Abstract
Background and Aims
Heteromorphy in flowers has a profound effect on breeding patterns within a species, but little is known about how it affects reproductive barriers between species. The heterostylous genus Primula is very diverse in the Himalaya region, but hybrids there have been little researched. This study examines in detail a natural hybrid zone between P. beesiana and P. bulleyana.
Methods
Chloroplast sequencing, AFLP (amplified fragment length polymorphism) markers and morphological comparisons were employed to characterize putative hybrids in the field, using synthetic F1s from hand pollination as controls. Pollinator visits to parent species and hybrids were observed in the field. Hand pollinations were conducted to compare pollen tube growth, seed production and seed viability for crosses involving different morphs, species and directions of crossing.
Key Results
Molecular data revealed all hybrid derivatives examined to be backcrosses of first or later generations towards P. bulleyana: all had the chloroplast DNA (cpDNA) of this species. Some individuals had morphological traits suggesting they were hybrids, but they were genetically similar to P. bulleyana; they might have been advanced generation backcrosses. Viable F1s could not be produced with P. bulleyana pollen on P. beesiana females, irrespective of the flower morphs used. Within-morph crosses for each species had very low (<10 %) seed viability, whereas crosses between pin P. bulleyana (female) and pin P. beesiana had a higher seed viability of 30 %. Thus genetic incompatibility mechanisms back up mechanical barriers to within-morph crosses in each species, but are not the same between the two species. The two species share their main pollinators, and pollinators were observed to fly between P. bulleyana and hybrids, suggesting that pollinator behaviour may not be an important isolating factor.
Conclusions
Hybridization is strongly asymmetric, with P. bulleyana the only possible mother and all detected hybrids being backcrosses in this direction. Partial ecological isolation and inhibition of heterospecific pollen, and possibly complete barriers to F1 formation on P. beesiana, may be enough to make F1 formation very rare in these species. Therefore, with no F1 detected, this hybrid zone may have a finite life span as successive generations become more similar to P. bulleyana.
Keywords: Unidirectional hybridization, reproductive barriers, F1 formation, primrose, Primula, heterostyly
INTRODUCTION
Hybridization potentially has several evolutionary consequences, including the origin and transfer of genetic adaptations, the origin of new ecotypes or species, and the reinforcement or breakdown of reproductive barriers (Rieseberg and Gerber, 1995; Arnold, 1997; Rieseberg, 1997; Rieseberg and Carney, 1998; Milne et al., 2003; Milne and Abbott, 2008; Soltis and Soltis, 2009; Ma et al., 2010; Abbott et al., 2013). Thus the phenomenon of hybridization has fascinated scientists for many decades (e.g. Haldane, 1922; Dobzhansky, 1937; Anderson, 1948; Mallet, 2005; Van et al., 2006; Arnold et al., 2012; Abbott et al., 2013). F1 formation is in most cases a rare event that, once achieved, can permit far larger numbers of later generation hybrid derivatives to form (Arnold, 1997; Rieseberg and Carney, 1998; Broyles, 2002; Ma et al., 2010), creating the potential for gene transfer between parental species via introgression (e.g. Arnold et al., 2012). Hence barriers to F1 formation may be the main barriers to interspecific gene flow, except in rare cases where F1s outnumber other hybrid derivatives (Milne et al., 2003; Kameyama et al., 2005; Zhou et al., 2008; Kamiya et al., 2011).
In the process of hybridization, the direction can be asymmetric as a result of differences in the strength of reproductive barriers between parental species (Bacilieri et al., 1996; Arnold, 1997; Tiffin et al., 2001; Field et al., 2010; Montgomery et al., 2010; Natalis and Wesselingh, 2012). The last decade has seen great progress in the study of individual components of reproductive isolation among many plant species pairs, including flower colour, morphology and odour, flowering phenology or chromosomal divergence (Bradshaw and Schemske, 2003; Marques et al., 2007; Pascarella, 2007; Yang et al., 2007; Waelti et al., 2008). However, reproductive isolation among most plant species pairs is not due to a single isolating factor, but is a consequence of a large number of different pre- and post-zygotic barriers (Bacilieri et al., 1996; Arnold, 1997; Tiffin et al., 2001; Field et al., 2010; Montgomery et al., 2010; Natalis and Wesselingh, 2012). Therefore, to understand clearly the factors controlling hybridization between any species pair, both pre- and post-zygotic barriers should be fully considered (Arnold, 1997; Coyne and Orr, 2004; Rieseberg and Willis, 2007; Widmer et al., 2009; De hert et al., 2012).
Primula is a large genus within which heterostyly promotes outcrossing, and whose breeding systems have been extensively studied (e.g. Mast and Conti, 2006; McCubbin, 2008; Keller et al., 2012). Hybridization within this genus has received only superficial attention, with little information available beyond morphological and/or molecular evidence to confirm the occurrence of hybridization (Woodell, 1969; Valentine, 1995; Kálmán et al., 2004; Gurney et al., 2007; Zhu et al., 2009; Wu and Zhang, 2010). Moreover, the evolutionary significance of heterostyly at the interspecific level in Primula remains poorly known (Keller et al., 2012). Over half of all Primula species occur in the Himalayas and western China (Richards, 1993, 2002; Arnold and Richards, 1998; Wu and Zhang, 2010), with many cases of species in sympatry (Hu and Kelso, 1996), but only two records of natural hybrids (Zhu et al., 2009; Wu and Zhang, 2010). One of these hybrids, between Primula beesiana Forrest and P. bulleyana Forrest, forms a large hybrid swarm at its only known site, and provides an opportunity to investigate breeding barriers in Primula, how they are broken and what happens afterwards.
In the present study, therefore, a large number of putative hybrid derivatives and parent individuals were examined from the hybrid zone between P. beesiana and P. bulleyana, using morphological and molecular markers and comparison with synthetic F1s. In addition, pollination experiments of intra- and interspecific crosses within and between different morphs were conducted on the parent species, from which pollen tube growth, fruit set, seed production and seed viability were recorded. Furthermore, visitation patterns of putative pollinators in the field were observed. We aimed to answer the following questions: what mechanisms restrict hybrid formation at the (1) pre-zygotic (i.e. distribution, floral characters, pollinators, pollen tube growth) and (2) post-zygotic stage (i.e. fruit set, seed number and seed viability)? (3) Is hybridization unidirectional? (4) What is the make-up of the hybrid swarm with respect to classes (i.e. F1, backcross and complex hybrid derivatives)?
MATERIALS AND METHODS
Plant species, field investigation and study sites
Primula beesiana and P. bulleyana are perennial heterostylous herbs. All populations of each species comprise two morphs: the pin morph bears flowers with a long style and short stamens, whereas the thrum morph bears flowers with short styles and long stamens. The flowering time of both species is completely overlapping, from late May until mid July (Wu and Zhang, 2010). These species are known from nine and three populations, respectively, in Yunnan and Sichuan, south-west China, with both species occurring at Heishui River, Lijiang, north-west Yunnan, China (Supplementary Data Table S1).
Between 2010 and 2012, we screened all known populations of P. beesiana and P. bulleyana for intermediate forms (Supplementary Data Table S1). At each site, the species forms a single, often broad population. As expected, morphologically intermediate forms were found only at a single locality on Jade Dragon Snow Mountain in Lijiang, where they were previously reported (Wu and Zhang, 2010). Both parental species and hybrids are diploid, and have the same chromosome number (2n = 22; Y. P. Ma, unpubl. res.). Here, a population of >500 flowering plants of P. beesiana grows in open habitat by a mountain road at 3024 m a.s.l., where they have been present since at least 2004 (Wu and Zhang, 2010). Down the slope, approx. 400 m away horizontally and 108 m lower down, a population exists of P. bulleyana, which comprises >1000 plants, in shady streamside habitats. In 2004, about 30 individuals of P. bulleyana were present within 10 m of the P. beesiana population (Z. K. Wu, pers. comm.), but fewer than ten remained there in 2012. However, putative hybrids were only observed among the main P. bulleyana group, both in 2004 (Wu, 2008) and in 2012. Within this mixed population, hybrids and P. bulleyana plants grow <1 m apart, although both types tend to grow in small groups; hence they are not evenly mixed.
Four sites were chosen for this study. Site 1 was a pure P. beesiana population near Wenhai village (27·00°N, 100°10′E), whereas Site 2 contained a pure P. bulleyana population near Heishui River (27·01°N, 100°10′E). Because controlled pollination experiments would be easily destroyed by serious grazing and tourism if performed in natural populations, these were done instead on cultivated material at Lijiang alpine botanical garden, which was designated Site 3 (26·88°N, 100°E). The sympatric population described above was designated Site 4 (see detailed information in Supplementary Data Table S1).
