Abstract
Background and Aims
The biomass-ratio hypothesis states that ecosystem properties are driven by the characteristics of dominant species in the community. In this study, the hypothesis was operationalized as community-weighted means (CWMs) of monoculture values and tested for predicting the decomposition of multispecies litter mixtures along an abiotic gradient in the field.
Methods
Decomposition rates (mg g−1 d−1) of litter from four herb species were measured using litter-bed experiments with the same soil at three sites in central France along a correlated climatic gradient of temperature and precipitation. All possible combinations from one to four species mixtures were tested over 28 weeks of incubation. Observed mixture decomposition rates were compared with those predicted by the biomass-ratio hypothesis. Variability of the prediction errors was compared with the species richness of the mixtures, across sites, and within sites over time.
Key Results
Both positive and negative prediction errors occurred. Despite this, the biomass-ratio hypothesis was true as an average claim for all sites (r = 0·91) and for each site separately, except for the climatically intermediate site, which showed mainly synergistic deviations. Variability decreased with increasing species richness and in less favourable climatic conditions for decomposition.
Conclusions
Community-weighted mean values provided good predictions of mixed-species litter decomposition, converging to the predicted values with increasing species richness and in climates less favourable to decomposition. Under a context of climate change, abiotic variability would be important to take into account when predicting ecosystem processes.
Keywords: Biomass-ratio hypothesis, BMRH, climatic gradient, community-aggregated traits, community-weighted means, Festuca rubra, litter decomposition, Lolium perenne, Taraxacum officinale, Trifolium repens
INTRODUCTION
Predicting ecosystem function in a changing environment is a fundamental challenge for ecologists. Ecosystems are characterized by complex interactions, both between component species and between these species and abiotic conditions. Consequently, ecosystem processes reflect the combined properties and performances of the constituent species in a changing environment; this complexity makes their study and prediction difficult in a general context.
One way to link the aggregated response of an ecosystem or a community property with the effects of each species is provided by Grime's ‘biomass-ratio hypothesis’ (Grime, 1998). This hypothesis states that ecosystem properties are driven by the characteristics of dominant species in the community. This has been shown in various ecosystem processes (e.g. Mokany et al., 2008). The biomass-ratio hypothesis was further operationalized by Garnier et al. (2004) via community-weighted (or ‘aggregated’) means. This is formalized in Eqn (1), in which eic is the average per capita effect of species i in community c, pic is the relative abundance of species i and Ec is the community-weighted effect on the ecosystem summed over all S species present. The average per capita effects of each species (eic) can be further decomposed into the contributions of different functional traits possessed by each species.
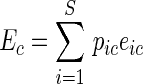 |
(1) |
As required by Garnier's version of the biomass-ratio hypothesis, this equation calculates the overall ecosystem response (E) as an additive function of individual species effects (piei). If synergistic or antagonistic interactions are present, then the biomass-ratio may not be an accurate predictor of the observed responses. If so, the biomass-ratio hypothesis would not hold because the effect of a particular species on the ecosystem would depend not only on its relative abundance, but also on the combination of species in the community.
Some studies have shown that litter decomposition rates can be correlated to functional traits such as leaf carbon and nitrogen content or leaf dry matter content (e.g. Kazakou et al., 2006; Santiago, 2007; Fortunel et al., 2009). In this context, Lavorel and Garnier (2002) and Suding et al. (2008) proposed a framework which differentiates between environmental responses (response traits) and effects of environmental change on ecosystem processes (effect traits). Our article refers to the decomposition rate as a single synthetic effect trait.
The decomposition of mixed-species plant litter, the subject of our study, provides an interesting model in this context because it constitutes an important part of the nutrient cycle and because there is already a large literature on this topic, although relatively few of these studies concern the effect of species richness in interspecific mixtures of litter (Wardle et al., 1997; reviewed by Gartner and Cardon, 2004; Hättenschwiler et al., 2005). Since litter decomposes in mixtures in most ecosystems, and since interactions occur frequently within communities, interspecific litter mixtures often show synergistic or antagonistic effects. Among the possible mechanisms for interspecific interactions, the review by Hättenschwiler et al. (2005) identified nutrient transfer, the effects of specific secondary compounds, interactions across trophic levels and improved microenvironmental conditions. Such non-additive effects have been reported in many decomposition experiments involving species mixtures (Gartner and Cardon, 2004). These non-additive interactions should therefore induce a bias in the prediction of mixed-species decomposition rates based on monoculture values (e.g. Wardle et al., 1997; Hector et al., 2000; Perez-Harguindeguy et al., 2008).
Considering this potential source of error, one could expect that predictions based on the biomass-ratio hypothesis would be incorrect since they implicitly assume that there are no interactions between species. However, some studies (Perez-Harguindeguy et al., 2008; Pakeman et al., 2011) showed that the use of the biomass-ratio hypothesis can still be accurate since the observed values did not produce any overall bias relative to predicted values. How can a hypothesis that assumes the absence of interspecific interactions provide accurate predictions of a phenomenon in which such interactions are common? Our study aims to explain such an apparent paradox.
Based on the ‘idiosyncratic hypothesis’ of Lawton (1994), an ‘idiosyncratic annulment’ hypothesis (Tardif and Shipley, 2013) was recently proposed to explain why the biomass-ratio hypothesis often provides good predictions of mixed-species litter decomposition despite the common presence of interactions between species. It assumes that interactions exist, but, being equally likely to be both positive or negative, they cancel each other out, leading to a global average rate around the predicted value. Furthermore, increasing the number of species increases the number of potential interactions which more fully cancel each other out, leading to a better prediction, i.e. through a decrease of the variation in observed decomposition rates between mixtures having the same number of species (Tardif and Shipley, 2013). However, Tardif and Shipley's (2013) empirical demonstration was conducted for tree species under controlled conditions, and so might not be applicable to ecosystems or communities in situ. Indeed, many environmental factors (e.g. temperature, humidity, soil pH, differing decomposer communities) affect litter decomposition rates (e.g. Berg et al., 1993; Aerts, 1997; Murphy et al., 1998; Cornwell et al., 2008; Zhang et al., 2008; Bontti et al., 2009) and environmental variability could strongly affect species interactions in mixture.
