family. Pflugers Arch. [DOI] [PubMed] [Google Scholar]
- 66.Rudman D, Dedonis JL, Fountain MT, Chandler JB, Gerron GG, et al. (1980) Hypocitraturia in patients with gastrointestinal malabsorption. N Engl J Med 303: 657–661 [DOI] [PubMed] [Google Scholar]
- 67.Baruch SB, Burich RL, Eun CK, King VF (1975) Renal metabolism of citrate. Med Clin North Am 59: 569–582 [DOI] [PubMed] [Google Scholar]
- 68.Nieth H, Schollmeyer P (1966) Substrate-utilization of the human kidney. Nature 209: 1244–1245 [DOI] [PubMed] [Google Scholar]
- 69.Pashley DH, Cohen JJ (1973) Substrate interconversion in dog kidney cortex slices: regulation by ECF-pH. Am J Physiol 225: 1519–1528 [DOI] [PubMed] [Google Scholar]
- 70.Nielsen TT (1983) Plasma citrate in relation to glucose and free fatty acid metabolism in man. Dan Med Bull 30: 357–378 [PubMed] [Google Scholar]
- 71.Dickens F (1941) The citric acid content of animal tissues, with reference to its occurrence in bone and tumour. Biochem J 35: 1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu S, Tang W, Fang J, Ren J, Li H, et al. (2009) Novel regulators of Fgf23 expression and mineralization in Hyp bone. Mol Endocrinol 23: 1505–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee SH, Davis EJ (1979) Carboxylation and decarboxylation reactions. Anaplerotic flux and removal of citrate cycle intermediates in skeletal muscle. J Biol Chem 254: 420–430 [PubMed] [Google Scholar]
- 74.Nielsen TT, Thomsen PE (1979) Leg and splanchnic arteriovenous differences of plasma citrate in exercising man. J Appl Physiol Respir Environ Exerc Physiol 46: 120–127 [DOI] [PubMed] [Google Scholar]
- 75.Nielsen TT, Henningsen P, Bagger JP, Thomsen PE, Eyjolfsson K (1980) Myocardial citrate metabolism in control subjects and patients with coronary artery disease. Scand J Clin Lab Invest 40: 575–580 [DOI] [PubMed] [Google Scholar]
- 76.Nielsen TT, Sorensen NS (1981) Daily plasma citrate rhythms in man during feeding and fasting. Scand J Clin Lab Invest 41: 281–287 [DOI] [PubMed] [Google Scholar]
- 77.Thomassen A, Nielsen TT, Bagger JP, Charles P, Lovgreen NA, et al. (1981) Circadian plasma citrate rhythms in juvenile diabetics. Acta Med Scand 210: 163–171 [DOI] [PubMed] [Google Scholar]
- 78.Neretti N, Wang PY, Brodsky AS, Nyguyen HH, White KP, et al. (2009) Long-lived Indy induces reduced mitochondrial reactive oxygen species production and oxidative damage. Proc Natl Acad Sci U S A 106: 2277–2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC (2003) Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med 54: 131–152 [DOI] [PubMed] [Google Scholar]
- 80.Fontana L, Klein S (2007) Aging, adiposity, and calorie restriction. JAMA 297: 986–994 [DOI] [PubMed] [Google Scholar]
- 81.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, et al. (2009) Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325: 201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lopez-Lluch G, Irusta PM, Navas P, de Cabo R (2008) Mitochondrial biogenesis and healthy aging. Exp Gerontol 43: 813–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, et al. (2012) Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 489: 318–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fei YJ, Inoue K, Ganapathy V (2003) Structural and functional characteristics of two sodium-coupled dicarboxylate transporters (ceNaDC1 and ceNaDC2) from Caenorhabditis elegans and their relevance to life span. J Biol Chem 278: 6136–6144 [DOI] [PubMed] [Google Scholar]
- 85.Shulman GI, Helfand SL (2011) Indy knockdown in mice mimics elements of dietary restriction. Aging (Albany NY) 3: 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruderman NB, Carling D, Prentki M, Cacicedo JM (2013) AMPK, insulin resistance, and the metabolic syndrome. J Clin Invest 123: 2764–2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang BB, Zhou G, Li C (2009) AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 9: 407–416 [DOI] [PubMed] [Google Scholar]
- 88.Jordan SD, Kruger M, Willmes DM, Redemann N, Wunderlich FT, et al. (2011) Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol 13: 434–446 [DOI] [PubMed] [Google Scholar]