Morphological characterization of intermediate forms in the hybrid zone
Several studies have established that the within-individual variation in flower characters was substantially smaller than the variance among individuals (e.g. Herrera et al., 2008; Gong and Huang, 2009). To determine the floral morphological traits of the two species, we therefore randomly selected 30 flowers (two flowers per plant) from 15 individuals of each morph in P. beesiana from Site 1, P. bulleyana from Site 2 and putative hybrids in Site 4, in 2010. Plants with yellow or magenta/rose flowers were putatively pure P. bulleyana or P. beesiana, respectively. Hybrids could be readily identified by corolla colour, but could be roughly divided into two colour morphs. The first, more common morph had light red flowers, whereas the second, less frequent morph had orange flowers. In total, 129 putative hybrids were identified by this method, among which 121 had light red flowers and eight had orange flowers. However, a similar or slightly smaller number of non-flowering rosettes, including plants at the seedling stage, were also present, whose flower morphology could not be assessed. From all individuals examined, we recorded floral colour and measured the stigma and anther heights (maximum height from the base of the ovary), corolla diameter, corolla tube length and relative anther–stigma distance (the latter to ±0·1 mm accuracy using a vernier caliper).
Flower visitor observation
Previous field observations during 2004–2005 confirmed that both P. beesiana and P. bulleyana are typical obligately outcrossing species, with bees and butterflies as the main pollinators (Wu, 2008). However, in the sympatric population, only one pollinator (Fabriciana adippe) was observed to visit both species, accounting for 10 % of all observed visits (Wu and Zhang, 2010). Pollinators were not observed to fly between hybrids and P. bulleyana; however, this might have been because few hybrids were included in the study. Hence Wu and Zhang's (2010) conclusion, that strong ethological isolation applies even where the species are sympatric, might not apply where large numbers of hybrids are present. To test this possibility, pollination visits to each species were first observed. Within Site 4, two plots were established, one containing ten individuals of P. beesiana (and no other Primula) and the other ten individuals of P. bulleyana (and no other Primula). Between 16 July and 31 July in 2012, the P. beesiana plot was observed for 7 d and the P. bulleyana plot for 9 d; each observation day lasted from 0900 h to 1730 h. It should be noted that if it was raining, field observations were stopped. We recorded a pollination visit if an insect contacted the plant's reproductive structures while actively searching for pollen and/or nectar (Memmott, 1999; Li and Huang, 2009; Ma et al., 2012). All types of flower visitors were photo recorded, identified and preserved in the insect collections of Kunming Institute of Botany, CAS.
To test whether insects transfer pollen between hybrids and P. bulleyana, a plot was set up in the hybrid zone, within which ten putative hybrids and ten P. bulleyana were replanted in a 4 × 5 grid structure, with plants 1 m apart and alternating between the two kinds. This plot was observed on two sunny days (12 and 13 July, 2012) for a total of 20 h, but insect visits were recorded only if they were observed to visit two individuals within the plot in succession.
Hand pollination experiments
Two types of pollination experiment were performed as follows.
Fruit set, seed number, detection of seed viability and synthesis of F1 hybrids
To investigate if reproductive barriers exist between the pollination and seed formation stage, within and between these species, a series of hand pollination experiments were conducted in July 2012. Each morph of each species was used as both a mother and father in all possible combinations, making 16 treatment types in total (Table 3). For each treatment, ten flowers from each of five plants of that species and morph were randomly selected while in bud, and bagged with nylon nets. Pollen of the chosen father species and morph was applied manually once each flower opened, then the bag was replaced. Some flowers were damaged before the process was completed; hence between 31 and 50 flowers were successfully pollinated for each treatment (Table 3; Supplementary Data Table S2).
Table 3.
Fruit set, seed number, seed viability and viable seeds per flower from 16 pollination treatments
| Treatment | Parent species* |
No. of fruit/no. of flowers (%) | Seed no. per fruit (s.e.) | Viability rate (%) | Viable seeds per flower† | |||
|---|---|---|---|---|---|---|---|---|
| P. beesiana | P. bulleyana | |||||||
| 1 | P ♀ | P ♂ | 6/40 (15) | 12·5 (5·6) | 6 | 0·1 | ||
| 2 | T ♀ | T ♂ | 4/45 (8·9) | 8·5 (3·3) | 9 | 0·1 | ||
| 3 | P ♀ | P ♂ | 4/31 (12·9) | 10·4 (4·2) | 4 | 0·1 | ||
| 4 | T ♀ | T ♂ | 4/50 (%) | 8·2 (3·3) | 8 | 0·1 | ||
| 5 | P ♀ | T ♂ | 39/43 (90·7) | 86·8 (7·4) | 99 | 78 | ||
| 6 | P ♂ | T ♀ | 42/50 (84) | 89·4 (8·9) | 97 | 73 | ||
| 7 | P ♀ | T ♂ | 47/50 (94) | 88·6 (7·7) | 94 | 78 | ||
| 8 | P ♂ | T ♀ | 34/39 (87·2) | 86·2 (7·6) | 96 | 72 | ||
| 9 | P ♀ | T ♂ | 23/44 (52·3) | 85 (7·7) | 0 | 0 | ||
| 10 | P ♀ | P ♂ | 18/45 (40) | 40·7 (7·6) | 0 | 0 | ||
| 11 | T ♀ | T ♂ | 27/45 (60) | 48 (8·9) | 0 | 0 | ||
| 12 | T ♀ | P ♂ | 46/50 (92) | 91·6 (3·3) | 0 | 0 | ||
| 13 | P ♂ | P ♀ | 10/50 (20) | 52·2 (7·4) | 30 | 3 | ||
| 14 | P ♂ | T ♀ | 29/50 (58) | 37 (2·9) | 80 | 17 | ||
| 15 | T ♂ | P ♀ | 15/50 (30) | 48·6 (5·5) | 6 | 1 | ||
| 16 | T ♂ | T ♀ | 0 (32) | 0 (0) | 0 | 0 | ||
*P, pin flower; T, thrum flower; ♀, female parent; ♂, male parent.
†Fruit set (no. of fruits/no. of flowers) × seed per fruit × viability rate.
In September 2012, fruits were harvested, and seed numbers per fruit were counted. For each fruit, seed viability tests were carried out via X-ray image systems (MX-20-DC12, Faxitron, USA). This has proved a successful means of detecting seed viability (Chen et al., 2011). The proportion of viable seeds was then calculated for each treatment.
Viable seeds from interspecific cross-pollinations were sown and incubated at 20 °C with 12 h of light per day. Those that germinated were then transferred to a greenhouse for cultivation. DNA was extracted from the leaves of these synthetic F1s for comparison with putative hybrids in the wild.
Pollen germination and pollen tube growth observation
Intramorph incompatibility occurs in heterostylous Primula species (Wu, 2008); hence, for interspecific crosses, between-morph crosses might be more successful than within-morph crosses, and have a better chance of successful pollen tube growth. Thus to examine the growth of pollen tubes in heterospecific crosses, two pollination treatments were performed: (1) P. beesiana (P) ♀ × P. bulleyana (T) ♂; and (2) P. bulleyana (P) ♀ × P. beesiana (T) ♂. While it would have been desirable to examine all pollen tubes similarly within all 16 types of crosses described above (Table 3), time and resources did not permit this. Hand pollination was conducted as described above, with 30 flowers in total per treatment. Stigmas from ten experimental flowers each were harvested after 3, 24 or 48 h. Each harvested stigma was sectioned and softened in 0·1 m NaOH at 60 °C for 1 h. Afterwards, the stigmas were incubated with 0·1 % aniline blue in phosphate buffer (pH 8·3) for 48 h. Then slides were examined under a fluorescence microscope with blue excitation (410 nm).
Data analysis
All morphological data were examined for normality and homogeneity of variance with a one-sample Kolmogorov–Smirnov test. Prior to analysis, morphological data from each plant were averaged and then analysed with one-way analysis of variance (ANOVA). Tukey post-hoc tests were performed to compare pairs of treatments.
Univariate ANOVA was performed for most of the pollination data. Specifically, we used a four-factor ANOVA to test the effects of four factors across all 16 cross pollination treatments: within vs. between species (WvBS), P. bulleyana vs. P. beesiana as mother (BvBM), pin vs. thrum as mother (PvTM) and pin vs. thrum as father (PvTF). This was done on each of four variables: fruit set, seed number per fruit, proportion of seed that are viable and viable seeds per flower. For intraspecific pollination treatments, a three-factor ANOVA was employed to test the effects of BvBM, PvTM and PvTF on fruit set per flower, seed number per fruit and proportion of viable seed. Interactions between these components were tested for fruit set per flower, and seed numbers per fruit, using five replicates per treatment, each replicate comprising results from an individual plant (Supplementary Data Table S2). Because seeds from all five plants of each treatment were pooled for seed viability analysis, it was not possible to do this for viability, or total viable seed per flower. Fruit sets and proportion of viable seeds were arcsine square-root transformed and viable seeds per flower were square-root transformed in order to improve ANOVA assumptions of normality and homogeneity. For detecting differences in seed viability percentages between treatments from interspecific pollinations (i.e. 13 vs. 14; 13 vs. 15), a χ2 test was employed. All statistical analyses were performed using SPSS 15·0 for Windows (SPSS, Chicago, IL, USA).
Molecular analyses
Sampling, DNA extraction and chloroplast sequencing
We sampled a total of 50 individuals from Site 4. These comprised 30 intermediate forms from the P. beesiana × P. bulleyana hybrid zone (22 with light red flowers, and eight with orange flowers; Fig. 2), plus ten individuals of each parent from the same site but at least 1000 m away from the hybrid zone, to provide indicators of morphology and molecular profiles of the pure parental species. Many studies routinely assume maternal cytoplasmic inheritance due to a lack of available information, despite evidence of bi-parental inheritance in many families (Mogensen, 1996; Zhang et al., 2003; Zhou et al., 2008; Vrancken and Wesselingh, 2010). Hence in this study we also examined eight of the synthetic F1s described above to test the hypothesis that chloroplast DNA (cpDNA) is normally maternally inherited in this cross. Genomic DNA was extracted from all collected leaves using a modified cetyltrimethyl ammonium bromide (CTAB) protocol (Doyle and Doyle, 1987).
