Abstract
Background and Aims
The arabinogalactan protein (AGP) gene family is involved in plant reproduction. However, little is known about the function of individual AGP genes in pollen development and pollen tube growth. In this study, Brassica campestris male fertility 8 (BcMF8), a putative AGP-encoding gene previously found to be pollen specific in Chinese cabbage (B. campestris ssp. chinensis), was investigated.
Methods
Real-time reverse transcription–PCR and in situ hybridization were used to analyse the expression pattern of BcMF8 in pistils. Prokaryotic expression and western blots were used to ensure that BcMF8 could encode a protein. Antisense RNA technology was applied to silence gene expression, and morphological and cytological approaches (e.g. scanning electron microscopy and transmission electron microscopy) were used to reveal abnormal phenotypes caused by gene silencing.
Key Results
The BcMF8 gene encoded a putative AGP protein that was located in the cell wall, and was expressed in pollen grains and pollen tubes. The functional interruption of BcMF8 by antisense RNA technology resulted in slipper-shaped and bilaterally sunken pollen with abnormal intine development and aperture formation. The inhibition of BcMF8 led to a decrease in the percentage of in vitro pollen germination. In pollen that did germinate, the pollen tubes were unstable, abnormally shaped and burst more frequently relative to controls, which corresponded to an in vivo arrest of pollen germination at the stigma surface and retarded pollen tube growth in the stylar transmitting tissues.
Conclusions
The phenotypic defects of antisense BcMF8 RNA lines (bcmf8) suggest a crucial function of BcMF8 in modulating the physical nature of the pollen wall and in helping in maintaining the integrity of the pollen tube wall matrix.
Keywords: AGP, arabinogalactan proteins, Brassica campestris, Chinese cabbage, aperture, intine, pollen tube, pollen wall development, aperture formation
INTRODUCTION
As a biological protector of male sperm, pollen is covered by the pollen wall. Despite the morphological diversity of the pollen wall, its fundamental structure shows a significant similarity among different species. The outer layer of the pollen wall, which is called the exine, is composed largely of sporopollenin, which usually comprises the outer sexine and inner nexine layers. The intine is the inner layer of the pollen wall underlying the exine, consisting of hydrolytic enzymes, hydrophobic proteins, cellulose, hemicellulose and pectic polymers (Ariizumi and Toriyama, 2011). Both layers of the pollen wall are important for pollen actions (Owen and Makaroff, 1995). Although the general pattern of pollen wall development and the components are clear (Ariizumi and Toriyama, 2011), the entire dynamic regulatory network of pollen wall synthesis is largely undefined, which may involve a considerable amount of different kinds of molecules (Seifert and Blaukopf, 2010).
Arabinogalactan proteins (AGPs), extensively glycosylated hydroxyproline-rich glycol-proteins, are abundant throughout the plant kingdom, mainly at cell surfaces, where they are thought to have important functions in plant growth and development, especially in plant reproduction (Showalter, 2001). By investigating the distribution of AGP epitopes and mRNA transcripts, results show that AGPs are highly expressed in male organs (pollen, pollen tube and sperm), female organs (stigma, style and ovary) and the embryo. In many species, such as Nicotiana alata, Lilium longiflorum, maize and tomato, AGPs have significant functions in anther development, discrimination and adhesion between the pollen and stigma, pollen tube germination and growth, fertilization and embryo development (Du et al., 1996; Geitmann and Steer, 2006; Lee et al., 2008, 2009). Although these studies showed that the reproduction process is associated with changes in the distribution of AGP epitopes or some AGP mRNA transcripts, they did not identify the precise function of a specific AGP protein or corresponding gene acting during reproduction. In recent years, several AGP mutants have been generated to understand the mechanisms of AGP function (van Hengel and Roberts, 2003; Gaspar et al., 2004; Sun et al., 2004; van Hengel et al., 2004; Seifert and Blaukopf, 2010). However, few studies directly support the functions of AGP-encoding genes in plant reproduction, except for the recent specific function found for AtAGP6, AtAGP11 and FLA3 in pollen development, and AtAGP18 in the initiation of female gametogenesis in arabidopsis (Garcia and Calzada, 2004; Pereira et al., 2006; Levitin et al., 2008; Coimbra et al., 2009, 2010; Li et al., 2010).
In a previous study, a genic male sterility (GMS) system named ‘Bcajh97-01A/B’ was constructed in B. campestris ssp. chinensis (Huang et al., 2008a). ‘Bcajh97-01A/B’ is a sibling line segregated in a 1:1 ratio with homozygous male-sterile plants (‘Bcajh97-01A’) and heterozygous male-fertile plants (‘Bcajh97-01B’). Moreover, the male-sterile plants do not produce any functional pollen, and exhibit dramatic changes in the transcriptional profile of downstream genes in flower buds, among which one gene encoding a putative classical AGP, namely BcMF8, was isolated (Huang et al., 2008a). BcMF8 was found to be pollen specific and started to be expressed at the uninucleate stage, which suggested its important function in pollen development (Huang et al., 2008b).
In the present study, the function of BcMF8 was determined by specifically degrading the endogenous BcMF8 transcript by antisense RNA technology. The pollen grains of the bcmf8 lines exhibited slipper-shaped and bilaterally sunken pollen. Most of the pollen tubes could not grow normally, whereas pollen germination at the stigma surface and pollen tube elongation in the style tissue and transmitting tract were retarded before 8 h after pollination (HAP) in vivo, and fewer pollen tubes could reach the base of the transmitting tract at 24 HAP. Furthermore, the downregulation of BcMF8 resulted in abnormal pollen intine development and pollen aperture formation, which possibly contributed to pollen tube growth. These results may provide valuable insight regarding AGP genes in pollen development and pollen tube growth.
MATERIALS AND METHODS
Plant material
The Brassica campestris ssp. chinensis (Chinese cabbage) GMS line system ‘Bcajh97-01A/B’ was cultivated in the experimental farm of Zhejiang University (Huang et al., 2008a).
Real-time RT–PCR
Total RNA was extracted from the closed flower buds from the whole inflorescence and pistils at 1, 2, 4, 8, 12 and 24 HAP using Trizol® reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's instructions. cDNA was then synthesized using a Reverse Transcription System A3500 (Promega, Madison, WI, USA) according to the manufacturer's instructions. Three technical repeats were performed. Real-time reverse transcription–PCR (RT–PCR) was achieved with the gene-specific primer pair P1 (5′-CCACCACGCAGACCAAGGCA-3') and P2 (5'-GTCCATCAACAACCTCTG-3') using GoTaq® qPCR Master Mix (Promega) on a BioRad CFX96 Real-time PCR Detection System according to the manufacturer's instructions. UBC-10 was used as the normalization control.
In situ hybridization
Flower buds and pistils of sterile plants at 1, 3, 10 and 24 HAP were fixed in 4 % paraformaldehyde PBS (phosphate-buffered saline) solution and embedded in Tissue-Tek® O.C.T. Compound (SAKURA, USA). A 14 µm section of tissue was hybridized to specific digoxigenin (DIG)-labelled RNA probes (Roche, Branchburg, Germany). Templates for the gene-specific probes were obtained through amplification with the primer pair P3 (5'-ATGGCACGTCAATTTGTCGTA-3') and P4 (5'-GAAGATGGTCCATCAACAACC-3'). The sense and antisense probes were synthesized using an Sp6/T7 transcription Kit (Roche).
Western blots
The coding sequence of BcMF8 was cloned into the pET32a(+) vector at SacI and HindIII sites with the gene-specific primer pair P5 (5'-CGAGCTCATGGCACGTCAATTTGTCGTATTG-3') and P6 (5'-CCCAAGCTTTTGGAGAGAGAAGAAGAAGAATCC-3'). A Trx-Tag, small His-Tag and S-Tag sequence were included in the fusion protein (LaVallie et al., 1993). The recombinant vector was then introduced into the Escherichia coli strain DE3. The expression of the BcMF8 fusion protein in E. coli cells was induced at 30 °C by adding isopropyl-β-d-thiogalactoside to a final concentration of 0·5 mmol L−1. Cultures were grown for an additional 4 h. The BcMF8 fusion protein was purified using a His-Bind Kit following the manufacturer's protocols (NovaGen, Madison, WI, USA), and detected by western blot using an anti-His antibody (Huaan, Hangzhou, China). The empty pET32a(+) vector was also induced under the same conditions.
