Abstract
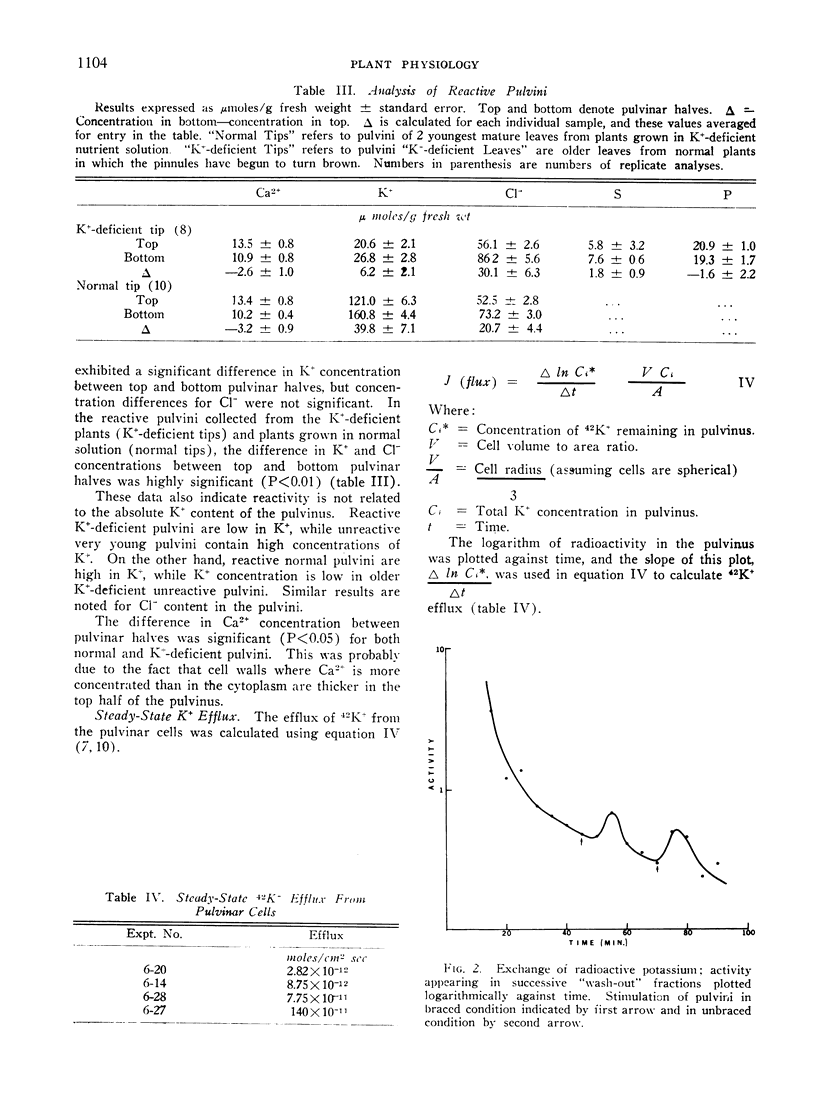
The efflux of K+ from the pulvinar cells of Mimosa pudica was shown to increase substantially during the seismonastic reaction. This result is shown to indicate a decrease in σ (reflection coefficient) of pulvinar cell membrane for potassium salts which could account for the pulvinar cell turgor decrease during the seismonastic reaction.