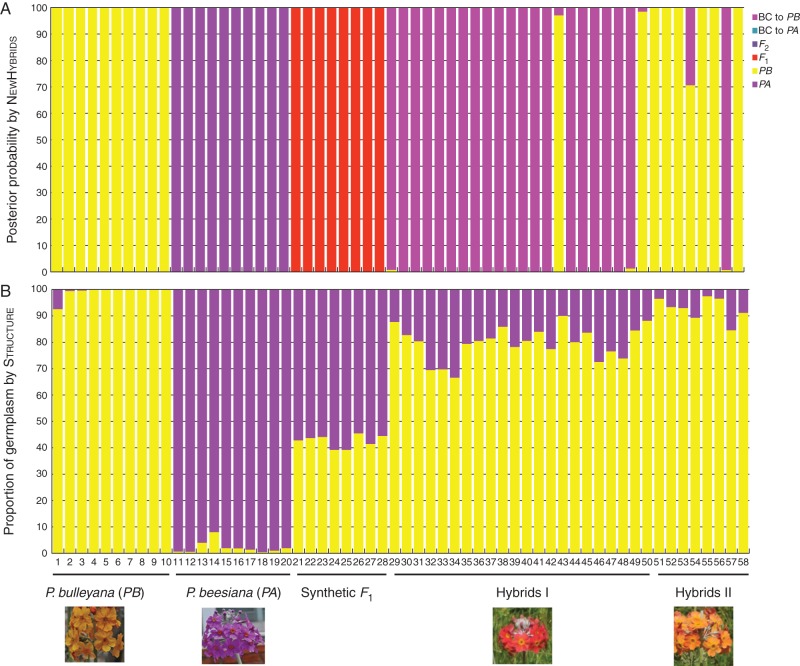
Fig. 2.
Genotype class assignment of all 58 individuals based on the programs NewHybrids (A) and Structure (B) using AFLP data.
To determine the direction of hybridization, the chloroplast TrnH-psbA spacer of all 58 individuals was amplified and sequenced using the universal primers of Kress et al. (2005). The reaction mix contained 0·625 U of AmpliTaq DNA polymerase, 1× PCR buffer, 1·5 mmol L−1 MgCl2, 0·2 mmol L−1 dNTP, 0·3 µmol L−1 primer and 20–60 ng of genomic DNA. Polymerase chain reactions were performed in a GeneAmp 9600 thermal cycler (Perkin Elmer, Norfolk, CT, USA). The PCR conditions were as follows: initial denaturation at 94 °C for 4 min; followed by 30 cycles of 1 min at 94 °C for template denaturation, 1 min at 50 °C for primer annealing, 1·5 min at 72 °C for extension; and finally an extension step of 10 min at 72 °C. The PCR products were purified using a Sangon Purification kit according to the manufacturer's protocol. DNA sequences were obtained using an ABI 3700 automated sequencer (Perkin Elmer). Contiguous DNA sequences were edited using the program SeqMan (DNASTAR package, Madison, WI, USA) and sequences aligned using Clustal 2·0 (Thompson et al., 1997).
AFLP analysis
To examine the putative hybrids in detail, we used amplified fragment length polymorphism (AFLP) markers that provide reliable diagnostic loci at varying taxonomical levels, and can be relatively easily generated in sufficient numbers to distinguish between genealogical classes in hybrid populations, especially in the case of weakly differentiated source populations (Miller, 2000; Campbell et al., 2003; Ma et al., 2010; Zha et al., 2010). Although co-dominant markers are twice as informative per locus as dominant markers, they are much more costly to produce, and it is possible to distinguish between F1, F2 and backcross hybrids with <5 % classification error using fewer than 30 diagnostic AFLP loci (Zha et al., 2010).
The AFLP procedure was carried out according to the Beckman Coulter protocol with only minor modifications, as described by Reisch (2007). Double digestion of genomic DNA was performed for 2 h at 37 °C in a 20 µL mix using 2 U of MseI and 10 U of EcoRI. Following this, adaptors were ligated to DNA in a 21 µL volume for 2 h at 37 °C using 2 U of T4 DNA Ligase (Shanghai Sangon Biological Engineering Technology, Shanghai, China). Pre-selective PCRs were run in a reaction volume of 25 µL. PCR parameters were chosen as follows: 2 min at 94 °C; 25 cycles of denaturing at 94 °C for 20 s, annealing at 56 °C for 30 s and extension at 72 °C for 2 min; followed by 2 min at 72 °C and ending with 30 min at 60 °C. Diluted 20× pre-selective products underwent selective PCR with the following three primer combinations: E-AAC/M-CAA, E-AAC/M-CTC and E-AAC/M-CTT. Selective amplifications were run in a 25 µL volume, and PCRs were performed with the following touchdown profile: 2 min at 94 °C; ten cycles of denaturing at 94 °C for 20 s, annealing for 30 s at 66 °C and then reduced by 1 °C for the next ten cycles, elongation at 72 °C for 2 min, followed by 25 cycles for denaturing at 94 °C for 20 s, annealing at 56 °C for 30 s, and 2 min elongation at 72 °C; ending with a final extension for 30 min at 60 °C. Finally, the PCR products were added to a mixture of Sample Loading Solution (Beckman Coulter, Fullerton, CA, USA) and CEQ Size Standard 400 (Beckman Coulter). The fluorescence labelled selective amplification products were separated by capillary gel electrophoresis on an automated sequencer (CEQ 8000, Beckman Coulter).
Raw data were collected and analysed with the CEQ Size Standard 400 using the CEQ 8000 software (Beckman Coulter). Individuals were scored for the presence or absence of each fragment in binary mode (1/0) in crv-files. Bins were built using the AutoBin option with a peak height of 800 and a bin width of 2. Fragments were then assigned to bins with a selective height and checked manually. In the AFLP data matrix, the presence of a band was scored as 1, whereas the absence of the band was coded as 0. From this was produced a binomial (0/1) data matrix, representing the scores for AFLP markers across all examined individuals.
The program NewHybrids employs a Bayesian analysis to identify hybrids within natural hybrid zones (Anderson and Thompson, 2002) and to determine their classes. This method can be used with dominant data such as AFLP markers and is capable of identifying hybrids even when the markers are not completely species specific. Using this program requires certain assumptions about the markers used: that they are unlinked, not subject to selection and were at linkage equilibrium in the parent species before hybridization (Milne and Abbott, 2008).
To provide an indication of the proportion of each parent's germplasm in each hybrid, the same data matrix was also analysed using the program Structure version 2·3·1 (Hubisz et al., 2009). We adopted the admixture model with correlated allele frequencies (Lepais et al., 2009; Zalapa et al., 2009; Ma et al., 2010). No prior knowledge of the species was included in the analysed data set. To determine the optimal number of groups (K), we ran Structure with K varying from 1 to 10, with five runs for each K value. Previous studies have found that, in many cases, the posterior probability for a given K increases slightly, even after the real K is reached (Dan et al., 2009). Therefore, we used the ad hoc statistic, ΔK, of Evanno et al. (2005) to determine the true value of K. Our parameters were 10 000 burn-in periods and 10 000 MCMC (Markov chain Monte Carlo) repetitions after burn-in.
RESULTS
Morphological characterization of intermediate forms in the hybrid zone
Floral colour differed between both parental species and the putative hybrids (Table 1). Primula beesiana had a significantly lower pin stigma than P. bulleyana, but there was no significant difference between the hybrids and either P. bulleyana or P. beesiana (Table 1). Anther–stigma distance in both pin and thrum flowers exhibited significant differences (pin flower, F2,42 = 4·358, P = 0·019; thrum flower, F2,42 = 7·639, P = 0·001). The stigma–anther distance in thrum flowers was significantly different between P. bulleyana and P. beesiana; however, no significant differences were detected between parental species and hybrids (Table 1). For pin flowers of hybrids, the stigma–anther distance in pin flowers of hybrids was significantly lower than in P. bulleyana but not significantly different from P. beesiana (Table 1). No other differences were significant.
Table 1.