In particular, if the abiotic conditions drive deviations in a particular direction (synergistic or antagonistic), and thus unbalance the equilibrium between positive and negative deviations, then the assumption of symmetrical bias around zero required by idiosyncratic annulment would be false. Moreover, as more limiting abiotic conditions reduce the overall rate of decomposition, a reduction of the variability between mixtures would be expected.
Among the abiotic conditions known to affect decomposition rates, mean annual temperature (MAT) and mean annual precipitation (MAP) are often used in litter decomposition experiments (e.g. Epstein et al., 2002; Zhang et al., 2008; Bontti et al., 2009). We therefore concentrate on variation in MAT and MAP. Thus, under natural conditions, the effects of abiotic environmental variability (i.e. climate) represent a second source of error in the prediction of decomposition rates, especially in species mixtures. We expect that abiotic conditions limiting to decomposition will reduce the potential variability of decomposition rates between species. In our study, we tested the ‘idiosyncratic annulment’ hypothesis on abiotic variability, applied to the decomposition of grassland litter. We measured both within- and between-site variability in decomposition, the latter based on a correlated gradient of both temperature and precipitation, which is described in more detail below.
Here, we report a litterbag experiment along a climatic gradient to examine the effects of the species richness and the abiotic conditions on litter decomposition. Specifically, we tested (1) whether the observed specific decomposition rates in mixed-species litters are equal to the community-weighted values obtained in monocultures (i.e. the biomass-ratio hypothesis); (2) whether the variation in observed specific decomposition rates between mixtures having the same number of species will decrease as the number of species in the mixture increases due to idiosyncratic annulment; and (3) whether the variation of decomposition rates between litter mixtures decreases as the abiotic conditions of the site become more limiting to decomposition, i.e. with lower measured average decomposition rates.
MATERIALS AND METHODS
Study sites
The experiment was conducted in three sites distributed along correlated altitudinal–climatic gradients near Clermont-Ferrand in central France: Laqueuille (45°38′33″N, 2°44′11″E, 1045 m a.s.l.), Theix (45°43′22″N, 3°1′21″E, 880 m a.s.l.) and Crouël (45°46′32″N, 3°8′48″E, 340 m a.s.l.). These sites have a contrasting climate. Laqueuille is the coolest (MAT = 8·0 °C) and wettest (MAP = 1100 mm), Theix is intermediate (MAT = 8·7 °C and MAP = 830 mm) and Crouël is hottest (MAT = 12·0 °C) and driest (MAP = 570 mm, with a drought in August). The climatic data were provided by CLIMATIK, an agroclimatic database (MétéoFrance and INRA convention). However, this experimental design was not made to test a single climatic factor such as temperature or precipitation, but rather to test a gradient from more favourable to more limiting abiotic conditions for decomposition.
Construction of litter bags
The experiment involved a set of four herb species: common dandelion (Taraxacum officinale F.H. Wigg), white clover (Trifolium repens L.), perennial ryegrass (Lolium perenne L.) and red fescue (Festuca rubra L.). The species were chosen in order to maximize the differences in leaf functional traits known to affect decomposition rates, especially leaf dry matter content (Kazakou et al., 2006; Fortunel et al., 2009; Pakeman et al., 2011) among the species present in our sites; leaf dry matter content values from Grime et al. (2007) are 137 (T. officinale), 179 (T. repens), 212 (L. perenne) and 267 mg g−1 (F. rubra). Litter of the four herbaceous species was gathered in autumn 2009 by cutting the senescent leaves of several individuals from monocultures grown in a field collection at Crouël (all species except red fescue) and Laqueuille (red fescue). All leaf litter was air-dried at 40 °C and then 1·5 g was gently placed in each litterbag with equal proportions for the mixed-species litters. Litterbags (10 × 11 cm) were made of fibreglass fabric (approx. 1 mm mesh size). After being thermosealed, each litterbag was weighed.
Experimental design
Litterbags were placed upon the soil surface of a standardized ‘litter bed’ (Cornelissen, 1996; Pérez-Harguindeguy et al., 2000), then covered and held down by chicken wire (approx. 25 mm mesh size).The same organic superficial layer of soil from Theix was placed into a 20 cm deep pit (4·6 × 4 m) in each site, after removing rocks and weeds. Since the four species tested in this experiment are naturally common in Theix, we expect this latter to contain appropriate micro- and mesofauna for decomposition. During the entire experiment, from 13 April to 26 October 2010, the ground was kept bare by manual weeding, to avoid interactions between vegetation and decomposition.
We used each litter monoculture and all possible combinations of two, three and four species, giving 15 different species compositions. Each species composition and harvest time was replicated in three blocks per site. Litterbags were randomly chosen within each block and destructively harvested after 15, 28, 42, 70, 126 and 196 d (i.e. six decomposition periods). At each harvest, litterbags were dried for 1 week at 40 °C, gently cleaned to remove dirt, and weighed. Thus the full experiment consisted of 3 sites × 3 replicates (blocks) per site × 6 litterbags per mixture type and 15 different mixture types.
Statistical analysis
Modelling the dynamics of litter decomposition
To model the dynamics of litter decomposition, we applied statistical analysis according to Tardif and Shipley (2013). The main points are provided here.