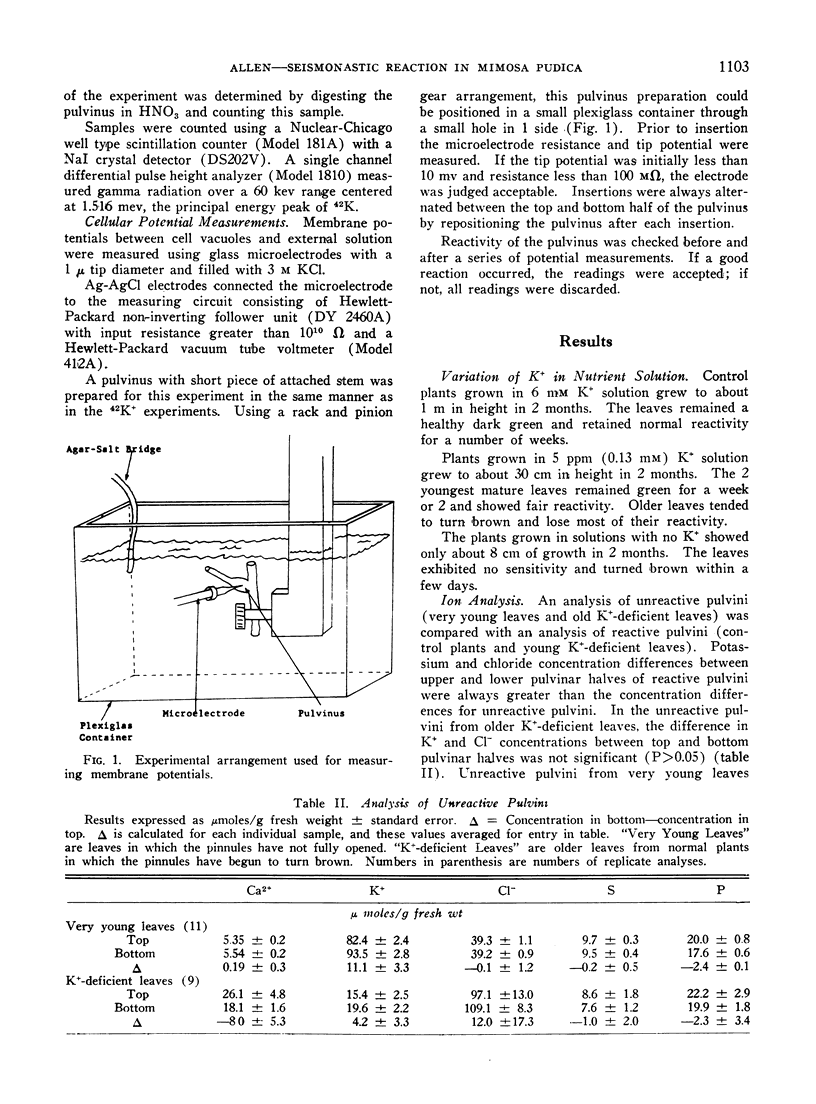
Membrane potentials and concentrations of Ca2+, K+, Cl-, S, and P were measured in the top and bottom halves of pulvini. Pulvinar cells showed a large negative membrane potential, cell vacuole relative to external solution, but no significant difference in membrane potential could be detected between upper and lower pulvinar cells. A large difference in K+ and Cl- concentration between top and bottom pulvinar halves was evident in reactive pulvini but not in unreactive pulvini. The effect of K+ concentration on plant growth and leaf reactivity was also investigated.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F., HODGKIN A. L., SHAW T. I. The effects of changes in internal ionic concentrations on the electrical properties of perfused giant axons. J Physiol. 1962 Nov;164:355–374. doi: 10.1113/jphysiol.1962.sp007026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAMOND J. M., SOLOMON A. K. Intracellular potassium compartments in Nitella axillaris. J Gen Physiol. 1959 May 20;42(5):1105–1121. doi: 10.1085/jgp.42.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dainty J., Ginzburg B. Z. Irreversible thermodynamics and frictional models of membrane processes, with particular reference to the cell membrane. J Theor Biol. 1963 Sep;5(2):256–265. doi: 10.1016/0022-5193(63)90063-8. [DOI] [PubMed] [Google Scholar]
- GAFFEY C. T., MULLINS L. J. Ion fluxes during the action potential in Chara. J Physiol. 1958 Dec 30;144(3):505–524. doi: 10.1113/jphysiol.1958.sp006116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Effect of External K, NH(4), Na, Ca, Mg, and H Ions on the Cell Transmembrane Electropotential of Avena Coleoptile. Plant Physiol. 1964 Mar;39(2):196–203. doi: 10.1104/pp.39.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. J. The influence of the intracellular potential on potassium uptake by beetroot tissue. J Gen Physiol. 1966 Jan;49(3):551–563. doi: 10.1085/jgp.49.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]



