Abstract
Background
Human rhinoviruses (HRVs) are a common cause of upper respiratory infection (URI) in hematopoietic stem cell transplant (HSCT) recipients; yet, their role in lower respiratory illness is not well understood.
Methods
We performed a retrospective chart review of HSCT recipients with HRV infection from the time molecular detection methods were implemented at our institution in 2008. Factors associated with proven or possible HRV pneumonia at the first HRV detection were evaluated by univariate and multivariate analysis. We then characterized all episodes of proven and possible HRV pneumonia from the initial HRV infection through a 1‐year follow‐up period.
Results
Between 2008 and 2011, 63 HSCT recipients had ≥1 documented HRV infections. At first HRV detection, 36 (57%) patients had HRV URI and 27 (43%) had proven or possible HRV pneumonia; in multivariate analysis, hypoalbuminemia (odds ratio [OR] 9.5, 95% confidence interval [CI] 1.3–71.7; P = 0.03) and isolation of respiratory co‐pathogen(s) (OR 24.2, 95% CI 2.0–288.4; P = 0.01) were independently associated with pneumonia. During the study period, 22 patients had 25 episodes of proven HRV pneumonia. Fever (60%), cough (92%), sputum production (61%), and dyspnea (60%) were common symptoms. Fifteen (60%) episodes demonstrated bacterial (n = 7), fungal (n = 5), or viral (n = 3) co‐infection. Among the remaining 10 (40%) cases of HRV monoinfection, patients’ oxygen saturations ranged from 80% to 97% on ambient air, and computed tomography scans showed peribronchiolar, patchy, ground glass infiltrates.
Conclusions
HRV pneumonia is relatively common after HSCT and frequently accompanied by bacterial co‐infection. As use of molecular assays for respiratory viral diagnosis becomes widespread, HRV will be increasingly recognized as a significant cause of pneumonia in immunocompromised hosts.
Keywords: human rhinovirus, viral pneumonia, hematopoietic stem cell transplantation, HSCT, lower respiratory infections
The human rhinoviruses (HRVs) are most commonly associated with upper respiratory infection (URI); however, HRV also plays an important role in lower respiratory tract infection (LRTI). In vitro studies have established that HRVs are capable of infecting human respiratory epithelial cell lines and inducing pro‐inflammatory cytokine and chemokine release 1. In healthy volunteers inoculated with HRV by intranasal aerosol insufflations, HRV is detected in bronchial biopsy specimens by in situ hybridization 2. HRVs are now recognized to have a major impact on asthma pathogenesis, including asthma development and exacerbations 3, 4, 5. Studies of hospitalized pediatric and adult patients demonstrate that HRV infection is associated with bronchiolitis and pneumonia 6, 7, 8, 9, 10. Furthermore, among elderly nursing home residents, HRVs have been implicated in outbreaks of severe acute respiratory illness leading to hospitalization and even death 11, 12.
Among hematopoietic stem cell transplant (HSCT) recipients, HRVs are the most common cause of URI 13; yet, their role in LRTI is not well understood. A recent prospective study of HRV and coronavirus infections in allogeneic HSCT recipients determined that rates of progression of HRV URI to pneumonia were low; however, 2 patients died of respiratory failure with HRV as the most likely etiologic agent 13, 14. In a retrospective study of HSCT recipients with acute pulmonary infiltrates, HRVs were detected in 8 bronchoalveolar lavage (BAL) fluid specimens from 6 patients, representing 6% of all BALs performed in HSCT recipients during the study period 15. To understand further the role of HRVs in LRTI in HSCT recipients, we conducted a review of all HRV infections in our institution's HSCT population since the implementation of molecular detection methods beginning in 2008.
Methods
Study population and data collection
We identified all adult (age >17 years of age) allogeneic, syngeneic, and autologous HSCT recipients testing positive for HRV at New York–Presbyterian Hospital/Weill Cornell Medical College in New York City from March 6, 2008 to April 20, 2011. Patients who tested positive for HRV during pre‐HSCT conditioning were also included. Clinical, laboratory, and radiographic data were abstracted from the existing electronic medical records. When available, lung tissue specimens from patients who underwent biopsies during episodes of proven HRV pneumonia were reviewed by one of the co‐authors (C.M.). The study was approved by the Institutional Review Board at Weill Cornell Medical College.
Specimen collection and detection of HRV
At our institution, HSCT recipients with URI or LRTI symptoms are routinely screened by culture and/or molecular methods for viral infection via nasopharyngeal (NP) swab or bronchoscopy with BAL. Beginning in March 2008, our laboratory implemented reference molecular testing for respiratory viruses by the xTAG Respiratory Viral Panel (RVP) (Luminex Molecular Diagnostics, Toronto, Canada) conducted by ViraCor Laboratories (Lee's Summit, Missouri, USA). The RVP assay comprises a multiplex real‐time polymerase chain reaction (PCR), followed by a multiplex target‐specific primer extension step 16, 17. The RVP assay detects 10 respiratory viruses (human metapneumovirus, influenza A, influenza B, parainfluenza viruses [PIV] 1–3, respiratory syncytial viruses [RSV] A and B, adenovirus, and HRV) and 2 additional subtypes (influenza A subtypes H1N1 and H3N2). In addition to the RVP assay, all BAL specimens from HSCT recipients with suspected pneumonia are evaluated by the following microbiologic studies: bacterial Gram stain and culture, fungal Calcofluor potassium hydroxide stain and culture, acid‐fast bacillus stain and culture, Legionella culture, Pneumocystis jirovecii direct fluorescent‐antibody testing, and respiratory virus culture including cytomegalovirus, influenza, PIV, RSV, and adenovirus.
Definitions
URI was defined as clinical symptoms including rhinorrhea, nasal congestion, pharyngitis or cough without clinical or radiographic evidence of lower respiratory involvement or hypoxia. Pneumonia was defined as new radiographic pulmonary infiltrates in patients with signs and symptoms of LRTI, including cough, dyspnea, sputum production, and fever. A separate, subsequent pneumonia episode required at least a 2‐week symptom‐free period between episodes. Pneumonia was further classified as (i) Proven HRV pneumonia if HRV was isolated from BAL fluid, (ii) Possible HRV pneumonia if HRV was isolated from an NP swab and no bronchoscopy was performed, and (iii) Non‐HRV pneumonia if HRV was not detected in the NP swab or BAL fluid during the episode of pneumonia. Neutropenia was defined as an absolute neutrophil count ≤500 cells/μL, and lymphopenia was defined as an absolute lymphocyte count ≤200 cells/μL occurring within 1 week before HRV infection. Hypoalbuminemia was defined as serum albumin ≤3.2 mg/dL. Invasive fungal infections were defined according to the European Organization for Research and Treatment of Cancer/Mycoses Study Group definitions 18.
