Abstract
Background and Aims
Although urban gardens provide opportunities for pollinators in an otherwise inhospitable environment, most garden plants are not native to the recipient biogeographical region and their value to local pollinators is disputed. This study tested the hypothesis that bumblebees foraging in English urban gardens preferentially visited sympatric Palaearctic-range plants over species originating outside their native range.
Methods
Twenty-seven surveys of flower availability and bumblebee visitation (Bombus spp.) were conducted over a 3-month summer period. Plants were categorized according to whether they were native British, Palaearctic or non-Palaearctic in origin. A phylogeny of the 119 plant species recorded was constructed and the relationship between floral abundance and the frequency of pollinator visits investigated by means of phylogenetically independent contrasts. Differentiation in utilization of plant species by the five bumblebee species encountered was investigated using niche overlap analyses.
Key Results
There was conflicting evidence for preferential use of native-range Palaearctic plant species by bumblebees depending on which plants were included in the analysis. Evidence was also found for niche partitioning between species based on respective preferences for native and non-native biogeographical range plants. Two bumblebees (Bombus terrestris and B. pratorum) concentrated their foraging activity on non-Palaearctic plants, while two others (B. hortorum and B. pascourum) preferred Palaearctic species.
Conclusions
The long-running debate about the value of native and non-native garden plants to pollinators probably stems from a failure to properly consider biogeographical overlap between plant and pollinator ranges. Gardeners can encourage pollinators without consideration of plant origin or bias towards ‘local’ biogeographical species. However, dietary specialist bumblebees seem to prefer plants sympatric with their own biogeographical range and, in addition to the cultivation of these species in gardens, provision of native non-horticultural (‘weed’) species may also be important for pollinator conservation.
Keywords: Biogeographical range, bumblebee, Bombus, exotic plants, foraging, horticulture, niche differentiation, pollinators, urban conservation, wildlife gardening
INTRODUCTION
Gardens constitute around one-quarter by area of the UK's urban landscape (Loram et al., 2008) and their value for maintaining and enhancing biodiversity has long been recognized (Gaston et al., 2005; Goddard et al., 2009). In particular, the presence of large densities and varieties of flowering plants supports a number of pollinating insects whose range and abundance has declined as a consequence of agricultural intensification and habitat loss (Corbet et al., 2001; Matteson and Langellotto, 2010). Bumblebees (Bombus spp.) are one of the pollinator groups to have suffered the most significant declines in recent decades (Goulson et al., 2005, 2006), yet some species remain relatively abundant in many urban environments (Osborne et al., 2008; Goulson et al., 2010). By virtue of their presumed role as one of the most important pollinator guilds in both semi-natural ecosystems and agro-ecosystems, a great deal of interest has recently focused on ways of halting further bumblebee decline. In arable landscapes the use of wildflower strips and cultivation of so-called mass-flowering crops (Carvell et al., 2007; Hanley et al., 2011) is widely held to benefit bumblebees and other pollinators, but increasing interest is now focused on the role of urban gardens in supporting threatened pollinators such as bumblebees (Osborne et al., 2008; Goulson et al., 2010).
One of the reasons why bumblebees and other pollinators continue to survive in urban landscapes is the provision of floral resources in parks, allotments and gardens. Indeed, the cultivation of exotic flowers by gardeners generally results in increased plant species richness within urban green spaces (Frankie et al., 2009; Goddard et al., 2009). Loram et al. (2008), for example, showed that over 70 % of the 1056 plant species recorded in urban gardens in five major UK cities were exotic in origin. Nonetheless, urbanization also tends to reduce the extent of native semi-natural habitat, leading to a decline in the diversity and richness of native flora (Goddard et al., 2009). One major consequence of this shift in plant species diversity may be that native floral specialist foragers no longer have access to the relatively limited range of native plants upon which they depend for pollen and nectar, and a number of studies have reported that floral specialists are relatively scarce in urban habitats (Cane, 2005; McFrederick and LeBuhn, 2006; Fetridge et al., 2008; Frankie et al., 2009).
Consequently, understanding the relative importance of exotic and native plant species for urban pollinators is pivotal to their conservation, yet there remains debate about the value of non-native plants to pollinators. On the one hand, Goddard et al. (2009) suggest that native plant abundance is rarely related to invertebrate species richness and that ‘wildlife-friendly’ gardens need not be dominated by native flora. Indeed, Shapiro (2002) notes how the loss of exotic plant species from urban California would greatly reduce food plant choice for over 40 % of the butterfly fauna, while more recently Hinners and Hjelmroos-Koski (2009) reported that 45 % of pollen loads collected from bees in suburban sites in Denver, Colorado, came from non-native plant species. Other authors, by contrast, have suggested that insect diversity is positively associated with the abundance of native plant species and that exotic species reduce forage opportunities for pollinators (Memmott and Waser, 2002; Burghardt et al., 2009). Corbet et al. (2001) examined the relative attractiveness of different garden plants to pollinators, suggesting that insect visits to ‘exotic’ species were generally much lower than to British native plant species. However, with the exception of Salvia splendens (Brazil), all ‘exotic’ species were in-fact native to mainland Europe.
