Key Points
Pomalidomide plus low-dose dexamethasone significantly improved PFS vs pomalidomide alone in relapsed and refractory multiple myeloma.
Pomalidomide plus low-dose dexamethasone is an important new treatment option for RRMM patients who have received multiple prior therapies.
Abstract
This multicenter, open-label, randomized phase 2 study assessed the efficacy and safety of pomalidomide (POM) with/without low-dose dexamethasone (LoDEX) in patients with relapsed/refractory multiple myeloma (RRMM). Patients who had received ≥2 prior therapies (including lenalidomide [LEN] and bortezomib [BORT]) and had progressed within 60 days of their last therapy were randomized to POM (4 mg/day on days 1-21 of each 28-day cycle) with/without LoDEX (40 mg/week). The primary end point was progression-free survival (PFS). In total, 221 patients (median 5 prior therapies, range 1-13) received POM+LoDEX (n = 113) or POM (n = 108). With a median follow-up of 14.2 months, median PFS was 4.2 and 2.7 months (hazard ratio = 0.68, P = .003), overall response rates (ORRs) were 33% and 18% (P = .013), median response duration was 8.3 and 10.7 months, and median overall survival (OS) was 16.5 and 13.6 months, respectively. Refractoriness to LEN, or resistance to both LEN and BORT, did not affect outcomes with POM+LoDEX (median PFS 3.8 months for both; ORRs 30% and 31%; and median OS 16 and 13.4 months). Grade 3-4 neutropenia occurred in 41% (POM+LoDEX) and 48% (POM); no grade 3-4 peripheral neuropathy was reported. POM+LoDEX was effective and generally well tolerated and provides an important new treatment option for RRMM patients who have received multiple prior therapies. This trial was registered at www.clinicaltrials.gov as #NCT00833833.
Introduction
Virtually all patients with multiple myeloma (MM) eventually relapse. Relapsed disease is characterized by increasingly shorter periods of remission following each salvage therapy.1 Survival among MM patients in whom novel agents (including bortezomib [BORT], lenalidomide [LEN], and/or thalidomide) have failed is especially poor.2 There is a clear unmet need for new treatments, particularly for patients who are relapsed and refractory to novel agents.
Pomalidomide (POM) is a distinct immunomodulatory drug with potent antimyeloma activity.3-5 POM plus dexamethasone (DEX) has synergistic antiproliferative effects in LEN-resistant myeloma cells.6 The activity of POM in cells resistant or refractory to LEN may be due to important differences in both the potency of the drugs and their respective mechanisms of action.3,7-11
POM has demonstrated efficacy in patients with relapsed/refractory MM (RRMM) who had received multiple prior therapies, either when given alone12-14 or with low-dose DEX (LoDEX).14-17 Here, we report the results of a multicenter, randomized, open-label, phase 2 trial. The phase 1 part of the study established the maximum tolerated dose of POM (4 mg/day on days 1-21 of a 28-day cycle).18 The phase 2 part evaluated the efficacy and safety of POM when given alone or in combination with LoDEX in RRMM patients.
Methods
MM-002 is a phase 1/2 trial conducted at 18 centers in the US and Canada, initiated in December 2009. This manuscript reports on the phase 2 part, which was an open-label, randomized trial; phase 1 results have already been reported.18 Eligible patients were aged ≥18 years, had RRMM, and had measurable M-paraprotein levels in serum or urine. All patients had received ≥2 prior antimyeloma therapies, including ≥2 cycles of LEN and ≥2 cycles of BORT, given separately or in combination. Patients had to have relapsed after having achieved at least stable disease (SD) for ≥1 cycle of treatment to ≥1 prior regimen, as well as have disease progression during or within 60 days (measured from the end of the last cycle) of completing treatment with the last regimen used prior to study entry (and thus had relapsed and refractory disease). Disease progression was defined as any of the following: increase in serum monoclonal paraprotein and/or urine paraprotein; increase in bone marrow plasmacytosis and plasma cells; appearance of new soft-tissue plasmacytomas or increase in size of existing plasmacytoma(s); new lytic bone lesions or an increase in the size of the existing bone lesions; or the development of hypercalcemia (serum calcium >11.5 mg/dL). Exclusion criteria were absolute neutrophil count <1000/µL; platelet count <75 000/µL or <30 000/µL for patients in whom <50% or ≥50% of bone marrow nucleated cells were plasma cells, respectively; serum creatinine ≥3.0 mg/dL; serum liver transaminase levels >3.0 × the upper limit of normal; or serum bilirubin >2.0 mg/dL. Concomitant intravenous amino-bisphosphonate therapy was permitted.
Patients were randomized (1:1) to POM (4 mg/day on days 1-21 of each 28-day cycle) alone or with LoDEX (40 mg/week), using an interactive voice response system. Treatment continued until disease progression or unacceptable toxicity. At progression, patients assigned to POM alone could add LoDEX. All patients received aspirin (81-100 mg/day) unless contraindicated. If aspirin was contraindicated, patients received another form of antithrombotic therapy according to local hospital guidelines or physician preference. Erythroid growth factors, bisphosphonates, platelet and/or red blood cell transfusions, and granulocyte colony-stimulating factor (G-CSF; if absolute neutrophil count <1000/µL) were allowed. The efficacy evaluable population included all patients who received ≥1 dose of study drug and had ≥1 postbaseline response assessment.