Sub-cellular localization of BcMF8–enhanced yellow fluorescent protein (eYFP) fusion protein in onion epidermal cells
The BcMF8-coding open reading frame (ORF) fragment with a modified stop codon was amplified with the gene-specific primer pair P7 (5'-CACCATGGCACGTCAATTTGTCGTATTG-3') and P8 (5'-TTGGAGAGAGAAGAAGAAGAATCC-3'). The resulting cDNA fragment was cloned into the Gateway entry vector pENTR (Invitrogen), and the entry vector was recombined with the destination binary vector pB7YWG2,0 (Karimi et al., 2002). The fusion vector of BcMF8–eYFP was transiently transformed into onion epidermal cells by particle bombardment (Gan, 1989). eYFP-dependent fluorescence was analysed 24 h after introduction under a fluorescence microscope (ECLIPSE 90i; Nikon). To visualize the eYFP distribution, onion epidermal cells were plasmolysed in 0·3 g mL−1 sucrose for 3 min.
Generation of antisense BcMF8 RNA constructs and plant transformation
A 566 bp partial cDNA sequence of BcMF8 was amplified using the oligonucleotide primer pair P9 (5'-AATGGCACGTCAATTTGTCGT-3', forward, with a BamHI restriction endonuclease site) and P10 (5'-CGCATCCAAAAATGTTACAA-3', reverse, with an XbaI restriction endonuclease site). The resulting BcMF8 fragment was then inserted in the antisense orientation into the binary vector pBI121 with the constitutive Cauliflower mosaic virus (CaMV) 35S promoter and the NPTII reporter gene. The fusion vector pBI35S::BcMF8A, as well as a control empty vector, pBI121, were transformed into the fertile Chinese cabbage mediated by Agrobacterium tumefaciens as described by Yu et al. (2004).
PCR analysis and Southern hybridization
For PCR and Southern hybridization, genomic DNA was extracted from fresh young leaves using the cetyl trimethyl ammonium bromide extraction method (McDonald and Martinez, 1990). PCR was performed to identify the transformed lines by detecting the reporter gene, NPTII, in the pBI121 vector, with the primer pair P11 (5'-CAGGATCTCCTGTCATCTCACC-3') and P12 (5'-GGCGATACCGTAAAGCACG-3'). Genomic DNA was then digested with EcoRI for Southern hybridization to confirm the integration of the antisense BcMF8 RNA fragment into the plant genome. Southern hybridization was performed according to Sambrook et al. (1989). The digested genomic DNA was electrophoresed in 1 % agarose gels, transferred by capillary action onto a nylon membrane (Amersham Biosciences, Buckinghamshire, UK) and hybridized to DIG-labelled DNA probes. The DNA probes were prepared by labelling the NPTII gene using a DIG High Prime DNA Labeling and Detection Starter Kit I (Roche) according to the manufacturer's instructions.
Phenotypic and cytological analysis of pollen
Anthers were dissected from unopened floral buds and subsequently dyed with aniline blue (0·1 % aniline blue in 0·1 m K2HPO4-KOH, pH 11) to stain callose in pollen. Alexander stain (Alexander, 1969) was used to detect the vitality of pollen, whereas 4',6-diamidino-2-phenylindole (DAPI) solution (Regan and Moffatt, 1990) was used to investigate the nuclei of pollen.
Electron microscopy
For scanning electron microscopy (SEM), individual pollen grains were spread on SEM carriers, coated with gold–palladium in an Eiko Model IB5 ion coater for 4–5 min, and observed in a Hitachi Model TM-1000 scanning electron microscope. Digital images were then taken. For transmission electron microscopy (TEM), anthers of different development stages were fixed with 2·5 % glutaraldehyde in phosphate buffer (pH 7·0) overnight, and post-fixed with 1 % OsO4 in phosphate buffer for 1 h. The specimens were then dehydrated through a graded series of ethanol, and embedded in Spurr resin. Ultrathin sections (70 nm) were obtained and stained with uranyl acetate, followed by alkaline lead citrate, and observed in a Hitachi Model H-7650 transmission electron microscope. Digital images were then recorded.
Pollen germination
Pollen grains for the pollen germination in vitro assay were collected and cultured in a medium containing 15 % sucrose, 0·4 mm HBO3, 0·4 mm Ca(NO3)2 and 0·1 % agar. The pH was adjusted to 5·8. The pollen grains were grown at 20 °C with 100 % relative humidity in the dark for 4 h. The germinating pollen grains were examined to calculate an average percentage germination and to what extent they were misshapen. To investigate pollen germination in vivo, the pre-emasculated mature flowers of the antisense transgenic and control plants were self-pollinated. Given that B. campestris is a typical sporophytic self-incompatibility species, selfing is induced using immature material in which the S phenotype is not yet expressed (bud pollination) to break down self-incompatibility (Mable, 2008). Flowers of the maternal parents were emasculated and pollinated at 2 d before anthesis. The pollinated pistils were collected at 0, 2, 8, 16 and 24 HAP, and fixed with a mixture of acetic acid and ethanol (3:1). The fixed pistils were rinsed three times with distilled water, softened with 8 m NaOH overnight, washed in distilled water and stained in decolorized aniline blue for 3 h in the dark. The stained pistils were observed and photographed with a Leica DMLB fluorescence microscope (Wetzlar, Germany) under UV light.
Analysis of seeds
Self-pollination tests were performed during the budding period to investigate the fertility of the transgenic plants. Flowers of the maternal parents were emasculated at 2 d before anthesis and pollinated with their own mature pollen. Ten siliques of each line were analysed to count the number of seeds.
RESULTS
BcMF8 is expressed in pollen tubes and pollinated stigma at the early stage of pollen tube growth
Results of previous work have shown that BcMF8 is pollen specific, and expressed at the onset of the uninucleate stage to the mature pollen stage (Huang et al., 2008b). In the present study, the expression pattern of BcMF8 in the pollinated pistils was supplemented. Real-time RT–PCR results showed that the BcMF8 expression level was significantly higher in the pollinated pistils than in those that were unpollinated, especially in the pollinated pistils at 1 and 2 HAP (Fig. 1).
Fig. 1.
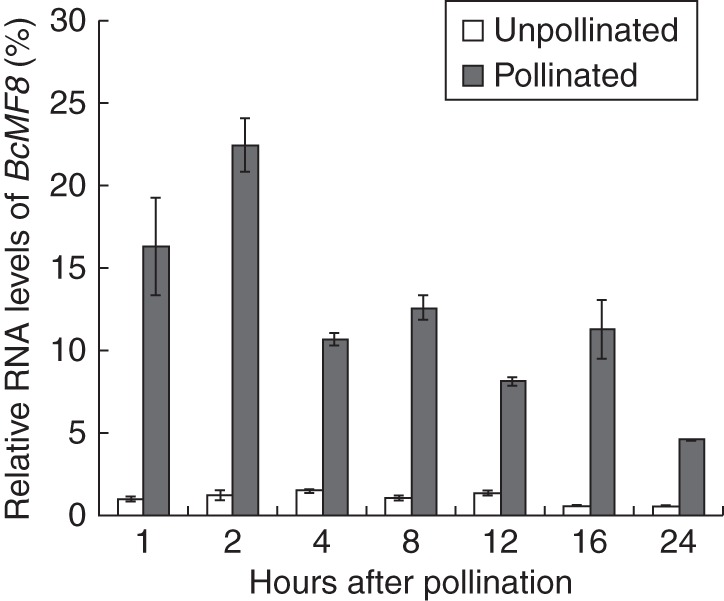
Analysis of the expression of BcMF8 in pistils of the Brassica campestris ssp. chinensis genic male-sterile line system (‘Bcajh97-01A/B’) using real-time RT–PCR. Increased expression levels in the pollinated pistils at 1, 2, 4, 8, 12 and 24 HAP were detected. Ubiquitously expressed UBC-10 was used as an internal control. Standard errors for three independent experiments are also shown.