It is common practice for gardeners everywhere to grow non-native plants, but by virtue of a benign climate British gardeners are able to cultivate plants from across the globe. As a result, the potential range of food plants for garden pollinators extends well beyond those considered by Corbet et al. (2001). However, the ability of different pollinator species to utilize resources from a wide variety of garden plants has seldom extended beyond a simple comparison of visits to ‘native’ versus ‘exotic’ species (Corbet et al., 2001; Shapiro, 2002), where ‘exotic’ loosely refers to any plant not naturally found in that region or country. Most British bumblebees are in fact found throughout the Palaearctic, a region extending from Europe into Asia and from where many now common British garden plant species originate. Consequently, pollinator distributions naturally overlap with the native ranges of many so-called exotic plant species. Moreover, bumblebees are themselves present in all major biogeographical regions except Australasia and sub-Saharan Africa (the Afrotropic region) and while British species may not naturally encounter plants from North and South America, and South-East Asia, functionally similar congeneric bumblebee species can and do forage upon them. Even in regions where bumblebees do not occur naturally, such as New Zealand, examination of their forage use following introduction shows that they will visit native as well as introduced (European, Asian and North American) plant species (Goulson and Hanley, 2004).
The aim of this study was to elucidate the relative attractiveness of garden flowers from different geographical regions to British bumblebees. Specifically, we test the hypothesis that British bumblebees discriminate between native-range Palaearctic and non-Palaearctic plant species and/or whether visits to plants are simply made on the basis of floral resource availability. In recording the forage preferences of different bumblebees across a remarkably wide range of potential food plants, we were also able to compare the dietary preferences of so-called generalist and specialist pollinators. Consequently, we also examine whether dietary specialists tend to confine their feeding to plant species from within their native range while generalists exhibit a more polylectic diet.
MATERIALS AND METHODS
Floral abundance and bumblebee visits
We monitored bumblebee activity along a 1 km × 2 m transect set out along a residential street running north-south in the central part of Plymouth, south-west England (50°23′86′′N, 4°8′16′′W). This survey method is similar to that used in studies of pollinator activity along arable crop margins (Carvell et al., 2007; Hanley et al., 2011). With occasional exceptions, the 116 properties on both sides of the street were terraced houses built around 1910 with small front gardens 2 m long × 6 m wide. The 2-m distance from house to footpath effectively constrained observations to a width that facilitated easy quantification and identification of all plant and bumblebee species. The transect was walked between 1000 and 1600 h each day on 27 separate days (approximately twice weekly) between early May and late July 2010 on days favourable to bumblebee activity (Goulson and Darvill, 2004). The observer walked one side of the road (i.e. half of the 1-km transect length), waiting at the northern end for 30 min before completing the transect along the opposite side of the road, and so limited double counting of individual bees.
On each survey occasion we estimated the number of flowers of each individual plant species encountered (except for wind-pollinated graminoids, we had no a priori expectations of which plants would be visited), including both horticultural plants and those considered to be weeds by most gardeners. We also identified and recorded all bumblebees observed actively foraging on plants (i.e. collecting pollen or nectar from an inflorescence), noting the identity of the plants upon which they foraged. Due to the difficulty of separating workers of the subgenus Bombus s. str. (i.e. Bombus terrestris, B. lucorum, B. magnus and B. cryptarum in the field (Williams et al., 2012)), we made no attempt to distinguish between these species and throughout refer to this group collectively as B. terrestris. Of the five bumblebee species encountered in this study, B. pratorum and B. terrestris are considered to be generalist foragers while B. hortorum is regarded as a specialist (Goulson and Darvill, 2004; Goulson et al., 2005, 2008; Hanley et al., 2008). In semi-natural grasslands B. lapidarius and B. pascuorum are pollen specialists, with a preference for Fabaceae species (Goulson et al., 2005; Hanley et al., 2008).
Data analysis
Following identification, all plant species were assigned to one of four geographical categories: 1, British native; 2, Palaearctic native; 3, non-Palaearctic sympatric (not Palaearctic but from regions where bumblebees occur naturally); 4, non-Palaearctic allopatric (regions where no bumblebees occur naturally). Plants assigned to category 3 included species from the Eastern and Western Nearctic, East, South and Western Neotropical and Oriental biogeographical regions; category 4 comprised garden plants from the Afrotropic and Australasian regions.
In order to take into account the degree of relatedness of plant species in the quantification of their use by the different bumblebee species, a phylogeny of the 119 plant species was constructed. A first approximation was obtained employing PHYLOMATIC (Webb and Donoghue, 2005; Phylomatic v3 is now at http://phylodiversity.net/phylomatic/). The tree thus obtained was resolved manually, employing specific phylogenetic studies of the different clades (full details are given in Supplementary Data Table S1). For the full tree, the genera Dianthus and Geranium could not be resolved and remained as polytomies, but this did not pose a problem when analysing the data for subsets of the tree (see below). Branch lengths were interpolated employing the routine bladj of PHYLOCOM (Webb et al., 2008), using Wikström et al.'s (2001) age estimates provided with this software.
The relationship between the estimated number of flowers per hectare and the extrapolated number of visits as observed from transects was investigated by means of phylogenetically independent contrasts (PICs). Both variables were log-transformed prior to analysis; in the case of visits, and in order to include non-visited plants, they were assigned a value of 1 before transformation. Due to the relatively low number of British native species we were unable to compare visits to these plants with those from the wider Palaearctic. However, we were able to test whether the relationship between floral abundance and bumblebee visits differed between Palaearctic and non-Palaearctic plants. We did this for all bumblebee species combined, including all 119 plant species, the 36 visited species or the subset excluding putative weeds. For the first two sets, we were also able to conduct the analyses for individual bumblebee species. When analysing subsets, this amounted to ‘pruning’ the phylogenetic tree to include only those plant species whose factor levels were under investigation. Phylogenetically independent contrasts were investigated with the PDAP routine (Midford et al., 2011) of MESQUITE (Maddison and Maddison, 2011). To test for differences in the slopes of the relationships thus obtained, forced through the origin, general linear models of the contrasts for different comparisons were fitted in SPSS 19.