We first plotted
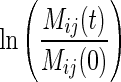 |
as a function of time, where Mij(t) is the mass of the litter mixture i in litterbag j at time t, and Mij(0) is the initial mass of litter mixture i (1·5 ± 0·1 g) in litterbag j. As we observed non-linear trends, we did not use the simple negative exponential model of Olson (1963) (i.e. linear on a semi-log scale) but instead we modelled the ln-transformed proportional mass loss as a quadratic function of time [Eqn (2)], which gave a better empirical fit to our data and where ai, bi and ci are coefficients of the equation for mixture i, t is the harvest date (days) and εij is the deviation of mixture i in litterbag j from its predicted value.
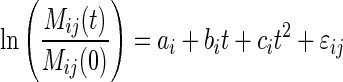 |
(2) |
Of course, this quadratic function makes no biological sense if extrapolated beyond our data, since it would eventually result in a mass gain. As in Tardif and Shipley (2013), we used mixed models, because the mass loss depends on both fixed (time) and random (the error between litterbags of a given mixture) effects.
Obtaining observed decomposition rates, and those predicted by the biomass-ratio hypothesis
A first derivative as a function of time of Eqn (2) provided ki(t), i.e. the observed decomposition rates of mixture i at each time t [Eqn (3)].
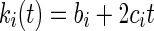 |
(3) |
A standard linear mixed model regression was fitted separately for monocultures and litter mixtures and each study site. We used the lmer function of the lme4 package of R (Pinheiro and Bates, 2000; Bates, 2005; Bates and Sarkar, 2006) in which the random components were ‘species’ and ‘mixture’, with separate analyses for monocultures and mixtures, respectively. The mixed model regression provided coefficients bi and ci of Eqn (2) and Eqn (3), allowing us to determine observed k-values for each monoculture and mixture at each harvest date, as decomposition rate varies with time.
For each litter mixture involving at least two species, the specific decomposition rate 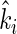 that would occur in that given mixture was calculated, assuming that the mass-ratio hypothesis is strictly true. Accordingly,
that would occur in that given mixture was calculated, assuming that the mass-ratio hypothesis is strictly true. Accordingly,
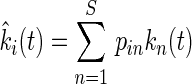 |
where pnj = 1/S is the initial mass proportion of species n in the mixture i, and kn is the empirically estimated (observed) specific decomposition rate of species n in mixture i when measured in monoculture at time t. These values were obtained by calculating the community-weighted values of bi and ci for mixture i, i.e.
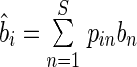 and
and 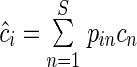 and substituting these into Eqn (3). We thus obtained Eqn (4), with the predicted decomposition rate of mixture i at time t.
and substituting these into Eqn (3). We thus obtained Eqn (4), with the predicted decomposition rate of mixture i at time t.
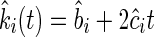 |
(4) |
Testing the mass-ratio hypothesis
The biomass-ratio hypothesis assumes that the mean observed decomposition of a mixture within a site is equal to the values predicted from a community-weighted approach [Eqn (5a)], with εij representing the residual variation around a single mixture.
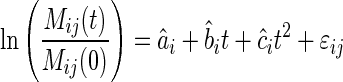 |
(5a) |
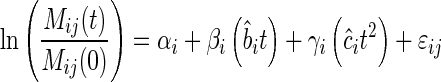 |
(5b) |
To test this hypothesis, two new variables were created for each mixture by multiplying the community-weighted values of each 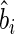 and
and 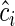 by time and its square, respectively. For each site, we then fitted a mixed-model regression following Eqn (5b) but also including the litter mixtures as the between-group random variable. If the biomass-ratio hypothesis is strictly true, then the estimated coefficients of β and γ are both equal to 1 and there will be no significant between-mixture variation associated with these parameters. If the biomass-ratio hypothesis is true on average, but not for every mixture, then the estimated coefficients of β and γ are both equal to 1 but there will be significant between-mixture variation associated with these parameters. If the biomass-ratio hypothesis is not true even on average, then one or both of β and γ will be significantly different from 1.
by time and its square, respectively. For each site, we then fitted a mixed-model regression following Eqn (5b) but also including the litter mixtures as the between-group random variable. If the biomass-ratio hypothesis is strictly true, then the estimated coefficients of β and γ are both equal to 1 and there will be no significant between-mixture variation associated with these parameters. If the biomass-ratio hypothesis is true on average, but not for every mixture, then the estimated coefficients of β and γ are both equal to 1 but there will be significant between-mixture variation associated with these parameters. If the biomass-ratio hypothesis is not true even on average, then one or both of β and γ will be significantly different from 1.
Testing the hypothesis of idiosyncratic annulment
Idiosyncratic annulment occurs when the biomass-ratio hypothesis is not strictly true, i.e. when the ecosystem response (mixed-species litter decomposition) is not simply the effect contributed by each species weighted by its relative abundance, but when the differences between the observed effect and that predicted by the biomass-ratio hypothesis tend to cancel out more completely as the species richness of the community increases. We tested this in two ways.
To test this hypothesis, we first used Monte Carlo simulations to describe the probability distribution of the standard deviation between the community-weighted decomposition rates of different mixtures having the same number of species, by assuming that this variation is due solely to sampling variation arising from different species compositions in the mixture; see supplementary data of Tardif and Shipley (2013). This is necessary because a decreasing variance with increasing species richness is not necessarily due to an idiosyncratic annulment, but could be a mathematical consequence of community-weighted means (CWMs) being sums of random variables. Given the same assumptions underlying the mixed-model regressions, the sampling distribution is found to be multivariate normal, the dimension of which is equal to the number of unique mixtures having the same number of species. The elements in the covariance matrix are a function of (1) the variance in monoculture decomposition rates; (2) the number of species in the mixtures; and (3) the number of species in common between pairs of mixtures. The Monte Carlo simulation generates random draws from this multivariate normal distribution using the mvrnorm function of the mass library of R (for details see supplementary data of Tardif and Shipley, 2013). This Monte Carlo distribution was then compared with the measured standard deviation of mixture decomposition rates. Next, the standard deviation of the observed deviations between observed and predicted (CWM) decomposition rates was regressed on the number of species per mixture (two, three or five), and a permutation test was used (Manly, 1997) to compare slopes, which were assumed to be independent of the species richness of the mixture. Simulations were done in R version 2·12·0 (R Development Core Team, 2010; see also Tardif and Shipley, 2013).