Floral characters of each morph of P. beesiana, P. bulleyana and putative hybrids (mean ± s.e., n = 15) with different superscript letters indicating a significant difference according to Tukey's post-hoc test at the 5 % level
| Characters | Pin flower |
Thrum flower |
||||
|---|---|---|---|---|---|---|
| P. bulleyana | P. beesiana | Hybrids | P. bulleyana | P. beesiana | Hybrids | |
| Floral colour | Yellow | Magenta | Orange to red | Yellow | Magenta | Orange to red |
| Corolla diameter | 20·62 ± 0·15 | 20·72 ± 0·24 | 20·35 ± 0·17 | 20·83 ± 0·21 | 21·00 ± 0·27 | 20·50 ± 0·26 |
| Corolla tube length | 12·01 ± 0·07 | 11·80 ± 0·22 | 11·67 ± 0·13 | 12·32 ± 0·10 | 12·03 ± 0·07 | 12·11 ± 0·19 |
| Anther height | 7·37 ± 0·06 | 7·35 ± 0·14 | 7·57 ± 0·10 | 11·92 ± 0·11 | 11·55 ± 0·11 | 11·89 ± 0·18 |
| Stigma heighta | 11·89 ± 0·14 | 11·29 ± 0·13 | 11·59 ± 0·10 | 7·37 ± 0·08 | 7·52 ± 0·09 | 7·61 ± 0·05 |
| Stigma–anther distanceb | 4·51 ± 0·09 | 4·01 ± 0·11 | 4·02 ± 0·10 | 4·54 ± 0·04 | 4·02 ± 0·05 | 4·28 ± 0·10 |
aPin flowers from parental species significantly different but hybrids not significantly different from either parent.
bPin flowers from P. bulleyana significantly different from P. bulleyana and hybrids but no significant difference observed between P. bulleyana and hybrids; thrum flowers from parental species significantly different but hybrids not significantly different from either parent.
Flower visitors
For the single-species plots, we observed 220 visits by insects to the P. bulleyana plot and 158 visits to the P. beesiana plot. The most frequent visitors to both were bees (Anthophora sp.), for which 164 visits were observed for P. bulleyana and 89 for P. beesiana. Butterflies (Aporia bieti, Pieris brassicae and Issoria lathonia) made 45 and 27 visits to P. bulleyana and P. beesiana, respectively. In contrast to both of these, bumble-bees visited P. beesiana (40 visits) far more frequently than they did P. bulleyana (ten visits). The remaining three visits were by hawkmoths (Macroglossum sp.), with two visits to P. beesiana and one to P. bulleyana (Fig. 1; Supplementary Data Fig. S1).
Fig. 1.
Shared pollinators among parental species and putative hybrids. Aporia bieti visiting P. bulleyana (A), a putative hybrid (B) and P. beesiana (C). Anthophora sp. visiting P. bulleyana (D), a putative hybrid (E) and P. beesiana (F). Scale bar = 2 cm.
For the plot containing 20 plants each of P. bulleyana and hybrids, 76 instances of bees (Anthophora sp.) flying from one Primula plant to another were observed. Among these, 53 instances (70 %) were between a parent and a hybrid (Table 2). Hence bee behaviour is no barrier to backcrossing. Only six instances of other pollinator species visiting more than one flower in this plot were observed (Table 2).
Table 2.
Observations of pollinator flights from one Primula individual to another, in the plot containing ten P. bulleyana and ten putative hybrids
| Visitation pattern |
Pollinator species |
||||
|---|---|---|---|---|---|
| First visit | Next visit | Anthophora sp. | Aporia bieti | Pieris brassicae | Issoria lathonia |
| P. bulleyana | P. bulleyana | 16 | 1 | 1 | 2 |
| P. bulleyana | Hybrid | 27 | 0 | 0 | 0 |
| Hybrid | P. bulleyana | 26 | 2 | 0 | 0 |
| Hybrid | Hybrid | 7 | 0 | 0 | 0 |
Hand pollination experiments
Fruit set, seed number and detection of seed abortion
Taking all these 16 hand pollination treatments together, which species was mother (BvBM) showed significant influences on fruit set per flower, seed per fruit, seed viability and, therefore, viable seed per flower (Table 4). In addition, paternal morph (PvTF) had a significant effect on fruit set, whereas a significant effect of within vs. between species (WvBS) indicated higher seed viability, both as a proportion of seed and per flower overall (Table 4). Additionally, interactions between two or more of the four factors were tested for combined effects on fruit set and seed numbers per fruit. On fruit set, significant effects occurred for all two-way interactions except BvBM × PvTF, and for two three-way interactions: WvBS × BvBM × PvTM and WvBS × PvTM × PvTF (Table 4). With regard to seed numbers per fruit, the same three-way interactions were significant, as were two two-way interactions (PvTM × PvTF and WvBS × BvBM) plus the four-way interaction WvBS × BvBM × PvTM × PvTF (Table 4).
Table 4.
Effect of cross-pollination treatments (within vs. between species, mother species, pin vs. thrum as mother, pin vs. thrum as father) on fruit set, seed production seed viability and viable seeds per flower
| Source | F-value | P-value |
|---|---|---|
| Fruit set* | ||
| Within–between species (WvBS) | 1·89 | 0·174 |
| Mother species (BvBM) | 38·436 | <0·001 |
| Pin/thrum as mother (PvTM) | 0·046 | 0·83 |
| Pin/thrum as father (PvTF) | 8·188 | 0·006 |
| BvBM × PvTM | 11·099 | 0·001 |
| PvTM × PvTF | 327·262 | <0·001 |
| WvBS × PvTM | 14·159 | <0·001 |
| BvBM × PvTF | 0·234 | 0·63 |
| WvBS × BvBM | 33·698 | <0·001 |
| WvBS × PvTF | 17·873 | <0·001 |
| BvBM × PvTM × PvTF | 1·599 | 0·211 |
| WvBS × BvBM × PvTM | 10·645 | 0·002 |
| WvBS × PvTM × PvTF | 50·649 | <0·001 |
| WvBS × BvBM × PvTF | 1·456 | 0·232 |
| WvBS × BvBM × PvTM × PvTF | 0·267 | 0·607 |
| Seed number* | ||
| WvBS | 0·599 | 0·442 |
| BvBM | 28·801 | <0·001 |
| PvTM | 3·862 | 0·054 |
| PvTF | 2·435 | 0·124 |
| BvBM × PvTM | 6·91 | 0·011 |
| PvTM × PvTF | 268·517 | <0·001 |
| WvBS × PvTM | 2·463 | 0·122 |
| BvBM × PvTF | 1·981 | 0·164 |
| WvBS × BvBM | 23·689 | <0·001 |
| WvBS × PvTF | 1·62 | 0·208 |
| BvBM × PvTM × PvTF | 2·868 | 0·095 |
| WvBS × BvBM × PvTM | 9·195 | 0·004 |
| WvBS × PvTM × PvTF | 62·947 | <0·001 |
| WvBS × BvBM × PvTF | 4·179 | 0·045 |
| WvBS × BvBM × PvTM × PvTF | 5·127 | 0·027 |
| Proportion of viable seed† | ||
| WvBS | 15·967 | 0·02 |
| BvBM | 30·354 | <0·001 |
| PvTM | 2·065 | 0·179 |
| PvTF | 0·114 | 0·742 |
| Number of viable seed per flower† | ||
| WvBS | 10·068 | 0·009 |
| BvBM | 18·004 | 0·001 |
| PvTM | 0·217 | 0·65 |
| PvTF | 0·021 | 0·888 |
*Interactions between ANOVA components are tested using five replicates for each treatment, each replicate comprising a single plant. Nominator d.f. = 1, denominator d.f. = 64.
†Interactions between ANOVA components cannot be tested for these variables because seeds for all five plants were pooled to test for viability. Nominator d.f. = 1, denominator d.f. = 11.
The three-way ANOVA analysis for intraspecific crosses (1–8) showed, however. that BvBM did not significantly affect fruit production (F1,32 = 0·053, P = 0·82), seed numbers (F1,32 = 0·207, P = 0·652) and seed viability (F1,4 = 0·003, P = 0·957), but the interaction between PvTM × PvTF significantly contributed to both fruit production (F1,32 = 214·56, P < 0·001) and seed numbers (F1,32 = 350·62, P < 0·001). Between-morph crosses (5–8 in Table 3) produced fruit from >80 % of flowers, compared with significantly decreased fruit set (8–15 %) for within-morph crosses (1–4 in Table 3). Likewise, all intraspecific between-morph crosses (1–4 in Table 3) averaged >80 seeds per fruit compared with >13 seeds per fruit for within-morph crosses, and had >93 % seed viability compared with <10 % in each within-morph cross (Table 3; Supplementary Data Fig. S2).
Therefore, the identity of the parent species (BvBM) has little or no effect in intraspecific crosses; hence, the significant effect of BvBM in all crosses from the four-way ANOVA must reflect a strong effect of mother species on interspecific crosses. Those interspecific crosses where P. beesiana was mother (9–12; Table 3) produced fruits and seeds, but seed viability was zero in all cases. Thrum–thrum crosses on P. bulleyana mothers (treatment 16; Table 3) also set no viable seed, though in this case it was because no fruits were produced. Conversely, pin–pin crosses on P. bulleyana mothers (treatment 13) produced fruit from 20 % of flowers and averaged 52 seed per fruit, with 30 % seed viability, giving an average of three viable seeds per flower (Table 3). Pin P. bulleyana × thrum P. beesiana crosses (treatment 14 in Table 3) had a far higher seed viability rate (80 %) than pin–pin crosses (treatment 13, χ2 = 375·503, P < 0·001) leading to on average 17 viable seeds per flower (Table 3). Conversely, thrum P. bulleyana × pin P. beesiana crosses (treatment 15; Table 3) had only 6 % viability, significantly lower than treatment 14 (χ2 = 16·457, P < 0·001); this led to an average of one viable seed per flower in treatment 15.