- 89.Birkenfeld AL, Lee HY, Majumdar S, Jurczak MJ, Camporez JP, et al. (2011) Influence of the hepatic eukaryotic initiation factor 2alpha (eIF2alpha) endoplasmic reticulum (ER) stress response pathway on insulin-mediated ER stress and hepatic and peripheral glucose metabolism. J Biol Chem 286: 36163–36170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bailly-Maitre B, Belgardt BF, Jordan SD, Coornaert B, von Freyend MJ, et al. (2010) Hepatic Bax inhibitor-1 inhibits IRE1alpha and protects from obesity-associated insulin resistance and glucose intolerance. J Biol Chem 285: 6198–6207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loffler MG, Birkenfeld AL, Philbrick KM, Belman JP, Habtemichael EN, et al. (2013) Enhanced fasting glucose turnover in mice with disrupted action of TUG protein in skeletal muscle. J Biol Chem 288: 20135–20150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kumashiro N, Beddow SA, Vatner DF, Majumdar SK, Cantley JL, et al. (2013) Targeting pyruvate carboxylase reduces gluconeogenesis and adiposity and improves insulin resistance. Diabetes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shulman GI (2000) Cellular mechanisms of insulin resistance. J Clin Invest 106: 171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, et al. (2004) Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 279: 32345–32353 [DOI] [PubMed] [Google Scholar]
- 95.Samuel VT, Petersen KF, Shulman GI (2010) Lipid-induced insulin resistance: unravelling the mechanism. Lancet 375: 2267–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, et al. (2007) Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest 117: 739–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Erion DM, Shulman GI (2010) Diacylglycerol-mediated insulin resistance. Nat Med 16: 400–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Birkenfeld AL, Shulman GI (2013) Non alcoholic fatty liver disease, hepatic insulin resistance and type 2 diabetes. Hepatology.epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Samuel VT, Shulman GI (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148: 852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jornayvaz FR, Jurczak MJ, Lee HY, Birkenfeld AL, Frederick DW, et al. (2010) A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am J Physiol Endocrinol Metab 299: E808–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee HY, Choi CS, Birkenfeld AL, Alves TC, Jornayvaz FR, et al. (2010) Targeted expression of catalase to mitochondria prevents age-associated reductions in mitochondrial function and insulin resistance. Cell Metab 12: 668–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jurczak MJ, Lee HY, Birkenfeld AL, Jornayvaz FR, Frederick DW, et al. (2011) SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes 60: 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jornayvaz FR, Birkenfeld AL, Jurczak MJ, Kanda S, Guigni BA, et al. (2011) Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc Natl Acad Sci U S A 108: 5748–5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee HY, Birkenfeld AL, Jornayvaz FR, Jurczak MJ, Kanda S, et al. (2011) Apolipoprotein CIII overexpressing mice are predisposed to diet-induced hepatic steatosis and hepatic insulin resistance. Hepatology 54: 1650–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pajvani UB, Shawber CJ, Samuel VT, Birkenfeld AL, Shulman GI, et al. (2011) Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nat Med 17: 961–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jurczak MJ, Lee AH, Jornayvaz FR, Lee HY, Birkenfeld AL, et al. (2012) Dissociation of inositol-requiring enzyme (IRE1alpha)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J Biol Chem 287: 2558–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Frankel S, Rogina B (2012) Indy mutants: live long and prosper. Front Genet 3: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schindler C (2012) New therapeutic efforts and upcoming developments in the field of diabetes medicine and endocrinology. Ther Adv Endocrinol Metab 3: 51–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aluvila S, Sun J, Harrison DH, Walters DE, Kaplan RS (2010) Inhibitors of the mitochondrial citrate transport protein: validation of the role of substrate binding residues and discovery of the first purely competitive inhibitor. Mol Pharmacol 77: 26–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Irwin JJ, Shoichet BK (2005) ZINC--a free database of commercially available compounds for virtual screening. J Chem Inf Model 45: 177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sun J, Aluvila S, Kotaria R, Mayor JA, Walters DE, et al. (2010) Mitochondrial and Plasma Membrane Citrate Transporters: Discovery of Selective Inhibitors and Application to Structure/Function Analysis. Mol Cell Pharmacol 2: 101–110 [PMC free article] [PubMed] [Google Scholar]