RESULTS
A quadratic function for modelling the dynamics of litter decomposition
Visual inspection of ln(mass loss) over time suggested a non-linear response. We therefore fitted a linear mixed model regressing the natural logarithm of observed mass ratio (remaining mass divided by initial mass) as a function of ‘site’, ‘time’, ‘time2’ and interactions (as fixed factors), and ‘species mixture’ nested in ‘time’ and ‘time2’ (as random terms). An analysis of variance (ANOVA) identified highly significant (P < 0·001) effects associated with all fixed effects factors: sites, harvest times, squared time and interactions with site. In particular, adding a quadratic term for time significantly improved the model (F-value = 127·72, P-value <0·001). As significant differences between sites were also found, we thus used a quadratic model to model the dynamic of decomposition, for each site separately. This implies a varying decomposition rate over time.
Empirical patterns of mass loss
Mass loss during the experimental period ranged from 16 to 86 % across mixtures (Fig. 1). There was an initial rapid rate of mass loss, which then decreased with time (Fig. 1). An initial examination of the data also showed a much stronger variability in the site of Laqueuille (the coolest and wettest site) than in Theix (the intermediate site) and Crouël (the hottest and driest site).
Fig. 1.
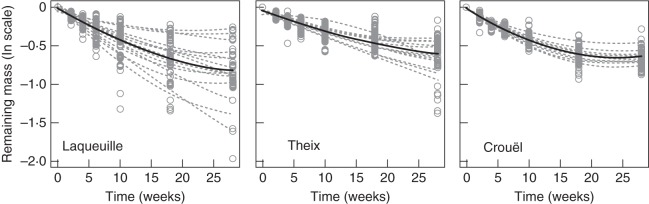
The natural logarithm of the observed remaining mass of leaf litter as a proportion of initial mass, plotted against time (weeks) from the beginning of the experiment, for each litterbag and each of three sites (open circles). Dotted grey and solid black lines represent the quadratic function of each mixture (mono- and polycultures) and their mean estimate, respectively.
The biomass-ratio hypothesis
Our first hypothesis was that the observed specific decomposition rates in mixed-species litter mixtures were equal to the community-weighted values (k CWM) obtained from monocultures (i.e. the biomass-ratio hypothesis), within each site. For a given site, the biomass-ratio hypothesis is strictly true if neither of the slope coefficients β and γ [Eqn (5b)] differs significantly from 1 and if there is also no significant between-mixture variance associated with them. The biomass-ratio hypothesis is true as an average claim but not true in every litter mixture if there is a significant between-mixture variance associated with either β or γ. Our results showed a significant between-mixture variance of the two fixed slopes β and γ for each of the tested sites in any site (P = 0·002, <0·001 and <0·001 for Crouël, Theix and Laqueuille, respectively). Notably, the fixed slopes estimates did not differ from unity in both Laqueuille and Crouël (Fig. 2), supporting the biomass-ratio hypothesis on average but not in every mixture. However, in Theix, β and γ were both different from unity. In this site, the biomass-ratio hypothesis was therefore rejected (Fig. 2).
Fig. 2.
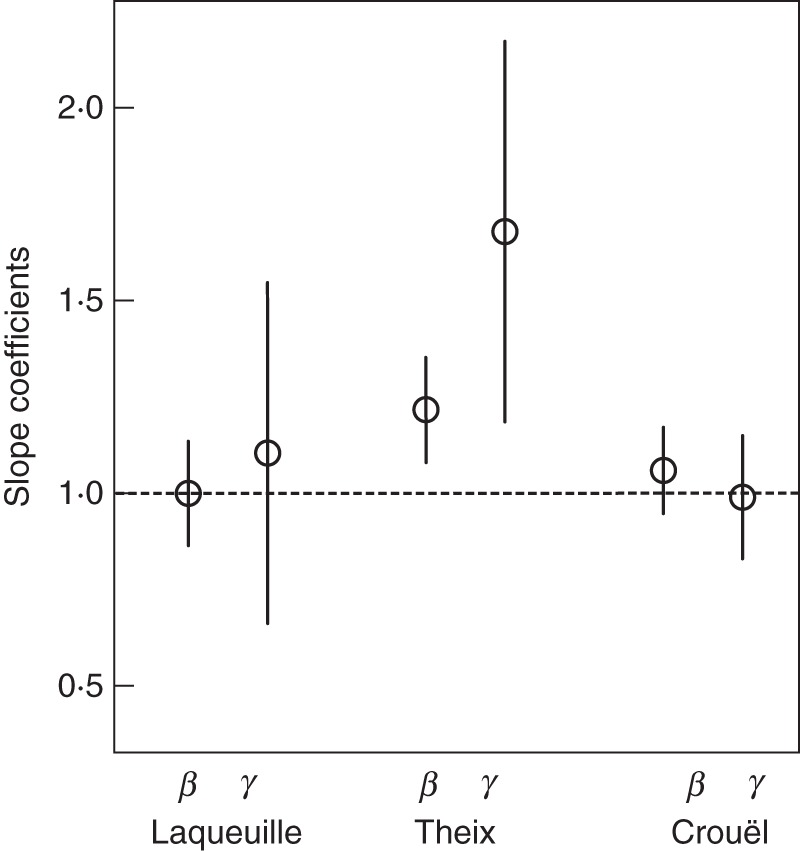
Estimated slope coefficients β and γ (circles) from the mixed-model regression testing of the biomass-ratio hypothesis for each site; vertical lines show the variation (± 2 s.e.). The horizontal broken line indicates the values predicted by the biomass-ratio hypothesis.