Pollen tube growth in interspecific pollination treatments
Pollen of each species can germinate on the stigma of the other within 3 h; after 24 h, pollen tubes had successfully extended and in some cases had reached ovules; after 48 h, most tubes were longer (Supplementary Data Fig. S3A–G) and more had reached the ovules (Supplementary Data Fig. S3H, I, R). In each case, >100 pollen grains germinated, but on average <30 reached the ovules, 28 for P. beesiana × P. bulleyana and 26 for P. bulleyana × P. beesiana, respectively. Hence most pollen tubes did not reach the ovules; most appear to have been inhibited by callus growth in different stages (Supplementary Data Fig. S3J–Q), representing a moderately strong but not complete barrier to F1 formation.
Molecular results
Chloroplast DNA TrnH-psbA sequences
Among ten individuals of P. beesiana and ten individuals of P. bulleyana, 17 variable sites were found in the TrnH-psbA sequences. These sites all distinguished the haplotype of P. beesiana (GenBank accession no. KF198513) from that of P. bulleyana (GenBank accession no. KF198512, Table 5). No variation within either species was detected. The synthesized F1 hybrids (GenBank accession no. KF198515), with P. bulleyana as maternal parent, all have the haplotype of this species, indicating maternal inheritance of cpDNA. Occasional paternal cpDNA inheritance is not excluded because the sample size of eight is small; the frequency of paternal inheritance per individual is somewhere between 0 and 31·2 % [based on the 95 % confidence level and the formula P = 1 – (1 – β)1/N; Vrancken and Wesselingh, 2010]. All of the 30 putative hybrids examined had cpDNA sequences identical to P. bulleyana (GenBank accession no. KF198514). Therefore, hybridization between these species could be unidirectional.
Table 5.
Chloroplast haplotypes of sequenced individuals and the codon positions at which they differ
| Species | No. of individuals | Sequence region and codon position of TrnH-psbA |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 137–138 | 150 | 154 | 176–181 | 252–253 | 292–293 | 296 | 380 | 382 | ||
| P. beesiana | 10 | AG | A | A | – | GA | TT | C | A | A |
| P. bulleyana | 10 | CT | T | T | AATGAT | TT | CC | – | – | T |
| Synthesized F1 | 8 | CT | T | T | AATGAT | TT | CC | – | – | T |
| Putative hybrids | 30 | CT | T | T | AATGAT | TT | CC | – | – | T |
AFLP analysis
We generated 144 polymorphic AFLP markers, of which 53 were present in all individuals of one parent species and absent from all individuals of the other examined, and a further 57 of which were present in one parent species only, though not in all individuals thereof.
Analysis of AFLP data using NewHybrids confirmed the identity of the P. beesiana and P. bulleyana individuals examined. Using the default NewHybrids settings, all eight synthetic F1 hybrids were identified as F1s with posterior probabilities of 100 %, suggesting that these markers were successful in generating unbiased results. Of the 30 putative hybrids examined, 21 individuals were identified as backcrosses to P. bulleyana with posterior probabilities of ≥98·8 %, whereas the other eight individuals were classified as P. bulleyana with posterior probabilities >97·2 % (Fig. 2A).
In the Structure analysis of AFLP data, the value of ΔK was 2309·18 for K = 2, 861·24 for K = 3, 803·8 for K = 4 and >200 for all values of K >4 (Supplementary Data Fig. S4). Therefore K = 2 best represents the data. Following ten independent Structure runs with K = 2, individuals morphologically identified as P. bulleyana were assigned to one cluster with high probability (q = 0·991 ± 0·023), whereas those morphologically identified as P. beesiana were assigned to the other cluster with similarly high probability (q = 0·978 ± 0·022). Therefore, these clusters were determined to represent P. bulleyana and P. beesiana, respectively. For eight synthetic F1 hybrids, the estimated proportion of P. beesiana was 0·576 on average (Fig. 2B). Among individuals morphologically identified as putative hybrids, the mean estimated proportion of P. beesiana was 0·170 (0·035–0·333). A first-generation backcross to P. bulleyana would have approx. 25 % P. beesiana germplasm, although, given the estimated 0·576 value for synthetic F1s, a value of around 0·29 would be likely from this analysis. For those individuals identified as backcrosses to P. bulleyana by NewHybrids, the mean proportion of P. beesiana germplasm was 21·1 %, with the lowest 12·3 % in individual 29 and the highest 33·3 % in individual 34 (Fig. 2B). The eight orange-flowered putative hybrids (individuals 51–58) had between 2 and 16 % P. beesiana germplasm according to Structure, much less than the average for putative hybrids and indicating a clear link between germplasm proportion and flower colour. However, all except one of these were identified as pure P. bulleyana by NewHybrids (100 % certainty in seven cases, only 70 % in the other), along with two other individuals. Given that no category was available to NewHybrids that fell between pure P. bulleyana and the first-generation backcross, the possibility exists that individuals assigned to either class might in reality be second- or later generation backcrosses to P. bulleyana.
DISCUSSION
The genus Primula includes >200 species in the Himalayas, but, despite this, to our knowledge only two natural hybrids have ever been reported from the region, i.e. P. secundiflora × P. poissonii (Zhu et al., 2009) and P. bulleyana × P. beesiana (Wu and Zhang, 2010). Despite range overlap, and complete overlap of flowering times, only a single hybrid population of the latter is known. Molecular data showed that at least 21 of the 30 putative hybrids examined from that population were backcrosses to P. bulleyana, and had the cpDNA of that species. Molecular data could not distinguish the remaining nine from P. bulleyana, although morphological markers such as orange or light red corollas, which do not occur in pure P. bulleyana populations, indicate that these are probably advanced generation backcrosses. The direction of hybridization here is highly asymmetric, and possibly unidirectional.
Inbreeding avoidance mechanisms in P. bulleyana and P. beesiana and their effects on hybrid formation
Our data show that inbreeding avoidance in both study species goes beyond the mechanical difficulty of transferring pollen between two pin flowers, or two thrum flowers. In both species, when crosses were made between two plants of the same morph, the seed viability rate was <10 % and, on average, far fewer than one viable seed was produced per flower (compared with >70 for between-morph crosses; Table 3). Both figures were substantially lower than for F1 seed on P. bulleyana mothers; indeed pin–pin crosses with a P. bulleyana mother and P. beesiana father had far higher seed viability (30 %) and viable seed per flower (three) than any pin–pin (or thrum–thrum) intraspecific cross (Table 3). The ANOVA (Table 4) reveals a complex pattern of effects, with the morph of the father affecting fruit set far more than that of the mother, whereas the reverse might be true for seed production and seed viability.
The heterostyly syndrome often entails a physiological response that inhibits self- and intramorph pollen tube germination and growth (Keller et al., 2012). Even with a relatively small sample, this appears to indicate that the genes that restrict pin–pin cross seed production are not the same in these species. Conversely, P. bulleyana thrum × P. beesiana thrum were the only crosses with no viable seeds. Further test crosses are needed to determine whether thrum–thrum incompatibility extends across the species barrier, and the extent to which pin–pin incompatibility does not. For hybrid formation, this is of limited significance as most interspecific pollination events are likely to involve between-morph crosses for mechanical reasons. However, if only between-morph crosses are considered, the mean F1 viability rate on P. bulleyana mothers rises to 43 %. Further work is needed to tease apart fully the effects of interspecific and heterospecific incompatibilities, and in particular whether they affect fitness after the zygote stage.
Barriers to hybrid formation
Pre-pollination barriers to hybridization are clearly incomplete in these taxa, as six kinds of pollinator visit both parent species. However, pollinator behaviour is equally important as each individual might potentially habituate to visiting only one species. We did observe that pollinators fly between hybrids and P. bulleyana, proving that pollinator behaviour is no barrier to backcrossing. However, evidence for how often pollinators might transfer pollen between the two parent species is lacking. Morphological differences between species, in terms of stigma height and especially anther–stigma distance in both morphs, might also create a mechanical barrier by depositing pollen on different parts of a pollinators' body (Keller et al., 2012). However, stigma–anther distance is very similar between P. beesiana and P. bulleyana (Table 1), so this barrier might be weak in this case. Spatial patterning of parent species may also affect pollinator behaviour (Natalis and Wesselingh, 2013).
Following interspecific pollination, the ability of pollen to germinate, form pollen tubes and fertilize ovules is one vital factor controlling F1 formation (Rieseberg, 1997; Howard, 1999; Rahmé et al., 2009; Natalis and Wesselingh, 2012); zygote viability is another. Our data show that when ample pollen is applied to the stigma, around 30 pollen tubes from each reciprocal cross type can reach the ovules within 48 h (Supplementary Data Fig. S3). Despite this, no viable F1 seeds were formed on P. beesiana mothers, indicating that unless heterospecific pollen is prevented from fertilizing once it reaches the ovule, the barrier to F1 formation in this direction might be post-zygotic. Hence certain P. bulleyana genes might be lethal in a P. beesiana cytoplasmic background (e.g. Trucco et al., 2009; Beatty et al., 2010), causing seed abortion at some point after fertilization.
