SUMMARY
Plasmodium falciparum malaria is responsible for over 250 million clinical cases every year worldwide. Severe malaria cases might present with a range of disease syndromes including acute respiratory distress, metabolic acidosis, hypoglycaemia, renal failure, anaemia, pulmonary oedema, cerebral malaria (CM) and placental malaria (PM) in pregnant women. Two main determinants of severe malaria have been identified: sequestration of parasitized red blood cells and strong pro-inflammatory responses. Increasing evidence from human studies and malaria infection animal models revealed the presence of host leucocytes at the site of parasite sequestration in brain blood vessels as well as placental tissue in complicated malaria cases. These observations suggested that apart from secreting cytokines, leucocytes might also contribute to disease by migrating to the site of parasite sequestration thereby exacerbating organ-specific inflammation. This evidence attracted substantial interest in identifying trafficking pathways by which inflammatory leucocytes are recruited to target organs during severe malaria syndromes. Chemo-attractant cytokines or chemokines are the key regulators of leucocyte trafficking and their potential contribution to disease has recently received considerable attention. This review summarizes the main findings to date, investigating the role of chemokines in severe malaria and the implication of these responses for the induction of pathogenesis and immunity to infection.
Key words: chemokines, severe malaria, pathogenesis
INTRODUCTION
Malaria is one the most serious infectious diseases of humans with over 250 million clinical cases every year worldwide. The infection is transmitted to humans by Anopheles mosquitoes that are infected with parasites of the genus Plasmodium. Most cases of severe disease are caused by Plasmodium falciparum, which is endemic in sub-Saharan Africa and throughout the tropics. The blood stage of the parasite is largely responsible for malaria-associated pathology (Miller et al. 2002). The fatalities are associated with a range of various disease syndromes including acute respiratory distress, metabolic acidosis, hypoglycaemia, renal failure, anaemia, pulmonary oedema and cerebral malaria (CM) (White and Ho, 1992). This disease syndrome is the most severe complication resulting from P. falciparum infection and accounts for nearly 1 million deaths every year (Murray et al. 2012). Children under the age of five are particularly susceptible to this condition, which is characterized by convulsions, seizures and coma. Similar to young children, pregnant women (particularly primigravid women) are at an increased risk of infection and might develop placental malaria (PM). This serious complication has been found to be associated with adverse pregnancy outcomes including premature labour, intrauterine growth retardation and low birth weight delivery, and is responsible for more than 75 000 infant deaths each year (McGregor, 1984; Steketee et al. 2001; Desai et al. 2007).
Mature forms of blood-stage malaria express parasitic proteins on the surface of the infected erythrocyte (such as P. falciparum Erythrocyte Membrane Protein 1), which allows them to bind to endothelial cells, sequester in vascular beds and avoid clearance in the spleen. Although the precise mechanisms leading to severe malaria syndromes are not completely understood, it is accepted that sequestration of parasitized red blood cells (pRBC) is a major determinant of disease development. Parasite sequestration is thought to induce obstructions in blood flow resulting in hypoxia and haemorrhages (Miller et al. 2002) that appear to be associated with the development of organ-specific syndromes such CM and PM. A large body of literature indicates that in addition to parasite sequestration, inflammatory responses mediated by cytokines such as TNF (Molyneux et al. 1993), IFN-γ and IL-1β (Pongponratn et al. 2003) are associated with disease severity in human malaria.
Much useful evidence on the inflammatory mechanisms contributing to the induction of severe malaria has been provided by the Plasmodium berghei ANKA model. This rodent infection has many features in common with human disease and is thus the best available model for certain aspects of clinical malaria (Schofield and Grau, 2005; Hansen, 2012). Like in humans, P. berghei-ANKA pRBC have been found to accumulate in brains of susceptible mice during infection. Moreover, recent evidence revealed that P. berghei-ANKA pRBC adhere to brain microvascular endothelial cells in a VCAM-1-dependent fashion (El-Assaad et al. 2013). A large body of work demonstrated that host immune responses elicited during P. berghei ANKA infection result in detrimental inflammation and contribute to cerebral disease induction. Host responses mediated by inflammatory cytokines such as TNF (Grau et al. 1987), LT-α (Engwerda et al. 2002), IFN-γ (Grau et al. 1989) and effector cells such as CD4+ (Grau et al. 1986; Yanez et al. 1996; Villegas-Mendez et al. 2012) and CD8+ T cells (Belnoue et al. 2002; Nitcheu et al. 2003), NKT cells (Hansen et al. 2003) and NK cells (Hansen et al. 2007; Ryg-Cornejo et al. 2013) have been shown to contribute to severe malaria in this model.
In addition to pRBC, human post-mortem studies revealed the presence of leucocytes and platelets within the brain microvasculature in a substantial proportion of CM cases (Porta et al. 1993; Patnaik et al. 1994; Grau et al. 2003; Hunt and Grau, 2003; Taylor et al. 2004). Interestingly, sequestered monocytes and macrophages were found to be more abundant in CM paediatric patients than in severe malarial anaemia (SMA) patients (Coltel et al. 2004). Similarly, although the sequestration of pRBC in the placenta appears to be responsible for initiation of pathology, histological evidence revealed the infiltration of various leucocyte populations including monocytes, macrophages, T cells and granulocytes (Ordi et al. 2001). An important intervillositis occurs in PM cases with evident sequestration of both pRBC and leucocytes preventing efficient blood flow across the placenta. Moreover, mononuclear intervillous inflammatory infiltration has been found to be associated with low birth weight and preterm delivery (Ordi et al. 1998, 2001).
In experimental mouse models, several leucocyte populations including macrophages, neutrophils, T cells, NK cells and platelets have been found in brain blood vessels of CM-affected mice during infection (Grau et al. 1987, 1993; Ma et al. 1996; Belnoue et al. 2002; Nitcheu et al. 2003; Hansen et al. 2007; Lundie et al. 2008). From these populations, CD8+ T cells comprise a high proportion of the brain-sequestered leucocyte pool of P. berghei-ANKA-infected mice and appear to mediate CM via a perforin-dependent mechanism (Belnoue et al. 2002; Nitcheu et al. 2003). Moreover, infection of β2-microglobulin−/− mice as well as antibody depletion studies demonstrated that CD8+ T cells contribute to the induction of experimental cerebral malaria (ECM) (Yanez et al. 1996).
The increasing body of evidence illustrating the presence of sequestered host cells together with infected erythrocytes in organs such as the brain or placenta during infection suggested that in addition to secreting cytokines and mediating systemic responses, leucocytes might also contribute to disease by migrating to the site of parasite sequestration, thereby exacerbating organ-specific inflammation. These observations attracted substantial interest in identifying trafficking pathways by which leucocytes are recruited to target organs during severe malaria syndromes and the potential contribution of chemokines to disease. In this review we have summarized the main findings to date investigating the role of chemokines during severe malaria including experimental infection in murine models as well as human field studies. The implications of those findings for the induction of pathogenesis and immunity to malaria are discussed.
THE ROLE OF CHEMOKINES IN HOMOEOSTASIS AND INFLAMMATION
Chemoattractant cytokines or chemokines are key regulators of leucocyte trafficking. Chemokine-guided movement allows leucocyte migration to various lymphoid tissues and deployment of immune cells to peripheral sites of pathogen challenge and inflammation. Chemokines are a superfamily of low molecular weight polypeptides of about 8–14 kDa, which bind and signal through seven-transmembrane spanning G-protein coupled receptors (GPCRs). To date, nearly 50 chemokines have been identified (Table 1) as well as numerous chemokine-binding proteins that do not induce cell-signalling events (Murphy, 2002). Different chemokines share a significant degree of sequence homology and are subdivided into CC, CXC, CX3C and C subfamilies based on the relative positions of conserved cysteine residues near the N-terminus (Luster, 1998).
Table 1.
Chemokines and chemokine receptors
| Cell type | Receptor | Chemokinea |
|---|---|---|
| Eosinophil Monocyte Activated T cell Dendritic cell | CCR1 | MCP-3 (CCL7), MCP-4 (CCL13), MIP-1α (CCL3), RANTES (CCL5), HCC-1 (CCL14) |
| Basophil Monocyte Activated T cell Dendritic cell NK cell | CCR2 | MCP-1 (CCL2), MCP-2 (CCL8), MCP-3 (CCL7), MCP-4 (CCL13), MCP-5 (CCL12) |
| Eosinophil Basophil Dendritic cell Activated T cell Resting T cell | CCR3 | Eotaxin-1 (CCL11), Eotaxin-2 (CCL24), MCP-3 (CCL7), MCP-4 (CCL13), RANTES (CCL5), PARC (CCL18) |
| Activated T cell Dendritic cell | CCR4 | TARC (CCL17), MDC (CCL22) |
| Monocyte Activated T cell Dendritic cell NK cell | CCR5 | MIP-1α (CCL3), MIP-1β (CCL4), RANTES (CCL5), HCC-1 (CCL14) |
| Dendritic cell | CCR6 | MIP-3α (CCL20) |
| Activated T cell Resting T cell | CCR7 | MIP-3β (CCL19), SLC (CCL21) |
| Monocyte | CCR8 | I-309 (CCL1) |
| Activated T cell Resting T cell Monocyte Dendritic cell | CCR9 | TECK (CCL25) |
| Monocyte activated T cell NK cell | CX3CR1 | Fractalkine (CX3CL1) |
| Neutrophil | CXCR1 | IL-8 (CXCL8), GCP-2 (CXCL6) |
| Neutrophil | CXCR2 | IL-8 (CXCL8), GCP-2 (CXCL6), GRO-α (CXCL1), GRO-β (CXCL2), GRO-γ (CXCL3), ENA-78 (CXCL5), NAP-2 (CXCL7) |
| Activated T cell NK cell | CXCR3 | IP-10 (CXCL10), MIG (CXCL9), I-TAC (CXCL11) |
| Monocyte Resting T cell Dendritic cell | CXCR4 | SDF-1 (CXCL12) |
| NK cell Activated T cell Dendritic cell | XCR1 | Lymphotactin (XCL1), SCM-1β (XCL2) |
Chemokines are identified with both common names and systematic names in parenthesis. Bold font identifies inflammatory/inducible chemokines. Homoeostatic chemokines are shown in italic font. Bold and italic font denotes chemokines belonging to both classifications.