At the Laqueuille and Crouël sites, the measured decomposition rates in mixtures showed no systematic bias and good correlations with respect to the predicted values based on the independently measured monoculture values and the biomass-ratio hypothesis (Fig. 3). Both positive and negative deviations were observed relative to the predicted rates, but a general 1:1 relationship exists even though there is no statistical necessity for this relationship. However, the site of Theix showed mainly synergistic deviations (decomposition rates with higher absolute values than those predicted by the CWM) which contradict the biomass-ratio hypothesis, even as an average claim.
Fig. 3.
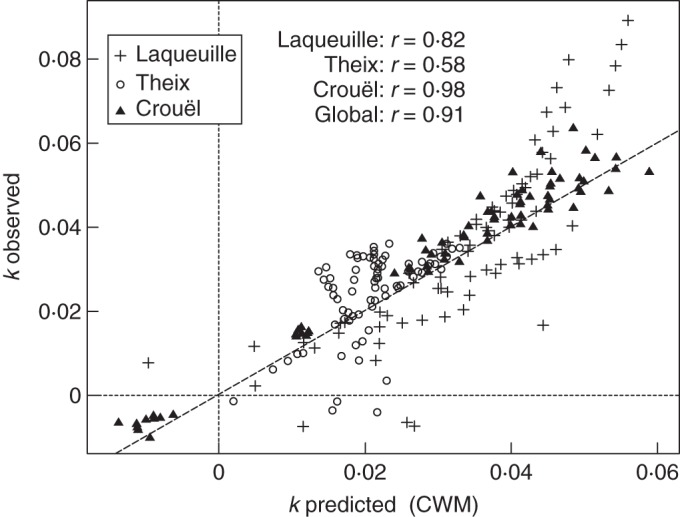
The observed decomposition rates (g g−1 week−1) of multispecies litter mixtures are plotted against the predicted decomposition rates based on monoculture decomposition rates weighted by relative abundance, for each site (CWM, in g g−1 week−1). The dashed line represents the 1:1 relationship, i.e. absence of deviation. Pearson's correlation coefficient (r) was calculated for each site and globally.
The ‘idiosyncratic annulment’ hypothesis
The variability of observed decomposition rates, measured as the standard deviation in k-values between mixtures having the same number of species, decreased with increasing number of species in the mixture (from one to three species, Fig. 4). Although results at Theix and Crouël showed similar variability with two- and three-species mixtures, the decreasing variability was clearest for the coolest and wettest site (Laqueuille), at all levels of species richness.
Fig. 4.
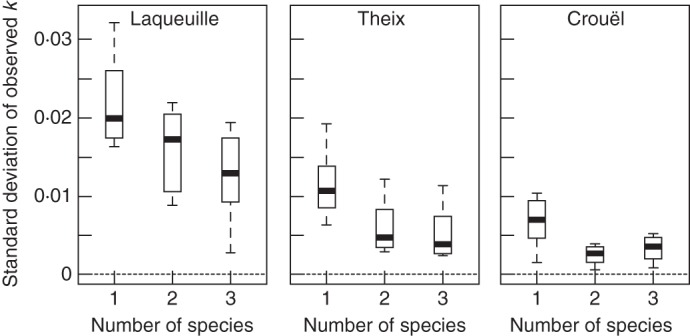
Standard deviation (g g−1 week−1) of observed decomposition rates (k-values) against the number of species, at each site and for monocultures, and two- and three-species mixtures. Boxes, horizontal bold line and whiskers represent the 25th and 75th percentiles, the median and the maximum values, respectively.
We then used Monte Carlo simulations to determine if the observed variability of the k-values was less than expected based purely on the fact that CMWs are sums of random variables. Using 20 000 Monte Carlo simulations for each, we compared the measured range of k-values for litters having the same number of species with the range of k-values expected, assuming that this variation was due solely to sampling variation arising from different species compositions, as explained in the Materials and Methods. In both Laqueuille and Theix, for two- and three-species mixtures and at all times, the variation of observed values was consistent with the values of Monte Carlo simulations (e.g. at 18 weeks, Laqueuille, P = 0·813, 0·177; Theix, P = 0·440, 0·785 for the two- and three-species mixtures, respectively). In Crouël, however, at 18 weeks for both two- and three-species mixtures and 28 weeks for two-species mixtures, the variations of observed values were significantly lower than the values of Monte Carlo simulations (e.g. P = 0·001 and 0·012 for the two- and three-species mixtures, respectively, at 18 weeks). Thus, the decrease in variability of decomposition rates between mixtures having the same number of species was consistent with mathematical expectations across sites and harvest dates, except at two harvest dates at Crouël; here the significantly lower variability indicates a biological effect.
To test further the idiosyncratic annulment sensu stricto, permutation tests were applied to determine if the observed variability significantly decreases as the number of species in the mixtures increases, at a given time. Analyses based on 50 000 permutations could not detect a significant decrease in the variability of the prediction errors with increase of species richness (e.g. at 18 weeks, P = 0·538, 0·462 and 0·621 for Laqueuille, Theix and Crouel, respectively).
The climate effect
The mean decomposition rates of Laqueuille (the coldest and wettest site) and Crouël (the hottest and driest site) were similar at the beginning of the experiment, then diverged (Fig. 5A). The site of Laqueuille showed the most favourable conditions for decomposition on average, with the highest mean k-values calculated over mixtures and dates. The climatically intermediate site of Theix had the slowest mean decomposition rate at the beginning of the experiment, but then increased to reach a rate similar to that of Laqueuille at the end of the experiment.