Based on seed set from intraspecific, between-morph crosses (Table 3), there will normally be >70 successful pollen tubes per stigma. From this, we can conclude that more than half of P. beesiana pollen tubes are prevented from reaching the ovules of P. bulleyana by callose tissue or other mechanisms, or at least are severely delayed. Hence, pollen tube inhibition reduces but does not prevent F1 formation. However, callose tissue might also slow down the development of heterospecific pollen tubes, ensuring that conspecific pollen, when present, reaches all the ovules first, hence preventing F1 formation unless only heterospecific pollen is available (e.g. Arnold et al., 1993; Carney et al., 1994, 1996).
Overall, therefore, various pre-zygotic barriers act to reduce F1 formation, but none appears complete. On P. beesiana mothers, a complete barrier applies at or after the fertilization stage, to which there seems to be no equivalent in the other direction. Post-zygotic fitness barriers might also have a role here, but we have no evidence regarding F1 fitness in the wild following germination, other than that at least one F1 must have reached adulthood at Site 4 for the hybrid zone to have formed.
Hybrid zone structure for P. bulleyana × P. beesiana
Every hybrid derivative detected was a backcross to P. bulleyana. We examined 30 out of 129 flowering putative hybrids at the site, so if other hybrid classes exist they are at very low frequency. Based on Structure analysis (Fig. 2), individuals 32, 33, 34, 46 and 48 might be first-generation backcrosses, while many of the others are likely to be second- or even later generation backcrosses; one individual (1) of P. bulleyana also had >5 % P. beesiana germplasm, whereas several individuals were putative hybrids based on corolla colour but barely differed from P. bulleyana for DNA (Fig. 2). Hence this hybrid zone comprises successive generations of backcrosses in one direction only, and possibly no other hybrid derivatives at all. Clearly at least one F1 must have been present originally, but if still extant it was not sampled. Hybrid zones can exist without F1s if the original F1 has died and no others have been formed (Arnold et al., 1993; Cruzan and Arnold, 1993; Chung et al., 2005). This raises two questions: first, how did the initial F1 formation event occur, and, secondly, why are all detected hybrids backcrosses?
Both cpDNA data and the inviability of F1 seeds on P. beesiana mothers strongly indicate a P. bulleyana mother for the initial F1 in this hybrid zone. As noted above, F1 formation appears highly unlikely where conspecific pollen is available due to impeding of heterospecific pollen, and hence may have been possible only when flowering individuals of one species were greatly outnumbered by those of another. From this, F1 formation might be most likely where a single P. bulleyana occurred surrounded by P. beesiana. This could have occurred in the population of P. beesiana further up the hill, which did have a small number of P. bulleyana close by. Alternatively, a P. beesiana population might have existed where the current hybrid zone is, although the habitat seems more suited to P. bulleyana. A third hypothesis of pollen transfer between populations seems highly unlikely, because, although transfer via pollinators might occur, again the pollen would be unlikely to succeed due to competition from conspecific pollen, as noted above. Hence the most likely hypothesis seems to be F1 formation on a P. bulleyana mother close to the P. beesiana population, followed by dispersal of the seed down the hill, perhaps via a small stream.
That hybrids are only present within the P. bulleyana population more or less proves that the F1 must have been there as well. Our data show that pollinators do make transitions between hybrids and P. bulleyana in both directions, so each can have received pollen from the other. However, with hybrids initially outnumbered, these are likely to have been the maternal parent to most backcrosses. Pollinators also fly from one hybrid to another, so some of the hybrid derivatives examined may be backcross to backcross crosses. The relative frequency of all these events will also have been affected by the degree of admixture between hybrids and P. bulleyana, which might affect pollinator behaviour (Natalis and Wesselingh, 2013). Hybrids and P. bulleyana plants each tended to form groups of their own kind in the field, hence potentially promoting within-type pollination events and so limiting backcrossing events and introgression.
The absence of backcrossing to P. beesiana is unsurprising given the absence of this species from the immediate hybrid zone, whereas the absence of F2s can be easily explained if there was only one F1, given that physical and genetic barriers to within-morph crosses make selfing difficult in Primula.
Breeding barriers between P. beesiana and P. bulleyana: a role for ecology?
Formation of F1s is clearly rare among these species, but, once it happens, large numbers of hybrid derivatives form, following a classic pattern (Arnold, 1997; Rieseberg and Carney, 1998; Brolyes, 2002). Regarding pre-zygotic barriers, the two species are only sympatric in one region (Heishui River), and within that region there is ecological isolation, with P. beesiana preferring open habitats while P. bulleyana favours shade. However, between 1990 and 2000, a tarmac road was built above where the hybrid zone currently exists. This created disturbance, which favoured hybrid formation and recruitment (Anderson, 1948; Semple and Semple, 1977; Levin et al., 1996; Rieseberg and Carney, 1998). In this case, it has brought large colonies of each species within 280 m of each other, but perhaps more significantly permitted a small number of P. bulleyana to grow close to P. beesiana. Pollinator behaviour might not be a barrier, because if pollinators fly between red-flowered hybrids and yellow-flowered P. bulleyana in a mixed population, then they are likely to do so in mixed interspecific populations. However, the presence of hybrids itself can reduce the faithfulness of pollinators, especially generalists (e.g. Wesselingh and Arnold, 2000), and this might account for why pollinators appeared to show more species-specific behaviour at this site in the past (Wu and Zhang, 2010). If so, pollinator behaviour might have been a significant barrier to F1 formation, even if it is not to subsequent backcrossing. The flowering periods entirely overlap. Therefore, ecological isolation might be the most significant barrier to pollination between the two species, coupled with obstacles to pollen tube growth for heterospecific pollen.
The past and future of the P. beesiana × P. bulleyana hybrid zone
Following F1 formation, some hybrid zones may be maintained indefinitely by backcrossing in both directions and the occasional formation of genetic intermediates by crosses between them (Arnold, 1997). Others are apparently maintained by regular formation of new F1s (e.g. Kyhos et al., 1981; Milne et al., 2003; Milne and Abbott, 2008). However, in this case, the hybrid zone appears to be young, based on a dramatic increase in the number of morphologically identifiable hybrid derivatives from very few plants in 2004 (Wu, 2008; Wu and Zhang, 2010) to at least 129 flowering plants in 2012. This fits with the hybrid zone having been initiated by disturbance due to road building after 1990, as the Jade Dragon area was developed for tourism. Curiously, despite this, all hybrid derivatives now occur down the slope, away from the disturbed roadside habitat. This could be because the F1s grew further down the slope, and seeds do not easily travel up the slope. Alternatively, backcrosses to P. bulleyana may be more suited to the shaded habitat lower down. Either way, the role of disturbance in this case seems to have been in promoting F1 formation (e.g. Semple and Semple, 1977; Lamont et al., 2003), rather than providing a habitat for hybrid derivatives (e.g. Abbott, 1992; Arnold, 1997; Rieseberg and Carney, 1998).
The hybrid zone is therefore changing over time, and, as noted above, pollinator behaviour may be changing with it, facilitating backcrossing. However, if no new F1s are formed and all new hybrid derivatives are backcrosses (with possibly some backcross to backcross crosses), then the increase in the overall number of hybrid derivatives will be accompanied by a decrease in the average proportion of P. beesiana germplasm among them, as more advanced backcrosses to P. bulleyana are generated while early generation backcrosses die off. Backcross to backcross crosses may delay but cannot reverse this process of diluting P. beesiana germplasm. This implies a finite life span for the hybrid zone, unless another F1 is formed. This in turn may depend on whether the influence of disturbance is receding; certainly if the small population of P. bulleyana close to the P. beesiana population disappears, F1 formation will become less likely at this location. Without new F1s, eventually only introgressed individuals of P. bulleyana will remain. Therefore, molecular examinations of the same hybrid population at periodic individuals might illustrate this process.
Introgression does appear to be occurring, with instances detected of P. bulleyana-like individuals containing P. beesiana germplasm, and individuals identified as P. bulleyana from AFLP data that had morphological traits of hybrids. Hence there is no clear dividing line between hybrids and P. bulleyana. This introgression might transfer new characters to P. bulleyana, particularly if gene flow from P. beesiana has not happened previously. Introgression can permit occupation of new habitats (Arnold, 1997; Arnold et al., 2012; Zulliger et al., 2013), and could help P. bulleyana to colonize habitats altered by local human activity. Future studies might therefore examine whether habitat differences can be detected between individuals with and without introgressed germplasm, and how this might change over time due to natural selection. From this, the hypothesis of a possible long-term evolutionary outcome from this hybridization event could be examined.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We are grateful to Elena Conti and Barbara Keller in the University of Zurich, as well as the handing editor Sophie Karrenberg and two anonymous reviewers for constructive comments on the manuscript. We thank Tobias Marczewski and Jane Droop from the Royal Botanic Garden, Edinburgh for help with written English, Yanhui Zhao for insect identification, and Tao Su for drawing the distribution map. This work was supported by the National Natural Science Foundation of China (grant no. 31200247), the Yunnan Natural Science Foundation (grant no. 312011FB103) and the Independent Research Program of the Chinese Academy of Sciences (grant no. KSCX2-EW-J-24).