The primary end point was progression-free survival (PFS), defined as the time from randomization to the first documentation of disease progression or death from any cause. Secondary end points included overall response rate (ORR; defined as partial response or better [≥PR]), time-to-response and duration of response (for patients who achieved ≥PR), overall survival (OS), and safety. PFS and responses were investigator assessed based on European Group for Blood and Marrow Transplantation criteria,19,20 and the ORR was assessed every 4 weeks and at discontinuation of study drug. ORR was calculated as the number of patients with a confirmed response (≥PR maintained for ≥6 weeks) divided by the number of efficacy evaluable patients. Time-to-response represents the interval between randomization and achievement of ≥PR; duration of response was defined as the time from achievement of ≥PR to first evidence of disease progression or death from any cause. OS was defined as the time from randomization to the time of death from any cause. Adverse event (AE) severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, v3.0. Data reported for the POM group include patients who received LoDEX at progression.
Subanalyses were performed in patients refractory to LEN, and in those refractory to both LEN and BORT, according to age (≤65 vs >65 years) and presence of modified high-risk cytogenetics (del[17p13] and/or t[4p16/14q32]). To determine the treatment effects on other disease parameters, change from baseline to best postbaseline value was retrospectively assessed for platelets, corrected calcium, creatinine clearance, hemoglobin, immunoglobulin, and Eastern Cooperative Oncology Group performance status scores; these analyses did not account for use of concomitant interventions, such as blood transfusion or G-CSF support.
For the PFS analysis, an improvement in median PFS from 6 to10 months with the addition of LoDEX was considered clinically relevant. Assuming a 10% drop-out rate, 96 patients in each treatment group would have 85% power to detect a hazard ratio (HR) rate of 1.67 using a one-sided log-rank test with an overall significance level of 0.025 (adjusted for one interim analysis), and a significance level of 0.0245 for the final analysis. The Kaplan-Meier method was used to estimate the survival distribution functions for each treatment group, and the log-rank test was used to compare Kaplan-Meier curves for both treatment groups. A Cox proportional hazards model was used to estimate the relative risk, HR rate, and 95% confidence intervals (CIs). For response assessment, a 25% response rate was considered clinically meaningful, whereas a 12% response was not. One-sample binomial test for ORR in each group was performed at the nominal one-sided 0.0125 significance level. The planned 96 patients in each arm provided 89% nominal power. All statistical analyses were conducted using SAS version 9.1 or higher. The intent-to-treat population (all randomized patients) was used for PFS and OS data, the efficacy evaluable population for response, and the safety population (patients who received ≥1 dose of study medication) for the safety data.
All patients provided written informed consent. The institutional review board at each participating center approved the study, which was conducted in accordance with the principles of Good Clinical Practice, the provisions of the Declaration of Helsinki, and other applicable local regulations. P.G.R., D.S.S., R.V., C.C.H., R.B., S.J., C.C., S. Lonial, N.B., A.B., N.R., C.S., M.L., J. Mikhael, D.V., M.C., C.J., Z.Y., and K.C.A. analyzed and interpreted the data; all authors had access to the primary clinical trial data.
Results
The protocol-specified final analysis was performed at 100% information, when 167 patients across both treatment arms had disease progression or had died during the study, with a median follow-up of 9.4 months (data cutoff April 1, 2011). Updated data were available after a median follow-up of 14.2 months (data cutoff February 1, 2013). Key results (PFS and OS) from the final analysis (cutoff April 1, 2011) are presented below. All other data (PFS, OS, ORR, safety, and subanalyses) are from the updated analysis (cutoff February 1, 2013).
Patients and treatment
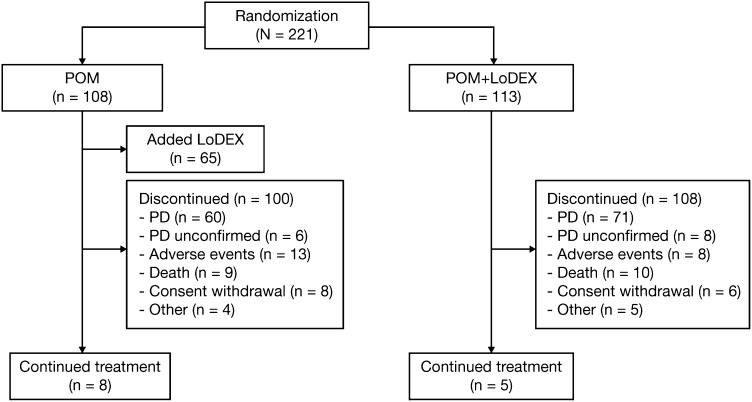
A total of 221 patients were randomized to POM+LoDEX (n = 113) or POM (n = 108) (Figure 1). Two patients were randomized but never received the study drug and were therefore excluded from the safety population and any subsequent analysis. At the time of the safety analysis (February 1, 2013), 208 patients (94%) had discontinued therapy.
Figure 1.
CONSORT diagram. PD, progressive disease.