Fig. 5.
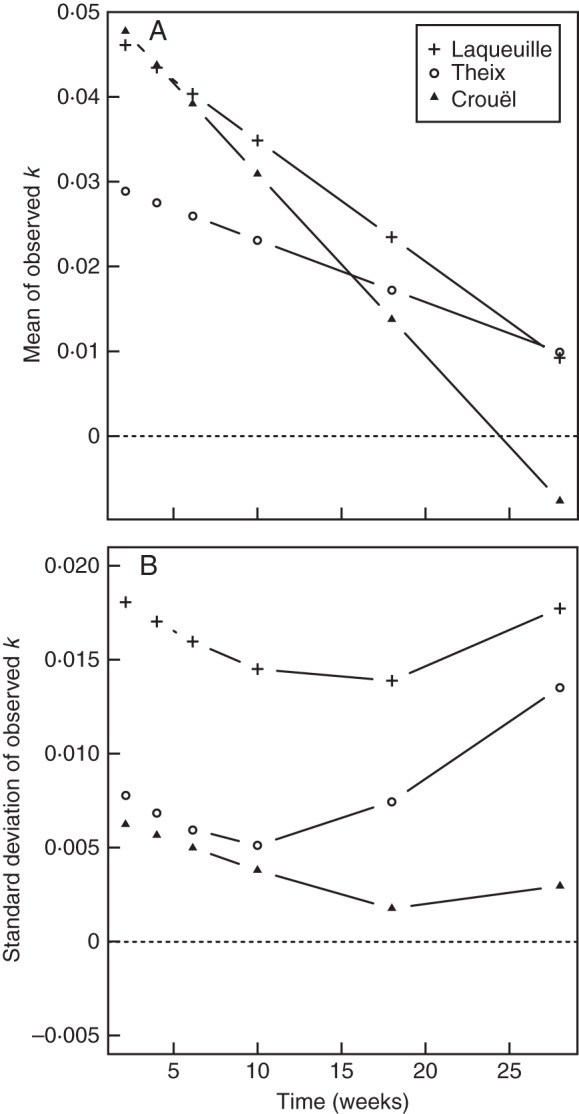
(A) Observed mean decomposition rate (litter mass loss, g g−1 week−1) and (B) observed standard deviation of the decomposition rate (g g−1 week−1) against the time for each site.
Figure 5B shows that the variability of observed decomposition rates was largest at the coolest and wettest site (Laqueuille) and lowest at the warmest and driest site (Crouel) at all times during the experiment. Within each site, we observed a U-shaped curve with two distinct and opposite patterns centred around 10–18 weeks (i.e. the fourth and the fifth harvest time; June 22 and August 17, respectively). During the first part of the experiment (from 0 to 10–18 weeks), the variability (standard deviation of the observed k-value between mixtures at each site) decreased to reach a minimum in July–August, then increased during the second part of the experiment (from 10–18 to 28 weeks). Before this shift, the observed variability of decomposition rates between mixtures (within sites) decreased with slowing rates over time. After the shift, observed variability increased even as decomposition rates continued to decrease. The fastest decomposing site (Laqueuille, the coolest and wettest site) then showed the highest level of variability, and the slowest decomposing site (Crouël, the hottest and driest site) showed the lowest variability.
DISCUSSION
The complexity of ecosystems makes it difficult to predict their process rates from information on their component parts. One suggested way of linking the properties of component species to ecosystem processes is to use community-weighted values of species-level traits (Garnier et al., 2004), a proposal derived from Grime's biomass-ratio hypothesis (Grime, 1998). Our main objective was to determine to what degree CWM values provide accurate predictions of ecosystem processes. Specifically, can monoculture decomposition rates, weighted by the relative abundance of each species in a mixed-species litter, be used to predict mixed-species decomposition rates in the field?
Here, we focused on two of the main sources of errors occurring in such predictions: interactions between species causing deviations from the linear sums predicted by community-weighted values and temporal variation in the abiotic environment (climate) causing correlated responses in such deviations.
Specifically, we tested the following hypotheses: (1) the decomposition rates of mixed-species litter agree with Grime's biomass-ratio hypothesis, (2) deviations from the predicted CWMs tend to cancel out as the number of species in the mixture increases (i.e. idiosyncratic annulment); and (3) the variation of decomposition rates between litter mixtures decreases as the abiotic conditions of the site become more limiting.
This study reveals contrasting results within and across sites. All sites considered, the CWM values of mixture decomposition rates based on abundance-weighted monoculture values predicted the actual mixture decomposition rates well (r = 0·91) and with no bias (Fig. 3). Thus, at this scale, the biomass-ratio hypothesis provides a good quantitative description of the mixed-species response. However, the results differ between sites. In the two climatically extreme sites (Laqueuille, the coolest and wettest; and Crouël, the hottest and driest), good support was found for the biomass-ratio hypothesis on average even though this was not true in every mixture. Furthermore, there was no statistical difference (Fig. 2) between values predicted by the biomass-ratio hypothesis and that actually observed. Indeed, we found abundant evidence of both positive and negative deviations in litter mixtures from the expected decomposition rates using CWMs, but such deviations were symmetrically distributed around zero. As a result, the average differences between observed and predicted rates of mixtures were approximately zero. This is consistent with results conducted under constant environmental conditions (Tardif and Shipley, 2013).
However, in the climatically intermediate site (Theix), the biomass-ratio hypothesis was not true, even on average (Fig. 2), and only 34 % of the variance in the observed decomposition rates was explained by the community-weighted values in this site. The equilibrium between positive and negative deviations from predictions that we found in Laqueuille and Crouël was not present in Theix, where most interactions were synergistic (Fig. 3).