Chemokines and chemokine receptors are essential in two distinct processes of leucocyte migration. The first is the directional migration and positioning of leucocytes within lymphoid organs as well as in peripheral tissues. In this context, cells encounter chemoattractant signals in a complex spatial and temporal pattern, in which cells follow a gradient of increasing concentration towards the source of the chemokine (reviewed in Rot and von Andrian (2004). The second trafficking process mediated by chemokines involves the arrest of migrating leucocytes on the vascular endothelium followed by extravasation from the blood vessel into lymphoid and inflamed tissues. In this process, chemokines are immobilized by sulphated sugars of the glycosaminoglycan (GAG) family on the luminal surface of vascular endothelial cells, which is essential for optimal adhesion of leucocytes to the endothelium (Rot, 1992; Tanaka et al. 1993).
Functionally, chemokines can be divided into two broad categories: constitutively expressed chemokines that maintain homoeostatic functions, and inducible chemokines that are usually upregulated in response to infection and/or inflammation (Sallusto et al. 2000; Moser et al. 2004). This distinction can be somewhat arbitrary as some chemokines fall into both categories depending on the biological context (Table 1). Inflammatory chemokines are produced by several cell types including stromal, endothelial and epithelial cells as well as leucocytes. Constitutive chemokines are produced in the thymus and lymphoid tissues and regulate homoeostatic functions of the immune system such as maintaining steady-state leucocyte homing and cell compartmentalization within lymphoid organs (Yoshie et al. 1997). Leucocytes have the capacity to switch the expression of receptors from constitutive to inflammatory chemokines, which allows for different migratory patterns. For example, upon antigen-specific activation and differentiation into effector function, T cells upregulate a repertoire of chemokine receptors and adhesion molecules, which facilitates their exit from secondary lymphoid organs and migration to peripheral inflamed tissues (Xie et al. 1999; Weninger et al. 2001).
Different effector cell subsets have been shown to express distinct chemokine receptor patterns, allowing them specific migration patterns to exert their function. Polarization of CD4+ T cells to TH1 and TH2 subsets is associated with the upregulation of a distinct set of chemokines and their chemokine receptors, which is influenced by the cytokine milieu during priming (Sallusto et al. 1998; Bromley et al. 2008). Typically, TH1 cells are predominantly characterized by the expression of CXCR3, CCR5 and CXCR6, whereas TH2 cells express CCR3, CCR4 and CCR8. However, the association of chemokine receptors with T-helper phenotypes in vivo shows a much more complex profile (Annunziato et al. 1999; Kim et al. 2001), suggesting that CD4+ T cell subsets cannot always be accurately identified solely on the basis of their chemokine receptor expression. Moreover, increasing evidence suggests that the chemokine receptor repertoire expressed on activated T cells shows a degree of dynamic plasticity, whereby several factors including strength of antigenic signals, tissue-specific imprinting by priming dendritic cells or cytokine milieu during priming can influence the trafficking phenotype of T cells (reviewed in Mora and von Andrian (2006).
ASSOCIATION OF CHEMOKINES WITH HUMAN SEVERE MALARIA SYNDROMES
Several inflammatory chemokines have been found to be associated with severe malaria syndromes in case-control studies (Table 2). Increased levels of CXCL8 and CXCL9 as well as reduced levels of CCL5 (also known as RANTES) have been observed in severe malaria patients (Burgmann et al. 1995; Ochiel et al. 2005; Ayimba et al. 2011; Lopera-Mesa et al. 2012). Moreover, CXCL8 and CXCL9 levels have been shown to correlate with parasite density (Burgmann et al. 1995; Ayimba et al. 2011) suggesting that increased parasitic loads might induce these responses. In support of that concept, in vitro studies demonstrated that P. falciparum-infected erythrocytes are able to induce CCL2, CCL20, CXCL1, CXCL2, CXCL6 and CXCL8 production by human endothelial cells (Viebig et al. 2005; Chakravorty et al. 2007; Tripathi et al. 2009).
Table 2.
Association between chemokines and the outcome of human malaria infections
| Chemokine | Production during infection | Association | Reference |
|---|---|---|---|
| CXCL8 | Increased in the serum and plasma of patients with severe malaria | Serum CXCL8 levels were shown to correlate with parasite density | (Burgmann et al. 1995; Lopera-Messa et al. 2012) |
| Increased in the CSF of children with CM | CSF CXCL8 levels were independently predictive of CM mortality | (Armah et al. 2007; John et al. 2008a, b) | |
| Increased in the placentae of women with PM | Placental CXCL8 levels were associated with placental monocyte infiltration and adverse pregnancy outcomes | (Abrams et al. 2003) | |
| CXCL9 | Increased in the plasma of patients with severe malaria | Serum CXCL9 levels were shown to correlate with parasite density | (Ayimba et al. 2011) |
| Increased in the placentae and peripheral blood of women with PM | Placental CXCL9 levels were negatively associated with low birth weight | (Muehlenbachs et al. 2007; Dong et al. 2012) | |
| CCL5 (RANTES) | Reduced in the serum of patients with severe malaria | Reduced RANTES levels were associated with suppression of erythropoiesis and thrombocytopenia | (Ochiel et al. 2005; Were et al. 2006) |
| Increased mRNA expression in brain samples from CM patients | Not determined | (Sarfo et al. 2004) | |
| CCL4 | Increased in the plasma of children with CM | Not determined | (Jain et al. 2008) |
| Increased in the CSF of children with CM | CSF CCL4 levels were independently predictive of CM mortality | (Armah et al. 2007) | |
| Increased in the placentae of women with PM | Placental plasma CCL4 levels were associated with low birth weight | (Abrams et al. 2003; Muehlenbachs et al. 2007) | |
| CXCL10 | Increased in the serum and plasma of children with CM | Circulating IP-10 levels were independently associated with CM mortality | (Armah et al. 2007; Jain et al. 2008; Wilson et al. 2011) |
| Increased in the CSF of children with CM | Not determined | (Armah et al. 2007) | |
| Increased in the placentae and peripheral blood of women with PM | Not determined | (Muehlenbachs et al. 2007; Boström et al. 2012) | |
| CCL2 | Increased in the placentae and peripheral blood of women with PM | Placental CCL2 levels were associated with placental monocyte infiltration | (Abrams et al. 2003; Dong et al. 2012) |
| CCL3 | Increased in the placentae and peripheral blood of women with PM | Placental and peripheral blood CCL3 levels were associated with placental monocyte infiltration | (Abrams et al. 2003) |
| CXCL13 | Increased in the placentae and peripheral blood of women with PM | Placental CXCL13 levels negatively correlated with low birth weight | (Muehlenbachs et al. 2007; Dong et al. 2012) |
| CXCL16 | Increased in the placentae of women with PM | Not determined | (Muehlenbachs et al. 2007) |
Severe malarial anaemia is one of the most common clinical manifestations of disease in children (Marsh et al. 1995). Both erythropoietic suppression (Kurtzhals et al. 1997) and RBC destruction (Looareesuwan et al. 1991) are thought to contribute to the development of SMA. Reduced circulating levels of CCL5 have been found to be associated with SMA in children (Ochiel et al. 2005; Were et al. 2006). Moreover, reduced CCL5 production was associated with the suppression of erythropoiesis and malaria-induced thrombocytopaenia (Were et al. 2006), suggesting that CCL5 may be involved in the regulation of the erythorpoietic response during malaria infection. It is thought that monocytic-acquisition of P. falciparum hemozoin (pfHz) may in part contribute to CCL5 suppression during SMA, as children with high levels of intramonocytic pfHz have been shown to have lower circulating CCL5 levels than children with only low levels of intramonocytic pfHz (Were et al. 2009). This is further supported by the observation that CCL5 production in IFN-γ-stimulated PBMCs from malaria-infected children decreased with increasing levels of intramonocytic pfHZ (Were et al. 2009).