Statistical analysis
Descriptive statistics were expressed as median values, range, and interquartile range (IQR). We evaluated factors associated with proven or possible HRV pneumonia at first detection of HRV post transplant by univariate analysis using chi‐square and Fisher's exact tests as appropriate for categorical variables and Mann–Whitney U‐test for continuous variables; P‐value ≤0.05 was considered significant. Variables with a P‐value ≤0.1 on univariate analysis were subsequently analyzed by multivariable logistic regression. Data were analyzed using STATA 12.1 (College Station, Texas, USA). Patients were followed up for 1 year after the first HRV infection.
Results
Study population
Between March 2008 and April 2011, 444 patients underwent HSCT (239 autologous, 202 allogeneic, and 3 syngeneic). Fifty‐four (12%) patients had 1 or more HRV infections at a median of 73.5 days (IQR 14–258) post transplant. An additional 9 patients transplanted before March 2008 also had 1 or more HRV infections during the study period. Therefore, a total of 63 HSCT recipients were included. Forty‐two (67%) patients received allogeneic HSCT, 20 (32%) patients received autologous HSCT, and 1 (2%) patient received syngeneic HSCT. The conditioning regimen was myeloablative in 42/63 (67%) patients. Additional patient demographics, transplant characteristics, and comorbidities are presented in Table 1. HRV infections, including URIs and proven and possible HRV pneumonias, occurred year‐round with peak infection rates observed in October, March, and April (Fig. 1).
Table 1.
Baseline characteristics of hematopoietic stem cell transplant (HSCT) recipients with ≥1 post‐transplant human rhinovirus (HRV) infections
| Variable | N = 63 (%) patients |
|---|---|
| Female | 25 (40) |
| Median age at HSCT (range) | 55 (21–71) |
| Primary hematologic disorder | |
| Acute leukemia | 26 (41) |
| Lymphoma | 20 (32) |
| Multiple myeloma | 10 (16) |
| Chronic myelogenous leukemia | 3 (5) |
| Other | 4 (6) |
| Transplant type | |
| Allogeneic | 42 (67) |
| Matched‐related donor | 19/42 (45) |
| Matched‐unrelated donor | 14/42 (33) |
| Mismatched‐unrelated donor | 1/42 (2) |
| Cord blood | 8/42 (19) |
| Autologous | 20 (32) |
| Syngeneic | 1 (2) |
| Conditioning regimen | |
| Myeloablative | 42 (67) |
| Reduced‐intensity | 21 (33) |
| Graft‐versus‐host disease (GVHD)a , b | 21/42 (50) |
| Comorbidities | |
| Chronic obstructive pulmonary disease/Asthma | 6 (10) |
| Other post‐transplant lung diseasec | 7 (11) |
| Tobacco use currentd | 8 (13) |
| Tobacco use ever | 27 (43) |
| Diabetes | 10 (16) |
| History of pneumonia within previous 30 days | 0 |
| Visit type at first HRV infection | |
| Inpatient | 36 (57) |
| Admission for HSCT | 17 (27) |
| Admission for acute reason | 19 (30) |
| Outpatient | 27 (43) |
Includes patients with acute GVHD grade 2–4 and/or chronic GVHD.
An additional 10 subjects were diagnosed with GVHD during the 1‐year follow‐up period.
Bronchiolitis obliterans (N = 5), Idiopathic pulmonary fibrosis (N = 1), Interstitial pneumonitis (N = 1).
Defined as tobacco use within the previous 12 months.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 1.
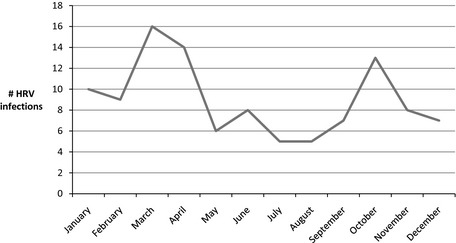
Seasonal epidemiology of human rhinovirus (HRV) infections among hematopoietic stem cell transplant recipients at New York Presbyterian Hospital/Weill Cornell Medical College, 2008–2011.
Clinical features at initial detection of HRV
The median age at initial detection of HRV post HSCT was 55 years (range 22–71). Seventeen (27%) patients were inpatients admitted for HSCT, 19 (30%) were inpatients admitted for an acute reason, and 27 (43%) were outpatients. Thirty‐six (57%) and 27 (43%) patients met the clinical criteria for URI and pneumonia, respectively. None of the patients had a history of pneumonia within 30 days prior to HRV infection. Eleven (17%) patients had concurrent bacteremia.
According to the pre‐specified definitions, the 27 episodes of HRV pneumonia were further sub‐classified as proven HRV pneumonia (n = 14) and possible HRV pneumonia (n = 13). Nine of the 14 patients with proven HRV pneumonia and 4 of the 13 patients with possible HRV pneumonia had respiratory co‐infection with bacterial, fungal, or viral pathogens. In univariate analysis, the following variables were associated with proven or possible HRV pneumonia at the first HRV detection: neutropenia (odds ratio [OR] 4.7, 95% confidence interval [CI] 1.3–17.2; P = 0.02), hypoalbuminemia (OR 12.7, 95% CI 3.7–43.6; P < 0.001), receipt of ≥24 h of antibiotic therapy within the previous 7 days (OR 7.1, 95% CI 2.3–21.8; P = 0.001), and isolation of respiratory co‐pathogen(s) (OR 32.5, 95% CI 3.9–272.5; P = 0.001). In multivariate analyses, hypoalbuminemia (OR 9.5, 95% CI 1.3–71.7; P = 0.03) and isolation of respiratory co‐pathogen(s) (OR 24.2, 95% CI 2.0–288.4; P = 0.01) remained independently associated with proven or possible HRV pneumonia at the first detection of HRV post transplant (Table 2). Transplant type and intensity of conditioning regimen were not associated with proven or possible HRV pneumonia.