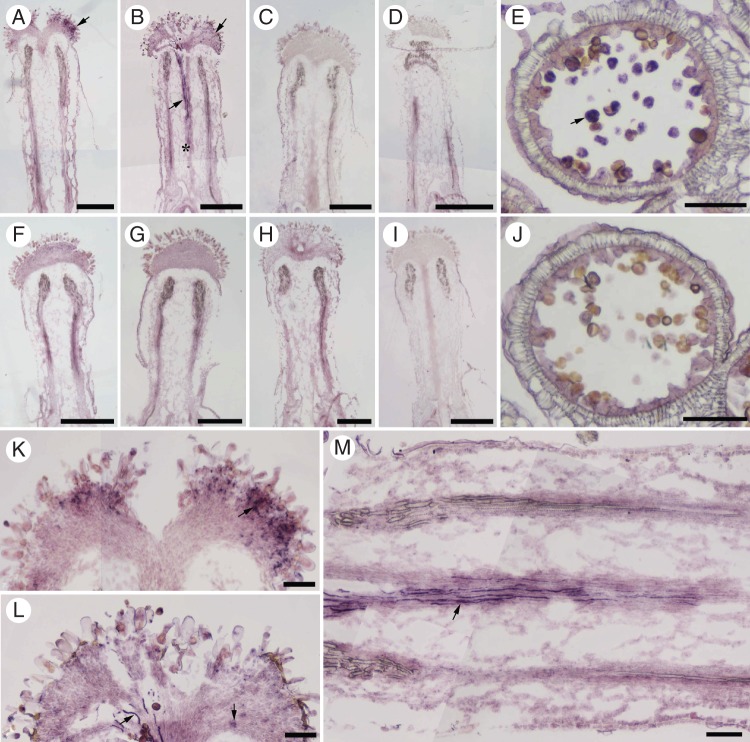
In situ hybridization was performed to visualize the precise location of BcMF8 mRNA in the pistils. A specific hybridization signal was detected in the pollen and stigma of the pollinated pistil at 1 HAP (Fig. 2A, K). A stronger hybridization signal was detected in the stigma at 3 HAP, and in the growing pollen tubes when the pollen tubes penetrated the style tissue and almost reached the upper end of the transmitting tract of the pistil (Fig. 2B, L, M). No signal could be detected in the pollinated pistils at 10 and 24 HAP (Fig. 2C, D), or in the unpollinated pistils (Fig. 2F–I) and sense control (Supplementary Data Fig. S1).
Fig. 2.
The expression of BcMF8 in pollen tubes of the Brassica campestris ssp. chinensis genic male-sterile line system (‘Bcajh97-01A/B’) based on in situ hybridization. (A–D) Longitudinal sections of the pollinated pistils at 1, 3, 10 and 24 HAP hybridized with a BcMF8 antisense probe. A specific hybridization signal (arrowhead) was detected at the stigma at 1 HAP (A). A stronger hybridization signal (arrowhead) was detected at the stigma at 3 HAP and in the growing pollen tubes, which penetrated the style tissue and almost reached the upper end of the transmitting tract of the pistil (asterisk) (B). No hybridization signal was detected in the pollinated pistils at 10 HAP (C) and 24 HAP (D). (E) A section of anther at the binucleate stage hybridized with a BcMF8 antisense probe. Specific hybridization signals were observed in the pollen (arrowhead). (F–I) Longitudinal sections of the unpollinated pistils at 1, 3, 10 and 24 HAP hybridized with a BcMF8 antisense probe. No hybridization signal was detected in the unpollinated pistils. (J) A section of anther at the binucleate stage hybridized with a BcMF8 sense probe as a negative control. (K and L) Magnified images of the corresponding stigma in A and B showing the hybridization signal (arrowhead). (M) Magnified image of the corresponding style in B showing the hybridization signal (arrowhead) in the pollen tubes. Scale bars = 500 µm (A–D, F–I); 100 µm (E, J–M).
BcMF8 protein exhibits extracellular localization
To establish that BcMF8 can encode a protein, the BcMF8 ORF was cloned and expressed in an E. coli strain DE3 T7 RNA polymerase expression system. Total protein was extracted from cultures and purified. Western blot analysis showed that the protein induced in the empty pET32a(+) vector, which contained a Trx-Tag, small His-Tag and S-Tag, was 20·5 kDa. The purified BcMF8 fusion protein, which also contained a Trx-Tag, small His-Tag and S-Tag, was approx. 40 kDa, which indicates that the molecular mass of the BcMF8 protein characterized by SDS–PAGE was approx. 20 kDa (Fig. 3). This mass was slightly higher than that predicted by the deduced amino acid sequence in ExPASy (http://web.expasy.org/compute_pi/), which is 13·2 kDa.
Fig. 3.
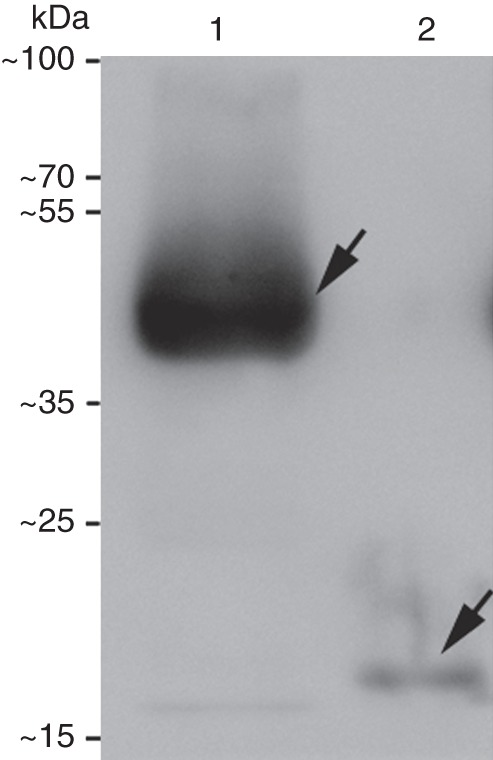
Western blot of the purified BcMF8 fusion protein. Lane 1, purified BcMF8 protein fused with a Trx-Tag, small His-Tag and S-Tag; lane 2, 20·5 kDa protein induced in the empty pET32a(+) vector, containing a Trx-Tag, small His-Tag and S-Tag.
Sequence analysis of BcMF8 using the SignalP 4·0 Server revealed a secretory signal sequence at the N-terminus, with a most probable cleavage site between amino acid residues 20 and 21. WoLF PSORT Protein Subcellular Localization Prediction showed that the BcMF8 protein was localized both intracellularly and extracellularly. An eYFP gene was fused to BcMF8 under the control of the constitutive CaMV 35S promoter to investigate the sub-cellular location of BcMF8. The fusion vector was then transformed into onion epidermal cells. The results showed that the BcMF8–eYFP transgenic cells, including the nuclei, displayed a widespread distribution of fluorescence (Fig. 4A, B). In the plasmolysed BcMF8–eYFP cells, fluorescence was observed in the plasma membrane and cell wall (Fig. 4C, D). These results indicated that the BcMF8 product was a secreted protein, and exhibited an extracellular localization.
Fig. 4.
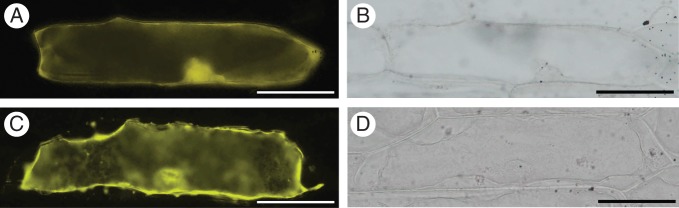
Subcellular localization of BcMF8–eYFP fusion protein in onion epidermal cells. (A) Fluorescence image of unplasmolysed BcMF8–eYFP transgenic cells. (C) Fluorescence image of plasmolysed BcMF8–eYFP transgenic cells. (B) and (D) Bright-field image. Scale bars = 100 µm.
Inhibition of BcMF8 expression results in a pollen morphological defect with ectopic intine development and abnormal aperture formation
To characterize the biological function of BcMF8 in pollen development and pollen tube growth, antisense RNA technology was used to reduce specifically BcMF8 expression in Chinese cabbage. Fusion vector (Supplementary Data Fig. S2) was obtained and transformed into fertile Chinese cabbage mediated by A. tumefaciens. PCR detection (Supplementary Data Fig. S3) and Southern hybridization analysis (Supplementary Data Fig. S4) identified eight independent bcmf8 lines (named bcmf8-1 to bcmf8-8). Control transformed plants were simultaneously obtained with empty vector pBI121 (named CK). Real-time RT–PCR analysis validated the significantly reduced expression of BcMF8 in antisense RNA lines (>60 % reduction compared with CK; Fig. 5).
Fig. 5.