The median age was 63 years (range, 34-88 years), with 59% of patients aged ≤65 years. Most patients (81%) were white, 54% were male, and 27% had high-risk cytogenetics (Table 1). The median number of prior therapies was 5 (range, 1-13); all patients had received prior LEN and BORT, 99% had received prior DEX, 67% had received prior thalidomide, 75% had undergone prior stem cell transplantation, and 23% had received prior carfilzomib (CFZ). Overall, 62% of patients were refractory to both LEN and BORT. A total of 219 patients received ≥1 dose of study drug and were included in the safety population. The median number of treatment cycles was 5 (range, 1-38); median treatment duration was 5 months. Of the 108 patients assigned to POM alone, 65 (60%) received LoDEX at disease progression.
Table 1.
Baseline patient characteristics
| POM+LoDEX (n = 113) | POM alone (n = 108) | Total (N = 221) | |
|---|---|---|---|
| Median age, y (range) | 64 (34-88) | 61 (37-88) | 63 (34-88) |
| Age distribution, % | |||
| ≤65 y | 55 | 64 | 59 |
| >65 y | 45 | 36 | 41 |
| ≤75 y | 88 | 88 | 88 |
| >75 y | 12 | 12 | 12 |
| Male, % | 55 | 53 | 54 |
| MM stage, % | |||
| I | 7 | 7 | 7 |
| II | 26 | 27 | 26 |
| III | 67 | 66 | 67 |
| ECOG performance status score, % | |||
| 0 | 28 | 22 | 25 |
| 1 | 60 | 66 | 63 |
| 2 | 12 | 10 | 11 |
| 3 | 0 | 2 | 1 |
| Cytogenetic profile, % | |||
| High-risk* | 27 | 28 | 27 |
| Standard-risk | 50 | 40 | 45 |
| Missing data | 23 | 32 | 28 |
| Median number of prior therapies (range) | 5 (2-13) | 5 (1-12) | 5 (1-13) |
| Number of prior therapies, % | |||
| ≤2 | 5 | 5 | 5 |
| >2 | 95 | 95 | 95 |
| Prior therapies, % | |||
| LEN and BORT | 100 | 100 | 100 |
| DEX | 99 | 99 | 99 |
| Thalidomide | 67 | 67 | 67 |
| Stem cell transplantation | 74 | 76 | 75 |
| CFZ | 17 | 29 | 23 |
| Prior thalidomide exposure, % | |||
| Yes | 67 | 67 | 67 |
| No | 33 | 33 | 33 |
| LEN as last prior therapy, % | 39 | 31 | 35 |
| Refractory to LEN, %† | 78 | 80 | 79 |
| Refractory to BORT, %‡ | 71 | 70 | 71 |
| Refractory to both LEN and BORT, %§ | 62 | 61 | 62 |
CFZ, carfilzomib; ECOG, Eastern Cooperative Oncology Group; PD, progressive disease.
High-risk cytogenetic profiles defined as del(17p13) and/or t(4p16/14q32).
Defined as patients who experienced PD during or within 60 d (measured from the last dose) of completing treatment with LEN.
Defined as patients who experienced PD during or within 60 d (measured from the last dose) of completing treatment with BORT.
Defined as patients who experienced PD during or within 60 d (measured from the last dose) of completing treatment with LEN and BORT.
Efficacy
Overall PFS, OS, and response.
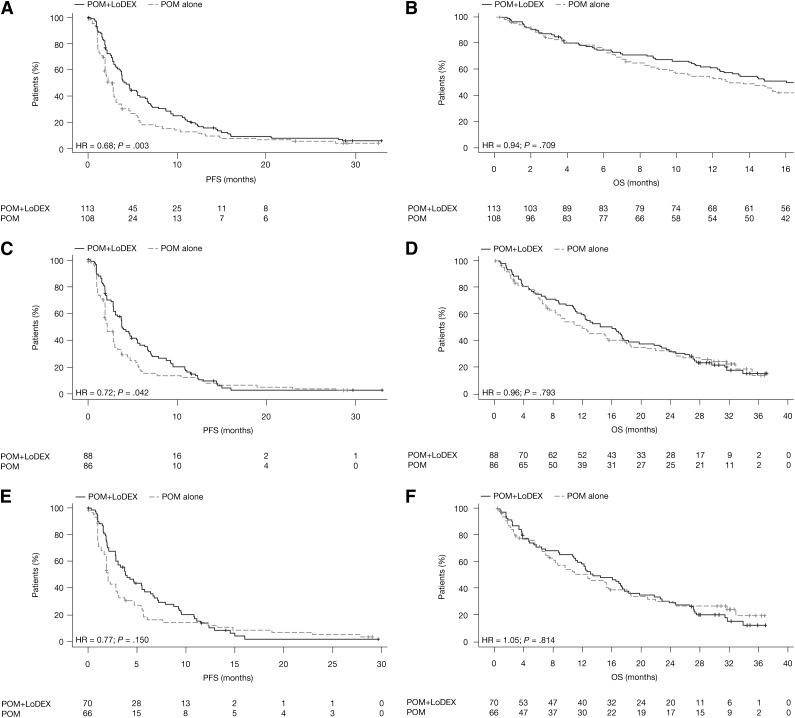
At the time of the final analysis (April 1, 2011), the median PFS was 4.6 and 2.6 months in the POM+LoDEX and POM alone groups, respectively (HR = 0.64, 95% CI = 0.47-0.86, P < .001). The median OS was 14.4 and 13.7 months, respectively (HR = 0.85, 95% CI = 0.57-1.29, P = .449).