Although reduced circulating levels of CCL5 have been shown to be associated with CM (John et al. 2006), increased transcription of this chemokine has been observed in brain samples from CM patients (Sarfo et al. 2004). Circulating levels of CCL4 (also known as MIP-1β), CXCL10 (also known as IP-10), CXCL4 and CXCL8 have been found to be significantly elevated in CM cases compared with mild malaria cases or healthy control individuals (John et al. 2006, 2008b; Armah et al. 2007; Jain et al. 2008; Wilson et al. 2011). Amongst these chemokines, CXCL10 appears to be the most accurate predictor of CM mortality (Jain et al. 2008; Wilson et al. 2011). Moreover, cerebrospinal fluid levels of CXCL10, CXCL8 and CCL4 have been found to be significantly higher in children with CM compared with children with SMA and non-malaria-infected individuals (Armah et al. 2007; John et al. 2008a).
CHEMOKINES AND HUMAN PLACENTAL MALARIA
Most of the available information to date on the role of chemokines in the pathogenesis of severe malaria in humans has been obtained from PM studies. During this serious pregnancy complication, parasite sequestration appears to induce secretion of pro-inflammatory cytokines and chemokines as well as the recruitment of macrophages and monocytes to the intervillous space (Rogerson et al. 2003a, b). Consistent with these observations, increased levels of CCL2 (also known as MCP-1), CCL3 (also known as MIP-1α), CCL4, CXCL8, CXCL9, CXCL13 and CXCL16 have been observed in the placentae of women with PM (Abrams et al. 2003; Chaisavaneeyakorn et al. 2003; Muehlenbachs et al. 2007; Dong et al. 2012). Reduced levels of CCL5 and increased levels of CXCL10, CXCL9 and CCL2 have also been observed in the peripheral blood of women with PM (Bostrom et al. 2012; Dong et al. 2012). However, only placental CCL4, CXCL8, CXCL9 and CXCL13 have been associated with adverse pregnancy outcomes such as low birth weight deliveries (Abrams et al. 2003; Muehlenbachs et al. 2007; Dong et al. 2012).
The β-chemokines CCL2, CCL3 and CCL4 are chemo-attractants for monocytes (Rollins, 1997), while CXCL8 is thought to promote monocyte adhesion (Gerszten et al. 1999). Thus, production of these chemokines in the placenta during infection may promote monocyte infiltration of the intervillous space. Consistent with this idea, it has been reported that placental CCL2, CCL3 and CXCL8 levels are associated with placental monocyte infiltration (Abrams et al. 2003). The intravillous infiltrate primarily consists of monocytes and macrophages. Small numbers of B cells, T cells and granulocytes have also been observed in this infiltrate (Ordi et al. 2001). Although it is unclear how these cells migrate to the intervillous space during infection, it is possible that recruitment of B and T cells may be mediated by CXCL13 and CXCL9 respectively.
Both maternal and fetal cells appear to contribute to chemokine production in the placenta during PM. Histopathological analysis of malaria-infected placentae has shown that maternal macrophages and fetal stromal cells contribute to CCL3, CCL4 and CXCL13 production in this organ (Abrams et al. 2003; Muehlenbachs et al. 2007), while CCL2, CCL3, CCL4 and CXCL10 have been found in intervillous blood monocular cells isolated from malaria-infected placentae (Suguitan et al. 2003). In addition, in vitro differentiated syncytiotrophoblasts have been shown to produce CCL3, CCL4 and CXCL8 in response to stimulation with malaria hemozoin (Lucchi et al. 2008, 2011), which is highly abundant in the placenta during PM (Galbraith et al. 1980).
THE ROLE OF CHEMOKINES IN SEVERE DISEASE AND IMMUNITY: LESSONS FROM MOUSE INFECTION MODELS
The initial observations supporting a role for inflammatory chemokines in the aetiology of ECM were provided by gene expression studies. Microarray analysis revealed that the expression of CCR5, CXCR3 and CCR1 and several of their chemokines including CXCL9, CXCL10, CCL2, CCL3 and CCL9 significantly increases in response to infection in CM-affected mice (Sexton et al. 2004; Hansen et al. 2005). CCL5, CCR1, CCR3 and CCR5 mRNA expression was also detected in brains of Swiss Webster mice and appeared to contribute to the inflammatory response that results in cellular degradation in the cerebellum during Plasmodium yoelii (17XL) infection (Sarfo et al. 2005). Further chemokine gene expression studies in brains of ECM-susceptible mice confirmed that CXCL10, CXCL9 and CCL5 and to lesser extent CCL2, CCL7, CCL3, CCL4 and CXCL2 are upregulated during infection with P. berghei ANKA (Hanum et al. 2003; Campanella et al. 2008; Miu et al. 2008; Van den Steen et al. 2008). Interestingly, the expression of CCL2, CCL5, CCL4, CXCL9 and CXCL10 is induced by either IFN-γ or TNF, which is consistent with the important role of these pro-inflammatory cytokines in ECM pathogenesis.
To study the mechanism of leucocyte recruitment to the brain during malaria infection several studies analysed the chemokine receptor usage of brain-sequestered leucocytes from infected mice. CD8+ T cells isolated from the spleen and brain of malaria-infected mice significantly upregulated CCR5, CCR2, CXCR4 and CXCR3 expression (Nitcheu et al. 2003), initially suggesting that trafficking via these chemokine receptors could be involved in the recruitment of inflammatory cells during severe disease. However, further studies indicated that CCR2 deficient mice (Table 3) are fully susceptible to ECM (Belnoue et al. 2003a). Although there has been conflicting evidence on the role of CCR5 in ECM with CCR5−/− mice reported to be either 80% resistant to P. berghei ANKA-mediated CM (Belnoue et al. 2003b) or to display only a delayed onset of cerebral disease symptoms (Nitcheu et al. 2003), mixed-Plasmodium species infection experiments support a role for this trafficking pathway in CM. Plasmodium berghei ANKA-induced CM is inhibited by the co-infection with the non-virulent P. yoelii yoelii 17X clone 1.1 (Table 3). Protection was found to be associated with reduced accumulation of CD8+ T cells in the brain vasculature as well as reduced CCL3, CCL4 and CCL5 levels in the brain (Clark and Phillips, 2011).
Table 3.
Effect of genetic deletion or neutralization of chemokines/chemokine receptors on the outcome of malaria infection in rodent models
| Chemokine/chemokine receptor | Parasite | Mouse strain | Effect on disease | Reference |
|---|---|---|---|---|
| CCR2 | P. berghei-ANKA | CCR2−/− | CCR2−/− mice were fully susceptible to CM | (Belnoue et al. 2003a) |
| P. chabaudi adami | CCR2−/− | No effect | (Weidnaz et al. 2010) | |
| P. chabaudi chabaudi AS | CCR2−/− | Parasite clearance was delayed and monocyte recruitment to the spleen was reduced in CCR2−/− mice | (Sponaas et al. 2009) | |
| CCR5 | P. berghei-ANKA | CCR5−/− | CCR5−/− mice were shown to be 80% resistant to CM | (Belnoue et al. 2003b) |
| P. berghei-ANKA | CCR5−/− | CCR5−/− mice only showed delayed onset of cerebral disease | (Nitcheu et al. 2003) | |
| CCL3 CCL4 CCL5 | P. berghei ANKA/ P. yoelii yoelii 17X clone 1.1 | P. berghei ANKA-mediated CM was inhibited by the co-infection P. yoelii yoelii 17X clone 1.1. Protection was associated with reduced CCL3, CCL4 and CCL5 levels in the brain | (Clark and Phillips, 2011) | |
| CXCL9 | P. berghei-ANKA | CXCL9−/− | CXCL9−/− mice were partially protected from CM | (Campanella et al. 2008) |
| CXCL10 | P. berghei-ANKA | CXCL10−/− | CXCL10−/− mice had reduced cerebral intravascular inflammation and peripheral parasitaemia, and were protected from CM | (Nie et al. 2009) |
| P. berghei-ANKA | C57BL/6 | CXCL10 neutralization during infection reduced cerebral intravascular inflammation and peripheral parasitaemia, and protected mice from CM | (Nie et al. 2009) | |
| P. berghei-ANKA | C57BL/6 | Pharmacological inhibition of CXCL10 in combination with anti-malarial therapy protected mice from CM | (Wilson et al. 2013) | |
| CXCL4 | P. berghei-ANKA | PAF4−/− | PAF4−/− mice were partially protected from CM and showed reduced T cell infiltration into the brain during infection | (Srivastava et al. 2008) |
| CXCR4 | P. chabaudi CR | C57BL/6 | CXCR4 blockade resulted in increased recrudescent parasitaemias | (Garnica et al. 2002) |
Further phenotypic characterization of leucocytes isolated from brain blood vessels of malaria-infected mice by flow cytometry revealed that 80–90% of NK cells and T cells expressed CXCR3, indicating that the expression of this chemokine receptor is strongly associated with lymphocyte trafficking during CM (Hansen et al. 2007). Moreover, in vitro chemotaxis assays revealed that whereas T cells from naive mice were unable to migrate in response to the CXCR3-ligand CXCL10, T cells from P. berghei ANKA-infected mice showed a 3-fold increase in their CXCL10-mediated chemotaxis (Hansen et al. 2007), indicating that during malaria, T cells acquire the capacity to migrate in response to this chemokine. This appears to be a feature specific of CM-susceptible mouse strains, as T cells from malaria-infected CM-resistant BALB/c animals were found to be unable to respond to CXCL10 chemotactic stimulus (Van den Steen et al. 2008). Together, this evidence supports a role for CXCR3-mediated trafficking in the migration of pathogenic T cells to the brain during ECM. In agreement, it has been found that 70–80% of CXCR3−/− mice are resistant to P. berghei-mediated CM (Campanella et al. 2008; Miu et al. 2008).