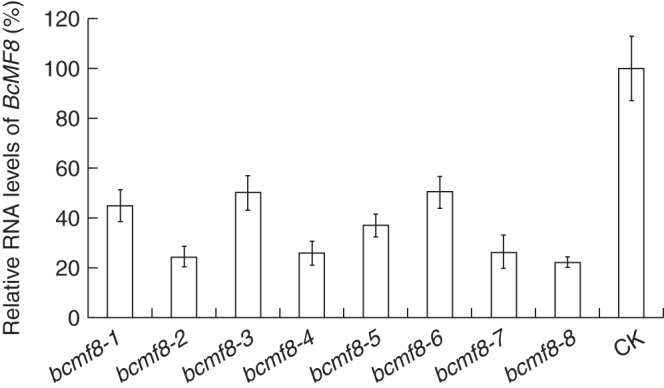
Analysis of the BcMF8 mRNA levels in the inflorescence of the bcmf8 lines by real-time RT–PCR. The expression levels of BcMF8 in the inflorescence in bcmf8 lines were significantly less than those in the empty vector pBI121 (CK), which was defined as 100 %. Ubiquitously expressed UBC-10 was used as an internal control. Standard errors for three independent experiments are also shown.
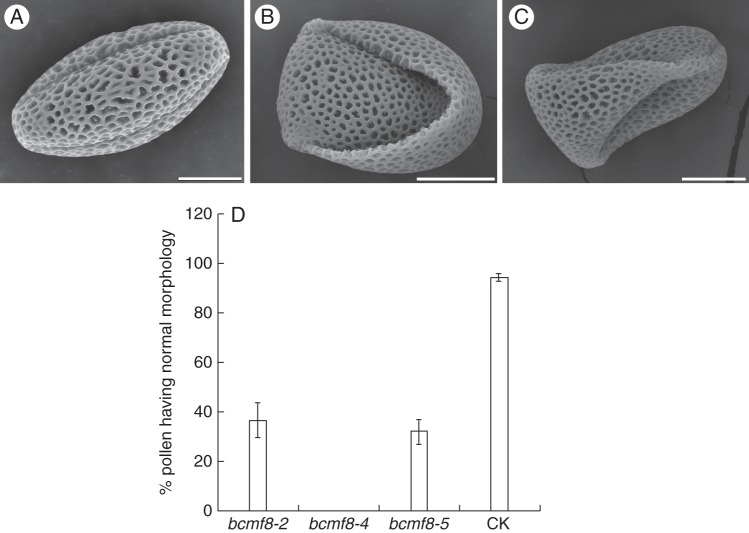
Further investigation revealed that these lines had consistent phenotypes. They displayed normal vegetative growth and flowered normally, with no defects in the sepal, petal, gynoecium, nectar or other floral organs (data not shown). However, obvious defects in pollen morphology in these lines were assessed by SEM (Fig. 6). Three lines, namely bcmf8-2, bcmf8-4 and bcmf8-5, displayed stronger and more obvious defects than the others. Compared with the normal-shaped pollen grains of CK (Fig. 6A), pollen grains from bcmf8 were slipper shaped and bilaterally sunken, and also lost the even aperture distribution pattern (Fig. 6B, C). Statistically, 64 and 69 % of pollen from bcmf8-2 and bcmf8-5, respectively, had morphological defects (Fig. 6D), and almost 100 % of the pollen from bcmf8-4 line was abnormal (Fig. 6D). However, DAPI staining showed that the bcmf8 pollen underwent normal karyokinesis. Aniline blue staining indicated that callose was synthesized normally at the pollen meiosis stage and was degraded at the onset of the uninucleate microspore stage in the bcmf8 pollen. Alexander staining also showed that the vitality of the bcmf8 pollen was not significantly different from that of the control (data not shown).
Fig. 6.
Morphological defects of pollen grains from the bcmf8 lines. (A) Scanning electron micrographs of normal pollen of empty vector pBI121-transformed plants (CK). (B and C) Scanning electron micrographs showing the slipper-shaped and bilaterally sunken pollen of the bcmf8 lines. (D) Histogram showing the normal percentage of pollen in bcmf8-2, bcmf8-4 and bcmf8-5. Standard errors are also shown. Scale bars = 100 µm.
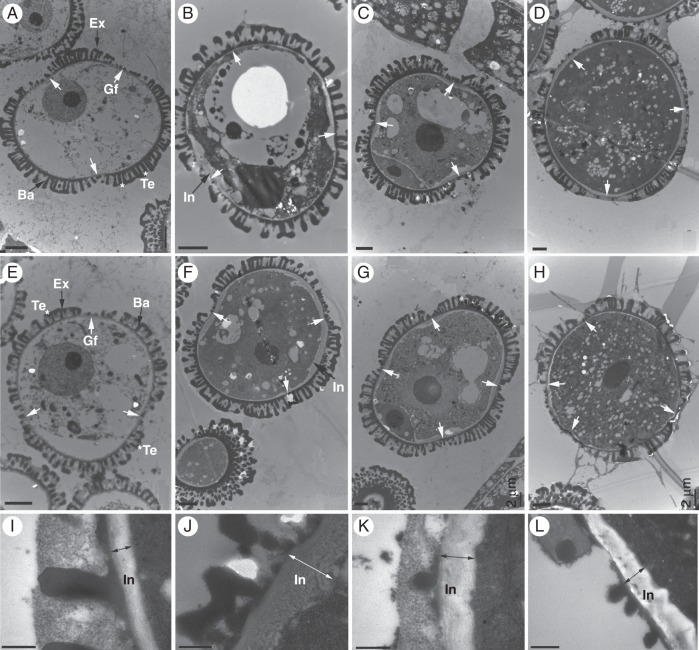
Further examinations of the anther and pollen development process were performed by TEM. All the pollen development stages, including the early uninucleate, late uninucleate, binucleate and trinucleate stages, could be observed (Fig. 7). The TEM results demonstrate that the cell nuclei exhibited normal division and differentiation, and the cell organelles, anther tapetum and other layers showed normal formation throughout the pollen development process (data not shown). These findings reinforced the previous results obtained by DAPI staining and aniline blue staining, which indicate that the bcmf8 pollen was capable of normal callose metabolism and karyokinesis. The only ectopic structure observed was the pollen wall. In normal control pollen, the exine was formed with visible tectum and baculum layers at the early uninucleate microscope stage (Fig. 7A). Three apertures started to form with the development of the pollen wall. At the late uninucleate microscope stage, the intine began to take shape inside the prospective aperture region, whereas no exine occurred (Fig. 7B). This even formation of intine led to a binucleate microscope stage with three evenly distributed apertures (Fig. 7C). Compared with the control pollen, the pollen from bcmf8-2, bcmf8-4 and bcmf8-5 displayed unusual but similar development processes. Prior to the late uninucleate microscope stage, no obvious distinction was found between the bcmf8 pollen (Fig. 7E) and CK pollen (Fig. 7A). However, at the late uninucleate microscope stage, a remarkable thickening in intine both inside and outside the aperture region was detected in the bcmf8 pollen (Fig. 7F, J, L). Moreover, at the binucleate microscope stage, rather than three evenly distributed apertures, four apertures were found in almost 80 % of pollen grains from the bcmf8 lines (Fig. 7G). When pollen developed to the trinucleate and maturation stages, the aperture distribution was still uneven (Fig. 7H). Previous results obtained by SEM revealed that the shape of the bcmf8 pollen was quite different from that of CK, and this difference could be associated with intine development and formation of four apertures.
Fig. 7.
Transmission electron micrographs of pollen development in the bcmf8 lines. (A–D) Micrographs of pollen from empty vector pBI121-transformed plants (CK) showing normal pollen morphology with three apertures and a normal development process from the early uninucleate microscope stage to the mature pollen stage. The pollen at the early uninucleate (A), late uninucleate (B), binucleate (C) and trinucleate (D) microscope stages was observed. (E) Micrograph of pollen from the bcmf8 lines showed normal morphology with three apertures at the early uninucleate microscope stage. (F) Micrograph of pollen from the bcmf8 lines at the late uninucleate microscope stage showing a remarkable thickening in intine (black arrow) both inside and outside the aperture region. (G) Micrograph of pollen from the bcmf8 lines at the binucleate microscope stage showing the number of potential apertures to increase to four instead of three as normal. (H) The uneven distribution of the apertures in the bcmf8 pollen at the mature pollen stage. (I) Mature intine outside the aperture region of the control pollen. (J) Mature intine outside the aperture region of the bcmf8 pollen. There was a remarkable thickening outside the aperture region, compared with the control pollen. (K) Mature intine inside the aperture region of the control pollen. (L) Mature intine inside the aperture region of the bcmf8 pollen. Gf, aperture; In, intine; Ex, exine; Te, tectum; Ba, baculum. Scale bars = 2 µm (A–H); 0·5 µm (I–L).