Updated data after a median follow-up of 14.2 months (February 1, 2013) continued to favor POM+LoDEX vs POM alone. The median PFS was 4.2 and 2.7 months in the groups, respectively (HR = 0.68, 95% CI = 0.51-0.90, P = .003) (Figure 2A). The median OS in the intent-to-treat population was 16.5 months with POM+LoDEX and 13.6 months with POM alone (HR = 0.94, 95% CI = 0.70-1.28, P = .709) (Figure 2B). Per prespecified one-sample binomial test, the response rate in the POM+LoDEX arm was considered to be statistically significantly effective, whereas the response rate in the POM arm was not. The ORR (≥PR) was 33% in the POM+LoDEX group and 18% in the POM alone group (odds ratio = 2.28, 95% CI = 1.21-4.29, P = .013); minimal response or better was achieved in 45% and 31% of patients, respectively (Table 2). The rate of complete response was 3% with POM+LoDEX and 2% with POM alone. The median time to response for POM+LoDEX and POM alone patients was 1.9 and 4.3 months, respectively, and the median duration of response in patients with ≥PR was 8.3 and 10.7 months, respectively (median follow-up time 16.1 and 12.3 months, respectively).
Figure 2.
PFS and OS. (A) PFS in the intent-to-treat population. (B) OS in the intent-to-treat population. (C) PFS in patients with disease refractory to LEN. (D) OS in patients with disease refractory to LEN. (E) PFS in patients with disease refractory to both LEN and BORT. (F) OS in patients with disease refractory to both LEN and BORT.
Table 2.
Efficacy parameters
| Intent-to-treat | LEN refractory | LEN and BORT refractory | ||||
|---|---|---|---|---|---|---|
| POM+LoDEX (n = 113) | POM alone (n = 108) | POM+LoDEX (n = 88) | POM alone (n = 86) | POM+LoDEX (n = 70) | POM alone (n = 66) | |
| Median PFS, months | 4.2 | 2.7 | 3.8 | 2.2 | 3.8 | 2.0 |
| Median OS, months | 16.5 | 13.6 | 16.0 | 12.0 | 13.4 | 12.5 |
| ORR (≥PR), % | 33 | 18 | 30 | 21 | 31 | 21 |
| ≥MR, % | 45 | 31 | 42 | 31 | 46 | 33 |
| CR | 3 | 2 | 0 | 1 | 0 | 1 |
| PR | 30 | 16 | 30 | 20 | 31 | 20 |
| MR | 12 | 13 | 13 | 11 | 14 | 12 |
| SD, % | 37 | 48 | 41 | 47 | 39 | 42 |
| Median time-to-response (≥PR), months | 1.9 | 4.3 | 1.9 | 4.6 | 1.6 | 4.6 |
| Median duration of response (≥PR), months | 8.3 | 10.7 | 7.7 | 8.8 | 6.5 | 11.4 |
| Median duration of ≥MR, months | 7.7 | 7.4 | 6.2 | 6.7 | 6.2 | 6.7 |
CR, complete response; MR, minimal response.
In the POM+LoDEX group, the median duration of study treatment was longer for patients who achieved ≥PR compared with their last prior therapy (11.9 vs 4.2 months, respectively), and responses were consistently observed regardless of refractoriness to LEN. Of the responders who had a longer duration of study treatment, 76% had a better response to POM+LoDEX than to their last prior therapy. Patients who achieved ≥PR in the POM alone group also had a longer median duration of study treatment than with their last prior therapy (16.6 vs 6.4 months, respectively). Of the responders who had a longer duration of study treatment, 86% had a better response to POM alone than to their last prior therapy.
Special populations.
Outcomes with POM+LoDEX were consistent regardless of age. The median PFS was 4.7 months in patients aged ≤65 years and 3.7 months in those >65 years; ORRs were 31% and 35%, and median response durations were 10.1 and 7.7 months, respectively. POM+LoDEX showed promising activity in the 30 patients with t(4;14) and/or del (17p) cytogenetic abnormalities: the median PFS was 3.1 months, ORR was 23%, and median response duration was 4.9 months.
LEN-refractory disease.
For patients with LEN-refractory disease (n = 174), the median PFS was 3.8 months with POM+LoDEX and 2.2 months with POM alone (HR = 0.72, 95% CI = 0.52-0.99, P = .042) (Figure 2C). ORRs were 30% and 21%, respectively (P = .224) (Table 2). The median duration of response was 7.7 and 8.8 months, and median OS was 16.0 and 12.0 months, respectively (HR = 0.96, 95% CI = 0.68-1.34, P = .793) (Figure 2D).
LEN- and BORT-refractory disease.
Among patients with disease refractory to both LEN and BORT (n = 136), the median PFS was 3.8 months with POM+LoDEX and 2.0 months with POM alone (HR = 0.77, 95% CI = 0.53-1.11, P = .150) (Figure 2E). ORRs were 31% and 21%, respectively (P = .243) (Table 2). The median duration of response was 6.5 and 11.4 months in the POM+LoDEX and POM alone groups, respectively. The median OS was 13.4 and 12.5 months, respectively (HR = 1.05, 95% CI = 0.71-1.54, P = .814) (Figure 2F).