The relative involvement of the 3 CXCR3 chemokines (CXCL9, CXCL10 and CXCL11) in the recruitment of inflammatory leucocytes to the brain of malaria-infected mice has been extensively investigated (Table 3). As CXCL11 is not normally expressed in CM-susceptible C57BL/6 mice (usually used in ECM experiments) a role for this chemokine in this model is not expected. CXCL9−/− mice were found to be partially protected from P. berghei-ANKA-mediated CM (Campanella et al. 2008). However, whether the increased survival rates to malaria infection in the absence of this chemokine result from reduced leucocyte recruitment to the brain and/or a differential induction of immune response to infection has not been examined.
The role of the CXCR3 chemokine CXCL10 has been studied in more detail. CXCL10−/− mice or mice receiving anti-CXCL10 neutralizing antibodies over the course of P. berghei-ANKA infection were found to have reduced cerebral intravascular inflammation and were protected against fatality (Nie et al. 2009). Moreover, pharmacological inhibition of CXCL10 together with anti-malarial therapy also protects infected animals from CM-mediated mortality (Wilson et al. 2013). Interestingly, CXCL10 blockade during infection resulted in significantly reduced peripheral parasitaemia, suggesting that the absence of this chemokine has a beneficial effect for the development of parasite-specific responses involved in the control of parasite replication (Nie et al. 2009). Since Plasmodium spp. are blood-borne parasites, the spleen constitutes a key site in the initiation of immune responses and control of parasite replication (Looareesuwan et al. 1993; Sayles et al. 1993; Yap and Stevenson, 1994; Chotivanich et al. 2002). Moreover, it has been proposed that the spleen is the site of initial induction of inflammatory cells that migrate to the site of parasite sequestration in target organs (Renia et al. 2006). Consistent with that concept, the increased resistance to infection observed in the absence of CXCL10-mediated cell trafficking was found to be associated with a preferential accumulation and subsequent expansion of parasite-specific CD4+ T cells in spleens of infected animals (Nie et al. 2009). Overall these findings are consistent with two concepts: (1) trafficking pathways might control the balance between pathogenic organ-specific inflammation and spleen-mediated protective immunity and (2) inflammatory processes that occur during infection are not only detrimental for their involvement in severe disease but can also compromise the induction of anti-parasite immunity by inducing leucocyte migration away from the spleen.
The cellular sources responsible for the secretion of CXCR3 chemokines during ECM have not been extensively investigated, with only a few studies focusing primarily on brain tissue. Gene expression analysis (Miu et al. 2008) as well as immunohistochemistry approaches (Campanella et al. 2008) revealed that CXCL9 is predominantly expressed by endothelial cells and surrounding microglial cells. There is conflicting evidence on the cellular sources of CXCL10 during infection. Whereas CXCL10 RNA was found to be upregulated in brain endothelial cells and astrocytes clustering around IP-10 positive microvessels (Miu et al. 2008), immunohistochemistry studies showed CXCL10 staining mainly in neurons throughout the brain parenchyma and only occasionally on endothelial cells, but not on astrocytes (Campanella et al. 2008). Whereas the endothelial and glial localization of CXCL9 and CXCL10 producing cells is consistent with a role for these chemokines in the recruitment of inflammatory leucocytes to the site of parasite sequestration (intravascular infiltration without extravasation into the brain parenchyma), further research is required to determine the effect that CXCL10 upregulation might have in neuronal tissue and the effector signals responsible for this response during infection.
In addition to CXCL9 and CXCL10, the platelet-derived chemokine, platelet factor 4 (PF4 or CXCL4), which binds CXCR3B, a spliced variant of the chemokine receptor CXCR3, was found to be induced in the brain during P. berghei-ANKA infection (Srivastava et al. 2008). Furthermore, PF4−/− mice showed reduced ECM mortality and T cell infiltration into the brain during infection (Srivastava et al. 2008). Thus CXCL4 might contribute to the recruitment of T cells to the brain in ECM pathogenesis. In addition, CXCL4 was also found to enhance monocyte activation in response to P. berghei-ANKA infection (Srivastava et al. 2010). The precise contribution of this response to disease severity requires further investigation.
Chemokines are not only mediators of cell trafficking during inflammation but are also involved in the recruitment of immune cells to secondary lymphoid organs whereby they enhance the development of protective responses to infection. A few studies have addressed the role that chemokines play in the control of parasite densities using rodent malaria infections. CCR2 is a receptor involved in the recruitment of various cells including activated T cells, NK cells, dendritic cells and monocytes. Whereas genetic deficiency of this chemokine does not appear to affect parasite growth during P. berghei-ANKA (Belnoue et al. 2003a) or Plasmodium chabaudi adami infection (Weidanz et al. 2010), parasite clearance was found be delayed in CCR2−/− mice infected with Plasmodium chabaudi chabaudi AS (Sponaas et al. 2009). CCR2 is involved in the recruitment of bone marrow-derived inflammatory monocytes in response to infection (Serbina et al. 2008). The reduced control of parasitaemia observed in CCR2−/− mice correlated with significantly lower numbers of inflammatory monocytes in the spleen of these animals, suggesting these cells play a role in controlling blood-stage malaria. Monocytes as well as dendritic cells are also capable of migrating in response to CXCL12, which recognizes the CXCR4 receptor. CXCR4 blockade during Plasmodium chabaudi CR infection has also been shown to result in increased recrudescent parasitaemias (Garnica et al. 2002), however the precise mechanism by which this trafficking pathway modulates parasite density in this model requires further investigation.
CONCLUDING REMARKS
Over the past few decades, most of the work on soluble factors associated with the development of severe malaria has mainly focused on the analysis of cytokine responses both in human studies and rodent infection models. The role of chemokines in severe disease induction has only recently started to become appreciated and receive more attention. Cytokines are primarily secreted by immune cells and their main function is modulation of immune responses. Unlike cytokines, chemokines are secreted by a broad range of cellular sources in lymphoid organs and/or inflamed tissue and they appear to control a range of diverse processes including leucocyte trafficking, angiogenesis and tissue remodelling. It is then possible that the cellular sources of chemokines produced in response to malaria differ in inflamed tissue and lymphoid organs and that they control different processes. Further research is needed to fully appreciate the contribution of these chemokine-mediated processes to severe malaria and the precise mechanisms by which inflammatory chemokines exacerbate disease.
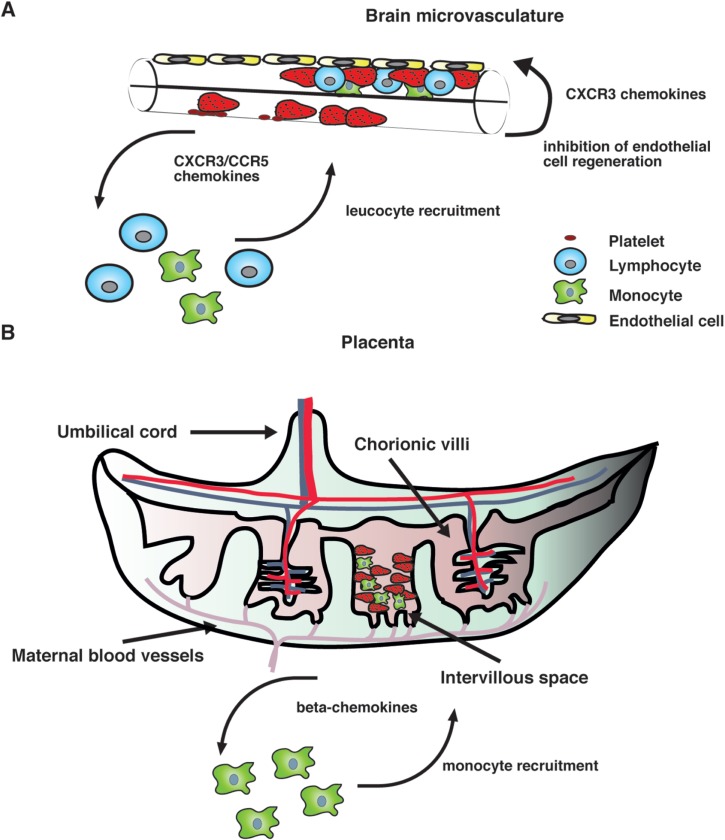
Our current knowledge on human PM supports the idea that the intravillous infiltrate that accompanies parasite sequestration mainly consists of monocytes and macrophages. Consistent with this observation, elevated levels of monocyte-attracting β-chemokines such as CCL2 and CCL3 have been found in infected placentae and their production appears to be associated with cellular infiltration (Abrams et al. 2003), strongly suggesting that chemokine secretion in the placenta during infection recruits monocytes to the intervillous space (Fig. 1).