Table 2.
Factors associated with proven or possible human rhinovirus (HRV) pneumonia at first post‐hematopoietic stem cell transplant (HSCT) HRV detection
| Variable | HRV URI only, N = 36 (%) | HRV pneumoniaa, N = 27 (%) | Univariate odds ratio (95% CI) | Univariate P‐value | Multivariate odds ratio (95% CI) | Multivariate P‐value |
|---|---|---|---|---|---|---|
| Median age in years at HSCT (range) | 54 (25–70) | 55 (21–65) | – | 0.9 | ||
| Female | 16 (44) | 9 (33) | 0.6 (0.2–1.8) | 0.4 | ||
| Transplant type | ||||||
| Allogeneic | 25 (69) | 17 (63) | 1.0 | – | ||
| Autologous | 11 (31) | 9 (33) | 1.2 (0.4–3.5) | 0.7 | ||
| Syngeneic | 0 | 1 (4) | – | 0.4 | ||
| Donor status | ||||||
| Matched‐related | 14/25 (56) | 6/18 (33) | 1.0 | – | ||
| Matched‐unrelated | 8/25 (32) | 6/18 (33) | 1.8 (0.4–7.3) | 0.5 | ||
| Mismatch‐unrelated | 0 | 1/18 (6) | – | 0.3 | ||
| Cord blood | 3/25 (12) | 5/18 (28) | 3.8 (0.7–21.7) | 0.2 | ||
| Conditioning regimen | ||||||
| Myeloablative | 23 (64) | 19 (70) | 1.0 | – | ||
| Reduced‐intensity | 13 (36) | 8 (30) | 0.8 (0.3–2.2) | 0.8 | ||
| Graft‐versus‐host disease (GVHD)b | 11 (31) | 10 (37) | 1.3 (0.5–3.8) | 0.6 | ||
| Corticosteroid use | 9 (25) | 12 (44) | 2.4 (0.8–7.0) | 0.1 | 4.5 (0.9–24.1) | 0.08 |
| Relapsed disease | 3 (8) | 3 (11) | 1.4 (0.3–7.4) | 0.7 | ||
| Comorbidities | ||||||
| COPD/asthma | 4 (11) | 2 (7) | 0.6 (0.1–3.8) | 0.6 | 0.4 (0.03–5.8) | 0.5 |
| Tobacco use within past 12 months | 7 (19) | 1 (4) | 0.2 (0.02–1.4) | 0.1 | ||
| Tobacco use ever | 15 (42) | 12 (44) | 1.1 (0.4–3.1) | 0.8 | ||
| Diabetes | 6 (17) | 4 (15) | 0.9 (0.2–3.4) | 0.8 | ||
| HRV infection within 100 days of HSCT | 19 (53) | 12 (44) | 0.7 (0.3–1.9) | 0.5 | ||
| Neutropenia (<500 cells/μL) | 4 (11) | 10 (37) | 4.7 (1.3–17.2) | 0.02 | 0.8 (0.1–10.0) | 0.8 |
| Lymphopenia (<200 cells/μL) | 7 (19) | 11 (41) | 2.8 (0.9–8.8) | 0.07 | 1.2 (0.1–12.7) | 0.9 |
| Albumin <3.2 mg/dL | 14 (40) | 22 (82) | 12.7 (3.7–43.6) | <0.001 | 9.5 (1.2–71.7) | 0.03 |
| Concurrent bacteremiac | 5 (14) | 8 (30) | 2.6 (0.7–9.2) | 0.1 | 0.8 (0.1–6.1) | 0.9 |
| Antibiotic therapy within previous 7 days | 9 (25) | 19 (70) | 7.1 (2.3–21.8) | 0.001 | 2.6 (0.4–15.6) | 0.3 |
| Co‐infection with ≥1 additional respiratory co‐pathogensd | 1 (3) | 13 (48) | 32.5 (3.9–272.5) | 0.001 | 24.2 (2.0–288.4) | 0.01 |
Bold values are significant.
Includes 14 cases of proven HRV pneumonia and 13 cases of possible HRV pneumonia.
Includes patients with acute GVHD grade 2–4 and/or chronic GVHD.
Coagulase‐negative staphylococcal bacteremia was defined as 2 positive blood cultures drawn within 72 h of each other.
Respiratory co‐pathogens: Stenotrophomonas maltophilia (2 cases); Influenza A; Escherichia coli; S. maltophilia, Acinetobacter baumannii; Vancomycin‐resistant Enterococcus faecium; Haemophilus influenzae; Parainfluenza virus 3 (3 cases); Possible fungal pneumonia; Proven fungal pneumonia; Respiratory Syncytial Virus A; Pseudomonas aeruginosa.
URI, upper respiratory infection; CI, confidence interval; COPD, chronic obstructive pulmonary disease.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Subsequent episodes of pneumonia
Figure 2 outlines all episodes of pneumonia and HRV association during a 1‐year period following initial detection of HRV post HSCT. Among the 36 patients who presented with HRV URI at the first HRV detection, 6 (17%) had ≥1 subsequent episodes of proven or possible HRV pneumonia, and 9 (25%) had ≥1 subsequent episodes of pneumonia in which HRV was not detected or not tested. The median time from HRV URI to the first episode of pneumonia was 61 days (range 22–310) in patients with proven or possible HRV pneumonia and 167.5 days (range 27–303) in patients with pneumonia in whom HRV was not detected or not tested.
Figure 2.
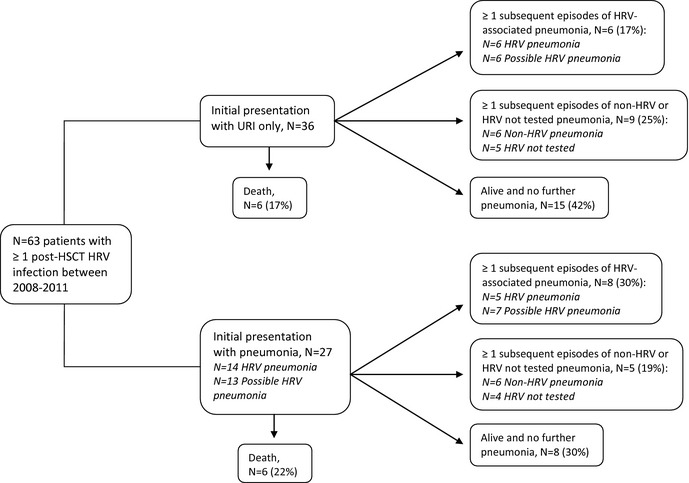
Flow diagram of all pneumonia episodes and human rhinovirus (HRV) association in patients with ≥1 post‐hematopoietic stem cell transplant (HSCT) HRV infection. URI, upper respiratory infection.