Considering the similarity of the responses in the two other sites and under controlled conditions, this result from the intermediate site is surprising. Given that our experiment under variable environmental conditions involved only three sites, it is difficult to identify a causal explanation for this contrasted behaviour in Theix. The same soil, species mixtures and sources of litter were used at all three sites. The same decomposer community existed in all three sites at the beginning of the experiment, although this probably changed over the course of the experiment. The most obvious difference between the sites was in the abiotic conditions.
Our results show that the biomass-ratio hypothesis can lead to biased predictions within particular sites due to correlated responses between mixtures in a particular direction, but still produce unbiased and relatively accurate predictions at a larger scale involving different sites. This is similar to what was found by Tardif and Shipley (2013): CWMs can both over- and underestimate observed rates within a given species mixture but, taken over many mixtures, they provide unbiased predictions. Therefore, it is essential to consider the scale of the study when using CWMs to predict decomposition rates.
The species richness of a mixture is also an important factor when predicting the global decomposition rate. As already found for example in Keith et al. (2008), Perez-Harguindeguy et al. (2008) and Tardif and Shipley (2013), the variability of observed decomposition rates in mixtures decreased as the number of species increased (Fig. 4). However, the magnitude of this decrease varied between sites. A part of this effect is a necessary mathematical consequence of (1) the reduction of variation of weighted random variables compared with the values of the component variables and (2) the covariance occuring when different mixtures contain species in common (Tardif and Shipley, 2013). However, beyond this purely statistical effect, we had expected a biological one, caused by a combinatorial increase in the number of possible species interactions with an increasing species richness of the litter (Tardif and Shipley, 2013), which we called ‘idiosyncratic annulment’. This biological effect would decrease the variability of the prediction errors even below that expected due to purely statistical reasons. We did not observe this except for at Crouël (the hottest and driest site) after 10–18 weeks of the decomposition experiment. Using permutation tests to test the idiosyncratic annulment sensu stricto, we could not detect any additional decrease in variance with increasing species richness of the litter mixture. This is consistent with our previous results in Tardif and Shipley (2013), observed under controlled conditions. Either the idiosyncratic annulment effect does not exist or it is too weak to be detected. Despite this, the decreasing variability of observed decomposition rates with an increasing number of species due to purely statistical effects still makes the predictions using CWMs more accurate in litter mixtures containing more species.
Our third hypothesis was that the variation between the decomposition rates measured in the litter mixtures decreases as the abiotic conditions of the site become more limiting to decomposition, i.e. when the average measured decomposition rates at the site are lower.
Our results supported this hypothesis, when comparing the responses both (1) across the sites differing in average conditions of temperature and precipitations and (2) within each site as the conditions became more or less favourable to decomposition (cf. figure of temperatures and precipitations in Supplementary Data Fig. S1).
First, the coldest and wettest site (Laqueuille) showed the most favourable conditions for litter decomposition along the gradient (Fig. 5A), with the highest mean k-values across the whole experiment (all mixtures considered) and also having the greatest variability in decomposition rates between litter mixtures. In contrast, the hottest and driest site (Crouel) had the poorest conditions for litter decomposition, the lowest mean k-values across the whole experiment (all mixtures considered) and also the least variability in decomposition rates between litter mixtures.
Then, as shown in Fig. 5B, we also found a clear effect of the climate on the variability of the observed k-values within sites: the variability decreased with the hotter and drier climate occuring in July–August, especially for the site of Crouël (cf. figure of temperatures and precipitations in Supplementary Data Fig. S1). Increased precipitation and/or decreasing temperatures during the autumn were associated with a rise in variability of decomposition rates between litter mixtures (especially in Theix and Laqueuille). This reduction in the variability of decomposition rates between litter mixtures at a given site and time that was induced by a hotter and drier climate was presumably due to a reduction in the activity of decomposers under such conditions which may have restricted the strength of the interactions between the species in the litter.
A consequence of our results is that we can expect better predictions using the CWM under a climate less favourable to decomposition. As proposed in Tardif and Shipley (2013), the spatial scale will also be a determinant in the prediction of species mixture decomposition rates because increasing spatial scales have higher species richness which would decrease the variability between different species mixtures in the litter. Considering the differences of structure (e.g. spatial pattern of species distribution) and species richness between communities (or biomes), we expect better predictions in communities (or biomes) with higher species richness, for a given spatial scale (e.g. temperate grasslands vs. temperate forest, or tropical vs. temperate forest). However, even at larger spatial scales, CWMs of monoculture decomposition rates would be expected to have lower predictive accuracy in climates more favourable to decomposition.
Our results are also important in the current context of climate change and should be included in the predictive ecosystem models. Indeed, IPPC predictions for the next decades of this study area (central France region) imply warming and summer droughts (IPCC, 2001). If our results are more generally true, then such warmer and drier conditions would result in better predictions of litter decomposition rates using the CWM.
Beyond the example of litter decomposition and as previously suggested in Tardif and Shipley (2013), these results support the idea that the biomass-ratio hypothesis, operationalized as CWMs, could offer the opportunity to predict ecosystem processes at larger spatial scales and in a changing environment. These initial results are encouraging, but other studies must be conducted to extrapolate to other plant types and climates. Also, it would be interesting to study the variations of functional diversity of the involved species, as it might be an important source of error in the predictions.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank David Shipley, Alexandre Salcedo and the technical staff of Crouël, Theix and Laqueuille for technical assistance, and Dr Katja Klumpp for revisions and helpful comments. We also thank the Centre for Forest Studies (CEF) in Quebec, Canada, and INRA Grassland Ecosystem Research Unit (UREP), France, for their collaboration. This research was funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada.