Differentiation in the utilization of plant species by the five bumblebee species encountered was investigated by niche overlap analyses conducted in EcoSim (Gotelli and Entsminger, 2001). The method consists in the calculation of niche overlap (similarity of resource use) between every pair of bumblebee species employing Pianka's and Czechanowski's (also known as Bray–Curtis) indices. The value of these two indices varies between 0 (when there is no overlap in plant species visited by a pair of bumblebee species) and 1 (when resource use is identical in both identity and degree of use/visits). Departure of the observed values of niche overlap from null model expectations (see below) was tested by randomizing the observed, untransformed, visitation values to the 36 plant species (or subsets thereof) employing all four different algorithms provided in EcoSim. These algorithms consist of the four combinations of the assumptions relating to ‘niche breadth’ (identity of plants visited) and ‘zero states’ (unvisited plant species). For niche breadth, visit values by each bumblebee species were either preserved and then shuffled across plant species (preserved niche breadth: bumblebees can only use the number of plant species that received visits, but plant identity is randomly determined in the simulated null communities) or allowed to vary with a probability between 0 and 1 of a uniform distribution across all plant species (relaxed niche breadth: all plant species can be used). For zero states, unvisited plants either remain unvisited by individual bumblebee species (retained zero states) or can be visited in the simulations (reshuffled zero states). These assumptions thus either limit or relax the observed resource use (i.e. the presumed niche of the bumblebees) and address the question of whether the observed value of niche overlap between each pair of bumblebee species differs from each of the four combinations of the assumptions (randomization algorithms RA1 to RA4). These are: RA1, niche breadth relaxed/zero states relaxed; RA2, niche breadth relaxed/zero states fixed; RA3, niche breadth fixed/zero states relaxed; RA4, niche breadth fixed/zero states fixed. The number of simulations was in each case 10 000, and ‘resource states’, the availabilities of each plant species, were set equal to their floral abundance expressed on a per hectare basis. The two indices produced similar values, but we only present Pianka's because it spanned a slightly wider range, separating species pairs more clearly.
RESULTS
Floral resource use by all bumblebees
We recorded a total of 119 flowering plant species, of which 36 were visited by foraging bumblebees. Although the proportion of the available British native plant species visited was relatively high (45·5 % for all bumblebee species combined; Table 1), it did not differ from visits to non-British Palaearctic, non-Palaearctic sympatric and non-Palaearctic allopatric plants (χ2 = 3·39(3); P = 0·342). Moreover, of the British native plants used by bumblebees, only Digitalis purpurea and Mecanopsis cambrica can be considered cultivated garden plants; the remainder were species such as Rubus fruticosus, Crepis capillaris and Ranunculus repens. A further two of the 49 Palaearctic flowering plant species (Cymbalaria muralis and Geranium pyrenaicum) were also unlikely to be deliberately cultivated by gardeners. When putative weed species were discarded, there was a remarkably similar proportion of garden plant use by bumblebees across all four biogeographic categories (Table 1; χ2 = 1·192(df = 3); P = 0·755).
Table 1.
Relative frequencies of flower visitation by bumblebees to plants in urban gardens in Plymouth, Devon, England
| Plant origin |
||||
|---|---|---|---|---|
| British | Palaearctic | Non-Palaearctic sympatric | Non-Palaearctic allopatric | |
| Total plant species available | 22 (9) | 49 (47) | 36 | 12 |
| Species visited by bees | 10 (3) | 12 (10) | 11 | 3 |
| Proportion of available visited (%) | 45·5 (33·3) | 24·5 (21·3) | 30·5 | 25·0 |
Non-Palaearctic species were divided into plants from regions where bumblebees naturally occur (sympatric) and those from outside the natural bumblebee evolutionary range (allopatric).
Figures in parentheses for British and Palaearctic plants show visitation to horticultural species only.
Of the six most commonly visited plants (together accounting for 71 % of all observed visits), only one (D. purpurea; 6 % of all bee visits) was a British native. Moreover, only half were of Palaearctic origin, including the most frequently visited species Campanula poscharskyana (20·6 % of visits); the remainder were from the Nearctic (Ceanothus spp., 11 % of visits) and Oriental (Deutzia spp.; 7 % of visits) regions, while the second most visited plant, Hebe × franciscana (18 % of visits) is a hybrid variety with parents from New Zealand (H. speciosa) and both Australasia and South America (H. elliptica). Full details of bumblebee visits to flowering garden plants are given in Supplementary Data Table S2.
When all 428 bumblebee visits were considered together for all 119 plant species, PIC analysis contrasts revealed that visits were positively related to the number of flowers present and that the slope of the relationship was similar for plants of Palaearctic (categories 1 and 2) and non-Palaearctic (categories 3 and 4) distribution (Fig. 1A). When only the 36 plant species that received bumblebee visits were taken into account, there was no correlation between total visits and floral abundance for Palaearctic plants, but the relationship was significant for non-Palaearctic species (Fig. 1B). However, when putative weeds were excluded from the analysis, the relationship for Palaearctic plants was re-established and region had no effect on the slope of the relationship (Fig. 1C).
Fig. 1.