LEN as last prior therapy.
For patients who had received LEN as their last prior therapy (n = 77), the median PFS was 3.8 months with POM+LoDEX and 1.8 months with POM alone (HR = 0.52, 95% CI = 0.32-0.85, P = .008). ORRs were 25% and 15%, respectively. The median response duration was 6.2 months with POM+LoDEX and 8.4 months with POM alone, and the median OS was 16.6 and 8.5 months, respectively (HR = 0.71, 95% CI = 0.42-1.21, P = .205).
Prior CFZ.
Among patients who had received prior CFZ (n = 50), the median PFS was 4.7 months with POM+LoDEX and 2.8 months with POM alone (HR = 0.53, 95% CI = 0.28-0.99, P = .040). ORRs were 37% (7 of 19) and 10% (3 of 31), respectively (P = .030). The median response duration was 14.1 and 10.7 months with POM+LoDEX and POM alone, respectively. Median OS was 17.7 and 9.9 months, respectively (HR = 0.64, 95% CI = 0.34-1.20, P = .159).
Extramedullary disease.
A total of 5 patients receiving POM+LoDEX were observed to have extramedullary plasmacytomas. Of these, 2 achieved PR, 1 achieved SD, 1 attained minimal response, and 1 had progressive disease.
Safety
The most common grade 3-4 AE was neutropenia, which occurred in 41% of patients treated with POM+LoDEX and 48% of patients treated with POM alone (Table 3). The incidence of grade 3-4 febrile neutropenia was low in the POM+LoDEX and POM alone groups (3% and 5%, respectively). The most common grade 3-4 nonhematologic AE was pneumonia (22% with POM+LoDEX and 15% with POM alone). In the POM+LoDEX group, 27% of the cases of any grade pneumonia were also associated with dyspnea (any grade). The incidence of any grade deep-vein thrombosis was low (2% with POM+LoDEX and 3% with POM alone), and there were no cases of grade 3-4 peripheral neuropathy.
Table 3.
Grade 3-4 AEs, AEs leading to treatment modifications, and supportive care
| POM+LoDEX (n = 112) | POM alone (n = 107) | |
|---|---|---|
| Hematologic AEs occurring in ≥5% of patients, % | ||
| Neutropenia | 41 | 48 |
| Anemia | 22 | 24 |
| Thrombocytopenia | 19 | 22 |
| Leukopenia | 10 | 7 |
| Lymphopenia | 7 | 2 |
| Febrile neutropenia | 3 | 5 |
| Nonhematologic AEs occurring in ≥5% of patients, % | ||
| Pneumonia | 22 | 15 |
| Fatigue | 14 | 11 |
| Dyspnea | 13 | 8 |
| Back pain | 10 | 14 |
| Urinary tract infection | 9 | 2 |
| Sepsis | 5 | 6 |
| Dehydration | 5 | 5 |
| Acute renal failure | 5 | 8 |
| Muscular weakness | 4 | 6 |
| Blood creatinine increase | 3 | 6 |
| Confusional state | 3 | 7 |
| Hypercalcemia | 1 | 10 |
| AEs leading to dose reduction in ≥5% of patients, % | ||
| Thrombocytopenia | 5 | 9 |
| Neutropenia | 4 | 7 |
| AEs leading to dose interruption in ≥5% of patients, % | ||
| Neutropenia | 9 | 14 |
| Thrombocytopenia | 5 | 11 |
| Pneumonia | 18 | 12 |
| Upper respiratory tract infection | 5 | 9 |
| Fatigue | 8 | 4 |
| Pyrexia | 6 | 2 |
| Most common AEs leading to discontinuation in patients, % | ||
| Increased blood creatinine | 1 | 1 |
| Acute renal failure | 1 | 2 |
| Supportive-care use during study treatment, % | ||
| G-CSF | 46 | 58 |
| Epoetin alfa | 18 | 23 |
| Darbepoetin alfa | 12 | 26 |
| Red blood cell transfusion | 45 | 49 |
| Platelet transfusion | 14 | 20 |
Treatment-emergent AEs are defined as any AE occurring or worsening on or after the first treatment of the study medication and within 30 days after treatment-phase end date.
During study treatment, ∼50% of patients received G-CSF and ∼20% received erythroid growth factors (Table 3). Platelet and red blood cell transfusions were required in 17% and 47% of patients. Approximately one-third of patients required ≥1 POM dose reduction (29% with POM+LoDEX and 36% with POM alone), although dose reductions or interruptions due to neutropenia and thrombocytopenia were infrequent (Table 3). The rate of POM discontinuation due to treatment-related AEs was 3% (2% in the POM+LoDEX group and 5% in the POM group). The most common AEs leading to discontinuation of POM+LoDEX were renal failure and increased blood creatinine.
Of the 19 deaths that occurred during the study period, 10 occurred in the POM+LoDEX group and 9 in the POM alone group; the majority of deaths were attributed to MM and disease progression.