BcMF8 inhibition retards pollen tube growth, resulting in significantly fewer seeds
In view of the defect of the bcmf8 pollen and upregulated BcMF8 transcript in the pollinated pistils, in vitro and in vivo pollen tube growth were investigated. The bcmf8 pollen tubes were unstable when cultured in vitro. Although an average pollen germination of 63 % could be obtained in optimized conditions, only a small portion exhibited normal pollen tube elongation (Fig. 8E). In the germinated bcmf8-2 pollen, 30·2 % of pollen tubes were bead like (Fig. 8C, E), whereas 17·3 % had burst (Fig. 8D, E). In the germinated bcmf8-4 pollen, 17·7 % of tubes were bead like (Fig. 8E), whereas 47·4 % had burst (Fig. 8E). In the germinated bcmf8-5 pollen, 3·3 % of tubes were balloon tipped (Fig. 8B, E), 6·4 % were bead like (Fig. 8E) and 18·8 % had burst (Fig. 8E). In contrast, >90 % of the control pollen tubes could elongate normally, and none burst or displayed abnormal shapes (Fig. 8A, E).
Fig. 8.
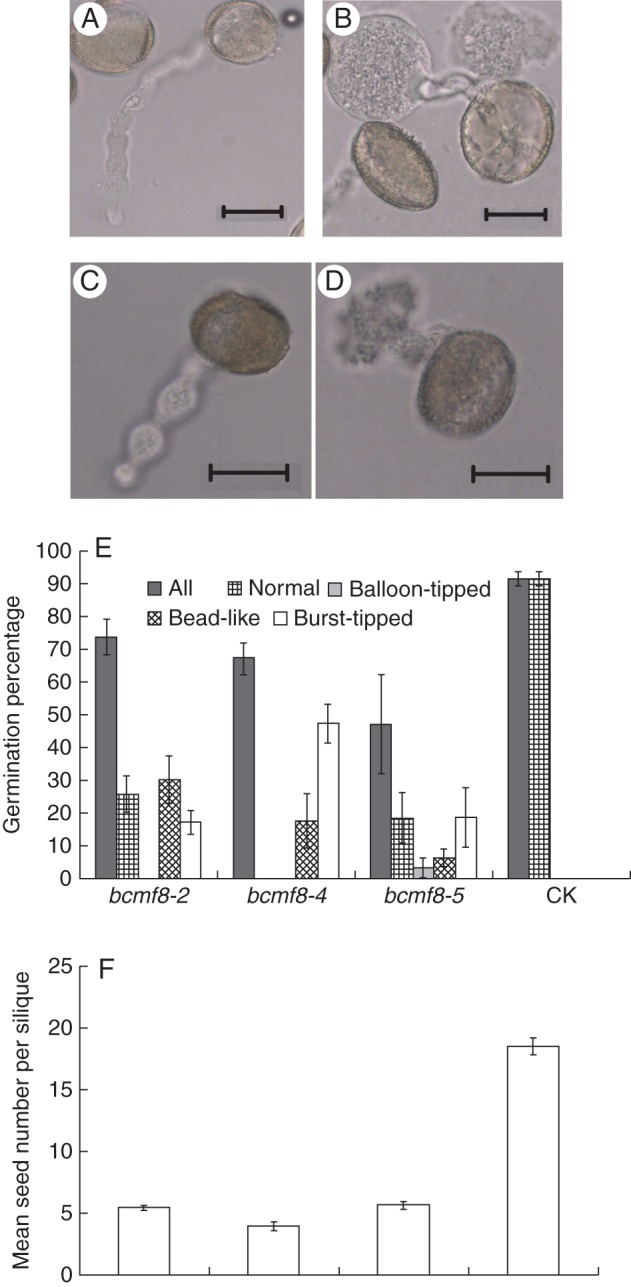
BcMF8 inhibition specifically disrupted the in vitro pollen tube growth and affected seed set. (A) Pollen from empty vector pBI121-transformed plants (CK) was normal. (B–D) Pollen from the bcmf8 lines was abnormal. The pollen tubes of the bcmf8 lines were balloon-tipped (B), bead-like (C) or burst-tipped (D). (E) The germination percentage (± s.e.) of the bcmf8 pollen. (F) The bcmf8 siliques produced significantly fewer seeds than those of CK. Mean seed numbers (± s.e.) per silique of the bcmf8 lines are shown. Scale bars = 20 µm (A–D).
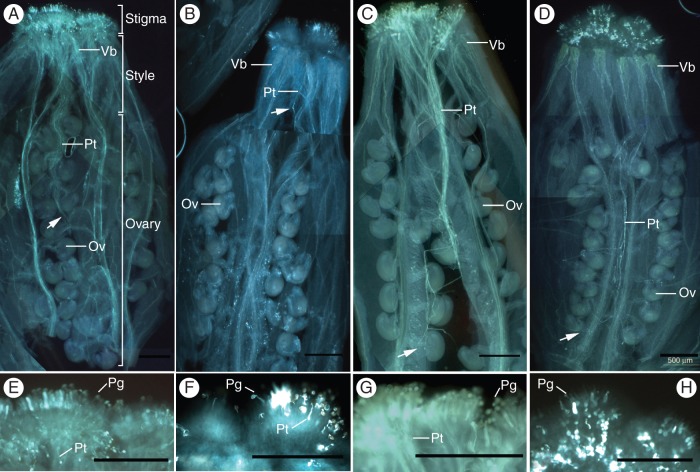
Self-pollination tests were performed during the budding period to investigate the fertility of the bcmf8 lines. The bcmf8 lines could bear fruit pods when pollinated with enough pollen grains. However, each silique of CK could produce about 18 seeds, whereas only around five seeds could be produced in the bcmf8 silique (Fig. 8F). To investigate the cause of the reduction in seeds, the in vivo germination of the bcmf8 pollen on the stigma was examined. The results showed an initial growth of bcmf8 tubes on the surface of stigmatic cells, and the style tissue was significantly affected (Fig. 9). At 8 HAP, the CK pollen tubes penetrated through the style tissue, exited the placenta, travelled along the funicular surface and finally entered as far as half of the length of the transmitting tract (Fig. 9A). The bcmf8 pollen tubes could not even penetrate through the style tissue completely (Fig. 9B). Despite this retardation, the bcmf8 tubes still managed to elongate after 8 HAP, and finally entered the ovule at the base of the transmitting tract (Fig. 9D), similar to the CK tubes (Fig. 9C). However, markedly fewer pollen tubes could be observed within the bcmf8 pistils (Fig. 9D) compared with those in CK (Fig. 9C) under the condition where all the pistils were pollinated with enough pollen grains (Fig. 9E–H). Numerous bcmf8 pollen grains could not germinate (Fig. 9F, H), and remained on the stigma surface. In contrast, most of the CK pollen grains could germinate, pass through the style and enter the ovules (Fig. 9E, G). This result confirms that BcMF8 influenced pollen germination at the stigma, and contributed to tube growth in the stylar transmitting tissues of pistils. Although the bcmf8 lines could produce seeds, the significant decrease in the number of fruit pods was caused by the retardation of pollen tube growth.
Fig. 9.
In vivo pollen germination and pollen tube growth in the bcmf8 lines. (A) Pollen tube growth in the CK female organs at 8 HAP. Pollen tubes had passed through the style tissue and reached approximately half the length of the transmitting tract. (B) Pollen tubes growth in the bcmf8 female organs at 8 HAP. Part of the pollen germinated at the stigma but could not penetrate through the style tissues. (C) Pollen tube growth in the CK female organs at 24 HAP. The pollen tubes had almost reached the base of the ovary. (D) Pollen tube growth in the bcmf8 female organs at 24 HAP. Markedly fewer pollen tubes could be observed within the bcmf8 pistils. (E–H) Magnified images of the corresponding stigma in A–D showing the pollen grains on the stigma. Numerous bcmf8 pollen grains could not germinate (F, H). Arrows show the position at which the pollen tubes arrived. Vb, vascular bundle; Pt, pollen tube; Ov, ovule; Pg, pollen grain. Scale bars = 500 µm.
DISCUSSION
BcMF8 is expressed in the pollen tube and activated in the stigma by pollination
Pollen, pollen wall function and pollen tube growth have been the focus of substantial research because of their biological importance and uniqueness (Becker and Feijo, 2007). In an increasing number of cases, genes with essential gametophytic functions in pollen, including AGP, PG and PME genes, have been discovered by characterizing loss-of-function phenotypes caused by knockout or knockdown mutations (Twell, 2010). AGPs are widely distributed within the plant kingdom. In the arabidopsis genome, 47 putative AGPs have been found (Gaspar et al., 2001; Borner et al., 2002, 2003). Much biochemical and immunohistochemical evidence indicates that AGPs contribute to different aspects of plant growth and development, specifically sexual reproduction (Garcia and Calzada, 2004; Pereira et al., 2006; Coimbra et al., 2007; Qin et al., 2007; Chen et al., 2008; Lee et al., 2009).