Fig. 1.
A hypothetical model of action of chemokines in human severe malaria syndromes. (A) After binding to the brain microvasculature, sequestered pRBC induce activation of vascular endothelial cells, which results in the release of inflammatory cytokines as well as CXCR3 and CCR5 binding chemokines. It is possible that local production of these chemokines stimulates the accumulation of CXCR3+ and CCR5+ leucocytes. In addition, some CXCR3 chemokines such as CXCL10 that have angiostatic activity could inhibit endothelial cell regeneration of the brain microvasculature, thereby compromising the integrity of the blood–brain barrier. (B) In the placenta, both maternal and fetal cells might contribute to the production of β-chemokines in response to infection. These mediators stimulate the recruitment of monocytes and macrophages to the intravillous space, which appears to be associated with adverse pregnancy outcomes.
On the other hand, mainly CXCR3 and CCR5 binding chemokines have been found to be associated with CM severity in humans (Armah et al. 2007). Although the precise mechanism by which these chemokines contribute to human disease remains unclear, it is possible that their local production in the brain during infection might stimulate the accumulation of CXCR3+ and CCR5+ leucocytes (Fig. 1). Consistent with this hypothesis, increased CXCR3 and CCR5 mRNA levels have been observed in brain samples from children that have succumbed to P. falciparum-mediated CM (Sarfo et al. 2004). In addition to their chemotactic function, some inflammatory chemokines such as CXCL10 have been shown to exert angiostatic activity (Angiolillo et al. 1995; Luster et al. 1995; Romagnani et al. 2001). It is then possible that locally produced CXCL10 may inhibit endothelial cell regeneration of the brain microvasculature, thereby compromising the integrity of the blood–brain barrier (Fig. 1). In support of this view, the angiogenic factor VEGF has been found to be protective against CM-associated mortality (Armah et al. 2007; Jain et al. 2008).
Although rodent malaria infection models are not perfect, CXCR3 and CCR5 chemokines (such as CXCL10 and CCL4) that were identified as biomarkers of CM in humans (Armah et al. 2007) have also been found to participate in ECM induction in mice (Campanella et al. 2008; Nie et al. 2009). Thus animal models appear to provide a fast and cost-effective resource to investigate chemokine-mediated mechanisms of disease and for assessment of potential therapeutic interventions (Nie et al. 2009; Wilson et al. 2013). Moreover, good mouse models of SMA and PM have been developed (Evans et al. 2006; Neres et al. 2008; Rodrigues-Duarte et al. 2012) and are now available to explore the involvement of chemokines in these disease syndromes.
Current views in the field propose that the same inflammatory responses that contribute to severe malaria might be also involved in the control of parasitaemia. This concept has discouraged the use of anti-cytokine treatments (van Hensbroek et al. 1996) as adjunctive therapy for complicated malaria cases as it might reduce inflammation but also result in immunosuppression, which could be detrimental for the control of infection. Anti-chemokine therapies (Nie et al. 2009) are emerging as potential safe therapeutic alternatives to improve outcomes of severe malaria cases during treatment with anti-malarial drugs, as they alleviate organ-specific inflammation without inducing a generalized immunosuppression of the host.
FINANCIAL SUPPORT
Supported by the Australian Government National Health and Medical Research Council IRIISS and Project Grant 1031212.
REFERENCES
- Abrams E. T., Brown H., Chensue S. W., Turner G. D. H., Tadesse E., Lema V. M., Molyneux M. E., Rochford R., Meshnick S. R. and Rogerson S. J. (2003). Host response to malaria during pregnancy: placental monocyte recruitment is associated with elevated beta chemokine expression. Journal of Immunology 170, 2759–2764 [DOI] [PubMed] [Google Scholar]
- Angiolillo A. L., Sgadari C., Taub D. D., Liao F., Farber J. M., Maheshwari S., Kleinman H. K., Reaman G. H. and Tosato G. (1995). Human interferon-inducible protein-10 is a potent inhibitor of angiogenesis in vivo. Journal of Experimental Medicine 182, 155–162. 10.1084/jem.182.1.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F., Cosmi L., Galli G., Beltrame C., Romagnani P., Manetti R., Romagnani S. and Maggi E. (1999). Assessment of chemokine receptor expression by human Th1 and Th2 cells in vitro and in vivo. Journal of Leukocyte Biology 65, 691–699 [DOI] [PubMed] [Google Scholar]
- Armah H. B., Wilson N. O., Sarfo B. Y., Powell M. D., Bond V. C., Anderson W., Adjei A. A., Gyasi R. K., Tettey Y., Wiredu E. K., Tongren J. E., Udhayakumar V. and Stiles J. K. (2007). Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malaria Journal 6, 147. 10.1186/1475-2875-6-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayimba E., Hegewald J., Segbena A. Y., Gantin R. G., Lechner C. J., Agosssou A., Banla M. and Soboslay P. T. (2011). Proinflammatory and regulatory cytokines and chemokines in infants with uncomplicated and severe Plasmodium falciparum malaria. Clinical and Experimental Immunology 166, 218–226. 10.1111/j.1365-2249.2011.04474.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belnoue E., Kayibanda M., Vigario A. M., Deschemin J. C., van Rooijen N., Viguier M., Snounou G. and Renia L. (2002). On the pathogenic role of brain-sequestered αβ CD8+ T cells in experimental cerebral malaria. Journal of Immunology 169, 6369–6375 [DOI] [PubMed] [Google Scholar]
- Belnoue E., Costa F. T., Vigario A. M., Voza T., Gonnet F., Landau I., Van Rooijen N., Mack M., Kuziel W. A. and Renia L. (2003a). Chemokine receptor CCR2 is not essential for the development of experimental cerebral malaria. Infection and Immunity 71, 3648–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belnoue E., Kayibanda M., Deschemin J. C., Viguier M., Mack M., Kuziel W. A. and Renia L. (2003b). CCR5 deficiency decreases susceptibility to experimental cerebral malaria. Blood 101, 4253–4259 [DOI] [PubMed] [Google Scholar]
- Boström S., Ibitokou S., Oesterholt M., Schmiegelow C., Persson J.-O., Minja D., Lusingu J., Lemnge M., Fievet N., Deloron P., Luty A. J. F. and Troye-Blomberg M. (2012). Biomarkers of Plasmodium falciparum infection during pregnancy in women living in Northeastern Tanzania. PLoS ONE 7, e48763. 10.1371/journal.pone.0048763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley S. K., Mempel T. R. and Luster A. D. (2008). Orchestrating the orchestrators: chemokines in control of T cell traffic. Nature Immunology 9, 970–980. 10.1038/ni.f.213 [DOI] [PubMed] [Google Scholar]
- Burgmann H., Hollenstein U., Wenisch C., Thalhammer F., Looareesuwan S. and Graninger W. (1995). Serum concentrations of MIP-1α and IL-8 in patients suffering from acute Plasmodium falciparum malaria. Clinical Immunology and Immunopathology 76, 32–36. 10.1006/clin.1995.1084 [DOI] [PubMed] [Google Scholar]
- Campanella G. S., Tager A. M., El Khoury J. K., Thomas S. Y., Abrazinski T. A., Manice L. A., Colvin R. A. and Luster A. D. (2008). Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proceedings of the National Academy of Sciences USA 105, 4814–4819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisavaneeyakorn S., Moore J. M., Mirel L., Othoro C., Otieno J., Chaiyaroj S. C., Shi Y. P., Nahlen B. L., Lal A. A. and Udhayakumar V. (2003). Levels of macrophage inflammatory protein 1α (MIP-1α) and MIP-1β in intervillous blood plasma samples from women with placental malaria and human immunodeficiency virus infection. Clinical and Diagnostic Laboratory Immunology 10, 631–636. 10.1128/cdli.10.4.631-636.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty S. J., Carret C., Nash G. B., Ivens A., Szestak T. and Craig A. G. (2007). Altered phenotype and gene transcription in endothelial cells, induced by Plasmodium falciparum-infected red blood cells: pathogenic or protective? International Journal for Parasitology 37, 975–987. 10.1016/j.ijpara.2007.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotivanich K., Udomsangpetch R., McGready R., Proux S., Newton P., Pukrittayakamee S., Looareesuwan S. and White N. J. (2002). Central role of the spleen in malaria parasite clearance. Journal of Infectious Diseases 185, 1538–1541 [DOI] [PubMed] [Google Scholar]
- Clark C. J. and Phillips R. S. (2011). Cerebral malaria protection in mice by species-specific Plasmodium co-infection is associated with reduced CC chemokine levels in the brain. Parasite Immunology 33, 637–641. 10.1111/j.1365-3024.2011.01329.x [DOI] [PubMed] [Google Scholar]