Discussion
POM, with or without LoDEX, an oral antimyeloma therapy, demonstrated clinical efficacy in this open-label, phase 2 part of a phase 1/2 study of patients with RRMM who had received multiple prior therapies, including LEN and BORT. As seen with LEN, the efficacy of POM was enhanced by the addition of DEX and the combination, compared with POM alone, significantly increased the median PFS (P = .003) and led to an impressive ORR of 33% (P = .013). Responses were durable with both POM+LoDEX and POM alone. The median OS was 16.5 and 13.6 months in the POM+LoDEX and POM groups, respectively, which compares favorably with historically reported 9-month survival rates for patients in whom currently approved novel therapies have failed.2
This study confirmed the efficacy and safety of POM when used at the maximum tolerated dose (4 mg/day on days 1-21 of each 28-day cycle) established in the phase 1 part of the study.18 Furthermore, the present study employed a “randomized design” to evaluate 2 experimental schedules.21 This showed ORRs of 33% with POM+LoDEX compared with 18% in the POM alone arm, which confirmed the synergistic effect of POM+LoDEX as observed in previous in vitro studies.6 LoDEX alone is not effective in this population of RRMM patients and therefore was not chosen as the comparator; POM+LoDEX was selected as the active arm in the subsequent phase 3 study of patients with advanced MM who had exhausted BORT and LEN treatment. The latter study further confirmed the benefits of POM+LoDEX in terms of PFS (4.0 months) and OS (12.7 months) vs high-dose DEX, the standard of supportive care (PFS 1.9 months and OS 8.1 months).22 Trials evaluating POM plus steroid-based regimens will assess whether the treatment of RRMM patients can be further advanced.23-28
The efficacy of POM+LoDEX was not affected by prior treatment; POM+LoDEX was as effective in patients who had received LEN-based treatment as their last prior therapy and who had disease that was refractory to LEN or both LEN and BORT.29 These findings replicate previous phase 2 studies15-17 and have now been confirmed by phase 3 data.22 Thus, there is currently no clinical evidence of cross-resistance between POM and LEN. We also found an ORR of 37% with POM+LoDEX in patients who had received previous CFZ. This is of particular interest, because in a similar population of RRMM patients (although less stringently defined because patients with SD on last therapy could be included), treatment with CFZ resulted in ORRs of 15% with a duration of response of 7.8 months and a median OS of 11.9 months.30
Patients with the cytogenetic abnormalities t(4;14) and/or del (17p) have a poor prognosis and represent an additional unmet medical need.31-33 POM+LoDEX resulted in an ORR of 23%, with a median response duration of 4.9 months in this subgroup, suggesting encouraging activity in patients with high-risk cytogenetic profiles and poor prognosis. The efficacy and safety of POM+LoDEX in this population will be further evaluated in IFM-2010-02 study (www.clinicaltrials.gov #NCT01745640). Further studies are warranted to evaluate the use of POM in combination with other novel agents, such as BORT or CFZ, in this high-risk population.
The primary AEs observed with POM, with or without LoDEX, were neutropenia, thrombocytopenia, and anemia. The incidence of infections was higher with POM+LoDEX than with POM, but the incidence of deep-vein thrombosis, which is generally increased when LEN or thalidomide are combined with DEX,34-37 was low (2%), with relatively simple thromboprophylaxis consisting mainly of oral aspirin (81-100 mg/day). Importantly, none of the patients developed grade 3-4 peripheral neuropathy, and other nonhematologic AEs were generally mild to moderate. With appropriate management, the rates of discontinuations due to treatment-related AEs were low (2% to 3%).
A potential limitation of this study is the open-label design, which may result in a bias in PFS assessment. A further limitation is that many patients (60%) assigned to POM received POM+LoDEX at progression, making it difficult to isolate the POM effect. In addition, the median PFS observed in this study (2.7-4.2 months) was also shorter than the original assumption of clinical relevance used for the power calculation (6.0-10.0 months). However, the sample size and power were based on comparison between the 2 groups, which was close to the protocol assumption (POM+LoDEX vs POM; HR = 0.60 per protocol vs observed HR = 0.68), and the difference remained statistically significant at the final analysis (P = .003).
In conclusion, this study confirms the synergistic action of POM+LoDEX and shows encouraging clinical efficacy in patients with RRMM who have exhausted multiple prior therapies, including BORT and LEN. The limited cross-resistance between POM and LEN supports the effectiveness of sequential use of immunomodulatory drugs, as well as combinations.38-40 Therefore, POM-based combination therapy regimens represent an important new treatment option for patients with RRMM for whom effective new therapies are urgently required.
Acknowledgments
The authors gratefully acknowledge the editorial support of Excerpta Medica (Adriana Stan and Eva Polk) in the preparation of this manuscript.
This study was funded by Celgene Corporation. The authors are fully responsible for all content and editorial decisions for this manuscript.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.G.R., D.S.S., R.B., S.J., J. Mikhael, M.C., M.H.Z., C.J., Z.Y., and K.C.A. conceived and designed the study; P.G.R., D.S.S., R.B., A.J., K.S., N.R., S. Lentzsch, J. Matous, M.C., and K.C.A. collected and assembled the data; P.G.R., D.S.S., R.V., C.C.H., R.B., S.J., C.C., S. Lonial, N.B., A.B., N.R., C.S., M.L., J. Mikhael, D.V., M.C., M.H.Z., C.J., Z.Y., and K.C.A. analyzed and interpreted the data; all authors contributed to writing of the manuscript and give final approval; R.B., A.J., N.B., N.R., C.S., S. Lentzsch, and J. Matous provided study material or patients.