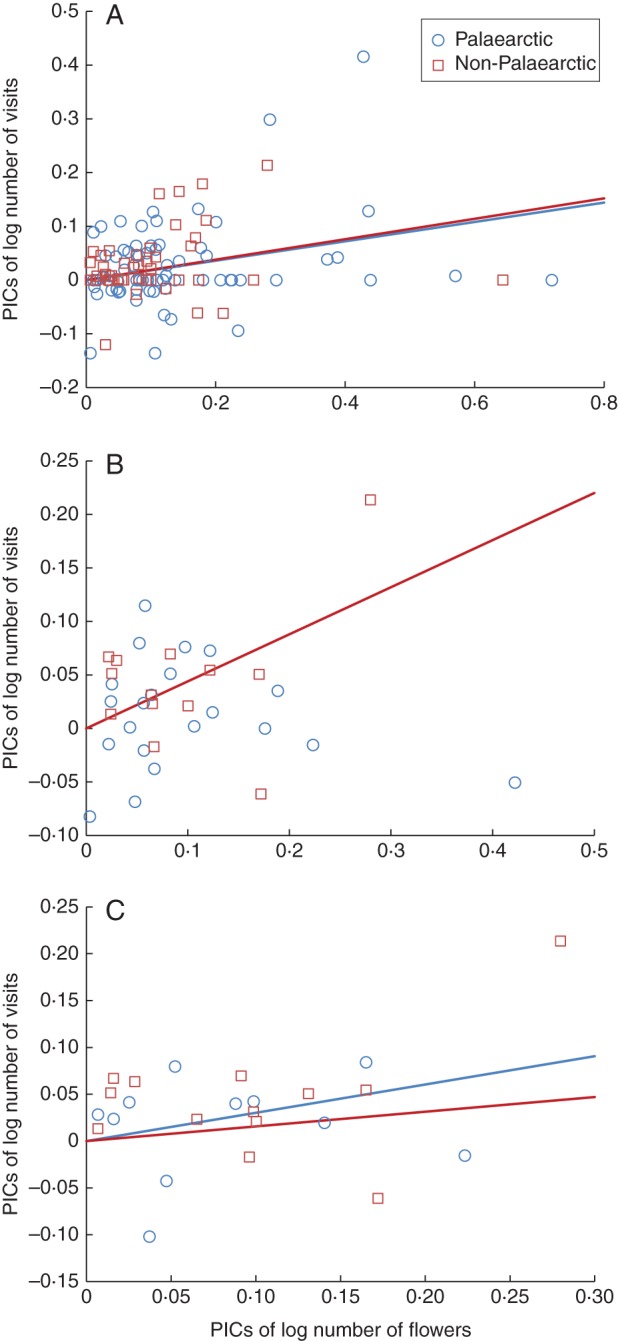
Relationship between the number of bumblebee visits (all five species combined) and the number of flowers available, expressed as phylogenetically independent contrasts (PICs) of log-transformed data, when (A) all 119 plant species with bee-visitation syndromes (B) 36 plant species with actual visits, and (C) only 26 horticultural (i.e., non-weed) plant species were included. Blue points and lines: PICs for plants of Palaearctic distribution and their least squares slope drawn through the origin, respectively; Red points and lines: PICs for plants from outside the Palaearctic Region and their least squares slope, respectively. Except for the relationship for Palaearctic plant species in (B), which was not significant (P > 0·05), and is thus omitted, all slopes were significantly different from zero (P > 0·01). The interaction term PICs of flower number × Region (Palearctic vs non-Palearctic), which tests slope differences between the two regions, had the following significance levels: (A) F1,114 = 0·071, P = 0·79; (B) F1,32 = 6·79, P = 0·014; (C) F1,21 = 0·60, P = 0·56). The proportion of variance accounted for by the covariate (PICs of flower number through the origin), plus separate slopes (Region × PICs of flower number) model was, respectively: (A) R2adj = 0·15; (B) R2adj = 0·23; (C) R2adj = 0·28. All three models were significant at P≤0·01.
Floral resource use by different bumblebee species
Bombus lapidarius was the most commonly encountered bumblebee (Table 2) and was the only species whose visits to Palaearctic (55·9 % of the species’ visits) and non-Palaearctic plants (44·1 %) did not contribute significantly to an overall difference in visitation to these two plant groups across all five bumblebee species [overall, χ2 = 60·12(4), P < 0·001; B. lapidarius, χ2 = 0·3(1) and 0·3(1) for Palaearctic and non-Palaearctic plants, respectively, P < 0·001]. When weeds were excluded, use by B. lapidarius of British native plants was confined to a single visit to M. cambrica.
Table 2.
Total number and relative frequencies of flower visits by five British bumblebee (Bombus) species recorded in urban gardens in Plymouth, Devon, England
| Bombus species | Palaearctic |
Non-Palaearctic |
Flower visits |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Native British plants |
Not British native (n = 12) |
Sympatric (n = 9) |
Allopatric (n = 5) |
|||||||||
| All plants (n = 10) |
Cultivated only (n = 2) |
|||||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| hortorum | 34 | 68·0 | 25 | 50·0 | 7 | 14·0 | 4 | 8·0 | 5 | 10·0 | 50 | 11·7 |
| lapidarius | 21 | 10·8 | 1 | 0·5 | 88 | 45·1 | 37 | 19·0 | 49 | 25·1 | 195 | 45·6 |
| pascuorum | 11 | 36·7 | 6 | 20·0 | 16 | 53·3 | 1 | 3·3 | 2 | 6·7 | 30 | 7·0 |
| pratorum | 0 | 0·0 | 0 | 0·0 | 12 | 27·3 | 28 | 63·6 | 4 | 9·1 | 44 | 10·3 |
| terrestris | 18 | 16·5 | 12 | 11·0 | 20 | 18·3 | 34 | 31·2 | 37 | 33·9 | 109 | 25·5 |
| Total visits | 84 | 19·6 | 44 | 10·3 | 143 | 33·4 | 104 | 24·3 | 97 | 22·7 | 428 | |
Non-Palaearctic species were divided into plants from regions where bumblebees naturally occur (sympatric) and those from outside the natural bumblebee evolutionary range (allopatric).