Several AGPs are expressed in a high tissue-specific manner, such as AtAGP31, which has high expression in vascular tissues (Liu and Mehdy, 2007); AtAGP30 is exclusively expressed in roots (van Hengel and Roberts, 2003); and AtAGP6 and AtAGP11 are expressed specifically in pollen (Pereira et al., 2006; Levitin et al., 2008).
Our previous work showed that BcMF8 is a pollen-specific gene, whose transcript is expressed at the uninucleate stage and maintained throughout the pollen at the pollination stage (Huang et al., 2008b). In the present study, real-time RT–PCR and in situ hybridization results reveal that BcMF8 was also expressed in pollen tubes in the pollinated pistils. Thus, BcMF8 is a late pollen gene according to the classification by Mascarenhas (1990), and may function in pollen development and pollen tube growth. Notably, BcMF8 was also activated by pollination and expressed in the stigma during 0 HAP to 3 HAP. During the process of pollen germination, many genes show significant changes (Wang et al., 2008; Qin et al., 2009), and the stigmas produce proteins secreted to the exudate for pollen–pistil interactions (Quiapim et al., 2009). AGPNa3, a Nicotiana stigma-specific gene, is the only AGP gene that functions in pollen–pistil interactions after pollination (Du et al., 1996; Quiapim et al., 2009). The prevalence of AGPs in reproductive tissues has led to the speculation that they have significant functions therein, ranging from serving as nutrient resources to involvement in cell–cell recognition in plant reproduction (Cheung and Wu, 1999). Taking into account the expression of BcMF8 in the stigma during the early stage of pollen germination, BcMF8 possibly participates in pollen–pistil interactions.
BcMF8 is a cell wall and secreted protein
A common feature among the diverse AGPs is that they are highly glycosylated cell wall or secreted proteins, in which >90 % (w/w) carbohydrates are covalently attached to a core protein backbone (Showalter, 2001; Liu and Mehdy, 2007). SDS–PAGE analysis showed that the molecular mass of the BcMF8 protein was approx. 20 kDa, which was 6·8 kDa larger than the molecular weight of 13·2 kDa calculated by ExPASy. Meanwhile, an isoelectric point (pI) of 4·31 was predicted by ExPASy, which may explain why the BcMF8 protein ran more slowly than predicted, as a charged protein can show a very different migration in the electric field even if the sequence is completely correct without any unexpected modifications (Takano et al., 1988). It is worth noting that the BcMF8 protein induced in the E. coli expression system was immature, which means that it was not decorated by polysaccharide units.
Arabinogalactan proteins are expressed throughout the plant kingdom, mainly at cell surfaces (Showalter, 2001). Sub-cellular localization of BcMF8 illustrated that BcMF8 was a secreted protein, and exhibited a plasma membrane and extracellular localization. Despite the expected GPI (glycophosphatidylinositol) anchor based on the deduced amino acid sequence (Huang et al., 2008b), the BcMF8 protein was still extracellularly secreted. Although GPI could anchor the AGP to the luminal face of the endoplasmic reticulum membrane and the Golgi membrane (Showalter, 2001), the BcMF8 protein could still be released from the plasma membrane for cell wall and extracellular destinations by the action of an endogenous phospholipase, similar to its homologous gene AGP11 in arabidopsis (Borner et al., 2003).
BcMF8 is important for pollen morphogenesis and aperture formation
Although some AGP genes are associated with plant vegetative growth or signalling, such as LeAGP1, AGP17, and AGP30 (van Hengel and Roberts, 2003; Gaspar et al., 2004; van Hengel et al., 2004; Sun et al., 2004), few molecular results demonstrate the involvement of specific AGPs in pollen function.
At the time of writing, one fasciclin-like AGP gene, FLA3, and two homologous members, AGP6 and AGP11, have been proven to contribute to pollen development. FLA3-RNA interference (RNAi) transgenic plants displayed pollen abortion during the transition from uninucleate microspores to bicellular pollen, with abnormal cellulose distribution and an abnormal intine layer, suggesting that FLA3 was involved in microspore development, and may affect pollen intine formation possibly by participating in cellulose deposition (Li et al., 2010). AGP6 and AGP11 are specifically expressed in stamens, pollen grains and pollen tubes (Levitin et al., 2008); their products have 68 % amino acid sequence similarity (Gaspar et al., 2001) and share functional redundancy because neither the agp6 nor the agp11 mutant shows obvious phenotypic alterations (Levitin et al., 2008; Coimbra et al., 2009). However, the pollen of plants harbouring mutated AGP6 and AGP11 and loss of function in AGP6 and AGP11 inside each locule were devoid of content and had a collapsed appearance, showing a condensed cytoplasm, membrane blebbing and the presence of small lytic vacuoles. In addition, the aborted pollen underwent incomplete mitotic division (Coimbra et al., 2009). Further study which revealed reduced germination and elongation of mutant agp6 agp11 pollen, as well as precocious germination inside the anthers, indicated that AGPs possibly interfere with the timing of pollen germination (Coimbra et al., 2010). However, these investigations could explain neither the mechanism by which AGPs influence the pollen function and sequential pollen tube growth, nor the exact process in which AGPs participate.
Despite the 80·29 % sequence similarity to AGP11, the single BcMF8 knockdown transgenic plants had obvious phenotypic alterations in B. campestris. The antisense BcMF8 transgenic lines produced slipper-shaped and bilaterally sunken pollen, which displayed abnormal intine programming. These results suggest that the BcMF8 product was one of the most important components of the pollen intine layer. Plasma membrane AGPs may help in maintaining the integrity of the plasma membrane and cell wall matrix (Johnson et al., 2003; MacMillan et al., 2010). BcMF8 functioned to maintain normal intine formation, providing a stable interior environment for microspore development.
Arabinogalactan proteins are complex compounds of hydroxyproline-rich core proteins, which are decorated by arabinose- and galactose-rich polysaccharide units (Showalter, 2001). Reversible glycosylation is a complicated process, which is implicated in polysaccharide biosynthesis through the conversion of sugar nucleotides into polysaccharides or other carbohydrates (Drakakaki et al., 2006). The core protein backbone and carbohydrate epitopes are essential in AGP function (Showalter, 2001). Therefore, we hypothesized that the defective pollen without a well-defined intine layer in bcmf8 may result from a lack of a specific protein backbone for the attachment of specific polysaccharides.
A remarkable thickening of bcmf8 intine was observed not only inside but also outside the prospective aperture region, where exine should occur, which resulted in the number of potential apertures increasing to four instead of three as normal. Although aperture number, positions and morphology vary greatly across different species, apertures usually have precise shapes, sizes, decorations and locations within a given species. The discovery of INAPERTURATE POLLEN1 (INP1) provided the first step in understanding at the molecular level of how such functional apertures took shape on the areas where no exine was deposited (Dobritsa and Coerper, 2012). INP1 was specifically involved in the formation of the pollen surface apertures, which resulted from the restriction of exine deposition at specific sites. The bcmf8 mutant described in this study underwent unregulated intine programming, as uneven intine thickening occurred at four areas both inside and outside the prospective aperture region. Following the uneven exine deposition at those corresponding domains, four unevenly distributed apertures were observed instead of three. The increase in aperture size and uneven location were indirectly caused by the inaccurate intine formation.
The pollen wall is more complex than other cell walls in plants. Although several genes are associated with pollen wall development, including the pollen mother cell primary wall (Rhee et al., 2003; Francis et al., 2006), callose (Enns et al., 2005) and exine (Sowders et al., 2001; Ariizumi and Toriyama, 2011), few studies have investigated the mechanism underlying intine development. Our previous work indicated the involvement of two other genes, BcMF2 and BcMF9, which belong to the PG gene family, in intine development and pollen tube growth through the regulation of dynamic pectin metabolism (Huang et al., 2009a, b). BcMF8 might have a redundant function with BcMF2 and BcMF9 in intine development but has special characteristics in aperture formation.
BcMF8 product is essential for pollen tube growth in female floral tissue
As the continuation of the intine layer, quite a lot is known about pollen tube growth (Stone et al., 2004). During this process, gene products involved in cell rescue (stress/abiotic/heat-related function), proteins with binding functions and cellular transport are over-represented, except for the pre-stored transcripts and proteins in pollen (Wang et al., 2008). Many genes are critical for pollen germination and tube growth (Shi and Yang, 2010). The AGP gene family makes up an important part of these genes (Pereira et al., 2006).