- Coltel N., Combes V., Hunt N. H. and Grau G. E. (2004). Cerebral malaria – a neurovascular pathology with many riddles still to be solved. Current Neurovascular Research 1, 91–110. 10.2174/1567202043480116 [DOI] [PubMed] [Google Scholar]
- Desai M., ter Kuile F. O., Nosten F., McGready R., Asamoa K., Brabin B. and Newman R. D. (2007). Epidemiology and burden of malaria in pregnancy. Lancet Infectious Diseases 7, 93–104. 10.1016/s1473-3099(07)70021-x [DOI] [PubMed] [Google Scholar]
- Dong S., Kurtis J. D., Pond-Tor S., Kabyemela E., Duffy P. E. and Fried M. (2012). CXCL9 response to malaria during pregnancy is associated with low-birth-weight deliveries. Infection and Immunity 80, 3034–3038. 10.1128/iai.00220-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Assaad F., Wheway J., Mitchell A. J., Lou J., Hunt N. H., Combes V. and Grau G. E. (2013). Cytoadherence of Plasmodium berghei-infected red blood cells to murine brain and lung microvascular endothelial cells in vitro. Infection and Immunity. 10.1128/iai.00428-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engwerda C. R., Mynott T. L., Sawhney S., De Souza J. B., Bickle Q. D. and Kaye P. M. (2002). Locally up-regulated lymphotoxin α, not systemic tumor necrosis factor α, is the principle mediator of murine cerebral malaria. Journal of Experimental Medicine 195, 1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans K. J., Hansen D. S., van Rooijen N., Buckingham L. A. and Schofield L. (2006). Severe malarial anemia of low parasite burden in rodent models results from accelerated clearance of uninfected erythrocytes. Blood 107, 1192–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith R. M., Faulk W. P., Galbraith G. M. P., Holbrook T. W. and Bray R. S. (1980). Human materno-foetal relationship in malaria. 1. Identification of pigment and parasites in the placenta. Transactions of the Royal Society of Tropical Medicine and Hygiene 74, 52–60. 10.1016/0035-9203(80)90011-5 [DOI] [PubMed] [Google Scholar]
- Garnica M. R., Souto J. T., Silva J. S. and de Andrade H. F. Jr. (2002). Stromal cell derived factor 1 synthesis by spleen cells in rodent malaria, and the effects of in vivo supplementation of SDF-1α and CXCR4 receptor blocker. Immunology Letters 83, 47–53 [DOI] [PubMed] [Google Scholar]
- Gerszten R. E., Garcia-Zepeda E. A., Lim Y. C., Yoshida M., Ding H. A., Gimbrone M. A., Luster A. D., Luscinskas F. W. and Rosenzweig A. (1999). MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 398, 718–723 [DOI] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Engers H. D., Louis J. A., Vassalli P. and Lambert P. H. (1986). L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. Journal of Immunology 137, 2348–2354 [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H. and Vassalli P. (1987). Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 237, 1210–1212 [DOI] [PubMed] [Google Scholar]
- Grau G. E., Heremans H., Piguet P. F., Pointaire P., Lambert P. H., Billiau A. and Vassalli P. (1989). Monoclonal antibody against interferon γ can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proceedings of the National Academy of Sciences USA 86, 5572–5574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Tacchini-Cottier F., Vesin C., Milon G., Lou J. N., Piguet P. F. and Juillard P. (1993). TNF-induced microvascular pathology: active role for platelets and importance of the LFA-1/ICAM-1 interaction. European Cytokine Network 4, 415–419 [PubMed] [Google Scholar]
- Grau G. E., Mackenzie C. D., Carr R. A., Redard M., Pizzolato G., Allasia C., Cataldo C., Taylor T. E. and Molyneux M. E. (2003). Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. Journal of Infectious Diseases 187, 461–466 [DOI] [PubMed] [Google Scholar]
- Hansen D. S. (2012). Inflammatory responses associated with the induction of cerebral malaria: lessons from experimental murine models. PLoS Pathogens 8, e1003045. 10.1371/journal.ppat.1003045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D. S., Siomos M. A., Buckingham L., Scalzo A. A. and Schofield L. (2003). Regulation of murine cerebral malaria pathogenesis by CD1d-restricted NKT cells and the natural killer complex. Immunity 18, 391–402 [DOI] [PubMed] [Google Scholar]
- Hansen D. S., Evans K. J., D'Ombrain M. C., Bernard N. J., Sexton A. C., Buckingham L., Scalzo A. A. and Schofield L. (2005). The natural killer complex regulates severe malarial pathogenesis and influences acquired immune responses to Plasmodium berghei-ANKA. Infection and Immunity 73, 2288–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D. S., Bernard N. J., Nie C. Q. and Schofield L. (2007). NK cells stimulate recruitment of CXCR3+ T cells to the brain during Plasmodium berghei-mediated cerebral malaria. Journal of Immunology 178, 5779–5788 [DOI] [PubMed] [Google Scholar]
- Hanum P. S., Hayano M. and Kojima S. (2003). Cytokine and chemokine responses in a cerebral malaria-susceptible or -resistant strain of mice to Plasmodium berghei-ANKA infection: early chemokine expression in the brain. International Immunology 15, 633–640 [DOI] [PubMed] [Google Scholar]
- Hunt N. H. and Grau G. E. (2003). Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends in Immunology 24, 491–499 [DOI] [PubMed] [Google Scholar]
- Jain V., Armah H. B., Tongren J. E., Ned R. M., Wilson N. O., Crawford S., Joel P. K., Singh M. P., Nagpal A. C., Dash A. P., Udhayakumar V., Singh N. and Stiles J. K. (2008). Plasma IP-10, apoptotic and angiogenic factors associated with fatal cerebral malaria in India. Malaria Journal 7, 83. 10.1186/1475-2875-7-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John C. C., Opika-Opoka R., Byarugaba J., Idro R. and Boivin M. J. (2006). Low levels of RANTES are associated with mortality in children with cerebral malaria. Journal of Infectious Diseases 194, 837–845. 10.1086/506623 [DOI] [PubMed] [Google Scholar]
- John C. C., Panoskaltsis-Mortari A., Opoka R. O., Park G. S., Orchard P. J., Jurek A. M., Idro R., Byarugaba J. and Boivin M. J. (2008a). Cerebrospinal fluid cytokine levels and cognitive impairment in cerebral malaria. American Journal of Tropical Medicine and Hygiene 78, 198–205 [PMC free article] [PubMed] [Google Scholar]
- John C. C., Park G. S., Sam-Agudu N., Opoka R. O. and Bolvin M. J. (2008b). Elevated serum levels of IL-1rα in children with Plasmodium falciparum malaria are associated with increased severity of disease. Cytokine 41, 204–208. 10.1016/j.cyto.2007.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. H., Rott L., Kunkel E. J., Genovese M. C., Andrew D. P., Wu L. J. and Butcher E. C. (2001). Rules of chemokine receptor association with T cell polarization in vivo. Journal of Clinical Investigation 108, 1331–1339. 10.1172/jci13543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzhals J. A. L., Rodrigues O., Addae M., Commey J. O. O., Nkrumah F. K. and Hviid L. (1997). Reversible suppression of bone marrow response to erythropoietin in Plasmodium falciparum malaria. British Journal of Haematology 97, 169–174. 10.1046/j.1365-2141.1997.82654.x [DOI] [PubMed] [Google Scholar]
- Looareesuwan S., Davis T. M. E., Pukrittayakamee S., Supanaranond W., Desakorn V., Silamut K., Krishna S., Boonamrung S. and White N. J. (1991). Erythrocyte survival in severe falciparum malaria. Acta Tropica 48, 263–270. 10.1016/0001-706x(91)90014-b [DOI] [PubMed] [Google Scholar]
- Looareesuwan S., Suntharasamai P., Webster H. K. and Ho M. (1993). Malaria in splenectomized patients: report of four cases and review. Clinical Infectious Diseases 16, 361–366 [DOI] [PubMed] [Google Scholar]
- Lopera-Mesa T. M., Mita-Mendoza N. K., van de Hoef D. L., Doumbia S., Konate D., Doumbouya M., Gu W., Traore K., Diakite S. A. S., Remaley A. T., Anderson J. M., Rodriguez A., Fay M. P., Long C. A., Diakite M. and Fairhurst R. M. (2012). Plasma uric acid levels correlate with inflammation and disease severity in Malian children with Plasmodium falciparum malaria. PLoS ONE 7, e46424. 10.1371/journal.pone.0046424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchi N. W., Peterson D. S. and Moore J. M. (2008). Immunologic activation of human syncytiotrophoblast by Plasmodium falciparum. Malaria Journal 7, 42–49. 10.1186/1475-2875-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchi N. W., Sarr A., Owino S. O., Mwalimu S. M., Peterson D. S. and Moore J. M. (2011). Natural hemozoin stimulates syncytiotrophoblast to secrete chemokines and recruit peripheral blood mononuclear cells. Placenta 32, 579–585. 10.1016/j.placenta.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundie R. J., de Koning-Ward T. F., Davey G. M., Nie C. Q., Hansen D. S., Lau L. S., Mintern J. D., Belz G. T., Schofield L., Carbone F. R., Villadangos J. A., Crabb B. S. and Heath W. R. (2008). Blood-stage Plasmodium infection induces CD8+ T lymphocytes to parasite-expressed antigens, largely regulated by CD8α+ dendritic cells. Proceedings of the National Academy of Sciences USA 105, 14509–14514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luster A. D. (1998). Chemokines – chemotactic cytokines that mediate inflammation. New England Journal of Medicine 338, 436–445 [DOI] [PubMed] [Google Scholar]