LITERATURE CITED
- Abbott RJ. Plant invasions, hybridization and the evolution of new plant taxa. Trends in Ecology and Evolution. 1992;7:401–405. doi: 10.1016/0169-5347(92)90020-C. [DOI] [PubMed] [Google Scholar]
- Abbott R, Albach D, Ansell S, et al. Hybridization and speciation. Journal of Evolutionary Biology. 2013;26:229–246. doi: 10.1111/j.1420-9101.2012.02599.x. [DOI] [PubMed] [Google Scholar]
- Anderson E. Hybridization of the habitat. Evolution. 1948;2:1–9. [Google Scholar]
- Anderson EC, Thompson EA. A model based method for identifying species hybrids using multilocus genetic data. Genetics. 2002;160:1217–1229. doi: 10.1093/genetics/160.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold ES, Richards AJ. On the occurrence of unilateral incompatibility in Primula sect. Aleuritia Duby and the origin of Primula scotica Hook. Botanical Journal of the Linnean Society. 1998;128:359–368. [Google Scholar]
- Arnold ML. Natural hybridization and evolution. New York: Oxford University Press; 1997. [Google Scholar]
- Arnold ML, Hamrick JL, Bennett BD. Interspecific pollen competition and reproductive isolation in Iris. Journal of Heredity. 1993;84:13–16. [Google Scholar]
- Arnold ML, Ballerini ES, Brothers AN. Hybrid fitness, adaptation and evolutionary diversification: lessons learned from Louisiana Irises. Heredity. 2012;108:159–166. doi: 10.1038/hdy.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacilieri R, Ducousso A, Petit R, Kremer A. Mating system and asymmetric hybridization in a mixed stand of European oaks. Evolution. 1996;50:900–908. doi: 10.1111/j.1558-5646.1996.tb03898.x. [DOI] [PubMed] [Google Scholar]
- Beatty GE, Philipp M, Provan J. Unidirectional hybridization at a species' range boundary: implications for habitat tracking. Diversity and Distributions. 2010;16:1–9. [Google Scholar]
- Bradshaw HD, Schemske DW. Allele substitution at a flower colour locus produces a pollinator shift in monkey flowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Broyles SB. Hybrid bridges to gene flow: a case study in milkweeds (Asclepias) Evolution. 2002;56:1943–1953. doi: 10.1111/j.0014-3820.2002.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Campbell D, Duchesne P, Bernatchez L. AFLP utility for population assignment studies: analytical investigation and empirical comparison with microsatellites. Molecular Ecology. 2003;12:1979–1991. doi: 10.1046/j.1365-294x.2003.01856.x. [DOI] [PubMed] [Google Scholar]
- Carney SE, Cruzan MB, Arnold ML. Reproductive interactions between hybridizing irises: analyses of pollen tube growth and fertilization success. American Journal of Botany. 1994;81:1169–1175. [Google Scholar]
- Carney SE, Hodges SA, Arnold ML. Effects of pollen-tube growth and ovule position on hybridization in the Louisiana irises. Evolution. 1996;50:1871–1878. doi: 10.1111/j.1558-5646.1996.tb03574.x. [DOI] [PubMed] [Google Scholar]
- Chen HY, Sun WB, Li WQ. Seed storage behavior of Formanodendron doichangensis (Fagaceae), a rare and endangered plant. Plant Diversity and Resources. 2011;33:540–546. [Google Scholar]
- Chung MY, Nason JD, Chung MG. Patterns of hybridization and population genetic structure in the terrestrial orchids Liparis kumokiri and Liparis makinoana (Orchidaceae) in sympatric populations. Molecular Ecology. 2005;14:4389–4402. doi: 10.1111/j.1365-294X.2005.02738.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- Cruzan MB, Arnold ML. Ecological and genetic associations in an Iris hybrid zone. Evolution. 1993;47:1432–1445. doi: 10.1111/j.1558-5646.1993.tb02165.x. [DOI] [PubMed] [Google Scholar]
- Dan T, Ikeda H, Mitsui Y, Isagi Y, Setoguchi H. Genetic structure of refugial populations of the temperate plant Shortia rotundifolia (Diapensiaceae) on a subtropical island. Conservation Genetics. 2009;10:859–867. [Google Scholar]
- De hert K, Jacquemyn H, Glabeke SV, et al. Reproductive isolation and hybridization in sympatric populations of three Dactylorhiza species (Orchidaceae) with different ploidy levels. Annals of Botany. 2012;109:709–720. doi: 10.1093/aob/mcr305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Genetics and the origin of species. New York: Columbia University Press; 1937. [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure for small quantities of fresh leaf material. Phytochemical Bulletin. 1987;19:11–15. [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Field DL, Ayre DJ, Whelan RJ, Young AG. The importance of pre-mating barriers and the local demographic context for contemporary mating patterns in hybrid zones of Eucalyptus aggregata and Eucalyptus rubida. Molecular Ecology. 2011;20:2367–2379. doi: 10.1111/j.1365-294X.2011.05054.x. [DOI] [PubMed] [Google Scholar]
- Gong YB, Huang SQ. Floral symmetry: pollinator-mediated stabilizing selection on flower size in bilateral species. Proceedings of the Royal Society B: Biological Sciences. 2009;276:4013–4020. doi: 10.1098/rspb.2009.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney M, Preston CD, Barrett J, Briggs D. Hybridisation between Oxlip Primula elatior (L.) Hill and Primrose P. vulgaris Hudson, and the identification of their variable hybrid P.×digenea A. Kerner. Watsonia. 2007;26:239–251. [Google Scholar]
- Haldane JBS. Sex ratio and unisexual sterility in hybrid animals. Journal of Genetics. 1922;12:101–109. [Google Scholar]
- Herrera J, Arista M, Ortiz PL. Perianth organization and intra-specific floral variability. Plant Biology. 2008;10:704–710. doi: 10.1111/j.1438-8677.2008.00091.x. [DOI] [PubMed] [Google Scholar]
- Howard DJ. Conspecific sperm and pollen precedence and speciation. Annual Review of Ecology and Systematics. 1999;30:109–132. [Google Scholar]
- Hu CM, Kelso S. Primulaceae. In: Wu ZY, Raven PH, editors. Flora of China. Vol. 15. Beijing: Science Press, and; 1996. St. Louis, MO: Missouri Botanical Garden Press, China, 100. [Google Scholar]
- Hubisz MJ, Falush D, Stephens M, Pritchard JK. Inferring weak population structure with the assistance of sample group information. Molecular Ecology Resources. 2009;9:1322–1332. doi: 10.1111/j.1755-0998.2009.02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kálmán K, Medvegy A, Mihalik E. Pattern of the floral variation in the hybrid zone of two distylous Primula species. Flora. 2004;199:218–227. [Google Scholar]
- Kameyama Y, Toyama M, Ohara M. Hybrid origins and F1 dominance in the free-floating sterile bladderwort, Utricularia australis f. australis (Lentibulariaceae) American Journal of Botany. 2005;92:469–476. doi: 10.3732/ajb.92.3.469. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Gan YY, Lum SKY, Khoo MS, Chua SC, Faizu NNH. Morphological and molecular evidence of natural hybridization in Shorea (Dipterocarpaceae) Tree Genetics and Genomes. 2011;7:297–306. [Google Scholar]
- Keller B, de Vos JM, Conti E. Decrease of sexual organ reciprocity between heterostylous primrose species, with possible functional and evolutionary implications. Annals of Botany. 2012;110:1233–1244. doi: 10.1093/aob/mcs199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH. Use of DNA barcodes to identify flowering plants. Proceedings of the National Academy of Sciences, USA. 2005;102:8369–8374. doi: 10.1073/pnas.0503123102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyhos DW, Clark C, Thompson WC. The hybrid nature of Encelia laciniata (Compositae: Heliantheae) and control of population composition by post-dispersal selection. Systematic Botany. 1981;6:399–411. [Google Scholar]
- Lamont BB, He T, Enright NJ, Krauss SL, Miller BP. Anthropogenic disturbance promotes hybridization between Banksia species by altering their biology. Journal of Evolutionary Biology. 2003;16:551–557. doi: 10.1046/j.1420-9101.2003.00548.x. [DOI] [PubMed] [Google Scholar]
- Lepais O, Petit RJ, Guichoux E, et al. Species relative abundance and direction of introgression in oaks. Molecular Ecology. 2009;18:2228–2242. doi: 10.1111/j.1365-294X.2009.04137.x. [DOI] [PubMed] [Google Scholar]
- Levin DA, Francisco-Ortega J, Jansen RK. Hybridization and the extinction of rare species. Conservation Biology. 1996;10:10–16. [Google Scholar]
- Li JK, Huang SQ. Effective pollinators of Asian sacred lotus (Nelumbo nucifera): contemporary pollinators may not reflect the historical pollination syndrome. Annals of Botany. 2009;104:845–851. doi: 10.1093/aob/mcp173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YP, Milne RI, Zhang CQ, Yang JB. Unusual patterns of hybridization involving a narrow endemic Rhododendron species in Yunnan (Ericaceae), China. American Journal of Botany. 2010;97:1749–1757. doi: 10.3732/ajb.1000018. [DOI] [PubMed] [Google Scholar]