The other four bumblebee species all showed some variation in their preference for Palaearctic and non-Palaearctic plants. Bombus hortorum and B. pascuorum (χ2 > 6·5(1); P < 0·01 in both cases) favoured Palaearctic over non-Palaearctic plants (82 and 90 % respectively). However, the exclusion of weed species reduced the use of British natives to 50 and 20 % for B. hortorum and B. pascuorum respectively; the latter figure was ascribed to the fact that half of the total B. hortorum observations were visits to D. purpurea. The opposite trend emerged for B. terrestris and B. pratorum (χ2 > 5·5(1); P < 0·02 in both cases) as both species favoured non-Palaearctic garden plants (65·1 and 72·7 % of all B. terrestris and B. pratorum visits, respectively). Most foraging visits for B. terrestris were to Hebe × franciscana (28·2 %), while B. pratorum did not visit any native British species.
When visits to garden flowers were separated for individual bumblebee species, PIC analysis revealed a positive relationship (with similar slopes for each species) between floral abundance and use of all 36 visited plants (Fig. 2). Due to the smaller subset of species utilized by each bumblebee species, it was not possible to use this method to test for differences in visitation to plants from different origins.
Fig. 2.
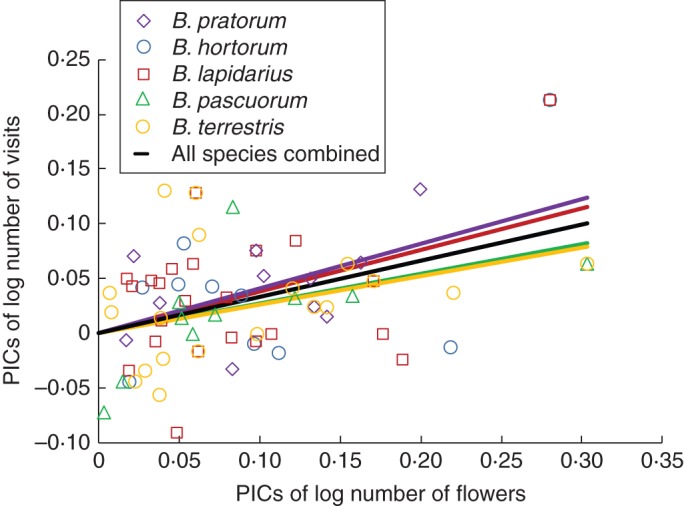
Relationship between the number of bumblebee visits and the number of flowers available, expressed as phylogenetically independent contrasts of log-transformed data, for each of five bumblebee species and for all five species combined. Species are as indicated in the key: note that the line for Bombus hortorum (blue) is hidden behind that of B. pratorum (purple). Whole-model R2adj = 0·34, F6,67 = 7·21, P < 0·001. The interaction PICs of flower number × bumblebee species, which tests for slope differences between bumblebee species, was not significant (F5,67 = 0·22, P = 0·95).
Niche overlap
When considering all 36 visited garden plants together, bumblebee niche overlap was generally small, but five species pairs (two involving B. pratorum) exhibited niche overlaps in excess of 25 % (this figure divides the lowest, tightly clustered 80 % niche overlap values from the remaining, scattered and trailing highest 20 %) (Table 3). The results from null models RA1 and RA4 suggested niche differentiation and niche overlap, respectively, one being too lax in its assumptions of likely resource use (anything can be used by anyone), and the other being too restrictive (high degree of specialization assumed) and thus prone to type II error (Winemiller and Pianka, 1990). These differences persisted between the more conservative models RA2 and RA3 (observed mean niche overlap = 17 %; RA2 simulated mean niche overlap = 34 %, Pobs<exp < 0·0001; RA3 simulated mean niche overlap = 8·4 %, Pobs<exp = 0·96; Table 4).
Table 3.
Observed niche overlap (Pianka's index expressed as a percentage) between pairs of bumblebee (Bombus) species in urban gardens in Plymouth, Devon, England
|
Bombus species |
|||||
|---|---|---|---|---|---|
| Bombus species | lapidarius | pascuorum | pratorum | terrestris | |
| 36 visited plant species | hortorum | 4·22 | 34·87 | 0·01 | 0·10 |
| lapidarius | 2·30 | 43·78 | 4·84 | ||
| pascuorum | 7·25 | 9·43 | |||
| pratorum | 52·86 | ||||
| 22 Palaearctic plant species | hortorum | 8·84 | 19·47 | 6·83 | 7·12 |
| lapidarius | 10·49 | 14·72 | 8·76 | ||
| pascuorum | 27·30 | 14·49 | |||
| pratorum | 25·35 | ||||
| 14 non-Palaearctic plant species | hortorum | 10·71 | 0·02 | 2·39 | 14·73 |
| lapidarius | 4·39 | 52·07 | 30·43 | ||
| pascuorum | 0·11 | 2·97 | |||
| pratorum | 31·98 | ||||
| 26 visited garden plant species | hortorum | 0·004 | 19·86 | 0·006 | 4·41 |
| lapidarius | 0·001 | 44·66 | 4·20 | ||
| pascuorum | 0·001 | 0·88 | |||
| pratorum | 52·22 | ||||
| 12 Palaearctic garden plant species | hortorum | 0·000 | 19·97 | 0·000 | 4·41 |
| lapidarius | 0·000 | 0·16 | 66·86 | ||
| pascuorum | 0·000 | 0·88 | |||
| pratorum | 58·39 | ||||
Values in bold represent the 20 % largest niche overlaps (those that were >25 %).