- Luster A. D., Greenberg S. M. and Leder P. (1995). The IP-10 chemokine binds to a specific cell-surface heparan-sulfate site shared with platelet factor-4 and inhibits endothelial cell proliferation. Journal of Experimental Medicine 182, 219–231. 10.1084/jem.182.1.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N. L., Hunt N. H., Madigan M. C. and ChanLing T. (1996). Correlation between enhanced vascular permeability, up-regulation of cellular adhesion molecules and monocyte adhesion to the endothelium in the retina during the development of fatal murine cerebral malaria. American Journal of Pathology 149, 1745–1762 [PMC free article] [PubMed] [Google Scholar]
- Marsh K., Forster D., Waruiru C., Mwangi I., Winstanley M., Marsh V., Newton C., Winstanley P., Warn P., Peshu N., Pasvol G. and Snow R. (1995). Indicators of life-threatening malaria in African children. New England Journal of Medicine 332, 1399–1404. 10.1056/nejm199505253322102 [DOI] [PubMed] [Google Scholar]
- McGregor I. A. (1984). Epidemiology, malaria and pregnancy. American Journal of Tropical Medicine and Hygiene 33, 517–525 [DOI] [PubMed] [Google Scholar]
- Miller L. H., Baruch D. I., Marsh K. and Doumbo O. K. (2002). The pathogenic basis of malaria. Nature 415, 673–679 [DOI] [PubMed] [Google Scholar]
- Miu J., Mitchell A. J., Muller M., Carter S. L., Manders P. M., McQuillan J. A., Saunders B. M., Ball H. J., Lu B., Campbell I. L. and Hunt N. H. (2008). Chemokine gene expression during fatal murine cerebral malaria and protection due to CXCR3 deficiency. Journal of Immunology 180, 1217–1230 [DOI] [PubMed] [Google Scholar]
- Molyneux M. E., Engelmann H., Taylor T. E., Wirima J. J., Aderka D., Wallach D. and Grau G. E. (1993). Circulating plasma receptors for tumour necrosis factor in Malawian children with severe falciparum malaria. Cytokine 5, 604–609 [DOI] [PubMed] [Google Scholar]
- Mora J. R. and von Andrian U. H. (2006). T-cell homing specificity and plasticity: new concepts and future challenges. Trends in Immunology 27, 235–243. 10.1016/j.it.2006.03.007 [DOI] [PubMed] [Google Scholar]
- Moser B., Wolf M., Walz A. and Loetscher P. (2004). Chemokines: multiple levels of leukocyte migration control. Trends in Immunology 25, 75–84. 10.1016/j.it.2003.12.005 [DOI] [PubMed] [Google Scholar]
- Muehlenbachs A., Fried M., Lachowitzer J., Mutabingwa T. K. and Duffy P. E. (2007). Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. Journal of Immunology 179, 557–565 [DOI] [PubMed] [Google Scholar]
- Murphy P. M. (2002). International Union of Pharmacology. Update on chemokine receptor nomenclature. Pharmacological Reviews 54, 227–229. 10.1124/pr.54.2.227 [DOI] [PubMed] [Google Scholar]
- Murray C. J. L., Rosenfeld L. C., Lim S. S., Andrews K. G., Foreman K. J., Haring D., Fullman N., Naghavi M., Lozano R. and Lopez A. D. (2012). Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379, 413–431 [DOI] [PubMed] [Google Scholar]
- Neres R., Marinho C. R. F., Goncalves L. A., Catarino M. B. and Penha-Goncalves C. (2008). Pregnancy outcome and placenta pathology in Plasmodium berghei-ANKA-infected mice reproduce the pathogenesis of severe malaria in pregnant women. PLoS ONE 3, e1608. 10.1371/journal.pone.0001608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie C. Q., Bernard N. J., Norman M. U., Amante F. H., Lundie R. J., Crabb B. S., Heath W. R., Engwerda C. R., Hickey M. J., Schofield L. and Hansen D. S. (2009). IP-10-mediated T cell homing promotes cerebral inflammation over splenic immunity to malaria infection. PLoS Pathogens 5, e1000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitcheu J., Bonduelle O., Combadiere C., Tefit M., Seilhean D., Mazier D. and Combadiere B. (2003). Perforin-dependent brain-infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. Journal of Immunology 170, 2221–2228 [DOI] [PubMed] [Google Scholar]
- Ochiel D. O., Awandare G. A., Keller C. C., Hittner J. B., Kremsner P. G., Weinberg J. B. and Perkins D. J. (2005). Differential regulation of beta-chemokines in children with Plasmodium falciparum malaria. Infection and Immunity 73, 4190–4197. 73/7/4190 10.1128/IAI.73.7.4190-4197.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordi J., Ismail M. R., Ventura P. J., Kahigwa E., Hirt R., Cardesa A., Alonso P. L. and Menendez C. (1998). Massive chronic intervillositis of the placenta associated with malaria infection. American Journal of Surgical Pathology 22, 1006–1011. 10.1097/00000478-199808000-00011 [DOI] [PubMed] [Google Scholar]
- Ordi J., Menendez C., Ismail M. R., Ventura P. J., Palacin A., Kahigwa E., Ferrer B., Cardesa A. and Alonso P. L. (2001). Placental malaria is associated with cell-mediated inflammatory responses with selective absence of natural killer cells. Journal of Infectious Diseases 183, 1100–1107. 10.1086/319295 [DOI] [PubMed] [Google Scholar]
- Patnaik J. K., Das B. S., Mishra S. K., Mohanty S., Satpathy S. K. and Mohanty D. (1994). Vascular clogging, mononuclear cell margination, and enhanced vascular-permeability in the pathogenesiss of human cerebral malaria. American Journal of Tropical Medicine and Hygiene 51, 642–647 [PubMed] [Google Scholar]
- Pongponratn E., Turner G. D., Day N. P., Phu N. H., Simpson J. A., Stepniewska K., Mai N. T., Viriyavejakul P., Looareesuwan S., Hien T. T., Ferguson D. J. and White N. J. (2003). An ultrastructural study of the brain in fatal Plasmodium falciparum malaria. American Journal of Tropical Medicine and Hygiene 69, 345–359 [PubMed] [Google Scholar]
- Porta J., Carota A., Pizzolato G. P., Wildi E., Widmer M. C., Margairaz C. and Grau G. E. (1993). Immunopathological changes in human cerebral malaria. Clinical Neuropathology 12, 142–146 [PubMed] [Google Scholar]
- Renia L., Potter S. M., Mauduit M., Rosa D. S., Kayibanda M., Deschemin J. C., Snounou G. and Gruner A. C. (2006). Pathogenic T cells in cerebral malaria. International Journal for Parasitology 36, 547–554 [DOI] [PubMed] [Google Scholar]
- Rodrigues-Duarte L., de Moraes L. V., Barboza R., Marinho C. R. F., Franke-Fayard B., Janse C. J. and Penha-Goncalves C. (2012). Distinct placental malaria pathology caused by different Plasmodium berghei lines that fail to induce cerebral malaria in the C57BL/6 mouse. Malaria Journal 11, 231. 10.1186/1475-2875-11-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson S. J., Brown H. C., Pollina E., Abrams E. T., Tadesse E., Lema V. M. and Molyneux M. E. (2003a). Placental tumor necrosis factor α but not γ interferon is associated with placental malaria and low birth weight in Malawian women. Infection and Immunity 71, 267–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogerson S. J., Pollina E., Getachew A., Tadesse E., Lema V. M. and Molyneux M. E. (2003b). Placental monocyte infiltrates in response to Plasmodium falciparum malaria infection and their association with adverse pregnancy outcomes. American Journal of Tropical Medicine and Hygiene 68, 115–119 [PubMed] [Google Scholar]
- Rollins B. J. (1997). Chemokines. Blood 90, 909–928 [PubMed] [Google Scholar]
- Romagnani P., Annunziato F., Lasagni L., Lazzeri E., Beltrame C., Francalanci M., Uguccioni M., Galli G., Cosmi L., Maurenzig L., Baggiolini M., Maggi E., Romagnani S. and Serio M. (2001). Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. Journal of Clinical Investigations 107, 53–63. 10.1172/jci9775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot A. (1992). Endothelial cell binding of NAP-1/IL-8 – role in neutrophil emigration. Immunology Today 13, 291–294. 10.1016/0167-5699(92)90039-a [DOI] [PubMed] [Google Scholar]
- Rot A. and von Andrian U. H. (2004). Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annual Review of Immunology 22, 891–928. 10.1146/annurev.immunol.22.012703.104543 [DOI] [PubMed] [Google Scholar]