Among the 27 patients who presented with proven or possible HRV pneumonia at the first HRV detection, 8 (30%) had ≥1 subsequent episodes of proven or possible HRV pneumonia, and 5 (19%) had ≥1 subsequent episodes of pneumonia in which HRV was not detected or not tested. The median time to the second episode of pneumonia was 53 days (range 41–253) among patients with proven or possible HRV pneumonia, and was 201 days (38–546 days) among patients with pneumonia in whom HRV was not detected or not tested.
In total, 25 episodes of proven HRV pneumonia and 26 episodes of possible HRV pneumonia occurred among 33 HSCT recipients during the study period (Table 3). One or more additional respiratory co‐pathogens were detected in 47% of pneumonia episodes (bacterial n = 12, fungal n = 7, viral n = 5).
Table 3.
Characteristics of subjects with proven or possible human rhinovirus (HRV) pneumonia
| Variable | Proven HRV pneumonia, N = 22 (%)a | Possible HRV pneumonia, N = 19 (%)b |
|---|---|---|
| Median age in years (range) at HSCT | 50 (22–65) | 55 (24–66) |
| Median days HSCT to first HRV detection (IQR) | 34 (10.5–337) | 100 (11–292) |
| Median days HSCT to HRV pneumonia (IQR) | 106 (20–346) | 154 (21.5–313) |
| Transplant type | ||
| Allogeneic | 18 (82) | 12 (63) |
| Autologous | 4 (18) | 7 (37) |
| Conditioning regimen | ||
| Myeloablative | 16 (73) | 15 (79) |
| Reduced‐intensity | 6 (27) | 4 (21) |
| GVHD at time of HRV detection | 11/18 (61) | 8/12 (67) |
| Relapse of underlying hematologic malignancy | 2 (10) | 3 (16) |
| COPD/Asthma | 1 (5) | 2 (11) |
| Antibiotic therapy within previous 7 days | 21/25 (84) | 15/26 (58) |
| Signs and symptomsc | ||
| Fever (≥38.0°C) | 15 (60) | 15 (58) |
| Cough | 22/24 (92) | 26 (100) |
| Sputum production | 14/23 (61) | 16/22 (73) |
| Dyspnea | 16 (64) | 16 (62) |
| Hypoxia (oxygen saturation <95% on room air) | 15 (60)d | 8 (31)d |
| Laboratory markersc | ||
| Neutropenia (<500 cells/μL) | 5 (20) | 10 (38) |
| Lymphopenia (<200 cells/μL) | 12 (48) | 10 (38) |
| Albumin <3.2 mg/dL | 24 (96) | 21 (81) |
| CT scan characteristics in patients with HRV mono‐infectione | ||
| Multilobar (≥2 lung segments) | 8 (100) | 12 (80) |
| Interstitial infiltrates | 1 (13) | 0 |
| Patchy ground glass infiltrates | 5 (63) | 5 (33) |
| Peribronchiolar infiltrates | 7 (88)d | 5 (33)d |
| Consolidation with air bronchograms | 5 (63) | 10 (67) |
| Nodular infiltrates with surrounding ground glass | 2 (25) | 2 (13) |
| Respiratory co‐pathogen(s) detectedc | 15 (60)d | 8 (31)d |
| Bacterial co‐pathogens | Stenotrophomonas maltophilia (2 cases); Methicillin‐resistant Staphylococcus aureus; Escherichia coli; S. maltophilia, Acinetobacter baumannii; Corynebacterium pseudodiphtheriticum | Pseudomonas aeruginosa; Escherichia coli, P. aeruginosa; P. aeruginosa; Haemophilus influenzae; Vancomycin‐resistant Enterococcus |
| Viral co‐pathogens | Parainfluenza virus 3 (2 cases); Respiratory syncytial virus A | Parainfluenza virus 3; Influenza A |
| Fungal co‐pathogens | Pneumocystis jirovecii (2 cases); Aspergillus calidoustus; Proven fungal pneumonia;f Probable fungal pneumonia;g Possible fungal pneumonia | Hormographiella aspergillata |
A total of 25 episodes of proven HRV pneumonia in 22 patients.
A total of 26 episodes of possible HRV pneumonia in 19 patients.
During N = 25 and N = 26 episodes of proven and possible HRV pneumonia, respectively; denominator indicated where data are missing.
P = 0.05, 0.03, and 0.05 for comparison of hypoxia, peribronchiolar infiltrates, and co‐pathogen(s) detected, respectively, using Fisher's exact test.
Among 10 episodes of proven HRV pneumonia and 18 episodes of possible HRV pneumonia in which HRV was the sole pathogen detected, there were 8 and 15 CT scans available for review, respectively.
Proven fungal pneumonia criteria met: bilateral pulmonary infiltrates, skin biopsy with angioinvasive septated hyphal forms.
Probable fungal pneumonia criteria met: positive result for Aspergillus antigen in ≥2 blood samples, lower respiratory tract fungal disease (dense, well‐circumscribed lesions without a halo sign).
HSCT, hematopoietic stem cell transplantation; IQR, interquartile range; GVHD, graft‐versus‐host disease; COPD, chronic obstructive pulmonary disease; CT, computed tomography.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Proven HRV pneumonia
During the study period, 22 patients had 25 episodes of pneumonia in which HRV was detected in BAL fluid. Among these 22 patients with proven HRV pneumonia, 82% were allogeneic HSCT recipients, of which 61% had graft‐versus‐host disease (GVHD), and 84% had received antibiotics within the previous 7 days. Fever (60%), cough (92%), sputum production (61%), and dyspnea (60%) were common symptoms. In 8 proven HRV pneumonia episodes, an upper respiratory tract sample was analyzed within the previous 2 weeks, and HRV was detected in all specimens.