Conflict-of-interest disclosure: P.G.R. has a consultancy/advisory role with Celgene Corporation, Millennium, J&J, Bristol-Myers Squibb, and Novartis; D.S.S. has a consultancy/advisory role and has received an honoraria payment from Celgene Corporation, Millennium, and Onyx; R.V. and N.R. have a consultancy/advisory role with Celgene Corporation; C.C.H. has a consultancy/advisory role with Celgene Corporation and has received research funding as well as an honoraria payment from Multiple Myeloma Research Foundation; R.B., A.B., and M.L. have received research funding from Celgene Corporation; S.J. has a consultancy/advisory role with Celgene Corporation, Millennium, and Merck; C.C. has received honoraria and research funding from Celgene Corporation; S. Lonial has a consultancy/advisory role with Celgene Corporation, Millennium, Bristol-Myers Squibb, Novartis, Onyx, and Sanofi; A.J., D.V., and J. Matous have a consultancy/advisory role with and have received an honoraria payment from Celgene Corporation; S.L. and N.B. have a consultancy/advisory role and have received an honoraria plus research funding from Celgene Corporation; K.S. has a consultancy/advisory role and has received an honoraria payment from Celgene Corporation and Janssen; C.S. has received an honoraria payment from Celgene Corporation; M.C., M.H.Z., C.J., and Z.Y., are employed by Celgene Corporation and are stock owners; and K.C.A. has a consultancy/advisory role with Celgene Corporation, Onyx, Gilead, and Sanofi and has stock options with Acetylon and Oncopep. The remaining author declares no competing financial interests.
Correspondence: Paul G. Richardson, Dana-Farber Cancer Insitute, 450 Brookline Ave, Boston, MA 02115; e-mail: paul_richardson@dfci.harvard.edu.
References
- 1.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79(7):867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Lee JH, Lahuerta JJ, et al. International Myeloma Working Group. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26(1):149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corral LG, Haslett PA, Muller GW, et al. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogues that are potent inhibitors of TNF-alpha. J Immunol. 1999;163(1):380–386. [PubMed] [Google Scholar]
- 4.Hideshima T, Chauhan D, Shima Y, et al. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96(9):2943–2950. [PubMed] [Google Scholar]
- 5.Mitsiades N, Mitsiades CS, Poulaki V, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99(12):4525–4530. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 6.Rychack E, Mendy D, Miller K, et al. Overcoming resistance; the use of pomalidomide (POM) and dexamethasone (DEX) in re-sensitizing lenalidomide (LEN)-resistant multiple myeloma (MM) cells. Haematologica. 2011;96(Suppl 1):S126. Abstract P-328. [Google Scholar]
- 7.Quach H, Ritchie D, Stewart AK, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24(1):22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escoubet-Lozach L, Lin IL, Jensen-Pergakes K, et al. Pomalidomide and lenalidomide induce p21 WAF-1 expression in both lymphoma and multiple myeloma through a LSD1-mediated epigenetic mechanism. Cancer Res. 2009;69(18):7347–7356. doi: 10.1158/0008-5472.CAN-08-4898. [DOI] [PubMed] [Google Scholar]
- 9.Koh KR, Janz M, Mapara MY, et al. Immunomodulatory derivative of thalidomide (IMiD CC-4047) induces a shift in lineage commitment by suppressing erythropoiesis and promoting myelopoiesis. Blood. 2005;105(10):3833–3840. doi: 10.1182/blood-2004-03-0828. [DOI] [PubMed] [Google Scholar]
- 10.Xu Y, Li J, Ferguson GD, et al. Immunomodulatory drugs reorganize cytoskeleton by modulating Rho GTPases. Blood. 2009;114(2):338–345. doi: 10.1182/blood-2009-02-200543. [DOI] [PubMed] [Google Scholar]
- 11.Anderson G, Gries M, Kurihara N, et al. Thalidomide derivative CC-4047 inhibits osteoclast formation by down-regulation of PU.1. Blood. 2006;107(8):3098–3105. doi: 10.1182/blood-2005-08-3450. [DOI] [PubMed] [Google Scholar]
- 12.Schey SA, Fields P, Bartlett JB, et al. Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol. 2004;22(16):3269–3276. doi: 10.1200/JCO.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 13.Streetly MJ, Gyertson K, Daniel Y, Zeldis JB, Kazmi M, Schey SA. Alternate day pomalidomide retains anti-myeloma effect with reduced adverse events and evidence of in vivo immunomodulation. Br J Haematol. 2008;141(1):41–51. doi: 10.1111/j.1365-2141.2008.07013.x. [DOI] [PubMed] [Google Scholar]
- 14.Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009;27(30):5008–5014. doi: 10.1200/JCO.2009.23.6802. [DOI] [PubMed] [Google Scholar]
- 15.Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma (MM). Leukemia. 2010;24(11):1934–1939. doi: 10.1038/leu.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacy MQ, Allred JB, Gertz MA, et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood. 2011;118(11):2970–2975. doi: 10.1182/blood-2011-04-348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leleu X, Attal M, Arnulf B, et al. Intergroupe Francophone du Myélome. Pomalidomide plus low-dose dexamethasone is active and well tolerated in bortezomib and lenalidomide-refractory multiple myeloma: Intergroupe Francophone du Myélome 2009-02. Blood. 2013;121(11):1968–1975. doi: 10.1182/blood-2012-09-452375. [DOI] [PubMed] [Google Scholar]