- Ryg-Cornejo V., Nie C. Q., Bernard N. J., Lundie R. J., Evans K. J., Crabb B. S., Schofield L. and Hansen D. S. (2013). NK cells and conventional dendritic cells engage in reciprocal activation for the induction of inflammatory responses during Plasmodium berghei-ANKA infection. Immunobiology 218, 263–271. 10.1016/j.imbio.2012.05.018 [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. and Mackay C. R. (1998). Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunology Today 19, 568–574 [DOI] [PubMed] [Google Scholar]
- Sallusto F., Mackay C. R. and Lanzavecchia A. (2000). The role of chemokine receptors in primary, effector, and memory immune responses. Annual Review of Immunology 18, 593–620. 10.1146/annurev.immunol.18.1.593 [DOI] [PubMed] [Google Scholar]
- Sarfo B. Y., Singh S., Lillard J. W., Quarshie A., Gyasi R. K., Armah H., Adjei A. A., Jolly P. and Stiles J. K. (2004). The cerebral-malaria-associated expression of RANTES, CCR3 and CCR5 in post-mortem tissue samples. Annals of Tropical Medicine and Parasitology 98, 297–303 [DOI] [PubMed] [Google Scholar]
- Sarfo B. Y., Armah H. B., Irune I., Adjei A. A., Olver C. S., Singh S., Lillard J. W. Jr. and Stiles J. K. (2005). Plasmodium yoelii 17XL infection up-regulates RANTES, CCR1, CCR3 and CCR5 expression, and induces ultrastructural changes in the cerebellum. Malaria Journal 4, 63. 10.1186/1475-2875-4-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayles P. C., Yanez D. M. and Wassom D. L. (1993). Plasmodium yoelii: splenectomy alters the antibody responses of infected mice. Experimental Parasitology 76, 377–384 [DOI] [PubMed] [Google Scholar]
- Schofield L. and Grau G. E. (2005). Immunological processes in malaria pathogenesis. Nature Reviews Immunology 5, 722–735 [DOI] [PubMed] [Google Scholar]
- Serbina N. V., Jia T., Hohl T. M. and Pamer E. G. (2008). Monocyte-mediated defense against microbial pathogens. Annual Reviews Immunology 26, 421–452. 10.1146/annurev.immunol.26.021607.090326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton A. C., Good R. T., Hansen D. S., D'Ombrain M. C., Buckingham L., Simpson K. and Schofield L. (2004). Transcriptional profiling reveals suppressed erythropoiesis, up-regulated glycolysis, and interferon-associated responses in murine malaria. Journal of Infectious Diseases 189, 1245–1256 [DOI] [PubMed] [Google Scholar]
- Sponaas A. M., Freitas do Rosario A. P., Voisine C., Mastelic B., Thompson J., Koernig S., Jarra W., Renia L., Mauduit M., Potocnik A. J. and Langhorne J. (2009). Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria. Blood 114, 5522–5531. 10.1182/blood-2009-04-217489 [DOI] [PubMed] [Google Scholar]
- Srivastava K., Cockburn I. A., Swaim A., Thompson L. E., Tripathi A., Fletcher C. A., Shirk E. M., Sun H., Kowalska M. A., Fox-Talbot K., Sullivan D., Zavala F. and Morrell C. N. (2008). Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe 4, 179–187. 10.1016/j.chom.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava K., Field D. J., Aggrey A., Yamakuchi M. and Morrell C. N. (2010). Platelet factor 4 regulation of monocyte KLF4 in experimental cerebral malaria. PLoS ONE 5, e10413. 10.1371/journal.pone.0010413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee R. W., Nahlen B. L., Parise M. E. and Menendez C. (2001). The burden of malaria in pregnancy in malaria-endemic areas. American Journal of Tropical Medicine and Hygiene 64, 28–35 [DOI] [PubMed] [Google Scholar]
- Suguitan A. L., Leke R. G. F., Fouda G., Zhou A. N., Thuita L., Metenou S., Fogako J., Megnekou R. and Taylor D. W. (2003). Changes in the levels of chemokines and cytokines in the placentas of women with Plasmodium falciparum malaria. Journal of Infectious Diseases 188, 1074–1082 [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Adams D. H. and Shaw S. (1993). Proteoglycans on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunology Today 14, 111–115. 10.1016/0167-5699(93)90209-4 [DOI] [PubMed] [Google Scholar]
- Taylor T. E., Fu W. J., Carr R. A., Whitten R. O., Mueller J. S., Fosiko N. G., Lewallen S., Liomba N. G. and Molyneux M. E. (2004). Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nature Medicine 10, 143–145 [DOI] [PubMed] [Google Scholar]
- Tripathi A. K., Sha W., Shulaev V., Stins M. F. and Sullivan D. J. Jr. (2009). Plasmodium falciparum-infected erythrocytes induce NF-κB regulated inflammatory pathways in human cerebral endothelium. Blood 114, 4243–4252. 10.1182/blood-2009-06-226415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Steen P. E., Deroost K., Aelst I. V., Geurts N., Martens E., Struyf S., Nie C. Q., Hansen D. S., Matthys P., Damme J. V. and Opdenakker G. (2008). CXCR3 determines strain susceptibility to murine cerebral malaria by mediating T lymphocyte migration toward IFN-γ-induced chemokines. European Journal of Immunology 38, 1082–1095 [DOI] [PubMed] [Google Scholar]
- van Hensbroek M. B., Palmer A., Onyiorah E., Schneider G., Jaffar S., Dolan G., Memming H., Frenkel J., Enwere G., Bennett S., Kwiatkowski D. and Greenwood B. (1996). The effect of a monoclonal antibody to tumor necrosis factor on survival from childhood cerebral malaria. Journal of Infectious Diseases 174, 1091–1097 [DOI] [PubMed] [Google Scholar]
- Viebig N. K., Wulbrand U., Forster R., Andrews K. T., Lanzer M. and Knolle P. A. (2005). Direct activation of human endothelial cells by Plasmodium falciparum-infected erythrocytes. Infection and Immunity 73, 3271–3277. 10.1128/iai.73.6.3271-3277.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Mendez A., Greig R., Shaw T. N., de Souza J. B., Gwyer Findlay E., Stumhofer J. S., Hafalla J. C., Blount D. G., Hunter C. A., Riley E. M. and Couper K. N. (2012). IFN-γ-producing CD4+ T cells promote experimental cerebral malaria by modulating CD8+ T cell accumulation within the brain. Journal of Immunology 189, 968–979. 10.4049/jimmunol.1200688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidanz W. P., LaFleur G., Brown A., Burns J. M. Jr., Gramaglia I. and van der Heyde H. C. (2010). γδ T cells but not NK cells are essential for cell-mediated immunity against Plasmodium chabaudi malaria. Infection and Immunity 78, 4331–4340. 10.1128/iai.00539-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weninger W., Crowley M. A., Manjunath N. and von Andrian U. H. (2001). Migratory properties of naive, effector, and memory CD8+ T cells. Journal of Experimental Medicine 194, 953–966. 10.1084/jem.194.7.953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Were T., Ouma C., Otieno R. O., Orago A. S. S., Ong'echa J. M., Vulule J. M., Keller C. C. and Perkins D. J. (2006). Suppression of RANTES in children with Plasmodium falciparum malaria. Haematologica – the Hematology Journal 91, 1396–1399 [PubMed] [Google Scholar]
- Were T., Davenport G. C., Yamo E. O., Hittner J. B., Awandare G. A., Otieno M. F., Ouma C., Orago A. S. S., Vulule J. M., Ong'echa J. M. and Perkins D. J. (2009). Naturally acquired hemozoin by monocytes promotes suppression of RANTES in children with malarial anemia through an IL-10-dependent mechanism. Microbes and Infection 11, 811–819. 10.1016/j.micinf.2009.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White N. J. and Ho M. (1992). The pathophysiology of malaria. Advances in Parasitology 31, 83–173 [DOI] [PubMed] [Google Scholar]
- Wilson N. O., Jain V., Roberts C. E., Lucchi N., Joel P. K., Singh M. P., Nagpal A. C., Dash A. P., Udhayakumar V., Singh N. and Stiles J. K. (2011). CXCL4 and CXCL10 predict risk of fatal cerebral malaria. Disease Markers 30, 39–49. 10.3233/dma-2011-0763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N. O., Solomon W., Anderson L., Patrickson J., Pitts S., Bond V., Liu M. and Stiles J. K. (2013). Pharmacologic inhibition of CXCL10 in combination with anti-malarial therapy eliminates mortality associated with murine model of cerebral malaria. PLoS ONE 8, e60898. 10.1371/journal.pone.0060898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H. J., Lim Y. C., Luscinskas F. W. and Lichtman A. H. (1999). Acquisition of selectin binding and peripheral homing properties by CD4+ and CD8+ T cells. Journal of Experimental Medicine 189, 1765–1775. 10.1084/jem.189.11.1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez D. M., Manning D. D., Cooley A. J., Weidanz W. P. and van der Heyde H. C. (1996). Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. Journal of Immunology 157, 1620–1624 [PubMed] [Google Scholar]
- Yap G. S. and Stevenson M. M. (1994). Differential requirements for an intact spleen in induction and expression of B-cell-dependent immunity to Plasmodium chabaudi AS. Infection and Immunity 62, 4219–4225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshie O., Imai T. and Nomiyama H. (1997). Novel lymphocyte-specific CC chemokines and their receptors. Journal of Leukocyte Biology 62, 634–644 [DOI] [PubMed] [Google Scholar]