Table 4.
Comparison of mean observed niche overlap and expected niche overlap employing Pianka's index, expressed as a percentage, for four null models of niche (RA1–4) breadth and resource suitability (‘zero states’) implemented in Ecosim v7 (Gotelli and Entsminger, 2001)
| Mean niche overlap and variance |
||||||
|---|---|---|---|---|---|---|
| Observed | RA1 | RA2 | RA3 | RA4 | ||
| 36 visited plant species | Mean | 16·96 | 75·24 (<0·0001) | 33·80 (<0·0001) | 7·62 (0·96) | 8·38 (0·93) |
| Variance | 3·71 | 0·25 (>0·9999) | 1·19 (0·9996) | 2·42 (0·7405) | 2·55 (0·7223) | |
| 22 Palaearctic plant species | Mean | 14·34 | 75·36 (<0·0001) | 32·24 (<0·0001) | 28·49 (<0·0001) | 30·24 (<0·0001) |
| Variance | 0·56 | 0·40 (0·8052) | 1·83 (0·0203) | 2·79 (0·0025) | 1·95 (0·0194) | |
| 14 non-Palaearctic plant species | Mean | 14·98 | 75·63 (<0·0001) | 36·07 (<0·0001) | 29·92 (<0·0001) | 31·90 (<0·0001) |
| Variance | 3·09 | 0·63 (>0·9999) | 3·70 (0·367) | 4·98 (0·1073) | 5·39 (0·0746) | |
| 26 visited garden plant species | Mean | 12·63 | 75·30 (<0·0001) | 34·82 (<0·0001) | 6·87 (0·8732) | 7·34 (0·8461) |
| Variance | 3·96 | 0·34 (>0·9999) | 2·27 (0·9588) | 3·41 (0·6489) | 3·52 (0·6410) | |
| 12 Palaearctic garden plant species | Mean | 15·07 | 75·74 (<0·0001) | 34·13 (0·0005) | 13·78 (0·6455) | 14·65 (0·6143) |
| Variance | 6·70 | 0·73 (>0·9999) | 3·74 (0·9658) | 6·72 (0·5215) | 6·84 (0·5303) | |
Model RA1 relaxed niche breadth/relaxed zero states; RA2 relaxed niche breadth/fixed zero states; RA3 retained niche breadth/relaxed zero states; RA4 retained niche breadth/fixed zero states.
Values in parentheses represent the randomization test probability that observed value ≤ expected value.
Values in bold indicate that observed mean or variance in niche overlap is significantly lower than model expectation at the P value shown.
Conducting the analyses separately for Palaearctic and non-Palaearctic plants revealed that two of two (Palaearctic) and two of three (non-Palaearctic) cases of niche overlap involved B. pratorum (Table 3). All four null models indicated strong niche differentiation in use of both Palaearctic and non-Palaearctic plants, and the variability of observed niche overlap was smaller than that expected in three out of four models (RA2–RA4; Table 4). However, the suggested niche differentiation in visits to non-Palaearctic plants was accompanied by non-significantly smaller variation of observed versus simulated niche overlap in all four models, which would be expected if niche differentiation occurred (Table 4). The results from the garden plants subsets were similar to those for all 36 plant species: niche differentiation was detected by models RA1 and RA2, but not by models RA3 and RA4 (Table 4).
DISCUSSION
Our analyses of garden plant use by bumblebees produced conflicting results depending on which plant species were included. Using all 119 available plant species, results suggested that, rather than visit species on the basis of provenance, bumblebees selected from the pool of available plants according to floral abundance. When we considered only those 36 plant species actually used by bumblebees, floral abundance alone ceased to influence visits to Palaearctic plants and there appeared to be discrimination between biogeographic regions. This relationship disappeared, however, when we excluded putative weed species from the analysis. Consequently, our first major conclusion is that the relative values of native and non-native garden plants vary according to how one defines the term ‘garden plant’.
Nonetheless, three bumblebee species (B. lapidarius, B. pratorum and B. terrestris) consistently exhibited little preference for British or even Palaearctic native plants. Indeed, in the cases of B. pratorum and B. terrestris, species considered to have relatively polylectic diets (Goulson and Darvill, 2004; Goulson et al., 2008), individual bees favoured non-Palaearctic plant species, with the former species never observed foraging on British natives. Bombus pratorum also showed the most niche overlap with other species, although given that this is an early-season species and most observations were made before early June, temporal partitioning of resource use is likely. Only B. hortorum and B. pascuorum showed any evidence of preference for native biogeographic range plants, the latter devoting over 70 % of its visits to Palaearctic species even when weeds were excluded. Bombus hortorum is a long-tongued species generally considered to have the most restricted dietary range of the five species encountered (Goulson et al., 2008; Hanley et al., 2008). In addition to a strong preference for Palaearctic plants, B. hortorum showed remarkably little niche overlap (whatever the model) with any species other than the medium-/long-tongued B. pascuorum. Our second conclusion is therefore that pollinator use of native and non-native range garden plants varies between specialist and generalist species. Interestingly, however, while over half of all recorded flower visits for B. hortorum were to the British native D. purpurea, nearly one-fifth were to non-Palaearctic plants, including the New Zealand native Cordyline australis. So even this supposedly specialist forager was still actively visiting garden plants from outside its native biogeographic range.