Real-time RT–PCR analysis and in situ hybridization illustrated that BcMF8 was expressed in the pollen tubes. The in vitro germination study showed that the BcMF8 pollen tube was highly unstable when grown in culture medium. Although it was able to germinate in vitro, the resulting pollen tube failed to elongate. This finding indicates that the antisense transgenic lines altered the mechanical characteristics of the pollen tube wall and created the imbalance of the apical wall dynamics.
In accordance with this conjecture, retarded pollen tube growth in vivo was also observed. Knockdown of BcMF8 not only led to the reduced germination rate of the pollen tubes on the surface of stigmatic cells, but also retarded the pollen tube penetrating the style tissue and transmitting tract. Despite the bcmf8 pollen tube entering the base of the transmitting tract, significantly fewer seeds of bcmf8 were produced, which resulted in a reduction in male fertility. Thus, this putative AGP, BcMF8, had a crucial function in modulating the physical nature of the growing pollen tube wall, and helped in maintaining the integrity of the pollen tube wall matrix. Previous studies indicated that the style and transmitting tract are composed of cells that secrete a specialized nutrient-rich extracellular matrix (ECM) (Sassen, 1974). Most AGPs are implicated in pollen tube nutrition and guidance (Cheung and Wu, 1999; Wu et al., 2000). Tissue-specific proteins display a gradient of increasing glycosylation from the stigma to the ovary, thereby inducing the pollen tube to elongate along the style and transmitting tracts. We hypothesized that the BcMF8 product has a special distribution in the pollen tubes. The inhibition of BcMF8 expression in bcmf8 may disturb the balance of polysaccharide distribution in the pollen tubes, as well as the interaction between the pollen tubes and ECM, resulting in retarded pollen tube growth and fewer seeds.
Given the efficiency of antisense technology, partial pollen tubes were suggested to reach the base of the ovary, enter the ovules and produce the seeds, because BcMF8 was not completely inhibited. The possibility that complete disruption of the BcMF8 gene would result in a stronger phenotype or null mutation, determined using using RNAi or artificial microRNA technology, which has higher efficiency of gene silencing, could not be eliminated. The AGP family in B. campestris had quite a substantial number of members, similar to the arabidopsis genome in which 47 putative AGPs have been found (Gaspar et al., 2001; Borner et al., 2002, 2003). Other AGP family members and genes that are critical for pollen germination and tube growth might share functional redundancy with BcMF8.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was supported by the Chinese Natural Science Foundation [no. 30800697, no. 31071805 and no. 31372078].
LITERATURE CITED
- Alexander MP. Differential staining of aborted and non-aborted pollen. Stain Technology. 1969;44:117–122. doi: 10.3109/10520296909063335. [DOI] [PubMed] [Google Scholar]
- Ariizumi T, Toriyama K. Genetic regulation of sporopollenin synthesis and pollen exine development. Annual Review of Plant Biology. 2011;62 doi: 10.1146/annurev-arplant-042809-112312. 1·1–1·24. [DOI] [PubMed] [Google Scholar]
- Becker JD, Feijo JA. How many genes are needed to make a pollen tube? Lessons from transcriptomics. Annals of Botany. 2007;100:1117–1123. doi: 10.1093/aob/mcm208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner GHH, Sherrier DJ, Stevens TJ, Arkin IT, Dupree P. Prediction of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A genomic analysis. Plant Physiology. 2002;129:486–499. doi: 10.1104/pp.010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner GHH, Lilley KS, Stevens TJ, Dupree P. Identification of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A proteomic and genomic analysis. Plant Physiology. 2003;132:568–577. doi: 10.1104/pp.103.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KM, Wu GL, Wang YH, et al. The block of intracellular calcium release affects the pollen tube development of Picea wilsonii by changing the deposition of cell wall components. Protoplasma. 2008;233:39–49. doi: 10.1007/s00709-008-0310-2. [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM. Arabinogalactan proteins in plant sexual reproduction. Protoplasma. 1999;208:87–98. [Google Scholar]
- Coimbra S, Almeida J, Junqueira V, Costa ML, Pereira LG. Arabinogalactan proteins as molecular markers in Arabidopsis thaliana sexual reproduction. Journal of Experimental Botany. 2007;58:4027–4035. doi: 10.1093/jxb/erm259. [DOI] [PubMed] [Google Scholar]
- Coimbra S, Costa M, Jones B, Mendes MA, Pereira LG. Pollen grain development is compromised in Arabidopsis agp6 agp11 null mutants. Journal of Experimental Botany. 2009;60:3133–3142. doi: 10.1093/jxb/erp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra S, Costa M, Mendes MA, Pereira AM, Pinto J, Pereira LG. Early germination of Arabidopsis pollen in a double null mutant for the arabinogalactan protein genes AGP6 and AGP11. Sexual Plant Reproduction. 2010;23:199–205. doi: 10.1007/s00497-010-0136-x. [DOI] [PubMed] [Google Scholar]
- Dobritsa AA, Coerper D. The novel plant protein INAPERTURATE POLLEN1 marks distinct cellular domains and controls formation of apertures in the Arabidopsis pollen exine. The Plant Cell. 2012;24:4452–4462. doi: 10.1105/tpc.112.101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Simpson RJ, Clarke AE, Bacic A. Molecular characterization of a stigma-specific gene encoding an arabinogalactan-protein (AGP) from Nicotiana alata. The Plant Journal. 1996;9:313–323. doi: 10.1046/j.1365-313x.1996.09030313.x. [DOI] [PubMed] [Google Scholar]
- Enns LC, Kanaoka MM, Torii KU, Comia L, Okada L, Cleland RE. Two callose synthases, GSL1 and GSL5, play an essential and redundant role in plant and pollen development and in fertility. Plant Molecular Biology. 2005;58:333–349. doi: 10.1007/s11103-005-4526-7. [DOI] [PubMed] [Google Scholar]
- Francis KE, Lam SY, Copenhaver GP. Separation of Arabidopsis pollen tetrads is regulated by QUARTET1, a pectin methylesterase gene. Plant Physiology. 2006;142:1004–1013. doi: 10.1104/pp.106.085274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan C. Gene gun accelerates DNA-coated particles to transform intact cells. The Scientist. 1989;3:25. [Google Scholar]
- Garcia GA, Calzada JPV. A classical arabinogalactan protein is essential for the initiation of female gametogenesis in Arabidopsis. The Plant Cell. 2004;16:2614–2628. doi: 10.1105/tpc.104.024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar Y, Johnson KL, McKenna JA, Bacic A, Schultz CJ. The complex structures of arabinogalactan-proteins and the journey towards understanding function. Plant Molecular Biology. 2001;47:161–176. [PubMed] [Google Scholar]
- Gaspar YM, Nam J, Schultz CJ, et al. Characterization of the Arabidopsis lysine-rich arabinogalactan-protein AtAGP17 mutant (rat1) that results in a decreased efficiency of agrobacterium transformation. Plant Physiology. 2004;135:2162–2171. doi: 10.1104/pp.104.045542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geitmann A, Steer MW. The architecture and properties of the pollen tube cell wall. In: Malhó R, editor. The pollen tube: a cellular and molecular perspective. vol 3. Berlin: Springer-Verlag; 2006. pp. 177–200. Plant Cell Monographs. [Google Scholar]
- van Hengel AJ, Roberts K. AtAGP30, an arabinogalactan-protein in the cell walls of the primary root, plays a role in root regeneration and seed germination. The Plant journal. 2003;36:256–270. doi: 10.1046/j.1365-313x.2003.01874.x. [DOI] [PubMed] [Google Scholar]
- van Hengel AJ, Barber C, Boberts K. The expression patterns of arabinogalactan protein AtAGP30 and GLABRA2 reveal a role for abscisic acid in the early stages of root epidermal patterning. The Plant Journal. 2004;39:70–83. doi: 10.1111/j.1365-313X.2004.02104.x. [DOI] [PubMed] [Google Scholar]
- Huang L, Cao J, Ye W, Liu T, Jiang L, Ye Y. Transcriptional differences between the male-sterile mutant bcms and wild-type Brassica campestris ssp. chinensis reveal genes related to pollen development. Plant Biology. 2008a;10:342–355. doi: 10.1111/j.1438-8677.2008.00039.x. [DOI] [PubMed] [Google Scholar]