Another respiratory pathogen was identified in 12 (48%) of 25 BALs. Three additional patients met criteria for proven, probable, and possible fungal pneumonia. Although the numbers were small, compared with patients with HRV alone detected in BAL fluid, patients with bacterial, fungal, or viral respiratory co‐infection were not significantly different in terms of age at HSCT, transplant type, conditioning regimen, GVHD, or receipt of antibiotics within the previous 7 days (data not shown).
Computed tomography images and radiology reports from 8 of the 10 patients with HRV monoinfection in BAL fluid were reviewed by 2 of the study investigators (S.E.J. and T.J.W.). In all 8 patients, pulmonary infiltrates were bilateral and involved 3 or more lung segments. The most common infiltrate appearance was patchy ground glass opacities (n = 5 cases); in 4 of the 5 cases, some of these opacities became more confluent to form consolidative lesions (Fig. 3). Small nodular consolidations with surrounding ground glass were the predominant appearance of HRV pneumonia in 2 additional cases. In all of the above 7 cases, infiltrates aggregated in a peribronchiolar distribution. Finally, 1 patient who presented from home to the emergency department with acute respiratory distress after a week of fever and upper respiratory symptoms had dense bilateral multilobar consolidations. Among all 10 patients, oxygen saturations ranged from 80% to 97% on room air, with 7 patients having an oxygen saturation <93% and 1 patient requiring mechanical ventilation.
Figure 3.
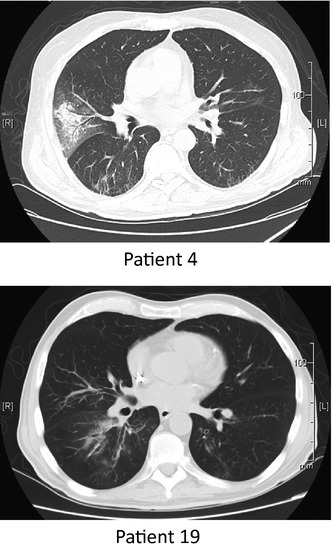
Representative computed tomography scans from 2 patients with pneumonia and human rhinovirus alone detected in bronchoalveolar lavage fluid. Infiltrates are bilateral, focal, and peribronchiolar.
During the study period, 5 tissue specimens obtained via transbronchial biopsy during episodes of proven HRV pneumonia were available for review (Fig. 4). Bacterial co‐infection was present by culture in BAL fluid in 2 cases (Escherichia coli and Staphylococcus aureus), fungal co‐infection was present in 1 case (Pneumocystis pneumonia), viral co‐infection was present in 1 case (RSVA), and the fifth case had no evidence of bacterial, fungal, or viral co‐infection. The 2 cases of HRV and bacterial co‐infection showed an acute neutrophil‐rich necrotizing bronchitis and interstitial pneumonitis, with cytopathic changes including effaced nuclear chromatin with preserved nuclear membrane and ciliocytophthoria primarily observed in bronchial epithelium. The case of HRV and Pneumocystis co‐infection showed a type II pneumocyte hyperplasia with some cellular atypia and an interstitial pneumonitis. Multiple aggregates of characteristic Pneumocystis organisms were also seen in the alveolar spaces. The case of HRV and RSVA co‐infection demonstrated subacute interstitial pneumonitis with type II pneumocyte hyperplasia unassociated with a significant inflammatory host response. In the fifth case, where HRV alone was isolated in BAL fluid, histopathologic features included slightly prominent pneumocytes and interstitial fibrin deposition, suggesting capillary injury. Cytoplasmic inclusions, multinucleation, and megaloblastic changes were not present in any of the 5 specimens.
Figure 4.
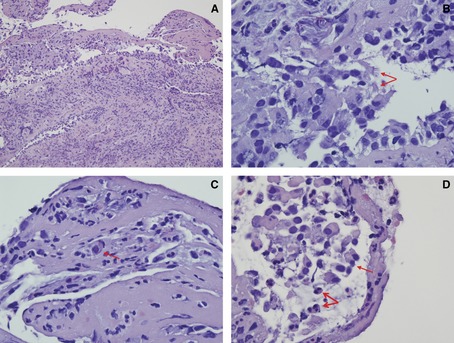
Trans‐bronchial biopsy specimens during 5 episodes of human rhinovirus pneumonia were reviewed. (A) There is marked inflammation in the bronchial wall with degenerative epithelial changes. The dominant inflammatory cell infiltrate is neutrophilic in nature (hematoxylin‐eosin [H‐E] stain, original magnification ×200). (B) There is ciliocytophthoria characterized by detached tufts of cilia separated from the remainder of the bronchial cell, a finding suggestive of a viral infection 34 (arrows) (H‐E stain, original magnification ×1000). (C) The chromatin is effaced and appears eosinophilic. These cytopathic changes are consistent with viral infection (arrow) (H‐E stain, original magnification ×400). (D) There is marked bronchial inflammation. The bronchial epithelial cells are detached (thin arrow). The chromatin is largely effaced. The nuclei have a homogeneous amphophilic quality likely indicative of viral effect (thick arrows) (H‐E stain, original magnification ×1000).
Mortality
Overall mortality in the total cohort of 63 patients was 38% during the 1 year following the first HRV infection. Among patients who presented with proven HRV pneumonia (n = 14), possible HRV pneumonia (n = 13), and HRV URI (n = 36) at the first HRV detection, 3‐month and 1‐year mortality was 21% and 50%, 38% and 38%, and 8% and 33%, respectively. Among patients whose first pneumonia was non‐HRV or HRV not tested pneumonia (n = 10), 3‐month and 1‐year mortality was 0% and 20%, respectively. Pneumonia was the immediate cause of death in 3 patients; microbiologic data for these patients were as follows: HRV, Stenotrophomonas maltophilia, Acinetobacter baumannii detected in BAL fluid (n = 1), HRV detected in NP swab, and bloodstream infection with A. baumannii (bronchoscopy not performed) (n = 1), and HRV detected in NP swab and vancomycin‐resistant Enterococcus faecium detected in BAL fluid (BAL fluid not sent for RVP testing) (n = 1).