Our final conclusion is that traditional garden plants alone may not fulfil all the dietary requirements of the urban pollinator community. British weed species like R. fruticosus, C. capillaris and R. repens attracted visits from all five bumblebee species. Perhaps the biggest potential impact that urbanization has had on pollinators is the loss of important groups of native food plants not normally cultivated in gardens (Goddard et al., 2009). The absence of high densities of important bumblebee food plants, particularly members of the Fabaceae and Lamiaceae, may influence pollinator abundance and feeding behaviour, particularly for specialist species like B. hortorum and B. pascuorum that have a close association with these plant families (Goulson et al., 2005). However, it is interesting to note that a comparison of bumblebee abundance from repeat surveys made along a 1-km-long grassland transect in the floristically diverse Salisbury Plain region of Wiltshire, England, in June 2004 and June 2005 (average of 20·8 bumblebees per survey; data from Hanley et al., 2008) is remarkably similar to the average June abundance of bumblebees along our urban transect (23·1 bees per survey). The only substantive difference in bumblebee species composition was the occurrence of B. humilis at Salisbury Plain. Even allowing for variation in bumblebee numbers between years, this comparison suggests that urban gardens can support an abundant bumblebee community.
A second interesting parallel with semi-natural grasslands is that (when weeds are considered) bumblebee use of native range plants is discriminating rather than proportional to availability, perhaps because floral resource use in the urban environment is also linked to specific dietary requirements for pollen and/or nectar, as it is in grasslands (Goulson et al., 2005; Hanley et al., 2008). The fact that visits to non-Palaearctic plants were consistently correlated with relative floral abundance may suggest that bees are simply exploring these relatively unfamiliar (in evolutionary terms) resources in proportion to the likelihood of encounter. That we found evidence of niche partitioning between bumblebees foraging on either Palaearctic or non-Palaearctic species accompanied with variance reduction in the former but not in the latter underscores these suggestions; long-term familiarity with native-range Palaearctic species would be more likely to facilitate specialist foraging behaviour and differentiation. This is reinforced by the analyses of niche differentiation when visiting garden plants: although two of the models in each of the two garden plant subsets (RA1 and RA2) suggested niche differentiation, the variance of observed niche overlap was higher than expected by all eight simulations (RA1–RA4 for both garden plant subsets) (Table 4). Together with the relaxed niche breadth assumed by these two models, this higher variance of observed niche overlap implies a wide realized niche, and indicates that active avoidance among the different species, rather than strict, ‘hard-wired’ fundamental-niche differentiation drives the specialization observed.
The key issue is perhaps whether declining pollinator species can be better sustained by the wide biogeographic range of plants grown in urban gardens. Our results suggest that it is not simply a question of growing species native to the particular biogeographic range in question, even if this is desirable for other reasons, but that any showily flowered plant is likely to offer some forage reward. There are caveats, however. While Bergerot et al. (2010) found no evidence that dietary preference played a role in determining butterfly distribution along urban gradients, they did note that dietary specialists were less abundant in urban than rural areas. While other aspects of habitat provision may be important to bumblebee success in the urban landscape (e.g. nesting and hibernation sites; Lye et al., 2012), for pollinators with specific dietary needs (e.g. the high-protein pollen offered by Fabaceae species; Hanley et al., 2008), a lack of certain food plants may be crucial in dictating their persistence in urban gardens. Without a major shift in attitude towards the cultivation of native grassland plant species, gardeners alone are unlikely to be able to offer the range of food plants required by specialist pollinators (Hinners et al., 2012). However, a combination of more effective use of urban green spaces, parks and derelict land to provide patches of flower-rich grassland (McFrederick and LeBuhn, 2006; Goddard et al., 2009; Fischer et al., 2013) with the wide biogeographic range of food plants offered by small gardens may offer an important conservation opportunity for urban pollinators.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank the householders of Trelawney Road, Peverell, Plymouth, for their patience and cooperation during our bumblebee observations.
LITERATURE CITED
- Bergerot B, Fontain B, Renard M, Cadi A, Julliard R. Preferences for exotic flowers do not promote urban life in butterflies. Landscape and Urban Planning. 2010;96:98–107. [Google Scholar]
- Burghardt KT, Tallamy DW, Shriver D. Impact of native plants on bird and butterfly biodiversity in urban landscapes. Conservation Biology. 2009;23:219–224. doi: 10.1111/j.1523-1739.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- Cane JH. Bees, pollination, and the challenges of sprawl. In: Johnson EA, Klemens MW, editors. Nature in fragments: the legacy of sprawl. New York: Columbia University Press; 2005. pp. 109–124. [Google Scholar]
- Carvell C, Meek WR, Pywell RF, Goulson D, Nowakowski M. Comparing the efficacy of agri-environment schemes to enhance bumble bee abundance and diversity on arable field margins. Journal of Applied Ecology. 2007;44:29–40. [Google Scholar]