- Huang L, Cao JS, Zhang AH, Ye YQ. Characterization of a putative pollen-specific arabinogalactan protein gene, BcMF8, from Brassica campestris ssp. chinensis. Molecular Biology Reports. 2008b;35:631–639. doi: 10.1007/s11033-007-9133-z. [DOI] [PubMed] [Google Scholar]
- Huang L, Cao J, Zhang A, Ye Y, Zhang Y, Liu T. The polygalacturonase gene BcMF2 from Brassica campestris is associated with intine development. Journal of Experimental Botany. 2009a;60:301–313. doi: 10.1093/jxb/ern295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Ye Y, Zhang Y, Zhang A, Liu T, Cao J. BcMF9, a novel polygalacturonase gene, is required for both Brassica campestris intine and exine formation. Annals of Botany. 2009b;104:1339–1351. doi: 10.1093/aob/mcp244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KL, Jones BJ, Bacic A, Schultz CJ. The fasciclin-like arabinogalactan proteins of Arabidopsis. A multigene family of putative cell adhesion molecules. Plant Physiology. 2003;133:1911–1925. doi: 10.1104/pp.103.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Inze D, Depicker A. GATEWAY™ vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science. 2002;7:193–195. doi: 10.1016/s1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- LaVallie ER, DiBlasio EA, Kovacic S, Grant KL, Schendel PF, McCoy JM. A thioredoxin gene fusion expression system that circumvents inclusion body formation in the E. coli cytoplasm. Bio/Technology. 1993;11:187–193. doi: 10.1038/nbt0293-187. [DOI] [PubMed] [Google Scholar]
- Lee CB, Kim S, McClure B. Pollen proteins bind to the C-terminal domain of Nicotiana alata pistil arabinogalactan proteins. Journal of Biological Chemistry. 2008;283:26965–26973. doi: 10.1074/jbc.M804410200. [DOI] [PubMed] [Google Scholar]
- Lee CB, Kim S, McClure B. A pollen protein, NaPCCP, that binds pistil arabinogalactan proteins also binds phosphatidylinositol 3-phosphate and associates with the pollen tube endomembrane system. Plant Physiology. 2009;149:791–802. doi: 10.1104/pp.108.127936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitin B, Richter D, Markovich I, Zik M. Arabinogalactan proteins 6 and 11 are required for stamen and pollen function in Arabidopsis. The Plant Journal. 2008;56:351–363. doi: 10.1111/j.1365-313X.2008.03607.x. [DOI] [PubMed] [Google Scholar]
- Li J, Yu M, Geng LL, Zhao J. The fasciclin-like arabinogalactan protein gene, FLA3, is involved in microspore development of Arabidopsis. The Plant Journal. 2010;64:482–497. doi: 10.1111/j.1365-313X.2010.04344.x. [DOI] [PubMed] [Google Scholar]
- Liu C, Mehdy MC. A nonclassical arabinogalactan protein gene highly expressed in vascular tissues, AGP31, is transcriptionally repressed by methyl jasmonic acid in Arabidopsis. Plant Physiology. 2007;145:863–874. doi: 10.1104/pp.107.102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mable BK. Genetic causes and consequences of the breakdown of self-incompatubility: case studies in the Brassicaceae. Gentical Research. 2008;90:47–60. doi: 10.1017/S0016672307008907. [DOI] [PubMed] [Google Scholar]
- MacMillan CP, Mansfield SD, Stachurski ZH, Evans R, Southerton SG. Fasciclin-like arabinogalactan proteins: specialization for stem biomechanics and cell wall architecture in Arabidopsis and Eucalyptus. The Plant Journal. 2010;62:689–703. doi: 10.1111/j.1365-313X.2010.04181.x. [DOI] [PubMed] [Google Scholar]
- Mascarenhas JP. Gene activity during pollen development. Annual Review of Plant Physiology and Plant Molecular Biology. 1990;41:317–338. [Google Scholar]
- McDonald BA, Martinez JP. Restriction fragment length polymorphisms in Septoria tritici occur at a high frequency. Current Genetics. 1990;17:133–138. [Google Scholar]
- Owen HA, Makaroff CA. Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. ecotype Wassilewskija (Brassicaceae) Protoplasma. 1995;185:7–21. [Google Scholar]
- Pereira LG, Coimbra S, Oliveira H, Monteiro L, Sottomayor M. Expression of arabinogalactan protein genes in pollen tubes of Arabidopsis thaliana. Planta. 2006;223:374–380. doi: 10.1007/s00425-005-0137-4. [DOI] [PubMed] [Google Scholar]
- Qin Y, Chen D, Zhao J. Localization of arabinogalactan proteins in anther, pollen, and pollen tube of Nicotiana tabacum L. Protoplasma. 2007;231:43–53. doi: 10.1007/s00709-007-0245-z. [DOI] [PubMed] [Google Scholar]
- Qin Y, Leydon AR, Manziello A, et al. Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genetics. 2009;5 doi: 10.1371/journal.pgen.1000621. pe1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiapim AC, Brito MS, Bernardes LAS, et al. Analysis of the Nicotiana tabacum stigma/style transcriptome reveals gene expression differences between wet and dry stigma species. Plant Physiology. 2009;149:1211–1230. doi: 10.1104/pp.108.131573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan SM, Moffatt BA. Cytochemical analysis of pollen development in wild-type Arabidopsis and a male sterile mutant. The Plant Cell. 1990;2:877–889. doi: 10.1105/tpc.2.9.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee SY, Osborne E, Poindexter PD, Somerville CR. Microspore separation in the quartet3 mutants of Arabidopsis is impaired by a defect in a developmentally regulated polygalacturonase required for pollen mother cell wall degradation. Plant Physiology. 2003;133:1170–1180. doi: 10.1104/pp.103.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sassen MMA. The stylar transmitting tissue. Acta Botanica Neerlandica. 1974;23:99–108. [Google Scholar]
- Seifert GJ, Blaukopf Irritable walls: the plant extracellular matrix and signaling. Plant Physiology. 2010;153:467–478. doi: 10.1104/pp.110.153940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi DQ, Yang WC. Pollen germination and tube growth. In: Pua EC, Davey MR, editors. Plant developmental biology – biotechnological perspectives. Vol. 1. Berlin: Springer-Verlag; 2010. pp. 245–282. [Google Scholar]
- Showalter AM. Arabinogalactan-proteins: structure, expression and function. Cellular and Molecular Life Sciences. 2001;58:1399–1417. doi: 10.1007/PL00000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowders DMP, Dodrill CH, Owen HA, Mararoff CA. DEX1, a novel plant protein, is required for exine pattern formation during pollen development in Arabidopsis. Plant Physiology. 2001;127:1739–1749. [PMC free article] [PubMed] [Google Scholar]
- Stone LM, Seaton KA, Kuo J, McComb JA. Fast pollen tube growth in Conospermum species. Annals of Botany. 2004;93:369–378. doi: 10.1093/aob/mch050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Kieliszewshi MJ, Showalter AM. Overexpression of tomato LeAGP1 arabinogalactan protein promotes lateral branching and hampers reproductive development. The Plant Journal. 2004;40:870–881. doi: 10.1111/j.1365-313X.2004.02274.x. [DOI] [PubMed] [Google Scholar]
- Takano E, Maki M, Mori H, et al. Pig heart calpastatin: identification of repetitive domain structures and anomalous behavior in polyacrylamide gel electrophoresis. Biochemistry. 1988;27:1964–1972. doi: 10.1021/bi00406a024. [DOI] [PubMed] [Google Scholar]
- Twell D. Male gametophyte development. In: Pua EC, Davey MR, editors. Plant developmental biology – biotechnological perspectives. Vol. 1. Berlin: Springer-Verlag; 2010. pp. 225–244. [Google Scholar]
- Wang Y, Zhang WZ, Song LF, Zou JJ, Su Z, Wu WH. Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in Arabidopsis. Plant Physiology. 2008;148:1201–1211. doi: 10.1104/pp.108.126375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HM, Wong E, Ogdahl J, Cheung AY. A pollen tube growth-promoting arabinogalactan proteins from Nicotiana alata is similar to the tobacco TTS protein. The Plant Journal. 2000;22:165–176. doi: 10.1046/j.1365-313x.2000.00731.x. [DOI] [PubMed] [Google Scholar]
- Yu X, Cao J, Ye W, Wang Y. Construction of an antisense CYP86MF gene plasmid vector and production of a male-sterile Chinese cabbage transformant by the pollen-tube method. Journal of Horticultural Science and Biotechnology. 2004;79:833–839. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.