Outcomes in patients with pre‐transplant HRV infection
During the study period, 3 allogeneic HSCT recipients had symptomatic HRV infection during pre‐transplant conditioning. One patient progressed to proven HRV pneumonia 16 days after HSCT. Her symptoms resolved within 2 weeks; ultimately, she died 6 months post HSCT from relapsed acute myelogenous leukemia. One patient had persistent URI symptoms and HRV positivity for 3 months post HSCT; he was alive at 1 year after the first HRV infection. The third patient resolved his pre‐HSCT URI symptoms and had no further episodes of URI or pneumonia during the next year.
Discussion
Since molecular methods of respiratory virus testing were implemented at our institution in 2008, a total of 63 HSCT recipients have had at least 1 HRV‐associated acute respiratory illness, including 51 episodes of proven or possible HRV pneumonia. HRV infection rates peaked during the fall and early spring, consistent with the seasonal epidemiology observed in other studies of temperate climates 19. Furthermore, 4% of all patients undergoing HSCT between March 2008 and April 2011 were diagnosed with HRV infection during their inpatient transplant admission; nearly half of these patients had pneumonia. To our knowledge, this is the largest described cohort of HSCT recipients with HRV‐associated LRTI. We suspect that as use of multiplex PCR assays for diagnosis of respiratory viral infections becomes widespread, HRV will be increasingly recognized as a significant cause of pneumonia in immunocompromised hosts.
In univariate analysis, we found that markers for increased immunosuppression and illness severity including neutropenia, hypoalbuminemia, receipt of antibiotics, and infection with respiratory co‐pathogen(s) were associated with pneumonia at first post‐transplant detection of HRV. Notably, in multivariate analyses, the presence of 1 or more additional respiratory pathogens remained independently associated with pneumonia. Furthermore, among all 25 episodes of proven HRV pneumonia, 60% demonstrated bacterial, fungal, or viral co‐infection. These findings are consistent with previous reports of HSCT recipients with pneumonia and HRV detection in BAL fluid that have also identified bacterial or fungal co‐infection in the majority of cases 15, 20. Among immunocompetent adults and children, HRVs have been detected in up to 17% of episodes of community‐acquired pneumonia using PCR 21, 22, often with concomitant bacterial infection. Several potential mechanisms through which HRV increases susceptibility to bacterial infection have been demonstrated in vitro. For example, HRVs stimulate Streptococcus pneumoniae adhesion to human tracheal epithelial cells via increases in platelet‐activating factor‐receptors 23, promote S. aureus internalization into non‐fully permissive cultured pneumocytes 24, and disrupt epithelial cell barrier function by dissociation of zona‐occludens 1 from the tight junction complex, thereby facilitating transmigration of bacteria 25. Therefore, HRVs may be similar to influenza in predisposing to bacterial superinfection. Other mechanisms by which influenza increases susceptibility to bacterial infection, including airway epithelial damage, surface receptor changes, and altered neutrophil function, warrant further study in models of HRV infection.
Viral co‐infection was uncommon in this study, occurring in 5 (9%) episodes of proven or possible HRV pneumonia (Influenza A [n = 1], PIV3 [n = 3], RSVA [n = 1]). This is notable because all patients were tested for a panel of 10 respiratory viruses using multiplex PCR during each HRV infection, and therefore, the likelihood of missed cases of viral co‐infection was low. Our findings are consistent with those of Greer et al. 26, who showed a statistically significant reduction in viral co‐detection compared with other respiratory viruses, perhaps because of mediation of interferon‐stimulating genes, thus inducing a protective antiviral state.
Our study also identified 10 cases of pneumonia in which HRV was the sole pathogen identified in BAL fluid. To date, little research has been performed on the pathogenesis of HRV pneumonia. However, studies focused on the mechanism of HRV‐induced exacerbations of chronic lung disease, including asthma 27, 28, 29 and chronic obstructive pulmonary disease (COPD) 30, 31, 32, may provide insights into the pathophysiology of HRV pneumonia. For example, in patients with asthma, impaired innate and adaptive immune responses, including deficient interferon production 29, 33 and augmented T‐helper (Th)2 or impaired Th1 or Interleukin‐10 immunity 27, have been implicated in increased bronchial inflammation and hyperreactivity. Through a similar mechanism identified in patients with asthma and COPD, HRV may potentially cause parenchymal destruction through direct cell injury as well as altered immune responses in immunocompromised hosts.
Although a limited number of our patients had lung tissue obtained during episodes of proven HRV pneumonia, the predominant histopathologic findings are associated with viral, rather than bacterial or fungal, infection. HRV infection appears to have 2 histological components: a necrotizing bronchitis and an interstitial process. These findings included a striking necrotizing bronchitis including ciliocytophthoria of bronchial epithelial cells 34 and an effaced nuclear chromatin amidst bronchial epithelial cells, although without unequivocal intranuclear and/or cytoplasmic inclusions. Similar nuclear changes were not seen within the alveolar pneumocytes. In the few reported cases of HRV LRTI with human histology, HRV is capable of causing both interstitial and alveolar processes. In these reports, pathologic findings included bronchiolitis obliterans with organizing pneumonia 14, interstitial pneumonitis 20, acute and chronic inflammation with fibrinopurulent alveolar debris 35, and hyperplasia and desquamation of alveolar cells 36. These findings collectively contribute to our understanding of the pathogenesis of pulmonary HRV infection.
This study has several limitations. First, as our data are observational and rely on clinical specimens, some patients with HRV‐associated acute respiratory illness may have been missed if they were not tested for HRV. Missed cases of HRV‐associated acute respiratory illness would be more likely to occur with mild URI than pneumonia, because most HSCT recipients with pneumonia are hospitalized and undergo thorough microbiologic evaluation. Therefore, we cannot infer the relative frequency with which HRV causes pneumonia compared to URI in the HSCT population. Concern may be raised that HRV detection in the lower airways reflects colonization rather than infection. However, the same argument could be made for other respiratory viruses, such as influenza A, PIV3 and RSV, which are known to cause both URI and LRTI. Several studies have demonstrated HRV replication in primary human airway epithelial cells 37, 38. Moreover, the ability of HRVs to replicate in bronchial epithelium after experimental upper airway infection of healthy human volunteers has been demonstrated using in situ hybridization 2. Another limitation is that molecular testing was not routinely performed for certain viral (coronavirus, PIV4, and human bocavirus) and atypical pathogens (Chlamydophila pneumonia and Mycoplasma pneumonia) during the study period, potentially missing additional cases of HRV co‐infection.