LITERATURE CITED
- Aerts R. Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos. 1997;79:439–449. [Google Scholar]
- Bates D. Fitting linear mixed models in R. R News. 2005;5:27–30. [Google Scholar]
- Bates D, Sarkar D. lme4: linear mixed-effects models using S4 classes. 2006 http://CRAN.R-project.org , R package version 0·99875-8. [Google Scholar]
- Berg B, Berg MP, Bottner P, et al. Litter mass-loss rates in pine forests of Europe and eastern United States – some relationships with climate and litter quality. Biogeochemistry. 1993;20:127–159. [Google Scholar]
- Bontti EE, Decant JP, Munson SM, et al. Litter decomposition in grasslands of Central North America (US Great Plains) Global Change Biology. 2009;15:1356–1363. [Google Scholar]
- Cornelissen JHC, Diez PC, Hunt R. Seedling growth, allocation and leaf attributes in a wide range of woody plant species and types. Journal of Ecology. 1996;84:755–765. [Google Scholar]
- Cornwell WK, Cornelissen JHC, Amatangelo K, et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecology Letters. 2008;11:1065–1071. doi: 10.1111/j.1461-0248.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- Epstein HE, Burke IC, Lauenroth WK. Regional patterns of decomposition and primary production rates in the US Great Plains. Ecology. 2002;83:320–327. [Google Scholar]
- Fortunel C, Garnier E, Joffre R, et al. Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology. 2009;90:598–611. doi: 10.1890/08-0418.1. [DOI] [PubMed] [Google Scholar]
- Garnier E, Cortez J, Billes G, et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004;85:2630–2637. [Google Scholar]
- Gartner TB, Cardon ZG. Decomposition dynamics in mixed-species leaf litter. Oikos. 2004;104:230–246. [Google Scholar]
- Grime JP. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. Journal of Ecology. 1998;86:902–910. [Google Scholar]
- Grime JP, Hodgson JG, Hunt R. Comparative plant ecology: a functional approach to common British species. 2nd edn. Castlepoint Press; 2007. [Google Scholar]
- Hättenschwiler S, Tiunov AV, Scheu S. Biodiversity and litter decomposition in terrestrial ecosystems. Annual Review of Ecology, Evolution, and Systematics. 2005;36:191–218. [Google Scholar]
- Hector A, Beale AJ, Minns A, Otway SJ, Lawton JH. Consequences of the reduction of plant diversity for litter decomposition: effects through litter quality and microenvironment. Oikos. 2000;90:357–371. [Google Scholar]
- McCarthy JJ, Canzani OF, Leary NA, Dokken DJ, White KS, editors. IPCC. Climate change 2001: contribution of the working group II to the third assessment report of the inter- governmental panel on climate change. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- Kazakou E, Vile D, Shipley B, Gallet C, Garnier E. Co-variations in litter decomposition, leaf traits and plant growth in species from a Mediterranean old-field succession. Functional Ecology. 2006;20:21–30. [Google Scholar]
- Keith AM, van der Wal R, Brooker RW, et al. Increasing litter species richness reduces variability in a terrestrial decomposer system. Ecology. 2008;89:2657–2664. doi: 10.1890/07-1364.1. [DOI] [PubMed] [Google Scholar]
- Lawton JH. What do species do in ecosystems? Oikos. 1994;71:367–374. [Google Scholar]
- Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology. 2002;16:545–556. [Google Scholar]
- Manly BFJ. Randomization, bootstrap and Monte Carlo methods in biology. 2nd edn. London: Chapman and Hall; 1997. [Google Scholar]
- Mokany K, Ash J, Roxburgh S. Functional identity is more important than diversity in influencing ecosystem processes in a temperate native grassland. Journal of Ecology. 2008;96:884–893. [Google Scholar]
- Murphy KL, Klopatek JM, Klopatek CC. The effects of litter quality and climate on decomposition along an elevational gradient. Ecological Applications. 1998;8:1061–1071. [Google Scholar]
- Olson JS. Energy storage and the balance of producers and decomposers in ecological systems. Ecology. 1963;44:322–331. [Google Scholar]
- Pakeman RJ, Eastwood A, Scobie A. Leaf dry matter content as a predictor of grassland litter decomposition: a test of the ‘mass ratio hypothesis. Plant and Soil. 2011;342:49–57. [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Cornelissen JHC, Vendramini F, Cabido M, Castellanos A. Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant and Soil. 2000;218:21–30. [Google Scholar]
- Pérez-Harguindeguy N, Blundo CM, Gurvich DE, Díaz S, Cuevas E. More than the sum of its parts? Assessing litter heterogeneity effects on the decomposition of litter mixtures through leaf chemistry. Plant and Soil. 2008;303:151–159. [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects models in S and S-PLUS. Berlin: Springer; 2000. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. 2010 R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/ [Google Scholar]
- Santiago LS. Extending the leaf economics spectrum to decomposition: evidence from a tropical forest. Ecology. 2007;88:1126–1131. doi: 10.1890/06-1841. [DOI] [PubMed] [Google Scholar]
- Suding KN, Lavorel S, Chapin FS, et al. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Global Change Biology. 2008;14:1125–1140. [Google Scholar]
- Tardif A, Shipley B. Using the biomass-ratio and idiosyncratic hypotheses to predict mixed-species litter decomposition. Annals of Botany. 2013;111:135–141. doi: 10.1093/aob/mcs241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle DA, Bonner KI, Nicholson KS. Biodiversity and plant litter: experimental evidence which does not support the view that enhanced species richness improves ecosystem function. Oikos. 1997;79:247–258. [Google Scholar]
- Zhang D, Hui D, Luo Y, Zhou G. Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. Journal of Plant Ecology. 2008;1:85–93. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