- 18.Richardson PG, Siegel D, Baz R, et al. Phase 1 study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121(11):1961–1967. doi: 10.1182/blood-2012-08-450742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bladé J, Samson D, Reece D, et al. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Br J Haematol. 1998;102(5):1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 20.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 21.Cannistra SA. Phase II trials in journal of clinical oncology. J Clin Oncol. 2009;27(19):3073–3076. doi: 10.1200/JCO.2009.23.1811. [DOI] [PubMed] [Google Scholar]
- 22.Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 23.Palumbo A, Larocca A, Montefusco V, et al. Pomalidomide cyclophosphamide and prednisone (PCP) treatment for relapsed/refractory multiple myeloma. Blood. 2012;120(21) Abstract 446. [Google Scholar]
- 24.Baz R, Shain KH, Alsina M, et al. Oral weekly cyclophosphamide in combination with pomalidomide and dexamethasone for relapsed and refractory myeloma: report of the dose escalation cohort. Blood. 2012;120(21) Abstract 4062. [Google Scholar]
- 25.Berenson JR, Hilger JD, Klein LM, et al. A phase 1/2 study of pomalidomide, dexamethasone and pegylated liposomal doxorubicin for patients with relapsed/refractory multiple myeloma. Blood. 2012;120(21) Abstract 2979. [Google Scholar]
- 26.Mark TM, Boyer A, Rossi AC, et al. ClaPD (clarithromycin, pomalidomide, dexamethasone) therapy in relapsed or refractory multiple myeloma. Blood. 2012;120(21) doi: 10.1182/bloodadvances.2018028027. Abstract 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson PG, Hofmeister CC, Siegel S, et al. MM-005: a phase 1, multicenter, open-label, dose-escalation study to determine the maximum tolerated dose for the combination of pomalidomide, bortezomib, and low-dose dexamethasone in subjects with relapsed or refractory multiple myeloma. Blood. 2012;120(21) Abstract 727. [Google Scholar]
- 28.Shah JJ, Stadtmauer EA, Abonour R, et al. A multi-center phase I/II trial of carfilzomib and pomalidomide with dexamethasone (Car-Pom-d) in patients with relapsed/refractory multiple myeloma. Blood. 2012;120(21) Abstract 74. [Google Scholar]
- 29.Vij R, Richardson PG, Jagannath S, et al. Pomalidomide (POM) with or without low-dose dexamethasone (LoDEX) in patients (pts) with relapsed/refractory multiple myeloma (RRMM): outcomes in pts refractory to lenalidomide (LEN) and/or bortezomib (BORT). J Clin Oncol. 2012;30(15) Abstract 8016. [Google Scholar]
- 30.Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munshi NC, Anderson KC, Bergsagel PL, et al. International Myeloma Workshop Consensus Panel 2. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117(18):4696–4700. doi: 10.1182/blood-2010-10-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hervé AL, Florence M, Philippe M, et al. Molecular heterogeneity of multiple myeloma: pathogenesis, prognosis, and therapeutic implications. J Clin Oncol. 2011;29(14):1893–1897. doi: 10.1200/JCO.2010.32.8435. [DOI] [PubMed] [Google Scholar]
- 33.Avet-Loiseau H, Attal M, Campion L, et al. Long-term analysis of the IFM 99 trials for myeloma: cytogenetic abnormalities [t(4;14), del(17p), 1q gains] play a major role in defining long-term survival. J Clin Oncol. 2012;30(16):1949–1952. doi: 10.1200/JCO.2011.36.5726. [DOI] [PubMed] [Google Scholar]
- 34.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. International Myeloma Working Group. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22(2):414–423. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- 35.von Lilienfeld-Toal M, Hahn-Ast C, Furkert K, et al. A systematic review of phase II trials of thalidomide/dexamethasone combination therapy in patients with relapsed or refractory multiple myeloma. Eur J Haematol. 2008;81(4):247–252. doi: 10.1111/j.1600-0609.2008.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dimopoulos M, Spencer A, Attal M, et al. Multiple Myeloma (010) Study Investigators. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 37.Weber DM, Chen C, Niesvizky R, et al. Multiple Myeloma (009) Study Investigators. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357(21):2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 38.Lonial S, Mitsiades CS, Richardson PG. Treatment options for relapsed and refractory multiple myeloma. Clin Cancer Res. 2011;17(6):1264–1277. doi: 10.1158/1078-0432.CCR-10-1805. [DOI] [PubMed] [Google Scholar]
- 39.Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist. 2007;12(6):664–689. doi: 10.1634/theoncologist.12-6-664. [DOI] [PubMed] [Google Scholar]
- 40.Laubach JP, Mahindra A, Mitsiades CS, et al. The use of novel agents in the treatment of relapsed and refractory multiple myeloma. Leukemia. 2009;23(12):2222–2232. doi: 10.1038/leu.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]