Of note, owing to sequence homology between human enterovirus (HEV) and HRV, the RVP molecular methods may not reliably distinguish HEV and HRV. However, because tests of clinical performance characteristics of the RVP assay found that 42 of 43 specimens testing positive for HEV/HRV were confirmed to be HRV (personal communication with the manufacturer), the specimens identified as HRV in our patients also have a high probability of being HRV. This conclusion is also consistent with 2 recent studies in which 100% of clinical specimens (n = 164) positive for HRV/HEV by RVP were subsequently confirmed positive for HRV and negative for HEV upon further molecular testing 39, 40.
There may be other risk factors for HRV pneumonia that we were unable to ascertain in this study. In 2006, molecular methods showed a significant genotypic variation in HRV and identified a novel HRV species named HRV‐C 41. In hospitalized patients, HRV‐C may be associated with wheezing, pneumonia 10, 42, and viremia 43 more often than HRV‐A and HRV‐B. However, rates of LRTI vary considerably among the 3 HRV species owing to different patient populations, primary outcomes, and geographic study locations 44, 45. In addition, HRV viral load may correlate with symptom severity in hospitalized, immunocompetent pediatric and adult patients, asthmatics, and lung transplant recipients 27, 46, 47. Because of unavailability of specimens and limitations of the testing methodology, we were unable to determine the HRV strain or quantify viral load in the current study to evaluate whether these factors may identify patients at risk for severe or recurrent infection.
In conclusion, this study provides definitive evidence that HRVs are a significant lower respiratory tract pathogen in HSCT recipients. Given the high morbidity and mortality associated with pneumonia in HSCT recipients, a better understanding of the molecular epidemiology and distinguishing clinical features of HRVs is needed in patients with underlying hematologic malignancies. Knowledge that certain strains and species of HRV are associated with severe respiratory infection in patients with hematologic malignancy may aid in risk stratification of patients with HRV URI before transplantation and improve infection control practices in hospitalized patients. A study of the molecular epidemiology of HRV infection in patients with hematologic malignancy and HSCT recipients is ongoing at our institution.
Acknowledgements
Thanks: The authors thank Ms. Xuming Sun for her assistance with statistical analysis.
Support: This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant T32 AI007613) and by the Clinical and Translational Science Center at Weill Cornell Medical College (grant UL1RR024996).
Disclosure: The authors report no financial support for this study or potential conflicts of interest.
Author contributions: S.E.J., R.S., M.J.S., and T.J.W. designed the study; S.E.J. performed data collection and drafted the article; S.E.J., T.B.S., C.M., and T.J.W. analyzed and interpreted data; R.S., T.B.S, M.J.S., A.N.S., C.M., S.G.J., and T.J.W. critically revised the article and approved the article.
Jacobs S.E., Soave R., Shore T.B., Satlin M.J., Schuetz A.N., Magro C., Jenkins S.G., Walsh T.J.. Human rhinovirus infections of the lower respiratory tract in hematopoietic stem cell transplant recipients. Transpl Infect Dis 2013: 15: 474–486. All rights reserved
References
- 1. Subauste MC, Jacoby DB, Richards SM, Proud D. Infection of a human respiratory epithelial cell line with rhinovirus: induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J Clin Invest 1995; 96: 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papadopoulos NG, Bates PJ, Bardin PG, et al. Rhinoviruses infect the lower airways. J Infect Dis 2000; 181 (6): 1875–1884. [DOI] [PubMed] [Google Scholar]
- 3. Gern JE. The ABCs of rhinoviruses, wheezing, and asthma. J Virol 2010; 84 (15): 7418–7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high‐risk children. Am J Respir Crit Care Med 2008; 178 (7): 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corne JM, Marshall C, Smith S, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non‐asthmatic individuals: a longitudinal cohort study. Lancet 2002; 359: 831–834. [DOI] [PubMed] [Google Scholar]
- 6. Mansbach JM, Piedra PA, Teach SJ, et al.; MARC‐30 Investigators . Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med 2012; 166 (8): 700–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Van Leeuwen JC, Goossens LK, Hendrix RMGR, Van Der Palen J, Lusthusz A, Thio BJ. Equal virulence of rhinovirus and respiratory syncytial virus in infants hospitalized for lower respiratory tract infection. Pediatr Infect Dis J 2012; 31 (1): 84–86. [DOI] [PubMed] [Google Scholar]
- 8. Renwick N, Schweiger B, Kapoor V, et al. A recently identified rhinovirus genotype is associated with severe respiratory‐tract infection in children in Germany. J Infect Dis 2007; 196 (6): 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lieberman D, Shimoni A, Shemer‐Avni Y, Keren‐Naos A, Shtainberg R, Lieberman D. Respiratory viruses in adults with community‐acquired pneumonia. Chest 2010; 138 (4): 811–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piralla A, Rovida F, Campanini G, et al. Clinical severity and molecular typing of human rhinovirus C strains during a fall outbreak affecting hospitalized patients. J Clin Virol 2009; 45 (4): 311–317. [DOI] [PubMed] [Google Scholar]
- 11. Hicks LA, Shepard CW, Britz PH, et al. Two outbreaks of severe respiratory disease in nursing homes associated with rhinovirus. J Am Geri Soc 2006; 54 (2): 284–289. [DOI] [PubMed] [Google Scholar]