- Ma YP, Wu ZK, Tian XL, Zhang CQ, Sun WB. Growth discrepancy between filament and style facilitates autonomous self-fertilization in Hedychium yunnanense. Plant Ecology and Evolution. 2012;145:185–189. [Google Scholar]
- Mallet J. Hybridization as an invasion of the genome. Trends in Ecology and Evolution. 2005;20:229–237. doi: 10.1016/j.tree.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Marques I, Rossello-Graell A, Draper D, Iriond JM. Pollination patterns limit hybridization between two sympatric species of Narcissus (Amaryllidaceae) American Journal of Botany. 2007;94:1352–1359. doi: 10.3732/ajb.94.8.1352. [DOI] [PubMed] [Google Scholar]
- Mast AR, Conti E. The primrose path to heterostyly. New Phytologist. 2006;171:439–442. doi: 10.1111/j.1469-8137.2006.01833.x. [DOI] [PubMed] [Google Scholar]
- McCubbin A. Heteromorphic self-incompatibility in Primula: 21st century tools promise to unravel a classic 19th century model system. In: Franklin-Tong VE, editor. Self-incompatibility in flowering plants – evolution, diversity, and mechanisms. Berlin: Springer-Verlag; 2008. pp. 289–308. [Google Scholar]
- Memmott J. The structure of a plant–pollinator food web. Ecology Letters. 1999;2:276–280. doi: 10.1046/j.1461-0248.1999.00087.x. [DOI] [PubMed] [Google Scholar]
- Miller LM. Classifying genealogical origins in hybrid populations using dominant markers. Journal of Heredity. 2000;91:46–49. doi: 10.1093/jhered/91.1.46. [DOI] [PubMed] [Google Scholar]
- Milne RI, Abbott RJ. Reproductive isolation among two interfertile Rhododendron species: low frequency of post-F1 hybrid genotypes in alpine hybrid zones. Molecular Ecology. 2008;17:1108–1121. doi: 10.1111/j.1365-294X.2007.03643.x. [DOI] [PubMed] [Google Scholar]
- Milne RI, Terzioglu S, Abbott RJ. A hybrid zone dominated by fertile F1s: maintenance of species barriers in Rhododendron. Molecular Ecology. 2003;12:2719–2729. doi: 10.1046/j.1365-294x.2003.01942.x. [DOI] [PubMed] [Google Scholar]
- Mogensen HL. The hows and whys of cytoplasmic inheritance in seed plants. American Journal of Botany. 1996;83:383–404. [Google Scholar]
- Montgomery BR, Soper DM, Delph LF. Asymmetrical conspecific seed-siring advantage between Silene latifolia and S. dioica. Annals of Botany. 2010;105:595–605. doi: 10.1093/aob/mcq013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natalis LC, Wesselingh RA. Post-pollination barriers and their role in asymmetric hybridization in Rhinanthus (Orobanchaceae) American Journal of Botany. 2012;99:1847–1856. doi: 10.3732/ajb.1200085. [DOI] [PubMed] [Google Scholar]
- Natalis LC, Wesselingh RA. Parental frequencies and spatial configuration shape bumblebee behavior and floral isolation in hybridizing Rhinanthus. Evolution. 2013;67:1692–1705. doi: 10.1111/evo.12044. [DOI] [PubMed] [Google Scholar]
- Pascarella JB. Mechanisms of prezygotic reproductive isolation between two sympatric species, Gelsemium rankinii and G. sempervirens (Gelsemiaceae), in the southeastern United States. American Journal of Botany. 2007;94:468–476. doi: 10.3732/ajb.94.3.468. [DOI] [PubMed] [Google Scholar]
- Rahmé J, Widmer A, Karrenberg S. Pollen competition as an asymmetric reproductive barrier between two closely related Silene species. Journal of Evolutionary Biology. 2009;22:1937–1943. doi: 10.1111/j.1420-9101.2009.01803.x. [DOI] [PubMed] [Google Scholar]
- Reisch C. Genetic structure of Saxifraga tridactylites (Saxifragaceae) from natural and man-made habitats. Conservation Genetics. 2007;8:893–902. [Google Scholar]
- Richards AJ. Primula. London: Batsford; 1993. [Google Scholar]
- Richards AJ. Primula. 2nd edn. London: Batsford; 2002. [Google Scholar]
- Rieseberg LH. Hybrid origins of plant species. Annual Review of Ecology and Systematics. 1997;28:359–389. [Google Scholar]
- Rieseberg LH, Carney SE. Plant hybridization. New Phytologist. 1998;140:599–624. doi: 10.1046/j.1469-8137.1998.00315.x. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Gerber D. Hybridization in the Catalina Island mountain mahogany (Cercocarpus traskiae): RAPD evidence. Conservation Biology. 1995;9:199–203. [Google Scholar]
- Rieseberg LH, Willis JH. Plant speciation. Science. 2007;317:910–914. doi: 10.1126/science.1137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple JC, Semple KS. Borrichia × cubana (B. frutescens × B. arborescens): interspecific hybridization in the Florida Keys. Systematic Botany. 1997;2:292–302. [Google Scholar]
- Soltis PS, Soltis DE. The role of hybridization in plant speciation. Annual Review of Plant Biology. 2009;60:561–588. doi: 10.1146/annurev.arplant.043008.092039. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins GD. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin P, Olson MS, Moyle MC. Asymmetrical crossing barriers in angiosperms. Proceedings of the Royal Society B: Biological Sciences. 2001;268:861–867. doi: 10.1098/rspb.2000.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco F, Tatum T, Rayburn AL, Tranel PJ. Out of the swamp: unidirectional hybridization with weedy species may explain the prevalence of Amaranthus tuberculatus as a weed. New Phytologist. 2009;184:819–827. doi: 10.1111/j.1469-8137.2009.02979.x. [DOI] [PubMed] [Google Scholar]
- Valentine DH. Studies in British Primulas. IV. Hybridization between Primula vulgaris Huds. and P. veris L. New Phytologist. 1955;54:70–80. [Google Scholar]
- Van Droogenbroeck B, Kyndt T, Romeijn-Peeters E, et al. Evidence of natural hybridization and introgression between Vasconcellea species (Caricaceae) from southern Ecuador revealed by chloroplast, mitochondrial and nuclear DNA markers. Annals of Botany. 2006;97:793–805. doi: 10.1093/aob/mcl038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrancken J, Wesselingh RA. Inheritance of the chloroplast genome in Rhinanthus angustifolius (Orobanchaceae) Plant Ecology and Evolution. 2010;143:239–242. [Google Scholar]
- Waelti MO, Muhlemann JK, Widmer A, Schiestl FP. Floral odour and reproductive isolation in two species of Silene. Journal of Evolutionary Biology. 2008;21:111–121. doi: 10.1111/j.1420-9101.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- Wesselingh RA, Arnold ML. Pollinator behavior and the evolution of Louisiana iris hybrid zones. Journal of Evolutionary Biology. 2000;13:171–180. [Google Scholar]
- Woodell SRJ. Natural hybridization in Britain between Primula vulgaris Huds (the primrose) and P. elatior (L.) Hill (the oxlip) Watsonia. 1969;7:115–127. [Google Scholar]
- Wu ZK. Systematics of Primula section Proliferae Pax (Primulaceae) Beijing, China: The Graduate School of Chinese Academy of Sciences; 2008. PhD dissertation. [Google Scholar]
- Wu ZK, Zhang CQ. Comparative study of pollination biology of two closely related alpine Primula species, namely Primula beesiana and P. bulleyana (Primulaceae) Journal of Systematics and Evolution. 2010;48:109–117. [Google Scholar]
- Yang CF, Gituru RW, Guo YH. Reproductive isolation of two sympatric louseworts, Pedicularis rhinanthoides and Pedicularis longiflora (Orobanchaceae): how does the same pollinator type avoid interspecific pollen transfer? Biological Journal of the Linnean Society. 2007;90:37–48. [Google Scholar]
- Zalapa JE, Brunet J, Guries RP. Patterns of hybridization and introgression between invasive Ulmus pumila and native U. rubra. American Journal of Botany. 2009;96:1116–1128. doi: 10.3732/ajb.0800334. [DOI] [PubMed] [Google Scholar]
- Zha HG, Milne RI, Sun H. Asymmetric hybridization in Rhododendron agastum: a hybrid taxon comprising mainly F1s in Yunnan, China. Annals of Botany. 2010;105:89–100. doi: 10.1093/aob/mcp267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Liu Y, Sodmergen Examination of the cytoplasmic DNA in male reproductive cells to determine the potential for cytoplasmic inheritance in 295 angiosperm species. Plant and Cell Physiology. 2003;44:941–951. doi: 10.1093/pcp/pcg121. [DOI] [PubMed] [Google Scholar]
- Zhou RC, Gong X, Boufford D, Wu CI, Shi SH. Testing a hypothesis of unidirectional hybridization in plants: observations on Sonneratia, Bruguiera and Ligularia. BMC Evolutionary Biology. 2008;8:149. doi: 10.1186/1471-2148-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XF, Li Y, Wu GL, Fang ZD, Li QJ, Liu JQ. Molecular and morphological evidence for natural hybridization between Primula secundiflora Franchet and P. poissonii Franchet (Primulaceae) Acta Biologica Cracoviensia Series Botanica. 2009;51:29–36. [Google Scholar]
- Zulliger D, Schnyder E, Gugerli F. Are adaptive loci transferable across genomes of related species? Outlier and environmental association analyses in Alpine Brassicaceae species. Molecular Ecology. 2013;22:1626–1639. doi: 10.1111/mec.12199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.