- Corbet SA, Bee J, Dasmahapatra K, et al. Native or exotic? Double or single? Evaluating plants for pollinator-friendly gardens. Annals of Botany. 2001;87:219–232. doi: 10.1006/anbo.2000.1322. [DOI] [PubMed] [Google Scholar]
- Fetridge ED, Ascher JS, Langellotto GA. The bee fauna of residential gardens in a suburb of New York City (Hymenoptera: Apoidea) Annals of the Entomological Society of America. 2008;101:1067–1077. [Google Scholar]
- Fischer LK, von der Lippe M, Kowarik I. Urban grassland restoration: which plant traits make desired species successful colonizers? Applied Vegetation Science. 2013;16:272–285. [Google Scholar]
- Frankie GW, Thorp RW, Hernandez J, et al. Native bees are a rich natural resource in urban California gardens. California Agriculture. 2009;63:113–120. [Google Scholar]
- Gaston KJ, Smith RM, Thompson K, Warren PH. Urban domestic gardens (II): experimental tests of methods for increasing biodiversity. Biodiversity and Conservation. 2005;14:395–413. [Google Scholar]
- Goddard MA, Dougill AJ, Benton TG. Scaling up from gardens: biodiversity conservation in urban environments. Trends in Ecology and Evolution. 2009;25:90–98. doi: 10.1016/j.tree.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Gotelli NJ, Entsminger GL. EcoSim: null models software for ecology. Version 7·0. 2001 Acquired Intelligence Inc. and Kesey-Bear. Discontinued but available as EcoSim 2004 at http://www.garyentsminger.com/ecosim/ (last accessed February 8, 2014) [Google Scholar]
- Goulson D, Darvill B. Niche overlap and diet breadth in bumblebees; are rare species more specialized in their choice of flowers? Apidologie. 2004;35:55–64. [Google Scholar]
- Goulson D, Hanley ME. Distribution and forage use of exotic bumblebees in South Island, New Zealand. New Zealand Journal of Ecology. 2004;28:225–232. [Google Scholar]
- Goulson D, Hanley ME, Darvill B, Ellis JS, Knight M. Causes of rarity in bumblebees. Biological Conservation. 2005;122:1–8. [Google Scholar]
- Goulson D, Hanley ME, Darvill B, Ellis J. Biotope associations and the decline of bumblebees (Bombus spp.) Journal of Insect Conservation. 2006;10:95–103. [Google Scholar]
- Goulson D, Lye GC, Darvill B. Diet breadth, coexistence and rarity in bumblebees. Biodiversity and Conservation. 2008;17:3269–3288. [Google Scholar]
- Goulson D, Lepais O, O'Connor S, et al. Effects of land use at a landscape scale on bumblebee nest density and survival. Journal of Applied Ecology. 2010;46:1207–1215. [Google Scholar]
- Hanley ME, Franco M, Pichon S, Darvill B, Goulson D. Breeding system, pollinator choice, and variation in pollen quality in British herbaceous plants. Functional Ecology. 2008;22:592–598. [Google Scholar]
- Hanley ME, Franco M, Dean CE, et al. Increased bumblebee abundance along the margins of a mass flowering crop: evidence for pollinator spill-over. Oikos. 2011;120:1618–1624. [Google Scholar]
- Hinners SJ, Hjelmroos-Koski MK. Receptiveness of foraging wild bees to exotic landscape elements. American Midland Naturalist. 2009;162:253–265. [Google Scholar]
- Hinners SJ, Kearns CA, Wessman CA. Roles of scale, matrix, and native habitat in supporting a diverse suburban pollinator assemblage. Ecological Applications. 2012;22:1923–1935. doi: 10.1890/11-1590.1. [DOI] [PubMed] [Google Scholar]
- Loram A, Thompson K, Warren PH, Gaston KJ. Urban domestic gardens (XII): the richness and composition of the flora in five UK cities. Journal of Vegetation Science. 2008;19:321–330. [Google Scholar]
- Lye GC, Osborne JL, Park KJ, Goulson D. Using citizen science to monitor Bombus populations in the UK: nesting ecology and relative abundance in the urban environment. Journal of Insect Conservation. 2012;16:697–707. [Google Scholar]
- Maddison WP, Maddison DR. Mesquite: a modular system for evolutionary analysis. Version 2·75. 2011 http://mesquiteproject.org. (20 January 2014) [Google Scholar]
- Matteson K, Langellotto GA. Determinates of inner city butterfly and bee species richness. Urban Ecosystems. 2010;13:333–347. [Google Scholar]
- McFrederick QS, LeBuhn G. Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biological Conservation. 2006;129:372–382. [Google Scholar]
- Memmott J, Waser NM. Integration of alien plants into a native flower-pollinator visitation web. Proceedings of the Royal Society of London, B. 2002;269:2395–2399. doi: 10.1098/rspb.2002.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midford PE, Garland T, Maddison WP. PDAP package of Mesquite. Version 1·16. 2011 http://mesquiteproject.org/pdap_mesquite/ (20 January 2014) [Google Scholar]
- Osborne JL, Martin AP, Shortall CR, et al. Quantifying and comparing bumblebee nest densities in gardens and countryside habitats. Journal of Applied Ecology. 2008;45:784–792. [Google Scholar]
- Shapiro AM. The Californian urban butterfly fauna is dependent on alien plants. Diversity and Distributions. 2002;8:31–40. [Google Scholar]
- Webb CO, Donoghue MJ. Phylomatic: tree assembly for applied phylogenetics. Molecular Ecology Notes. 2005;5:181–183. [Google Scholar]
- Webb CO, Ackerly DD, Kembel SW. Phylocom: software for the analysis of phylogenetic community structure and trait evolution. Bioinformatics. 2008;24:2098–2100. doi: 10.1093/bioinformatics/btn358. [DOI] [PubMed] [Google Scholar]
- Wikström N, Savolainen V, Chase MW. Evolution of the angiosperms: calibrating the family tree. Proceedings of the Royal Society of London, B. 2001;268:221–2220. doi: 10.1098/rspb.2001.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PH, Brown MJF, Carolan JC, et al. Unveiling cryptic species of the bumblebee subgenus Bombus s. str. world-wide with COI barcodes (Hymenoptera: Apidae) Systematics and Biodiversity. 2012;10:21–56. [Google Scholar]
- Winemiller KO, Pianka ER. Organization in natural assemblages of desert lizards and tropical fishes. Ecological Monographs. 1990;60:27–55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