- 12. Louie JK, Yagi S, Nelson FA, et al. Rhinovirus outbreak in a long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis 2005; 41 (2): 262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Milano F, Campbell AP, Guthrie KA, et al. Human rhinovirus and coronavirus detection among allogeneic hematopoietic stem cell transplantation recipients. Blood 2010; 115 (10): 2088–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gutman JA, Peck AJ, Kuypers J, Boeckh M. Rhinovirus as a cause of fatal lower respiratory tract infection in adult stem cell transplantation patients: a report of two cases. Bone Marrow Transplant 2007; 40 (8): 809–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ison MG, Hayden FG, Kaiser L, Corey L, Boeckh M. Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clin Infect Dis 2003; 36 (9): 1139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Merante F, Yaghoubian S, Janeczko R. Principles of the xTAG respiratory viral panel assay (RVP Assay). J Clin Virol 2007; 1: S31–S35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raymaekers M, de Rijke B, Pauli I, Van den Abeele A‐M, Cartuyvels R. Timely diagnosis of respiratory tract infections: evaluation of the performance of the Respifinder assay compared to the xTAG respiratory viral panel assay. J Clin Virol 2011; 52 (4): 314–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Pauw B, Walsh T, Donnelly J. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive. Clin Infect Dis 2008; 46 (12): 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobs SE, Lamson DM, St. George K, Walsh TJ. Human rhinoviruses. Clin Microbiol Rev 2013; 26 (1): 135–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghosh S, Champlin R, Couch R. Rhinovirus infections in myelosuppressed adult blood and marrow transplant recipients. Clin Infect Dis 1999; 29 (3): 528–532. [DOI] [PubMed] [Google Scholar]
- 21. Jennings LC, Anderson TP, Beynon KA, et al. Incidence and characteristics of viral community‐acquired pneumonia in adults. Thorax 2008; 63 (1): 42–48. [DOI] [PubMed] [Google Scholar]
- 22. Templeton KE, Scheltinga SA, van den Eeden WC, Graffelman AW, van den Broek PJ, Claas ECJ. Improved diagnosis of the etiology of community‐acquired pneumonia with real‐time polymerase chain reaction. Clin Infect Dis 2005; 41 (3): 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishizuka S, Yamaya M, Suzuki T, et al. Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J Infect Dis 2003; 188 (12): 1928–1939. [DOI] [PubMed] [Google Scholar]
- 24. Passariello C, Schippa S, Conti C, et al. Rhinoviruses promote internalisation of Staphylococcus aureus into non‐fully permissive cultured pneumocytes. Microbes Infect 2006; 8 (3): 758–766. [DOI] [PubMed] [Google Scholar]
- 25. Sajjan U, Wang Q, Zhao Y, Gruenert DC, Hershenson MB. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am J Respir Crit Care Med 2008; 178 (12): 1271–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greer RM, McErlean P, Arden KE, et al. Do rhinoviruses reduce the probability of viral co‐detection during acute respiratory tract infections? J Clin Virol 2009; 45 (1): 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kelly JT, Busse WW. Host immune responses to rhinovirus: mechanisms in asthma. J Allergy Clin Immunol 2008; 122 (4): 671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Message SD, Laza‐Stanca V, Mallia P, et al. Rhinovirus‐induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL‐10 production. Proc Natl Acad Sci USA 2008; 105 (36): 13562–13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wark PA, Johnston SL, Bucchieri F, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005; 201 (6): 937–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mallia P, Message SD, Kebadze T, Parker HL, Kon OM, Johnston SL. An experimental model of rhinovirus induced chronic obstructive pulmonary disease exacerbations: a pilot study. Respir Res 2006; 7: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mallia P, Message SD, Gielen V, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med 2011; 183 (6): 734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singh M, Lee S‐H, Porter P, et al. Human rhinovirus proteinase 2A induces TH1 and TH2 immunity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2010; 125 (6): 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Contoli M, Message SD, Laza‐Stanca V, et al. Role of deficient type III interferon‐lambda production in asthma exacerbations. Nat Med 2006; 12 (9): 1023–1026. [DOI] [PubMed] [Google Scholar]
- 34. Powers CN. Diagnosis of infectious diseases: a cytopathologist's perspective. Clin Microbiol Rev 1998; 11 (2): 341–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Malcolm E, Arruda E, Hayden FG, Kaiser L. Clinical features of patients with acute respiratory illness and rhinovirus in their bronchoalveolar lavages. J Clin Virol 2001; 21 (1): 9–16. [DOI] [PubMed] [Google Scholar]
- 36. Imakita M, Shiraki K, Yutani C, Ishibashi‐Ueda H. Pneumonia caused by rhinovirus. Clin Infect Dis 2000; 30: 611–612. [DOI] [PubMed] [Google Scholar]
- 37. Schroth MK, Grimm E, Frindt P, et al. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol 1999; 20 (6): 1220–1228. [DOI] [PubMed] [Google Scholar]
- 38. Vareille M, Kieninger E, Alves MP, et al. Impaired type I and type III interferon induction and rhinovirus control in human cystic fibrosis airway epithelial cells. Thorax 2012; 67 (6): 517–525. [DOI] [PubMed] [Google Scholar]
- 39. Chandrasekaran A, Manji R, Joseph A, Zhang F, Ginocchio CC. Broad reactivity of the Luminex xTAG Respiratory Virus Panel (RVP) assay for the detection of human rhinoviruses. J Clin Virol 2012; 53 (3): 272–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Longtin J, Marchand‐Austin A, Winter AL, et al. Rhinovirus outbreaks in long‐term care facilities, Ontario, Canada. Emerg Infect Dis 2010; 16 (9): 1463–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lamson D, Renwick N, Kapoor V, et al. MassTag polymerase‐chain‐reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza‐like illness in New York State during 2004‐2005. J Infect Dis 2006; 194 (10): 1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lau SKP, Yip CCY, Lin AWC, et al. Clinical and molecular epidemiology of human rhinovirus C in children and adults in Hong Kong reveals a possible distinct human rhinovirus C subgroup. J Infect Dis 2009; 200 (7): 1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fuji N, Suzuki A, Lupisan S, et al. Detection of human rhinovirus C viral genome in blood among children with severe respiratory infections in the Philippines. PLoS One 2011; 6 (11): e27247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iwane MK, Prill MM, Lu X, et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young U.S. children. J Infect Dis 2011; 204 (11): 1702–1710. [DOI] [PubMed] [Google Scholar]
- 45. Linsuwanon P, Payungporn S, Samransamruajkit R, et al. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J Infect 2009; 59 (2): 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Costa C, Bergallo M, Astegiano S, et al. Detection of human rhinoviruses in the lower respiratory tract of lung transplant recipients. Arch Virol 2011; 156 (8): 1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gerna G, Piralla A, Rovida F, et al. Correlation of rhinovirus load in the respiratory tract and clinical symptoms in hospitalized immunocompetent and immunocompromised patients. J Med Virol 2009; 81 (8): 1498–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]


